Abstract
We have developed a method for determining the serotypes of poliovirus isolates by PCR. Three sets of serotype-specific antisense PCR-initiating primers (primers seroPV1A, seroPV2A, and seroPV3A) were designed to pair with codons of VP1 amino acid sequences that are conserved within but that differ across serotypes. The sense polarity primers (primers seroPV1S, seroPV2S, and seroPV3S) matched codons of more conserved capsid sequences. The primers contain mixed-base and deoxyinosine residues to compensate for the high rate of degeneracy of the targeted codons. The serotypes of all polioviruses tested (48 vaccine-related isolates and 110 diverse wild isolates) were correctly identified by PCR with the serotype-specific primers. None of the genomic sequences of 49 nonpolio enterovirus reference strains were amplified under equivalent reaction conditions with any of the three primer sets. These primers are useful for the rapid screening of poliovirus isolates and for determining the compositions of cultures containing mixtures of poliovirus serotypes.
The World Health Organization has established the year 2000 as the target date for the global eradication of wild polioviruses (12). Good progress has been achieved toward meeting this goal (12, 29). As polio eradication is approached, increased emphasis has been placed upon strengthening the field (3) and virologic (24) components of wild poliovirus surveillance, prompting the development of more specific and sensitive methods for the detection and identification of polioviruses in clinical specimens or environmental samples (28).
The three poliovirus serotypes were originally defined by their patterns of reactivity with neutralizing antibodies (4). The current standard methods for typing poliovirus isolates are neutralization tests with pools of type-specific antisera (19) or enzyme-linked immunosorbent assays with antisera specific for individual serotypes (28). Serologic methods have also been used for the intratypic differentiation of poliovirus isolates (21, 28). Although the serologic methods are generally reliable, they require the preparation of batches of specific antisera, whose specificities have been approached, but not matched, by panels of monoclonal antibodies (28).
In recent years, serologic approaches have been supplemented with a variety of powerful molecular methods. Polioviruses can be cultured selectively from most nonpolio enteroviruses (NPEVs) in recombinant murine cells containing the human receptor for polioviruses (11). Alternatively, polioviruses can be distinguished from NPEVs in PCR assays with poliovirus-specific primers (14). Specific RNA probes and PCR primers recognizing Sabin vaccine-related isolates (5, 30) or different wild poliovirus genotypes (6, 31) have been developed for routine diagnostic use. Poliovirus isolates have also been identified by restriction fragment length polymorphism analysis of the products amplified with broadly reacting primers (1, 26). The molecular methods generally have the combined advantages of high specificities, good selectivities, rapid performance, and ease of use.
When genotype-specific molecular reagents are available, identification of poliovirus isolates is rapid and straightforward and does not require virus typing (5, 6, 30, 31). However, specific probes and primer sets are not yet available for all contemporary wild poliovirus genotypes (13). Wild polioviruses can be identified by exclusion by using Sabin strain-specific molecular reagents (5, 30), but this approach is dependent upon the accurate typing of the virus isolates. Consequently, we sought to develop rapid methods for differentiating poliovirus serotypes by PCR. By extension of an approach we had previously taken for the development of poliovirus group-specific PCR primers (14), we designed degenerate PCR primers targeted to codons of VP1 amino acid sequences that are conserved within but not across poliovirus serotypes. In this report we describe the properties and potential application of poliovirus serotype-specific PCR primers.
MATERIALS AND METHODS
Viruses.
The poliovirus isolates used in this study (Table 1) had previously been characterized by neutralization with hyperimmune equine sera, in vitro amplification with panPV PCR primers (14), probe hybridization (5), and partial genomic sequencing (13). Vaccine-related isolates were also directly identified by PCR with Sabin strain-specific primer pairs (30). Viruses were propagated in HEp-2 cell (a human larynx epidermoid carcinoma cell line; ATCC CCL23) or RD cell (a human rhabdomyosarcoma cell line; ATCC CCL136) monolayers to produce high-titer inoculation stocks.
TABLE 1.
