Abstract
Mesenchymal stem cell secretome or conditioned medium (MSC-CM) is a combination of biomolecules and growth factors in cell culture growth medium, secreted by mesenchymal stem cells (MSCs), and the starting point of several derived products. MSC-CM and its derivatives could be applied after injuries and could mediate most of the beneficial regenerative effects of MSCs without the possible side effects of using MSCs themselves. However, before the clinical application of these promising biopharmaceuticals, several issues such as manufacturing protocols and quality control must be addressed. This review aims to underline the influence of the procedure for conditioned medium production on the quality of the secretome and its derivatives and highlights the questions considering cell sources and donors, cell expansion, cell passage number and confluency, conditioning period, cell culture medium, microenvironment cues, and secretome-derived product purification. A high degree of variability in MSC secretomes is revealed based on these parameters, confirming the need to standardize and optimize protocols. Understanding how bioprocessing and manufacturing conditions interact to determine the quantity, quality, and profile of MSC-CM is essential to the development of good manufacturing practice (GMP)-compliant procedures suitable for replacing mesenchymal stem cells in regenerative medicine.
Keywords: mesenchymal stem cell, secretome, manufacturing, tissue regeneration
1. Introduction
The regenerative effects promoted by mesenchymal stem cells (MSCs) are mainly associated with the secretion of bioactive molecules, which are endowed with paracrine activity, including soluble factors (proteins, nucleic acids, lipids) and extracellular vesicles (EVs) [1], and defined as the secretome or mesenchymal stem cell-conditioned medium (MSC-CM) [2,3]. The preparation of MSC-CM consists of leaving the cells in culture for a certain period, before using centrifugation to collect their growth medium containing all their secretions.
The number of peer-reviewed articles focusing on the use of MSC-CM has increased exponentially in the last few years [4,5]. MSC-CM is emerging as an alternative to direct MSC therapy and has a promising prospect to be produced as pharmaceuticals for regenerative medicine [4]. Indeed, the use of CM has several advantages compared to the use of stem cells. As it is devoid of cells, there is no need to match the donor and the recipient to avoid rejection problems [4], and the absence of replicating (allogeneic) cells in secretome fractions significantly improves patient safety profile.
The concentrations of factors contained in MSC-CM are relatively low. Thus, the use of MSC-CM does not induce any severe histological inflammatory responses as could be observed with the clinical use of recombinant factors [6]. The low metabolic activity allows more efficient quality controls and quality assurance. Moreover, CM can be manufactured, freeze-dried, packaged, and transported more easily than cells. The simplicity of storage provides the basis for cost-efficient shipping of this potentially therapeutic substance [7]. Although all studies converge around the regenerative potential of MSC-CM and its derivatives as the therapeutically active components of MSCs, the literature reveals high variability in terms of MSC sources and manufacturing processes. This highlights the challenges to the clinical translation of MSC-CM and its derivatives and underlines the importance of method and protocol standardization for MSC secretome-derived products of GMP grade [8,9,10,11,12,13,14]. Process optimization and standardization are needed to avoid changes in protein secretion profiles, properly compare studies, and ensure effective quality control [15]. Furthermore, understanding how cell sources, donors, and culture conditions interact to determine the quantity, quality, and types of biomolecules secreted by MSCs in conditioned medium and its derivatives is essential to the development of bioprocesses aimed at scaling up the production of secretome-derived products [11].
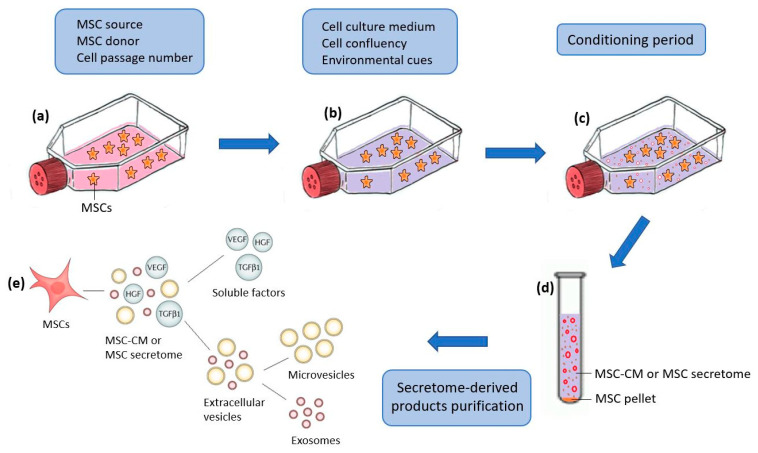
Secretome characterization is required to confirm the reproducibility of the manufacturing method and because of regulatory requirements concerning quality, safety, and efficacy. The tools mostly used for studying the expression of the MSC secretome in vitro include protein/peptide separation techniques followed by protein identification by mass spectrometry and immunological assays [16]. The characterization of the MSC-CM using antibody-based techniques such as Elisa and antibody arrays provides only a narrow window of factors secreted by MSCs. Global proteomic approaches would better clarify the potential complexity of MSC secretome, with the ultimate aim of obtaining clear and defined MSC-CM profiles for appropriate use of each MSC-CM according to its profile. However, all these techniques can play an essential role in the standardization of the production of MSC-CM and its derivatives, which are hampered by variations in MSC source, donors, cell expansion, cell passage number, conditioning period, cell culture medium, microenvironment cues, and secretome-derived product purification (Figure 1). Here, we discuss these different variations and illustrate their effect on the MSC secretome profile and protein concentrations using microarray analysis, protein electrophoresis, and the Bradford assay.
Figure 1.
Schematic representation of the procedure, followed in the literature, for obtaining the secretome from mesenchymal stem cells (MSC-CM) and the different variations that can affect its production at the different stages. (a) MSC in culture; (b) changing the culture medium of adherent cells after reaching a certain degree of confluency; (c) MSCs, incubated for some time, release their secretome in the growing medium; (d) MSC-CM obtained after collection and centrifugation of the supernatant; (e) purification of MSC-CM-derived products.
2. Cell Sources
The MSC secretome varies significantly according to the source tissue [17]. Baglio et al. demonstrated that stem cells from bone marrow (BMSCs) and adipose tissue (ASCs) secreted different tRNA species that may be relevant for clinical applications [18]. Pires et al. illustrated the difference in profiles and efficiencies of secretomes produced from BMSCs, umbilical cord mesenchymal stem cells (UMSCs), and ASCs. Although important changes were observed within the secretome of the cell populations that were analyzed, all cell populations shared the capability of secreting important regulatory molecules [19]. Du et al. demonstrated the heterogeneous proangiogenic properties of BMSCs, ASCs, UMSCs, and placental chorionic villi (PMSCs) and suggested that BMSCs and PMSCs might be preferred in clinical applications for therapeutic angiogenesis [20]. A study conducted by Kehl et al. compared the angiogenic potential of MSC secretomes from ASCs, BMSCs, and UMSCs and suggested UMSCs as the most potent MSC source for inflammation-mediated angiogenesis induction, while the potency of ASC secretomes was the lowest [21]. In contrast, Hsiao et al. suggested that ASCs may be the preferred source, after a comparison of the expression of angiogenic paracrine factors in ASCs, BMSCs, and MSCs from dermal tissues [22]. Ribeiro et al. also detected more factors in ASC-CM than in UMSC-CM [23]. Hsieh et al. suggested that UMSCs, because of their secreted factors involved in angiogenesis and neurogenesis, were better than BMSCs for promoting in vivo neuro-restoration and endothelium repair [24].
