Abstract
Streptococcus intermedius is frequently associated with brain and liver abscesses, while pleuropulmonary infections are considered rarer. Even less frequent is the association of lung and brain abscesses due to this agent with infective endocarditis. We describe the case of a 40-year-old man complaining of cough, fever, and headache who was diagnosed with a brain abscess due to S. intermedius, a concomitant lung abscess, and aortic native valve endocarditis. He was treated with surgical drainage of the brain abscess and a 4-week course of intravenous ceftriaxone, followed by oral amoxicillin/clavulanate, obtaining healing of the lesions without relapse of the infection.
Keywords: Streptococcus intermedius, brain abscess, lung abscess, endocarditis, case report
1. Introduction
Streptococcus intermedius is a gram positive, catalase-negative coccus belonging to the Streptococcus anginosus group (SAG), which is often referred to as the Streptococcus milleri group, including S. intermedius, S. anginosus, and S. constellatus. SAG bacteria have been detected in the mouth, the gastrointestinal and upper respiratory tracts, and the vagina [1,2].
Streptococcus intermedius has been frequently associated with brain and liver abscesses, in rare cases related to endocarditis or congenital heart diseases [3,4,5,6,7,8]. Pleuropulmonary infections by this agent are uncommon [9], but are increasingly recognized. Absolutely anecdotal is the association of lung and brain abscesses due to S. intermedius with endocarditis [8]. We describe the case of a 40-year-old man with a brain abscess due to S. intermedius, a concomitant lung abscess, and aortic native valve endocarditis.
2. Case Presentation
The patient was a 40-year-old male of Egyptian origin who had resided in Italy for about 15 years and worked as a bartender. He had no allergies, previous hospitalizations, or surgical interventions; furthermore, he denied smoking habits, drug abuse, or alcohol consumption. For about 20 days, he experienced persistent cough, asthenia, intermittent fever, intense headache, and worsening back and lumbar pain that was partially responsive to therapy with paracetamol and non-steroidal anti-inflammatory drugs. Due to the worsening of these symptoms, he went to the Emergency Department (ED) of a district Hospital, where his vital signs were as follows: blood pressure 140/80 mmHg, heart rate 105 bpm, respiratory rate 17 breaths per minute, oxygen saturation 97% in room air, body temperature 38 °C, and Glasgow Coma Scale 15. There were no focal neurological deficits or meningeal signs. On thoracic objective examination, vesicular murmur was normally transmitted throughout the pulmonary field with no pathological moist noises; heart tones were paraphonic with poorly assessable pauses. On abdominal objective examination, the abdomen appeared flat, treatable, and non-tender on deep palpation, with negative Blumberg’s and Giordano’s signs. No major lymph nodes were palpable. The results of the oral cavity examination in the ED were not reported. Blood tests showed neutrophilic leukocytosis (white blood cells 12,900/mmc; neutrophils 77.7%) and a slight increase in C-reactive protein (1.2 mg/dL). The antigen test for SARS-CoV-2 was negative. No blood cultures were collected at that time. A non-contrast cranial computed tomography (CT) scan revealed a necrotic-colliquative expansive lesion measuring 36 mm in diameter in the left subcortical occipital area, with an associated perilesional oedema. The patient started treatment with ceftriaxone 2 g intravenously (IV) and methylprednisolone 4 mg as anti-oedema therapy.
