Abstract
Sepsis is a serious disease with high mortality and has been a hot research topic in medical research in recent years. With the continuous reporting of in-depth research on the pathological mechanisms of sepsis, various compounds have been developed to prevent and treat sepsis. Natural small-molecule compounds play vital roles in the prevention and treatment of sepsis; for example, compounds such as resveratrol, emodin, salidroside, ginsenoside, and others can modulate signaling through the NF-κB, STAT3, STAT1, PI3K, and other pathways to relieve the inflammatory response, immunosuppression, and organ failure caused by sepsis. Here, we discuss the functions and mechanisms of natural small-molecule compounds in preventing and treating sepsis. This review will lay the theoretical foundation for discovering new natural small-molecule compounds that can potentially prevent and treat sepsis.
Keywords: sepsis, small molecule compounds, inflammatory response, organ failure
1. Introduction
Sepsis is a serious disease with relatively high morbidity and mortality rates, involving life-threatening organ dysfunction caused by the host’s abnormal response to infection [1]. With the delisting of Eli Lilly’s (Indianapolis, IN, USA) Xigris in 2011, there is currently an absence of a specific pharmaceutical intervention for the management of sepsis; therefore, identifying effective drugs for the prevention and treatment of sepsis has become a research hotspot.
The pathogenesis of sepsis is complex. The initial acute response of the host to an invasive pathogen activates macrophages and produces a series of cytokines (pro-inflammatory and anti-inflammatory factors) that trigger apoptosis, necroptosis, and pyroptosis and activate damage-associated molecular patterns or pathogen-associated molecular patterns [2], which in turn trigger a cytokine storm [3]. A major change in the levels of cytokines in the body can cause damage to multiple organs, including the kidneys [4], lungs [5], liver [6], and heart [7], and eventually affect the immune function of the body and cause immune disorders. Inflammatory responses and immunosuppression occur sequentially during sepsis [8], and severe inflammatory responses in the body can lead to coagulation disorders [9]. Therefore, reducing inflammation, immunosuppression, and coagulation disorders are key parts of sepsis treatment. The drugs developed to regulate inflammatory responses include cytokine antagonists, pattern-recognition receptor inhibitors, recombinant human APC (Activated Protein C), and recombinant human soluble thrombosis regulators for regulating the blood coagulation system. Additionally, several anti-immunosuppressive drugs, such as cytokines (granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor) and co-repressor molecule inhibitors, have been developed [10]. In addition to these drugs, this review will also focus on some natural medicinal substances, such as kombucha [11] and black mulberry [12], which exert antioxidant and anti-inflammatory effects in sepsis models and improve the immunity and survival rates of septic mice. Other small-molecule compounds, such as Fucoxanthin [13] and bis-N-norgliovictin [14], have also been reported to improve survival by reducing inflammation levels.
Natural small-molecule compounds (usually compounds with a molecular weight < 1000 Da) have attracted wide attention in drug research because of their characteristics and the advantages of rapid diffusion into cells to reach the target. Drugs can broadly be divided into several categories based on their structure and properties, such as polyphenols, anthraquinones, glycosides, flavonoids, and biogenic amines. Through a thorough investigation of the literature, this paper summarizes and discusses the functions and mechanisms of natural small-molecule compounds in preventing and treating sepsis.
2. Polyphenol Compounds
Polyphenols are compounds with multiple phenolic groups. Polyphenols can inhibit the activity of nuclear factor kappa-B (NF-κB), which in turn inhibits cell proliferation, angiogenesis, and metastasis and promotes apoptosis [15]. Currently, the main small-molecule polyphenols used for treating sepsis are resveratrol, curcumin, and tetrahydrocurcumin.
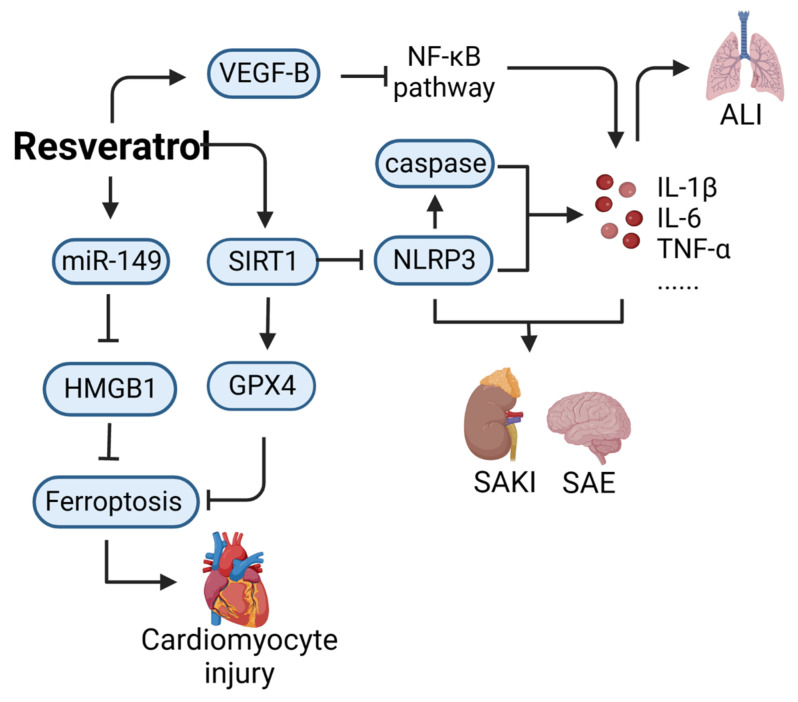
Resveratrol (CAS: 501-36-0) is a non-flavonoid polyphenol compound with anti-inflammatory, antiviral, antibacterial, and antitumor properties [16]. Thus far, research on the use of resveratrol for treating sepsis has mostly involved animal experiments; this compound has not yet entered the stage of clinical research regarding sepsis treatment. As shown in Figure 1, resveratrol can mitigate the acute kidney injury induced by sepsis. Sirtuin1 (SIRT1) is an NAD-dependent protein deacetylase, which is considered the main regulator of sepsis-induced acute kidney injury because it reduces oxidative stress and inflammation [17]. Activation of SIRT1 can inhibit the inflammatory response and oxidative stress. Resveratrol, as an SIRT1 activator, alleviates acute kidney injury in cecal ligation and puncture (CLP) septic mice by activating SIRT1 to promote deacetyl-mediated autophagy [18]. Resveratrol can also alleviate damage to other organs. For instance, it ameliorates the cardiomyocyte injury induced by lipopolysaccharide (LPS) by upregulating miR-149 and downregulating high mobility group protein B1 (HMGB1) [19]. Resveratrol also improves sepsis-associated encephalopathy by inhibiting the expression of the nucleotide-binding oligomerization domain, leucine-rich repeat, NOD-like receptor protein 3 (NLRP3), and interleukin-1β (IL-1β) [20] and can activate vascular endothelial growth factor-B (VEGF-B) expression and inhibit the NF-κB pathway to alleviate sepsis-induced acute lung injury [21]. However, high doses of resveratrol can increase intracellular oxidation, enhance mitochondrial membrane depolarization, and induce endothelial cell death [22].
Figure 1.
Action mechanism of resveratrol in the treatment of sepsis. ALI, acute lung injury; SAKI, sepsis-induced acute kidney injury; SAE, sepsis-associated encephalopathy; VEGF-B, vascular endothelial growth factor-B; SIRT1, Sirtuin1; NLRP3, NOD-like receptor protein 3; HMGB1, high mobility group protein B1; GPX4, glutathione Peroxidase 4.
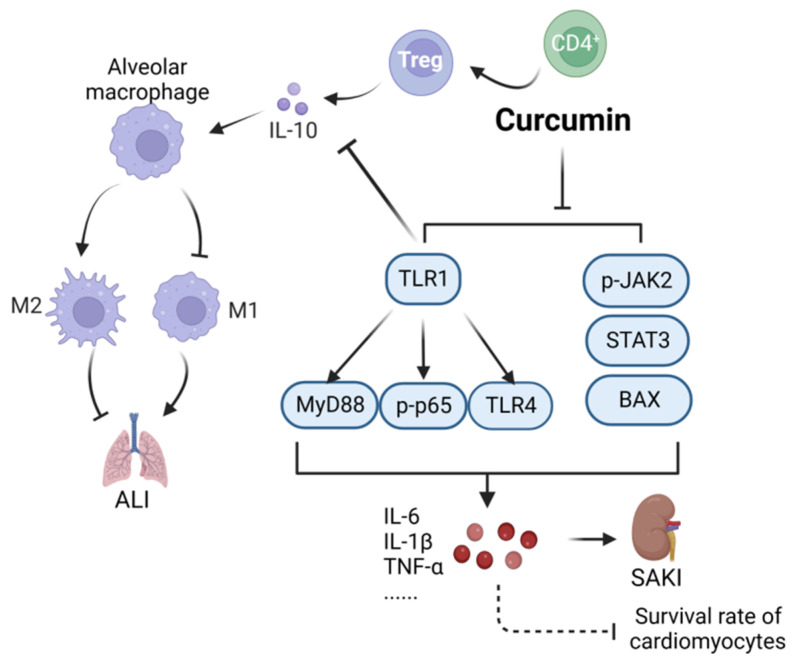
Curcumin (CAS: 458-37-7) is a natural polyphenol compound with anti-inflammatory, anti-infection, and other biological properties [23]. As shown in Figure 2, curcumin alleviates lung injury in CLP model mice by regulating the differentiation of CD4+ T cells into Tregs, promoting the transformation of macrophages, and exerting anti-apoptosis, anti-inflammatory, and immunoregulatory effects [24]. Curcumin can also downregulate Toll-like receptor 1 (TLR1), inhibit the phosphorylation of NF-κB, and improve the survival rate of cardiomyocytes treated with lipopolysaccharides (LPS) [25]. Curcumin has also been reported to inhibit NF-κB and janus kinase2 (JAK2)/signal transducer and activator of Transcription 3 (STAT3) signaling and the expression of p-JAK2/STAT3, p-p65, and BCL2-Associated X (BAX) in mice with acute kidney injury to alleviate septic acute kidney injury effectively in CLP mouse models [26]. Curcuma longa extract is rich in turmeric. The main compound present in turmeric is β-turmerone (CAS: 19693-54-0). In a previous study, treatment with Curcuma longa extract had an anti-inflammatory effect and reduced the production of NO in an inflammation model induced by LPS [27]. In another study, targeted inhibition of toll-likereceptor4(TLR4) mediated downstream information transmission, thereby effectively preventing brain injury caused by neuroinflammation in LPS model mice [28].
Figure 2.
Action mechanism of curcumin in the treatment of sepsis. ALI, acute lung injury; SAKI, sepsis-induced acute kidney injury; MyD88, myeloid differentiation factor 88; BAX, BCL2-Associated X; STAT3, Signal Transducer and Activator of Transcription 3. Solid line: direct action; Dashed line: indirect action.
