Abstract
Post-transcriptional modifications of tRNA are crucial for their core function. The inosine (I; 6-deaminated adenosine) at the first position in the anticodon of tRNAArg(ICG) modulates the decoding capability and is generally considered essential for reading CGU, CGC, and CGA codons in eubacteria. We report here that the Bacillus subtilis yaaJ gene encodes tRNA-specific adenosine deaminase and is non-essential for viability. A β−galactosidase reporter assay revealed that the translational activity of CGN codons was not impaired in the yaaJ-deletion mutant. Furthermore, tRNAArg(CCG) responsible for decoding the CGG codon was dispensable, even in the presence or absence of yaaJ. These results strongly suggest that tRNAArg with either the anticodon ICG or ACG has an intrinsic ability to recognize all four CGN codons, providing a fundamental concept of non-canonical wobbling mediated by adenosine and inosine nucleotides in the anticodon. This is the first example of the four-way wobbling by inosine nucleotide in bacterial cells. On the other hand, the absence of inosine modification induced +1 frameshifting, especially at the CGA codon. Additionally, the yaaJ deletion affected growth and competency. Therefore, the inosine modification is beneficial for translational fidelity and proper growth-phase control, and that is why yaaJ has been actually conserved in B. subtilis.
Keywords: tRNA, post-transcriptional modification, inosine, four-way wobbling, Bacillus subtilis
1. Introduction
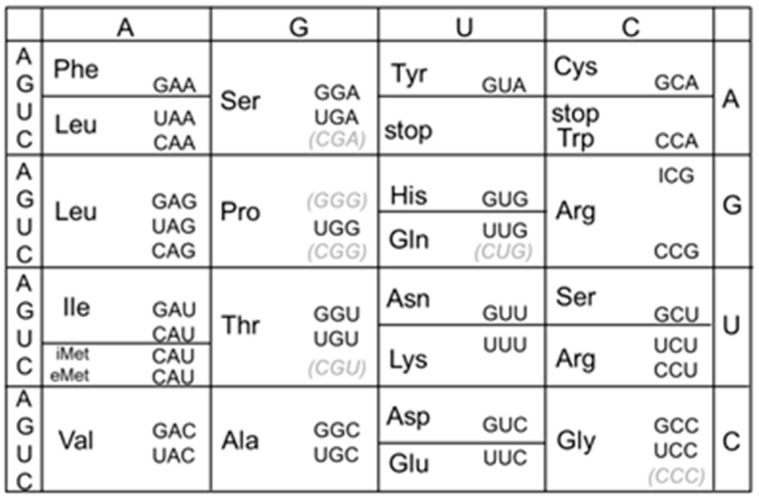
A productive codon–anticodon pairing on the ribosome is crucial for efficient and accurate protein synthesis [1,2,3,4]. Post-transcriptional modifications of tRNA molecules are functionally important, and nucleotide modification at the wobble position of the anticodon (position 34) plays a key role in codon recognition [5,6,7,8]. Inosine (denoted as I) is the 6-deaminated form of adenosine, and in this form, its chemical structure is similar to guanosine because the amino group (hydrogen donor) at position 6 is substituted for the keto group (hydrogen acceptor) [9,10]. In eubacteria, inosine modification of RNA molecules is usually identified at the wobble position of the anticodon of tRNAArg(ICG), which is one of the tRNA repertoires responsible for decoding the CGN four-codon family (Figure 1) [6,7,10,11,12,13]. Well-studied eubacteria, including Escherichia coli and B. subtilis, contain two species of tRNA for decoding CGN codons. According to the orthodox base-pairing theory, it is generally assumed that tRNAArg(ICG) recognizes CGU, CGC, and CGA codons, whereas tRNAArg(CCG) recognizes the CGG codon (Figure 2, upper right) [13,14,15,16,17,18].
Figure 1.
Anticodons in B. subtilis and E. coli. All anticodons are shown in the 5′ to 3′ direction. Modified nucleotides other than the inosine of tRNAArg are omitted. iMet and eMet indicate initiator and elongator tRNAMet(CAU), respectively. The anticodon CAU responsible for isoleucine is modified to LAU (L; lysidine) in B. subtilis and E. coli [5,6,7]. Anticodons that are not found in B. subtilis but occur in E. coli are in parentheses [11,18].
Figure 2.
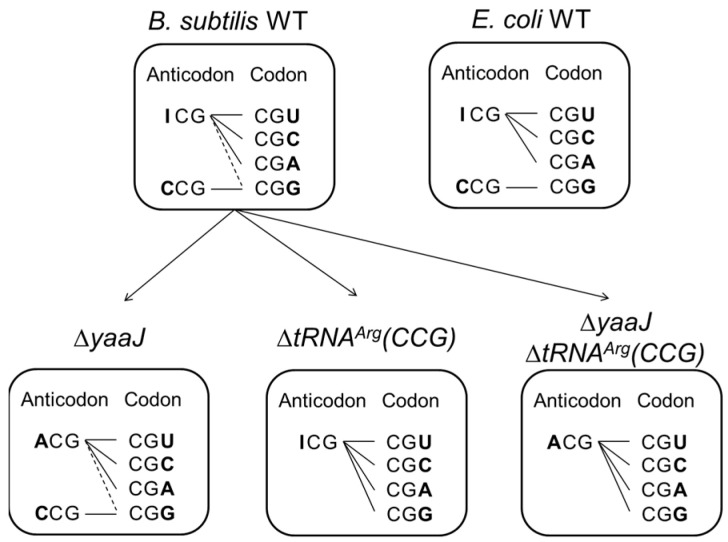
Decoding model of CGN codon family in B. subtilis and E. coli. All codons and anticodons are shown in the 5′ to 3′ direction. For most eubacteria, tRNAArg(ICG) dominantly decodes three arginine codons, namely CGU, CGC, and CGA, whereas tRNAArg(CCG) decodes the CGG codon. In B. subtilis and E. coli, tRNAArg(ACG) and tRNAArg(CCG) are encoded by four and a single gene, respectively. The genes encoding tRNAArg(ACG), tRNAArg(CCG), and tRNA adenosine deaminase (TadA) are all essential for the viability of E. coli. In B. subtilis, as indicated by dashed lines, CGG codons may be redundantly recognized by two tRNAs, namely tRNAArg(ICG) and tRNAArg(CCG) in wild-type cells (WT), or by tRNAArg(ACG) and tRNAArg(CCG) in the yaaJ-deletion mutant cells (ΔyaaJ). All four CGN codons can be decoded by either (1) tRNAArg(ICG) in the trnQ-Arg(CCG)-deletion mutant (ΔtRNAArg(CCG)), or by (2) tRNAArg(ACG) in the double-deletion mutant of yaaJ and trnQ-Arg(CCG) (ΔyaaJΔtRNAArg(CCG)).
Base pairings between unmodified A34 and N(III) (where N denotes U, C, A, or G at the third position of the codon) are theoretically possible, though they are unstable or nonproductive, and sequencing analyses of tRNA gene sets from various organisms have revealed deductively that unmodified A34 can recognize all four nucleotides at the wobble position of the codon [11,13,14,19,20,21,22,23,24,25,26,27,28,29]. Pairings between A34 and R(III) (where R denotes a purine nucleotide) are particularly unstable, and they have been predicted to form only with non-canonical conformations of nucleotides. As for A34-Y(III) pairings (where Y denotes a pyrimidine nucleotide), in vitro and in vivo analyses have shown their instability and decoding inefficiency. Furthermore, it has been reported that an unmodified A34 on the ribosomal P site destabilizes the next A site codon–anticodon duplex during translation [22], suggesting that unmodified A34 is not preferred for decoding. Inosine modification would alleviate such adverse effects of A34 and modulate its reading of codons. A34 is post-transcriptionally converted to I34 in nature, and unmodified A34 in mature tRNAs has been found in some mycoplasmas and organelles that contain compact genomes encoding a reduced set of tRNA repertoires [11,30,31,32].
Crystallization of the 30S subunit of the Thermus thermophilus ribosome complexed with an anticodon stem-loop oligonucleotide containing inosine at the wobble position of the anticodon has confirmed the presence of two hydrogen bonds in the I34-A(III) pairing with the anti-conformation geometry of sugars, as proposed by Crick [1,15,24]. In this context, it is generally thought that I34 modification is crucial for decoding three codons by a single species of tRNAArg and that this modification has evolved as a solution to decoding the A-ending codons [5,6,7,10,13].