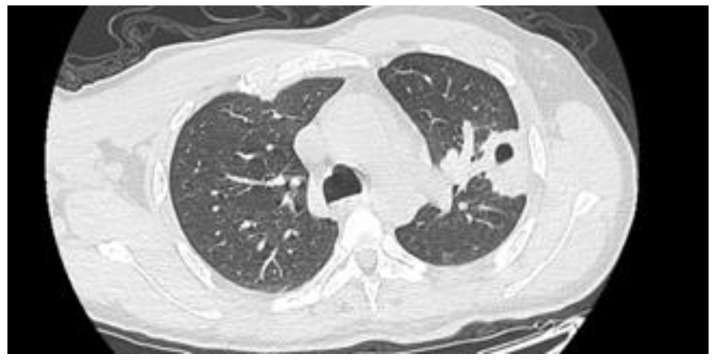
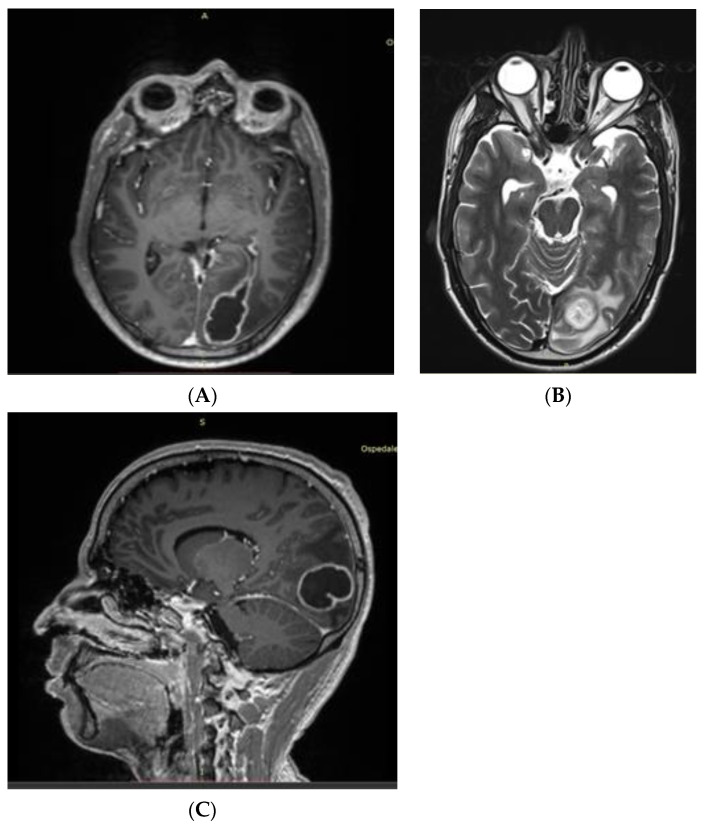
He was then transferred to the ED of a second-level Center with Neurosurgical facilities, where a contrast-enhanced chest CT scan showed a pleural-based consolidative lesion in the left lung lower lobe measuring 63 mm in diameter, associated with a 16 mm internal cavity (Figure 1). The patient was admitted to the Oncologic Pneumology department. After an Infectious Diseases consultation, the antibiotic therapy was modified to include meropenem 2 g three times/day (TID) and vancomycin 500 mg four times/day. QuantiFERON-TB (QTF) Gold Plus and blood cultures resulted in negative results. A nuclear magnetic resonance (NMR) scan (Figure 2) confirmed the abscess nature of the brain lesion and revealed its communication with the trigone of the left lateral ventricle, along with perilesional oedema.
Figure 1.
Chest CT scan showing a consolidated area with a thick-walled cavity lesion in the left lung lower lobe.
Figure 2.
(A) First axial contrast-enhanced T1-weighted NMR showing an oval-shaped enhanced lesion in the left occipital lobe with ipsilateral ventricular communication. (B) T2-weighted NMR showing ventricular communication of the lesion with T2 hyperintense fluid collection. (C) T1-weighted sagittal view of the abscess.
He was then transferred to the Neurosurgery department, where a bronchial aspiration was performed for direct microscopic examination, culture, and DNA testing for Mycobacterium tuberculosis complex, non-tuberculous mycobacteria, and Aspergillus. A full-spine NMR scan was also conducted but was negative for focal lesions. Pending microbiologic results, considering the possible tuberculous etiology of the disease, empirical combination therapy with linezolid 600 mg twice daily, meropenem 2 g TID, rifampicin 600 mg once daily (QD), isoniazid 300 mg QD, ethambutol 1200 mg QD, and levofloxacin 750 mg QD was started; dexamethasone 6 mg TID was also administered as suggested in the therapy of tuberculous meningitis [10].
Subsequently, a left occipital parasagittal craniotomy procedure was performed, resulting in drainage of the abscess and partial removal of the abscess capsule, along with sampling of biological material. The culture of the cerebral abscess samples resulted in a positive result for Streptococcus intermedius, which is sensitive to ampicillin, ceftriaxone, clindamycin, teicoplanin, and vancomycin. Thus, the patient started targeted therapy with ceftriaxone (2 g IV BID) and dexamethasone (12 mg IV BID). Following the QTF and PCR results, which tested negative for mycobacteria and fungi from bronchoalveolar lavage and from brain abscess specimens, the previous anti-tubercular therapy was discontinued.