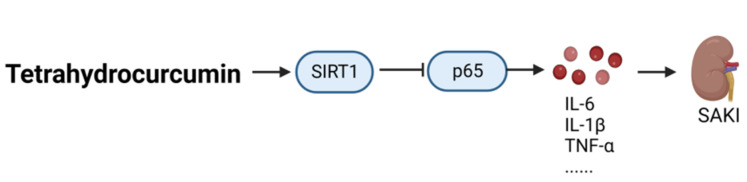
After hydrogenation of curcumin, tetrahydrocurcumin (CAS: 36062-04-1) is obtained, which contains fewer unsaturated C double bonds in its structure; thus, tetrahydrocurcumin has higher stability, stronger antioxidation effects, and higher bioavailability than curcumin. As shown in Figure 3, tetrahydrocurcumin significantly increased the expression of SIRT1 and inhibited inflammation and oxidative stress, thereby preventing sepsis-induced acute kidney injury in a CLP mouse model [29].
Figure 3.
Therapeutic mechanism of tetrahydrocurcumin in the management of sepsis. SAKI, sepsis-induced acute kidney injury; SIRT1, Sirtuin1.
3. Anthraquinone Compounds
Many natural anthraquinones have anticancer, anti-inflammatory, antioxidant, anti-osteoporosis, and other physiological properties [30]. The natural small-molecule anthraquinones used in sepsis treatment include emodin and aloin.
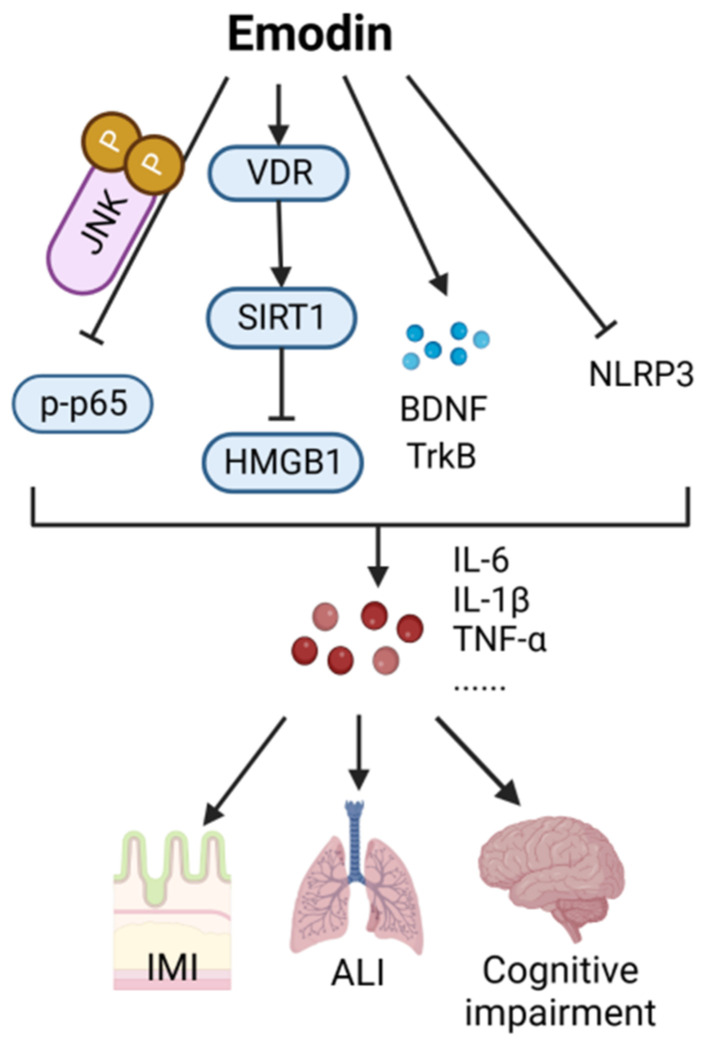
Emodin (CAS: 518-82-1) is a natural anthraquinone compound with numerous pharmacological effects, including anticancer, antiviral, anti-inflammatory, antibacterial, and hepatoprotective activities [31]. Thus far, research on the utility of emodin in preventing and treating sepsis has primarily focused on in vitro cell and animal experiments. Emodin can relieve lung injury, intestinal mucosal barrier injury, and cognitive impairment caused by sepsis through the scorch signaling pathway and the Vitamin D receptor (VDR)/NF-E2-related factor 2 (Nrf2) pathway. As shown in Figure 4, emodin alleviates NLRP3-induced acute lung injury by inhibiting LPS-dependent scorch death signaling [32]. It is known that VDR can activate the SIRT1/Nrf-2 pathway [33]. Emodin inhibits SIRT1-mediated HMGB1 protein expression by increasing the mRNA and protein expression of VDR and its downstream molecules [34], thus alleviating the lung injury caused by sepsis [32]. In addition, emodin can bind to c-Jun N-terminal kinase (JNK2), inhibit the activation of NF-κB signaling [35], induce a protective effect against sepsis-associated intestinal mucosal barrier injury, increase the expression of tyrosine kinase receptor B (TrkB) and brain-derived neurotrophic factor (BDNF), and significantly inhibit the inflammatory response in CLP mice, thereby improving cognitive impairment and reducing pathological damage [36].
Figure 4.
Action mechanism of emodin in the treatment of sepsis. IMI, intestinal mucosal barrier injury; ALI, acute lung injury; VDR, Vitamin D receptor; SIRT1, Sirtuin1; HMGB1, high mobility group protein B1; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B.
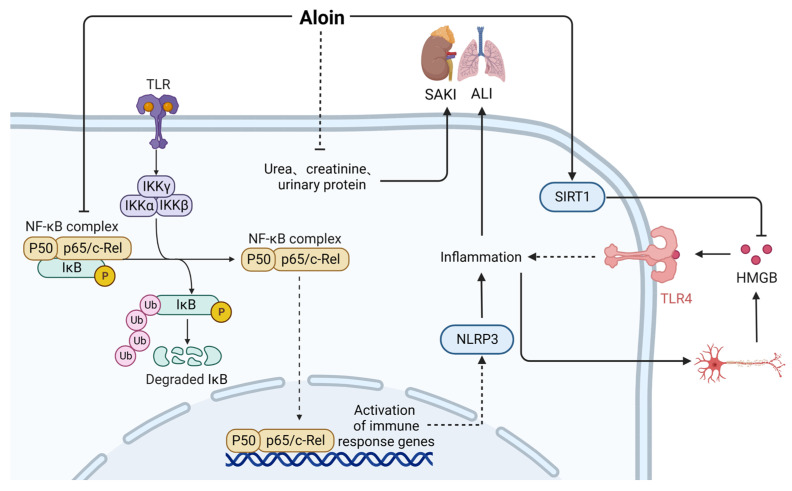
Aloin (CAS: 1415-73-2) is an anthraquinone compound with antitumor, anti-inflammatory, antiosteoporosis, antiviral, antibacterial, and other pharmacological properties [37]. As shown in Figure 5, aloin treatment can alleviate LPS-induced inflammation by inhibiting NF-κB signaling; blocking the phosphorylation, acetylation, and nuclear transport of p65 subunits; and downregulating stress-related genes [38,39]. At the same time, aloin significantly inhibits the activation of NLRP3 inflammatory bodies to improve LPS-induced acute lung injury and increase the expression of SIRT1 [40]. In LPS cell models and CLP-induced sepsis mouse models, deacetylation of HMGB1 achieved by activating SIRT1 reduces the release of HMGB1 and sepsis-related mortality [41]. Treatment with aloin has been shown to significantly reduce the levels of harmful renal functional substances, such as urea, creatinine, and urinary protein, and protect mice from sepsis-induced acute kidney injury [42]. Therefore, aloin, as a small-molecule drug, is effective in alleviating sepsis-induced acute kidney and lung injury.
Figure 5.
Action mechanism of aloin in the treatment of sepsis. SAKI, sepsis-induced acute kidney injury; ALI, acute lung injury; SIRT1, Sirtuin1; Solid line: direct action; Dashed line: indirect action.
4. Glucoside Compounds
Glycosides connect the end-group carbon atoms of sugars or sugar derivatives with non-sugar substances. Currently, the main glycosides used for treating sepsis are salidroside and geniposide.
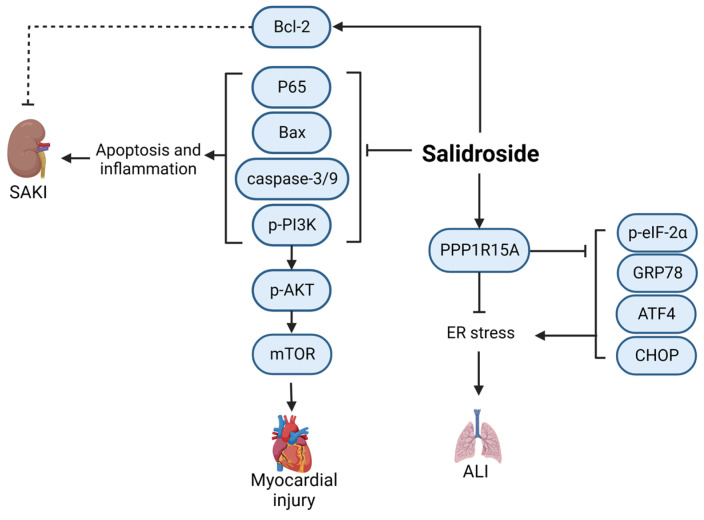
Salidroside (CAS: 10338-51-9) is a constituent of herbal Rhodiola rosea L., extensively employed in the adjunctive therapy of cardiovascular and cerebrovascular disorders and certain neoplasms [43]. The use of salidroside in sepsis treatment is still being investigated through in vivo and in vitro experiments, and the drug has not yet entered clinical research. As shown in Figure 6, salidroside inhibits the production of caspase-3/9 by upregulating Bcl-2 and downregulating Bax, thereby inhibiting the phosphorylation of Phosphoinositide-3 kinase (PI3K) and AKT and reducing the levels of pro-inflammatory cytokines and apoptosis [44]. Salidroside can significantly reduce the expression of p65 in kidney tissue, reduce the levels of pro-inflammatory factors in the plasma and kidney, and alleviate sepsis-induced acute kidney injury in CLP models. It has also been shown to significantly reduce the mortality of septic rats [45]. Salidroside can enhance the expression of PPP1R15A and downregulate endoplasmic reticulum stress-related proteins, thereby inhibiting endoplasmic reticulum stress and improving lung injury in septic mice [46]. Salidroside can also inhibit the phosphorylation of NF-κB and PI3K/AKT/mTOR, significantly reduce the expression levels of ROS, CAT, SOD, GSH-px, TNF-α, IL-6, and IL-1β in cells, and have obvious cardioprotective effects on LPS-treated rats [47]. Thus, further investigation of the clinical applications of salidroside will be beneficial.
