Abstract
Patients with non-alcoholic steatohepatitis (NASH) show significantly faster progress in the stages of fibrosis compared to those with non-alcoholic fatty liver (NAFL) disease. The non-invasive diagnosis of NASH remains an unmet clinical need. Preliminary data have shown that sphingolipids, especially ceramides, fatty acids, and other lipid classes may be related to the presence of NASH and the histological activity of the disease. The aim of our study was to assess the association of certain plasma lipid classes, such as fatty acids, acylcarnitines, and ceramides, with the histopathological findings in patients with NASH. The study included three groups: patients with NASH (N = 12), NAFL (N = 10), and healthy [non non-alcoholic fatty liver disease (NAFLD)] controls (N = 15). Plasma samples were collected after 12 h of fasting, and targeted analyses for fatty acids, acylcarnitines, and ceramides were performed. Baseline clinical and demographic characteristics were collected. There was no significant difference in baseline characteristics across the three groups or between NAFL and NASH patients. Patients with NASH had increased levels of several fatty acids, including, among others, fatty acid (FA) 14:0, FA 15:0, FA 18:0, FA 18:3n3, as well as Cer(d18:1/16:0), compared to NAFL patients and healthy controls. No significant difference was found between NAFL patients and healthy controls. In conclusion, patients with NASH exhibited a distinctive plasma lipid profile that can differentiate them from NAFL patients and non-NAFLD populations. More data from larger cohorts are needed to validate these findings and examine possible implications for diagnostic and management strategies of the disease.
Keywords: plasma lipids, acylcarnitines, fatty acids, ceramides, non-alcoholic steatohepatitis, non-alcoholic fatty liver disease, diagnostic markers, non-invasive diagnosis
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is considered a modern pandemic affecting more than 25% of the general population worldwide [1]. Its prevalence is constantly rising in parallel with the prevalence of οbesity and metabolic syndrome (MetS). NAFLD is considered the hepatic component of the latter. The spectrum of the disease includes non-alcoholic fatty liver (NAFL), which is characterized by steatosis with or without mild inflammation, and the more progressive non-alcoholic steatohepatitis (NASH), which is characterized by the coexistence of steatosis, inflammation, and hepatocellular ballooning [1,2] with or without fibrosis.
Regarding the prognosis of the disease, the most important prognostic indicator is the presence of advanced fibrosis. Steatohepatitis seems to be an important driver for the progression of more advanced stages of fibrosis. It has been demonstrated that patients with NAFL progress by one stage of fibrosis on average every 14 years, while patients with NASH show faster disease progression of one fibrosis stage every 7 years. It is evident that the early diagnosis of NASH is clinically important, as it can prompt early interventions in the disease course, leading to a subsequent reduction in morbidity and mortality in a significant part of the general population [3,4]. Even though there are reliable non-invasive modalities to assess liver fibrosis, there are currently no non-invasive modalities to assess for the presence and grade of NASH. Transient elastography and several non-invasive serological tests (NITs), such as FIB-4 and NAFLD fibrosis score, can rule out or rule in clinically significant liver fibrosis with high sensitivity and specificity [5,6]. On the other hand, the diagnosis of NASH can only be made histologically with a liver biopsy. The limitations of liver biopsy include increased costs (due to the need for hospitalization and follow-up) and a small risk of life-threatening complications. It is evident that there is an increasing need for a reliable non-invasive diagnostic biomarker for NASH.
It has been recently proposed that plasma lipids may have a role in the non-invasive diagnosis of NASH [7,8]. Recent studies found notable alterations in lipid composition in liver biopsies of patients with NAFLD (such as phospholipids, fatty acids, and sphingolipids), indicating that disturbances in the metabolism of specific lipid species could be a potential contributor to the pathogenesis of NAFLD/NASH. Puri et al. [7] assessed hepatic lipid composition in 27 liver biopsies and found that NAFLD is associated with alterations in the hepatic lipid profile. Moreover, changes in the circulating plasma lipid species might also be associated with disease progression. Apostolopoulou et al. [9] conducted a prospective study including bariatric patients. They analysed liver, serum, and adipose tissue ceramides and found that sphingolipid levels were higher in patients with insulin resistance and NASH, and their levels correlated with the degree of hepatic inflammation and oxidative stress. A small number of further lipidomic studies have been performed in recent years [10,11,12,13,14,15,16] with important methodological limitations. Most of these studies included morbidly obese/bariatric patients and often heterogeneous populations. The majority of these studies examined only hepatic and not plasma lipids, which potentially could be used for the non-invasive diagnosis of NASH. Finally, the studies that examined both plasma and hepatic lipids showed inconsistency between measured parameters.
The aim of our study was to investigate potential differences in plasma lipids in patients with NASH, NAFL, and healthy controls that may help differentiate NASH from NAFL and non-invasively assess the histological activity of the disease. To achieve this goal, we applied multitargeted analyses by liquid chromatography–mass spectrometry (LC–MS) and gas chromatography–mass spectrometry (GC–MS) for the quantification of plasma fatty acids, acylcarnitines, and ceramides. Moreover, state-of-the-art statistical machine learning methods were used for the analysis of the data.
2. Results
2.1. Characteristics of the Study Population
Our study included 37 participants: 15 healthy volunteers, 10 subjects with NAFL, and 12 subjects with NASH. The median age was 54 years, and 22 (59.4%) were male. There were no differences across the three groups in terms of age and genre. The median BMI of the total population was 29.3 kg/m2. Patients with NASH and NAFL had significantly higher BMIs compared to healthy controls, but the difference between the former two groups was not significant. MetS and its individual components (arterial hypertension, T2DM, increased WC, and dyslipidemia) were more common in NASH than in NAFL patients and were more common in NAFL patients than healthy controls. AST, HbA1c, and triglyceride values were higher in NASH compared to NAFL patients. Baseline characteristics and biochemical parameters are summarized in Table 1.