The patient was then transferred to our Infectious Diseases Center in stable and afebrile conditions. During the hospitalization, a transthoracic echocardiogram was performed to rule out heart valve involvement. An image with an uncertain interpretation was noticed in the aortic valve, so, in agreement with the cardiologists, this lesion was further investigated. A transesophageal color Doppler echocardiogram (TEE) was performed, revealing an aortic valve thickening with a prolapsing attitude of the right cusp and a nodular hyperechogenic image between the right and left cusps, along with mild valve insufficiency. The nodule was 8 × 6 mm in diameter without echocardiographic features suggesting a risk of embolization, possibly because embolization had already occurred. TEE was done after the diagnosis of a cerebral abscess and after 2 weeks of antibiotic therapy. There were no signs of left ventricular dilatation. An ultrasound scan of the abdomen was negative for embolic lesions. According to the 2023 Duke-ISCVID criteria [11], this case can be considered a definite endocarditis according to clinical criteria, as one major criterion (TEE positive) and three minor criteria (fever, cerebral abscess, positive culture from an embolus) were concomitant. Due to the presence of multiple carious lesions, a dental CT scan was performed, showing periapical bone resorption at tooth 14 in the upper arch and a large carious lesion at tooth 36, accompanied by an inflammatory periapical bone resorption area.
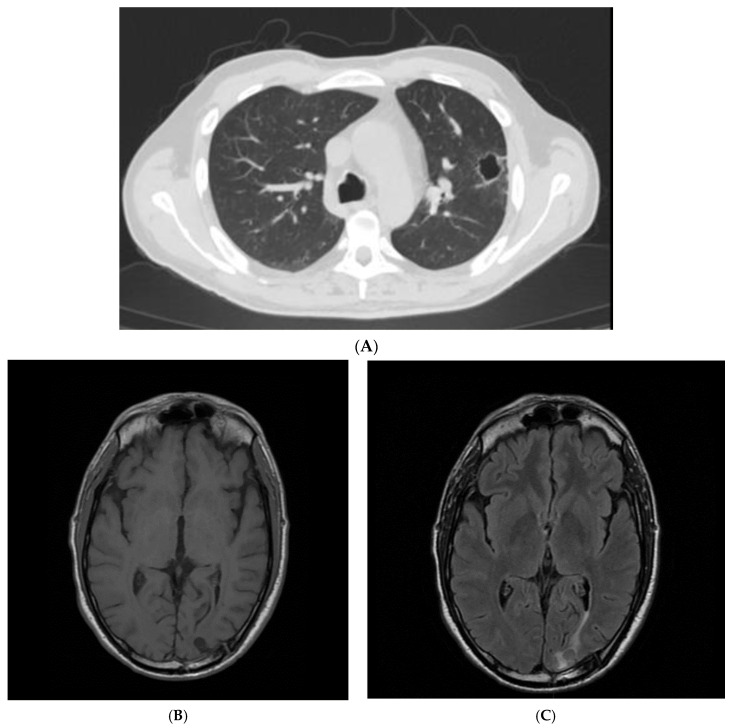
The patient received a four-week course of ceftriaxone 2 g IV BID, resulting in progressive improvement of his clinical, laboratory, and radiological findings (Figure 3). He was discharged in good general condition with instructions to continue oral therapy with amoxicillin/clavulanate (875/125 mg TID) for another 10 days and a program of cardiological, neurosurgical, and dental outpatient visits. After a three-month follow-up, the patient is still in good health with no signs of infection relapse, as assessed in our outpatient clinic.
Figure 3.
(A) A follow-up CT scan showing the reduction of the left lung lesion after a 4-week antibiotic course. (B) Follow-up T1-weighted brain NMR after a 4-week antibiotic course. (C) Follow-up T2-weighted brain NMR after a 4-week antibiotic course and surgical drainage.
3. Discussion
S. intermedius is a known cause of brain abscesses and endocarditis. Research conducted by PubMed with the keywords “Streptococcus intermedius” AND “brain abscess” obtained 123 articles, while the keywords “Streptococcus intermedius” AND “endocarditis” yielded 57 papers. However, the association between S. intermedius endocarditis and brain abscess is rarely reported. In fact, using the keywords “Streptococcus intermedius” AND “endocarditis” AND “brain abscess”, we found 12 works, of which just 4 actually described cases of S. intermedius endocarditis with brain abscess [3,4,6,8], and 1 reported a case of S. intermedius brain abscess related to a patent foramen ovale [5] (Table 1).
Table 1.