Figure 6.
Mechanism of salidroside in the treatment of sepsis. ALI, acute lung injury; SAKI, sepsis-induced acute kidney injury; BAX, BCL2-Associated X; Bcl-2, B-cell lymphoma-2; mTOR, mammalian target of rapamycin; GRP78, glucose-regulated protein 78; ATF4, activating transcription factor 4; CHOP, C/EBP-homologous protein; Solid line: direct action; Dashed line: indirect action.
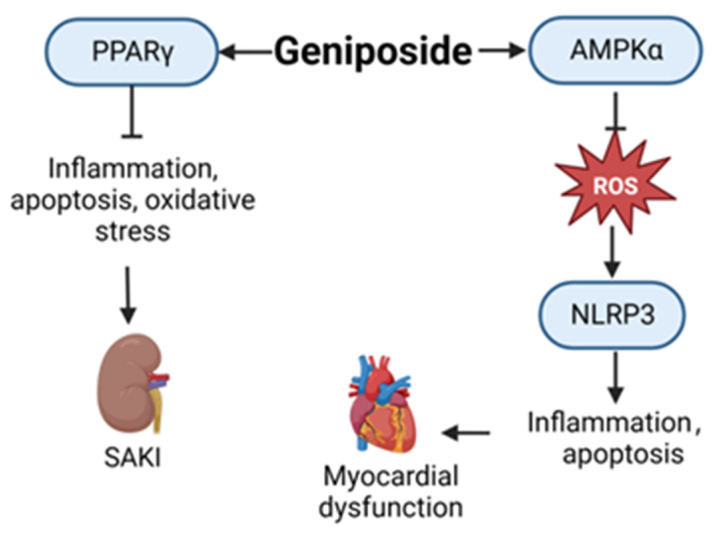
As a new iridoid glycoside, geniposide (CAS: 24512-63-8) has many biological activities, such as anti-inflammation, anti-oxidation, and anti-apoptosis properties [48]. As shown in Figure 7, in a mouse model of septic myocardial dysfunction induced by LPS, geniposide can activate AMPKα and inhibit myocardial reactive oxygen species (ROS) production, block NLRP3-mediated cardiomyocyte apoptosis and pyrolysis, and improve septic-induced myocardial dysfunction [49]. Additionally, in LPS-induced cell and CLP-induced sepsis mouse models, geniposide significantly inhibits the inflammatory response, apoptosis, oxidative stress, and vascular permeability associated with sepsis-induced acute kidney injury by activating Peroxisome proliferator-activated receptor γ(PPARγ) [50]. There are few reports of the use of geniposide in sepsis treatment, and its specific mechanism in this context requires further study.
Figure 7.
Action mechanism of geniposide in the treatment of sepsis. SAKI, sepsis-induced acute kidney injury; ROS, reactive oxygen species; PPARγ, Peroxisome proliferator-activated receptor γ.
5. Sterol Compounds
Sterols, also known as steroids, are lipid compounds. Currently, ginsenosides are the main steroids used for sepsis treatment. They are used in LPS- and CLP-induced sepsis models.
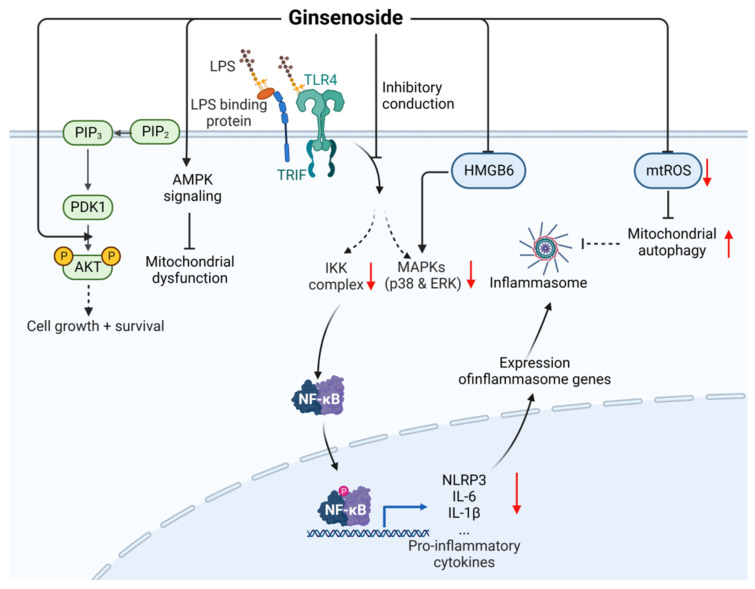
Ginsenosides are also known as triterpenoid saponins and are divided into many categories. Among them are Rb1 (CAS: 41753-43-9) [51], Rb3 (CAS: 68406-26-8) [52,53], Rd (CAS: 52705-93-8) [54], Re (CAS: 52286-59-6) [55], Rg1 (CAS: 22427-390) [56,57], Rg5 (CAS: 186763-78-0) [58], Rg6 (CAS: 147419-93-0) [59], and Rh1 (CAS: 63223-86-9) [60] reduce the expression of pro-inflammatory cytokines through the TLR4/NF-κB/MAPK signaling pathway to reduce inflammation and organ damage and improve the survival rate.
Furthermore, as shown in Figure 8, Rh1 can potentially attenuate the activation of TNF-α and IL-6 mediated by HMGB6 [61] and minimize tissue damage. Additionally, Rh1 and Rg2 can inhibit the production of mitochondrial reactive oxygen species (mtROS) [62], and Rg3 can activate the AMPK signaling pathway to promote mitochondrial autophagy, maintain mitochondrial homeostasis, and alleviate the subsequent inflammatory response, thereby reducing the cell and organ damage caused by sepsis and increasing the survival rate of septic rats [63]. Rg4 can activate PI3K/p-AKT signal transduction, inhibit the septic kidney inflammation induced by CLP, and improve survival [64]. In clinical treatment, the combination of total ginsenosides and ulinastatin has been shown to be effective against septic acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [65]. Thus, ginsenosides have great potential for the prevention and treatment of sepsis.
Figure 8.
Mechanism underlying the therapeutic effects of ginsenoside against sepsis. LPS, lipopolysaccharides; HMGB6, high mobility group protein B6; mtROS, mitochondrial reactive oxygen species; Solid line: direct action; Dashed line: indirect action.
6. Flavonoid Compounds
Flavonoids are compounds resulting from the linkage of two benzene rings with three carbon atoms. The main flavonoids used in sepsis treatment are breviscapine, baicalein, and diosmtin.
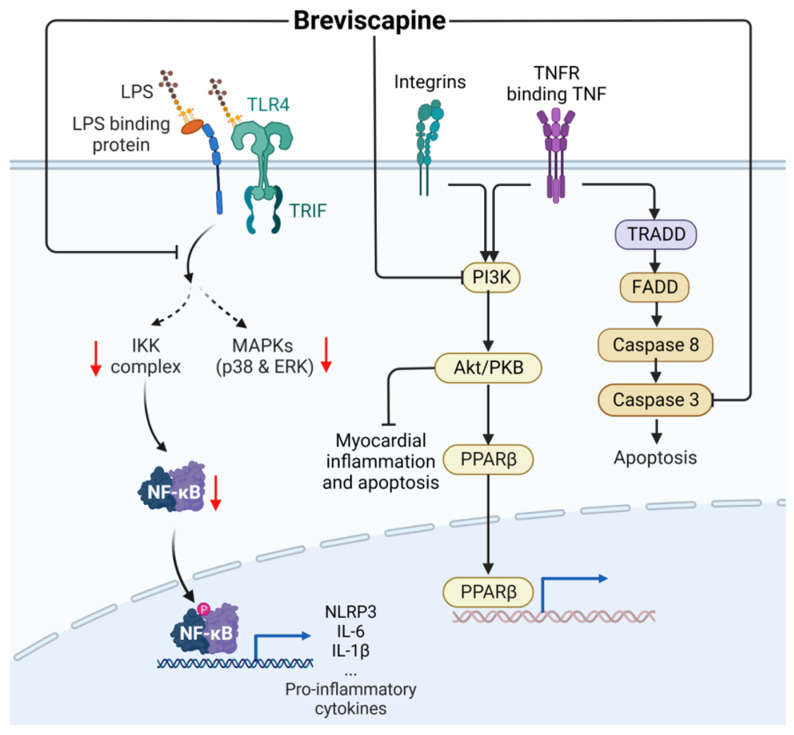
As shown in Figure 9, breviscapine (BS, CAS: 116122-36-2) inhibits the overactivation of the TLR4/NF-κB, caspase-3/PARP, and MAPK signaling pathways, thereby inhibiting the expression of proinflammatory cytokines and chemokines [66]. Breviscapine can also regulate the PI3K/Akt/glycogen synthase kinase-3β (GSK-3β) pathway and inhibit myocardial inflammation and apoptosis of coronary microembolization (CME) to achieve cardiac protection [67].
Figure 9.
Action mechanism of breviscapine in the treatment of sepsis. LPS, lipopolysaccharides; TRADD, TNF receptor-associated death domain; FADD, fas-associated protein with death domain; PPARβ, Peroxisome proliferator-activated receptor β. Solid line: direct action; Dashed line: indirect action.
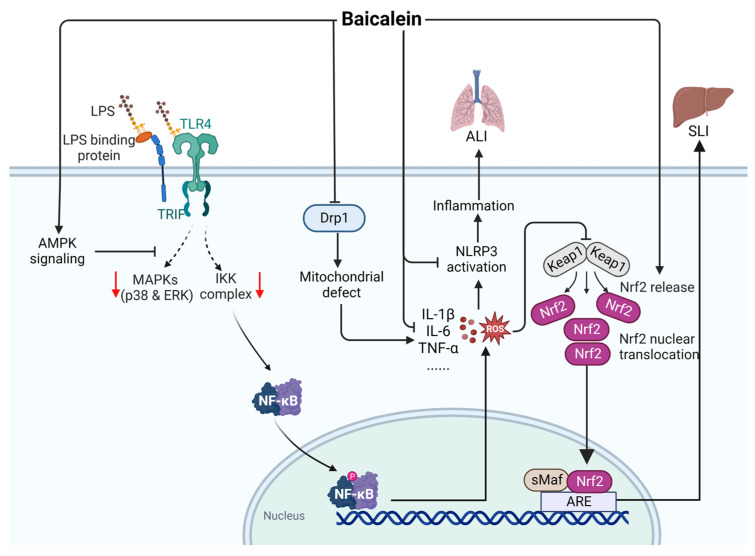
Baicalein (CAS: 491-67-8) is an important component extracted from Scutellaria baicalensis. The use of baicalein in the treatment of sepsis has not yet been investigated clinically. As shown in Figure 10, baicalein can activate the AMPK pathway and inhibit downstream MAPK/NF-κB signal transduction and chemokines to inhibit the expression of ROS [68,69], thus inhibiting the activation of NLRP3 inflammatory bodies [70]. Baicalein inhibits the expression of dynamic protein-associated protein 1 (Drp1), reduces the levels of ROS, and reduces the production of TNF-α, MIP-1, and IL-6 to inhibit LPS-induced acute lung injury [71]. Moreover, baicalein can improve the sepsis-induced liver injury induced by LPS and CLP in septic mice by activating Nrf2 signaling in hepatocytes, which regulates antioxidation and pro-inflammatory signal transduction [72]. The above-mentioned findings suggest that baicalein may be a candidate drug for treating sepsis.