Table 1.
Baseline characteristics and biochemical parameters of the study population.
| Variables | Total Population | Control Group | NAFL | NASH | NAFL vs. Control $ | NASH vs. Control $ | NASH vs. NAFL $ |
|---|---|---|---|---|---|---|---|
| N (Male) | 37 (22) | 15 (8) | 10 (6) | 12 (8) | 0.7422 | 0.4835 | 0.7462 |
| Age (years) | 54.0 (46.0–60.0) | 53.0 (41.5–55.0) | 57.0 (53.2–60.0) | 57.5 (45.8–65.5) | 0.1261 | 0.3046 | 0.9211 |
| BMI (kg/m2) | 29.3 (24.9–31.8) | 25.3 (24.0–28.7) | 31.1 (26.6–33.0) | 31.6 (28.6–35.2) | 0.0158 * | 0.0023 ** | 0.4483 |
| Waist circumference (cm) | 100 (95.0–112) | 95.0 (83.5–98.0) | 106 (97.0–118) | 111 (100–120) | 0.0105 * | 0.0030 ** | 0.6678 |
| HOMA-IR | 3.50 (1.90–6.20) | 1.90 (1.05–2.55) | 3.30 (1.92–8.00) | 6.10 (4.80–8.88) | 0.0958 | <0.0001 ** | 0.1378 |
| T2DM | 13 (35.1%) | 3 (20%) | 3 (30%) | 7 (58.3%) | 0.5663 | 0.0404 * | 0.1839 |
| Arterial hypertension | 12 (32.4%) | 3 (20%) | 4 (40%) | 5 (41.7%) | 0.2752 | 0.2205 | 0.9369 |
| Metabolic syndrome | 20 (54.1%) | 3 (20%) | 6 (60%) | 11 (91.7%) | 0.0412 * | 0.0002 ** | 0.0776 |
| NAS | 2 (0–3) | 0 | 2.5 (2–3) | 5 (4–6) | - | <0.0001 ** | <0.0001 ** |
| ALT (U/L) | 28.0 (22.0–51.0) | 20.0 (15.5–26.0) | 35.5 (23.2–50.5) | 48.5 (39.0–92.5) | 0.0211 * | 0.0001 ** | 0.1134 |
| AST (U/L) | 27.0 (20.0–39.0) | 20.0 (18.0–23.5) | 27.0 (23.8–31.0) | 43.5 (35.8–52.2) | 0.0224 * | 0.0001 ** | 0.0111 * |
| GGT (U/L) | 23.0 (13.0–54.0) | 13.0 (11.0–20.0) | 25.0 (18.5–56.2) | 52.5 (35.0–93.5) | 0.0169 * | 0.0021 ** | 0.1378 |
| ALP (U/L) | 72.0 (55.0–93.0) | 58.0 (53.5–89.0) | 77.5 (62.8–96.8) | 86.5 (64.8–94.2) | 0.1741 | 0.2040 | 0.9212 |
| Platelets(×103) (K/μL) | 226 (182–274) | 217 (206–255) | 253 (187–314) | 215 (175–251) | 0.4708 | 0.4208 | 0.3068 |
| HBA1C (%) | 5.50 (5.20–6.00) | 5.40 (5.10–5.70) | 5.50 (5.22–5.75) | 6.15 (5.72–7.02) | 0.5969 | 0.0335 * | 0.0407 * |
| FBG (mg/dl) | 96.0 (88.0–110) | 89.0 (84.5–96.5) | 94.5 (85.0–111) | 109 (94.8–134) | 0.4370 | 0.0167 * | 0.2611 |
| Insulin (μIU/mL) | 12.3 (7.40–25.8) | 9.50 (4.40–11.2) | 14.4 (7.45–31.6) | 25.4 (21.9–27.8) | 0.1492 | <0.0001 ** | 0.2913 |
| Total cholesterol (mg/dl) | 185 (147–202) | 177 (155–200) | 192 (125–215) | 192 (168–200) | 0.8243 | 0.6605 | 0.8174 |
| LDL (mg/dl) | 101 (77.0–126) | 99.0 (76.0–128) | 114 (60.5–126) | 96.0 (81.0–120) | 0.7602 | 0.9611 | 1.0000 |
| HDL (mg/dl) | 50.0 (42.0–60.0) | 56.0 (53.5–64.0) | 45.5 (32.2–54.8) | 43.0 (40.8–49.2) | 0.0324 * | 0.0024 ** | 0.7659 |
| Triglycerides (mg/dl) | 116 (85.0–178) | 84.0 (60.0–100) | 128 (95.2–150) | 192 (144–283) | 0.0116 * | 0.0002 ** | 0.0478 * |
| Ferritin (ng/mL) | 149 (79.7–249) | 97.0 (65.2–160) | 136 (84.4–293) | 200 (140–564) | 0.1341 | 0.0104 * | 0.3068 |
| Uric acid (mg/dl) | 4.90 (4.30–5.80) | 4.80 (4.00–5.10) | 4.85 (4.70–5.92) | 5.60 (4.40–6.75) | 0.2549 | 0.0427 * | 0.4092 |
| Albumin (gr/dl) | 4.58 (4.38–4.70) | 4.60 (4.36–4.70) | 4.57 (4.51–4.68) | 4.50 (4.36–4.62) | 0.8225 | 0.5571 | 0.5521 |
Continuous variables are presented as median (25th–75th percentile). Categorical variables are presented as counts and percentage for each variable’s category. In the categorical variables, subjects are presented that have the disease. $: In the comparison between the groups, the p values were calculated using the Mann–Whitney U test for the continuous variables and the Chi2 (χ2) test for the categorical variables. *: p value < 0.05, **: p value < 0.01, statistically significant.
2.2. Serum Lipids Concentrations
The concentrations of individual lipid species were considered in the three groups, as well as lipid sums, ratios, and indexes that are indicative of de novo lipogenesis (DNL), which is highly related to NAFLD and NASH pathogenesis. As hepatic steatosis is consistently observed with an attenuation of the polyunsaturated to monounsaturated fatty acids ratio [17], the sum of monounsaturated fatty acids (MUFA), non-essential fatty acids (NEFA), saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), and the lipogenic index calculated as the ratio of palmitic acid (16:0) to the essential omega-6 linoleic acid (18:2), reflecting DNL rates, was assessed. In addition, desaturation and elongation indices were evaluated. The abbreviations of the plasma lipids determined, used in the tables and figures of this manuscript are presented in supplementary Table S1.
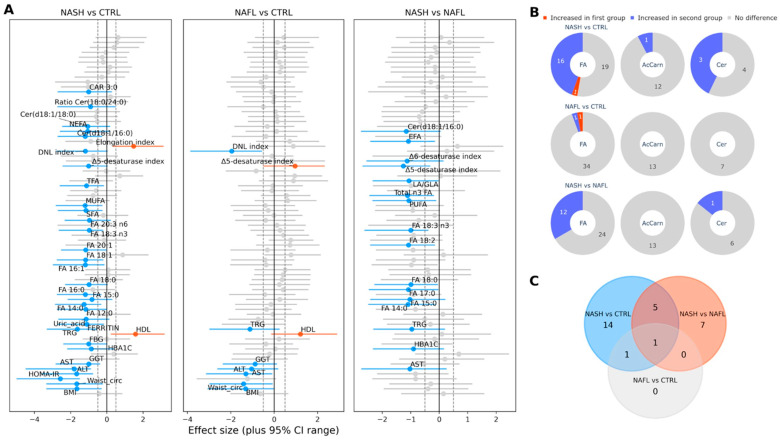
Fatty acid (FA) composition was significantly different in NASH patients compared to NAFL patients. Specifically, fatty acids FA 14:0, FA 15:0, FA 17:0, FA 18:0, FA 18:2, and FA 18:3n3 were significantly elevated in the NASH group. The LA/GLA ratio, levels of essential fatty acids (EFA), total polyunsaturated fatty acids (PUFA), and total n3-fatty acids were also significantly increased. The NASH group had higher Δ5 and Δ6 desaturase indexes. Regarding ceramide levels, NASH patients had significantly higher levels of Cer(d18:1/16:0) in comparison to NAFL patients. Similarly, in NASH patients, fatty acids FA 12:0, FA 14:0, FA 15:0, FA 16:0, FA 16:1, FA 18:0, FA 18:3n3, FA 20:1, FA 20:3n6, and FA 18:1 were upregulated in comparison to healthy controls. Essential fatty acids (EFA), non-essential fatty acids (NEFA), total fatty acids (TFA), total saturated fatty acids (SFA), total monounsaturated fatty acids (MUFA), the Δ5-desaturase index, the de novo lipogenesis (DNL) index, and the elongation index were elevated in NASH patients compared to controls. Levels of Cer(d18:1/16:0), Cer(d18:1/18:0), and the Cer(d18:1/18:0)/Cer(d18:1/24:0) ratio were significantly different in the NASH group compared to healthy controls. NAFL patients had no significant differences in fatty acid levels compared to healthy controls, with the exception of the Δ5-desaturase index and DNL index values. No significant difference in acylcarnitines levels was found between the NASH group and the other two groups, with the exception of CAR 3:0, which differed significantly between NASH and the controls. The plasma lipids, where statistically significant changes were found, are shown in Table 2. The detailed plasma lipid analysis is presented in supplementary Table S2. Figure 1 presents the differences among the three study groups in an “effect size” analysis.
Table 2.
Plasma lipid levels in the three study groups.