The pairing of the ICG anticodon with the CGG codon does not occur because the I34-G(III) pairing likely causes steric hindrance, although it is possible only when it involves unusual tautomerization or geometry of nucleotides under specific conditions [13,24,27,28,29]. Thus, tRNAArg(CCG) should be required for decoding CGG codons (Figure 2), and both tRNAArg(ICG) and tRNAArg(CCG) have been shown indispensable for the viability of E. coli [33].
Inosine formation generally occurs through hydrolytic deamination of genomically encoded adenosine, a process that is catalyzed by adenosine deaminase [9,10,17]. Inosine synthase in the E. coli K12 strain was identified as the first prokaryotic tRNA-specific adenosine deaminase (TadA) [34]. It has been shown that tadA is broadly conserved among eubacteria with exceptions in some mollicutes [31]. The deletion mutant of E. coli tadA could not be obtained using the direct gene disruption method [34], supporting the early prediction that I34 is crucial for translation in eubacteria. In contrast, we found that yaaJ, the B. subtilis homolog of tadA, does not appear on published lists of B. subtilis essential genes [10,35,36,37], implying that the I34 modification of tRNAArg(ACG) is not essential for viability. This observation encouraged us to elucidate the function of the inosine modification of tRNAArg(ICG) and the decoding strategy of the CGN codon family in B. subtilis.
Here, we show that yaaJ is the only gene responsible for the I34 formation of tRNAArg and that it is completely dispensable for the growth and translation in B. subtilis. We also found that tRNAArg(CCG) responsible for the CGG codon is non-essential both in the presence and absence of yaaJ. These findings demonstrate the decoding of all four CGN codons by a single species of tRNA involving non-canonical A34-N(III) and I34-N(III) pairings; this is the first example of non-discriminating four-way wobbling by inosine nucleotide in bacterial cells. On the other hand, the yaaJ deletion caused a marked increase in frameshifting at the CGA codon, indicating that I34 contributes to translational fidelity. Additionally, the growth of the yaaJ-deletion mutant was affected in the poor medium at higher temperatures and was significantly impaired in natural competence. These results suggest the physiological importance of I34 in stress responses.
2. Materials and Methods
Construction of B. subtilis strains of the yaaJ and tRNAArg(CCG)
Deletion mutants of yaaJ and tRNAArg(CCG) listed in Table 1 were constructed by replacement of each gene in the genome of the B. subtilis 168 strain with a PCR fragment that was designed to contain the antibiotic-resistance gene flanked by the target gene region (Figure S1) and generated using the primers listed in Table S1. In all cases, the correct introduction of the deletion mutation was confirmed by back-crossing into strain 168, followed by PCR and DNA sequencing as a second verification.
Table 1.
B. subtilis strains used in this study.
| Strain | Genotype (Characteristics) | Source or Reference |
|---|---|---|
| 168 | trpC2 (Wild-type) | Laboratory stock |
| KUB10 | ΔyaaJ::cat trpC (yaaJ-deletion) | This study |
| SOM1 | ΔtrnQ-Arg(CCG)::cat trpC2 (trnQ-deletion) | This study |
| SOM2 | ΔyaaJ::tet ΔtrnQ-Arg(CCG)::cat trpC2 (yaaJ, trnQ-double deletion) |
This study |
| SOM3 | ΔtrnQ-Arg(CCG)::spc trpC2 (trnQ-deletion) | This study |
tRNA isolation
Total RNA was prepared from late-log phase B. subtilis cells cultured in LB at 37 °C, using ISOGEN (Nippon Gene, Toyama, Toyama, Japan), as described previously [38]. Then, crude tRNA was abstracted from the total RNA by fractionation on a Bio-scale DEAE2 column (Bio-Rad, Hercules, CA, USA) with a linear gradient of NaCl consisting of solvent A (200 mM NaCl, 20 mM Hepes-KOH [pH 7.5] and 8 mM MgCl2) and solvent B (700 mM NaCl, 20 mM Hepes-KOH [pH 7.5] and 8 mM MgCl2) at a flow rate of 2 mL/min. tRNAArg(I/ACG) was isolated from crude tRNA according to the solid-phase DNA probe method described previously [39] using the 3′-biotinylated probe (Table S1). Denaturing PAGE was used for further purification of the isolated tRNAArg molecule.
Mass spectrometry
The isolated RNAs were digested into nucleosides and analyzed by LC/MS using ion-trap mass spectrometry as described previously [40], with the following slight modifications. An LCQDUO ion-trap (IT) mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an electrospray ionization (ESI) source and HP1100 liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA) were used to analyze the nucleosides. The isolated tRNAArg(I/ACG) was digested with P1 nuclease (Yamasa, Noda, Chiba, Japan) and alkaline phosphatase (E. coli C75, Takara, Kusatsu, Shiga, Japan) in a 25 μL reaction mixture containing 20 mM HEPES-KOH (pH 7.6) at 37 °C for 3 h and then analyzed by LC/MS. The hydrolysate was fractionated by using an Inertsil ODS-3 column, 250 × 2.1 mm (GL Science, Shinjuku-ku, Tokyo, Japan). The solvent system consisted of 5 mM NH4OAc (pH 5.3) (A) and 60% acetonitrile (B), used as follows: 1–35% B in 0–35 min, 35–99% B in 35–40 min, and 99% B in 40–50 min. The chromatographic effluent (150 μL/min) was directly conducted into the ion source without prior splitting. Positive ions were scanned over an m/z range of 103–700 throughout the separation under the following conditions: flow rate of sheath gas, 95 arb; capillary temperature, 245 °C; and spray voltage, 5 kV.
Sporulation assay
B. subtilis cells were inoculated at OD600 of ca. 0.03–0.05 and grown in 2× SG medium (2× Schaeffer’s sporulation medium supplemented with 0.1% glucose, [41]) for 24 h at 37 °C with shaking. To assay for cells that had produced heat-resistant spores, the culture was heated at 80 °C for 10 min, plated on LB agar plates, and incubated at 37 °C for 24 h. The number of cell colonies, both those subjected and not subjected to heat treatment, was then compared.
Transformation efficiency assay
Trp+ transformation activity of strains was determined using 168W (trp+) chromosomal DNA. Cells were grown in a competence-inducing (CI) medium consisting of Spizizen’s minimal glucose (0.5%) (MMG) medium supplemented with 0.03% yeast extract instead of casein acid hydrolysate [42] at 37 °C with shaking until the OD600 reached 1.0. An aliquot (0.1 mL) of the culture was withdrawn and mixed with the DNA solution at a final concentration of 2 μg ml−1. After incubation for 30 min at 37 °C with shaking, the cells were plated on MMG agar plates. Trp+ transformants were counted after 2 days of incubation at 37 °C.
Northern blot analysis of B. subtilis tRNAArg(I/ACG) and tRNAArg(CCG)
Total RNA prepared from late-log phase B. subtilis cells cultured in LB at 37 °C, using ISOGEN (Nippon Gene, Toyama, Toyama, Japan), was separated on an 8% polyacrylamide gel containing 8 M urea. The gel was blotted onto Hybond N+ (GE Healthcare Bio-science AB, Uppsala, Sweden) for Northern blotting analysis. Hybridization was performed by using 5′ 32P-labeled synthetic DNA oligonucleotides (Table S1) as probes [38].
Reverse-transcription polymerase chain reaction (RT-PCR) and DNA sequencing
RT-PCR and sequencing analysis followed the previous study [43]. Total RNA prepared from B. subtilis cells as described above was reverse-transcribed with ReverTraAce (TOYOBO, Osaka, Osaka, Japan) and PCR-amplified with Blend Taq (TOYOBO, Osaka, Osaka, Japan). Pimers used for RT-PCR are listed in Table S1. PCR products were gel purified and cloned using the TA cloning kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Between 50 and 100 clones were sequenced for each product.