Specific detection of each poliovirus serotype with serotype-specific PCR primers
| Isolate type and PCR primer pair | No. positive/no. tested
|
||
|---|---|---|---|
| Type 1 | Type 2 | Type 3 | |
| Vaccine-related isolatesa | |||
| PV1,2S + PV1A | 16/16 | 0/16 | 0/16 |
| PV1,2S + PV2A | 0/16 | 16/16 | 0/16 |
| PV3S + PV3A | 0/16 | 0/16 | 16/16 |
| Wild isolatesb | |||
| PV1,2S + PV1A | 60/60 | 0/20 | 0/30 |
| PV1,2S + PV2A | 0/60 | 20/20 | 0/30 |
| PV3S + PV3A | 0/60 | 0/20 | 30/30 |
Vaccine-related isolates tested include poliovirus serotype 1 3875/MOG91, 0074/PER88, 5498/USA84, 2800/HON91, 0584/GUT91, 9825/USA89, 9703/ELS89, 9360/VEN89, 9240/HON89, 8315/MEX88, 8284/HON88, 8221/GUT87, 7245/COL86; 6529/CHI86, 6440/ARG85, and 6258/MORP85; poliovirus type 2, 0042/ELS90, 9364/GUT89, 7653/SOA86, 0636/ELS91, 9897/GUT90, 0078/PER89, 9819/BRA89, 9818/PER89, 9579/USA89, 8378/PER88, 8238/GUT87, 8018/GUT87, 7170/MEX86, 6700/HON86; 7837/PER84, and 6886/GUT83; poliovirus type 3, 6114/LAO94, 1063/USA91, 0131/MEX89, 0644/HON91, 0642/ELS91, 0405/GUT90, 0040/ELS90, 9896/GUT89, 9847/MEX89, 9442/NIC89, 9441/GUT89, 1339/CHN89, 0044/GUT89, 8774/TRT88, 6880/COL86, and 7149/MEX84.
Wild isolates tested, in addition to those in Fig. 2 to 4, include poliovirus type 1, 6306/TKM95, 6103/THA94, 5740/CHN93, 6118/NEP92, 6111/MMR92, 6015/CAM94, 6001/VTN94, 3940/THA92, 3706/MAA92, 3677/CYP92, 0004/TJK91, 18655/PAK91, 16838/TUR90, 11270/EGY91, 8649/IND91, 8645/IND91, 7362/PAK91, 7169/BUL91, 3643/CHN91, 2609/ETH91, 1184/ROM91, 0427/SSR91, 0124/CHN91, 0062/PER91, 6701/TUR90, 3727/AZE90, 2786/VTN90, 9475/ZAI89, 9366/SAA89, 2758/VTN89, 1338/CHN89, 0006/CHN89, 8771/OMA88, 5145/UZB88, 8425/ISR88, 1607/SOA88, 0955/SRL88, 8223/GUT87, 2662/COL87, 0941/SRL87, 0289/POR87, 7054/IND86, 6750/SEN86, 0285/INO86, 0109/CHN86, 9258/TUN85, 6224/ZIM85, and 3638/CHN85; poliovirus type 2, 8654/IND91, 7354/PAK91, 3869/IND91, 6876/COL86, 0302/YUG81, 2996/SWE77, 0290/TUR73, and 0710/KEN71; poliovirus type 3, 6112/MMR93, 6093/THA93, 6113/LAO92, 3904/SYR91, 8668/IND91, 7350/PAK91, 3838/EGY91, 15953/FRA90, 4076/ARM90, 2619/MOL90, 0010/TJK90, 0380/MEX90, 2732/UZB89, 9259/TUN88, 9035/BRA88, 0324/INO86, 8178/VEN87, and 7840/PER86. Country abbreviations are as defined by the World Health Organization (5). The RNAs of the reference strains (19) of 49 NPEVs (coxsackievirus A types 3 to 6, 8 to 10, 12, 14, 21, and 24; coxsackievirus B types 1 to 6; echovirus types 3 to 9, 11 to 21, 24 to 27, and 29 to 34; and enterovirus types 68 to 71) were also analyzed in PCR assays containing the seroPV PCR primer pairs. All assays were negative for specific amplification products.
Oligonucleotide synthesis.