Figure 10.
Action mechanism of baicalein in the treatment of sepsis. ALI, acute lung injury; SLI, sepsis-induced liver injury; LPS, lipopolysaccharides; Drp1, dynamic protein-associated protein 1; ROS, reactive oxygen species; Keap1, kelch like ECH associated protein 1; Nrf2, NF-E2-related factor 2; ARE, AU-rich element; sMaF, synthetic music mobile application format. Solid line: direct action; Dashed line: indirect action.
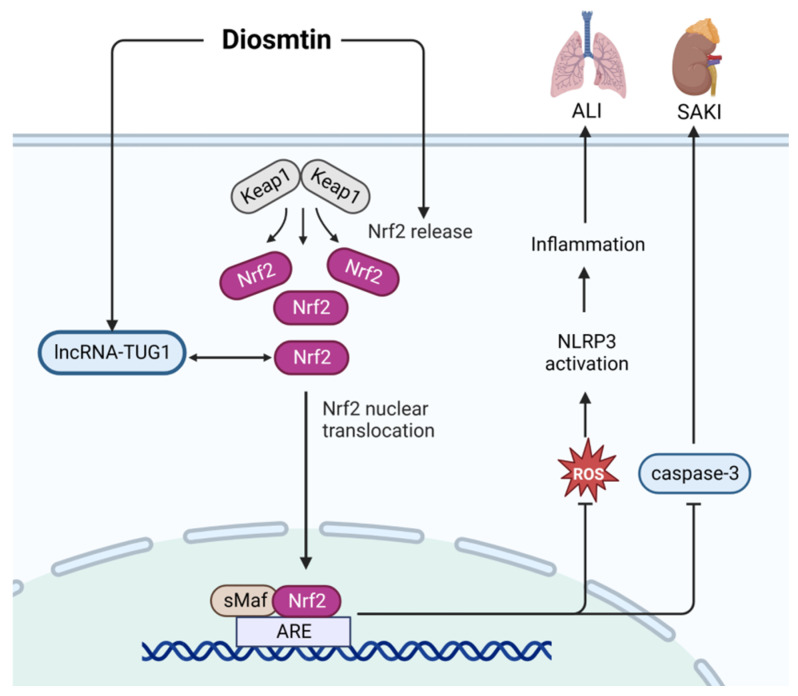
Diosmtin (Dio, CAS: 520-27-4) has anti-inflammation, anti-oxidation, and anti-apoptosis properties [73]. It is still being studied in the laboratory as a treatment for sepsis. As shown in Figure 11, in an LPS-induced cell model, Dio can alleviate sepsis-induced acute kidney injury by enhancing the activity of the Nrf2 pathway, increasing the expression of lncRNA-TUG1, and inhibiting the expression of caspase-3 [74]. In addition, vanilla lignin can activate Nrf2 signaling to clear ROS and inhibit the activation of NLRP3 to limit the development of inflammation, which can alleviate LPS-induced acute lung injury [75].
Figure 11.
Action mechanism of diosmtin in the treatment of sepsis. ALI, acute lung injury; SAKI, sepsis-induced acute kidney injury; TUG1, Taurine Up-Regulated 1; Keap1, kelch like ECH associated protein 1; Nrf2, NF-E2-related factor 2; ARE, AU-rich element; sMaF, synthetic music mobile application format; ROS, reactive oxygen species. Solid line: direct action; Dashed line: indirect action.
7. Biogenic Amines
Biogenic amines, which are organic compounds with low molecular weights, possess biological activity and have a high nitrogen content.
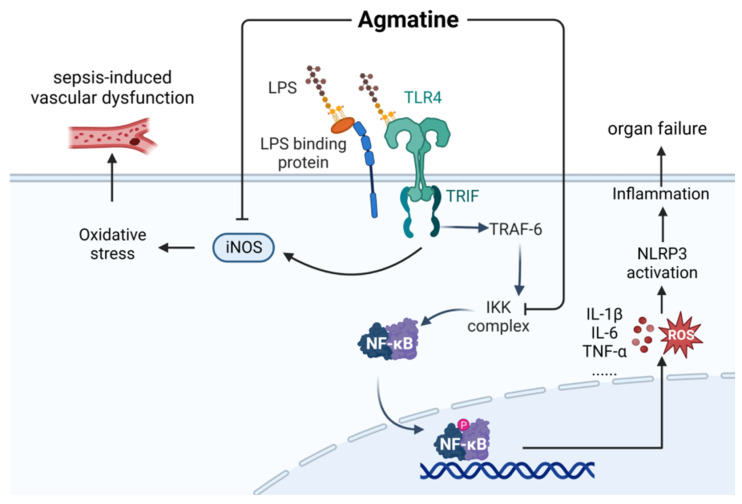
Agmatine (AGM, CAS: 306-60-5) is a naturally occurring polyamine synthesized by the enzyme L-arginine decarboxylase within the central nervous system. AGM is broadly distributed within the liver and central nervous system [76]. As shown in Figure 12, agmatine alleviates vascular dysfunction in LPS-treated rats by inhibiting the expression of inducible nitric oxide synthase (iNOS) and oxidative stress [77]. Agmatine can also inhibit the phosphorylation and degradation of IκB, thereby inhibiting the activation of NF-κB signal transduction and reducing systemic inflammation and organ failure in LPS mice [78].
Figure 12.
Mechanism underlying the therapeutic effects of agmatine in the management of sepsis. LPS, lipopolysaccharides; ROS, reactive oxygen species; iNOS, inducible nitric oxide synthase.
8. Alkaloid Compounds
Alkaloids are a class of basic organic compounds containing nitrogen. Most alkaloids have a complex ring structure and a range of biological activities. The main alkaloids used for the prevention and treatment of sepsis are kukoamine B, matrine, anisodamine hydrobromide, berberine, and leonurine.
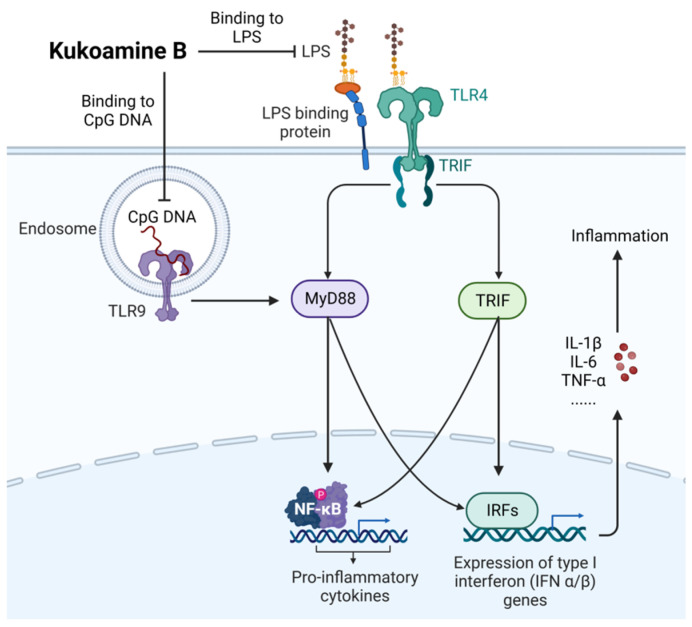
As shown in Figure 13, kukoamine B (CAS: 164991-67-7), as a novel dual inhibitor of LPS and CpG DNA, regulates the downstream signal pathway by directly binding and neutralizing LPS and CpG DNA, thus significantly inhibiting the inflammatory response in LPS-induced septic mice [79]. Wang et al. [80] used an exposure-response model to optimize dose selection in phase IIb clinical trials and recommended a 0.24 mg/kg regimen. A randomized, double-masked, placebo-controlled, multi-dose phase I study also demonstrated that single and multiple intravenous infusions of 0.06–0.24 mg/kg were safe and tolerable in healthy volunteers [81].
Figure 13.
Action mechanism of kukoamine B in the treatment of sepsis. LPS, lipopolysaccharides; MyD88, myeloid differentiation factor 88.
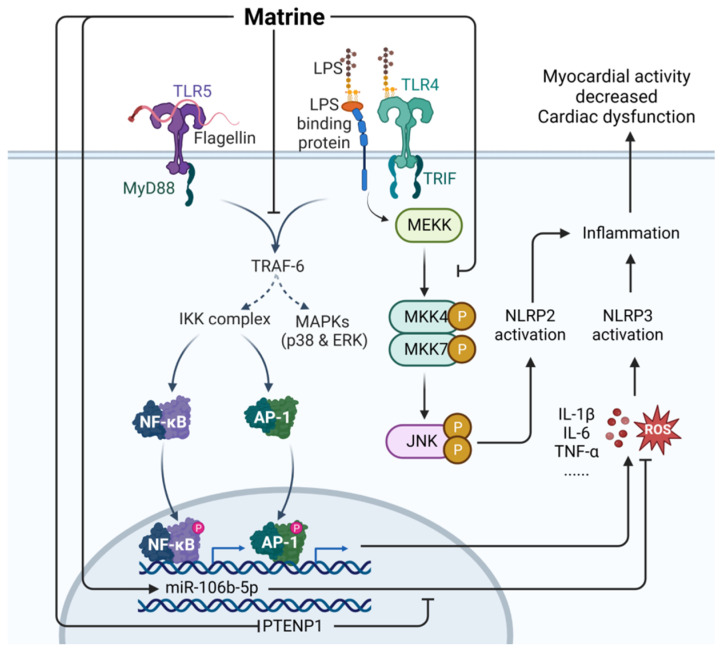
Matrine (CAS: 519-02-8) is the main alkaloid used in traditional Chinese herbal medicine and is extracted from Sophora flavescens (Leguminosae) [82]. Matrine has many biological activities, such as anti-tumor, anti-inflammation, analgesia, anti-fibrosis, anti-viral, and anti-arrhythmia properties, and can enhance immune function [83]. Matrine is still being studied in the laboratory as a sepsis treatment. As shown in Figure 14, matrine has been shown to restore the levels of miR-9 reduced by LPS by inhibiting the JNK and NF-κB pathways, thereby protecting cells from tissue damage induced by LPS [82]. Matrine can inhibit the TLR4/MyD88/NF-κB pathway, NLRP3 inflammatory body activation, and the secretion of proinflammatory cytokines [84] and can regulate the JNK signaling pathway to inhibit the activation of NLRP2 inflammatory bodies, thereby effectively alleviating the symptoms of CLP-induced sepsis in mice [85]. Matrine can also downregulate PTENP1 and upregulate miR-106b-5p to enhance the vitality of cardiac myoblasts and reduce the inflammatory response, thus alleviating the cardiac insufficiency caused by sepsis [86].