β−Galactosidase assay
The lacZ-coding region of pMC1871 [44] was transferred to pAPNC213 [45] to construct the reporter plasmid pTOM20c, as described previously [38]. The peptide sequence DERRKLRR, which is encoded by two sets of tandem CGN (i.e., CGU, CGC, CGA, CGG, or AGA) codons, was inserted between the first and second amino acid residues of lacZ in pTOM20c to construct the plasmid pTOM21, pTOM22, pTOM23, pTOM24, and pTOM25, respectively. pTOM26 encoding the peptide sequence DEAAKLAA was also used as a control. Synthetic DNA oligonucleotides used for plasmid constructions are listed in Table S1. The B. subtilis strains transformed with these plasmid DNA linearized by ScaI digestion to integrate the lacZ gene into the aprE site of the genome of the wild-type or the deletion mutants of yaaJ and trnQ-Arg(CCG) were used for the β−gal assay (KUB11-32 in Table S2). Precultures of each strain were inoculated into fresh LB media with 1 mM IPTG and cultured at 37 °C until the OD600 reached 0.6. The β−gal assay was performed according to the references [38,46].
Frameshift assay
Our methods were based on previous studies of the E. coli release factor 2 (RF-2) programmed frameshift mechanism [47,48]. Plasmids encoding lacZ, which contains the CGU, CGC, CGA, CGG, or UGA codon at the frameshifting regulatory site at the N-terminus of lacZ were constructed and designated pKUB20, pKUB21, pKUB22, pKUB23, or pKUB24, respectively. As a control, pKUB25 without the frameshifting regulatory site was used. Synthetic DNA oligonucleotides used for plasmid constructions are listed in Table S1. The B. subtilis strains transformed with these plasmids to integrate the lacZ gene into the aprE site of the genome, as described above, were used for β−gal assay (KUB33-44 in Table S2).
Quantitative real-time PCR (qPCR)
B. subtilis cells grown in LB at 37 °C were harvested when the OD600 reached 0.6; total RNA was prepared using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Next, cDNA was prepared from total RNA by reverse transcription using random hexamer primer and ReverTraAce (TOYOBO, Osaka, Osaka, Japan) according to the manufacturer’s instructions. Expression of the target genes was determined by qPCR, using the primers listed in Table S1, with Power SYBR Green PCR Master Mix and a 7500 Real-Time PCR System (Applied Biosystems, University Park, IL, USA). Transcript abundance was normalized to 16S rRNA. The qPCR reactions were carried out using RNA isolated from at least three independent cultures.
3. Results
B. subtilis yaaJ encodes a tRNA adenosine deaminase and is dispensable but beneficial for growth and competency
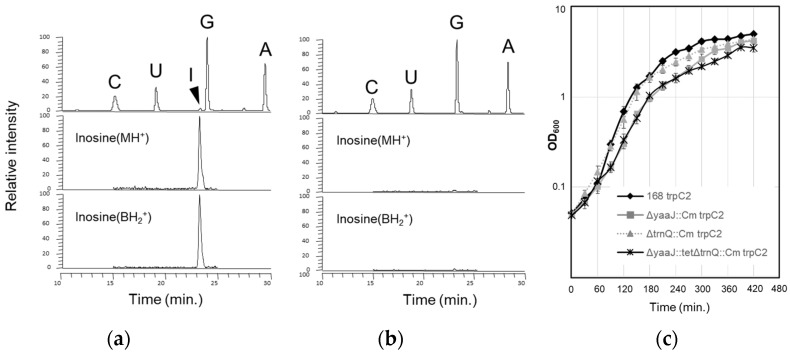
B. subtilis YaaJ, a homolog of E. coli TadA, possibly encodes a tRNA-specific adenosine deaminase [34,35,36]. The deletion mutant of yaaJ has been constructed by the B. subtilis genome project [36], suggesting that yaaJ is non-essential for viability. To confirm that yaaJ is completely dispensable and responsible for inosine formation, a deletion mutant strain (KUB10) was constructed by replacing the coding region of yaaJ with a gene encoding chloramphenicol acetyltransferase (Figure S1a). The genes located downstream of yaaJ (scr-dnaX-yaaK-recR-yaaL-bofA) harbor their own promoters, and thus the deletion of yaaJ does not inhibit the expression of the downstream genes. Next, the solid-phase DNA probe method [39] was used to isolate tRNAArg(ICG) or tRNAArg(ACG) from wild-type (strain 168) and KUB10 cells cultured in LB medium at 37 °C (a typical laboratory growth condition); inosine formation of tRNAArg was analyzed by mass spectrometry (Figure 3a,b). Analysis of the total nucleosides derived from the digested tRNAArg samples revealed the specific disappearance of inosine from the KUB10 cells, indicating that yaaJ is required for the inosine modification of tRNAArg. The sequence of the anticodon was confirmed by a reverse transcription polymerase chain reaction (RT-PCR) of tRNAArg(I/ACG) followed by a sequencing analysis. Since inosine is recognized as guanosine by reverse transcriptase, “I” was represented as “G” at the corresponding position in the sequencing outputs. The results revealed that tRNAArg from the KUB10 cells harbored the anticodon sequence ACG (Figure S2b), whereas those from the wild-type harbored the anticodon sequence GCG only (Figure S2a). Northern blotting showed that tRNAArg with unmodified A34 is as stable as that with I34 in wild-type cells (Figure S3).
Figure 3.
Mass spectrometric analyses of tRNAArg(I/ACG) and growth of B. subtilis strains. LC/MS nucleoside analysis of purified tRNAArg(I/ACG) from (a) the wild-type (strain 168) and (b) KUB10 strains. The upper panel shows the UV trace at 254 nm, and the position of each nucleoside is indicated. The middle and lower panels show mass chromatograms detecting proton adduct (MH+, m/z 269) and the base-related ion (BH2+, m/z 137) of inosine, respectively. (c) Growth of the wild-type (strain 168; diamonds with black line), yaaJ-deletion mutant (KUB10; squares with gray line), trnQ-Arg(CCG)-deletion mutant (SOM1; triangles with gray dashed line), and a double deletion mutant of trnQ-Arg(CCG) and yaaJ (SOM2; crosses with black line) strains in LB medium at 37 °C with shaking. Data are represented as mean ± SD, n = 3.
Growth of KUB10 strain in LB medium at 37 °C was slowed during the log phase (Figure 3c), and such a tendency was even clearer when grown at 45 °C (Figure S4a). This observation seems to be due to the limiting effect of increased temperature on the KUB10 growth rate. The growth rate of the wild-type strain increased with the temperature both in rich (LB, Figure S4a) and poor media (CSM; competence and sporulation medium, Figure S4b). However, this was not observed for KUB10. It was intriguing that the growth curves of KUB10 in CSM were almost the same at 37 °C and 45 °C (Figure S4b).
B. subtilis cells respond to a range of stress by spore formation. This process involves up to 500 genes [49,50,51,52,53] that tend to use minor codons including the CGA codon [54,55]. We then examined the effect of yaaJ deletion on heat-resistant spore formation. KUB10 showed only a slight decrease in sporulation efficiency (Table 2) suggesting that inosine modification of tRNAArg is not essential for the expression of sporulation genes. On the path toward sporulation, some of the B. subtilis cells become naturally competent and thus have the opportunity to adapt to their environment [51,53]. We found that the competency of KUB10 decreased by 100-fold (Table 3), indicating the significance of the yaaJ gene. The development of competency in B. subtilis is regulated by a complex and sophisticated signal transduction network. Expression of the competence genes that mediate DNA uptake and recombination are driven mostly by a master regulator comK during transformation. Then, we performed a reporter assay to monitor the expression of comK. We also checked the expression of srfA, which is located upstream of comK in the regulatory cascade and positively regulates the stability of the comK protein. The translational fusion of the regulatory region and the first six codons (not arginine codons) of comK or srfA to the N-terminus of lacZ [56] was integrated into the amyE site of the wild-type or KUB10 genome, yielding strains that express β−gal under the control of comK or srfA regulatory sequences. When the KUB10 strains harboring comK-lacZ (Figure S5, middle) or srfA-lacZ (Figure S5, top) were grown on the CI plate medium (with X−gal) which induces competence, they turned white or blue, respectively. Therefore, the inhibitory effect of the yaaJ-deletion seems attributable to the defect of the comK synthesis. To understand the detailed mechanism for this phenomenon, further analysis will be required.
Table 2.
Sporulation efficiency of B. subtilis wild-type and KUB10 strains.
| Strain | Viable Bacteria Count (c.f.u. mL−) | Frequency (%) | |
|---|---|---|---|
| Total | Spore | ||
| Wild-type (trpC2) | (2.30 ± 0.63) × 108 | (2.06 ± 0.23) × 108 | 89.6 |
| KUB10 (ΔyaaJ trpC2) | (1.55 ± 0.14) × 108 | (1.09 ± 0.87) × 108 | 70.0 |
Colony formation on plate medium was counted. The values are the mean of three independent experiments (p < 0.05). Means ± SD are shown.