| Study Groups | p Values $ | ||||||
|---|---|---|---|---|---|---|---|
| Total (N = 37) | Controls (n = 15) | NAFL (n = 10) | NASH (n = 12) | NAFL vs. Control $ | NASH vs. Control $ | NASH vs. NAFL $ | |
| Fatty Acids, Summary of Fatty Acids and Ratios | |||||||
| FA 12:0 μmol/L | 73.4 (71.4–75.3) | 71.7 (71.2–72.6) | 72.6 (70.7–75.0) | 75.2 (73.8–85.9) | 0.4540 | 0.0002 ** | 0.0602 |
| FA 14:0 μmol/L | 156 (147–171) | 148 (143–160) | 149 (143–165) | 176 (162–209) | 0.8029 | 0.0014 ** | 0.0134 * |
| FA 15:0 μmol/L | 24.0 (22.7–30.5) | 23.5 (22.2–28.8) | 23.1 (21.0–26.0) | 26.9 (25.1–34.9) | 0.6774 | 0.0429 * | 0.0272 * |
| FA 16:0 μmol/L | 1716 (1531–2240) | 1537 (1492–1786) | 1694 (1610–1836) | 2281 (1687–2944) | 0.3899 | 0.0137 * | 0.0927 |
| FA 17:0 μmol/L | 28.5 (25.4–35.8) | 28.5 (25.2–34.2) | 25.8 (24.6–32.3) | 32.9 (28.0–41.6) | 0.5603 | 0.0673 | 0.0134 * |
| FA 18:0 μmol/L | 650 (577–727) | 650 (570–684) | 601 (565–674) | 753 (601–804) | 0.7184 | 0.0429 * | 0.0229 * |
| FA 16:1 μmol/L | 145 (129–184) | 134 (121–144) | 144 (128–174) | 228 (154–334) | 0.4212 | 0.0058 ** | 0.0518 |
| FA 18:1 μmol/L | 1333 (1134–1715) | 1244 (1016–1394) | 1316 (1149–1512) | 1714 (1305–2750) | 0.4212 | 0.0137 * | 0.1062 |
| FA 20:1 μmol/L | 9.42 (8.82–9.99) | 9.06 (8.41–9.55) | 9.03 (8.66–10.3) | 9.91 (9.41–11.3) | 0.5603 | 0.0058 ** | 0.0806 |
| FA 18:2 μmol/L | 1522 (1374–1775) | 1519 (1412–1672) | 1326 (1277–1628) | 1699 (1513–2380) | 0.0907 | 0.1021 | 0.0161 * |
| FA 18:3 n3 μmol/L | 66.0 (64.9–69.9) | 65.3 (63.7–67.1) | 65.3 (64.9–65.6) | 70.8 (66.9–81.4) | 0.7603 | 0.0043 ** | 0.0062 ** |
| FA 20:3 n6 μmol/L | 206 (196–218) | 199 (193–207) | 205 (196–215) | 214 (205–232) | 0.5982 | 0.0381 * | 0.1985 |
| SFA mmol/L | 2.80 (2.63–3.49) | 2.64 (2.54–2.94) | 2.72 (2.64–3.03) | 3.55 (2.76–4.28) | 0.4540 | 0.0104 * | 0.0602 |
| MUFA mmol/L | 1.53 (1.37–1.99) | 1.46 (1.24–1.62) | 1.53 (1.39–1.74) | 2.08 (1.50–3.23) | 0.4212 | 0.0137 * | 0.1062 |
| PUFA mmol/L | 2.70 (2.45–2.94) | 2.70 (2.49–2.84) | 2.44 (2.38–2.80) | 2.87 (2.64–3.58) | 0.2555 | 0.1128 | 0.0134 * |
| Total n3 FA mmol/L | 0.32 (0.31–0.34) | 0.32 (0.30–0.33) | 0.31 (0.29–0.32) | 0.35 (0.31–0.36) | 0.4540 | 0.1500 | 0.0378 * |
| TFA mmol/L | 6.85 (6.50–8.52) | 6.85 (6.14–7.40) | 6.73 (6.44–7.40) | 8.76 (6.82–11.09) | 0.9337 | 0.0264 * | 0.0518 |
| LA/GLA | 20.0 (18.0–22.0) | 19.6 (18.9–20.7) | 17.8 (15.6–20.0) | 22.0 (19.9–26.2) | 0.0806 | 0.1021 | 0.0321 * |
| Δ5-desaturase index | 2.95 (2.63–3.31) | 2.88 (2.79–3.12) | 2.61 (2.40–2.90) | 3.35 (3.07–4.33) | 0.0429 * | 0.0264 * | 0.0062 ** |
| Δ6-desaturase index | 10.5 (9.74–12.0) | 10.5 (9.93–11.5) | 9.56 (8.64–10.9) | 11.2 (10.4–14.7) | 0.0631 | 0.2319 | 0.0378 * |
| DNL index | 1.13 (1.04–1.27) | 1.06 (1.02–1.09) | 1.26 (1.18–1.34) | 1.16 (1.11–1.33) | 0.0017 ** | 0.0264 * | 0.6209 |
| Elongation index | 0.36 (0.34–0.38) | 0.37 (0.36–0.40) | 0.36 (0.32–0.38) | 0.33 (0.28–0.36) | 0.1741 | 0.0078 ** | 0.2766 |
| EFA μmol/L | 1696 (1506–1921) | 1661 (1548–1816) | 1464 (1423–1778) | 1853 (1681–2542) | 0.0907 | 0.0923 | 0.0192 * |
| NEFA μmol/L | 3833 (3470–4935) | 3620 (3202–4005) | 3831 (3484–4005) | 5029 (3620–6912) | 0.5235 | 0.0180 * | 0.1213 |
| Ceramides and Ceramides Ratio | |||||||
| Cer(d18:1/16:0) μmol/L | 0.59 (0.44–0.65) | 0.49 (0.42–0.63) | 0.56 (0.38–0.62) | 0.70 (0.54–0.76) | 0.8461 | 0.0232 * | 0.0378 * |
| Cer(d18:1/18:0) μmol/L | 0.24 (0.18–0.31) | 0.18 (0.13–0.29) | 0.23 (0.18–0.30) | 0.29 (0.25–0.37) | 0.2917 | 0.0264 * | 0.1211 |
| Ratio Cer(18:0/24:0) | 0.02 (0.02–0.03) | 0.02 (0.01–0.02) | 0.02 (0.02–0.03) | 0.03 (0.02–0.030) | 0.1924 | 0.0205 * | 0.3390 |
| Acylcarnitines | |||||||
| CAR 3:0 μmol/L | 0.57 (0.51–0.72) | 0.53 (0.50–0.60) | 0.54 (0.42–0.70) | 0.66 (0.58–0.77) | 0.8461 | 0.0157 * | 0.1379 |
Values of the plasma lipids are presented as median (25th–75th percentile). $: In the comparison between the groups, the p values were calculated using the Mann–Whitney U test. *: p value < 0.05, **: p value < 0.01: statistically significant.
Figure 1.
Effect size analysis of the differences among the study groups. (A) Effect size plots of all the parameters studied: The calculated effect size of each variable is depicted along with the corresponding 95% CI. Variables that had an effect size value beyond the +/− 0.5 limit (gray dashed vertical lines) and simultaneously present a statistically significant difference with a Mann–Whitney U test p value < 0.05 are colored red and blue, respectively. (B) Pie chart with a comparison of the number of plasma lipid species (total fatty acids (FA), acylcarnitines (AcCarn), and ceramides (Cer)) that showed significant changes among the 3 groups: For each pair of groups compared, we have a record of the category of the plasma lipids compared. The criteria for a significant change and the color code are the same as those in Figure 1A. (C) Venn diagram showing the number of common significantly differentiated lipid species in the paired comparisons of the three groups of subjects: The common lipids for the NASH vs. NAFLD and NASH vs. control pairs are FA 14:0, 15:0, 18:0, 18:3n3, Δ5-desaturase index, and Cer(d18:1/16:0) μmol/L.
2.3. Subgroup Analysis Based on HOMA IR
A subgroup analysis was performed of the total study population based on the homeostasis model assessment of insulin resistance (HOMA-IR) values. The study population was further stratified into two groups, a low (<3) and a high HOMA-IR (>3) group. The “high HOMA-IR” group had a higher BMI (31.8 vs. 25.3 kg/m2) and WC. The presence of MetS and its individual components were more common in the same group. Patients with NASH had higher HOMA-IR values, while only 20% of normal controls had high HOMA-IR values. The “high HOMA-IR” group had increased levels of liver function tests (ALT, AST, GGT, ALP), ferritin, and uric acid. Characteristics of the two groups are presented in Table 3.
Table 3.
Baseline characteristics and biochemical parameters according to HOMA-IR.