Articles describing S. intermedius brain abscesses associated with endocarditis or PFO.
| Article | n. of Patients |
Sex | Age (y) | Infection Site | Outcome |
|---|---|---|---|---|---|
| Carrena et al., 2018 [8] | 1 | M | 61 | Lung, brain, Chiari network endocarditis | Recovered |
| Honnorat et al., 2016 [4] | 1 | M | 56 | Brain, endocarditis on surgical patch | Recovered |
| Syros et al., 2011 [5] | 1 | M | 20 | Brain; concomitant PFO | Recovered |
| Nakaya et al., 1998 [6] | 1 | M | 60 | Brain, mitral valve endocarditis | Recovered |
| Melo et al., 1978 [3] | 1 | M | 69 | Liver, brain and presumptive endocarditis | Death |
PFO—patent foramen ovale.
Pleuropulmonary infections due to Streptococcus intermedius are also considered uncommon [12]; however, in recent years, their importance has been increasingly recognized. We reviewed the available medical literature by PubMed with the keywords “Streptococcus intermedius” AND “lung abscess”, “pleural effusion” and “pleural empyema” from 1993 to 2023, published in English. We found 34 articles meeting the inclusion criteria (Table 2), of which 4 described concomitant brain and lung abscesses, but none reported the presence of lung and brain abscesses due to S. intermedius associated with left sided endocarditis [1,2,8,9,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Only one paper [8] described the case of a lung and brain abscess associated with Chiari network endocarditis in the right heart.
Table 2.
Articles describing lung abscesses and/or pleural infections due to S. intermedius.
| Article | n. of Patients |
Sex | Age (y) | Infection Site | Outcome |
|---|---|---|---|---|---|
| Shinzato et al., 1995 [1] | 9 | M = 8 F = 1 |
Mean 61.3 | Lung, pleura | NA |
| Noguchi et al., 2015 [2] | 14 | M = 10 F = 4 |
Mean 77.3 | Lung/pleura | Recovered = 12 Death = 2 |
| Bueno et al., 2023 [9] | 1 | F | 25 | Lung | Recovered |
| Erne et al., 2010 [12] | 1 | M | 61 | Lung, brain | Recovered |
| Jerng et al., 1997 [13] | 17 | NA | NA | Lung/pleura | NA |
| Chandy et al., 2001 [14] | 1 | M | 21 | Blood, lung, frontal sinus, epidural abscess | Recovered |
| May et al., 2010 [15] | 1 | M | 53 | Lung | Death |
| Van Laren et al., 2011 [16] | 1 | F | 29 | Blood, lung, genital | Recovered |
| De Cruif et al., 2012 [17] | 1 | F | 63 | Lung, dental abscess | Recovered |
| Trabue et al., 2014 [18] | 1 | M | 36 | Lung, brain | NA |
| Maeda et al., 2012 [19] | 1 | F | 46 | Chest wall abscess, pleura | Recovered |
| Armendariz-Guezala et al., 2017 [20] | 1 | M | 33 | Brain, lung | Recovered |
| Sakurai et al., 2020 [21] | 1 | M | 80 | Pleura, iliopsoas abscess | Recovered |
| Carrena et al., 2018 [8] | 1 | M | 61 | Lung, brain, Chiari network endocarditis | Recovered |
| Fujihara et al., 2021 [22] | 1 | M | 64 | Lung, pleura | Death |
| Tasleem et al., 2021 [23] | 1 | M | 54 | Lung, pleura | Death |
| Manasrah et al., 2021 [24] | 1 | M | 54 | Lung, pleura, vertebrae, and discitis | Recovered |
| Nakagawa et al., 2022 [25] | 1 | M | 6 mo | Lung, pleura | Recovered |
| Christensen et al., 1993 [26] | 1 | M | 56 | Lung | Recovered |
| Patail et al., 2020 [27] | 1 | M | 30 | Lung, pleura | NA |
| Takahashi et al., 2019 [28] | 1 | F | 83 | Pleura | Recovered |
| Dyrhovden et al., 2019 [29] | 16 | NA | NA | Pleura | NA |
| Cobo et al., 2018 [30] | 9 | M = 7 F = 2 |
Mean 63.9 | Lung, pleura | Recovered = 8 Death = 1 |
| Crespo Valades et al., 2005 [31] | 1 | M | 60 | Pleural effusion, subphrenic abscess | Recovered |
| Noguchi et al., 2014 [32] | 1 | M | 79 | Lung, pleura | Recovered |
| Hannoodi et al., 2016 [33] | 1 | F | 52 | Lung, pleura | Recovered |
| Mautner et al., 2000 [34] | 1 | M | 80 | Lung, pleura | Recovered |
| Huang et al., 2022 [35] | 1 | M | 10 | Lung, pleura | Recovered |
| Kurkowski et al., 2022 [36] | 1 | M | 39 | Liver, pleura, blood | Recovered |
| Jud et al., 2019 [37] | 1 | M | 59 | Lung, pleura | Recovered |
| Lescan et al., 2013 [38] | 1 | M | 74 | Lung, pleura, epidural abscess | Recovered |
| Stelzmueller et al., 2006 [39] | 2 | NA | NA | Pleural empyema | Recovered |
| Iskandar et al., 2006 [40] | 1 | NA | NA | Pleural empyema | NA |
| Lau et al., 2002 [41] | 1 | M | 32 | Pleural empyema, blood | Recovered |
NA—not available; mo—months.