Taken together, all these studies indicate that MSC secretomes differ between cell sources. Thus, it is important, before the clinical translation of MSC secretome-based products, to determine which cellular sources provide the most potential for each application associated with tissue regeneration.
3. Donors
Mesenchymal stem cells are used as a starting material for CM manufacturing, and their properties crucially influence the composition of CM. Therefore it is necessary to standardize MSC recovery, and, also, to take into account the variability of MSC donor characteristics during manufacturing [14].
The effect of donor variability on the MSC-CM profile is currently not well understood. The composition of MSC-CM appears to be influenced by donor variability in some studies [25], as well as their effect in vitro, including opposite effects sometimes [17]. However, other studies suggested that the trophic nature of MSCs and their cytokine profiles do not depend on donor individuality [26]. They showed a similar set of proteins expressed in the MSC secretomes of two different donors, while donor-dependent variations were just reflected by the different expression levels of each protein [27].
Donor-related factors may be responsible for the impact of donor variability on MSC function and corresponding secretome. These characteristics are age, gender, metabolic state, and disease. Whilst very few studies have reported gender dimorphism of MSC effects [28], functions, and secretions [29], the age of donors has been shown to have an impact on the properties and functions of MSCs [30,31], as well as their secretome profiles [32] and potentials [33].
Further understanding of the impact of age and other donor-related factors will be crucial to the development and application of secretome-derived products [15]. To date, age’s effect on MSC potential and secretomes has not been demonstrated [34]; MSCs’ functional properties and factor secretion status were not essentially determined by age, despite their dissimilarity between different human MSC donors and preparations [35].
Another explanation for this possible variation in MSC secretomes among donors is MSC populations. The fraction of ‘‘stem-like’’ cells in a population of MSCs appears to be quite heterogeneous and can vary in proportion depending on the donor (interpopulation heterogeneity). Variability in the secretion of several proteins from cultured MSCs of individual subjects suggests that these cells exist as a heterogeneous population containing functionally distinct subtypes, which differ in numbers between donors [36]. Variation can be found even when the same donor is utilized: a significant difference exists between secretomes of size-sorted MSC subpopulations from the same donor (intrapopulation heterogeneity) [27]. A significantly higher trophic factor-producing capacity is attributed to the large MSC subpopulations [27,37,38]. The majority of factors are pro-osteogenic, pro-senescence, and anti-chondrogenic. Some factors associated with pro-chondrogenic and anti-osteogenic functions are found at a higher level in the small and medium-size subpopulations, and a more significant impact of the size-dependent MSC secretome could be expected in long-term cultures [27]. Current MSC-based clinical trials rarely select for competent subpopulations after culture expansion, which might be an important cause for their inconsistent therapeutic outcomes. Identification and sorting of subpopulations from culture-expanded MSCs may lead to substantial improvements in the therapeutic outcomes, by selecting subpopulations with the most suitable secretome profile for each specific therapeutic application [27].
4. Cell Passage Number
Understanding the differences in properties of MSCs at early versus late passage will help refine MSC treatment strategies. Early passage cell populations increase the likelihood of heterogeneity whilst late passage cells retain characteristic markers for MSC phenotype in a selectively more homogeneous population [39]. Moreover, the immunomodulatory properties of MSCs in a long-term culture have been reported [40]. Nevertheless, prolonged in vitro culture of MSCs leads to a changed MSC phenotype and multipotency [30,41,42,43], attenuating their stemness and contributing to reduced therapeutic potential [44]. Stem cells with low passage numbers can secrete larger amounts of therapeutic paracrine factors, which are required for tissue regeneration, as compared to stem cells with high passage numbers [45,46,47,48]. Serra et al. characterized the secretomes obtained from different passages (from passage 3 to passage 12) using proteomic analysis. They revealed that different passages present distinct profiles, with no significant variation in the composition of proteins associated with neuroprotection/differentiation and axonal growth [49].
5. Culture Medium
Studies showed that cell culture media could have an impact on the potential of MSCs for adhesion and growth and can be positively selective for specific MSC subpopulations [50,51]. Sagaradze et al. observed that the concentrations of factors are different between MSC-CMs where two different growth media were used [13]. In contrast, Czapla et al. found that MSCs cultured in three different media exhibited similar secretion profiles [52].
Furthermore, some researchers use fetal bovine serum (FBS) in the culture medium for MSC-CM production. Contamination risk from animal proteins is normally present in FBS [53], and thus, immunologic reactions are expected when MSC-CM is used in vivo. Concerns already exist with FBS use such as the variability of FBS from batch to batch [53] due to geographical and seasonal variations [54] and the ill-defined mixture of its components that contains thousands of constituents and can contain contaminants, such as endotoxins, mycoplasma, viruses, or prion proteins [54]. A short culture period may leave some growth factors derived from serum that has not been consumed by the cells [4]. These factors will be added to the growth factors secreted by the cells themselves. Different percentages of FBS result in different amounts of growth factors present in a culture; thus, some MSC manufacturers emphasize the importance of qualifying FBS lots to facilitate product comparability between manufacturing runs [8]. The most common alternative for FBS is human serum and its derivatives such as human platelet lysate [8,55]. However, the effect of the human platelet lysate on the immunomodulatory capacity of MSCs and its reproducibility (donor-to-donor variability) are still contradictory [53]. All these issues make it difficult for MSC secretome-based products prepared in the presence of serum to validate GMP-compliant processes. The most acceptable alternative is serum-free or preferably chemically defined medium, the latter not only serum-free but also lacking any hydrolysates or supplements of unknown composition [55]. Interestingly, it has been shown that serum-deprived cultures of MSCs secreted a higher level of angiogenic factors [56,57]. Also, MSC-CM collected under serum conditions was toxic to cells when used undiluted (100% concentration) and, when diluted, did not have the positive effects of MSC-CM collected under serum deprivation conditions [58].
6. Cell Confluency and Conditioning Period
There is significant controversy in the literature regarding the optimal conditions and time points for MSC-CM collection [58]. Cell confluency as well as the period of medium conditioning with MSCs could affect the concentration of secreted factors. The secretome could be richer in factors when there are more cells, or when cells are kept longer in the culture so they become more confluent. However, the expression levels of stemness genes reduce with high cell density [59], with an impact on their secretome. Thus, MSC confluency and conditioning period should be determined carefully before starting an experiment. Mizukami et al. showed that the majority of interesting proteins from the MSC secretome were enriched through time in culture [60]. Sagaradze et al. analyzed the total concentrations of four growth factors in MSC-CM on certain days (until 14 days). Peak factor concentrations were mostly reached on days 7 or 10 for all of them [13]. Most commonly, MSC-CM is collected from 70–90% of MSC confluency during the first three days of culture [4].
7. Microenvironment Cues
The use of microenvironment cues to manipulate MSC potency and the MSC secretome in cultures has been extensively explored [61]. They are used to change the MSC secretome profile or to increase growth factor secretion, to enhance the therapeutic capacity/potential of MSC-CM. A variety of different factors were used, in the literature, to change the microenvironment or induce an in vitro preconditioning of MSCs, including the following: 3D culture [62,63,64], hypoxia [65], biochemical stimuli (including cytokines, growth factors, hormones, and pharmacological agents), mechanical stimuli [15,66], electrical stimuli [67], and photobiomodulation [68,69].