Synthetic oligodeoxynucleotides were prepared, purified, and analyzed as described previously (30). The degenerate primers used for amplifying individual serotypes were as follows: seroPV1,2S (positions 2459 to 2477), 5′-TGCGIGA(C/T)ACIACICA(C/T)AT-3′; seroPV1A (positions 2528 to 2509), 5′-ATCATICT(C/T)TCIA(A/G)CAT(C/T)TG-3′; seroPV2A (positions 2537 to 2518), 5′-A(C/T)ICC(C/T)TCIACI(A/G)CICC(C/T)TC-3′; seroPV3S (positions 3037 to 3056), 5′-AA(C/T)CCITCI(A/G)TITT(C/T)TA(C/T)AC-3′; and seroPV3A (positions 3176 to 3157), 5′-CCIAI(C/T)TGITC(A/G)TTIG(C/T)(A/G)TC-3′. Primer polarities are indicated by A (antisense or antigenome polarity) or S (sense or genome polarity). The numbers in parentheses indicate the genomic sequences matching the primers (Fig. 1 and 2) according to the consensus numbering system of Toyoda et al. (27). Deoxyinosine residues are indicated by the letter I. Primer positions having equimolar amounts of two different nucleotides are enclosed in parentheses.
FIG. 1.
The locations along poliovirus genome of the sequences targeted by seroPV PCR primers are depicted in the diagram at the top. Untranslated regions are indicated as lines; the region of the translated polyprotein is represented by the rectangle. Shaded areas indicate locations of surface loops forming neutralization antigenic sites 1, 2a, and 3 (20). Arrows indicate the locations and polarities of the seroPV PCR primers. The alignment of the amino acid residues (in boldface type) whose codons are specifically bound by the seroPV PCR primers is indicated below. The surface residues forming neutralizing antigenic site 2a (20) are underlined. Amino acid differences among the target sites within and across serotypes are shown for 20 independent poliovirus isolates (13). The locations of capsid amino acid residues are given by four-digit numbers (15): the first digit identifies the virion protein and the next three digits specify residue position (e.g., 1001 indicates residue 1 of VP1). Country abbreviations are as defined by the World Health Organization (5).
FIG. 2.
Alignment of seroPV PCR primers with poliovirus target sequences. The targeted amino acid sequences (top) are aligned with all possible encoding nucleotide sequences (middle) and the sequences of the seroPV PCR primers (bottom). Abbreviations for nucleotides follow the International Union of Biochemistry nomenclature (22): H, adenine, cytosine, or thymine; I, inosine; N, adenine, cytosine, guanine, or thymine; R, adenine or guanine; Y, cytosine or thymine.
PCR amplification and analysis.
In vitro amplification by PCR was performed by a modification of methods described previously (30). Amplification reactions were carried out in 50-μl reaction mixtures containing 1 μl of each individual virus cell culture lysate in 67 mM Tris-HCl (pH 8.8), 17 mM NH4SO4, 6 μM EDTA, 2 mM MgCl2, 1 mM 2-mercaptoethanol, 80 pmol of each degenerate primer, 100 μM (each) dATP, dCTP, dGTP, and dTTP (Pharmacia Biotech, Piscataway, N.J.), 5 U of placental RNase inhibitor (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), 1.5 U of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim), and 1.25 U of Taq DNA polymerase (Perkin-Elmer Cetus, Norwalk, Conn.). The components of the reaction mixtures (excluding the RNase inhibitor, avian myeloblastosis virus reverse transcriptase, and Taq DNA polymerase) were prepared, overlaid with mineral oil, heated for 5 min at 95°C to release the virion RNA, and chilled on ice. The excluded components were then added, and the samples were incubated at 42°C for 30 min before 30 cycles of programmed amplification (denaturation at 94°C for 1 min, annealing at 42°C for 1 min, and extension at 60°C for 1 min) in a DNA thermal cycler (Perkin-Elmer Cetus). Conditions for polyacrylamide gel electrophoresis and detection of amplified products by ethidium bromide staining were as described previously (30).
Nucleic acid sequence analysis.
VP1 sequences of wild poliovirus isolates were determined in cycle sequencing reactions (14) containing fluorescent dye-labeled dideoxynucleotide chain terminators (Applied Biosystems, Foster City, Calif.). The amplification products were further characterized in similar reactions by using the seroPV PCR sets as primers. Nucleotide sequences were determined with the aid of an automated sequenator (model 373A; Applied Biosystems).