Table 3.
Transformation efficiency of B. subtilis wild-type and KUB10 strains.
| Strain | Viable Bacteria Count (c.f.u. mL−) | Transformation Frequency (%) | |
|---|---|---|---|
| Total | Transformant | ||
| Wild-type (trpC2) | (4.36 ± 0.17) × 108 | (1.16 ± 0.30) × 105 | 0.027 |
| KUB10 (ΔyaaJ trpC2) | (4.01 ± 0.22) × 108 | (1.17 ± 0.01) × 103 | 0.0003 |
Colony formation on plate medium was counted. The values are the mean of three independent experiments (p < 0.05). Means ± SD are shown.
Based on these observations, we concluded that (1) B. subtilis yaaJ encodes a tRNA-specific adenosine deaminase, (2) inosine formation at the wobble position of tRNAArg(ICG) is dispensable yet beneficial for the growth, and (3) yaaJ is important for the development of competency.
Translational activity without I34 of B. subtilis tRNAArg
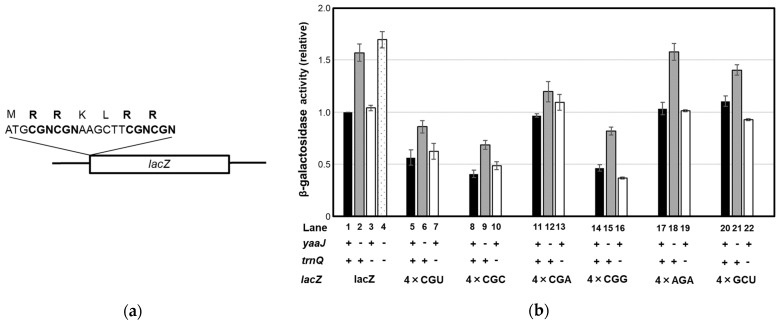
The results described above raised the question of how the CGN codon family, particularly CGC and CGA codons, are decoded by tRNAArg without I34 in yaaJ-deletion mutant cells. Decoding both CGC and CGA codons by tRNAArg(CCG), another repertoire for the CGN codon box is generally implausible. Alternatively, tRNAs responsible for amino acids other than arginine may recognize those codons resulting in mistranslation. However, CGC and CGA codons are frequently used in essential genes in the B. subtilis genome: genes for DNA polymerases (e.g., polC and dnaE), RNA polymerases (e.g., rpoA and rpoB), aminoacyl-tRNA synthetases (e.g., metS and argS), and ribosomal proteins (e.g., rplS, and rpsO) [35,36,55]. Therefore, in KUB10 cells, tRNAArg(ACG) acting cooperatively with tRNAArg(CCG) is expected to translate all CGN codons mostly as arginine. To analyze the fundamental decoding property of CGN codons in B. subtilis, a reporter assay was performed using the β−gal, which originally contains 20, 36, 3, and 7 CGT, CGC, CGA, and CGG codons, respectively. We also used the lacZ derivatives (4 × CGN−lacZ) where two pairs of tandem CGN codons, which are intervened by two non-arginine codons, are fused to the N-terminus of lacZ (Figure 4a). Each lacZ gene was integrated into the amyE locus of the wild-type and KUB10 genomes (Table S2). In the wild-type strain, the β−gal activity of the original lacZ and the 4 × CGA−lacZ was comparable to each other (lanes 1 and 11 in Figure 4b), showing that continual CGA codons do not impede translation in B. subtilis cells. In KUB10, the β−gal activity of the original and lacZ derivatives was not impaired but rather higher (lanes 2, 6, 9, 12, 15, 18, and 21 in Figure 4b) than that in the wild-type strain (lanes 1, 5, 8, 11, 14, 17, and 20). The increase of β−gal activity was smallest when the 4 × CGA−lacZ was used (lanes 11 and 20), suggesting that a tract of CGA codons affects the production of β−gal protein in KUB10 cells. Quantitative real-time PCR analysis of lacZ−mRNAs showed that the amount of mRNA of the original lacZ in KUB10 cells is higher (lanes 1 and 2 in Figure S6), while that of the 4 × CGA−lacZ is almost the same as the wild-type strain (lanes 3 and 4 in Figure S6). Therefore, the small increase of β−gal activity of the 4 × CGA−lacZ in the absence of I34 was possibly derived from the reduced amount of mRNA. The exact mechanism for the increases in the β−gal activity is unclear. Translational activity sometimes affects mRNA stability [57,58,59], and thus active translation caused close spacing of translating ribosomes on the β−gal mRNA to protect mRNA from cleavage. It is also possible that the slower translation in the absence of yaaJ rather stabilized the lacZ−mRNA and resulted in a higher expression of the β−galactosidase protein.
Figure 4.
β−galactosidase assay with or without I34 modification and tRNAArg(CCG). (a) Schematic illustration of the lacZ reporter genes used in the β−gal assays, showing the inserted nucleotide and peptide sequences. The two tandem arginine (R) residues encoded by the CGN codons in the inserted peptide are indicated in bold. Negative controls were also used in which the CGN codons are substituted by AGA (non-CGN arginine codon) or GCU for alanine (non-arginine codon). (b) β−gal activity of the unmodified lacZ, 4 × CGN-lacZ, 4 × AGA-lacZ, or 4 × GCU-lacZ reporter genes in B. subtilis strains with (+) or without (−) yaaJ and trnQ-Arg(CCG).
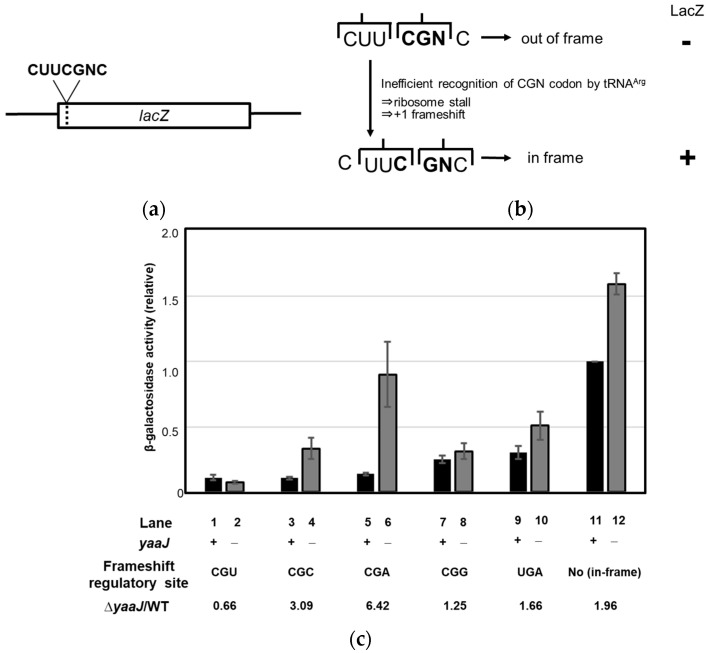
Lack of I34 increases the rate of frameshifting at the CGA and CGC codons
Even though the β−gal assay showed increased activity in the yaaJ-deletion mutant, I34 was still preferable for cell growth. Therefore, we hypothesized that I34 contributes to translational fidelity. To evaluate this possibility, a reporter assay based on the E. coli release factor 2 (RF-2) programmed frameshift mechanism [47,48] was performed in B. subtilis strains. In our analysis, the frameshift regulatory UGA codon in the lacZ/RF-2 fusion was replaced with a CGN codon, such that the generation of β−gal activity required frameshifting (Figure 5a,b). At this site, frameshifting and in-phase translation of the codon were competing reactions, and frameshift-dependent β−gal activity would then be inversely related to the rate of aminoacyl-tRNA selection. Thus, the frequency of frameshifting could be used to estimate the A site binding of tRNA at CGN codons.
Figure 5.
The frameshift-dependent lacZ/RF-2 reporter assay with or without I34 modification. (a) Schematic illustration of the lacZ/RF-2 reporter genes used in the β−gal assays showing the inserted nucleotides. (b) In the lacZ/RF-2 fusions, the regulatory UGA codon at the E. coli RF-2 frameshift site was replaced by the CGN codon, such that the generation of β−gal activity required frameshifting. In-phase translation (upper pathway) and +1 frameshift (lower pathway) were competing reactions at the regulatory frameshift site. (c) β−gal activity of B. subtilis strains with the lacZ/RF-2 gene with (+) or without (−) yaaJ (Table S2). The relative β−gal activity in the KUB10 strain versus the wild-type strain is indicated by KUB10/WT.