Using 3D culture is a more complete modeling of the MSC natural microenvironment, allowing MSC proliferation and differentiation potential to be retained for a longer time [14]. The microenvironment established within the spheroids acts in an autocrine fashion favoring an enhanced secretion of paracrine factors [70]. The composition of the resulting CM is significantly different from that obtained with 2D microenvironments [64,71]. A 3D cell culture is a typical example of microgravity application [72] that significantly increases the anti-apoptotic and anti-inflammatory effects of MSC-CM [73]. Changing the microenvironment in vitro can be produced also via MSC preconditioning. There are different strategies for MSC preconditioning to stimulate growth factor secretion into the culture CM. The application of stress factors, such as serum deprivation or hypoxic conditions, has been widely used for this purpose, to stimulate the stress environment found in damage conditions in the in vivo situation. MSC stimulation with hypoxia triggers an increase in growth factor secretion with enhanced paracrine activity [74,75]. Other factors, treatment with pharmacological molecules or cytokines, and induction of thermal shock have been also applied [58]. For example, we showed in our previous publication that an MSC culture with a B-27 supplement drastically changes the secretome’s profile, further stimulating neurite outgrowth [48]. Also, Park et al. showed that treatment with bFGF and selenium stimulates the production of paracrine factors from MSCs, promoting cell proliferation and migration and the wound-healing process [76]. MSC differentiation affects the secretome profile of MSCs [77]. MSC exposure to mechanical stimuli is another strategy to influence MSC behavior and MSCs’ secretome profile. MSC secretomes respond to the mechanical properties of their substrate, including stiffness [78]. Surface topographies can change the shape of stromal cells and quantitatively influence their cytokine secretion profile. However, qualitative stromal cell secretory characteristics are preserved irrespective of microenvironmental surface factors [79]. A CO2 laser enhanced the secretion of pro-angiogenic molecules and increased the regenerative capacity of MSCs [80]. Interestingly, the cell-conditioned medium of an extrinsic microenvironment can modify the age-related properties of tissue-specific stem cells [81].
Each preconditioning regimen induces an individual expression profile with a wide variety of factors, including several growth factors and cytokines [82]. Further studies are required to evaluate the best preconditioning regimen for each specific application of MSC-CM in human regenerative medicine.
8. Secretome-Derived Product Purification
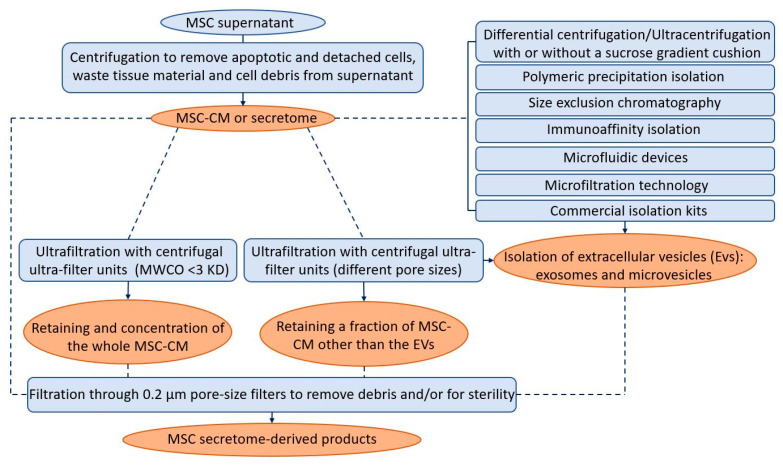
The MSC-CM or secretome is primarily prepared by centrifuging the expended medium of MSCs. The resulting product can be used directly, or by adding concentration, fractionation, and/or filtration steps. The preparation procedure of secretome products derived from an MSC culture supernatant differs between studies. It consists of one or a combination of these steps. The centrifugation is essential to remove apoptotic and detached cells, waste tissue material, and cell debris from the supernatant. The centrifugal speed and time are very different between studies. Ultrafiltration technology allows the choice of filtration modules with different molecular weight cut-offs (MWCOs), thus allowing the retention of only parts or the whole secretome [10]. Centrifugal ultra-filter units that have an MWCO of <3 KD are used to retain and concentrate the whole CM [83,84]. MSC-CMs were used concentrated in some studies [85,86] and diluted in others [58,87,88], possibly due to the achievement of the optimum balance between metabolic inhibitory by-products and paracrine stimulatory products [58,89]. Centrifugal ultra-filter units with other pore sizes are used when just a fraction of the CM needs to be retained. By fractionating CM, it is possible to correlate a particular molecular subset or CM fractions with a specific measured effect [15]. Many studies have linked CM paracrine effects to specific molecular size fractions. For example, a CM containing products >1000 kDa was described as having a cardio-protection effect in a murine model of ischemia and reperfusion injury [85]. Protein fractions with a molecular weight in the range of 10kDa-3kDa were the only fraction that could protect neurons against induced neurodegeneration, suggesting that these microproteins could be responsible for the neuroprotection of DPSC-CM [90]. Among four fractions of SHED-CM, only the fraction of <6 kDa could promote neurite outgrowth from dorsal root ganglion neurons [91]. Finally, it is important to note that a filtration with a 0.2 μm pore-size filter is usually performed to remove debris from CM and/or for sterility.
Extracellular vesicles (EVs) represent an important fraction of the cell secretome [92], containing cellular proteins, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), exosomes, and microvesicles (MVs). These EVs are heterogeneous, membranous, cell-derived vesicles with a diameter of approximately 40 to 5000 nm that are released by a variety of cells into their microenvironment [93]. Some confusion still exists in the literature regarding the distinction between exosomes and MVs. The difference between these two terms is based on the vesicle size [94]: exosomes are less than 100 nm (30–100 nm), while microvesicles range from 100 to 1000 nm, but these definitions are flexible as this is still quite a novel research field [95].
The qualitative/quantitative composition, and thus the biological activity of MSC-derived products, is strongly influenced by the isolation method chosen (complete CM, soluble factors, exosomes, or MVs). The isolation of EVs is a challenging procedure. Several methods were introduced and utilized for the isolation and purification of EVs: differential centrifugation/ultracentrifugation with or without sucrose gradient cushion, polymeric precipitation isolation, size exclusion chromatography, immunoaffinity isolation, ultrafiltration and microfiltration technologies, microfluidic devices, and exosome isolation reagents [93,96]. The most widely applied method for concentrating and purifying EVs is isolation by differential centrifugation/ultracentrifugation. It consists of several centrifugations that sequentially increase in speed and time and thus sequentially pellet smaller particles [97]. Ultracentrifugation remains the most commonly used isolation method (81%) [98], even if some authors reported some limitations [99,100,101]. Differential centrifugation/ultracentrifugation only sees recovery rates of up to 25% [102]. There is also evidence that the high forces involved (typically 100,000× g) can affect the bioactivity of the EVs themselves [103]. Indeed, each of these isolation methods has advantages and limitations. None of them can offer a high recovery together with high specificity [9,104]; wherefore, 59% of respondents use a combination of methods [98] to achieve a higher quality of EVs. They can be filter-sterilized at the end of the isolation process [12]. Data available in the literature tend to state that secretome (or EV) sterilization is possible using filtration, without apparent loss of efficacy [93]. Figure 2 summarizes the different protocols and techniques followed in the literature to obtain the secretome-derived products used for their regenerative potentials.
Figure 2.