Streptococcus intermedius is a Gram-positive, catalase-negative, nonmotile, and facultative anaerobe coccus that colonizes the mouth and the upper respiratory tract [2,33]. SAG streptococci are oral bacteria and may be unable to grow significantly on ordinary aerobic cultures; as a consequence, conventional cultivation may underestimate the role of these pathogens [32] in respiratory infections. The significance of anaerobes and oral bacteria in patients with community-acquired pneumonia and pleuritis has previously been reported [42,43]. In some cases, a 16S rRNA gene sequencing analysis of bronchoalveolar or pleural effusion specimens was able to identify pathogens that are generally difficult to isolate using ordinary cultivation methods [32].
Risk factors for S. intermedius pleuropulmonary disease include smoking, alcoholism, dental diseases, chronic obstructive pulmonary disease, malignant neoplasms, liver cirrhosis, and diabetes [9]. Our patient had dental lesions that could have caused bacteremia and aortic valve endocarditis, with a brain abscess as a hematogenous spread complication. The lung abscess could have been caused by aspiration or hematogenous spread. Teramoto et al. [44] reported that aspiration may contribute to the pathogenesis of pneumonia in elderly patients and that an increased age is associated with the risk of developing aspiration pneumonia. Given the young age of our patient, the aspiration in this case is less likely, whereas the hematogenous spread could have caused the lung abscess. Bacteraemic venous blood following the venous draining system to the right ventricle of the heart is pumped into the pulmonary arteries, the capillary network of alveoli, and parts of the visceral pleura. Indeed, several works describe the simultaneous occurrence of brain abscess, and lung abscess or pleural empyema [29]. In their recent work, Dyrhovden and coll. suggest that facultative and anaerobic oral bacteria, able to spread by deoxygenated venous blood to establish purulent infections in brain tissue, could also be able to reach and establish pyogenic infections in the lung parenchyma or pleural cavity [29].
S. intermedius is reported as a causative pathogen in only 2–5% of cases of bacterial pneumonia but in 13–44% of pulmonary abscesses/empyema [28]. S. intermedius infections are commonly associated with abscess development. Virulence factors contributing to tissue invasion and abscess formation by this agent have recently been described by Issa and coll [45] and include antigens I/II surface proteins; hydrolytic enzymes such as hyaluronidase, chondroitin sulfatase, and deoxyribonuclease; biofilm formation and defensive genes to oppose the human immune system, such as superantigens that cause lymphocyte apoptosis; the polysaccharidic capsule formation; the genes of the Streptococcus Invasion locus system; the intermedilysin, which initiates pore complex formation in host cell membranes, and the sialidase A that contributes to pathogenicity by controlling interbacterial communication and host-bacterial interactions. Abscess drainage and surgery should be considered on a case-by-case basis in order to successfully achieve adequate source control of the infection.
The major strengths of this case include the involvement of many different specialists in the management of this complex bacterial infection (ED clinicians, neurosurgeons, pneumologists, cardiologists, radiologists, infectious disease physicians, bacteriologists, and dentists) and the diagnostic work-up leading to the identification of the probable source of infection (dental lesions) and the relevant complications that followed. One important limitation is the lack of blood cultures drawn on hospital admission, which could have further strengthened the diagnosis.
4. Conclusions
In summary, we described a case of brain abscess caused by S. intermedius, associated with lung abscess and aortic valve endocarditis, in a young patient with dental disorders. In addition to being a well-known cause of brain abscesses, S. intermedius is increasingly recognized as a causative agent of pleuropulmonary disease.