| Variables | Total Population | Low HOMA-IR | High HOMA-IR | Low vs. High HOMA-IR (p Value) $ |
|---|---|---|---|---|
| N (Male) | 37 (22) | 18 (10) | 19 (12) | 0.6378 |
| Healthy controls | 15 | 12 (80%) | 3 (20%) | |
| NAFL | 10 | 5 (50%) | 5 (50%) | |
| NASH | 12 | 0 | 12 (100%) | |
| Age (years) | 54.0 (46.0–60.0) | 53.0 (44.0–58.2) | 56.0 (52.0–62.5) | 0.1318 |
| BMI (kg/m2) | 29.3 (24.9–31.8) | 25.3 (24.2–29.0) | 31.8 (29.4–34.4) | 0.0003 ** |
| Waist circumference (cm) | 100 (95.0–112) | 95.0 (85.0–99.0) | 112 (102–119) | 0.0002 ** |
| HOMA-IR | 3.50 (1.90–6.20) | 1.80 (0.92–2.28) | 6.20 (4.65–8.95) | <0.0001 ** |
| T2DM | 13 (35.1%) | 4 (22.2%) | 9 (47.4%) | 0.1093 |
| Arterial hypertension | 12 (32.4%) | 3 (16.7%) | 9 (47.4% | 0.0462 * |
| Metabolic syndrome | 20 (54.1%) | 2 (11.1%) | 18 (94.7%) | <0.0001 ** |
| ALT (U/L) | 28.0 (22.0–51.0) | 21.5 (16.0–27.5) | 44.0 (29.0–80.5) | 0.0002 ** |
| AST (U/L) | 27.0 (20.0–39.0) | 20.0 (18.0–26.8) | 36.0 (27.0–49.5) | 0.0002 ** |
| GGT (U/L) | 23.0 (13.0–54.0) | 15.0 (11.2–20.8) | 51.0 (28.0–94.0) | 0.0003 ** |
| ALP (U/L) | 72.0 (55.0–93.0) | 58.5 (53.2–69.8) | 93.0 (74.5–108) | 0.0013 ** |
| Platelets (×103) (K/μL) | 226 (182–274) | 223 (208–280) | 245 (177–265) | 0.6054 |
| HBA1C (%) | 5.50 (5.20–6.00) | 5.40 (5.12–5.60) | 5.90 (5.35–6.60) | 0.0571 |
| FBG (mg/dL) | 96.0 (88.0–110) | 91.0 (87.2–97.8) | 102 (91.0–126) | 0.1136 |
| Insulin (μIU/mL) | 12.3 (9.60–22.5) | 7.20 (4.40–9.90) | 25.8 (20.3–28.3) | <0.0001 ** |
| Total cholesterol (mg/dL) | 185 (164–199) | 172 (149–199) | 194 (176–202) | 0.5333 |
| LDL (mg/dL) | 101 (77.0–126) | 96.5 (75.5–125) | 110 (80.0–124) | 0.6815 |
| HDL (mg/dL) | 50.0 (42.0–60.0) | 56.0 (52.5–64.0) | 42.0 (37.5–49.5) | 0.0008 ** |
| Triglycerides (mg/dL) | 116 (85.0–178) | 84.5 (62.8–111) | 178 (129–262) | <0.0001 ** |
| Ferritin (ng/mL) | 149 (79.7–249) | 99.0 (66.3–169) | 181 (105–502) | 0.0193 * |
| Uric acid (mg/dL) | 4.90 (4.30–5.80) | 4.75 (3.92–5.00) | 5.60 (4.65–6.60) | 0.0075 ** |
| Albumin (gr/dL) | 4.58 (4.38–4.70) | 4.60 (4.43–4.70) | 4.53 (4.34–4.65) | 0.2926 |
Continuous variables are presented as median (25th–75th percentile). Categorical variables are presented as counts and percentage for each variable’s category. In the categorical variables, subjects that have the disease are presented. -$: In the comparison between the groups, the p values were calculated using the Mann–Whitney U test for the continuous variables and the Chi2 (χ2) test for the categorical variables. -*: p value < 0.05, **: p value < 0.01, statistically significant.
Serum lipid concentrations were elevated in high HOMA-IR compared to low HOMA-IR patients. Particularly, fatty acids FA 12:0, FA 14:0, FA 15:0, FA 16:0, FA 17:0, FA 18:0, FA 16:1, FA 18:1, FA 18:2, and FA 20:1 were significantly increased in the high HOMA-IR group. The LA/GLA and AA/EPA ratios, as well as SFA, MUFA, PUFA, and TFA, were significantly increased. The DNL and elongation index were higher in the HOMA-IR group. In the low HOMA-IR group, Cer(d18:1/18:0), the Cer(d18:1/18:0)/Cer(d18:1/24:0) ratio, CAR 3:0, and CAR 5:0 were lower compared to the high HOMA-IR group. Lipid profiles for the two groups are summarized in Table 4 and are presented in more detail in supplementary Table S3.
Table 4.
Plasma lipid composition in high and low HOMA-IR groups.
| Total (N = 37) | Low HOMA-IR (n = 18) | High HOMA-IR (n = 19) | Low vs. High HOMA-IR $ | |
|---|---|---|---|---|
| Fatty Acids, Summary of Fatty Acids, and Ratios | ||||
| FA 12:0 μmol/L | 73.4 (71.5–75.3) | 71.7 (71.2–72.7) | 75.2 (73.7–80.4) | 0.0010 ** |
| FA 14:0 μmol/L | 156 (147–171) | 148 (143–156) | 167 (156–192) | 0.0030 ** |
| FA 15:0 μmol/L | 24.0 (22.7–30.5) | 23.0 (21.4–24.0) | 26.6 (23.9–33.2) | 0.0217 * |
| FA 16:0 μmol/L | 1716 (1531–2240) | 1612 (1514–1718) | 2095 (1684–2664) | 0.0085 ** |
| FA 17:0 μmol/L | 28.5 (25.4–35.8) | 25.9 (24.8–32.5) | 33.8 (27.5–38.6) | 0.0373 * |
| FA 18:0 μmol/L | 650 (577–727) | 600 (565–672) | 719 (601–780) | 0.0200 * |
| FA 16:1 μmol/L | 145 (129–184) | 131 (120–144) | 179 (144–274) | 0.0033 ** |
| FA 18:1 μmol/L | 1333 (1134–1715) | 1272 (1097–1384) | 1582 (1193–2232) | 0.0275 * |
| FA 20:1 μmol/L | 9.42 (8.82–9.99) | 9.01 (8.49–9.56) | 9.90 (9.19–10.82) | 0.0144 * |
| FA 18:2 μmol/L | 1522 (1374–1775) | 1430 (1328–1618) | 1666 (1473–2140) | 0.0465 * |
| SFA mmol/L | 2.80 (2.63–3.49) | 2.64 (2.57–2.85) | 3.36 (2.76–3.98) | 0.0065 ** |
| MUFA mmol/L | 1.53 (1.37–1.99) | 1.48 (1.29–1.60) | 1.94 (1.44–2.57) | 0.0235 * |
| PUFA mmol/L | 2.70 (2.45–2.94) | 2.56 (2.41–2.77) | 2.86 (2.55–3.34) | 0.0298 * |
| TFA mmol/L | 6.85 (6.50–8.52) | 6.70 (6.39–7.22) | 8.42 (6.81–9.48) | 0.0157 * |
| LA/GLA | 20.0 (18.0–22.0) | 18.9 (17.6–20.2) | 20.6 (19.4–24.4) | 0.0465 * |
| AA/EPA | 5.86 (5.63–6.27) | 5.72 (5.33–5.94) | 6.26 (5.77–6.42) | 0.0157 * |
| DNL index | 1.13 (1.04–1.27) | 1.07 (1.02–1.23) | 1.17 (1.09–1.32) | 0.0402 * |
| Elongation index | 0.36 (0.34–0.38) | 0.37 (0.35–0.39) | 0.34 (0.29–0.37) | 0.0157 * |
| EFA μmol/L | 1696 (1506–1921) | 1571 (1467–1770) | 1814 (1615–2301) | 0.0465 * |
| NEFA μmol/L | 3833 (3470–4935) | 3577 (3339–3964) | 4935 (3588–5903) | 0.0144 * |
| Ceramides and Ceramides Ratio | ||||
| Cer (d18:1/18:0) μmol/L | 0.24 (0.18–0.31) | 0.18 (0.13–0.23) | 0.29 (0.25–0.35) | 0.0038 ** |
| Ratio Cer (18:0/24:0) | 0.02 (0.02–0.03) | 0.02 (0.01–0.02) | 0.03 (0.02–0.03) | 0.0040 ** |
| Acylcarnitines | ||||
| CAR 3:0 μmol/L | 0.57 (0.51–0.72) | 0.53 (0.50–0.60) | 0.62 (0.56–0.78) | 0.0465 * |
| CAR 5:0 μmol/L | 0.06 (0.05–0.07) | 0.05 (0.05–0.07) | 0.07 (0.06–0.09) | 0.0235 * |
Values of the plasma lipids are presented as the median (25th–75th percentile). $: In the comparison between the groups, the p values were calculated using the Mann–Whitney U test. *: p value < 0.05, **: p value < 0.01, statistically significant.
2.4. Correlation of Lipid Profiles with the Histologic Activity of NAFLD
We assessed correlations between clinical and biochemical characteristics and plasma lipid profiles, and NAS score and fibrosis score in NAFLD patients.
There was a positive correlation between the NAS score and transaminase, triglyceride, HOMA-IR, and uric acid levels. Among plasma lipid parameters, levels of several fatty acids, including FA 16:1, FA 16:0, FA 14:0, the elongation index, NEFA, MUFA, SFA, and FA 18:1, had a strong correlation with the NAS score. Regarding fibrosis stage, HOMA-IR, FBG, HBA1C, GGT, and FA 12:0 were strongly correlated with the fibrosis score. These data are presented in Table 5, and the detailed correlations are presented in supplementary Table S4.