The different techniques (in blue) and protocols (dotted arrows) followed in the literature to obtain the secretome-derived products (in orange) used for their regenerative potential.
EVs have been studied widely in the last few years and are described as key regulators of stem cell paracrine activity [104]. Subsequently, many studies have been conducted to identify whether MSC-CM functions are mainly associated or not with the enriched fractions of EVs. Kumar et al. demonstrated that MSC-CM mediates cardioprotection during myocardial ischemia/reperfusion injury through the exosomes [105]. MVs contributed along with soluble factors to the regenerative effect of MSCs in a study conducted by Ahmed et al. [106]. In contrast, Walter et al. showed that the major paracrine angiogenic effect of MSCs is associated with their soluble factors and not with their EVs [107]. Further studies should be conducted to confirm the benefits of MSC secretome fractionation and determine which fraction of MSC-CM is most effective for each regenerative application, especially since the isolation and characterization of EVs are costly and time-consuming, which impairs their use in clinical practice [104].
The secretome is composed of various elements secreted into the extracellular space. These elements include proteins and extracellular vesicles. These extracellular vesicles can contain apoptotic bodies, which appear during the programmed cell death process [108,109]. The various steps of centrifugation are required to remove these apoptotic bodies.
9. Other Manufacturing Conditions
In addition to all the above-mentioned points, the development of a reproducible, scalable, and well-controlled platform is an important key factor in the standardization of the production of MSC-CM and its derivatives. Adherent cell expansion has traditionally been performed on planar surfaces such as well plates and tissue culture flasks for simplicity and easy handling when large numbers of cells are not required. For larger-scale expansion, the transition from planar-based culture to microcarrier-based systems not only allows for higher-density culture (thus reducing the cost of goods) but also for more stringent culture control and monitoring [110]. This also avoids the high risk of contamination due to manual interventions of the manual process. Bioreactors improve the predictability of the composition and function of secretome-derived products. Suspension bioreactors offer a higher level of homogeneity and process control, serving to reduce both batch-to-batch and within-batch variability of cell cultures. Furthermore, bioreactors are used sometimes to provide a specific physiological in vitro environment [111]. Stirred suspension bioreactors are highly scalable, and several variables such as dissolved oxygen, pH, and temperature can be computer-controlled to provide a high level of process control, resulting in more uniform product batches [15].
In addition to manufacturing conditions, storage and transport of MSC secretome-derived products should be considered as they play an important role in maintaining characteristics and functions. It is important to consider the effects of freeze–thaw, stability at various temperatures, and the effects of freeze-drying components [15]. Cells cultured with CM stored at 4 °C before use maintained their viability. However, cell death increases with storage time, and the conservation of CM for more than 2 months decreases the properties that keep the cells viable. This may be due to the degeneration of hormones and growth factors in CM, or a concentration of these factors that is too low for long-term preservation of the target cells [112]. CM can reportedly be stored at −20 °C for several months without experiencing functional deterioration [113]. A study on urinary exosomes showed that freezing at −20 °C resulted in major losses, and freezing at −80 °C enabled almost complete recovery after up to 7 months of storage. Further, protease inhibitors are essential for proper preservation, and extensive vortexing (i.e., 90 s) enabled maximum recovery of thawed exosome samples [114]. Lyophilization is used to guarantee the long-term stability and easy storage and reconstitution of products. This process generates a variety of freezing and drying stresses that can alter the stability of the biological samples [10].
10. Future Trends and Recommendations
Cell therapy presents several limitations, such as safety issues, handling difficulties, and elevated cost. Therefore, MSC-CM is a therapeutic alternative, as it brings similar effects to the cells of origin, and it allows for avoiding the issues of cell grafting [115]. Taking into account the possibility to standardize conditioned media, MSC-CM clearly appears as a new therapeutic strategy. The use of such cell-free therapy in regenerative medicine has several advantages over stem-cell-based applications: safety considerations, control of protein content, storage without toxic cryopreservative agents, economical and practical aspects, and the possibility to orientate CM potential by preconditioning [108]. This cell-free approach will guide and promote revascularization and endogenous cell homing [109], with the possibility to specifically orientate tissue regeneration [116]. The use of MSC-CM is locally applied to reproduce stem cells’ intercellular signaling, in order to reproduce the biological effects of the original cells [117]. The topical application of a secretome requires a carrier, such as degradable particles, or can be accomplished using a hydrogel. Hydrogels are colloidal structures that present similitude to extracellular matrices and are fully biocompatible. They appear to be excellent carriers for MSC-CM applications in regenerative medicine [118].
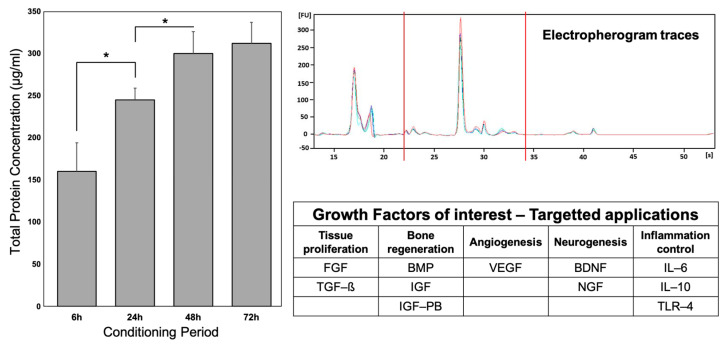
For further clinical applications of MSC-CM, strict controls will be mandatory and enhance the necessity of standardization to produce safe and efficient MSC-CM. Several potential points should be considered: (1) control of total protein concentration and protein profiles and (2) control of key protein presence (according to the targeted therapy). A summary of these controls and potential key proteins is presented in Figure 3.
Figure 3.
Control of MSC-CM profile and key protein identification. Left graph represents total protein concentrations with BCA Protein Assay kit, according to conditioning period. * indicates significant difference (p < 0.05) Right graph represents electropherogram traces of MSC-CM, using Agilent Bioanalyzer. The table summarizes key proteins to consider, according to the therapeutic target. Adapted from [48,116].
-
(1).
Total protein concentrations and protein profiles can be assessed and controlled by protein assay kits, such as the Pierce bicinchoninic acid BCA Protein Assay Kit (Thermo Fisher) and the Bradford Protein Assay (Thermo Fisher) [48]. Protein profiles can be controlled by electrophoresis with a bioanalyzer (i.e., Agilent 2100 Bioanalyzer) [116].
-
(2).
Specific proteins have to be considered to determine the potential efficiency of MSC-CM for specific applications. For tissue proliferation, FGF and TGF-ß have been described in MSC-CM and could be proposed as interesting candidates [119,120,121,122]. For bone regeneration, the BMP family, IGF, and IGF-BP could be considered [48,119,122]. For angiogenesis, VEGF is an obvious key protein, as it has been described several times in MSC-CM [119,121,122]. For neurogenesis, NGF and BDNF can naturally be used as controls [48,120]. Finally, for clinical applications targeting inflammatory control, proteins involved in the inflammation process could serve as key proteins, such as IL-6, IL-10, and TLR-4 [120,122].
11. Conclusions
The effects of MSC-CM are multiple, and even the minimal variability of its composition can strongly affect its activity [14]. Moreover, the process of producing human MSC secretome-derived products is a major consideration in developing standardized criteria to define and qualify the preparation of these products for clinical applications [123]. In this review, we discussed the essential origins of variability in MSC secretome-derived products. Based on literature data and experimental results, to standardize the manufacturing of these products, we recommend the preparation of secretomes from MSCs at a low cell passage number, obtained from relatively young and healthy donors. We also recommend DPSC-CM preparation under serum-free conditions, and collection during the first days of conditioning. Further work should be performed to determine which cell sources, MSC populations, environmental cues, and MSC-CM fractions provide the most potent MSC secretome-based product for each specific application in the field of human regenerative medicine.