Figure 14.
Mechanism of matrine in the treatment of sepsis. LPS, lipopolysaccharides; PTENP1, phosphatase and tensin homolog pseudogene 1; MyD88, myeloid differentiation factor 88; ROS, reactive oxygen species; Solid line: direct action; Dashed line: indirect action.
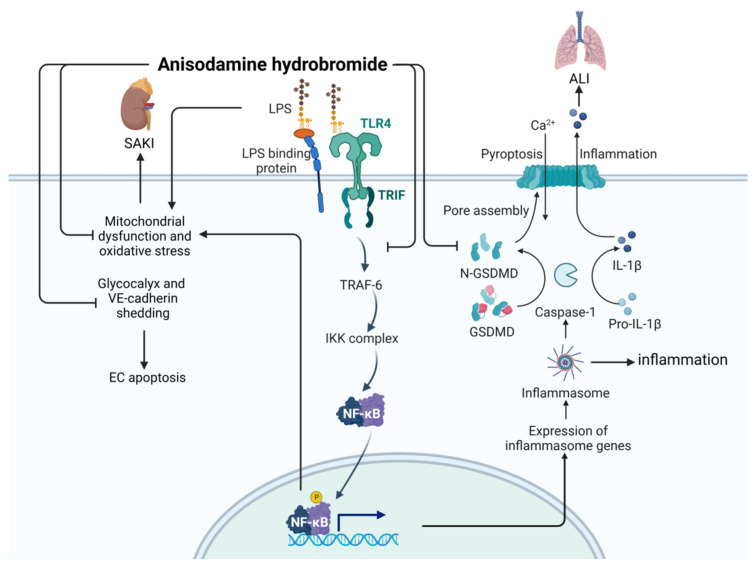
Anisodamine hydrobromide (CAS: 55449-49-5) is an alkaloid first extracted from Scopolia tangutica root in 1956 [87]. Tablets and injections made from anisodamine hydrobromide are often used as anticholinergic drugs in the clinic. As shown in Figure 15, anisodamine hydrobromide inhibits NF-κB signaling, thereby inhibiting LPS-induced apoptosis and inflammation [88], and alleviates LPS-induced acute kidney failure by inhibiting mitochondrial dysfunction and oxidative stress [87]. At the same time, anisodamine hydrobromide can inhibit cell death and apoptosis by inhibiting the gasdermin D (GSDMD) pathway, thereby reducing acute lung injury [89]. In addition, anisodamine hydrobromide can impede the degradation process of vascular endothelial cadherin, thereby preserving the integrity of the vascular endothelial barrier and enhancing microcirculation [90].
Figure 15.
Action mechanism of anisodamine hydrobromide in the treatment of sepsis. ALI, acute lung injury; SAKI, sepsis-induced acute kidney injury; LPS, lipopolysaccharides; GSDMD, gasdermin D.
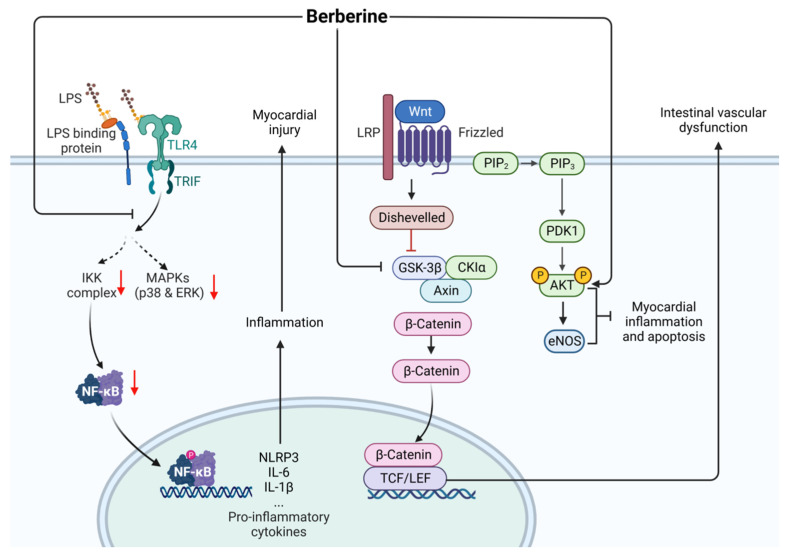
Berberine (CAS: 2086-83-1) is the main bioactive component extracted from the bark of Phellodendron chinensis and Coptis chinensis in traditional Chinese medicine. Clinical adverse events are rarely reported [91]. Berberine has a therapeutic effect against cardiac dysfunction, myocardial injury, and intestinal vascular barrier dysfunction caused by sepsis. As shown in Figure 16, berberine increases the activity of total nitric oxide synthase (NOS) in the heart, increases the protein expression of p-Akt and phosphorylated endothelial NOS, decreases the expression of inflammatory factors such as TNF-α and IL-1β by inhibiting the activation of the TLR4/NF-κB signaling pathway, and alleviates the cardiac dysfunction and myocardial injury caused by sepsis in LPS rat and mouse models [92,93]. In addition, berberine exhibits a protective effect against the intestinal vascular barrier dysfunction induced by sepsis in both LPS cell models and CLP rat models, which is related to berberine-induced downregulation of Wnt/β-catenin signaling [94].
Figure 16.
Action mechanism of berberine in the treatment of sepsis. LPS, lipopolysaccharides; LRP, lung resistance protein; eNOS, endothelial nitric oxide; Solid line: direct action; Dashed line: indirect action.
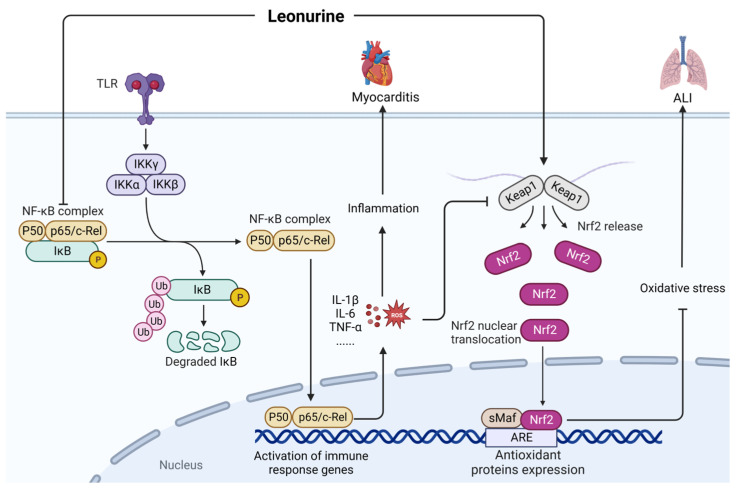
Leonurine (CAS: 24697-74-3) is a natural alkaloid with anti-inflammatory and antioxidant properties [95]. As shown in Figure 17, leonurine can alleviate LPS-induced myocarditis by inhibiting the expression of p-IκBα and p-p65 [96]. Furthermore, leonurine can mitigate the LPS-induced acute lung injury in mice by inhibiting oxidative stress and inflammation, which are regulated by the Nrf2 signaling pathway [95]. However, the application of leonurine in the treatment of sepsis is a relatively new area of research and has mostly been limited to experimental animal research.
Figure 17.
Mechanism of leonurine in the treatment of sepsis. ALI, acute lung injury; ROS, reactive oxygen species; Keap1, kelch like ECH associated protein 1; Nrf2, NF-E2-related factor 2; ARE, AU-rich element; sMaF, synthetic music mobile application format.
9. Amide Compounds
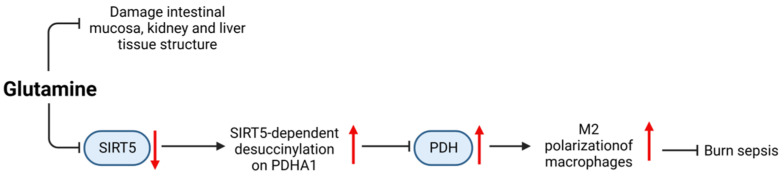
Glutamine (CAS: 56-85-9) is a type of l-α-amino acid containing five kinds of carbon and is considered the most abundant amino acid in the human body. As a single nutrient supplement, a reasonable dose of glutamine is considered safe [97]. As shown in Figure 18, glutamine supplementation in the abdominal cavity can reduce sepsis-induced damage to the intestinal mucosa, kidney, and liver tissues in CLP rat models [98]. Glutamine can inhibit the expression of SIRT5 to inhibit the desuccinylation of pyruvate dehydrogenase (PDH), which leads to an increase in oxidative phosphorylation, thereby promoting M2 polarization of macrophages to reduce burn sepsis in mice [99]. In clinical trials, intravenous administration and enteral combined administration of glutamine effectively improved transferrin, creatine/height index, and nitrogen balance in patients with dystrophic sepsis, with the best effects on days 7 and 15 [100].
Figure 18.
Mechanism of glutamine in the treatment of sepsis.
10. Discussion and Prospects
This review provides a comprehensive overview of the functions and mechanisms of natural small-molecule compounds in the prevention and treatment of sepsis. These natural small-molecule compounds can modulate many signaling pathways, such as NF-κB, TLR4, MAPK, NLRP3, AMPK, and PI3KAKT, to prevent and treat sepsis. Most of these compounds target proteins to inhibit pathways that cause inflammation; this prevents more serious organ damage and failure and can alleviate sepsis-related damage to the heart, liver, kidney, intestinal tract, and lung. However, the application of most natural small-molecule compounds in the treatment of sepsis is still in the early stages of laboratory research, and there are few reports on dose, administration time, treatment cycle, and adverse reactions. Therefore, many experimental studies are needed to further explore the clinical application of these compounds for treating sepsis in patients. While some small-molecule compounds, such as the alkaloid kukoamine B, have entered the clinical treatment stage, none have been approved or are widely used. Table 1 summarizes the experimental models reported in this paper for evaluating the use of small-molecule compounds in sepsis treatment, the improvements and adverse reactions in the experimental models, and the research stage.
Table 1.