Sequence relationships among poliovirus genomes were compared by using the CLUSTAL computer program (10) from version 7 of the PC/Gene nucleic acid analysis package (IntelliGenetics, Mountain View, Calif.). The thermal stabilities of primer-template hybrids were estimated by using the OLIGO 5.0 program (National Biosciences, Plymouth, Minn. [7]).
RESULTS
Design of serotype-specific PCR primers.
Serotype specificity is determined by the interaction of neutralizing antibodies with the virion surface (20). The polypeptide loops forming the virion surface show much higher variability than the residues forming the internal framework of the capsid (23, 27). The alignments of the VP1 amino acid sequences of diverse wild polioviruses revealed four sites of high variability: the amino-terminal region (residues 1001 to 1032 [type 1]), neutralization antigenic site 1 (residues 1091 to 1102 [type 1]), neutralization antigenic site 2a (residues 1221 to 1226 [type 1]), and neutralization antigenic site 3 (residues 1287 to 1292 [type 1]) (Fig. 1) (20). The amino-terminal residues of VP1 form the longest variable interval within the poliovirus capsid (27). Short sequences within this interval are largely conserved within each serotype: QMLESMI (residues 1004 to 1010; type 1), EGVVEGT (residues 1007 to 1013; type 2), and VAQGALA (residues 1009 to 1015; type 3) (Fig. 1). We reasoned that primers complementary to the genomic sequences encoding these amino acids might form hybrids in a serotype-specific manner. The initiating primers for types 1 and 2 targeted codons in this region. We prepared a candidate initiating primer for type 3 that was complementary to the codons of the VAQGALA sequence. However, false-negative results were obtained with some type 3 templates in PCR assays with this primer (data not shown). We attributed this problem to the high level of degeneracy of the 3′ donor end of the primer, which may have reduced the efficiency of initiation of primer extension by reverse transcriptase (2, 16). Therefore, we targeted codons of another VP1 site, the amino acid sequence DANDQVG (residues 1218 to 1224; Fig. 1), which forms a surface loop that has been identified as poliovirus neutralization antigenic site 2a (20). The type 3 initiating primer complementary to the nucleotides encoding this site had a lower level of degeneracy at the 3′ end, and this primer supported efficient amplification of all type 3 templates tested (see below).
We had previously observed that the specificities of the PCR assays were determined primarily by the capacities of the initiating (antisense polarity) primers to pair with the single-stranded RNA template (14). Consequently, our return (sense polarity) primers were designed to match the codons of sites that are internalized in the native virion (8) and that are conserved among polioviruses and related NPEVs (23). The same return primer was used for both the type 1 and type 2 PCR assays, matching codons of the VP3 sequence LRDTTHI (residues 3225 to 3231; Fig. 1). The type 3 return primer matched codons of the VP1 sequence NPSIFYT (residues 1178 to 1184; Fig. 1).
Although the targeted amino acid sequences were well conserved, the amino acid-encoding nucleotide sequences of different wild poliovirus isolates were highly variable. Most of the variability occurred at degenerate codon positions, such that the potential number of synonymous nucleotide sequence combinations was very large (Fig. 2) (14). To match the various codon combinations for our target sites, our primers contained either mixed-base or deoxyinosine residues at positions of codon degeneracy (Fig. 2). Primers were designed to minimize degeneracy at their 3′ donor ends to ensure good pairing of the bases at the locus of primer extension (16). Deoxyinosine was used at positions of fourfold degeneracy to limit the total number of oligodeoxynucleotide species in the primer mixtures (2, 14).
Serotype specificities of seroPV PCR primers.
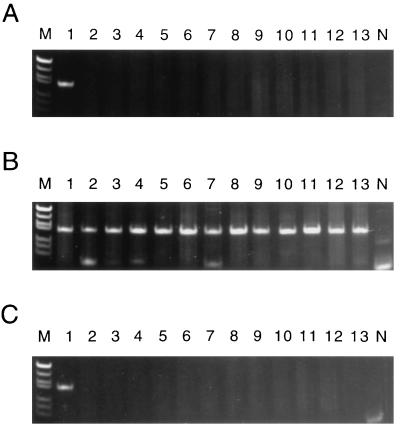
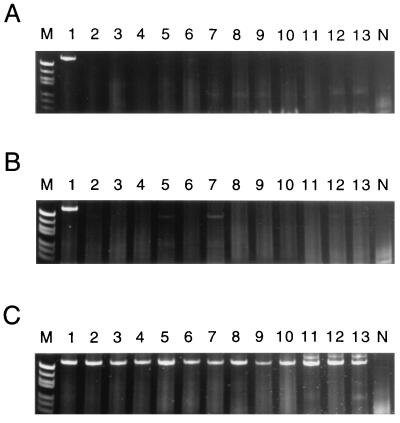
The specificities of the seroPV PCR primers were tested against 158 poliovirus isolates (48 vaccine-related isolates and 110 wild polioviruses; Table 1) of all three serotypes. The wild poliovirus isolates included representatives of most, if not all, of the genotypes known to have been in circulation in recent years (13, 14). All isolates were tested with each seroPV PCR primer pair. When the seroPV1 PCR primer pair (seroPV1A plus seroPV1,2S) was used in the amplification reactions, the predicted 70-bp product was generated only in reactions containing RNA from type 1 polioviruses (Fig. 3; Table 1). Similarly, when the seroPV2 PCR primer pair (seroPV2A plus seroPV1,2S) was used, the predicted 79-bp product was generated only in the presence of type 2 poliovirus RNA (Fig. 4; Table 1). High specificities were also observed for the seroPV3 PCR primer pair (seroPV3A plus seroPV3S) (Fig. 5; Table 1). None of the genomic sequences of 49 NPEV reference strains (19) were amplified under equivalent reaction conditions with any of the three seroPV PCR primer sets (Table 1). Minor products were occasionally generated with some templates, appearing as faint background bands in the polyacryamide gels (e.g., Fig. 3B, lane 5, and Fig. 3C, lane 8). The mobilities of the background bands could easily be distinguished from those of the specific product bands, such that serotype identifications were unambiguous.
FIG. 3.
Specificity of PCR amplification with the seroPV1 PCR primers, which yields a 70-bp product. Products were visualized after polyacrylamide gel electrophoresis by ethidium bromide fluorescence as described previously (30). (A to C) Lanes 1, amplicon from Sabin 1 template (positive control); lanes N, negative template control; lanes M, molecular weight marker V (57 to 587 bp) from Boehringer Mannheim; lanes 2 to 13, amplification products obtained with templates of different wild poliovirus isolates. (A) Poliovirus type 1. Lanes 2, 6070/CHN94; 3, 5558/NIE94; 4, 5386/ANG94; 5, 5058/THA93; 6, 3894/EGY92; 7, 3866/IND92; 8, 3862/TOG92; 9, 3647/CHN91; 10, 2781/VTN91; 11, 0467/COL91; 12, 0919/GEO90; and 13, 9188/BRA88. (B) Poliovirus type 2. Lanes 2, 3874/IND92; 3, 3825/PAK91; 4, 3845/EGY91; 5, 2613/PAK89; 6, 0176/PER89; 7, 7079/IND86; 8, 1534/IND82; 9, 0301/CAE80; 10, 0298/EGY79; 11, 0298/ISR78; 12, MEF-1/EGY42; and 13, Lansing/USA37. (C) Poliovirus type 3. Lanes: 2, 6071/CHN93; 3, 5376/PHL93; 4, 3899/EGY92; 5, 3873/IND92; 6, 0780/OMA91; 7, 3997/TKM90; 8, 2723/TUR90; 9, 9288/MEX89; 10, 9035/BRA88; 11, 8854/COL88; 12, 23127/FIN84; and 13, Saukett/USA52.
FIG. 4.
PCR amplification with the seroPV2 PCR primers, which yields a 79-bp product. (A to C) Lanes 1, amplicon from Sabin 2 template. Samples in all other lanes are as described in the legend to Fig. 3.
FIG. 5.
PCR amplification with the seroPV3 PCR primers, which yields a 140-bp product. (A to C) Lanes 1, amplicon from Sabin 3 template. Samples in all other lanes are as described in the legend to Fig. 3.
Several clinical isolates that were initially thought to contain a single poliovirus serotype were found by PCR to be heterotypic poliovirus mixtures. Because the oral poliovirus vaccine is usually given in the trivalent form, isolates containing vaccine-related poliovirus mixtures are common. However, some heterotypic mixtures of wild and vaccine-related polioviruses were also detected by PCR. The presence of the underlying vaccine-related strains was confirmed by using Sabin strain-specific PCR primers (30) or by sequencing the serotype-specific PCR products (data not shown).
DISCUSSION
This is the first report of primers that permit the serotype-specific identification of polioviruses by PCR. These new primers fill the gap between group-specific PCR primers that recognize all polioviruses (14) and genotype-specific primers that recognize Sabin vaccine strain-related isolates (30) or particular wild poliovirus genotypes (18, 31). The typing of polioviruses by PCR offers several advantages over typing by the standard serologic methods (19, 28). First, the primers are chemically defined reagents having uniform and predictable properties. Second, the primers can be prepared in effectively inexhaustable quantities. Third, the PCR typing assays are rapid, highly specific, and readily standardized. Finally, the exceptional sensitivity of PCR permits the detection of very low amounts of underlying polioviruses in mixtures of poliovirus or NPEV serotypes.
PCR offers unsurpassed flexibility for the systematic development of molecular diagnostic reagents and methods. The use of degenerate and inosine-containing primers targeting codons of conserved amino acid sequences is a general approach to the design of reagents conferring a breadth of specificity unattainable by other molecular methods (14), including nucleic acid probe hybridization (5, 6). Because amplification reactions can be initiated by the transient binding of primer to template, unstable pairings that would give low hybrid yields at equilibrium in probe hybridization assays can give high amplicon yields by PCR. The chief disadvantage of PCR as a routine diagnostic tool is the need to adhere to strict protocols to prevent carryover of amplified templates (16, 30).
The approach that we took for the development of poliovirus serotype-specific PCR primers followed that taken earlier to develop poliovirus group-specific primers (14). We first identified amino acid sequences that were characteristic for each serotype. We then prepared sets of candidate primers for testing against a large collection of wild poliovirus isolates representing all known contemporary genotypes. The primers showing the best specificities and sensitivities were used in routine characterizations of recent wild isolates from many different countries. When a template was inefficiently amplified in our PCR assays, its target sequences were determined, and the design of the primers was further optimized.
As with the poliovirus group-specific primers (14), the target nucleotide sequences were highly degenerate. For example, if all possible codon combinations in Fig. 2 are used, then the number of sequence combinations for the most degenerate target, bound by seroPV2A, is 18,432, and the number for the least degenerate target, bound by seroPV1A, is 288. To accommodate this high degree of variability, degenerate codon positions on the template were matched by mixed-base or deoxyinosine residues on the primer. Primers were designed to have the highest fidelity of pairing of bases at their 3′ donor ends. Primer residues 1, 2, 4 (except for seroPV3A; Fig. 2), and 5 from the 3′ ends matched the conserved first and second codon positions, while residue 3 was a twofold base mixture matching a twofold degenerate third codon position. Fourfold degenerate codon positions were matched to deoxyinosine residues located no closer than 6 nucleotides from the 3′ end of the primer. Because the deoxyinosine residues can pair with all four nucleotide bases, they can be used to replace fourfold base mixtures, thereby reducing the complexities of the primer mixtures. However, the bond strengths of the different pairings vary (2), so that pairs formed with deoxyinosine residues were stabilized on both sides by perfectly matched base pairs (16).
Only the seroPV3A initiating primer targeted codons of a known neutralization antigenic site resident on the native virion (20). The seroPV1A and seroPV2A PCR primers targeted codons of sequences near the amino terminus of VP1, in a domain containing a T-helper epitope for type 1 poliovirus (15). It may seem surprising that type-specific sequences are found in this highly variable domain (6, 27, 31) located in the interior of the native virion (8). However, the poliovirus capsid is conformationally dynamic at physiological temperature, with the VP1 amino terminus exposed at times in solution (17) and irreversibly extruded upon virus binding to the poliovirus receptor (8). The externalized amino-terminal sequences are bound by antibodies (17) found in human immune sera (25). Evolution of the amino-terminal domain appears to be constrained by the requirement that an amphipathic helical structure be conserved (8). An additional constraint on variability might be maintenance of serotype-specific interactions during conformational transitions, much as the variability of poliovirus neutralization antigenic sites appears to be restricted by the requirement that serotype-specific interactions be maintained for docking to the poliovirus receptor (9).
The ability to determine poliovirus serotypes by PCR should increase the speed and accuracy of routine poliovirus characterization. We use the seroPV PCR primers in conjunction with the panPV (14) and enterovirus group (31) PCR primers to detect polioviruses in clinical isolates from patients with acute flaccid paralysis. These reagents are especially useful for isolates of those wild poliovirus genotypes for which no genotype-specific reagents have yet been developed and when isolates contain mixtures of poliovirus serotypes or high titers of an NPEV.
ACKNOWLEDGMENTS
We thank Yvonne Stone and Kathy Crane for preparing the poliovirus isolates and Edwin George, Brian Holloway, and Melissa Olson of the Scientific Resources Program, Centers for Disease Control and Prevention, for preparing the synthetic oligodeoxynucleotide primers. We thank Mick Mulders (National Institute of Public Health and Environmental Protection [RIVM], Bilthoven, The Netherlands), Galina Lipskaya (Moscow State University, Moscow, Russia), and Sharon Bloom for collaboration in the development of the sequence database for wild polioviruses. Our thanks to the virologists and epidemiologists who contributed poliovirus samples for our studies. The cooperation and assistance of The Task Force for Child Survival and Development is appreciated.
REFERENCES
- 1.Balanant J, Guillot S, Candréa A, Delpeyroux F, Crainic R. The natural genome variability of poliovirus analyzed by a restriction fragment length polymorphism assay. Virology. 1991;184:645–654. doi: 10.1016/0042-6822(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 2.Batzer M A, Carlton J E, Deininger P L. Enhanced evolutionary PCR using oligonucleotides with inosine at the 3′-terminus. Nucleic Acids Res. 1991;19:5081. doi: 10.1093/nar/19.18.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birmingham, M. E., R. W. Linkins, B. P. Hull, and H. F. Hull. 1997. Poliomyelitis surveillance: the compass for eradication. J. Infect. Dis. 175(Suppl. 1):S146–S150. [DOI] [PubMed]
- 4.Bodian D, Morgan I M, Howe H A. Differentiation of types of poliomyelitis viruses. Am J Hyg. 1949;49:234–245. [PubMed] [Google Scholar]
- 5.De L, Nottay B, Yang C-F, Holloway B P, Pallansch M, Kew O. Identification of vaccine-related polioviruses by hybridization with specific RNA probes. J Clin Microbiol. 1995;33:562–571. doi: 10.1128/jcm.33.3.562-571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De L, Yang C-F, da Silva E, Boshell J, Cáceres P, Gómez J R, Pallansch M, Kew O. Genotype-specific RNA probes for the direct identification wild polioviruses by blot hybridization. J Clin Microbiol. 1997;35:2834–2840. doi: 10.1128/jcm.35.11.2834-2840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freier S M, Kierzek R, Jaeger J A, Sugimoto N, Caruthers M H, Nelson T, Turner D H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci USA. 1986;83:9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fricks C E, Hogle J M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harber J, Bernhardt G, Lu H H, Sgro J Y, Wimmer E. Canyon rim residues, including antigenic determinants, modulate serotype-specific binding of polioviruses to mutants of the poliovirus receptor. Virology. 1995;214:559–570. doi: 10.1006/viro.1995.0067. [DOI] [PubMed] [Google Scholar]
- 10.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 11.Hovi T, Stenvik M. Selective isolation of poliovirus in recombinant murine cell line expressing the human poliovirus receptor gene. J Clin Microbiol. 1994;32:1366–1368. doi: 10.1128/jcm.32.5.1366-1368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hull, H. F., M. E. Birmingham, B. Melgaard, and J. W. Lee. 1997. Progress towards global polio eradication. J. Infect. Dis. 175(Suppl. 1):S4–S9. [DOI] [PubMed]
- 13.Kew O M, Mulders M N, Lipskaya G Y, da Silva E E, Pallansch M A. Molecular epidemiology of polioviruses. Semin Virol. 1995;6:401–414. [Google Scholar]