Acknowledgments
This research was possible thanks to the research award granted to Batoul Chouaib by Lebanese University.
Author Contributions
Conceptualization, B.C. and M.H.-S.; methodology, B.C.; formal analysis, B.C.; investigation, B.C. and M.H.-S.; resources, F.C. (Franck Chaubron) and F.C. (Frederic Cuisinier); writing—original draft preparation, B.C.; writing—review and editing, P.-Y.C.-D.; supervision, P.-Y.C.-D.; project administration, F.C. (Franck Chaubron) and F.C. (Frederic Cuisinier); funding acquisition, P.-Y.C.-D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The raw/processed data required to reproduce these findings cannot be shared at this time due to technical limitations.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Fondation Des Gueules Cassees, grant number “22-2017”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kichenbrand C., Velot E., Menu P., Moby V. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng. Part B Rev. 2019;25:78–88. doi: 10.1089/ten.teb.2018.0168. [DOI] [PubMed] [Google Scholar]
- 2.Baraniak P.R., McDevitt T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerrose T., Olson S., Pontow S., Kalomoiris S., Jung Y., Annett G., Bauer G., Nolta J.A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 2010;62:1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawitan J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014;2014:965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunawardena T.N.A., Rahman M.T., Abdullah B.J.J., Abu Kasim N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019;13:569–586. doi: 10.1002/term.2806. [DOI] [PubMed] [Google Scholar]
- 6.Katagiri W., Osugi M., Kawai T., Ueda M. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int. J. Oral Maxillofac. Implant. 2013;28:1009–1016. doi: 10.11607/jomi.3036. [DOI] [PubMed] [Google Scholar]
- 7.Presen D.M., Traweger A., Gimona M., Redl H. Mesenchymal stromal cell-based bone regeneration therapies: From cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front. Bioeng. Biotechnol. 2019;7:352. doi: 10.3389/fbioe.2019.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendicino M., Bailey A.M., Wonnacott K., Puri R.K., Bauer S.R. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Bari E., Ferrarotti I., Torre M.L., Corsico A.G., Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release. 2019;309:11–24. doi: 10.1016/j.jconrel.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Bari E., Perteghella S., Di Silvestre D., Sorlini M., Catenacci L., Sorrenti M., Marrubini G., Rossi R., Tripodo G., Mauri P., et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells. 2018;7:190. doi: 10.3390/cells7110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crivelli B., Chlapanidas T., Perteghella S., Lucarelli E., Pascucci L., Brini A.T., Ferrero I., Marazzi M., Pessina A., Torre M.L., et al. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J. Control. Release. 2017;262:104–117. doi: 10.1016/j.jconrel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Gimona M., Pachler K., Laner-Plamberger S., Schallmoser K., Rohde E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017;18:1190. doi: 10.3390/ijms18061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagaradze G., Grigorieva O., Nimiritsky P., Basalova N., Kalinina N., Akopyan Z., Efimenko A. Conditioned medium from human mesenchymal stromal cells: Towards the clinical translation. Int. J. Mol. Sci. 2019;20:1656. doi: 10.3390/ijms20071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagaradze G.D., Nimiritsky P.P., Akopyan Z.A., Makarevich P.I., Efimenko A.Y. Cell-Free Therapeutics” from Components Secreted by Mesenchymal Stromal Cells as a Novel Class of Biopharmaceuticals. Biopharmaceuticals. 2018;47:47–61. [Google Scholar]
- 15.Phelps J., Sanati-Nezhad A., Ungrin M., Duncan N.A., Sen A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018;2018:9415367. doi: 10.1155/2018/9415367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Assoni A., Coatti G., Valadares M.C., Beccari M., Gomes J., Pelatti M., Mitne-Neto M., Carvalho V.M., Zatz M. Different donors mesenchymal stromal cells secretomes reveal heterogeneous profile of relevance for therapeutic use. Stem Cells Dev. 2017;26:206–214. doi: 10.1089/scd.2016.0218. [DOI] [PubMed] [Google Scholar]
- 18.Baglio S.R., Rooijers K., Koppers-Lalic D., Verweij F.J., Perez Lanzon M., Zini N., Naaijkens B., Perut F., Niessen H.W., Baldini N., et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pires A.O., Mendes-Pinheiro B., Teixeira F.G., Anjo S.I., Ribeiro-Samy S., Gomes E.D., Serra S.C., Silva N.A., Manadas B., Sousa N. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: A proteomic analysis. Stem Cells Dev. 2016;25:1073–1083. doi: 10.1089/scd.2016.0048. [DOI] [PubMed] [Google Scholar]
- 20.Du W.J., Chi Y., Yang Z.X., Li Z.J., Cui J.J., Song B.Q., Li X., Yang S.G., Han Z.B., Han Z.C. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 2016;7:163. doi: 10.1186/s13287-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehl D., Generali M., Mallone A., Heller M., Uldry A.C., Cheng P., Gantenbein B., Hoerstrup S.P., Weber B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019;4:8. doi: 10.1038/s41536-019-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiao S.T., Asgari A., Lokmic Z., Sinclair R., Dusting G.J., Lim S.Y., Dilley R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro C.A., Fraga J.S., Graos M., Neves N.M., Reis R.L., Gimble J.M., Sousa N., Salgado A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012;3:18. doi: 10.1186/scrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh J.Y., Wang H.W., Chang S.J., Liao K.H., Lee I.H., Lin W.S., Wu C.H., Lin W.Y., Cheng S.M. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE. 2013;8:e72604. doi: 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter M.N., Kohli N., Khan N., Major T., Fuller H., Wright K.T., Kuiper J.-H., Johnson W.E. Human mesenchymal stem cells stimulate EaHy926 endothelial cell migration: Combined proteomic and in vitro analysis of the influence of donor-donor variability. J. Stem Cells Regen. Med. 2015;11:18. doi: 10.46582/jsrm.1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park C.W., Kim K.-S., Bae S., Son H.K., Myung P.-K., Hong H.J., Kim H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells. 2009;2:59. doi: 10.15283/ijsc.2009.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin L., Yang Z., Wu Y., Denslin V., Yu C.C., Tee C.A., Lim C.T., Han J., Lee E.H. Label-free separation of mesenchymal stem cell subpopulations with distinct differentiation potencies and paracrine effects. Biomaterials. 2020;240:119881. doi: 10.1016/j.biomaterials.2020.119881. [DOI] [PubMed] [Google Scholar]
- 28.Pasanen I., Pietilä M., Lehtonen S., Lehtilahti E., Hakkarainen T., Sequeiros R.B., Lehenkari P., Kuvaja P. Mesenchymal stromal cells from female donors enhance breast cancer cell proliferation in vitro. Oncology. 2015;88:214–225. doi: 10.1159/000368556. [DOI] [PubMed] [Google Scholar]