Natural small-molecule drugs for sepsis treatment.
| Compound | Model | Improvement | Adverse Reaction | Research Progress |
|---|---|---|---|---|
| Resveratrol | CLP/LPS | SAKI/SAE/ALI/cardiomyocyte injury | High doses can increase intracellular oxidation | Animal experiment |
| Curcumin | CLP/LPS | SAKI/ALI | Animal experiment | |
| Tetrahydrocurcumin | CLP | SAKI | Animal experiment | |
| Emodin | CLP/LPS | ALI/IMI/cognitive dysfunction | Animal experiment | |
| Aloin | CLP/LPS | SAKI/ALI | Animal experiment | |
| Salidroside | CLP/LPS | SAKI/ALI/myocardial injury | Animal experiment | |
| Geniposide | CLP/LPS | SAKI/myocardial dysfunction | Animal experiment | |
| Ginsenoside | CLP/LPS | Inflammatory response and organ damage | Animal experiment | |
| Breviscapine | CME | Myocardium inflammation | Animal experiment | |
| Baicalein | CLP/LPS | ALI/SLI | Animal experiment | |
| Diosmtin | LPS | SAKI/ALI | Cell experiment | |
| Agmatine | LPS | Vascular dysfunction/systemic inflammation and organ failure | Animal experiment | |
| Kukoamine B | LPS | Inflammation | clinical trials | |
| Matrine | CLP/LPS | Cardiac insufficiency | Animal experiment | |
| Anisodamine hydrobromide | LPS | SAKI/ALI | Animal experiment | |
| Berberine | CLP/LPS | Cardiac dysfunction, myocardial injury, and intestinal vascular barrier dysfunction | Animal experiment | |
| Leonurine | LPS | ALI/myocarditis | Animal experiment | |
| Glutamine | CLP | Damage to the intestinal mucosa, kidney, and liver tissues | Clinical trials |
Studying therapeutic drugs for sepsis has always been a hotspot in medical research. Owing to the in-depth study of the pathogenesis of sepsis in recent years, various medicinal drugs have continued to emerge. However, there is still a long way to go to find effective and safe drugs for sepsis treatment. To better serve the clinic, we must explore the specific molecular mechanisms underlying the effects of these compounds in the context of sepsis and identify any adverse reactions. We have reason to believe that with further elucidation of the molecular mechanism of the occurrence and development of sepsis, the in-depth study of animal models, and the development of more clinical trials, more and more natural small-molecule compounds and other types of drugs can be developed as effective drugs for the prevention and treatment of sepsis.
Acknowledgments
We would like to thank Songying Ouyang and Qi Chen, who provided suggestions regarding the manuscript.
Author Contributions
Conceptualization and design, J.S. and F.Z.; analysis of the data and writing of the manuscript, S.W. and Z.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31770948, 31570875, and 81803547), the Natural Science Foundation of Fujian Province, China (Grant No. 2021J01204), and the Fujian Provincial Regional Development Project (Grant No. 2021N3005).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venet F., Monneret G. Advances in the Understanding and Treatment of Sepsis-Induced Immunosuppression. Nat. Rev. Nephrol. 2018;14:121–137. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 3.Jarczak D., Nierhaus A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022;23:11740. doi: 10.3390/ijms231911740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peerapornratana S., Manrique-Caballero C.L., Gómez H., Kellum J.A. Acute Kidney Injury From Sepsis: Current Concepts, Epidemiology, Pathophysiology, Prevention and Treatment. Kidney Int. 2019;96:1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woźnica E.A., Inglot M., Woźnica R.K., Łysenko L. Liver Dysfunction in Sepsis. Adv. Clin. Exp. Med. 2018;27:547–551. doi: 10.17219/acem/68363. [DOI] [PubMed] [Google Scholar]
- 7.L’Heureux M., Sternberg M., Brath L., Turlington J., Kashiouris M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020;22:35. doi: 10.1007/s11886-020-01277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas H. Sepsis: Bile Acids Promote Inflammation in Cholestasis-Associated Sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017;14:324–325. doi: 10.1038/nrgastro.2017.55. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y., Zhou Y., Wu F., Ji W., Zhang J., Wang X. The Bidirectional Interactions Between Inflammation and Coagulation in Fracture Hematoma. Tissue Eng. Part B Rev. 2019;25:46–54. doi: 10.1089/ten.teb.2018.0157. [DOI] [PubMed] [Google Scholar]
- 10.Huang M., Cai S., Su J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019;20:5376. doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P., Feng Z., Sang X., Chen W., Zhang X., Xiao J., Chen Y., Chen Q., Yang M., Su J. Kombucha Ameliorates LPS-Induced Sepsis in a Mouse Model. Food Funct. 2021;12:10263–10280. doi: 10.1039/D1FO01839F. [DOI] [PubMed] [Google Scholar]
- 12.de Pádua Lúcio K., Rabelo A.C.S., Araújo C.M., Brandão G.C., de Souza G.H.B., da Silva R.G., de Souza D.M.S., Talvani A., Bezerra F.S., Cruz Calsavara A.J., et al. Anti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxid. Med. Cell. Longev. 2018;2018:5048031. doi: 10.1155/2018/5048031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su J., Guo K., Huang M., Liu Y., Zhang J., Sun L., Li D., Pang K.L., Wang G., Chen L., et al. Fucoxanthin, a Marine Xanthophyll Isolated From Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front. Pharmacol. 2019;10:906. doi: 10.3389/fphar.2019.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y., Dou H., Gong W., Liu X., Yu Z., Li E., Tan R., Hou Y. Bis-N-norgliovictin, a Small-Molecule Compound From Marine Fungus, Inhibits LPS-Induced Inflammation in Macrophages and Improves Survival in Sepsis. Eur. J. Pharmacol. 2013;705:49–60. doi: 10.1016/j.ejphar.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Rasmi R.R., Sakthivel K.M., Guruvayoorappan C. NF-κB Inhibitors in Treatment and Prevention of Lung Cancer. Biomed. Pharmacother. 2020;130:110569. doi: 10.1016/j.biopha.2020.110569. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Zeng X., Yang F., Wang L., Luo X., Liu R., Zeng F., Lu S., Huang X., Lei Y., et al. Resveratrol: Potential Application in Sepsis. Front. Pharmacol. 2022;13:821358. doi: 10.3389/fphar.2022.821358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imperatore F., Maurizio J., Vargas Aguilar S., Busch C.J., Favret J., Kowenz-Leutz E., Cathou W., Gentek R., Perrin P., Leutz A., et al. SIRT1 Regulates Macrophage Self-Renewal. EMBO J. 2017;36:2353–2372. doi: 10.15252/embj.201695737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Z., Sun M., Wu J., Fang H., Cai S., An S., Huang Q., Chen Z., Wu C., Zhou Z., et al. SIRT1 Attenuates Sepsis-Induced Acute Kidney Injury via Beclin1 Deacetylation-Mediated Autophagy Activation. Cell Death Dis. 2021;12:217. doi: 10.1038/s41419-021-03508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Simayi A., Fu J., Zhao X., Xu G. Resveratrol Mediates the miR-149/HMGB1 Axis and Regulates the Ferroptosis Pathway to Protect Myocardium in Endotoxemia Mice. Am. J. Physiol. Endocrinol. Metab. 2022;323:E21–E32. doi: 10.1152/ajpendo.00227.2021. [DOI] [PubMed] [Google Scholar]
- 20.Sui D.M., Xie Q., Yi W.J., Gupta S., Yu X.Y., Li J.B., Wang J., Wang J.F., Deng X.M. Resveratrol Protects Against Sepsis-Associated Encephalopathy and Inhibits the NLRP3/IL-1β Axis in Microglia. Mediators Inflamm. 2016;2016:1045657. doi: 10.1155/2016/1045657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L., Zhang Z., Zhuo Y., Cui L., Li C., Li D., Zhang S., Cui N., Wang X., Gao H. Resveratrol Alleviates Sepsis-Induced Acute Lung Injury by Suppressing Inflammation and Apoptosis of Alveolar Macrophage Cells. Am. J. Transl. Res. 2018;10:1961–1975. [PMC free article] [PubMed] [Google Scholar]
- 22.Posadino A.M., Cossu A., Giordo R., Zinellu A., Sotgia S., Vardeu A., Hoa P.T., Nguyen L.H.V., Carru C., Pintus G. Resveratrol Alters Human Endothelial Cells Redox State and Causes Mitochondrial-Dependent Cell Death. Food Chem. Toxicol. 2015;78:10–16. doi: 10.1016/j.fct.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Noureddin S.A., El-Shishtawy R.M., Al-Footy K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019;182:111631. doi: 10.1016/j.ejmech.2019.111631. [DOI] [PubMed] [Google Scholar]
- 24.Chai Y.S., Chen Y.Q., Lin S.H., Xie K., Wang C.J., Yang Y.Z., Xu F. Curcumin Regulates the Differentiation of Naïve CD4+ T Cells and Activates IL-10 Immune Modulation Against Acute Lung Injury in Mice. Biomed. Pharmacother. 2020;125:109946. doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- 25.Chen D., Wang H., Cai X. Curcumin Interferes With Sepsis-Induced Cardiomyocyte Apoptosis via TLR1 Inhibition. Rev. Port. Cardiol. 2023;42:209–221. doi: 10.1016/j.repc.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H., Wang X., Wang X., Liu B., Yuan Y., Zuo X. Curcumin Attenuates Inflammation and Cell Apoptosis Through Regulating NF-κB and JAK2/STAT3 Signaling Pathway Against Acute Kidney Injury. Cell Cycle. 2020;19:1941–1951. doi: 10.1080/15384101.2020.1784599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cárdenas Garza G.R., Elizondo Luévano J.H., Bazaldúa Rodríguez A.F., Chávez Montes A., Pérez Hernández R.A., Martínez Delgado A.J., López Villarreal S.M., Rodríguez Rodríguez J., Sánchez Casas R.M., Castillo Velázquez U., et al. Benefits of Cardamom (Elettaria cardamomum (L.) Maton) and Turmeric (Curcuma longa L.) Extracts for Their Applications as Natural Anti-Inflammatory Adjuvants. Plants. 2021;10:1908. doi: 10.3390/plants10091908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M., Chang Y.Y., Huang S., Xiao L.H., Zhou W., Zhang L.Y., Li C., Zhou R.P., Tang J., Lin L., et al. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. Mol. Nutr. Food Res. 2018;62:10. doi: 10.1002/mnfr.201700281. [DOI] [PubMed] [Google Scholar]