The β−gal activities of both the original lacZ gene (with no frameshift site) and the gene with the UGA codon at the frameshift regulatory site in the KUB10 strain were higher than in the wild-type strain (lanes 9–12 in Figure 5c); these increases were the background as observed in Figure 4b. Insertion of the CGA codon at the regulatory site in the KUB10 strain showed a six-fold increase over the wild-type strain (lanes 5 and 6 in Figure 5c), indicating that the absence of I34 impeded A site selection and increased the frameshifting rate at the CGA codon. The second highest increase in frameshifting caused by the yaaJ deletion occurred when the CGC codon was at the regulatory site (lanes 3 and 4 in Figure 5c). This finding is explained by the chemical similarity between inosine and guanine, and also by the fact that unmodified A34 makes an unstable interaction with C(III) of the CGC codon in the A site. A slight decrease in the frameshifting rate in KUB10 cells was observed when the CGU codon was present at the regulatory site (lanes 1 and 2 in Figure 5c), suggesting that A34 is preferable for the stable recognition of U(III). The increase in β−gal activity of the KUB10 strain was slightly smaller when the CGG codon was contained at the regulatory site; the frameshifting at the CGG codons was repressed in the KUB10 strain. This implies that tRNAArg(ACG) participates in decoding of the CGG codon, which is generally considered to be recognized exclusively by tRNAArg(CCG).
Overall, these results indicate that I34 of tRNAArg ensures a smooth and stable interaction with CGN codons on the A site and that I34 contributes to the maintenance of the decoding fidelity, especially at the CGA codons in B. subtilis.
tRNAArg(CCG) is dispensable, and both tRNAArg(ICG) and tRNAArg(ACG) can each decode all four CGN codons in B. subtilis
The efficient translational activity independent of I34 suggests that the recognition of CGN codons is relatively relaxed in B. subtilis. We then attempted to construct a deletion mutant of tRNAArg(CCG). tRNAArg(CCG) encoded by a single gene; trnQ-Arg(CCG) was replaced with the antibiotic-resistance gene (Figure S1b). The resulting trnQ-Arg(CCG)-deletion mutant strains (SOM1 and SOM3 in Table 1) that lack tRNAArg(CCG), as confirmed by Northern blotting (Figure S3a), were viable. The growth of SOM1 in LB at 37 °C was slowed in the stationary phase (Figure 3c). The β−gal activities of original and 4 × CGG-lacZ in SOM1 (lanes 3 and 16 in Figure 4b) were almost identical to that in the wild-type strain (lanes 1 and 14 in Figure 4b), showing that tRNAArg(CCG) is not essential even for the decoding of the CGG codon.
Some species of mycoplasma and plant chloroplasts lack tRNAArg(CCG) genes [18,31,32,60,61]. In mycoplasmas, the wobble position of transcripts derived from the gene encoding tRNAArg(ACG) remains partially unmodified and is likely to decode CGG codons in the cell; it recruits both I34 and A34 to decode CGN codons [31]. There would be the possibility of a similar situation in our B. subtilis trnQ-Arg(CCG)-deletion mutant if a fraction of tRNAArg(ACG) remained unmodified at the wobble position of the anticodon. We thus determined the anticodon sequence of tRNAArg(I/ACG) from SOM1 cells by RT-PCR and sequencing analysis. As a result, all clones analyzed harbored I34 but not A34 (Figure S2c), suggesting that mature tRNAArg(I/ACG) is completely inosine-modified in SOM1, and thus tRNAArg with I34 is solely responsible for the decoding all four CGN codons even in the absence of tRNAArg(CCG). We also constructed a double deletion mutant of tRNAArg(CCG) and yaaJ, and the resulting strain (SOM2 in Table 1) that contains only tRNAArg(ACG) was viable. SOM2 grew more slowly in LB at 37 °C relative to the KUB10 or SOM1 strains (Figure 3c), whereas the β−gal activity in SOM2 was still higher than that in the wild-type strain (lanes 1 and 4 in Figure 4b). SOM2 exhibited cold sensitivity at 28 °C and below in LB medium (unpublished data).
The above results showed that a single repertoire, tRNAArg(ICG) or tRNAArg(ACG) can decode all four CGN codons in B. subtilis, suggesting that non-canonical recognition of G(III) by A34 or I34, A(III) by A34, and C(III) by A34 are possible in vivo (Figure 2).
Decoding of the CGN codon family is not accomplished by C34
For further analysis, we attempted to construct a deletion mutant of tRNAArg(ACG), encoded by four copies of the gene in the B. subtilis genome: trnB-Arg(ACG), trnE-Arg(ACG), trnI-Arg(ACG) and trnJ-Arg(ACG). A mutant strain lacking three genes of tRNAArg(ACG) was generated, but a mutant lacking all four gene copies could not be obtained even when tRNAArg(CCG) was located under the strong promoter (unpublished data), suggesting that the existence of tRNAArg(ICG) or tRNAArg(ACG) is crucial for viability and that tRNAArg(CCG) is unable to decode all four CGN codons. We cannot exclude the possibility that the mutant with complete deletion of the tRNAArg(ACG) genes was not obtained due to technical difficulties. But it is more plausible that tRNAArg(CCG) cannot recognize all four CGN codons in B. subtilis.
4. Discussion
We have determined that B. subtilis yaaJ encodes the tRNA-adenosine deaminase (TadA) for the I34 formation of tRNAArg. Our results showed that yaaJ is completely dispensable and thus tRNAArg(ACG) with unmodified A34 plays an adequate role during translation. Intriguingly, β−gal activity in the yaaJ-deletion mutant was not impaired but slightly higher than that in the wild-type strain. These observations contrast with that of E. coli, in which the downregulation of the expression of tadA caused growth inhibition and severe reduction in β−gal activity (unpublished data). The exact mechanism for the increases in the β−gal activity was not clarified in this study. A systematic analysis such as RNA-seq or proteosome analysis would provide a clue to better understand the effect of I34-deletion in tRNAArg on mRNA stability, codon recognition, and overall translational efficiency. Besides being responsible for decoding the CGG codon, tRNAArg(CCG) is non-essential whether in the presence or absence of yaaJ; it was unexpected that a single species of tRNAArg with either anticodon ICG or ACG decodes all four CGN codons in vivo (Figure 2). On the other hand, the tRNAArg(I/ACG)-deletion mutant could not be obtained, indicating that tRNAArg(CCG) is not adequate for the non-discriminating recognition of CGN codons. These results indicate that I34 and A34 of tRNAArg have an intrinsic ability to recognize all four nucleotides at the wobble position of the codon. The gaps in the β−gal activity among CGN codons in each strain suggest some direct interactions between I/A34 and N(III) in their base pairings.
Non-discriminating and extended codon recognition in nature have been reported for unmodified U34- or A34-containing tRNAs derived from specific bacteria and organelles that contain compact genomes encoding a reduced set of tRNA repertoires or that show a biased codon usage in which the corresponding codons are rarely used [13,21,31,60,61]. For example, in plant chloroplasts, the unmodified U34 of tRNAGly(UCC) can decode all four GGN codons in the absence of tRNAGly(GCC), while tRNAGly(GCC) cannot assume that role [62]. In the mitochondria of fungi and nematodes, tRNAArg with unmodified ACG anticodon is the only tRNA that recognizes the CGN codon family, whereas CGA codon is rarely used in fungi mitochondria [31,32,63]. For such decoding systems, the “super-wobbling” (also known as “four-way wobbling” or “hyper-wobbling”) hypothesis has been suggested as a possible mechanism. In super-wobbling, a single tRNA species with an unmodified U34 or A34 reads all four nucleotides in the third position of the codon [61,62,64,65,66]. This hypothesis may apply to the ability of B. subtilis tRNAArg(I/ACG) to decode CGN codons. Considering the existing idea that the I34-G(III) pairing is nonproductive, it was unpredictable that CGN codons are efficiently recognized solely by tRNAArg(ICG) in the absence of tRNAArg(CCG). A previous study has reported the recognition of all four nucleotides at the wobble position of the codon by I34 in yeast cells; tRNASer(IGA), responsible for the UCU, UCC, and UCA codons encoding serine, also recognizes the artificial non-sense codons, ΨAA and ΨAG, to suppress [67]. Our result showed the first example of non-discriminating recognition by I34 in bacterial cells and supports such unexpected ability of accommodation by the ribosomal decoding center in vivo.