Table 5.
Significant correlations between baseline characteristics and plasma lipids values and the histological activity of NAFLD.
| Variables | NAS Score * | Fibrosis Score * |
|---|---|---|
| ALT | 0.6929 | 0.2742 |
| HOMA-IR | 0.6532 | 0.6312 |
| AST | 0.6384 | 0.3078 |
| TRG | 0.6075 | 0.3859 |
| FA 16:1 | 0.5265 | 0.325 |
| Elongation index | −0.5236 | −0.4253 |
| NEFA | 0.5196 | 0.3184 |
| MUFA | 0.5194 | 0.3385 |
| FA 16:0 | 0.5185 | 0.305 |
| Uric acid | 0.5162 | 0.0502 |
| FA 14:0 | 0.5105 | 0.3631 |
| SFA | 0.5101 | 0.3015 |
| FA 18:1 | 0.5065 | 0.3503 |
| FBG | 0.3981 | 0.5594 |
| HBA1C | 0.3171 | 0.5154 |
| GGT | 0.3668 | 0.5084 |
| FA 12:0 | 0.3998 | 0.5277 |
* Correlations are presented with Pearson’s correlation coefficient (r), which ranges from −1 to +1.
2.5. Diagnostic Performance of Clinical, Biochemical, and Plasma Lipid Profiles in Predicting NASH
We calculated the area under the curve (AUC) to assess the ability of different parameters in discriminating NASH from NAFL/healthy controls. The index of Δ5-desaturase had an AUC of 0.854 (0.64–0.99), FA 18:3 n3 (ALA) had an AUC of 0.85 (0.69–0.99), and FA 14:0, FA 17:0, PUFA, EFA, and FA 18:2 had AUC values greater than 0.8. AST values had an AUC of 0.825 (0.63–0.99).
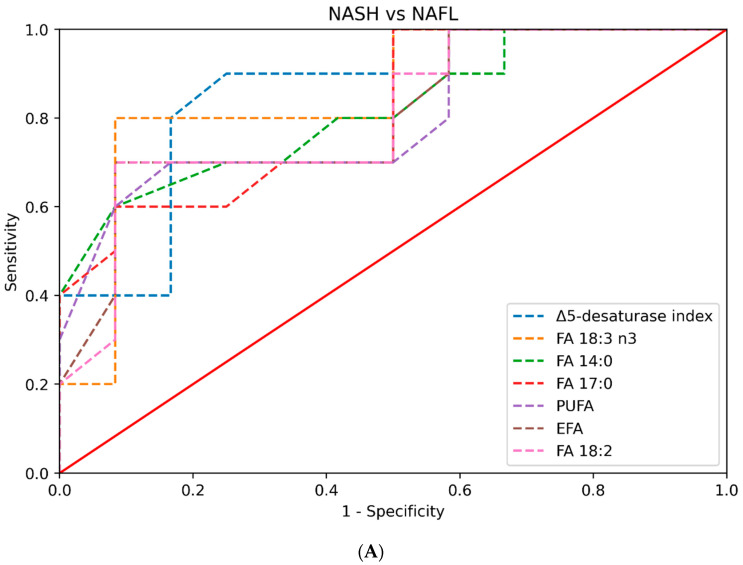
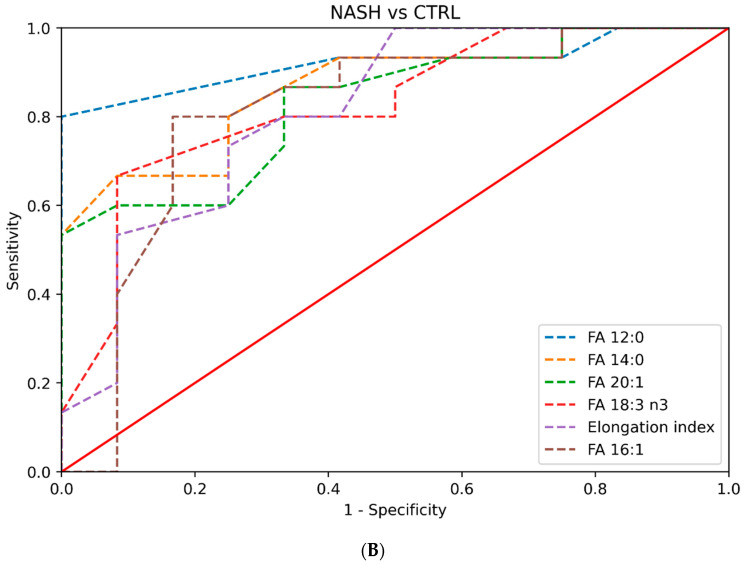
When we assessed the ability of plasma lipid parameters to discriminate between NASH and healthy controls, FA 12:0 had an AUC value of 0.92 (0.81–1); FA 14:0 had an AUC value of 0.87 (0.71–0.98); and FA 20:1, FA 18:3 n3, the elongation index, and FA 16:1 had AUC values greater than 0.8. The AUC values of the plasma lipids with an AUC > 0.8 for each pair of groups compared are presented in Table 6 and Figure 2. The AUC values for all the plasma lipids are presented in Supplementary Table S5.
Table 6.
AUC values of the plasma lipids with an AUC > 0.8 for each pair of groups compared.
| NASH vs. Control Comparison | Cut-Offs | |||||
|---|---|---|---|---|---|---|
| Lipids | AUROC (95% CI) | p Value | Cut-Off Point | AUROC (95% CI) | Sensitivity | Specificity |
| FA 12:0 | 0.92 (0.80–1) | <0.001 ** | 73.3 | 0.90 (0.79–1) | 0.800 | 1 |
| FA 14:0 | 0.87 (0.72–0.97) | <0.001 ** | 152 | 0.79 (0.63–0.93) | 0.667 | 0.917 |
| FA 20:1 | 0.83 (0.67–0.96) | <0.001 ** | 9.72 | 0.77 (0.60–0.93) | 0.867 | 0.667 |
| FA 18:3 n3 | 0.83 (0.66–0.97) | <0.001 ** | 66.1 | 0.79 (0.64–0.92) | 0.667 | 0.917 |
| Elongation index | 0.81 (0.63–0.96) | 0.132 | 0.324 | 0.75 (0.61–0.88) | 1 | 0.500 |
| FA 16:1 | 0.81 (0.61–0.96) | <0.001 ** | 145 | 0.82 (0.67–0.96) | 0.800 | 0.833 |
| NASH vs. NAFL Comparison | Cut-Offs | |||||
| Lipids | AUROC (95% CI) | p Value | Cut-Off Point | AUROC (95% CI) | Sensitivity | Specificity |
| Δ5-desaturase index | 0.85 (0.66–1) | <0.001 ** | 3.08 | 0.82 (0.64–0.96) | 0.900 | 0.750 |
| FA 18:3 n3 | 0.85 (0.67–1) | <0.001 ** | 66.1 | 0.86 (0.66–1) | 0.800 | 0.917 |
| FA 14:0 | 0.82 (0.59–0.96) | <0.001 ** | 152 | 0.76 (0.55–0.94) | 0.600 | 0.917 |
| FA 17:0 | 0.81 (0.61–0.94) | <0.001 ** | 26.5 | 0.76 (0.59–0.93) | 0.600 | 0.917 |
| PUFA | 0.80 (0.57–0.97) | <0.001 ** | 2.52 | 0.77 (0.58–0.95) | 0.700 | 0.833 |
| EFA | 0.80 (0.57–0.96) | <0.001 ** | 1.503 | 0.81 (0.62–0.96) | 0.700 | 0.917 |
| FA 18:2 | 0.80 (0.57–0.96) | 0.074 | 1.382 | 0.81 (0.65–0.96) | 0.700 | 0.917 |
The table contains (for the selected lipids and for each pair of groups compared): (a) The AUC values (95% CI) as well as the corresponding p value. (b) A defined cut-off point for each lipid, which is a threshold below from which the samples are classified in one category and above from this to the other. For these specific cut-offs, the AUC values (95% CI), as well as the sensitivity and specificity values, were recalculated. **: p value < 0.01, statistically significant.
Figure 2.
(A) Plot of the AUC values of the plasma lipid species in the prediction of NASH in comparison to NAFL (only AUC values greater than 0.8 are depicted). (B) Plot of the AUC values of the plasma lipid species in the prediction of NASH in comparison to healthy controls (only AUC values greater than 0.8 are depicted).