- 29.Li L., Liu X., Li S., Wang Q., Wang H., Xu M., An Y. Tetrahydrocurcumin Protects Against Sepsis-Induced Acute Kidney Injury via the SIRT1 Pathway. Ren. Fail. 2021;43:1028–1040. doi: 10.1080/0886022X.2021.1942915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Jiang J.G. Health Functions and Structure-Activity Relationships of Natural Anthraquinones from Plants. Food Funct. 2018;9:6063–6080. doi: 10.1039/C8FO01569D. [DOI] [PubMed] [Google Scholar]
- 31.Dong X., Zeng Y., Liu Y., You L., Yin X., Fu J., Ni J. Aloe-Emodin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Phytother. Res. 2020;34:270–281. doi: 10.1002/ptr.6532. [DOI] [PubMed] [Google Scholar]
- 32.Liu F.J., Gu T.J., Wei D.Y. Emodin Alleviates Sepsis-Mediated Lung Injury via Inhibition and Reduction of NF-kB and HMGB1 Pathways Mediated by SIRT1. Kaohsiung J. Med. Sci. 2022;38:253–260. doi: 10.1002/kjm2.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao B., He J., Yin X., Shi Y., Wan J., Tian Z. The Protective Effect of Lithocholic Acid on the Intestinal Epithelial Barrier Is Mediated by the Vitamin D Receptor via a SIRT1/Nrf2 and NF-κB Dependent Mechanism in Caco-2 Cells. Toxicol. Lett. 2019;316:109–118. doi: 10.1016/j.toxlet.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Shang L., Liu Y., Li J., Pan G., Zhou F., Yang S. Emodin Protects Sepsis Associated Damage to the Intestinal Mucosal Barrier Through the VDR/Nrf2/HO-1 Pathway. Front. Pharmacol. 2021;12:724511. doi: 10.3389/fphar.2021.724511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M., Lian B., Zhang R., Guo Y., Zhao J., He S., Bai Y., Wang N., Lin Y., Wang X., et al. Emodin Ameliorates Intestinal Dysfunction by Maintaining Intestinal Barrier Integrity and Modulating the Microbiota in Septic Mice. Mediat. Inflamm. 2022;2022:5026103. doi: 10.1155/2022/5026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao L.L., Wang Z.H., Mu Y.H., Liu Z.L., Pang L. Emodin Promotes Autophagy and Prevents Apoptosis in Sepsis-Associated Encephalopathy Through Activating BDNF/TrkB Signaling. Pathobiology. 2022;89:135–145. doi: 10.1159/000520281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao J., Chen S., Chen Y., Su J. The Potential Health Benefits of Aloin From Genus Aloe. Phytother. Res. 2022;36:873–890. doi: 10.1002/ptr.7371. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y., Wang J., Yang W., Qi X., Lan L., Luo L., Yin Z. Bergapten Prevents Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells Through Suppressing JAK/STAT Activation and ROS Production and Increases the Survival Rate of Mice After LPS Challenge. Int. Immunopharmacol. 2017;48:159–168. doi: 10.1016/j.intimp.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Luo X., Zhang H., Wei X., Shi M., Fan P., Xie W., Zhang Y., Xu N. Aloin Suppresses Lipopolysaccharide-Induced Inflammatory Response and Apoptosis by Inhibiting the Activation of NF-κB. Molecules. 2018;23:517. doi: 10.3390/molecules23030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei J., Shen Y., Xv G., Di Z., Li Y., Li G. Aloin Suppresses Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting NLRP3/NF-κB via Activation of SIRT1 in Mice. Immunopharmacol. Immunotoxicol. 2020;42:306–313. doi: 10.1080/08923973.2020.1765373. [DOI] [PubMed] [Google Scholar]
- 41.Yang S., Lee W., Lee B.S., Lee C., Park E.K., Ku S.K., Bae J.S. Aloin Reduces HMGB1-Mediated Septic Responses and Improves Survival in Septic Mice by Activation of the SIRT1 and PI3K/Nrf2/HO-1 Signaling Axis. Am. J. Chin. Med. 2019;47:613–633. doi: 10.1142/S0192415X19500320. [DOI] [PubMed] [Google Scholar]
- 42.Lee W., Jeong G.S., Baek M.C., Ku S.K., Bae J.S. Renal Protective Effects of Aloin in a Mouse Model of Sepsis. Food Chem. Toxicol. 2019;132:110651. doi: 10.1016/j.fct.2019.110651. [DOI] [PubMed] [Google Scholar]
- 43.Xu F., Xu J., Xiong X., Deng Y. Salidroside Inhibits MAPK, NF-κB, and STAT3 Pathways in Psoriasis-Associated Oxidative Stress via SIRT1 Activation. Redox Rep. 2019;24:70–74. doi: 10.1080/13510002.2019.1658377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu M., Hu R., Wang J., An Y., Lu L., Long C., Yan L. Salidroside Suppresses IL-1β-Induced Apoptosis in Chondrocytes via Phosphatidylinositol 3-Kinases (PI3K)/Akt Signaling Inhibition. Med. Sci. Monit. 2019;25:5833–5840. doi: 10.12659/MSM.917851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan H., Su B.J., Le J.W., Zhu J.H. Salidroside Protects Acute Kidney Injury in Septic Rats by Inhibiting Inflammation and Apoptosis. Drug Des. Dev. Ther. 2022;16:899–907. doi: 10.2147/DDDT.S361972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang L., Xu L., Zheng L., Wang Y., Zhuang M., Yang D. Salidroside Attenuates Sepsis-Associated Acute Lung Injury Through PPP1R15A Mediated Endoplasmic Reticulum Stress Inhibition. Bioorg. Med. Chem. 2022;71:116865. doi: 10.1016/j.bmc.2022.116865. [DOI] [PubMed] [Google Scholar]
- 47.Chen L., Liu P., Feng X., Ma C. Salidroside Suppressing LPS-Induced Myocardial Injury by Inhibiting ROS-Mediated PI3K/Akt/mTOR Pathway In Vitro and In Vivo. J. Cell. Mol. Med. 2017;21:3178–3189. doi: 10.1111/jcmm.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan T., Shi X., Chen H., Chen R., Wu D., Lin Z., Zhang J., Pan J. Correction to: Geniposide Suppresses Interleukin-1β-Induced Inflammation and Apoptosis in Rat Chondrocytes via the PI3K/Akt/NF-κB Signaling Pathway. Inflammation. 2019;42:404–405. doi: 10.1007/s10753-018-0897-1. [DOI] [PubMed] [Google Scholar]
- 49.Song P., Shen D.F., Meng Y.Y., Kong C.Y., Zhang X., Yuan Y.P., Yan L., Tang Q.Z., Ma Z.G. Geniposide Protects Against Sepsis-Induced Myocardial Dysfunction Through AMPKα-Dependent Pathway. Free Radic. Biol. Med. 2020;152:186–196. doi: 10.1016/j.freeradbiomed.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., Zhao N., Shi G., Wang H. Geniposide Ameliorated Sepsis-Induced Acute Kidney Injury by Activating PPARγ. Aging. 2020;12:22744–22758. doi: 10.18632/aging.103902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu L.L., Jia B.H., Sun J., Chen J.X., Liu Z.Y., Liu Y. Protective Effects of Ginsenoside Rb1 on Septic Rats and Its Mechanism. Biomed. Environ. Sci. 2014;27:300–303. doi: 10.3967/bes2014.053. [DOI] [PubMed] [Google Scholar]
- 52.Sun M., Ji Y., Li Z., Chen R., Zhou S., Liu C., Du M. Ginsenoside Rb3 Inhibits Pro-inflammatory Cytokines via MAPK/AKT/NF-κB Pathways and Attenuates Rat Alveolar Bone Resorption in Response to Porphyromonas gingivalis LPS. Molecules. 2020;25:4815. doi: 10.3390/molecules25204815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H., Liu M., Chen G., Wu Y., Xie L., Han X., Zhang G., Tan Z., Ding W., Fan H., et al. Anti-Inflammatory Effects of Ginsenoside Rb3 in LPS-Induced Macrophages Through Direct Inhibition of TLR4 Signaling Pathway. Front. Pharmacol. 2022;13:714554. doi: 10.3389/fphar.2022.714554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang S.J., Wang J.H., Lee J.S., Kang J.Y., Baek D.C., Kim G.H., Ahn Y.C., Son C.G. Ginseng Sprouts Attenuate Mortality and Systemic Inflammation by Modulating TLR4/NF-κB Signaling in an LPS-Induced Mouse Model of Sepsis. Int. J. Mol. Sci. 2023;24:1583. doi: 10.3390/ijms24021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu T., Tang Y., Zhang F., Zhang L. Roles of Ginsenosides in Sepsis. J. Ginseng Res. 2023;47:1–8. doi: 10.1016/j.jgr.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q.L., Yang L., Peng Y., Gao M., Yang M.S., Xing W., Xiao X.Z. Ginsenoside Rg1 Regulates SIRT1 to Ameliorate Sepsis-Induced Lung Inflammation and Injury via Inhibiting Endoplasmic Reticulum Stress and Inflammation. Mediat. Inflamm. 2019;2019:6453296. doi: 10.1155/2019/6453296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo M., Yan D., Sun Q., Tao J., Xu L., Sun H., Zhao H. Ginsenoside Rg1 Attenuates Cardiomyocyte Apoptosis and Inflammation via the TLR4/NF-kB/NLRP3 Pathway. J. Cell. Biochem. 2020;121:2994–3004. doi: 10.1002/jcb.29556. [DOI] [PubMed] [Google Scholar]
- 58.Kim T.W., Joh E.H., Kim B., Kim D.H. Ginsenoside Rg5 Ameliorates Lung Inflammation in Mice by Inhibiting the Binding of LPS to Toll-Like Receptor-4 on Macrophages. Int. Immunopharmacol. 2012;12:110–116. doi: 10.1016/j.intimp.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 59.Paik S., Choe J.H., Choi G.E., Kim J.E., Kim J.M., Song G.Y., Jo E.K. Rg6, a Rare Ginsenoside, Inhibits Systemic Inflammation through the Induction of Interleukin-10 and microRNA-146a. Sci. Rep. 2019;9:4342. doi: 10.1038/s41598-019-40690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen T.L.L., Huynh D.T.N., Jin Y., Jeon H., Heo K.S. Protective Effects of Ginsenoside-Rg2 and -Rh1 on Liver Function Through Inhibiting TAK1 and STAT3-Mediated Inflammatory Activity and Nrf2/ARE-Mediated Antioxidant Signaling Pathway. Arch. Pharm. Res. 2021;44:241–252. doi: 10.1007/s12272-020-01304-4. [DOI] [PubMed] [Google Scholar]
- 61.Lee W., Cho S.H., Kim J.E., Lee C., Lee J.H., Baek M.C., Song G.Y., Bae J.S. Suppressive Effects of Ginsenoside Rh1 on HMGB1-Mediated Septic Responses. Am. J. Chin. Med. 2019;47:119–133. doi: 10.1142/S0192415X1950006X. [DOI] [PubMed] [Google Scholar]