- 29.Crisostomo P.R., Wang M., Herring C.M., Markel T.A., Meldrum K.K., Lillemoe K.D., Meldrum D.R. Gender differences in injury induced mesenchymal stem cell apoptosis, expression of VEGF, TNF, and IL-6 and abrogation via TNFR1 ablation. J. Mol. Cell. Cardiol. 2007;42:142. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretlow J.D., Jin Y.-Q., Liu W., Zhang W.J., Hong T.-H., Zhou G., Baggett L.S., Mikos A.G., Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., An Y., Gao L.N., Zhang Y.J., Jin Y., Chen F.M. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials. 2012;33:6974–6986. doi: 10.1016/j.biomaterials.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Efimenko A., Dzhoyashvili N., Kalinina N., Kochegura T., Akchurin R., Tkachuk V., Parfyonova Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 2014;3:32–41. doi: 10.5966/sctm.2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horibe H., Murakami M., Iohara K., Hayashi Y., Takeuchi N., Takei Y., Kurita K., Nakashima M. Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS ONE. 2014;9:e98553. doi: 10.1371/journal.pone.0098553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dufrane D. Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant. 2017;26:1496–1504. doi: 10.1177/0963689717721203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalinina N., Kharlampieva D., Loguinova M., Butenko I., Pobeguts O., Efimenko A., Ageeva L., Sharonov G., Ischenko D., Alekseev D. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015;6:221. doi: 10.1186/s13287-015-0209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Wang Z., Lu W.W., Zhen W., Yang D., Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017;9:e435. doi: 10.1038/am.2017.171. [DOI] [Google Scholar]
- 38.Poon Z., Lee W.C., Guan G., Nyan L.M., Lim C.T., Han J., Van Vliet K.J. Bone marrow regeneration promoted by biophysically sorted osteoprogenitors from mesenchymal stromal cells. Stem Cells Transl. Med. 2015;4:56–65. doi: 10.5966/sctm.2014-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tropel P., Noël D., Platet N., Legrand P., Benabid A.-L., Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp. Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 40.Chapman V., Markides H., Sagar D.R., Xu L., Burston J.J., Mapp P., Kay A., Morris R.H., Kehoe O., El Haj A.J. Therapeutic benefit for late, but not early, passage mesenchymal stem cells on pain behaviour in an animal model of osteoarthritis. Stem Cells Int. 2017;2017:2905104. doi: 10.1155/2017/2905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igarashi A., Segoshi K., Sakai Y., Pan H., Kanawa M., Higashi Y., Sugiyama M., Nakamura K., Kurihara H., Yamaguchi S. Selection of common markers for bone marrow stromal cells from various bones using real-time RT-PCR: Effects of passage number and donor age. Tissue Eng. 2007;13:2405–2417. doi: 10.1089/ten.2006.0340. [DOI] [PubMed] [Google Scholar]
- 42.Bonab M.M., Alimoghaddam K., Talebian F., Ghaffari S.H., Ghavamzadeh A., Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Requicha J., Viegas C.A., Albuquerque C., Azevedo J., Reis R., Gomes M.E. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. Rep. 2012;8:1211–1222. doi: 10.1007/s12015-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 44.Jiang T., Xu G., Wang Q., Yang L., Zheng L., Zhao J., Zhang X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017;8:e2851. doi: 10.1038/cddis.2017.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Im G.-B., Kim Y.H., Kim Y.-J., Kim S.-W., Jung E., Jeong G.-J., Wang K., Kim J., Kim D.-I., Kim T.-H. Enhancing the Wound Healing Effect of Conditioned Medium Collected from Mesenchymal Stem Cells with High Passage Number Using Bioreducible Nanoparticles. Int. J. Mol. Sci. 2019;20:4835. doi: 10.3390/ijms20194835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crisostomo P.R., Wang M., Wairiuko G.M., Morrell E.D., Terrell A.M., Seshadri P., Nam U.H., Meldrum D.R. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 47.Sears V., Ghosh G. Harnessing mesenchymal stem cell secretome: Effect of extracellular matrices on proangiogenic signaling. Biotechnol. Bioeng. 2020;117:1159–1171. doi: 10.1002/bit.27272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chouaib B., Collart-Dutilleul P.Y., Blanc-Sylvestre N., Younes R., Gergely C., Raoul C., Scamps F., Cuisinier F., Romieu O. Identification of secreted factors in dental pulp cell-conditioned medium optimized for neuronal growth. Neurochem. Int. 2021;144:104961. doi: 10.1016/j.neuint.2021.104961. [DOI] [PubMed] [Google Scholar]
- 49.Serra S.C., Costa J.C., Assuncao-Silva R.C., Teixeira F.G., Silva N.A., Anjo S.I., Manadas B., Gimble J.M., Behie L.A., Salgado A.J. Influence of passage number on the impact of the secretome of adipose tissue stem cells on neural survival, neurodifferentiation and axonal growth. Biochimie. 2018;155:119–128. doi: 10.1016/j.biochi.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Riis S., Nielsen F.M., Pennisi C.P., Zachar V., Fink T. Comparative analysis of media and supplements on initiation and expansion of adipose-derived stem cells. Stem Cells Transl. Med. 2016;5:314–324. doi: 10.5966/sctm.2015-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagmann S., Moradi B., Frank S., Dreher T., Kämmerer P.W., Richter W., Gotterbarm T. Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC Musculoskelet. Disord. 2013;14:223. doi: 10.1186/1471-2474-14-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czapla J., Matuszczak S., Kulik K., Wisniewska E., Pilny E., Jarosz-Biej M., Smolarczyk R., Sirek T., Zembala M.O., Zembala M., et al. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res. Ther. 2019;10:235. doi: 10.1186/s13287-019-1331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panchalingam K.M., Jung S., Rosenberg L., Behie L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015;6:225. doi: 10.1186/s13287-015-0228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang C.-Y., Wu C.-C., Fang C.-L., Chen W.-Y., Chen C.-L. Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS ONE. 2017;12:e0178960. doi: 10.1371/journal.pone.0178960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barekzai J., Petry F., Zitzmann J., Czermak P., Salzig D. New Advances on Fermentation Processes. IntechOpen; London, UK: 2019. Bioprocess Development for Human Mesenchymal Stem Cell Therapy Products. [Google Scholar]
- 56.Qu C., Brohlin M., Kingham P.J., Kelk P. Evaluation of growth, stemness, and angiogenic properties of dental pulp stem cells cultured in cGMP xeno-/serum-free medium. Cell Tissue Res. 2020;380:93–105. doi: 10.1007/s00441-019-03160-1. [DOI] [PubMed] [Google Scholar]
- 57.Oskowitz A., McFerrin H., Gutschow M., Carter M.L., Pochampally R. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res. 2011;6:215–225. doi: 10.1016/j.scr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paschalidis T., Bakopoulou A., Papa P., Leyhausen G., Geurtsen W., Koidis P. Dental pulp stem cells’ secretome enhances pulp repair processes and compensates TEGDMA-induced cytotoxicity. Dent. Mater. 2014;30:e405–e418. doi: 10.1016/j.dental.2014.08.377. [DOI] [PubMed] [Google Scholar]