Author Contributions
Each author has made substantial contributions to the conception of the work, to the acquisition of data and to the revision of the paper. Each author agrees to be personally accountable for the author’s own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. Conceptualization, F.G. and P.C.; Data Curation, G.C. and G.O.; Methodology, A.C. and F.T.; Writing—Original Draft Preparation, P.C. and G.G.; Writing—Review and Editing, S.A.M. and E.C.; Supervision, F.T. and P.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethical Committee of National Institute for Infectious Diseases “L. Spallanzani”, Rome (Italy); protocol code E2-2023, approved on 17 July 2023.
Informed Consent Statement
Written informed consent for publication was obtained from the patient for the case report and imaging.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Conflicts of Interest
F.T. received grants from Tillots, MSD, Menarini, Angelini, Gilead. All other authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Shinzato T., Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin. Infect. Dis. 1995;21((Suppl. S3)):S238–S243. doi: 10.1093/clind/21.supplement_3.s238. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi S., Yatera K., Kawanami T., Yamasaki K., Naito K., Akata K., Shimabukuro I., Ishimoto H., Yoshii C., Mukae H. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015;15:133. doi: 10.1186/s12890-015-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo J.C., Raff M.J. Brain abscess due to Streptococcus MG-intermedius (Streptococcus milleri) J. Clin. Microbiol. 1978;7:529–532. doi: 10.1128/jcm.7.6.529-532.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honnorat E., Seng P., Riberi A., Habib G., Stein A. Late infectious endocarditis of surgical patch closure of atrial septal defects diagnosed by 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT): A case report. BMC Res. Notes. 2016;9:416. doi: 10.1186/s13104-016-2223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syros G., Briasoulis A., Psevdos G. Suspicious seizures: Uncommon complication of PFO/ASA. J. Cardiol. Cases. 2011;3:e170–e172. doi: 10.1016/j.jccase.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakaya M., Okimoto M., Abe H., Sato A., Watanabe Y., Nakajima N. A mitral valve reconstruction of infective endocarditis with brain abscess and intracranial mycotic aneurysm. Jpn. J. Thorac. Cardiovasc. Surg. 1998;46:647–650. doi: 10.1007/BF03217796. [DOI] [PubMed] [Google Scholar]
- 7.Tran M.P., Caldwell-McMillan M., Khalife W., Young V.B. Streptococcus intermedius causing infective endocarditis and abscesses: A report of three cases and review of the literature. BMC Infect. Dis. 2008;8:154. doi: 10.1186/1471-2334-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrena O., Oluoha O., Wahba A., Eshun D., Endsley M., Okafor H. Complicated Infective Endocarditis Limited to a Chiari Network. Case Rep. Cardiol. 2018;2018:3837825. doi: 10.1155/2018/3837825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueno C.O.P., Trillos S.J.G., Rosales D.J.C., García E.A.B. Lung abscess due to Streptococcus intermedius associated with SARS-CoV-2 infection in pregnancy: Unusual presentation and complication of a commensal bacteria during pregnancy. Clin. Case Rep. 2023;11:e6763. doi: 10.1002/ccr3.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thwaites G.E., Nguyen D.B., Nguyen H.D., Hoang T.Q., Do T.T., Nguyen T.C., Nguyen Q.H., Nguyen T.T., Nguyen N.H., Nguyen T.N., et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 11.Fowler V.G., Durack D.T., Selton-Suty C., Athan E., Bayer A.S., Chamis A.L., Dahl A., DiBernardo L., Durante-Mangoni E., Duval X., et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023:ciad271. doi: 10.1093/cid/ciad271. [DOI] [Google Scholar]
- 12.Erne B.V., Exner C., Frauenfelder T., Schmid S., Weder W. A curious case of convulsion. Lancet. 2010;375:2050. doi: 10.1016/S0140-6736(10)60592-2. [DOI] [PubMed] [Google Scholar]