3. Discussion
The non-invasive diagnosis of NASH remains a clinical challenge, as there is currently no available biomarker that can discriminate NASH from NAFL. The diagnosis of NASH can only be made histologically. However, the large number of NAFLD patients and the invasive nature of liver biopsy render this approach problematic. The aim of this study was to characterize lipid profiles (plasma fatty acids, acylcarnitines, and ceramides) in patients with NASH and NAFL and to investigate potential correlations with histological activity and fibrosis.
Patients were classified as having NASH or NAFL based on liver histology. Patients with NAFL and NASH had similar metabolic profiles, but had more metabolic risk factors compared to controls, as expected. A major finding of our study is that patients with NASH had a distinctive plasma lipid profile from patients with NAFL, although the two groups did not differ in terms of established metabolic risk factors. In addition, patients with NAFL and healthy controls had similar plasma lipid profiles, even though the former had significantly more metabolic risk factors.
Patients with NASH had significant differences in the values of several species of fatty acids, such as FA 12:0, FA 14:0, FA 15:0, FA 16:1, FA 17:0, FA 18:0, FA 18:3n3, FA 18:2, the LA/GLA ratio, EFA, TFA, SFA, total n3-FAs, and others, compared to NAFL patients and healthy controls. These results indicate that specific lipotoxic fatty acids, mainly saturated fatty acids, may play a role in the pathogenesis of NASH. The intrahepatic accumulation of saturated fatty acids probably induces oxidative stress and activates the inflammasome in the liver, resulting in hepatocyte injury and apoptosis [18]. Given the fact that the samples in our study were taken after an overnight fasting, it is unlikely that this finding is an effect of dietary intake, but it probably is a result of increased DNL and increased peripheral lipolysis (due to insulin resistance). The fact that the products of DNL are exclusively saturated fatty acids further supports this rationale. This trend was also demonstrated in a previous study carried out by Puri et al. [19], although this study had important methodological limitations, such as differences between the study groups. Similar findings were also observed in a study conducted by Walle et al. [16], which found increased serum concentrations of saturated fatty acids, mainly FA 14:0, FA16:0, and FA 18:0, in patients with NASH compared to patients with NAFL. Another study that was conducted by Luukonen et al. [12] showed increased levels of saturated fatty acids in the livers of adults with NASH. Similar results have also been found in recent animal studies [20]. The existence of a genetic predisposition probably contributes to these findings. This was seen in a previous study conducted by our group, which concluded that the rs738409 polymorphism of the PNPLA3 gene affects the composition of the blood fatty acids [21]. This polymorphism resulted in increased concentrations of blood saturated fatty acids such as palmitic, stearic, oleic, and linoleic acids, the values of which were found to be significantly elevated in NASH patients in our study. In contrast to previous lipidomic studies, the levels of PUFA in our study were increased [8,19].
Regarding ceramides, in our study, Cer(d18:1/16:0) levels were significantly increased in NASH patients compared to NAFL patients and healthy controls, and Cer(d18:1/18:0) levels and the Cer(d18:1/18:0)/Cer(d18:1/24:0) ratio were significantly higher in NASH patients compared to healthy controls. Increased concentrations of C 16:0 ceramide were also observed both in recent animal studies [22,23], which found that this specific ceramide’s concentrations were increased in obesity-related insulin resistance, and in a human study carried out by Luukonen et al. [12] in patients with NASH and insulin resistance. These findings indicate that the “de novo” ceramide synthesis pathway is probably upregulated in NASH. Ceramides are bioactive molecules that play an important role in many cellular functions and have been associated with insulin resistance, altered insulin signaling, as well as inflammatory and apoptotic processes [24]. These lipotoxic intermediates are considered important mediators in hepatocellular injury in NASH.
Another important finding of our study is the association between several fatty acid levels and histological activity, as assessed using the NAS score. In addition, several lipids, such as Δ5-desaturase index, FA 14:0, FA 17:0, FA 18:2, FA 18:3 n3, and PUFA, exhibit high accuracy in discriminating NASH from NAFL. This suggests that the plasma lipid profile might prove a useful tool in the non-invasive diagnosis of NASH. In contrast, the plasma lipid profile did not correlate with the fibrosis stage, except for fatty acid FA 12:0. However, safe conclusions could not be drawn due to the relatively small sample size of our study.
Several previous lipidomic/metabolomic studies attempted to further investigate the complex pathophysiology of NAFLD [7,8,9,10,11,12,13,14,15]. These studies yielded encouraging results regarding the different qualitative and quantitative hepatic lipid compositions in NAFLD patients in comparison to healthy subjects. However, most of these studies had several limitations. Most of these studies included morbidly obese patients undergoing bariatric surgery, which raises concerns as to the effect that extreme BMI levels might have on the circulating plasma lipidomic footprint [7,9,12,13,14,15]. Moreover, the absence of steatosis was confirmed histologically in some of the studies, whereas others relied on the combination of normal liver ultrasound scans and normal liver biochemistry. Ultrasonography has the limitation that it can detect hepatic steatosis when at least 20–30% of the liver parenchyma is affected [25]. In addition, performing a liver biopsy in a patient with a normal liver raises bioethical issues when there is an equivalent, safe, and accurate non-invasive way to assess for hepatic steatosis, such as MRI-PDFF, which was used in our study [26,27]. Moreover, most of the studies focused on hepatic lipid composition, which might reflect more precisely the alterations in lipid metabolism that occur in NAFLD/NASH, but liver tissue is a biological material that is not easily accessible in daily clinical practice. In the studies that examined both plasma and hepatic lipids, the results were inconsistent in plasma and liver tissue. Finally, discrepancies were found in the results between the different studies that examined plasma lipids, possibly because of the different methods used, the heterogeneity in the populations examined, and the study designs.
Our study had substantial strengths. Firstly, we included three well-characterized groups. NAFL and NASH were confirmed histologically, and the absence of steatosis was confirmed by means of MRI-PDFF. Participants were common NAFLD patients seen in hepatology clinics and not patients at extreme ends of the MetS, such as morbidly obese patients (median BMI of NAFL and NASH patients were 31.1 and 31.6 kg/m2, respectively, and 25.3 kg/m2 in the control group). Moreover, a subgroup analysis was performed according to the HOMA-IR, which added further to our understanding of the role of lipids in NAFLD [28]. State-of-the-art statistical methods were also used. Regarding the methodology followed in our study for the plasma lipid profiles, it should be noted that it was specifically developed in our lab with the aim to assess dysregulated lipid profiles in clinical samples and to ensure the accurate determination of the certain lipid species.
Our study has some limitations. The relatively small number of participants is the main limitation. Another limitation is that a targeted lipidomic analysis was conducted that included lipid species that are thought to contribute to NASH pathogenesis according to currently available data. An untargeted lipidomic analysis that could identify more (all detectable) lipid species that potentially contribute to the pathogenesis of the disease could provide a more comprehensive pattern. This is the next study to be performed in these samples, and results will be soon published to build on the present data.
In summary, the results of our study indicate that NASH patients exhibit a distinct plasma lipid profile that is distinctive from NAFL patients and non-NAFLD controls. This profile seems to correlate with histological activity. Based on these results, plasma lipids may provide a useful biomarker for the diagnosis of NASH. Our findings might have significant implications, facilitating earlier diagnosis and the personalized management of NASH, leading to improved patient outcomes and reduced disease burden. The results of our study require validation in larger cohorts.
4. Materials and Methods
4.1. Study Design
The current study was a case-control study that included three well characterized groups: patients with biopsy-proven NAFL, patients with biopsy-proven NASH, and healthy controls. All participants were enrolled in our study between June 2021 and June 2023 after providing written informed consent. The study was performed according to the principles of the Declaration of Helsinki. The study was approved by the Institutional Review Board and the Bioethics Board of Medical School of Aristotle University of Thessaloniki (protocol number 4.399/26/01/2021).
4.2. Study Cohort
Consecutive adult subjects with a recent (or suspected) diagnosis of NAFLD (within 6 months) that attended the hepatology outpatient clinic at AHEPA University Hospital (Thessaloniki, Greece) during the study period were screened for eligibility after providing written informed consent. Inclusion criteria were age > 18 years and a probable diagnosis of NAFLD based on the presence of hepatic steatosis on imaging studies (ultrasound scan, CT, or MRI scan), the presence of metabolic risk factors, and the exclusion of other causes for hepatic steatosis. Exclusion criteria were alcohol consumption > 20 gr/d in women and >30 gr/day in men. Patients with concomitant liver diseases (chronic viral hepatitis, autoimmune and cholestatic liver diseases, Wilson’s disease, hemochromatosis, a-1-antitrypsin deficiency, and drug-induced liver disease) were also excluded. Finally, subjects with underlying severe systemic diseases (such as cancer and end-stage liver, kidney, and heart disease) were excluded from our study.