Each of the CGN codons in non-pathogenic E. coli is biasedly used and the CGA codon is rarely found in essential genes, including ribosomal protein genes (Table S3). In B. subtilis, bias in CGN codon usage is relatively mild, and the CGA codon appears in ribosomal protein genes with a frequency comparable to those of the other CGN codons. This tendency is observed in other codon families [54,63], and such unbiased codon usage may correlate with the non-discriminating decoding property of B. subtilis. Additionally, there are fewer species of tRNA responsible for four-codon families in B. subtilis than in E. coli. For example, in B. subtilis, only one tRNAPro with the anticodon UGG of which U34 is modified to mo5U (5-methoxyuridine) decodes all four CCN codons, whereas three anticodons with cmo5U34 (5-carboxymethoxyuridine), G34 and C34 are assigned in E. coli (Figure 1) [11,18,68]. We have constructed a mutant strain of B. subtilis in which most of the four-codon family is decoded by a single species of anticodon (unpublished data). Therefore, a limited anticodon repertoire suffices for translation in B. subtilis.
Why is the super-wobbling, I34-N(III) and A34 -N(III) possible in B. subtilis? Codon–anticodon interaction dominantly depends on the hydrogen bonds between bases but it also involves other interactions to fit the overall shape of base pairings into the Watson–Crick geometry [69,70,71,72,73,74]. It has been suggested that non-discriminating codon recognition results from a combination of factors including the structural context of tRNA, competition with other tRNAs, and the nature of the ribosome. Sequences outside the anticodon including nucleotides at positions 32, 33, 37, and 38 in the anticodon-loop [26,75,76,77], and nucleotides at positions 9 and 24 in the D-arm [78,79,80], have been known to contribute greatly to decoding capacity and efficiency. Several differences found in sequence and modifications in tRNAArg(ICG) from B. subtilis and E. coli may affect the stabilization of unorthodox wobbling. However, the introduction of B. subtilis tRNAArg(ICG), which contains recognition elements for E. coli arginyl-tRNA synthetase [81], could not suppress the essentiality of tadA in E. coli (unpublished data), implying that the difference in the decoding ability of those bacteria is not derived solely from the tRNA. Previous studies have also indicated the intrinsic difference between the Gram-positive and Gram-negative bacteria in the decoding property [82,83,84]. Identification of the characteristics underlying the decoding property of B. subtilis is expected.
While the inosine modification of B. subtilis tRNAArg was dispensable, it was still preferable for translational fidelity, growth, and competency; this study partly answered the basic question of why the inosine modification has been actually conserved in this microbe. I34 modification would have a physiological role under stress conditions. However, sporulation was not affected, whereas competency was significantly inhibited in the absence of yaaJ. These observations seem to contradict each other, and further investigations are required to understand the respective mechanism. It is also possible that yaaJ has unidentified substrates other than tRNA. Recently, inosine modifications in mRNA of non-essential genes have been found in E. coli [10]. Although inosine modifications in mRNA have not been reported in B. subtilis, mRNA editing may have physiological functions in such a soil bacterium to survive harsh environments.
Acknowledgments
We express our deep thanks to K. Kobayashi, and N. Ogasawara for the distribution of B. subtilis strains and kind advice. We thank K. Watanabe, A. Muto, S. Yokobori, K. Takai, H. Himeno, S. Goto, K. Miyauchi, K. Ishiguro, and C. Ushida for insightful comments and valuable discussions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081515/s1, Figure S1: Genetic maps of the B. subtilis yaaJ- and trnQ-Arg(CCG)-deletion mutants; Figure S2: Detection of inosine modification of the B. subtilis tRNAArg(I/ACG) by DNA sequencing analysis of RT-PCR products; Figure S3: Northern blot analysis of B. subtilis tRNAArg(I/ACG) and tRNAArg(CCG); Figure S4: Growth of B. subtilis strains under various culture conditions; Figure S5: X−gal plate assay of srfA or comK-induced lacZ translation; Figure S6: Real-time PCR analysis of β−gal mRNA in wild-type and KUB10 strains; Table S1: Primers and probes used in this study; Table S2: B. subtilis strains used in β−gal assay; Table S3: Codon usage in B. subtilis and E. coli. References [11,56,63,85] are cited in the supplementary materials.
Author Contributions
Conceptualization, A.S., Y.S. (Yasuhiko Sekine) and T.S.; methodology, A.S., Y.S. (Yasuhiko Sekine), T.S., Y.I., F.K. and H.Y.; investigation, A.S., A.K., D.T., Y.I., H.A., F.K., Y.S. (Yuh Shiwa) and Y.K.; resources, F.K., H.N. and H.Y.; data curation, A.S., Y.I., Y.S. (Yasuhiko Sekine), F.K., T.S., Y.S. (Yuh Shiwa) and Y.K.; writing—original draft preparation, A.S.; writing—review and editing, Y.S. (Yasuhiko Sekine), T.S., Y.I., Y.K., Y.S. (Yuh Shiwa) and F.K.; supervision, A.S., Y.I., Y.S. (Yasuhiko Sekine) and T.S.; project administration, A.S., Y.S. (Yasuhiko Sekine) and T.S.; funding acquisition, A.S., Y.S. (Yasuhiko Sekine), T.S. and H.Y. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a grant from the Nagase Science and Technology Foundation (to A.S), the Ohsumi Frontier Science Foundation (to A.S. and F.K.), the Noda Institute for Scientific Research (to A.S.), a Grant-in-Aid for Young Scientists B (17770147 to A.S.), for scientific research on priority areas from the Ministry of Education, Science, Sports, and Culture of Japan (17GS0314 to Y.S. (Yasuhiko Sekine)), a Grant-in-Aid for Scientific Research on Innovative Areas (Research in a proposed research area) (26113003 to T.S.), Initiative for Realizing Diversity in the Research Environment in Chiba University (to A.S.), and the MEXT-supported Program for Strategic Research Foundation at Private Universities (S1311017 to H.Y., Y.S. (Yuh Shiwa), and Y.K.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Crick F.H. Codon—Anticodon pairing: The wobble hypothesis. J. Mol. Biol. 1966;19:548–555. doi: 10.1016/S0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 2.Grosjean H., Westhof E. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res. 2016;44:8020–8040. doi: 10.1093/nar/gkw608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wohlgemuth I., Pohl C., Mittelstaet J., Konevega A.L., Rodnina M.V. Evolutionary optimization of speed and accuracy of decoding on the ribosome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:2979–2986. doi: 10.1098/rstb.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusupova G.Z., Yusupov M.M., Cate J.H., Noller H.F. The path of messenger RNA through the ribosome. Cell. 2001;106:233–241. doi: 10.1016/S0092-8674(01)00435-4. [DOI] [PubMed] [Google Scholar]
- 5.Björk G.R. Biosynthesis and Function of Modified Nucleosides. In: Söll D.R., RajBhandary U.L., editors. tRNA, Structure, Biosynthesis, and Function. American Society for Microbiology; Washington, DC, USA: 1995. pp. 165–205. [DOI] [Google Scholar]
- 6.Suzuki T. Biosynthesis and function of tRNA wobble modifications. In: Grosjean H., editor. Fine-Tuning of RNA Functions by Modification and Editing. Volume 12. Springer; Berlin/Heidelberg, Germany: 2005. pp. 23–69. [DOI] [Google Scholar]
- 7.Agris P.F., Eruysalb E.R., Narendranb A., Väre V.Y.P., Vangavetia S., Ranganathan S.V. Celebrating wobble decoding: Half a century and still much is new. RNA Biol. 2018;15:537–553. doi: 10.1080/15476286.2017.1356562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell. Biol. 2021;22:375–392. doi: 10.1038/s41580-021-00342-0. [DOI] [PubMed] [Google Scholar]
- 9.Gerber A.P., Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science. 1999;286:1146–1149. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- 10.Liao W., Nie W., Ahmad I., Chen G., Zhu B. The occurrence, characteristics, and adaptation of A-to-I RNA editing in bacteria: A review. Front. Microbiol. 2023;14:1143929. doi: 10.3389/fmicb.2023.1143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jühling F., Mörl M., Hartmann R.K., Sprinzl M., Stadler P.F., Pütz J. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su A.A., Randau L. A-to-I and C-to-U editing within transfer RNAs. Biochemistry. 2011;76:932–937. doi: 10.1134/S0006297911080098. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama S., Nishimura S. Modified Nucleotides and Codon Recognition. In: Söll D.R., RajBhandary U.L., editors. tRNA, Structure, Biosynthesis, and Function. American Society for Microbiology; Washington, DC, USA: 1995. pp. 207–224. [DOI] [Google Scholar]