- 13.Jerng J.S., Hsueh P.R., Teng L.J., Lee L.N., Yang P.C., Luh K.T. Empyema thoracis and lung abscess caused by viridans streptococci. Am. J. Respir. Crit. Care Med. 1997;156:1508–1514. doi: 10.1164/ajrccm.156.5.97-03006. [DOI] [PubMed] [Google Scholar]
- 14.Chandy B., Todd J., Stucker F.J., Nathan C.O. Pott’s puffy tumor and epidural abscess arising from dental sepsis: A case report. Laryngoscope. 2001;111:1732–1734. doi: 10.1097/00005537-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 15.May A.M., Riede F.N., Riede U.N. Acute subepicardial infarction associated with severe septic shock--insight in myocardial perfusion. Pathol. Res. Pract. 2010;206:401–404. doi: 10.1016/j.prp.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Van Laren M., van Walree N.C., Kluytmans J.A. Multiple lung abscesses secondary to a uterine empyema caused by an intrauterine device. Infection. 2011;39:385–387. doi: 10.1007/s15010-011-0118-4. [DOI] [PubMed] [Google Scholar]
- 17.De Kruif M.D., van Gorp E.C., Bel E.H., Gerlag D.M., Kunst P.W. Streptococcal lung abscesses from a dental focus following tocilizumab: A case report. Clin. Exp. Rheumatol. 2012;30:951–953. [PubMed] [Google Scholar]
- 18.Trabue C., Pearman R., Doering T. Pyogenic brain and lung abscesses due to Streptococcus intermedius. J. Gen. Intern. Med. 2014;29:407. doi: 10.1007/s11606-013-2565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S., Sado T., Sakurada A., Okada Y., Kondo T. Successful closure of an open-window thoracostomy wound by negative-pressure wound therapy: Report of a case. Surg. Today. 2012;42:295–298. doi: 10.1007/s00595-011-0008-5. [DOI] [PubMed] [Google Scholar]
- 20.Armendariz-Guezala M., Undabeitia-Huertas J., Samprón-Lebed N., Michan-Mendez M., Ruiz-Diaz I., Úrculo-Bareño E. Actinomycotic brain abscess in immunocompetent patient. Cir. Cir. 2017;85((Suppl. S1)):103–107. doi: 10.1016/j.circir.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai M., Nagasawa H., Takeuchi I., Yanagawa Y. A Case of an 80-Year-Old Man with Empyema and Psoas Abscess. Case Rep. Emerg. Med. 2020;2020:8895785. doi: 10.1155/2020/8895785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujihara T., Itoh N., Yamamoto S., Kurai H. Lateral thoracic artery aneurysm with lung abscess and empyema caused by Streptococcus intermedius. J. Gen. Fam. Med. 2021;22:296–297. doi: 10.1002/jgf2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasleem A., Mahmood A., Sharma R. Streptococcus intermedius Pleuropulmonary Disease: A Not So Commonly Seen Radiological Picture. Cureus. 2021;13:17385. doi: 10.7759/cureus.17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manasrah N., Nanja Reddy S., Al Sbihi A., Hafeez W. Streptococcus intermedius: Unusual presentation and complication of lung abscess. BMJ Case Rep. 2021;14:e245675. doi: 10.1136/bcr-2021-245675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa Y., Otake S., Oue T., Ryu H., Kasai M. Case of infant invasive Streptococcus intermedius infection suggesting the need for anaerobic cultures. J. Infect. Chemother. 2022;28:437–439. doi: 10.1016/j.jiac.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Christensen P.J., Kutty K., Adlam R.T., Taft T.A., Kampschroer B.H. Septic pulmonary embolism due to periodontal disease. Chest. 1993;104:1927–1929. doi: 10.1378/chest.104.6.1927. [DOI] [PubMed] [Google Scholar]
- 27.Patail H., Patail H., Ahmad S. A Man in His 30s Presenting with Shortness of Breath and Productive Cough After a Recent Pneumonia. Chest. 2020;157:e91–e93. doi: 10.1016/j.chest.2019.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi S., Ishitsuka T., Namatame K., Nakamura M. A false-positive pneumococcal rapid urinary antigen test in Streptococcus intermedius infection. Respirol. Case Rep. 2019;7:e00466. doi: 10.1002/rcr2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyrhovden R., Nygaard R.M., Patel R., Ulvestad E., Kommedal Ø. The bacterial aetiology of pleural empyema. A descriptive and comparative metagenomic study. Clin. Microbiol. Infect. 2019;25:981–986. doi: 10.1016/j.cmi.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Cobo F., Sampedro A., Rodríguez-Granger J., Aliaga-Martínez L., Navarro-Marí J.M. Clinical and microbiologic characteristics of pleuro-pulmonary infection due to Streptococcus intermedius. Rev. Esp. Quimioter. 2018;31:146–151. [PMC free article] [PubMed] [Google Scholar]