Healthy controls were recruited from June 2021 to June 2023. In this group, the absence of hepatic steatosis was determined by normal liver biochemistry and a liver fat fraction < 5% on MRI-PDFF [26,27]. All healthy controls had a complete liver screen that was negative for chronic liver diseases.
Participants had clinical assessment, physical examination, and blood tests at baseline. The anthropometric and demographic parameters that were recorded were age, gender, weigh, height, body mass index (BMI), and waist circumference (WC). Baseline biochemistry parameters included: alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGt), alkaline phosphatase (ALP), billrubin, serum albumin, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides, fasting blood glucose (FBG), insulin, HbA1c, and ferritin.
Patients with NAFLD, who had indications according to current clinical practice guidelines [1,2], underwent ultrasound-guided percutaneous liver biopsy [1,2]. Liver biopsies were examined by an expert liver pathologist, who was blinded to patient characteristics. The definitive diagnosis of NAFLD was established by the presence of steatosis in at least 5% of the hepatocytes. The NAFLD activity score (NAS) and fibrosis score by the NASH Clinical Research Network (CRN) were used to grade inflammatory activity and stage fibrosis, respectively [29]. The NAS score consists of three components: steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2), and it ranges from 0 to 8. The fibrosis score ranges from 0 (absence of fibrosis) to 4 (cirrhosis). Subjects with NAFLD were further divided into NAFL and NASH based on histological findings. Subjects with steatosis with no or mild inflammation were classified as NAFL. Subjects with at least 1 grade of each component of the NAS score were classified as NASH [30].
Blood samples were taken from all participants for plasma lipid analysis by three different methods (in subjects with NAFLD on the morning of the liver biopsy) after overnight fasting and a low-fat diet in the past 24 h. Blood samples were centrifugated, and plasma was separated and immediately stored at −80 °C.
4.3. Acylcarnitines, Ceramides and Fatty Acids Analyses
For the analysis of acylcarnitines and ceramides, two LC-MS methods developed in our lab were applied. For acylcarnitines, an hydrophilic interaction liquid chromatography tandem mass spectrometry (HILIC–MS/MS) method was applied quantifying 13 acylcarnitine analogues, namely Acetyl-L-Carnitine (CAR 2:0); Propionyl-L-Carnitine (CAR 3:0); Butyryl-L-Carnitine (CAR 4:0); Valeryl-L-Carnitine (CAR 5:0); Hexanoyl-L-Carnitine (CAR 6:); Octanoyl-L-Carnitine (CAR 8:0); Decanoyl-L-Carnitine (CAR 10:0); Lauroyl-L-Carnitine (CAR 12:0); Myristoyl-L-Carnitine (CAR 14:0); Palmitoyl-L-Carnitine (CAR 16:0); Stearoyl-L-Carnitine (CAR 18:0); Oleoyl-L-carnitine (CAR 18:1); and Linoleoyl-L-Carnitine (CAR 18:2) [31,32].
For ceramides, the reverse phase LC–MS/MS method, quantifying four species, namely N-Palmitoyl-D-erythro-sphingosine (Cer d18:1/16:0); N-stearoyl-D-erythro-sphingosine (Cer d18:1/18:0); N-lignoceroyl-D-erythro-sphingosine (Cer d18:1/24:0); and N-nervonoyl-D-erythro-sphingosine (Cer d18:1/24:1), was applied [33]. Analysis was performed on an Acquity UPLC System (Waters Corporation, Milford, CT, USA) coupled on a XEVO TQD Mass Spectrometer (Waters Corporation, Milford, CT, USA). Data acquisition and analysis were performed by Waters MassLynx version 4.1 and TargetLynx (Waters, Milford, MA 01757, USA).
Total fatty acid analysis was performed on an Agilent Technologies 8860 GC, combined with a 5977 MSD (Agilent Technologies, Santa Clara, CA, USA). Fatty acid methyl esters were separated on an Agilent 100 m HP-88 column (0.25 μm, i.d. of 0.25 μm). Data were processed by MassHunter Workstation (Version 10.0) software. Fatty acids were extracted from serum samples using a modified Folch protocol followed by methanolysis/methylation under acidic conditions, as described in our previous study [21]. In total, 20 fatty acid methyl esters were quantified, namely Lauric (FA 12:0), Myristic (FA 14:0), Pentadecanoic (FA 15:0), Palmitic (FA 16:0), Heptadecanoic (FA 17:0), Stearic (FA 18:0), Arachidic (FA 20:0), Behenic (FA 22:0), Lignoceric (FA 24:0), Palmitoleic (FA 16:1), cis-9-Oleic (FA 18:1), cis-11-Eicosenoic (FA 20:1), Nervonic (FA 24:1), Linoleic (FA 18:2), Gamma Linolenic (FA 18:3n6), Alpha Linolenic (FA 18:3n3), Dihomogamma Linolenic (FA 20:3n6), Arachidonic (FA 20:4n6), cis-5,8,11,14,17 Eicosapentaenoic (FA 20:5n3), cis-4,7,10,13,16,19 Docosahexaenoic (FA 22:6n3) acids.
4.4. Data and Statistical Analysis
The Python (v. 3.10.6) programming language was used for statistical computations and the visualization of the results on a Linux OS based PC. The continuous variables are expressed as medians with interquartile range (IQR, 25th–75th percentile), while the categorical variables are expressed as counts and percentages for each variable’s category. The differences between the variables’ categories were examined using the Mann–Whitney U test for the continuous variables and the Chi-square (χ2) test for the categorical variables. Receiver operating characteristic (ROC) curves were plotted, and the area under the ROC (AUROC) and the corresponding p value were calculated to determine the diagnostic accuracy of the lipids to differentiate NASH from NAFL and CTRL. The sensitivity and the specificity for cutoffs were also calculated for specific lipids. Two-tailed p values < 0.05 were considered to indicate statistically significant differences in variables between groups. In addition, the effect size offering valuable information about the practical significance of a finding, independent of sample size, was calculated, along with its 95% confidence interval range, as a metric to quantify the magnitude of the observed differences in the study [34]. Cohen’s d was used as an effect size measure, which calculated the standardized mean difference between two groups by dividing the difference in means by the pooled standard deviation. Effect size values greater than 0.5 and 0.8 indicated medium and large effects, respectively. Confidence intervals were calculated using the bootstrapping method, which involves the repeated random resampling of the original dataset with the replacement [35]. Correlations between lipids and histological features were assessed by Pearson’s correlation.
4.5. Predetermined Subgroup Analysis
A subgroup analysis was performed based on the values of the homeostasis model assessment of insulin resistance (HOMA-IR) to examine patients that were insulin-resistant and, therefore, at a higher risk of suffering from NASH. Due to the lack of a globally accepted cut-off value of HOMA-IR that can differentiate insulin-resistant from insulin-sensitive subjects, a cut-off value of 3 was used. The total study population was divided into two groups: individuals with HOMA-IR values > 3 were classified into the “High HOMA-IR” group, while individuals with HOMA-IR values < 3 were classified into the “Low HOMA-IR” group.
5. Conclusions
In conclusion, our study demonstrated that subjects with NASH have a distinct plasma lipid profile compared to subjects with NAFL and healthy controls. This finding has multiple clinical, diagnostic, and therapeutic implications; however, due to the relatively small sample size of our study, larger-scale studies are needed to validate our results.
Acknowledgments
This work was a part of the fulfillment of the requirements for Georgios Kalopita’s PhD Degree.
Abbreviations
| ALA | Alfa-linoleic acid |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| BMI | Body mass index |
| CRN | Clinical Research Network |
| CT | Computed tomography |
| CVD | Cardiovascular disease |
| DGLA | Dihomo-γ-linolenic acid |
| FA | Fatty acid |
| FBG | Fasting blood glucose |
| GC–MS | Gas chromatography tandem mass spectrometry |
| GGT | Gamma-glutamyl transferase |
| HDL | High-density lipoprotein |
| HILIC-MS/MS | Hydrophilic interaction liquid chromatography tandem mass spectrometry |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| LA | Linoleic acid |
| LC–MS | Liquid chromatography–mass spectrometry |
| LDL | Low-density lipoprotein |
| MetS | Metabolic syndrome |
| MS | Mass spectrometry |
| NAFL | Non-alcoholic fatty liver |
| NAFLD | Non-alcoholic fatty liver disease |
| NAS | NAFLD activity score |
| NASH | Non-alcoholic steatohepatitis |
| NITs | Non-invasive tests |
| RPLC–MS/MS | Reversed phase liquid chromatography tandem mass spectrometry |
| T2DM | Type 2 diabetes mellitus |
| WC | Waist circumference |
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241612717/s1.