- 59.Kim D.S., Lee M.W., Lee T.H., Sung K.W., Koo H.H., Yoo K.H. Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed. Rep. 2017;6:300–306. doi: 10.3892/br.2017.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizukami A., Thomé C.H., Ferreira G.A., Lanfredi G.P., Covas D.T., Pitteri S.J., Swiech K., Faça V.M. Proteomic identification and time-course monitoring of secreted proteins during expansion of human mesenchymal stem/stromal in stirred-tank bioreactor. Front. Bioeng. Biotechnol. 2019;7:154. doi: 10.3389/fbioe.2019.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kusuma G.D., Carthew J., Lim R., Frith J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26:617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 62.Redondo-Castro E., Cunningham C.J., Miller J., Brown H., Allan S.M., Pinteaux E. Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res. Ther. 2018;9:11. doi: 10.1186/s13287-017-0753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa M.H.G., McDevitt T.C., Cabral J.M.S., da Silva C.L., Ferreira F.C. Tridimensional configurations of human mesenchymal stem/stromal cells to enhance cell paracrine potential towards wound healing processes. J. Biotechnol. 2017;262:28–39. doi: 10.1016/j.jbiotec.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Carter K., Lee H.J., Na K.S., Fernandes-Cunha G.M., Blanco I.J., Djalilian A., Myung D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019;99:247–257. doi: 10.1016/j.actbio.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribeiro T.O., Silveira B.M., Meira M.C., Carreira A.C.O., Sogayar M.C., Meyer R., Fortuna V. Investigating the potential of the secretome of mesenchymal stem cells derived from sickle cell disease patients. PLoS ONE. 2019;14:e0222093. doi: 10.1371/journal.pone.0222093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira J.R., Teixeira G.Q., Santos S.G., Barbosa M.A., Almeida-Porada G., Gonçalves R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beugels J., Molin D.G.M., Ophelders D., Rutten T., Kessels L., Kloosterboer N., Grzymala A.A.P., Kramer B.W.W., van der Hulst R., Wolfs T. Electrical stimulation promotes the angiogenic potential of adipose-derived stem cells. Sci. Rep. 2019;9:12076. doi: 10.1038/s41598-019-48369-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim K., Lee J., Jang H., Park S., Na J., Myung J.K., Kim M.J., Jang W.S., Lee S.J., Kim H., et al. Photobiomodulation Enhances the Angiogenic Effect of Mesenchymal Stem Cells to Mitigate Radiation-Induced Enteropathy. Int. J. Mol. Sci. 2019;20:1131. doi: 10.3390/ijms20051131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park I.S., Chung P.S., Ahn J.C. Enhanced angiogenic effect of adipose-derived stromal cell spheroid with low-level light therapy in hind limb ischemia mice. Biomaterials. 2014;35:9280–9289. doi: 10.1016/j.biomaterials.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 70.Santos J.M., Camões S.P., Filipe E., Cipriano M., Barcia R.N., Filipe M., Teixeira M., Simões S., Gaspar M., Mosqueira D. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015;6:90. doi: 10.1186/s13287-015-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miceli V., Pampalone M., Vella S., Carreca A.P., Amico G., Conaldi P.G. Comparison of immunosuppressive and angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem Cells Int. 2019;2019:7486279. doi: 10.1155/2019/7486279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imura T., Nakagawa K., Kawahara Y., Yuge L. Stem cell culture in microgravity and its application in cell-based therapy. Stem Cells Dev. 2018;27:1298–1302. doi: 10.1089/scd.2017.0298. [DOI] [PubMed] [Google Scholar]
- 73.Otsuka T., Imura T., Nakagawa K., Shrestha L., Takahashi S., Kawahara Y., Sueda T., Kurisu K., Yuge L. Simulated microgravity culture enhances the neuroprotective effects of human cranial bone-derived mesenchymal stem cells in traumatic brain injury. Stem Cells Dev. 2018;27:1287–1297. doi: 10.1089/scd.2017.0299. [DOI] [PubMed] [Google Scholar]
- 74.Binch A.L., Richardson S.M., Hoyland J.A., Barry F.P. Combinatorial conditioning of adipose derived-mesenchymal stem cells enhances their neurovascular potential: Implications for intervertebral disc degeneration. JOR Spine. 2019;2:e1072. doi: 10.1002/jsp2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia X., Chiu P.W.Y., Lam P.K., Chin W.C., Ng E.K.W., Lau J.Y.W. Secretome from hypoxia-conditioned adipose-derived mesenchymal stem cells promotes the healing of gastric mucosal injury in a rodent model. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018;1864:178–188. doi: 10.1016/j.bbadis.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Park J., Lee J.H., Yoon B.S., Jun E.K., Lee G., Kim I.Y., You S. Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018;9:293. doi: 10.1186/s13287-018-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mussano F., Genova T., Petrillo S., Roato I., Ferracini R., Munaron L. Osteogenic differentiation modulates the cytokine, chemokine, and growth factor profile of ASCs and SHED. Int. J. Mol. Sci. 2018;19:1454. doi: 10.3390/ijms19051454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nasser M., Wu Y., Danaoui Y., Ghosh G. Engineering microenvironments towards harnessing pro-angiogenic potential of mesenchymal stem cells. Mater. Sci. Eng. C. 2019;102:75–84. doi: 10.1016/j.msec.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leuning D.G., Beijer N.R.M., du Fosse N.A., Vermeulen S., Lievers E., van Kooten C., Rabelink T.J., Boer J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018;8:7716. doi: 10.1038/s41598-018-25700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Constantin A., Dumitrescu M., Mihai Corotchi M.C., Jianu D., Simionescu M. CO2 laser increases the regenerative capacity of human adipose-derived stem cells by a mechanism involving the redox state and enhanced secretion of pro-angiogenic molecules. Lasers Med. Sci. 2017;32:117–127. doi: 10.1007/s10103-016-2093-6. [DOI] [PubMed] [Google Scholar]
- 81.Ma D., Ma Z., Zhang X., Wang W., Yang Z., Zhang M., Wu G., Lu W., Deng Z., Jin Y. Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J. Endod. 2009;35:1546–1553. doi: 10.1016/j.joen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 82.Baer P.C., Overath J.M., Urbschat A., Schubert R., Koch B., Bohn A.A., Geiger H. Effect of different preconditioning regimens on the expression profile of murine adipose-derived stromal/stem cells. Int. J. Mol. Sci. 2018;19:1719. doi: 10.3390/ijms19061719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lotfinia M., Kadivar M., Piryaei A., Pournasr B., Sardari S., Sodeifi N., Sayahpour F.A., Baharvand H. Effect of Secreted Molecules of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells on Acute Hepatic Failure Model. Stem Cells Dev. 2016;25:1898–1908. doi: 10.1089/scd.2016.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee S.C., Kim J.O., Kim S.J. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia-reperfusion injury. Surgery. 2015;157:934–943. doi: 10.1016/j.surg.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 85.Timmers L., Lim S.K., Arslan F., Armstrong J.S., Hoefer I.E., Doevendans P.A., Piek J.J., El Oakley R.M., Choo A., Lee C.N., et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Ho C.H., Lan C.W., Liao C.Y., Hung S.C., Li H.Y., Sung Y.J. Mesenchymal stem cells and their conditioned medium can enhance the repair of uterine defects in a rat model. J. Chin. Med. Assoc. 2018;81:268–276. doi: 10.1016/j.jcma.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 87.Sun H., Benardais K., Stanslowsky N., Thau-Habermann N., Hensel N., Huang D., Claus P., Dengler R., Stangel M., Petri S. Therapeutic potential of mesenchymal stromal cells and MSC conditioned medium in Amyotrophic Lateral Sclerosis (ALS)--in vitro evidence from primary motor neuron cultures, NSC-34 cells, astrocytes and microglia. PLoS ONE. 2013;8:e72926. doi: 10.1371/journal.pone.0072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isele N.B., Lee H.S., Landshamer S., Straube A., Padovan C.S., Plesnila N., Culmsee C. Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons. Neurochem. Int. 2007;50:243–250. doi: 10.1016/j.neuint.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 89.Hu L., Zhao J., Liu J., Gong N., Chen L. Effects of adipose stem cell-conditioned medium on the migration of vascular endothelial cells, fibroblasts and keratinocytes. Exp. Ther. Med. 2013;5:701–706. doi: 10.3892/etm.2013.887. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Venugopal C., Rai K.S., Pinnelli V.B., Kutty B.M., Dhanushkodi A. Neuroprotection by human dental pulp mesenchymal stem cells: From billions to nano. Curr. Gene Ther. 2018;18:307–323. doi: 10.2174/1566523218666180913152615. [DOI] [PubMed] [Google Scholar]