- 31.Crespo Valadés E., Fernández Blanco J.M., Troya García J., Malmierca Corral M. Primary left subphrenic abscess due to Streptococcus intermedius. Ann. Med. Intern. 2005;22:202–203. doi: 10.4321/s0212-71992005000400016. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi S., Yatera K., Kawanami T., Yamasaki K., Fukuda K., Naito K., Akata K., Nagata S., Ishimoto H., Taniguchi H., et al. Pneumonia and empyema caused by Streptococcus intermedius that shows the diagnostic importance of evaluating the microbiota in the lower respiratory tract. Intern. Med. 2014;53:47–50. doi: 10.2169/internalmedicine.53.0971. [DOI] [PubMed] [Google Scholar]
- 33.Hannoodi F., Ali I., Sabbagh H., Kumar S. Streptococcus intermedius Causing Necrotizing Pneumonia in an Immune Competent Female: A Case Report and Literature Review. Case Rep. Pulmonol. 2016;2016:7452161. doi: 10.1155/2016/7452161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mautner G.H., Lu I., Ort R.J., Grossman M.E. Transverse leukonychia with systemic infection. Cutis. 2000;65:318–320. [PubMed] [Google Scholar]
- 35.Huang M., Li S., Wu X., Xu D., Tang L., Chen Z. An isolated pulmonary nodule secondary to Streptococcus intermedius infection in an otherwise healthy 10-year-old boy: A case report and literature review. Front. Pediatr. 2022;10:921258. doi: 10.3389/fped.2022.921258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurkowski S.C., Thimmesch M.J., Jha P., Abdelgadir Y.H. Streptococcus intermedius Bacteremia and Pyogenic Liver Abscess in a Patient with No Risk Factors. Cureus. 2022;14:e26786. doi: 10.7759/cureus.26786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jud P., Fink-Neuboeck N., Lindenmann J. Massive ventilator-associated pleural empyema. Korean J. Intern. Med. 2019;34:942–943. doi: 10.3904/kjim.2017.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lescan M., Lepski G., Steger V., Schlensak C., Walker T. Rapidly progressive paraplegia and pleural empyema: How does that correlate? Gen. Thorac. Cardiovasc. Surg. 2013;61:640–642. doi: 10.1007/s11748-012-0199-8. [DOI] [PubMed] [Google Scholar]
- 39.Stelzmueller I., Berger N., Wiesmayr S., Eller M., Tabarelli W., Fille M., Margreiter R., Bonatti H. Group milleri streptococci: Significant pathogens in solid organ recipients. Transpl. Int. 2007;20:51–56. doi: 10.1111/j.1432-2277.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Iskandar S.B., Al Hasan M.A., Roy T.M., Byrd R.P., Jr. Streptococcus intermedius: An unusual cause of a primary empyema. Tenn. Med. 2006;99:37–39. [PubMed] [Google Scholar]
- 41.Lau S.K., Woo P.C., Chan B.Y., Fung A.M., Que T.L., Yuen K.Y. Haemophilus segnis polymicrobial and monomicrobial bacteraemia identified by 16S ribosomal RNA gene sequencing. J. Med. Microbiol. 2002;51:635–640. doi: 10.1099/0022-1317-51-8-635. [DOI] [PubMed] [Google Scholar]
- 42.Kawanami T., Fukuda K., Yatera K., Kido M., Mukae H., Taniguchi H. A higher significance of anaerobes: The clone library analysis of bacterial pleurisy. Chest. 2011;139:600–608. doi: 10.1378/chest.10-0460. [DOI] [PubMed] [Google Scholar]
- 43.Yamasaki K., Kawanami T., Yatera K., Fukuda K., Noguchi S., Nagata S., Nishida C., Kido T., Ishimoto H., Taniguchi H., et al. Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS ONE. 2013;8:e63103. doi: 10.1371/journal.pone.0063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teramoto S., Fukuchi Y., Sasaki H., Sato K., Sekizawa K., Matsuse T., Japanese Study Group on Aspiration Pulmonary Disease High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: A multicenter, prospective study in Japan. J. Am. Geriatr. Soc. 2008;56:577–579. doi: 10.1111/j.1532-5415.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 45.Issa E., Salloum T., Tokajian S. From Normal Flora to Brain Abscesses: A Review of Streptococcus intermedius. Front. Microbiol. 2020;11:826. doi: 10.3389/fmicb.2020.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.