Author Contributions
Conceptualization: G.K., T.M., G.G. and H.G.; methodology: G.K., T.M., T.L. and H.G.; software: G.K., T.M. and T.L.; formal analysis: G.K., T.M., T.L. and H.G.; investigation: G.K., K.A., A.I., K.M. and G.G.; data curation: G.K. and T.M.; writing–original draft preparation: G.K., T.M., T.L., K.A., K.M., E.T. and E.S.; writing review and editing: M.C., H.G. and G.G.; supervision: H.G. and G.G.; project administration: H.G. and G.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marchesini G., Day C.P., Dufour J.F., Canbay A., Nobili V., Ratziu V., Tilg H., Roden M., Gastaldelli A., Yki-Jarvinen H., et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., Abdelmalek M.F., Caldwell S., Barb D., Kleiner D.E., Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. [(accessed on 1 July 2023)];Hepatology. 2023 77:1797–1835. doi: 10.1097/HEP.0000000000000323. Available online: https://journals.lww.com/hep/Fulltext/2023/05000/AASLD_Practice_Guidance_on_the_clinical_assessment.31.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor R.S., Taylor R.J., Bayliss S., Hagström H., Nasr P., Schattenberg J.M., Ishigami M., Toyoda H., Wai-Sun Wong V., Peleg N., et al. Association between Fibrosis Stage and Outcomes of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611–1625.e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Simon T.G., Roelstraete B., Khalili H., Hagström H., Ludvigsson J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut. 2021;70:1375–1382. doi: 10.1136/gutjnl-2020-322786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui M.S., Yamada G., Vuppalanchi R., Van Natta M., Loomba R., Guy C., Brandman D., Tonascia J., Chalasani N., Neuschwander-Tetri B., et al. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019;17:1877–1885.e5. doi: 10.1016/j.cgh.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O., Sargeant C., Contos M.J., Sanyal A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 8.Araya J., Rodrigo R., Videla L.A., Thielemann L., Orellana M., Pettinelli P., Poniachik J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 9.Apostolopoulou M., Gordillo R., Koliaki C., Gancheva S., Jelenik T., De Filippo E., Herder C., Markgraf D., Jankowiak F., Esposito I., et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care. 2018;41:1235–1243. doi: 10.2337/dc17-1318. [DOI] [PubMed] [Google Scholar]
- 10.Barr J., Caballería J., Martínez-Arranz I., Domínguez-Díez A., Alonso C., Muntané J., Pérez-Cormenzana M., García-Monzón C., Mayo R., Martín-Duce A., et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J. Proteome Res. 2012;11:2521–2532. doi: 10.1021/pr201223p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang R.-X., Hu C.-X., Sun W.-L., Pan Q., Shen F., Yang Z., Su Q., Xu G.-W., Fan J.-G. Serum Monounsaturated Triacylglycerol Predicts Steatohepatitis in Patients with Non-alcoholic Fatty Liver Disease and Chronic Hepatitis B. Sci. Rep. 2017;7:10517. doi: 10.1038/s41598-017-11278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luukkonen P.K., Zhou Y., Sädevirta S., Leivonen M., Arola J., Orešič M., Hyötyläinen T., Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Gorden D.L., Myers D.S., Ivanova P.T., Fahy E., Maurya M.R., Gupta S., Min J., Spann N.J., McDonald J.G., Kelly S.L., et al. Biomarkers of NAFLD progression: A lipidomics approach to an epidemic. J. Lipid Res. 2015;56:722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotronen A., Seppänen-Laakso T., Westerbacka J., Kiviluoto T., Arola J., Ruskeepää A.-L., Oresic M., Yki-Järvinen H. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58:203–208. doi: 10.2337/db08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anjani K., Lhomme M., Sokolovska N., Poitou C., Aron-Wisnewsky J., Bouillot J.-L., Lesnik P., Bedossa P., Kontush A., Clement K., et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J. Hepatol. 2015;62:905–912. doi: 10.1016/j.jhep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Walle P., Takkunen M., Männistö V., Vaittinen M., Lankinen M., Kärjä V., Käkelä P., Ågren J., Tiainen M., Schwab U., et al. Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism. 2016;65:655–666. doi: 10.1016/j.metabol.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Paglialunga S., Dehn C.A. Clinical assessment of hepatic de novo lipogenesis in non-alcoholic fatty liver disease. Lipids Health Dis. 2016;15:159. doi: 10.1186/s12944-016-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda T., Matsuoka S., Yamazaki M., Shibata T., Nirei K., Takahashi H., Kaneko T., Fujisawa M., Higuchi T., Nakamura H., et al. Apoptosis and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2018;24:2661–2672. doi: 10.3748/wjg.v24.i25.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri P., Wiest M.M., Cheung O., Mirshahi F., Sargeant C., Min H.-K., Contos M.J., Sterling R.K., Fuchs M., Zhou H., et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiappini F., Desterke C., Bertrand-Michel J., Guettier C., Le Naour F. Hepatic and serum lipid signatures specific to nonalcoholic steatohepatitis in murine models. Sci. Rep. 2016;6:31587. doi: 10.1038/srep31587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouskeftara T., Goulas A., Ioannidou D., Ntenti C., Agapakis D., Assimopoulou A., Gika H. A Study of Blood Fatty Acids Profile in Hyperlipidemic and Normolipidemic Subjects in Association with Common PNPLA3 and ABCB1 Polymorphisms. Metabolites. 2021;11:90. doi: 10.3390/metabo11020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M., Mauer J., Xu E., Hammerschmidt P., Brönneke H.S., et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Hla T., Kolesnick R. C16:0-ceramide signals insulin resistance. Cell Metab. 2014;20:703–705. doi: 10.1016/j.cmet.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagadala M., Kasumov T., McCullough A.J., Zein N.N., Kirwan J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraioli G., Soares Monteiro L.B. Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 2019;25:6053–6062. doi: 10.3748/wjg.v25.i40.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noureddin M., Lam J., Peterson M.R., Middleton M., Hamilton G., Le T.-A., Bettencourt R., Changchien C., Brenner D.A., Sirlin C., et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caussy C., Reeder S.B., Sirlin C.B., Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. [(accessed on 1 July 2023)];Hepatology. 2018 68:763–772. doi: 10.1002/hep.29797. Available online: https://europepmc.org/articles/PMC6054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horáková D., Štěpánek L., Janout V., Janoutová J., Pastucha D., Kollárová H., Petráková A., Štěpánek L., Husár R., Martiník K. Optimal Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Cut-Offs: A Cross-Sectional Study in the Czech Population. Medicina. 2019;55:158. doi: 10.3390/medicina55050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.-C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 30.Hjelkrem M., Stauch C., Shaw J., Harrison S.A. Validation of the non-alcoholic fatty liver disease activity score. Aliment. Pharmacol. Ther. 2011;34:214–218. doi: 10.1111/j.1365-2036.2011.04695.x. [DOI] [PubMed] [Google Scholar]
- 31.Meikopoulos T., Deda O., Karagiannidis E., Sianos G., Theodoridis G., Gika H. A HILIC-MS/MS method development and validation for the quantitation of 13 acylcarnitines in human serum. Anal. Bioanal. Chem. 2022;414:3095–3108. doi: 10.1007/s00216-022-03940-9. [DOI] [PubMed] [Google Scholar]
- 32.Deda O., Panteris E., Meikopoulos T., Begou O., Mouskeftara T., Karagiannidis E., Papazoglou A.S., Sianos G., Theodoridis G., Gika H. Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules. 2022;12:354. doi: 10.3390/biom12030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begou O.A., Deda O., Karagiannidis E., Sianos G., Theodoridis G., Gika H.G. Development and validation of a RPLC-MS/MS method for the quantification of ceramides in human serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021;1175:122734. doi: 10.1016/j.jchromb.2021.122734. [DOI] [PubMed] [Google Scholar]
- 34.Ellis P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results. Cambridge University Press; Cambridge, UK: 2010. [Google Scholar]
- 35.Efron B., Tibshirani R.J. An Introduction to the Bootstrap. 1st ed. Chapman and Hall/CRC; Boca Raton, FL, USA: 1994. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable.