Abstract
Background
Two Centuries from today, Karl Friedrich Burdach attributed the nomenclature “arcuate fasciculus” to a white matter (WM) pathway connecting the frontal to the temporal cortices by arching around the Sylvian fissure. Although this label remained essentially unvaried, the concepts related to it and the characterization of the structural properties of this bundle evolved along with the methodological progress of the past years. Concurrently, the functional relevance of the arcuate fasciculus (AF) classically restricted to the linguistic domain has extended to further cognitive abilities. These features make it a relevant structure to consider in a large variety of neurosurgical procedures.
Objective
Herein, we build on our previous review uncovering the connectivity provided by the Superior Longitudinal System, including the AF, and provide a handy representation of the structural organization of the AF by considering the frequency of defined reports in the literature. By adopting the same approach, we implement an account of which functions are mediated by this WM bundle. We highlight how this information can be transferred to the neurosurgical field by presenting four surgical cases of glioma resection requiring the evaluation of the relationship between the AF and the nearby structures, and the safest approaches to adopt.
Conclusions
Our cumulative overview reports the most common wiring patterns and functional implications to be expected when approaching the study of the AF, while still considering seldom descriptions as an account of interindividual variability. Given its extension and the variety of cortical territories it reaches, the AF is a pivotal structure for different cognitive functions, and thorough understanding of its structural wiring and the functions it mediates is necessary for preserving the patient's cognitive abilities during glioma resection.
Keywords: arcuate fasciculus, function, glioma resection, structure, surgical approaches, white matter
The descriptions attributed to the term “Arcuate Fasciculus” considerably evolved since its first mention in Burdach's work; The extension of the Arcuate Fasciculus accounts for its large functional potentiality and underpins the necessity of its preservation during surgical procedures; A thorough understanding of its structural wiring and functional implications is of foremost importance for surgical planning.
1. INTRODUCTION
Most of the vocabulary that we use to refer to the components of the nervous system and the related mechanisms comes from early anatomical studies performed between the 18th and the 19th Century. Karl Friedrich Burdach (1776–1847) is among the main contributors to our established neuroanatomical lexicon (Swanson, 2015). Considering his studies on the cerebral white matter (WM), his legacy entails the identification and labeling of the main association bundles of the human brain (Burdach, 1826). The second volume of “Vom Baue und Leben des Gehirns,” published in 1822, contains the first mention of the name “arcuate fasciculus” (AF)—originally “Bogenbündel” (i.e., arcuate fascicle) (Burdach, 1822). Owing to this description, Burdach's name has historically been associated to this WM bundle, to the extent that the Dejerines referred to it as “faisceau arqué de Burdach” (i.e., arcuate fascicle of Burdach (Dejerine & Dejerine‐Klumpke, 1895, 1901).
Two hundred years went by, and we still use Burdach's denomination. What changed is the definition of this WM structure, including the attributed partonomies, subdivisions, and the respective nomenclatures (Mandonnet et al., 2018; Porto de Oliveira et al., 2021; Vavassori et al., 2021). Indeed, the introduction and fast evolution of neuroimaging techniques (Leemans, 2019), the revisitation of early neuroanatomical discoveries (De Benedictis et al., 2014; Hope et al., 2016; Sarubbo et al., 2016), and the renovation of Klingler's microdissection approach (Agrawal et al., 2011; Klingler, 1935; Martino et al., 2011) led to the production of increasingly more detailed descriptions of the human WM pathways. In particular, the dissemination of diffusion magnetic resonance imaging‐based tractography (Basser et al., 2000) enabled the first characterization of the arcuate fascicle in vivo (Catani et al., 2002), leading off a series of innovative studies aimed to achieve the most accurate and comprehensive characterization of the wiring and extension of this bundle. Concurrently with these efforts, the ensuing functional relevance of the AF has become steadily more evident: damages of this bundle, both in terms of altered microstructural features or disconnection, have been implicated in a broad spectrum of syndromes, spanning from psychiatric symptoms (Jiang et al., 2017; Psomiades et al., 2016) to neurological diseases (Nakajima et al., 2018). Moreover, its volume in the right hemisphere has been indicated as a reliable predictor of aphasia recovery after stroke (Forkel et al., 2014). The involvement of the AF in such a variety of syndromes, and therefore of associated cognitive processes, relates to its large volume and long‐range extension. Indeed, the AF transverses three lobes (i.e., the frontal, parietal, and temporal cortices) and resides in close relationship with the insula, with longitudinal associative fascicles laterally (Mandonnet et al., 2018) and projection fibers on the medial side. These characteristics make it a fundamental mediator of different cognitive abilities and explain why it must inevitably be considered in many diverse surgical procedures.
The continuous methodological evolutions and the interest of the scientific community in investigating the properties of this specific WM bundle surely contributed to the achievement of a more complete depiction of the AF, but they also led to the production of an intricate and non‐univocal picture (Becker et al., 2022; Mandonnet et al., 2018; Porto de Oliveira et al., 2021; Vavassori et al., 2021). Therefore, an overview of the complex literature about this bundle is essential to highlight its multiple structural and functional facets that need to be considered when approaching the study of this bundle, especially in the surgical practice. By analyzing the controversies regarding the Superior Longitudinal System's subdivisions and the respective nomenclatures, our recent literature review (Vavassori et al., 2021) already exposed the current knowledge about the structural wiring of the AF intended as a whole ensemble of fibers connecting the frontal and the temporal lobes of the same hemisphere by passing above the insula (Mandonnet et al., 2018). The present work builds on this previous examining the architecture of frontotemporal dorsal associational connections independently from their partonomy or labeling and integrates it by addressing the functional roles attributed to the AF. We adopt a cumulative approach to reshape the structural definition of the bundle and to present the cognitive processes it mediates. This new perspective provides an easy‐to‐access general characterization of the AF, and by supplying supplementary information about the frequency of certain descriptions accounts for the most common as well as for the rarest patterns of connectivity/functional responses that should be considered in the surgical framework when dealing with this fasciculus. Indeed, emerging knowledge in the field of connectomics has become relevant for surgical approaches to the resection of tumors, highlighting the importance of the AF preservation while balancing between the extension of resection and the upkeep of the patient's quality of life (Bunevicius et al., 2014; Hervey‐Jumper & Berger, 2016; Sanai et al., 2011; Zarino et al., 2020; Zigiotto et al., 2020). With this aim, we append to our literature review a description of surgical approaches to tumor resection that require the careful consideration of this bundle extension, its relationships with the nearby anatomical structures and the related functional implications, and provide a collection of four different surgical cases to highlight how, in practice, extensive knowledge of the AF features can be applied in this field.
2. OLD AND NEW INSIGHTS ABOUT THE AF
From its first accounts…
The classification of the association pathways of the human brain was fostered by the works of pioneering dissectionists who, starting from the early 19th Century up to the beginning of the 20th Century, carried out a systematic exploration of the WM structural organization (Burdach, 1826; Dejerine & Dejerine‐Klumpke, 1895, 1901; Mayo, 1823; Reil, 1809, 1812; Monakow, 1897). In this instance, different anatomists reported that an orderly ensemble of fibers analogous to the more medial cingulum bundle could be isolated on the lateral aspect of each cerebral hemisphere (Burdach, 1822; Dejerine & Dejerine‐Klumpke, 1895, 1901). This WM pathway was described and finely illustrated as a connection running between the temporal and the frontal lobes, passing through the parietal lobe, and arching around the posterior limit of the Sylvian fissure (Dejerine & Dejerine‐Klumpke, 1895; Reil, 1812) (Figure 1a,b). Its characteristic arched shape granted it the name “arcuate fasciculus” (Burdach, 1822). In this same period, based on the observation of clinical signs related to specific anatomical lesions (Kumar et al., 2011), Carl Wernicke proposed a putative model for the localization of linguistic abilities in the brain: following a previous description of the faculty of speech production being located in the third frontal convolution of the left hemisphere (i.e., pars triangularis (IFGtri) and pars opercularis (IFGop) of the inferior frontal gyrus (IFG)) (Broca, 1865), he advanced that a second area, later established to be represented by the superior temporal gyrus (STG), would support words’ sound memory, and therefore language comprehension (Wernicke, 1874). This characterization of the distribution of linguistic abilities led Wernicke himself to postulate that a specific syndrome characterized by spared comprehension with repetition deficits, and defined as conduction aphasia, could arise from a disconnection between these two cortical regions (Figure 1c). In the 1960s, Geschwind formalized that the anatomical substrate for the interaction between Wernicke and Broca's areas is represented by the AF (Geschwind, 1965) (Figure 1d).
FIGURE 1.
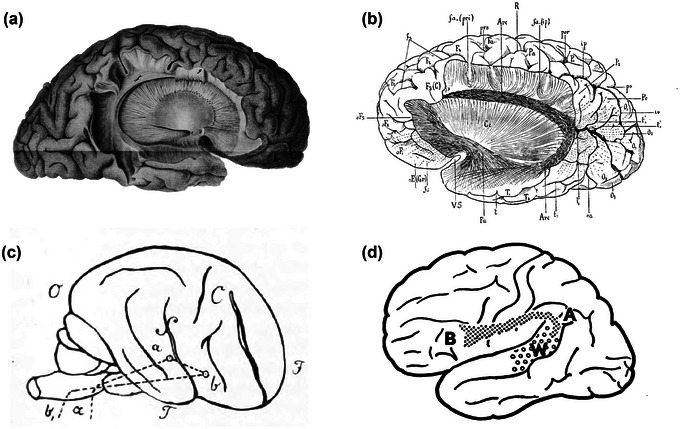
Historical drawings and diagrammatic representations of the arcuate fasciculus (AF) from the original works of (a) Reil (1812), (b) Dejerine and Dejerine‐Klumpke (1895), (c) Wernicke (1874), and (d) Geschwind (1970).
… to its current definition
Nowadays, it would be reductive to define the AF as a connection running exclusively between the IFG and the STG and purely devoted to language comprehension. Especially throughout the last 20 years, the creation and progressive fine‐tuning of innovative techniques for the in vivo representation of the brain's WM and the testing of cognitive functions dramatically enriched the characterization of the AF.
As mentioned above, an exhaustive account of the current definition of the AF structural wiring can be found in Vavassori et al. (2021) considering all the connections running between the frontal and temporal lobes, independently from the adopted nomenclature. Herein, we set the debate on the bundle's partitioning aside and build on these data by analyzing how frequently defined connections have been attributed to the AF description across studies. The present approach aims to highlight the most characterizing patterns of connectivity established by the AF (i.e., those that are more likely to be found when approaching the study of this bundle), while still representing those that have been reported only in few instances. This provides an account of the possible differences in the AF extension across individuals, an important variable that should be accounted for especially in the clinical setting (Forkel et al., 2021). Since very few studies explicitly analyze and report a specific pattern of connectivity between two precise cortical regions and the majority of them rather indicate the overall anterior and posterior terminations of the bundle, we assume all the areas listed in the same description to be mutually interconnected unless differently specified (Figure 2, see Table 1 for references).
FIGURE 2.
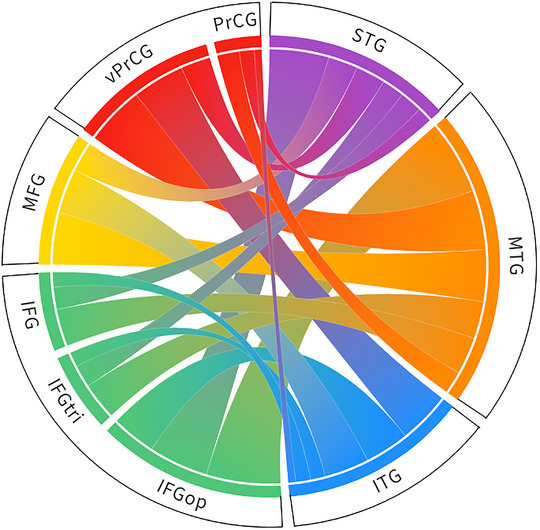
Representation of the frontotemporal connectivity provided by the arcuate fasciculus (AF) according to the literature reviewed in Vavassori et al. (2021). In line with the present cumulative perspective, the width of each link is proportional to the number of studies referring to the AF and mentioning a certain pattern of inter‐gyral connectivity. IFG, inferior frontal gyrus; IFGop, pars opercularis; IFGtri, pars triangularis; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; PrCG, precentral gyrus; STG, superior temporal gyrus; vPrCG ventral precentral gyrus.
TABLE 1.
Patterns of the arcuate fasciculus (AF) structural connectivity as defined in the studies reviewed in Vavassori et al. (2021) and classified according to the present cumulative approach.
| Frontal termination | Temporal terminations | Number mentions | References |
|---|---|---|---|
| IFGop | ITG | 6 | Sarubbo et al. (2016) |
| Martino et al. (2011) | |||
| Barrick et al. (2006) | |||
| Rojkova et al. (2016) | |||
| Glasser and Rilling (2008) | |||
| Yagmurlu et al. (2016) | |||
| MTG | 9 | Vassal et al. (2016) | |
| Sarubbo et al. (2016) | |||
| Barrick et al. (2006) | |||
| Lawes et al. (2008) | |||
| Rojkova et al. (2016) | |||
| Zemmoura et al. (2014) | |||
| Glasser and Rilling (2008) | |||
| Fernández‐Miranda et al. (2015) | |||
| Yagmurlu et al. (2016) | |||
| STG | 8 | Vassal et al. (2016) | |
| Sarubbo et al. (2016) | |||
| Barrick et al. (2006) | |||
| Rojkova et al. (2016) | |||
| Glasser and Rilling (2008) | |||
| Thiebaut de Schotten et al. (2012) | |||
| Fernández‐Miranda et al. (2015) | |||
| Yagmurlu et al. (2016) | |||
| IFGtri | ITG | 2 | Martino et al. (2011) |
| Glasser and Rilling (2008) | |||
| MTG | 5 | Vassal et al. (2016) | |
| Martino et al. (2011) | |||
| Zemmoura et al. (2014) | |||
| Glasser and Rilling (2008) | |||
| Yagmurlu et al. (2016) | |||
| STG | 3 | Vassal et al. (2016) | |
| Thiebaut de Schotten et al. (2012) | |||
| Yagmurlu et al. (2016) | |||
| IFG | ITG | 2 | Martino, Silva‐Freitas et al. (2013) |
| Thiebaut de Schotten et al. (2012) | |||
| MTG | 5 | Catani et al. (2005) | |
| Forkel et al. (2020) | |||
| Martino, Silva‐Freitas et al. (2013) | |||
| Glasser and Rilling (2008) | |||
| Yagmurlu et al. (2016) | |||
| STG | 3 | Catani et al. (2005) | |
| Forkel et al. (2020) | |||
| Gharabaghi et al. (2009) | |||
| MFG | ITG | 6 | Sarubbo et al. (2016) |
| Martino, Silva‐Freitas et al. (2013) | |||
| Glasser and Rilling (2008) | |||
| Thiebaut de Schotten et al. (2012) | |||
| Fernández‐Miranda et al. (2015) | |||
| Yagmurlu et al. (2016) | |||
| MTG | 7 | Vassal et al. (2016) | |
| Sarubbo et al. (2016) | |||
| Martino, Silva‐Freitas et al. (2013) | |||
| Glasser and Rilling (2008) | |||
| Thiebaut de Schotten et al. (2012) | |||
| Fernández‐Miranda et al. (2015) | |||
| Yagmurlu et al. (2016) | |||
| STG | 4 | Frey et al. (2008) | |
| Vassal et al. (2016) | |||
| Sarubbo et al. (2016) | |||
| Gharabaghi et al. (2009) | |||
| vPrCG | ITG | 7 | Sarubbo et al. (2016) |
| Martino et al. (2011) | |||
| Martino, Silva‐Freitas et al. (2013) | |||
| Glasser and Rilling (2008) | |||
| Thiebaut de Schotten et al. (2012) | |||
| Fernández‐Miranda et al. (2015) | |||
| Yagmurlu et al. (2016) | |||
| MTG | 8 | Vassal et al. (2016) | |
| Sarubbo et al. (2016) | |||
| Martino et al. (2011) | |||
| Martino, Silva‐Freitas et al. (2013) | |||
| Glasser and Rilling (2008) | |||
| Thiebaut de Schotten et al. (2012) | |||
| Fernández‐Miranda et al. (2015) | |||
| Yagmurlu et al. (2016) | |||
| STG | 4 | Vassal et al. (2016) | |
| Sarubbo et al. (2016) | |||
| Glasser and Rilling (2008) | |||
| Yagmurlu et al. (2016) | |||
| PrCG | ITG | 1 | Barrick et al. (2006) |
| MTG | 3 | Barrick et al. (2006) | |
| Lawes et al. (2008) | |||
| Forkel et al. (2020) | |||
| STG | 2 | Barrick et al. (2006) | |
| Forkel et al. (2020) |
Abbreviations: IFG, inferior frontal gyrus; IFGop, pars opercularis; IFGtri, pars triangularis; MFG, middle frontal gyrus; PrCG, precentral gyrus; vPrCG ventral precentral gyrus; STG, superior temporal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus.
For what concerns the functional counterpart, we adopt the exact same cumulative approach already illustrated for the structural description to present the functional literature about the AF. Indeed, one single bundle is very likely to support multiple cognitive processes by playing different roles in different networks (Forkel et al., 2021). Since to date there is no recent review paper that summarizes the functional literature of the AF, we sampled the experimental works testing the involvement of this fiber pathway in a defined function, using both direct techniques (i.e., direct electrical stimulation (DES)) and correlational neuroimaging‐behavioral measurements (see Table 2 for references), with the aim to highlight the main different cognitive domains whose integration is mediated by this bundle. For this purpose, we conducted a literature search in Google Scholar using a combination of the strings “arcuate fasciculus” and “function”, “cognitive functions”, “behavioral correlates”, “linguistic”, “non‐linguistic”, and again, as the research highlighted more specific domains, “verbal memory”, “social cognition”, “spatial cognition”, “music perception”. Those works that imply a putative functional role of the bundle as a consequence of their findings but with no direct testing were not included.
TABLE 2.
List of the studies reviewed that outlined the functional involvement of the arcuate fasciculus (AF) in specific functional classes and the respective domains classified according to the present cumulative approach.
| Functional class | Domain | References | Specification | Hemisphere |
|---|---|---|---|---|
| Language | Speech production | Bates et al. (2003) | Verbal fluency deficit | LH |
| Hope et al. (2016) | Verbal fluency deficit | LH | ||
| Ivanova et al. (2016) | LH | |||
| Marchina et al. (2011) | LH | |||
| Wang et al. (2013) | LH | |||
| Speech comprehension | Geva et al. (2015) | LH | ||
| Ivanova et al. (2016) | LH | |||
| Wilson et al. (2011) | LH | |||
| Pragmatics | Chen et al. (2018) | Speech tone perception | LH | |
| Geva et al. (2015) | Rhyme judgment | LH | ||
| Glasser and Rilling (2008) | Prosody processing | RH | ||
| Phonology | Bello et al. (2007) | Phonemic paraphasia | BI, RH only for left‐handed | |
| Benzagmout et al. (2007) | Phonemic paraphasia | LH | ||
| Chan‐Seng et al. (2014) | Phonemic paraphasia | LH | ||
| De Benedictis et al. (2014) | Phonemic paraphasia | LH | ||
| Leclercq et al. (2010) | Phonemic paraphasia | BI, RH only for left‐handed | ||
| Maldonado, Moritz‐Gasser, de Champfleur et al. (2011) | Phonemic paraphasia | LH | ||
| Maldonado, Moritz‐Gasser, Duffau (2011) | Phonemic paraphasia | LH | ||
| Sarubbo et al. (2016) | Phonemic paraphasia | LH | ||
| Vassal et al. (2013) | Phonemic paraphasia | LH | ||
| Saygin et al. (2013) | Phonological awareness | LH | ||
| Vandermosten et al. (2012) | Phonological awareness | LH | ||
| Yeatman et al. (2011) | Phonological awareness | LH | ||
| Breier et al. (2008) | Repetition deficits | LH | ||
| Catani et al. (2005) | Repetition deficits | LH | ||
| Ellmore et al. (2009) | Repetition deficits | LH | ||
| Fridriksson et al. (2010) | Repetition deficits | LH | ||
| Geva et al. (2015) | Repetition deficits | LH | ||
| Kümmerer et al. (2013) | Repetition deficits | LH | ||
| Moritz‐Gasser and Duffau (2013) | Repetition deficits | LH | ||
| Sierpowska et al. (2017) | Nonword repetition deficits | LH | ||
| Janssen et al. (2023) | Nonword repetition | LH | ||
| Saur et al. (2008) | Repetition | LH | ||
| Glasser and Rilling (2008) | LH | |||
| Mullen et al. (2011) | BI, developping children | |||
| Phillips et al. (2011) | LH | |||
| Sarubbo et al. (2015) | LH | |||
| Sarubbo et al. (2020) | LH | |||
| Syntax | Friederici (2009) | LH | ||
| Griffiths et al. (2013) | LH | |||
| Leclercq et al. (2010) | BI, RH only for left‐handed | |||
| Papoutsi et al. (2011) | LH | |||
| Wilson et al. (2011) | LH | |||
| Semantics | Leclercq et al. (2010) | Semantic paraphasia | BI, RH only for left‐handed | |
| Teubner‐Rhodes et al. (2016) | Vocabulary knowledge | LH | ||
| Janssen et al. (2023) | Verb generation | LH | ||
| Zigiotto et al. (2022) | LH | |||
| Glasser and Rilling (2008) | LH | |||
| Articulatory | Leclercq et al. (2010) | Speech arrest | BI, RH only for left‐handed | |
| Word learning | López‐Barroso et al. (2013) | LH | ||
| Naming | Bello et al. (2007) | Anomia | BI, RH only for left‐handed | |
| Duffau et al. (2002) | Anomia | LH | ||
| Sarubbo et al. (2015) | Anomia | LH | ||
| Geva et al. (2015) | LH | |||
| Hope et al. (2016) | LH | |||
| Marchina et al. (2011) | LH | |||
| McDonald et al. (2008) | BI | |||
| Moritz‐Gasser and Duffau (2013) | LH | |||
| Reading | Saygin et al. (2013) | LH | ||
| Yeatman et al. (2011) | LH | |||
| Yeatman, Dougherty, Ben‐Shachar et al. (2012) | LH | |||
| Yeatman, Dougherty, Myall et al. (2012) | LH | |||
| Verbal memory | Verbal memory | Reijmer et al. (2012) | LH | |
| McDonald et al. (2008) | BI | |||
| Verbal working memory | Meyer et al. (2014) | LH | ||
| Verbal short‐term memory | Papagno et al. (2017) | Digit span order errors | LH | |
| Social cognition | Social cognition | Chiang et al. (2017) | RH | |
| False‐belief understanding | Grosse Wiesmann et al. (2017) | ToM development | BI, developping children | |
| Face‐based mentalizing | Yordanova et al. (2017) | RH | ||
| Cabinio et al. (2015) | RH | |||
| Herbet et al. (2014) | RH | |||
| Nakajima et al. (2018) | RH | |||
| Music perception | Tone discrimination | Loui et al. (2009) | RH | |
| Pitch‐related grammar learning | Loui et al. (2011) | RH | ||
| Rhythm modulation detection | Vaquero et al. (2021) | LH | ||
| Spatial cognition | Visuospatial attention | He et al. (2007) | Spatial neglect | RH |
| Rolland et al. (2018) | Spatial neglect | RH | ||
| Roux et al. (2011) | Spatial neglect | RH | ||
| Umarova et al. (2010) | Change detection | RH | ||
| Spatial perception | Sarubbo et al. (2015) | RH |
Abbreviations: BI, bilateral; LH, left hemisphere; RH, right hemisphere.
In line with the renowned notion of the left and right AF contributing to different cognitive domains (Nakajima et al., 2019), we will report findings according to the hemisphere they are related to. Although most of the studies considered focused their investigation of AF functionality on a specific hemisphere, there are some works that carried out their analysis on the whole brain and were therefore able to relate different functions to this bundle depending on its hemispheric location, or, on the contrary, to specify that the same function is mediated by the AF from both hemispheres. Those studies that found the same function to be mediated by bilateral AF will be considered as one evidence for each hemisphere.
The frontal gyri innervated by the AF are the IFG, the precentral gyrus (PrCG) and the middle frontal gyrus (MFG), reported in decreasing order based on how many times connections to these areas have been described. When addressing the anterior terminations of the AF in the PrCG many authors specified that only the most ventral part of this area is reached by the AF. Similarly, for what concerns the IFG, different works refer to terminations in IFGop, in IFGtri or in the whole gyrus. On the other hand, the temporal region which has more often been described to be connected by the AF is the middle temporal gyrus (MTG), followed by the inferior (ITG) and the superior (STG) temporal gyri.
Overall, all the cortical areas reported above seem to be mutually interconnected, but there is a great variability in how many works described the existence of defined wiring patterns. The connections that have more commonly been described as part of the AF run between the various gyri of the frontal cortex and the MTG: most of the reviewed studies describe IFGop–MTG connectivity, whereas connections between this temporal area and the vPrCG and the MFG have less frequently been reported. Although connections between IFGop and the STG seem to represent a typical layout of AF's wiring in the works we sampled, less of them report connections linking both the vPrCG and the MFG to the STG or to the ITG. On the contrary, less descriptions of connections from the IFGop to the ITG have been reported compared to connectivity between this gyrus and the STG. As mentioned in the previous section, few works among the ones we considered found anterior terminations of the AF in IFGtri. Still, the reported involvement of this region reflects the proportion of descriptions made for IFGop, with less mentions of connectivity from IFGtri to MTG, STG, and ITG, respectively. The same applies to those works that referred to IFG in its entirety; however, among those that described AF connectivity to the PrCG by considering the whole gyrus, a description of connections running from this area to the MTG, the STG, or to the ITG are also reported, in slight countertrend compared to the number of authors that specified the involvement of the sole ventral part of this region. The structural connections and the list of studies considered are reported in Table 1.
As we described in the historical section, the AF has been implied in linguistic functions since its first characterizations (Broca, 1865; Geschwind, 1965; Wernicke, 1874). This initial assumption based on anatomo‐clinical correlation studies was validated with the advent of functional MRI, intraoperative mapping with DES, and correlational studies of tractography‐derived bundles’ parameters with behavioral testing. Indeed, 41 out of the 59 studies we hereby reviewed support the involvement of direct dorsal frontotemporal connections in the linguistic domain. The congregate analysis of these works enlightens the complexity of language as a multifaceted ability, composed of several components whose coordinated interplay gives rise to more general abilities. Given this complexity and the magnitude of the number of works specifically investigating the role of the AF in language, findings regarding this function and nonlinguistic cognitive domains will be presented separately (Figure 3 for linguistic abilities and Figure 4 for the other domains, for references see Table 2).
FIGURE 3.
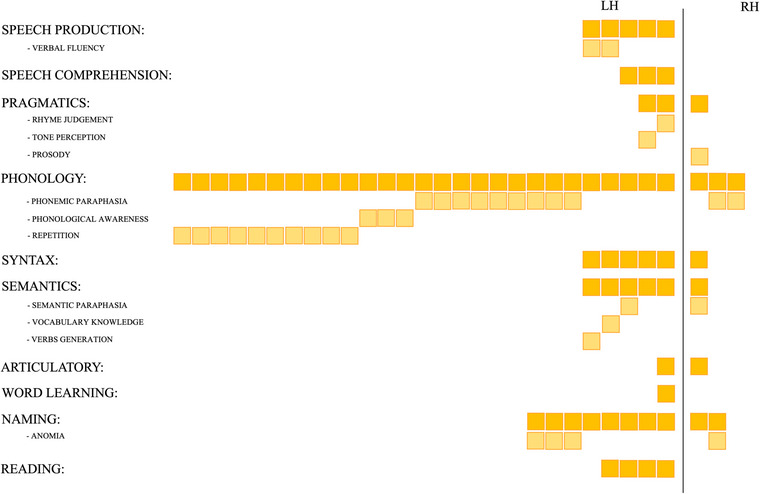
Schematic cumulative representation of the number of studies that described the role of the arcuate fasciculus (AF) in mediating linguistic abilities. All the revised works have been classified depending on the general ability, linguistic domain, or specific language‐related skills that they tackle (see text for a more detailed description). Each solid‐colored square represents one account for the defined category; shaded squares have been added below each of these broad categories in the case of studies specifying a precise subcategory (being it a particular deficit related to AF damage or a defined ability correlating with its features).
FIGURE 4.
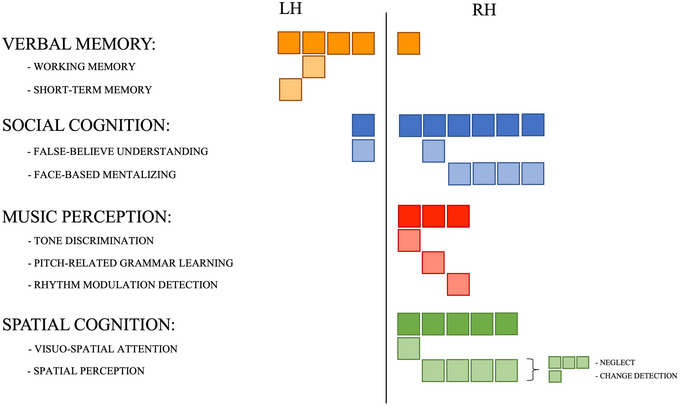
Schematic cumulative representation of the number of studies that described the role of the arcuate fasciculus (AF) in mediating nonlinguistic abilities. Each solid‐colored square represents one account for the defined category; shaded squares have been added below each of these broad categories in the case of studies specifying a precise subcategory (being it a particular deficit related to AF damage or a defined ability correlating with its features).
Different works among the ones we surveyed generally stated a relationship between structural features of the left AF, such as the degree of tract lesioning or the decrease of its microstructural diffusivity in pathology, and a general impairment of speech production and comprehension. Still, most of them focused on specific aspects of language, expressed both in terms of a defined cognitive impairment associated with a lesion or in terms of associations between structural/functional and behavioral evidence in healthy participants. These instances can be summarized considering four out of the five domains of language (i.e., pragmatics, phonology, syntax, and semantics). Although only few of the works reviewed reported the involvement of the bilateral AF in functions related to the pragmatics domain—the left hemisphere bundle was related to rhyme judgment and speech tone perception, and the right one was proposed to support prosody processing—most of them stressed an association between this WM pathway in the left hemisphere and phonological functions. Among these, studies using DES mapping described phonemic paraphasia in correspondence of AF stimulation; studies conducted both on healthy participants and with intraoperative stimulation in patients reported an association with repetition abilities, whereas studies conducted on a normative population stressed the role of the AF in phonological awareness, a predictor of reading skills. Moreover, the left AF is reported to play a role in the syntactic domain and in semantic aspects of language. In particular, one of the studies considered reported an association between the bundle's microstructural tractography‐derived measures and vocabulary knowledge, whereas another investigation with DES mapping observed semantic paraphasia following AF stimulation.
Up to now, we discussed the involvement of the AF in linguistic domains‐related functions mainly in the left hemisphere. Nevertheless, we also identified some reports of linguistic functions being mediated by the right AF (see Figure 3). The evident imbalance between the number of descriptions of an association between left and right AF and language is in line with the notorious strong left‐lateralization of this function in the normative population (Frost, 1999; Malik‐Moraleda et al., 2022; Wang et al., 2019). Moreover, it is worth considering that all the reports herein reviewed that attribute linguistic abilities to the right AF are studies relating a bilateral activation for that function. If we regard these works more carefully, it can be inferred that the relationship of the right AF with phonemic paraphasia, general syntactic abilities, and semantic paraphasia is found in left‐handed patients for which language is more likely to be lateralized in the right hemisphere compared to the general population (Szaflarski et al., 2002). The only non‐neurosurgical description of a correlation between this bundle's tractography‐derived measurements in the right hemisphere and phonological abilities was conducted on a cohort of developing children, for whom the fine wiring of linguistic functional networks might still be in progress (Holland et al., 2007).
In the collection of studies we reviewed, there are some other linguistic abilities that, although not being domain‐specific but rather arising from the interplay of multiple macrodomains, have been found to be modulated by the AF, namely, word learning and the development of reading abilities. Finally, a very complex ability that relies on different linguistic domains, that is, naming, has been defined to be supported by the AF mainly in the left hemisphere: indeed, few studies report anomia after AF stimulation during DES subcortical mapping.
Although it is evident that the left AF mainly mediates linguistic abilities, our review revealed an involvement of this bundle also in verbal memory. However, out of this sample, one work focuses its definition of verbal memory on working memory, whereas one other study specifies that the left AF might be involved in verbal short‐term memory since its direct stimulation during surgery causes item errors during a digit span task. Another cognitive function that has been attributed to the AF is social cognition, with a role in false‐belief understanding in developing children (bilateral AF) and in face‐based mentalizing (right AF). According to the review we carried out, there are two other nonlinguistic cognitive functions that have been attributed to the right AF: music perception (tone discrimination and grammar learning in the domain of pitch as well as rhythm modulation detection) and spatial cognition (spatial perception and visuospatial attention). The full lists of studies, separated according to specific functions, are reported in Table 2.
3. AF TOPOGRAPHICAL ANATOMY AND SURGICAL IMPLICATIONS
Our literature review upholds the intricacy of the AF structural wiring as the foundation of a likewise considerable functional potentiality. Despite WM pathways have themselves limited structural plastic potential once connections are established, the extension of this bundle constitutes the anatomical substrate of a possibly enormous postlesional functional reorganization (De Benedictis & Duffau, 2011; Duffau, 2009, 2014, 2018). Being it a mediator of the communication between frontal and temporal lobes by crossing the parietal one, thorough knowledge of this bundle is mandatory to plan and perform safe surgeries involving all these regions, to avoid severe postsurgical sequelae. Indeed, a recent work contains considerable reports of transient or permanent language deficits (in 11.7%–54.5% and 1.7%–18.2% of cases, respectively) for tumors’ resections involving these cortices (Fang et al., 2021). In particular, the worst language prognosis was reported for lesions located around the postcentral (PoCG) and the supramarginal (SMG) gyri, that correspond, in fact, to the segment of the AF course with the highest fiber density, and therefore the lowest compensating capacity in respect to its anterior and posterior portions (Fang et al., 2021; Herbet et al., 2016; Plaza et al., 2009; van Geemen et al., 2014). Moreover, and as previously mentioned, disconnection of the right AF has been related to impairment in face‐based mentalizing (Herbet et al., 2014; Yordanova et al., 2017).
Consequently, a careful and patient‐tailored surgical planning for the preservation of eloquent subcortical structures, including the AF, is of foremost importance. This last section reports four different surgical cases of glioma resection involving the AF territories. Each of them addresses not only how the functional information collected during the procedure drives the definition of the resection boundaries but also how knowledge about the AF possible extension and functional implications drives surgical planning. This envisages (i) an accurate preoperative neuropsychological assessment for the selection of the most adequate tasks to be administered during systematic cortical and subcortical electrical mapping in awake conditions based on the location of the lesion (Bu et al., 2021; Martino, Gomez et al., 2013; Peraud et al., 2004; Rolland et al., 2018; Sarubbo et al., 2020); (ii) the evaluation of which WM pathways will possibly be impacted by the specific procedure, coupled with a tractographic 3D reconstruction of the WM bundles of interest.
For what concerns intraoperative neuropsychological assessment, the denomination task is normally used for procedures concerning the AF territories within the left language‐dominant hemisphere to test the emergence of phonemic paraphasia or pure anomia with DES. Electrostimulation of the right AF can evoke transient mentalizing troubles or spatial neglect: The Reading the Mind in the Eyes (Baron‐Cohen et al., 2001; Herbet et al., 2014; Sarubbo et al., 2020; Vigneau et al., 2006; Yordanova et al., 2017) and the line bisection tasks (Bartolomeo et al., 2007; He et al., 2007; Rolland et al., 2018; Roux et al., 2011; Sarubbo et al., 2015, 2020; Thiebaut de Schotten, 2005) are the preferential neuropsychological evaluation tools adopted in this case. In general, intraoperative cognitive testing gives real‐time feedback to the operator about the functional relevance of a given structure and, therefore, represents a precious directive on whether it is safe to resect it.
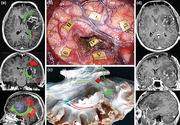
As briefly mentioned above, different surgical procedures require to carefully consider the patient's AF wiring and the related functional networks. For instance, when approaching a tumor located in the frontal lobe, namely, under the IFG, the MFG, and the dorsolateral prefrontal cortex, it should be considered that the AF courses horizontally at this level, and that its fibers are strongly intertwined with the deep layer of the inferior fronto‐occipital fasciculus (IFOF), which locally follows a vertical temporo‐frontal orientation. In this case, the fibers of the AF that extend to the IFG represent the deep and posterior functional boundary. At this level, AF terminations overlap with the most ventral segment of the superior longitudinal fasciculus (SLF III) and with the superficial layer of the IFOF (De Benedictis et al., 2012; Duffau, 2018; Dziedzic et al., 2022). Figure 5 illustrates the case of a 42‐year‐old man, who underwent resection of a high‐grade glioma located within the left dominant fronto‐insular region. Although cortical DES in awake condition allowed to identify one site eliciting speech arrest when stimulated, subcortical mapping during resection revealed eloquent functional sites evoking anomia, semantic paraphasia, and perseveration during denomination task, in correspondence to the IFOF's frontal projections.
FIGURE 5.
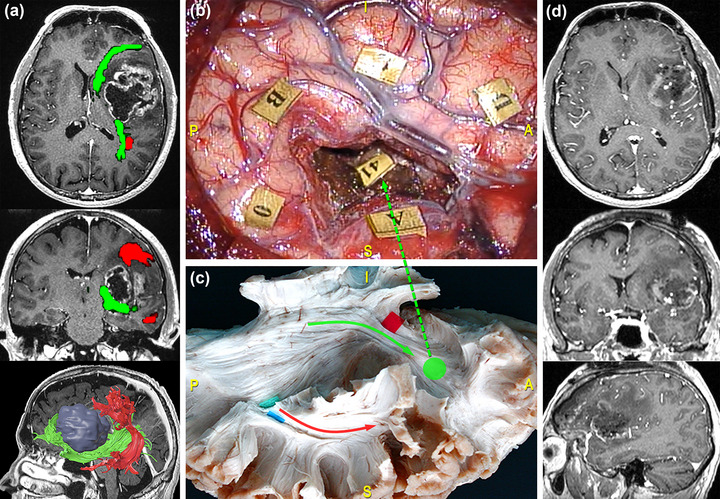
Surgical case concerning a 42‐year‐old man who underwent resection of a high‐grade glioma located within the left dominant fronto‐insular region. (a) Preoperative magnetic resonance imaging (MRI) (from top to bottom: axial, coronal, and 3D‐sagittal sequences) combined with tractographic reconstruction of the left arcuate fasciculus (AF) (red) and inferior fronto‐occipital fasciculus (IFOF) (green). (b) Intraoperative picture showing the tumor's resection performed with “asleep‐awake‐asleep” technique. Tag 0 refers to speech arrest site at the cortical level, while stimulation in correspondence of tag 41, representing IFOF's frontal projections elicited anomia, semantic paraphasia, and perseveration during denomination task. (c) Dissection of the perisylvian region with Klingler technique. The specimen has been oriented according to the surgical perspective. The green circle corresponds to the site of direct electrical stimulation (DES) stimulation along the course of the frontal projections of the IFOF (green arrow). (d) Postoperative MRI showing the resection on the axial, coronal, and sagittal plane from top to bottom.
When dealing with insular tumors, many studies support the safety and reliability of the transopercular approach, although it requires passing through IFGtri and IFGop (classically known as Broca's area). For this type of procedure, the AF constitutes the deep, superior, and posterior functional limit of resection (Dziedzic et al., 2022).
At the level of the temporo‐parieto‐occipital junction, the AF participates in a complex system of connections involving many eloquent pathways, and it is therefore relevant in the case of lesions involving the inferior parietal lobule. Indeed, at this level, the AF forms a compact vertical stem that runs deep and parallel to the temporoparietal component of the SLF, crossing even deeper tracts characterized by a horizontal anterior‐to‐posterior course, namely, the middle and inferior longitudinal fasciculi (MdLF and ILF), the IFOF, and the optic radiation (Martino et al., 2011; Sarubbo et al., 2016). Here, the AF is located at the anterolateral wall of the cavity (De Benedictis et al., 2014). Figure 6 reports the case of a 69‐year‐old man, who underwent resection of a high‐grade glioma located within the left dominant frontoparietal region. Functional responses were found at the cortical level when stimulating the PrCG, eliciting facial and arm contractions, the ventral premotor cortex (VPMC), eliciting speech arrest, the IFGtri, eliciting anomia, and the posterior third of the STG, eliciting phonemic paraphasia. No functional responses were found during stimulation of the superior parietal lobule. On the other hand, subcortical mapping in correspondence of the AF was related to phonemic paraphasia. Figure 7 shows the case of a 41‐year‐old man, who underwent resection of a high‐grade glioma located within the superior parietal lobule of the left dominant hemisphere. DES allowed to identify eloquent cortical sites, including the PoCG, eliciting right facial paresthesia when stimulated, and the SMG, related to speech arrest and anomia during picture naming task. No functional response was found for neither motor nor visuospatial tasks in the left parietal lobule. During tumor resection, subcortical mapping evoked semantic paraphasia along the dorsal component of the IFOF and verbal apraxia during the denomination task in correspondence of the temporoparietal component of the SLF and the AF.
FIGURE 6.
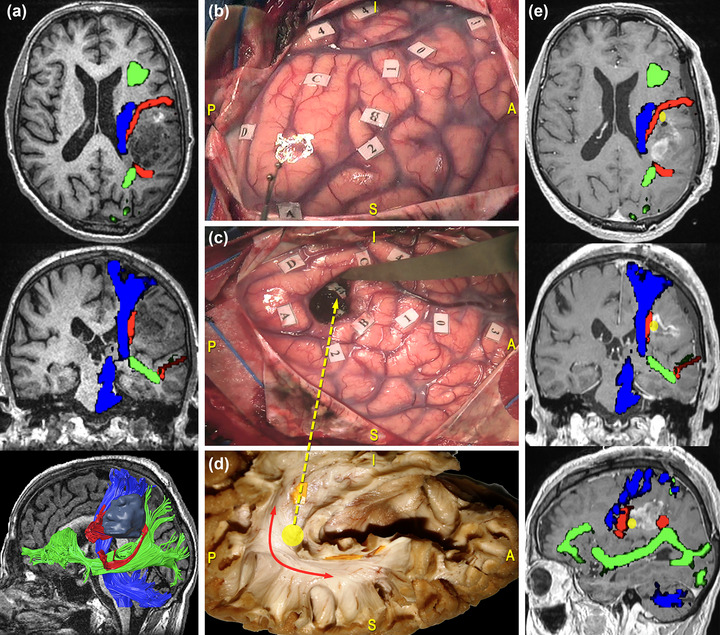
Surgical case concerning a 69‐year‐old man who underwent resection of a high‐grade glioma located within the left dominant frontoparietal region. (a) Preoperative magnetic resonance imaging (MRI) (from top to bottom: axial, coronal, and 3D‐sagittal sequences) combined with tractographic reconstruction of the left arcuate fasciculus (AF) (red), the inferior fronto‐occipital fasciculus (IFOF) (green) and the corticospinal tract (CST) (blue). (b) Intraoperative picture showing the cortical mapping performed in awake condition with direct electrical stimulation (DES). Functional responses were found at the level of the precentral gyrus (PrCG), eliciting facial, and arm contractions (tag 1 and 2); of the ventral premotor cortex (VPMC), eliciting speech arrest (tag 0); of the pars triangularis (IFGtri), eliciting anomia (tag 3); of the posterior third of the superior temporal gyrus (STG), eliciting phonemic paraphasia (tag 4). (c) Intraoperative picture showing the results of subcortical mapping. Phonemic paraphasia was elicited at the level of the AF (tag 44). (d) Anatomical specimen of a left hemisphere oriented according to the surgical perspective and showing the AF course corresponding to the stimulation site (yellow circle and arrow) (e) Postoperative MRI (from top to bottom: axial, coronal, and sagittal sequences) combined with tractographic reconstruction of the left AF (red), the IFOF (green), and the CST (blue). The yellow circle corresponds to the site of elicitation of phonemic paraphasia (tag 44), matching with the AF course.
FIGURE 7.
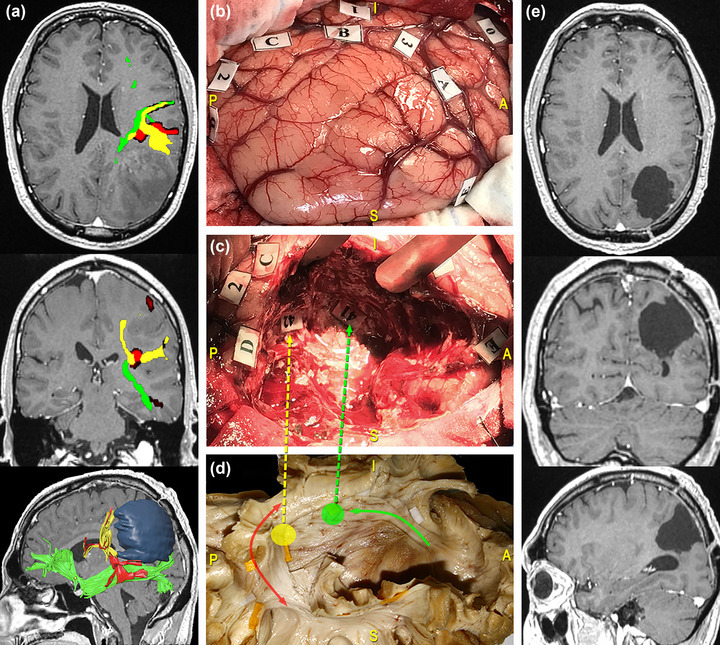
Surgical case concerning a 41‐year‐old man who underwent resection of a high‐grade glioma located within the superior parietal lobule of the left dominant hemisphere. (a) Preoperative magnetic resonance imaging (MRI) (from top to bottom: axial, coronal, and 3D‐sagittal sequences) combined with tractographic reconstruction of the left arcuate fasciculus (AF) (red), the inferior fronto‐occipital fasciculus (IFOF) (green), and the temporoparietal component of the superior longitudinal fasciculus (SLF) (yellow). (b) Intraoperative picture showing the tumor's resection performed according to the “asleep‐awake‐asleep” protocol. Direct electrical stimulation (DES) allowed to identify eloquent cortical sites including: the postcentral gyrus (PoCG), eliciting right facial paresthesia when stimulated (tag 0); the supramarginal gyrus (SMG), eliciting speech arrest (tag 1) and anomia (tag 2 and 3) during naming tasks. (c) During tumor resection, subcortical mapping allowed to evoke semantic paraphasia at the level of the dorsal part of the IFOF (tag 41) and verbal apraxia during the denomination task, along the temporoparietal component of the SLF and the AF (tag 42). (d) Dissection of the perisylvian region with Klingler technique. The specimen has been oriented according to the surgical perspective. The colored tags correspond to the IFOF (green circle and arrow) and the temporoparietal SLF/AF (yellow circle, red, and yellow arrows). Postoperative MRI shows complete tumor resection.
The AF also represents an important functional limit when performing a temporal lobectomy for tumor removal or epilepsy surgery, especially in the dominant hemisphere. In these cases, the AF temporal terminations constitute the subcortical posterolateral boundary of resection (Duffau et al., 2008). When approaching the ventral left temporal region, corresponding to the visual word form area, DES mapping is recommended to differentiate the course of the AF from the one of the ILF: Stimulation of the left AF at this level would induce pure anomia by interfering with the integration of visual information into the language network (Duffau et al., 2008; Mandonnet et al., 2017; Sarubbo et al., 2020). At this level, also the course of the IFOF should be considered. Figure 8 illustrates the case of a 47‐year‐old woman who underwent resection of a high‐grade glioma located within the posterior third of the MTG of the left dominant hemisphere. DES in awake condition allowed to identify the ventral motor strip of the face, and cortical stimulation of the VPMC elicited speech arrest. No functional response was found at denomination, reading comprehension and motor tasks during stimulation of the middle and posterior parts of the temporal region. Subcortical mapping allowed to identify the resection boundaries represented by the anteroinferior portion of the AF, that once stimulated led to phonemic paraphasia during denomination task, and the IFOF, whose stimulation led to semantic paraphasia.
FIGURE 8.
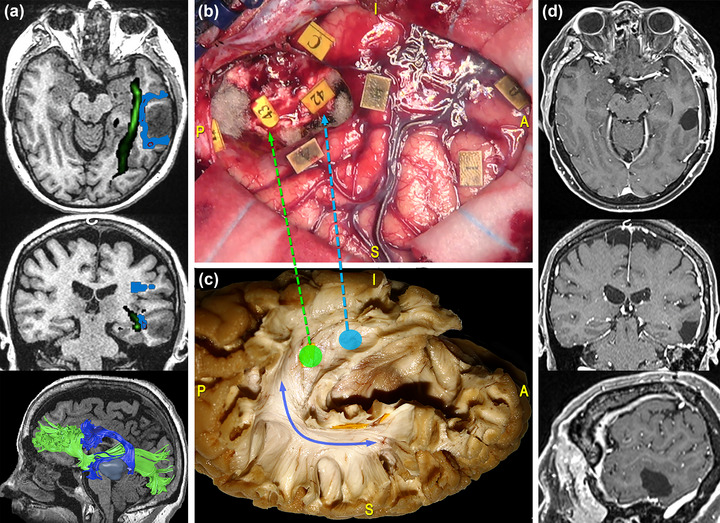
Surgical case concerning a 47‐year‐old woman who underwent resection of a high‐grade glioma located within the posterior third of the middle temporal gyrus (MTG) of the left dominant hemisphere. (a) Preoperative magnetic resonance imaging (MRI) (from top to bottom: axial, coronal, and 3D‐sagittal sequences) combined with tractographic reconstruction of the left arcuate fasciculus (AF) (blue) and inferior fronto‐occipital fasciculus (IFOF) (green). (b) Intraoperative picture showing the tumor's resection performed with “asleep‐awake‐asleep” technique. Direct electrical stimulation (DES) in awake condition allowed to identify eloquent cortical sites, including the ventral premotor cortex (VPMC), eliciting speech arrest (tag 0), and the ventral motor strip of the face (tag 1). During tumor's resection, the subcortical mapping allowed to identify the resection boundaries. These were established by eliciting phonemic paraphasia during the denomination task at the level of the anteroinferior portion of the AF (tag 42) and sematic paraphasia at the level of the IFOF (tag 43). (c) Dissection of the perisylvian region with Klingler technique. The specimen has been oriented according to the surgical perspective. The blue line corresponds to the AF stem and the light‐blue and green circles and arrows to the site where the IFOF and the AF were stimulated, respectively. (d) Postoperative MRI showed the complete tumor resection on the axial, coronal, and sagittal planes from top to bottom.
Finally, the AF is strictly related to the ventricular system. Indeed, the superior part of the AF runs lateral to the superior two thirds of the frontal horn and to the body of the lateral ventricle; its central segment runs lateral to the anterior two thirds of the atrium, whereas its inferior portion runs from the level of the posterior insular point (i.e., the junction between the inferior and the superior insular sulci) to the anterior tip of the temporal horn (Güngör et al., 2017). The understanding of these anatomical relationships is crucial to perform safe accesses to ventricular tumors, in particular when adopting an anterior frontal transcortical, posterior transcortical, or posterior transtemporal approach (De Benedictis et al., 2022).
4. CONCLUSIONS
The name “arcuate fasciculus” encloses two Centuries of neuroanatomical discoveries and evolutions. Since its first mention in Karl Friedrich Burdach's work (Burdach, 1822), this nomenclature became a pillar for the definition of the human brain's WM, and the associated knowledge increased concurrently with the methodological advancements of the past years. The investigations conducted on the AF concurred that this bundle is a crucial structural pathway for the integration of cognitive functions. Its shape, the large territories it connects, and the critical course it follows—interfacing with eloquent portions of the Superior and Inferior Longitudinal Systems as well as with projection fibers—make it one of the most critical association WM structures that require monitoring during surgical procedures. On the functional counterpart, many works demonstrate that the AF is critical for cognitive processing beyond language on both left and right sides. Indeed, this property can only be featured by a long‐range associative bundle connecting multimodal and highly distributed cortical areas, and therefore subserving the integration of different networks.
Given these premises, extensive knowledge about the AF structural extension, its relationship with other nearby WM structures, and the consequent implication in cognitive processes is essential to plan safe surgical approaches, and to consider all the variables that could affect the patient's outcome. We therefore summarized and reported multimodal evidence accounting for the current definition of the overall wiring and the functional relevance of the AF. The additional description of surgical cases of tumor resection considers how this knowledge can be translated into the surgical practice and describes practical tools and technical nuances for planning and performing safe resections with direct mapping of this critical structure.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.3107
ACKNOWLEDGMENTS
This study was supported by Provincia Autonoma of Trento through “NeuSurPlan: an integrated approach to neurosurgery planning based on multimodal data.” funding granted to Azienda Provinciale per i Servizi Sanitari (ID 2021‐D337‐00094). The Authors are grateful to the Health Department of the Provincia Autonoma of Trento and to the Direction Team of the Azienda Provinciale per i Servizi Sanitari for their support to NeuSurPlan Project.
Vavassori, L. , Venturini, M. , Zigiotto, L. , Annicchiarico, L. , Corsini, F. , Avesani, P. , Petit, L. , De Benedictis, A. , & Sarubbo, S. (2023). The arcuate fasciculus: Combining structure and function into surgical considerations. Brain and Behavior, 13, e3107. 10.1002/brb3.3107
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Agrawal, A. , Kapfhammer, J. P. , Kress, A. , Wichers, H. , Deep, A. , Feindel, W. , Sonntag, V. K. H. , Spetzler, R. F. , & Preul, M. C. (2011). Josef Klinglerʼs models of white matter tracts: Influences on neuroanatomy, neurosurgery, and neuroimaging. Neurosurgery, 69(2), 238–254. 10.1227/NEU.0b013e318214ab79 [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen, S. , Wheelwright, S. , Hill, J. , Raste, Y. , & Plumb, I. (2001). The “Reading the Mind in the Eyes” test revised version: A study with normal adults, and adults with Asperger syndrome or high‐functioning autism. Journal of Child Psychology and Psychiatry, 42(2), 241–251. 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- Barrick, T. R. , Lawes, I. N. , Mackay, C. E. , & Clark, C. A. (2006). White matter pathway asymmetry underlies functional lateralization. Cerebral Cortex, 17(3), 591–598. 10.1093/cercor/bhk004 [DOI] [PubMed] [Google Scholar]
- Bartolomeo, P. , Thiebaut de Schotten, M. T. , & Duffau, H. (2007). Mapping of visuospatial functions during brain surgery. Neurosurgery, 61(6), E1340–E1340. [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , Pajevic, S. , Pierpaoli, C. , Duda, J. , & Aldroubi, A. (2000). In vivo fiber tractography using DT‐MRI data. Magnetic Resonance in Medicine, 44, 625–632. [DOI] [PubMed] [Google Scholar]
- Bates, E. , Wilson, S. M. , Saygin, A. P. , Dick, F. , Sereno, M. I. , Knight, R. T. , & Dronkers, N. F. (2003). Voxel‐based lesion–symptom mapping. Nature Neuroscience, 6(5), 3. [DOI] [PubMed] [Google Scholar]
- Becker, Y. , Loh, K. K. , Coulon, O. , & Meguerditchian, A. (2022). The arcuate fasciculus and language origins: Disentangling existing conceptions that influence evolutionary accounts. Neuroscience & Biobehavioral Reviews, 134, 104490. 10.1016/j.neubiorev.2021.12.013 [DOI] [PubMed] [Google Scholar]
- Benzagmout, M. , Gatignol, P. , & Duffau, H. (2007). Resection of world health orhanization grade II gliomas involving Broca's area: Methodological and functional considerations. Neurosurgery, 61(4), 13. [DOI] [PubMed] [Google Scholar]
- Breier, J. I. , Hasan, K. M. , Zhang, W. , Men, D. , & Papanicolaou, A. C. (2008). Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. American Journal of Neuroradiology, 29(3), 483–487. 10.3174/ajnr.A0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca, P. (1865). Sur le siège de la faculté du langage articulé. Bulletins et Mémoires de La Société d'Anthropologie de Paris, 6, 337–393. [Google Scholar]
- Bu, L.‐H. , Zhang, J. , Lu, J.‐F. , & Wu, J.‐S. (2021). Glioma surgery with awake language mapping versus generalized anesthesia: A systematic review. Neurosurgical Review, 44(4), 1997–2011. 10.1007/s10143-020-01418-9 [DOI] [PubMed] [Google Scholar]
- Bunevicius, A. , Tamasauskas, S. , Deltuva, V. , Tamasauskas, A. , Radziunas, A. , & Bunevicius, R. (2014). Predictors of health‐related quality of life in neurosurgical brain tumor patients: Focus on patient‐centered perspective. Acta Neurochirurgica, 156(2), 367–374. 10.1007/s00701-013-1930-7 [DOI] [PubMed] [Google Scholar]
- Burdach, K. F. (1822). Vom Baue und Leben des Gehirns (Vol. 2). Dyk. [Google Scholar]
- Burdach, K. F. (1826). Vom Baue und Leben des Gehirns (Vol. 3). Dyk. [Google Scholar]
- Cabinio, M. , Rossetto, F. , Blasi, V. , Savazzi, F. , Castelli, I. , Massaro, D. , Valle, A. , Nemni, R. , Clerici, M. , Marchetti, A. , & Baglio, F. (2015). Mind‐reading ability and structural connectivity changes in aging. Frontiers in Psychology, 6, 1808. 10.3389/fpsyg.2015.01808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , Howard, R. J. , Pajevic, S. , & Jones, D. K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage, 17(1), 77–94. 10.1006/nimg.2002.1136 [DOI] [PubMed] [Google Scholar]
- Catani, M. , Jones, D. K. , & ffytche, D. H. (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57(1), 8–16. 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Chan‐Seng, E. , Moritz‐Gasser, S. , & Duffau, H. (2014). Awake mapping for low‐grade gliomas involving the left sagittal stratum: Anatomofunctional and surgical considerations. Journal of Neurosurgery, 120(5), 1069–1077. 10.3171/2014.1.JNS132015 [DOI] [PubMed] [Google Scholar]
- Chen, X. , Zhao, Y. , Zhong, S. , Cui, Z. , Li, J. , Gong, G. , Dong, Q. , & Nan, Y. (2018). The lateralized arcuate fasciculus in developmental pitch disorders among mandarin amusics: Left for speech and right for music. Brain Structure and Function, 223, 2013–2024. 10.1007/s00429-018-1608-2 [DOI] [PubMed] [Google Scholar]
- Chiang, H. , Chen, Y. , Lin, H. , Tseng, W. I. , & Gau, S. S. (2017). Disorder‐specific alteration in white matter structural property in adults with autism spectrum disorder relative to adults with ADHD and adult controls. Human Brain Mapping, 38(1), 384–395. 10.1002/hbm.23367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis, A. , & Duffau, H. (2011). Brain hodotopy: From esoteric concept to practical surgical applications. Neurosurgery, 68(6), 1703–1723. 10.1227/NEU.0b013e3182124690 [DOI] [PubMed] [Google Scholar]
- De Benedictis, A. , Duffau, H. , Paradiso, B. , Grandi, E. , Balbi, S. , Granieri, E. , Colarusso, E. , Chioffi, F. , Marras, C. E. , & Sarubbo, S. (2014). Anatomo‐functional study of the temporo‐parieto‐occipital region: Dissection, tractographic and brain mapping evidence from a neurosurgical perspective. Journal of Anatomy, 225(2), 132–151. 10.1111/joa.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis, A. , Marras, C. E. , Petit, L. , & Sarubbo, S. (2022). The inferior fronto‐occipital fascicle: A century of controversies from anatomy theaters to operative neurosurgery. Journal of Neurosurgical Sciences, 65(6), 605–615. 10.23736/S0390-5616.21.05360-1 [DOI] [PubMed] [Google Scholar]
- De Benedictis, A. , Sarubbo, S. , & Duffau, H. (2012). Subcortical surgical anatomy of the lateral frontal region: Human white matter dissection and correlations with functional insights provided by intraoperative direct brain stimulation: Laboratory investigation. Journal of Neurosurgery, 117(6), 1053–1069. 10.3171/2012.7.JNS12628 [DOI] [PubMed] [Google Scholar]
- Dejerine, J. , & Dejerine‐Klumpke, A. (1895). Anatomie des centres nerveux. Tome 1. Rueff et Cie. [Google Scholar]
- Dejerine, J. , & Dejerine‐Klumpke, A. (1901). Anatomie des centres nerveux. Tome 2. Rueff et Cie. [Google Scholar]
- Duffau, H. (2009). Does post‐lesional subcortical plasticity exist in the human brain? Neuroscience Research, 65(2), 131–135. 10.1016/j.neures.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Duffau, H. (2014). The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 58, 325–337. 10.1016/j.cortex.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Duffau, H. (2018). The error of Broca: From the traditional localizationist concept to a connectomal anatomy of human brain. Journal of Chemical Neuroanatomy, 89, 73–81. 10.1016/j.jchemneu.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Duffau, H. , de Schotten, M. T. , & Mandonnet, E. (2008). White matter functional connectivity as an additional landmark for dominant temporal lobectomy. Journal of Neurology, Neurosurgery & Psychiatry, 79(5), 492–495. 10.1136/jnnp.2007.121004 [DOI] [PubMed] [Google Scholar]
- Duffau, H. , Lopes, M. , Arthuis, F. , Bitar, A. , & Sichez, J.‐P. (2005). Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. Journal of Neurology, Neurosurgery, and Psychiatry, 76, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic, T. A. , Bala, A. , & Marchel, A. (2022). Anatomical aspects of the insula, opercula and peri‐insular white matter for a transcortical approach to insular glioma resection. Neurosurgical Review, 45(1), 793–806. 10.1007/s10143-021-01602-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmore, T. M. , Beauchamp, M. S. , O'Neill, T. J. , Dreyer, S. , & Tandon, N. (2009). Relationships between essential cortical language sites and subcortical pathways. Journal of Neurosurgery, 111(4), 755–766. 10.3171/2009.3.JNS081427 [DOI] [PubMed] [Google Scholar]
- Fang, S. , Liang, Y. , Li, L. , Wang, L. , Fan, X. , Wang, Y. , & Jiang, T. (2021). Tumor location‐based classification of surgery‐related language impairments in patients with glioma. Journal of Neuro‐Oncology, 155(2), 143–152. 10.1007/s11060-021-03858-9 [DOI] [PubMed] [Google Scholar]
- Fernández‐Miranda, J. C. , Wang, Y. , Pathak, S. , Stefaneau, L. , Verstynen, T. , & Yeh, F.‐C. (2015). Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Structure and Function, 220(3), 1665–1680. 10.1007/s00429-014-0751-7 [DOI] [PubMed] [Google Scholar]
- Forkel, S. J. , Friedrich, P. , Thiebaut de Schotten, M. , & Howells, H. (2021). White matter variability, cognition, and disorders: A systematic review. Brain Structure and Function, 227, 529–544. 10.1007/s00429-021-02382-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel, S. J. , Rogalski, E. , Drossinos Sancho, N. , D'Anna, L. , Luque Laguna, P. , Sridhar, J. , Dell'Acqua, F. , Weintraub, S. , Thompson, C. , Mesulam, M.‐M. , & Catani, M. (2020). Anatomical evidence of an indirect pathway for word repetition. Neurology, 94(6), e594–e606. 10.1212/WNL.0000000000008746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel, S. J. , Thiebaut de Schotten, M. , Dell'Acqua, F. , Kalra, L. , Murphy, D. G. M. , Williams, S. C. R. , & Catani, M. (2014). Anatomical predictors of aphasia recovery: A tractography study of bilateral perisylvian language networks. Brain, 137(7), 2027–2039. 10.1093/brain/awu113 [DOI] [PubMed] [Google Scholar]
- Frey, S. , Campbell, J. S. W. , Pike, G. B. , & Petrides, M. (2008). Dissociating the human language pathways with high angular resolution diffusion fiber tractography. Journal of Neuroscience, 28(45), 11435–11444. 10.1523/JNEUROSCI.2388-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Kjartansson, O. , Morgan, P. S. , Hjaltason, H. , Magnusdottir, S. , Bonilha, L. , & Rorden, C. (2010). Impaired speech repetition and left parietal lobe damage. Journal of Neuroscience, 30(33), 11057–11061. 10.1523/JNEUROSCI.1120-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici, A. D. (2009). Pathways to language: Fiber tracts in the human brain. Trends in Cognitive Sciences, 13(4), 175–181. 10.1016/j.tics.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Frost, J. A. (1999). Language processing is strongly left lateralized in both sexes: Evidence from functional MRI. Brain, 122(2), 199–208. 10.1093/brain/122.2.199 [DOI] [PubMed] [Google Scholar]
- Geschwind, N. (1965). Disconnexion syndromes in animals and man. II. Brain, 88(3), 585–644. [DOI] [PubMed] [Google Scholar]
- Geschwind, N. (1970). The organization of language and the brain. [DOI] [PubMed]
- Geva, S. , Correia, M. M. , & Warburton, E. A. (2015). Contributions of bilateral white matter to chronic aphasia symptoms as assessed by diffusion tensor MRI. Brain and Language, 150, 117–128. 10.1016/j.bandl.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharabaghi, A. , Kunath, F. , Erb, M. , Saur, R. , Heckl, S. , Tatagiba, M. , Grodd, W. , & Karnath, H.‐O. (2009). Perisylvian white matter connectivity in the human right hemisphere. BMC Neuroscience, 10(1), 15. 10.1186/1471-2202-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser, M. F. , & Rilling, J. K. (2008). DTI tractography of the human brain's language pathways. Cerebral Cortex, 18(11), 2471–2482. 10.1093/cercor/bhn011 [DOI] [PubMed] [Google Scholar]
- Griffiths, J. D. , Marslen‐Wilson, W. D. , Stamatakis, E. A. , & Tyler, L. K. (2013). Functional organization of the neural language system: Dorsal and ventral pathways are critical for syntax. Cerebral Cortex, 23(1), 139–147. 10.1093/cercor/bhr386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Wiesmann, C. , Schreiber, J. , Singer, T. , Steinbeis, N. , & Friederici, A. D. (2017). White matter maturation is associated with the emergence of Theory of Mind in early childhood. Nature Communications, 8(1), 14692. 10.1038/ncomms14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güngör, A. , Baydin, S. , Middlebrooks, E. H. , Tanriover, N. , Isler, C. , & Rhoton, A. L. (2017). The white matter tracts of the cerebrum in ventricular surgery and hydrocephalus. Journal of Neurosurgery, 126(3), 945–971. 10.3171/2016.1.JNS152082 [DOI] [PubMed] [Google Scholar]
- He, B. J. , Snyder, A. Z. , Vincent, J. L. , Epstein, A. , Shulman, G. L. , & Corbetta, M. (2007). Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron, 53(6), 905–918. 10.1016/j.neuron.2007.02.013 [DOI] [PubMed] [Google Scholar]
- Herbet, G. , Lafargue, G. , Bonnetblanc, F. , Moritz‐Gasser, S. , de Champfleur, N. M. , & Duffau, H. (2014). Inferring a dual‐stream model of mentalizing from associative white matter fibres disconnection. Brain, 16, 944–959. [DOI] [PubMed] [Google Scholar]
- Herbet, G. , Maheu, M. , Costi, E. , Lafargue, G. , & Duffau, H. (2016). Mapping neuroplastic potential in brain‐damaged patients. Brain, 139(3), 829–844. 10.1093/brain/awv394 [DOI] [PubMed] [Google Scholar]
- Hervey‐Jumper, S. L. , & Berger, M. S. (2016). Maximizing safe resection of low‐ and high‐grade glioma. Journal of Neuro‐Oncology, 130(2), 269–282. 10.1007/s11060-016-2110-4 [DOI] [PubMed] [Google Scholar]
- Holland, S. K. , Vannest, J. , Mecoli, M. , Jacola, L. M. , Tillema, J.‐M. , Karunanayaka, P. R. , Schmithorst, V. J. , Yuan, W. , Plante, E. , & Byars, A. W. (2007). Functional MRI of language lateralization during development in children. International Journal of Audiology, 46(9), 533–551. 10.1080/14992020701448994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope, T. M. H. , Seghier, M. L. , Prejawa, S. , Leff, A. P. , & Price, C. J. (2016). Distinguishing the effect of lesion load from tract disconnection in the arcuate and uncinate fasciculi. Neuroimage, 125, 1169–1173. 10.1016/j.neuroimage.2015.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, M. V. , Isaev, D. Y. U. , Dragoy, O. V. , Akinina, Y. S. , Petrushevskiy, A. G. , Fedina, O. N. , Shklovsky, V. M. , & Dronkers, N. F. (2016). Diffusion‐tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 85, 165–181. 10.1016/j.cortex.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Janssen, N. , Kessels, R. P. C. , Mars, R. B. , Llera, A. , Beckmann, C. F. , & Roelofs, A. (2023). Dissociating the functional roles of arcuate fasciculus subtracts in speech production. Cerebral Cortex, 33(6), 2539–2547. 10.1093/cercor/bhac224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Zhao, Y.‐J. , Hu, X.‐Y. , Du, M.‐Y. , Chen, Z.‐Q. , Wu, M. , Li, K.‐M. , Zhu, H.‐Y. , Kumar, P. , & Gong, Q.‐Y. (2017). Microstructural brain abnormalities in medication‐free patients with major depressive disorder: A systematic review and meta‐analysis of diffusion tensor imaging. Journal of Psychiatry & Neuroscience, 42(3), 150–163. 10.1503/jpn.150341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler, J. (1935). Erleichterung des makroskopischen Praeparation des Gehirns durch den Gefrierprozess. Schweizer Archiv Für Neurologie Und Psychiatrie, 36, 247–256. [Google Scholar]
- Kumar, D. R. , Aslinia, F. , Yale, S. H. , & Mazza, J. J. (2011). Jean‐Martin Charcot: The father of neurology. Clinical Medicine & Research, 9, 49–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer, D. , Hartwigsen, G. , Kellmeyer, P. , Glauche, V. , Mader, I. , Klöppel, S. , Suchan, J. , Karnath, H.‐O. , Weiller, C. , & Saur, D. (2013). Damage to ventral and dorsal language pathways in acute aphasia. Brain, 136(2), 619–629. 10.1093/brain/aws354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawes, I. N. C. , Barrick, T. R. , Murugam, V. , Spierings, N. , Evans, D. R. , Song, M. , & Clark, C. A. (2008). Atlas‐based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage, 39(1), 62–79. 10.1016/j.neuroimage.2007.06.041 [DOI] [PubMed] [Google Scholar]
- Leclercq, D. , Duffau, H. , Delmaire, C. , Capelle, L. , Gatignol, P. , Ducros, M. , Chiras, J. , & Lehéricy, S. (2010). Comparison of diffusion tensor imaging tractography of language tracts and intraoperative subcortical stimulations. Journal of Neurosurgery, 112(3), 503–511. 10.3171/2009.8.JNS09558 [DOI] [PubMed] [Google Scholar]
- Leemans, A. (2019). Diffusion MRI of the brain: The naked truth. NMR in Biomedicine, 32(4), e4084. 10.1002/nbm.4084 [DOI] [PubMed] [Google Scholar]
- López‐Barroso, D. , Catani, M. , Ripollés, P. , Dell'Acqua, F. , Rodríguez‐Fornells, A. , & de Diego‐Balaguer, R. (2013). Word learning is mediated by the left arcuate fasciculus. Proceedings of the National Academy of Sciences, 110(32), 13168–13173. 10.1073/pnas.1301696110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loui, P. , Alsop, D. , & Schlaug, G. (2009). Tone deafness: A new disconnection syndrome? Journal of Neuroscience, 29(33), 10215–10220. 10.1523/JNEUROSCI.1701-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loui, P. , Li, H. C. , & Schlaug, G. (2011). White matter integrity in right hemisphere predicts pitch‐related grammar learning. Neuroimage, 55(2), 500–507. 10.1016/j.neuroimage.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, I. L. , Moritz‐Gasser, S. , de Champfleur, N. M. , Bertram, L. , Moulinié, G. , & Duffau, H. (2011). Surgery for gliomas involving the left inferior parietal lobule: New insights into the functional anatomy provided by stimulation mapping in awake patients. Journal of Neurosurgery, 115(4), 770–779. 10.3171/2011.5.JNS112 [DOI] [PubMed] [Google Scholar]
- Maldonado, I. L. , Moritz‐Gasser, S. , & Duffau, H. (2011). Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Structure and Function, 216(3), 263–274. 10.1007/s00429-011-0309-x [DOI] [PubMed] [Google Scholar]
- Malik‐Moraleda, S. , Ayyash, D. , Gallée, J. , Affourtit, J. , Hoffmann, M. , Mineroff, Z. , Jouravlev, O. , & Fedorenko, E. (2022). An investigation across 45 languages and 12 language families reveals a universal language network. Nature Neuroscience, 25(8), 1014–1019. 10.1038/s41593-022-01114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet, E. , Martino, J. , Sarubbo, S. , Corrivetti, F. , Bouazza, S. , Bresson, D. , Duffau, H. , & Froelich, S. (2017). Neuronavigated fiber dissection with pial preservation: Laboratory model to simulate opercular approaches to insular tumors. World Neurosurgery, 98, 239–242. 10.1016/j.wneu.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Mandonnet, E. , Sarubbo, S. , & Petit, L. (2018). The nomenclature of human white matter association pathways: Proposal for a systematic taxonomic anatomical classification. Frontiers in Neuroanatomy, 12, 94. 10.3389/fnana.2018.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchina, S. , Zhu, L. L. , Norton, A. , Zipse, L. , Wan, C. Y. , & Schlaug, G. (2011). Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke; A Journal of Cerebral Circulation, 42(8), 2251–2256. 10.1161/STROKEAHA.110.606103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, J. , De Witt Hamer, P. C. , Vergani, F. , Brogna, C. , de Lucas, E. M. , Vázquez‐Barquero, A. , García‐Porrero, J. A. , & Duffau, H. (2011). Cortex‐sparing fiber dissection: An improved method for the study of white matter anatomy in the human brain: Cortex‐sparing fiber dissection. Journal of Anatomy, 219(4), 531–541. 10.1111/j.1469-7580.2011.01414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, J. , Gomez, E. , Bilbao, J. L. , Dueñas, J. C. , & Vázquez‐Barquero, A. (2013). Cost‐utility of maximal safe resection of WHO grade II gliomas within eloquent areas. Acta Neurochirurgica, 155(1), 41–50. 10.1007/s00701-012-1541-8 [DOI] [PubMed] [Google Scholar]
- Martino, J. , da Silva‐Freitas, R. , Caballero, H. , de Marco Lucas, E. , García‐Porrero, J. A. , & Vázquez‐Barquero, A. (2013). Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Operative Neurosurgery, 72(1), ons87–ons98. 10.1227/NEU.0b013e318274294b [DOI] [PubMed] [Google Scholar]
- Mayo, H. (1823). Anatomical and physiological commentaries, Number II. Underwood. [Google Scholar]
- McDonald, C. R. , Ahmadi, M. E. , Hagler, D. J. , Tecoma, E. S. , Iragui, V. J. , Gharapetian, L. , Dale, A. M. , & Halgren, E. (2008). Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology, 71(23), 1869–1876. 10.1212/01.wnl.0000327824.05348.3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, L. , Cunitz, K. , Obleser, J. , & Friederici, A. D. (2014). Sentence processing and verbal working memory in a white‐matter‐disconnection patient. Neuropsychologia, 61, 190–196. 10.1016/j.neuropsychologia.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Moritz‐Gasser, S. , & Duffau, H. (2013). The anatomo‐functional connectivity of word repetition: Insights provided by awake brain tumor surgery. Frontiers in Human Neuroscience, 7, 00–00. 10.3389/fnhum.2013.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, K. M. , Vohr, B. R. , Katz, K. H. , Schneider, K. C. , Lacadie, C. , Hampson, M. , Makuch, R. W. , Reiss, A. L. , Constable, R. T. , & Ment, L. R. (2011). Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage, 54(4), 2563–2570. 10.1016/j.neuroimage.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, R. , Kinoshita, M. , Shinohara, H. , & Nakada, M. (2019). The superior longitudinal fascicle: Reconsidering the fronto‐parietal neural network based on anatomy and function. Brain Imaging and Behavior, 14, 2817–2830. 10.1007/s11682-019-00187-4 [DOI] [PubMed] [Google Scholar]
- Nakajima, R. , Yordanova, Y. N. , Duffau, H. , & Herbet, G. (2018). Neuropsychological evidence for the crucial role of the right arcuate fasciculus in the face‐based mentalizing network: A disconnection analysis. Neuropsychologia, 115, 179–187. 10.1016/j.neuropsychologia.2018.01.024 [DOI] [PubMed] [Google Scholar]
- Papagno, C. , Comi, A. , Riva, M. , Bizzi, A. , Vernice, M. , Casarotti, A. , Fava, E. , & Bello, L. (2017). Mapping the brain network of the phonological loop: The phonological loop brain network. Human Brain Mapping, 38(6), 3011–3024. 10.1002/hbm.23569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi, M. , Stamatakis, E. A. , Griffiths, J. , Marslen‐Wilson, W. D. , & Tyler, L. K. (2011). Is left fronto‐temporal connectivity essential for syntax? Effective connectivity, tractography and performance in left‐hemisphere damaged patients. Neuroimage, 58(2), 656–664. 10.1016/j.neuroimage.2011.06.036 [DOI] [PubMed] [Google Scholar]
- Peraud, A. , Ilmberger, J. , & Reulen, H.‐J. (2004). Surgical resection of gliomas WHO grade II and III located in the opercular region. Acta Neurochirurgica, 146(1), 9–18. 10.1007/s00701-003-0165-4 [DOI] [PubMed] [Google Scholar]
- Phillips, O. R. , Clark, K. A. , Woods, R. P. , Subotnik, K. L. , Asarnow, R. F. , Nuechterlein, K. H. , Toga, A. W. , & Narr, K. L. (2011). Topographical relationships between arcuate fasciculus connectivity and cortical thickness. Human Brain Mapping, 32(11), 1788–1801. 10.1002/hbm.21147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza, M. , Gatignol, P. , Leroy, M. , & Duffau, H. (2009). Speaking without Broca's area after tumor resection. Neurocase, 15(4), 294–310. 10.1080/13554790902729473 [DOI] [PubMed] [Google Scholar]
- Porto de Oliveira, J. V. M. , Raquelo‐Menegassio, A. F. , & Maldonado, I. L. (2021). What's your name again? A review of the superior longitudinal and arcuate fasciculus evolving nomenclature. Clinical Anatomy, 34(7), 1101–1110. 10.1002/ca.23764 [DOI] [PubMed] [Google Scholar]
- Psomiades, M. , Fonteneau, C. , Mondino, M. , Luck, D. , Haesebaert, F. , Suaud‐Chagny, M.‐F. , & Brunelin, J. (2016). Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: A DTI‐tractography study. NeuroImage: Clinical, 12, 970–975. 10.1016/j.nicl.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer, Y. D. , Leemans, A. , Heringa, S. M. , Wielaard, I. , Jeurissen, B. , Koek, H. L. , & Biessels, G. J. (2012). Improved sensitivity to cerebral white matter abnormalities in Alzheimer's disease with spherical deconvolution based tractography. PLoS One, 7(8), e44074. 10.1371/journal.pone.0044074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reil, J. C. (1809). Die Sylvische Grube oder das Thal, das gestreifte grobe hirnganglium, dessen kapsel und die seitentheile des grobn gehirns. Archiv Für Die Physiologie, 9, 195–208. [Google Scholar]
- Reil, J. C. (1812). Die vördere commissur im groben gehirn. Archiv Für Die Physiologie, 11, 89–100. [Google Scholar]
- Rojkova, K. , Volle, E. , Urbanski, M. , Humbert, F. , Dell'Acqua, F. , & Thiebaut de Schotten, M. (2016). Atlasing the frontal lobe connections and their variability due to age and education: A spherical deconvolution tractography study. Brain Structure and Function, 221(3), 1751–1766. 10.1007/s00429-015-1001-3 [DOI] [PubMed] [Google Scholar]