- 14.Curran J.F. Decoding with the A:I wobble pair is inefficient. Nucleic Acids Res. 1995;23:683–688. doi: 10.1093/nar/23.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy F.V.T., Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat. Struct. Mol. Biol. 2004;11:1251–1252. doi: 10.1038/nsmb866. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson E.M., Alexander R.W. Bacterial wobble modifications of NNA-decoding tRNAs. IUBMB Life. 2019;71:1158–1166. doi: 10.1002/iub.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres A.G., Pineyro D., Filonava L., Stracker T.H., Batlle E., Ribas de Pouplana L. A-to-I editing on tRNAs: Biochemical, biological and evolutionary implications. FEBS Lett. 2014;588:4279–4286. doi: 10.1016/j.febslet.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Abe T., Inokuchi H., Yamada Y., Muto A., Iwasaki Y., Ikemura T. tRNADB-CE: tRNA gene database well-timed in the era of big sequence data. Front. Genet. 2014;5:114. doi: 10.3389/fgene.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter W.N., Brown T., Anand N.N., Kennard O. Structure of an adenine-cytosine base pair in DNA and its implications for mismatch repair. Nature. 1986;320:552–555. doi: 10.1038/320552a0. [DOI] [PubMed] [Google Scholar]
- 20.Boren T., Elias P., Samuelsson T., Claesson C., Barciszewska M., Gehrke C.W., Kuo K.C., Lustig F. Undiscriminating codon reading with adenosine in the wobble position. J. Mol. Biol. 1993;230:739–749. doi: 10.1006/jmbi.1993.1196. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki Y., Kojima A., Bessho Y., Hori H., Ohama T., Osawa S. Translation of synonymous codons in family boxes by Mycoplasma capricolum tRNAs with unmodified uridine or adenosine at the first anticodon position. J. Mol. Biol. 1995;251:486–492. doi: 10.1006/jmbi.1995.0450. [DOI] [PubMed] [Google Scholar]
- 22.Lim V. Analysis of action of the wobble adenine on codon reading within the ribosome. J. Mol. Biol. 1995;252:277–282. doi: 10.1006/jmbi.1995.0494. [DOI] [PubMed] [Google Scholar]
- 23.Takai K., Takaku H., Yokoyama S. In vitro codon-reading specificities of unmodified tRNA molecules with different anticodons on the sequence background of Escherichia coli tRNASer. Biochem. Biophys. Res. Commun. 1999;257:662–667. doi: 10.1006/bbrc.1999.0538. [DOI] [PubMed] [Google Scholar]
- 24.Takai K. Classification of the possible pairs between the first anticodon and the third codon positions based on a simple model assuming two geometries with which the pairing effectively potentiates the decoding complex. J. Theor. Biol. 2006;242:564–580. doi: 10.1016/j.jtbi.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Aldinger C.A., Leisinger A.K., Gaston K.W., Limbach P.A., Igloi G.L. The absence of A-to-I editing in the anticodon of plant cytoplasmic tRNA(Arg) ACG demands a relaxation of the wobble decoding rules. RNA Biol. 2012;9:1239–1246. doi: 10.4161/rna.21839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantara W.A., Bilbille Y., Kim J., Kaiser R., Leszczynska G., Malkiewicz A., Agris P.F. Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNA(Arg1,2) J. Mol. Biol. 2012;416:579–597. doi: 10.1016/j.jmb.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 27.Das G., Lyngdoh R.H. Role of wobble base pair geometry for codon degeneracy: Purine-type bases at the anticodon wobble position. J. Mol. Model. 2012;18:3805–3820. doi: 10.1007/s00894-012-1385-4. [DOI] [PubMed] [Google Scholar]
- 28.Westhof E. Isostericity and tautomerism of base pairs in nucleic acids. FEBS Lett. 2014;588:2464–2469. doi: 10.1016/j.febslet.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 29.Westhof E., Yusupov M., Yusupova G. Recognition of Watson-Crick base pairs: Constraints and limits due to geometric selection and tautomerism. F1000Prime Rep. 2014;6:19. doi: 10.12703/P6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K., Osawa S. tRNA Sequences and Variations in the Genetic Code. In: Söll D.R., RajBhandary U.L., editors. tRNA. American Society for Microbiology; Washington, DC, USA: 1995. pp. 225–250. [DOI] [Google Scholar]
- 31.Yokobori S., Kitamura A., Grosjean H., Bessho Y. Life without tRNAArg-adenosine deaminase TadA: Evolutionary consequences of decoding the four CGN codons as arginine in Mycoplasmas and other Mollicutes. Nucleic Acids Res. 2013;41:6531–6543. doi: 10.1093/nar/gkt356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igloi G.L., Aldinger C.A. Where have all the inosines gone? Conflicting evidence for A-to-I editing of the anticodon of higher eukaryotic tRNAACGArg questions the dogma of a universal wobble-mediated decoding of CGN codons. IUBMB Life. 2016;68:419–422. doi: 10.1002/iub.1497. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki Y., Niki H., Kato J. Profiling of Escherichia coli chromosome database. Methods Mol. Biol. 2008;416:385–389. doi: 10.1007/978-1-59745-321-9_26. [DOI] [PubMed] [Google Scholar]
- 34.Wolf J., Gerber A.P., Keller W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J. 2002;21:3841–3851. doi: 10.1093/emboj/cdf362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogasawara N. Systematic function analysis of Bacillus subtilis genes. Res. Microbiol. 2000;151:129–134. doi: 10.1016/S0923-2508(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi K., Ehrlich S.D., Albertini A., Amati G., Andersen K.K., Arnaud M., Asai K., Ashikaga S., Aymerich S., Bessieres P., et al. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo B.M., Kritikos G., Farelli J.D., Todor H., Tong K., Kimsey K., Wapinski I., Galardini M., Cabal A., Peters J.M., et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 2016;12:13. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soma A., Ikeuchi Y., Kanemasa S., Kobayashi K., Ogasawara N., Ote T., Kato J., Watanabe K., Sekine Y., Suzuki T. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell. 2003;12:689–698. doi: 10.1016/S1097-2765(03)00346-0. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko T., Suzuki T., Kapushoc S.T., Rubio M.A., Ghazvini J., Watanabe K., Simpson L. Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: Implication for tRNA sorting mechanism. EMBO J. 2003;22:657–667. doi: 10.1093/emboj/cdg066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Leighton T.J., Doi R.H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J. Biol. Chem. 1971;25:3189–3195. doi: 10.1016/S0021-9258(18)62213-6. [DOI] [PubMed] [Google Scholar]
- 42.Anagnostopoulos C., Spizizen J. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soma A., Onodera A., Sugahara J., Kanai A., Yachie N., Tomita M., Kawamura F., Sekine Y. Permuted tRNA genes expressed via a circular RNA intermediate in Cyanidioschyzon merolae. Science. 2007;318:450–453. doi: 10.1126/science.1145718. [DOI] [PubMed] [Google Scholar]
- 44.Casadaban M.J., Martinez-Arias A., Shapira S.K., Chou J. β−galactosidase gene fusions for analyzing gene expression in Escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto T., Loh P.C., Hirai T., Asai K., Kobayashi K., Moriya S., Ogasawara N. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology. 2002;148:3539–3552. doi: 10.1099/00221287-148-11-3539. [DOI] [PubMed] [Google Scholar]
- 46.Miller J.H. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. 4th ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 1992. [Google Scholar]