- 14.Kilpatrick D R, Nottay B, Yang C-F, Yang S-J, Mulders M N, Holloway B P, Pallansch M A, Kew O M. Group-specific identification of polioviruses by PCR using primers containing mixed-based or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1996;34:2990–2996. doi: 10.1128/jcm.34.12.2990-2996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutubuddin M, Simons J, Chow M. Identification of T-helper epitopes in the VP1 capsid protein of poliovirus. J Virol. 1992;66:3042–3047. doi: 10.1128/jvi.66.5.3042-3047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok S, Kellogg D E, McKinney N, Spasic D, Goda L, Levenson C, Sninsky J J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990;18:999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Yafal A G, Lee Y M, Hogle J, Chow M. Poliovirus neutralizing antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol. 1994;68:3965–3970. doi: 10.1128/jvi.68.6.3965-3970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipskaya G Y, Chervonskaya E A, Belova G I, Maslova S V, Kutateladze T N, Drozdov S G, Mulders M, Pallansch M A, Kew O M, Agol V I. Geographical genotypes (geotypes) of poliovirus case isolates from the former Soviet Union: relatedness to other known poliovirus genotypes. J Gen Virol. 1995;76:1687–1699. doi: 10.1099/0022-1317-76-7-1687. [DOI] [PubMed] [Google Scholar]
- 19.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 655–712. [Google Scholar]
- 20.Minor P D. Antigenic structure of picornaviruses. Curr Top Microbiol Immunol. 1990;161:121–154. doi: 10.1007/978-3-642-75602-3_5. [DOI] [PubMed] [Google Scholar]
- 21.Nakano J H, Hatch M H, Thieme M L, Nottay B. Parameters for differentiating vaccine-derived and wild poliovirus strains. Prog Med Virol. 1978;24:178–206. [PubMed] [Google Scholar]
- 22.Nomenclature Committee for the International Union of Biochemistry. Nomenclature for incompletely specified bases in nucleic acid sequences. Eur J Biochem. 1985;150:1–5. doi: 10.1111/j.1432-1033.1985.tb08977.x. [DOI] [PubMed] [Google Scholar]
- 23.Palmenberg A C. Sequences of picornavirus capsid proteins. In: Semler B, Ehrenfeld E, editors. Molecular aspects of picornavirus infection and detection. Washington, D.C: American Society for Microbiology; 1989. pp. 215–230. [Google Scholar]
- 24.Pinheiro, F. P., O. M. Kew, M. H. Hatch, C. M. da Silveira, and C. A. de Quadros. 1997. Eradication of wild polioviruses from the Americas: wild poliovirus surveillance—laboratory issues. J. Infect. Dis. 175(Suppl. 1):S43–S49. [DOI] [PubMed]
- 25.Roivainen M, Narvanen A, Korkolainen M, Huhtala M-L, Hovi T. Antigenic regions of poliovirus type 3/Sabin capsid proteins recognized by human sera in the peptide scanning technique. Virology. 1991;180:99–107. doi: 10.1016/0042-6822(91)90013-2. [DOI] [PubMed] [Google Scholar]
- 26.Schweiger B, Schreier E, Böthig B, Lopez-Pila J M. Differentiation of vaccine and wild-type polioviruses using polymerase chain reaction and restriction enzyme analysis. Arch Virol. 1994;134:39–50. doi: 10.1007/BF01379105. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda H, Kohara M, Kataoka Y, Suganuma T, Omata T, Imura N, Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes: implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984;174:561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- 28.Van der Avoort H G A M, Hull B P, Hovi T, Pallansch M A, Kew O M, Crainic R, Wood D J, Mulders M N, Van Loon A M. Comparative study of five methods for intratypic differentiation of polioviruses. J Clin Microbiol. 1995;33:2562–2566. doi: 10.1128/jcm.33.10.2562-2566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Expanded Programme on Immunization. Progress towards the global eradication of poliomyelitis, 1995. Weekly Epidemiol Rec. 1996;71:189–194. [PubMed] [Google Scholar]
- 30.Yang C-F, De L, Holloway B P, Pallansch M A, Kew O M. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 1991;20:159–179. doi: 10.1016/0168-1702(91)90107-7. [DOI] [PubMed] [Google Scholar]
- 31.Yang C-F, De L, Yang S-J, Gómez J R, Cruz J R, Holloway B P, Pallansch M A, Kew O M. Genotype-specific in vitro amplification of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Res. 1992;24:277–296. doi: 10.1016/0168-1702(92)90124-r. [DOI] [PubMed] [Google Scholar]