- Rolland, A. , Herbet, G. , & Duffau, H. (2018). Awake surgery for gliomas within the right inferior parietal lobule: New insights into the functional connectivity gained from stimulation mapping and surgical implications. World Neurosurgery, 112, e393–e406. 10.1016/j.wneu.2018.01.053 [DOI] [PubMed] [Google Scholar]
- Roux, F.‐E. , Dufor, O. , Lauwers‐Cances, V. , Boukhatem, L. , Brauge, D. , Draper, L. , Lotterie, J.‐A. , & Démonet, J.‐F. (2011). Electrostimulation mapping of spatial neglect. Neurosurgery, 69(6), 1218–1231. 10.1227/NEU.0b013e31822aefd2 [DOI] [PubMed] [Google Scholar]
- Sanai, N. , Polley, M.‐Y. , McDermott, M. W. , Parsa, A. T. , & Berger, M. S. (2011). An extent of resection threshold for newly diagnosed glioblastomas. Journal of Neurosurgery, 115(1), 3–8. 10.3171/2011.2.JNS10998 [DOI] [PubMed] [Google Scholar]
- Sarubbo, S. , De Benedictis, A. , Merler, S. , Mandonnet, E. , Balbi, S. , Granieri, E. , & Duffau, H. (2015). Towards a functional atlas of human white matter: Functional atlas of white matter. Human Brain Mapping, 36(8), 3117–3136. 10.1002/hbm.22832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo, S. , De Benedictis, A. , Merler, S. , Mandonnet, E. , Barbareschi, M. , Dallabona, M. , Chioffi, F. , & Duffau, H. (2016). Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation: Anatomo‐functional integration of language. Human Brain Mapping, 37(11), 3858–3872. 10.1002/hbm.23281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo, S. , Tate, M. , De Benedictis, A. , Merler, S. , Moritz‐Gasser, S. , Herbet, G. , & Duffau, H. (2020). Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: An original functional atlas of the human brain. Neuroimage, 205, 116237. 10.1016/j.neuroimage.2019.116237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur, D. , Kreher, B. W. , Schnell, S. , Kümmerer, D. , Kellmeyer, P. , Vry, M.‐S. , Umarova, R. , Musso, M. , Glauche, V. , Abel, S. , Huber, W. , Rijntjes, M. , Hennig, J. , & Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences, 105(46), 18035–18040. 10.1073/pnas.0805234105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin, Z. M. , Norton, E. S. , Osher, D. E. , Beach, S. D. , Cyr, A. B. , Ozernov‐Palchik, O. , Yendiki, A. , Fischl, B. , Gaab, N. , & Gabrieli, J. D. E. (2013). Tracking the roots of reading ability: White matter volume and integrity correlate with phonological awareness in prereading and early‐reading kindergarten children. Journal of Neuroscience, 33(33), 13251–13258. 10.1523/JNEUROSCI.4383-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierpowska, J. , Gabarrós, A. , Fernandez‐Coello, A. , Camins, À. , Castañer, S. , Juncadella, M. , Morís, J. , & Rodríguez‐Fornells, A. (2017). Words are not enough: Nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. Journal of Neurosurgery, 126(2), 435–445. 10.3171/2016.2.JNS151592 [DOI] [PubMed] [Google Scholar]
- Swanson, L. W. (2015). Neuroanatomical terminology: A lexicon of classical origins and historical foundations. Oxford University Press. [Google Scholar]
- Szaflarski, J. P. , Binder, J. R. , Possing, E. T. , McKiernan, K. A. , Ward, B. D. , & Hammeke, T. A. (2002). Language lateralization in left‐handed and ambidextrous people: FMRI data. Neurology, 59(2), 238–244. 10.1212/WNL.59.2.238 [DOI] [PubMed] [Google Scholar]
- Teubner‐Rhodes, S. , Vaden, K. I. , Cute, S. L. , Yeatman, J. D. , Dougherty, R. F. , & Eckert, M. A. (2016). Aging‐resilient associations between the arcuate fasciculus and vocabulary knowledge: Microstructure or morphology? Journal of Neuroscience, 36(27), 7210–7222. 10.1523/JNEUROSCI.4342-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten, M. (2005). Direct evidence for a parietal‐frontal pathway subserving spatial awareness in humans. Science, 309(5744), 2226–2228. 10.1126/science.1116251 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten, M. , Dell'Acqua, F. , Valabregue, R. , & Catani, M. (2012). Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 48(1), 82–96. 10.1016/j.cortex.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Umarova, R. M. , Saur, D. , Schnell, S. , Kaller, C. P. , Vry, M.‐S. , Glauche, V. , Rijntjes, M. , Hennig, J. , Kiselev, V. , & Weiller, C. (2010). Structural connectivity for visuospatial attention: Significance of ventral pathways. Cerebral Cortex, 20(1), 121–129. 10.1093/cercor/bhp086 [DOI] [PubMed] [Google Scholar]
- Vandermosten, M. , Boets, B. , Poelmans, H. , Sunaert, S. , Wouters, J. , & Ghesquiere, P. (2012). A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain, 135(3), 935–948. 10.1093/brain/awr363 [DOI] [PubMed] [Google Scholar]
- van Geemen, K. , Herbet, G. , Moritz‐Gasser, S. , & Duffau, H. (2014). Limited plastic potential of the left ventral premotor cortex in speech articulation: Evidence From intraoperative awake mapping in glioma patients: Ventral Premotor Cortex and Speech. Human Brain Mapping, 35(4), 1587–1596. 10.1002/hbm.22275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero, L. , Ramos‐Escobar, N. , Cucurell, D. , François, C. , Putkinen, V. , Segura, E. , Huotilainen, M. , Penhune, V. , & Rodríguez‐Fornells, A. (2021). Arcuate fasciculus architecture is associated with individual differences in pre‐attentive detection of unpredicted music changes. Neuroimage, 229, 117759. 10.1016/j.neuroimage.2021.117759 [DOI] [PubMed] [Google Scholar]
- Vassal, F. , Schneider, F. , Boutet, C. , Jean, B. , Sontheimer, A. , & Lemaire, J.‐J. (2016). Combined DTI tractography and functional MRI study of the language connectome in healthy volunteers: Extensive mapping of white matter fascicles and cortical activations. PLoS One, 11(3), e0152614. 10.1371/journal.pone.0152614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassal, F. , Schneider, F. , Sontheimer, A. , Lemaire, J.‐J. , & Nuti, C. (2013). Intraoperative visualisation of language fascicles by diffusion tensor imaging‐based tractography in glioma surgery. Acta Neurochirurgica, 155(3), 437–448. 10.1007/s00701-012-1580-1 [DOI] [PubMed] [Google Scholar]
- Vavassori, L. , Sarubbo, S. , & Petit, L. (2021). Hodology of the superior longitudinal system of the human brain: A historical perspective, the current controversies, and a proposal. Brain Structure and Function, 226, 1363–1384. [DOI] [PubMed] [Google Scholar]
- Vigneau, M. , Beaucousin, V. , Hervé, P. Y. , Duffau, H. , Crivello, F. , Houdé, O. , Mazoyer, B. , & Tzourio‐Mazoyer, N. (2006). Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage, 30(4), 1414–1432. 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Monakow, C. (1897). Gehirnpathologie: Allgemeine Einleitung, Localisation, Gehirnblutungen, Verstopfung der Hirnarterien.
- Wang, J. , Marchina, S. , Norton, A. C. , Wan, C. Y. , & Schlaug, G. (2013). Predicting speech fluency and naming abilities in aphasic patients. Frontiers in Human Neuroscience, 7, 831. 10.3389/fnhum.2013.00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Van der Haegen, L. , Tao, L. , & Cai, Q. (2019). Brain functional organization associated with language lateralization. Cerebral Cortex, 29(10), 4312–4320. 10.1093/cercor/bhy313 [DOI] [PubMed] [Google Scholar]
- Wernicke, C. (1874). Der aphasische Symptomencomplex: Eine psychologische Studie auf anatomischer Basis. Breslau, Cohn & Weigert. [Google Scholar]
- Wilson, S. M. , Galantucci, S. , Tartaglia, M. C. , Rising, K. , Patterson, D. K. , Henry, M. L. , Ogar, J. M. , DeLeon, J. , Miller, B. L. , & Gorno‐Tempini, M. L. (2011). Syntactic processing depends on dorsal language tracts. Neuron, 72(2), 397–403. 10.1016/j.neuron.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagmurlu, K. , Middlebrooks, E. H. , Tanriover, N. , & Rhoton, A. L. (2016). Fiber tracts of the dorsal language stream in the human brain. Journal of Neurosurgery, 124(5), 1396–1405. 10.3171/2015.5.JNS15455 [DOI] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Ben‐Shachar, M. , & Wandell, B. A. (2012). Development of white matter and reading skills. Proceedings of the National Academy of Sciences, 109(44), E3045–E3053. 10.1073/pnas.1206792109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Myall, N. J. , Wandell, B. A. , & Feldman, H. M. (2012). Tract profiles of white matter properties: Automating fiber‐tract quantification. PLoS One, 7(11), e49790. 10.1371/journal.pone.0049790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Rykhlevskaia, E. , Sherbondy, A. J. , Deutsch, G. K. , Wandell, B. A. , & Ben‐Shachar, M. (2011). Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience, 23(11), 3304–3317. 10.1162/jocn_a_00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova, Y. N. , Duffau, H. , & Herbet, G. (2017). Neural pathways subserving face‐based mentalizing. Brain Structure and Function, 222(7), 3087–3105. 10.1007/s00429-017-1388-0 [DOI] [PubMed] [Google Scholar]
- Zarino, B. , Di Cristofori, A. , Fornara, G. A. , Bertani, G. A. , Locatelli, M. , Caroli, M. , Rampini, P. , Cogiamanian, F. , Crepaldi, D. , & Carrabba, G. (2020). Long‐term follow‐up of neuropsychological functions in patients with high grade gliomas: Can cognitive status predict patient's outcome after surgery? Acta Neurochirurgica, 162(4), 803–812. 10.1007/s00701-020-04230-y [DOI] [PubMed] [Google Scholar]
- Zemmoura, I. , Serres, B. , Andersson, F. , Barantin, L. , Tauber, C. , Filipiak, I. , Cottier, J.‐P. , Venturini, G. , & Destrieux, C. (2014). FIBRASCAN: A novel method for 3D white matter tract reconstruction in MR space from cadaveric dissection. Neuroimage, 103, 106–118. 10.1016/j.neuroimage.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Zigiotto, L. , Annicchiarico, L. , Corsini, F. , Vitali, L. , Falchi, R. , Dalpiaz, C. , Rozzanigo, U. , Barbareschi, M. , Avesani, P. , Papagno, C. , Duffau, H. , Chioffi, F. , & Sarubbo, S. (2020). Effects of supra‐total resection in neurocognitive and oncological outcome of high‐grade gliomas comparing asleep and awake surgery. Journal of Neuro‐Oncology, 148, 97–108. [DOI] [PubMed] [Google Scholar]
- Zigiotto, L. , Vavassori, L. , Annicchiarico, L. , Corsini, F. , Avesani, P. , Rozzanigo, U. , Sarubbo, S. , & Papagno, C. (2022). Segregated circuits for phonemic and semantic fluency: A novel patient‐tailored disconnection study. NeuroImage: Clinical, 36, 103149. 10.1016/j.nicl.2022.103149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