- 47.Curran J.F., Yarus M. Base substitutions in the tRNA anticodon arm do not degrade the accuracy of reading frame maintenance. Proc. Natl. Acad. Sci. USA. 1988;83:6538–6542. doi: 10.1073/pnas.83.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curran J.F. Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res. 1993;21:1837–1843. doi: 10.1093/nar/21.8.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Errington J. Bacillus subtilis sporulation: Regulation of gene expression and control of morphogenesis. Microbiol. Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stragier P., Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 51.Schultz D., Wolynes P.G., Ben Jacob E., Onuchic J.N. Deciding fate in adverse times: Sporulation and competence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2009;106:21027–21034. doi: 10.1073/pnas.0912185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galperin M.Y., Mekhedov S.L., Puigbo P., Smirnov S., Wolf Y.I., Rigden D.J. Genomic determinants of sporulation in Bacilli and Clostridia: Towards the minimal set of sporulation-specific genes. Environ. Microbiol. 2012;14:2870–2890. doi: 10.1111/j.1462-2920.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta R., Gupta N. Fundamentals of Bacterial Physiology and Metabolism. Springer, Singapore; Tannery Lane, Singapore: 2021. Competence and Sporulation in Bacillus subtilis; pp. 653–670. [DOI] [Google Scholar]
- 54.Ogasawara N. Markedly unbiased codon usage in Bacillus subtilis. Gene. 1985;40:145–150. doi: 10.1016/0378-1119(85)90035-6. [DOI] [PubMed] [Google Scholar]
- 55.Kanaya S., Yamada Y., Kudo Y., Ikemura T. Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: Gene expression level and species-specific diversity of codon usage based on multivariate analysis. Gene. 1999;238:143–155. doi: 10.1016/S0378-1119(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 56.Kong J.H., Dubnau D. The regulation of competence transcription factor synthesis constitutes a critical control point in the regulation of competence in Bacillus subtilis. J. Bacteriol. 1994;176:5753–5761. doi: 10.1128/jb.176.18.5753-5761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iost I., Dreyfus M. The stability of Escherichia coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 1995;14:3252–3261. doi: 10.1002/j.1460-2075.1995.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharp J.S., Bechhofer D.H. Effect of translational signals on mRNA decay in Bacillus subtilis. J. Bacteriol. 2003;185:5372–5379. doi: 10.1128/JB.185.18.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duviau M.P., Chen F., Emile A., Cocaign-Bousquet M., Girbal L., Nouaille S. When translation elongation is impaired, the mRNA is uniformly destabilized by the RNA degradosome, while the concentration of mRNA is altered along the molecule. Nucleic Acids Res. 2023;51:2877–2890. doi: 10.1093/nar/gkad104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oba T., Andachi Y., Muto A., Osawa S. CGG: An unassigned or nonsense codon in Mycoplasma capricolum. Proc. Natl. Acad. Sci. USA. 1991;88:921–925. doi: 10.1073/pnas.88.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delannoy E., Le Ret M., Faivre-Nitschke E., Estavillo G.M., Bergdoll M., Taylor N.L., Pogson B.J., Small I., Imbault P., Gualberto J.M. Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation. Plant Cell. 2009;21:2058–2071. doi: 10.1105/tpc.109.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogalski M., Karcher D., Bock R. Superwobbling facilitates translation with reduced tRNA sets. Nat. Struct. Mol. Biol. 2008;15:192–198. doi: 10.1038/nsmb.1370. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian K., Payne B., Feyertag F., Alvarez-Ponce D. The codon statistics database: A database of codon usage bias. Mol. Biol. Evol. 2022;39:msac157. doi: 10.1093/molbev/msac157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alkatib S., Scharf L.B., Rogalski M., Fleischmann T., Matthes A., Seeger S., Schöttler M.A., Ruf S., Bock R. The contributions of wobbling and superwobbling to the reading of the genetic code. PLoS Genet. 2012;8:e1003076. doi: 10.1371/journal.pgen.1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yarus M. Crick wobble and superwobble in standard genetic code evolution. J. Mol. Evol. 2021;89:50–61. doi: 10.1007/s00239-020-09985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lei L., Burton Z.F. “Superwobbling” and tRNA-34 wobble and tRNA-37 anticodon loop modifications in evolution and devolution of the genetic code. Life. 2022;12:252. doi: 10.3390/life12020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karijolich J., Yu Y.T. Modifying the genetic code: Converting nonsense codons into sense codons by targeted pseudouridylation. Nature. 2011;474:395–398. doi: 10.1038/nature10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamada Y., Matsugi J., Ishikura H., Murao K. Bacillus subtilis tRNA(Pro) with the anticodon mo5UGG can recognize the codon CCC. Biochim. Biophys. Acta. 2005;1728:143–149. doi: 10.1016/j.bbaexp.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Ogle J.M., Ramakrishnan V. Structural insights into translational fidelity. Annu. Rev. Biochem. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- 70.Demeshkina N., Jenner L., Westhof E., Yusupov M., Yusupova G. A new understanding of the decoding principle on the ribosome. Nature. 2012;484:256–259. doi: 10.1038/nature10913. [DOI] [PubMed] [Google Scholar]
- 71.Rozov A., Demeshkina N., Khusainov I., Westhof E., Yusupov M., Yusupova G. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 2016;7:10457. doi: 10.1038/ncomms10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rozov A., Demeshkina N., Westhof E., Yusupov M., Yusupova G. New structural insights into translational miscoding. Trends Biochem. Sci. 2016;41:798–814. doi: 10.1016/j.tibs.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Hoernes T.P., Faserl K., Juen M.A., Kremser J., Gasser C., Fuchs E., Shi X., Siewert A., Lindner H., Kreutz C., et al. Translation of non-standard codon nucleotides reveals minimal requirements for codon-anticodon interactions. Nat. Commun. 2018;9:4865. doi: 10.1038/s41467-018-07321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ou X., Cao J., Cheng A., Peppelenbosch M.P., Pan Q. Errors in translational decoding: tRNA wobbling or misincorporation? PLoS Genet. 2019;15:e1008017. doi: 10.1371/journal.pgen.1008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Claesson C., Lustig F., Boren T., Simonsson C., Barciszewska M., Lagerkvist U. Glycine codon discrimination and the nucleotide in position 32 of the anticodon loop. J. Mol. Biol. 1995;247:191–196. doi: 10.1006/jmbi.1994.0132. [DOI] [PubMed] [Google Scholar]
- 76.Li J., Esberg B., Curran J.F., Bjork G.R. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 1997;271:209–221. doi: 10.1006/jmbi.1997.1176. [DOI] [PubMed] [Google Scholar]
- 77.Olejniczak M., Uhlenbeck O.C. tRNA residues that have coevolved with their anticodon to ensure uniform and accurate codon recognition. Biochimie. 2006;88:943–950. doi: 10.1016/j.biochi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J. Mol. Biol. 1971;58:439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- 79.Cochella L., Green R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science. 2005;308:1178–1180. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmeing T.M., Voorhees R.M., Kelley A.C., Ramakrishnan V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat. Struct. Mol. Biol. 2011;18:432–436. doi: 10.1038/nsmb.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephen P., Ye S., Zhou M., Song J., Zhang R., Wang E.D., Giegé R., Lin S.X. Structure of Escherichia coli Arginyl-tRNA synthetase in complex with tRNAArg: Pivotal role of the D-loop. J. Mol. Biol. 2018;430:1590–1606. doi: 10.1016/j.jmb.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 82.Fabret C., Dervyn E., Dalmais B., Guillot A., Marck C., Grosjean H., Noirot P. Life without the essential bacterial tRNA Ile2-lysidine synthetase TilS: A case of tRNA gene recruitment in Bacillus subtilis. Mol. Microbiol. 2011;80:1062–1074. doi: 10.1111/j.1365-2958.2011.07630.x. [DOI] [PubMed] [Google Scholar]
- 83.Köhrer C., Mandal D., Gaston K.W., Grosjean H., Limbach P.A., Rajbhandary U.L. Life without tRNAIle-lysidine synthetase: Translation of the isoleucine codon AUA in Bacillus subtilis lacking the canonical tRNA2Ile. Nucleic Acids Res. 2014;42:1904–1915. doi: 10.1093/nar/gkt1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taniguchi T., Miyauchi K., Nakane D., Miyata M., Muto A., Nishimura S., Suzuki T. Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res. 2013;41:2621–2631. doi: 10.1093/nar/gks1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murayama R., Akanuma G., Makino Y., Nanamiya H., Kawamura F. Spontaneous transformation and its use for genetic mapping in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2004;68:1672–1680. doi: 10.1271/bbb.68.1672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.