- 91.Miura-Yura E., Tsunekawa S., Naruse K., Nakamura N., Motegi M., Nakai-Shimoda H., Asano S., Kato M., Yamada Y., Izumoto-Akita T. Secreted factors from cultured dental pulp stem cells promoted neurite outgrowth of dorsal root ganglion neurons and ameliorated neural functions in streptozotocin-induced diabetic mice. J. Diabetes Investig. 2020;11:28–38. doi: 10.1111/jdi.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Codispoti B., Marrelli M., Paduano F., Tatullo M. NANOmetric BIO-Banked MSC-Derived Exosome (NANOBIOME) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018;7:357. doi: 10.3390/jcm7100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Momen-Heravi F., Balaj L., Alian S., Mantel P.-Y., Halleck A.E., Trachtenberg A.J., Soria C.E., Oquin S., Bonebreak C.M., Saracoglu E. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013;394:1253–1262. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franquesa M., Hoogduijn M.J., Ripoll E., Luk F., Salih M., Betjes M.G., Torras J., Baan C.C., Grinyo J.M., Merino A.M. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Front. Immunol. 2014;5:525. doi: 10.3389/fimmu.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katsuda T., Kosaka N., Takeshita F., Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 96.Yu L.-L., Zhu J., Liu J.-X., Jiang F., Ni W.-K., Qu L.-S., Ni R.-Z., Lu C.-H., Xiao M.-B. A comparison of traditional and novel methods for the separation of exosomes from human samples. BioMed Res. Int. 2018;2018:3634563. doi: 10.1155/2018/3634563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006;30:3–29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 98.Gardiner C., Vizio D.D., Sahoo S., Théry C., Witwer K.W., Wauben M., Hill A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lobb R.J., Becker M., Wen S.W., Wong C.S., Wiegmans A.P., Leimgruber A., Moller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cvjetkovic A., Lötvall J., Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles. 2014;3:23111. doi: 10.3402/jev.v3.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Witwer K.W., Buzas E.I., Bemis L.T., Bora A., Lasser C., Lotvall J., Nolte-’t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamparski H.G., Metha-Damani A., Yao J.-Y., Patel S., Hsu D.-H., Ruegg C., Le Pecq J.-B. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods. 2002;270:211–226. doi: 10.1016/S0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 103.Helwa I., Cai J., Drewry M.D., Zimmerman A., Dinkins M.B., Khaled M.L., Seremwe M., Dismuke W.M., Bieberich E., Stamer W.D., et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE. 2017;12:e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S., Salto-Tellez M., Timmers L., Lee C.N., El Oakley R.M., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Bruno S., Grange C., Deregibus M.C., Calogero R.A., Saviozzi S., Collino F., Morando L., Busca A., Falda M., Bussolati B., et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Merckx G., Hosseinkhani B., Kuypers S., Deville S., Irobi J., Nelissen I., Michiels L., Lambrichts I., Bronckaers A. Angiogenic Effects of Human Dental Pulp and Bone Marrow-Derived Mesenchymal Stromal Cells and their Extracellular Vesicles. Cells. 2020;9:312. doi: 10.3390/cells9020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costa L.A., Eiro N., Vaca A., Vizoso F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering. 2022;10:4. doi: 10.3390/bioengineering10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schnitzler A.C., Verma A., Kehoe D.E., Jing D., Murrell J.R., Der K.A., Aysola M., Rapiejko P.J., Punreddy S., Rook M.S. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem. Eng. J. 2016;108:3–13. doi: 10.1016/j.bej.2015.08.014. [DOI] [Google Scholar]
- 111.Hansmann J., Groeber F., Kahlig A., Kleinhans C., Walles H. Bioreactors in tissue engineering—Principles, applications and commercial constraints. Biotechnol. J. 2013;8:298–307. doi: 10.1002/biot.201200162. [DOI] [PubMed] [Google Scholar]
- 112.Vajrabhaya L.O., Vongphan N., Hongskul P., Suwannawong S.K. The effect of age of refrigerated conditioned medium on cell survivability in vitro. Dent. Traumatol. 2003;19:41–44. doi: 10.1034/j.1600-9657.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 113.Turksen K. Embryonic Stem Cell Protocols. Springer; Berlin/Heidelberg, Germany: 2006. [Google Scholar]
- 114.Zhou H., Yuen P.S., Pisitkun T., Gonzales P.A., Yasuda H., Dear J.W., Gross P., Knepper M.A., Star R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471–1476. doi: 10.1038/sj.ki.5000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eiro N., Fraile M., González-Jubete A., González L.O., Vizoso F.J. Mesenchymal (stem) stromal cells based as new therapeutic alternative in inflammatory bowel disease: Basic mechanisms, experimental and clinical evidence, and challenges. Int. J. Mol. Sci. 2022;23:8905. doi: 10.3390/ijms23168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chouaib B., Cuisinier F., Collart-Dutilleul P.-Y. Dental stem cell-conditioned medium for tissue regeneration: Optimization of production and storage. World J. Stem Cells. 2022;14:287. doi: 10.4252/wjsc.v14.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fraile M., Eiro N., Costa L.A., Martín A., Vizoso F.J. Aging and mesenchymal stem cells: Basic concepts, challenges and strategies. Biology. 2022;11:1678. doi: 10.3390/biology11111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sendon-Lago J., Rio L.G.-d., Eiro N., Diaz-Rodriguez P., Avila L., Gonzalez L.O., Vizoso F.J., Perez-Fernandez R., Landin M. Tailored hydrogels as delivery platforms for conditioned medium from mesenchymal stem cells in a model of acute colitis in mice. Pharmaceutics. 2021;13:1127. doi: 10.3390/pharmaceutics13081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Osugi M., Katagiri W., Yoshimi R., Inukai T., Hibi H., Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A. 2012;18:1479–1489. doi: 10.1089/ten.tea.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cantinieaux D., Quertainmont R., Blacher S., Rossi L., Wanet T., Noël A., Brook G., Schoenen J., Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS ONE. 2013;8:e69515. doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhang S.H., Lee S., Shin J.-Y., Lee T.-J., Jang H.-K., Kim B.-S. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol. Ther. 2014;22:862–872. doi: 10.1038/mt.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen C., Lie P., Miao T., Yu M., Lu Q., Feng T., Li J., Zu T., Liu X., Li H. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015;12:20–30. doi: 10.3892/mmr.2015.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Witwer K.W., Van Balkom B.W.M., Bruno S., Choo A., Dominici M., Gimona M., Hill A.F., De Kleijn D., Koh M., Lai R.C., et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The raw/processed data required to reproduce these findings cannot be shared at this time due to technical limitations.