- 62.Wang F., Park J.S., Ma Y., Ma H., Lee Y.J., Lee G.R., Yoo H.S., Hong J.T., Roh Y.S. Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation. Nutrients. 2021;13:856. doi: 10.3390/nu13030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xing W., Yang L., Peng Y., Wang Q., Gao M., Yang M., Xiao X. Ginsenoside Rg3 Attenuates Sepsis-Induced Injury and Mitochondrial Dysfunction in Liver via AMPK-Mediated Autophagy Flux. Biosci. Rep. 2017;37:BSR20170934. doi: 10.1042/BSR20170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim G.O., Kim N., Song G.Y., Bae J.S. Inhibitory Activities of Rare Ginsenoside Rg4 on Cecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2022;23:10836. doi: 10.3390/ijms231810836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun R., Li Y., Chen W., Zhang F., Li T. Total Ginsenosides Synergize With Ulinastatin Against Septic Acute Lung Injury and Acute Respiratory Distress Syndrome. Int. J. Clin. Exp. Pathol. 2015;8:7385–7390. doi: 10.3109/9780203912034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Wen P.H., Zhang X.X., Dai Y., He Q. Breviscapine Ameliorates CCl4-Induced Liver Injury in Mice Through Inhibiting Inflammatory Apoptotic Response and ROS Generation. Int. J. Mol. Med. 2018;42:755–768. doi: 10.3892/ijmm.2018.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Z.Q., Zhou Y., Chen F., Huang J.W., Zheng J., Li H.L., Li T., Li L. Breviscapine Pretreatment Inhibits Myocardial Inflammation and Apoptosis in Rats After Coronary Microembolization by Activating the PI3K/Akt/GSK-3β Signaling Pathway. Drug Des. Dev. Ther. 2021;15:843–855. doi: 10.2147/DDDT.S293382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinda B., Dinda S., Dassharma S., Banik R., Chakraborty A., Dinda M. Therapeutic Potentials of Baicalin and Its Aglycone, Baicalein Against Inflammatory Disorders. Eur. J. Med. Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X., Qin Y., Ruan W., Wan X., Lv C., He L., Lu L., Guo X. Targeting Inflammation-Associated AMPK/Mfn-2/MAPKs Signaling Pathways by Baicalein Exerts Anti-atherosclerotic Action. Phytother. Res. 2021;35:4442–4455. doi: 10.1002/ptr.7149. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y., Jing Y.Y., Zeng C.Y., Li C.G., Xu L.H., Yan L., Bai W.J., Zha Q.B., Ouyang D.Y., He X.H. Scutellarin Suppresses NLRP3 Inflammasome Activation in Macrophages and Protects Mice Against Bacterial Sepsis. Front. Pharmacol. 2017;8:975. doi: 10.3389/fphar.2017.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang C., Zhang J., Xie H., Guan H., Li R., Chen C., Dong H., Zhou Y., Zhang W. Baicalein Suppresses Lipopolysaccharide-Induced Acute Lung Injury by Regulating Drp1-Dependent Mitochondrial Fission of Macrophages. Biomed. Pharmacother. 2022;145:112408. doi: 10.1016/j.biopha.2021.112408. [DOI] [PubMed] [Google Scholar]
- 72.Dai J.M., Guo W.N., Tan Y.Z., Niu K.W., Zhang J.J., Liu C.L., Yang X.M., Tao K.S., Chen Z.N., Dai J.Y. Wogonin Alleviates Liver Injury in Sepsis Through Nrf2-Mediated NF-κB Signalling Suppression. J. Cell. Mol. Med. 2021;25:5782–5798. doi: 10.1111/jcmm.16604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Y., Gong X.B., Huang L.G., Wang Z.X., Wan R.Z., Zhang P., Zhang Q.Y., Chen Z., Zhang B.S. Diosmetin Exerts Anti-oxidative, Anti-Inflammatory and Anti-apoptotic Effects to Protect against Endotoxin-Induced Acute Hepatic Failure in Mice. Oncotarget. 2017;8:30723–30733. doi: 10.18632/oncotarget.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W., Zhang S., Yang F., Xie J., Chen J., Li Z. Diosmetin Alleviates Acute Kidney Injury by Promoting the TUG1/Nrf2/HO-1 Pathway in Sepsis Rats. Int. Immunopharmacol. 2020;88:106965. doi: 10.1016/j.intimp.2020.106965. [DOI] [PubMed] [Google Scholar]
- 75.Liu Q., Ci X., Wen Z., Peng L. Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury Through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome. Biomol. Ther. 2018;26:157–166. doi: 10.4062/biomolther.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotagale N.R., Taksande B.G., Inamdar N.N. Neuroprotective Offerings by Agmatine. Neurotoxicology. 2019;73:228–245. doi: 10.1016/j.neuro.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 77.El-Awady M.S., Nader M.A., Sharawy M.H. The Inhibition of Inducible Nitric Oxide Synthase and Oxidative Stress by Agmatine Attenuates Vascular Dysfunction in Rat Acute Endotoxemic Model. Environ. Toxicol. Pharmacol. 2017;55:74–80. doi: 10.1016/j.etap.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Li X., Zhu J., Tian L., Ma X., Fan X., Luo L., Yu J., Sun Y., Yang X., Tang W., et al. Agmatine Protects Against the Progression of Sepsis Through the Imidazoline I2 Receptor-Ribosomal S6 Kinase 2-Nuclear Factor-κB Signaling Pathway. Crit. Care Med. 2020;48:e40–e47. doi: 10.1097/CCM.0000000000004065. [DOI] [PubMed] [Google Scholar]
- 79.Liu X., Zheng X., Wang N., Cao H., Lu Y., Long Y., Zhao K., Zhou H., Zheng J. Kukoamine B, a Novel Dual Inhibitor of LPS and CpG DNA, Is a Potential Candidate for Sepsis Treatment. Br. J. Pharmacol. 2011;162:1274–1290. doi: 10.1111/j.1476-5381.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H., Hu X., Wang T., Cui C., Jiang J., Dong K., Chen S., Jin C., Zhao Q., Du B., et al. Exposure-Response Modeling to Support Dosing Selection for Phase IIb Development of Kukoamine B in Sepsis Patients. Front. Pharmacol. 2021;12:645130. doi: 10.3389/fphar.2021.645130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Q., Liu H., Wang Z., Wang T., Cui C., Wang H., Li L., Zhong W., Jiang J., Dong K., et al. Safety, Tolerability, and Pharmacokinetics of Kukoamine B in Healthy Volunteers: A Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Phase I Study. Adv. Ther. 2023;40:3186–3198. doi: 10.1007/s12325-023-02521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai X.H., Guo H., Xie B. Structural Modifications of Matrine-Type Alkaloids. Mini Rev. Med. Chem. 2018;18:730–744. doi: 10.2174/1389557516666161104150334. [DOI] [PubMed] [Google Scholar]
- 83.Lin Y., He F., Wu L., Xu Y., Du Q. Matrine Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review. Drug Des. Dev. Ther. 2022;16:533–569. doi: 10.2147/DDDT.S349678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun P., Sun N., Yin W., Sun Y., Fan K., Guo J., Khan A., He Y., Li H. Matrine Inhibits IL-1β Secretion in Primary Porcine Alveolar Macrophages Through the MyD88/NF-κB Pathway and NLRP3 Inflammasome. Vet. Res. 2019;50:53. doi: 10.1186/s13567-019-0671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Wu F.P., Huang Y.R., Li H.D., Cao X.Y., You Y., Meng Z.F., Sun K.Y., Shen X.Y. Matrine Suppresses NLRP3 Inflammasome Activation via Regulating PTPN2/JNK/SREBP2 Pathway in Sepsis. Phytomedicine. 2023;109:154574. doi: 10.1016/j.phymed.2022.154574. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y., Liu L., Zhang J. Protective Role of Matrine in Sepsis-Associated Cardiac Dysfunction Through Regulating the lncRNA PTENP1/miR-106b-5p Axis. Biomed. Pharmacother. 2021;134:111112. doi: 10.1016/j.biopha.2020.111112. [DOI] [PubMed] [Google Scholar]
- 87.Wan F., Du X., Liu H., He X., Zeng Y. Protective Effect of Anisodamine Hydrobromide on Lipopolysaccharide-Induced Acute Kidney Injury. Biosci. Rep. 2020;40:BSR20201812. doi: 10.1042/BSR20201812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z., Xu C., Tao Y., Liang Y., Liang Q., Li J., Li R., Ye H. Anisodamine Alleviates Lipopolysaccharide-Induced Pancreatic Acinar Cell Injury Through NLRP3 Inflammasome and NF-κB Signaling Pathway. J. Recept. Signal Transduct. Res. 2020;40:58–66. doi: 10.1080/10799893.2020.1713808. [DOI] [PubMed] [Google Scholar]
- 89.Zeng Y., Du X., Jiang W., Qiu Y. Anisodamine Hydrobromide Protects Against Acute Lung Injury in Septic Rats Induced by Lipopolysaccharide or Cecal Ligation and Puncture via Inhibiting Apoptosis and Pyroptosis. FASEB. J. 2021;35 doi: 10.1096/fasebj.2021.35.S1.02246. [DOI] [Google Scholar]
- 90.Du X., Liu H., Yue Y., Wu Q., Jiang W., Qiu Y., Zeng Y. Anisodamine Hydrobromide Protects Glycocalyx and Against the Lipopolysaccharide-Induced Increases in Microvascular Endothelial Layer Permeability and Nitric Oxide Production. Cardiovasc. Eng. Technol. 2021;12:91–100. doi: 10.1007/s13239-020-00486-8. [DOI] [PubMed] [Google Scholar]
- 91.Takahara M., Takaki A., Hiraoka S., Adachi T., Shimomura Y., Matsushita H., Nguyen T.T.T., Koike K., Ikeda A., Takashima S., et al. Berberine Improved Experimental Chronic Colitis by Regulating Interferon-γ- and IL-17A-producing Lamina Propria CD4+ T Cells Through AMPK Activation. Sci. Rep. 2019;9:11934. doi: 10.1038/s41598-019-48331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H., Wu X., Tao Y., Lu G. Berberine Attenuates Sepsis-Induced Cardiac Dysfunction by Upregulating the Akt/eNOS Pathway in Mice. Exp. Ther. Med. 2022;23:371. doi: 10.3892/etm.2022.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen H., Liu Q., Liu X., Jin J. Berberine Attenuates Septic Cardiomyopathy by Inhibiting TLR4/NF-κB Signalling in Rats. Pharm. Biol. 2021;59:121–128. doi: 10.1080/13880209.2021.1877736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He Y., Yuan X., Zuo H., Sun Y., Feng A. Berberine Exerts a Protective Effect on Gut-Vascular Barrier via the Modulation of the Wnt/Beta-Catenin Signaling Pathway During Sepsis. Cell. Physiol. Biochem. 2018;49:1342–1351. doi: 10.1159/000493412. [DOI] [PubMed] [Google Scholar]
- 95.Zhang G., Wang L. Leonurine: A Compound With the Potential to Prevent Acute Lung Injury. Exp. Ther. Med. 2022;23:358. doi: 10.3892/etm.2022.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang R., Li D., Ouyang J., Tian X., Zhao Y., Peng X., Li S., Yu G., Yang J. Leonurine Alleviates LPS-Induced Myocarditis Through Suppressing the NF-κB Signaling Pathway. Toxicology. 2019;422:1–13. doi: 10.1016/j.tox.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10:1564. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stonoga E.T.S., Bueno R.Z., Nagano T.A., Martins V., Rocha S.L. Effects of Intraperitoneal Glutamine in the Treatment of Experimental Sepsis. Arq. Bras. Cir. Dig. 2019;32:e1431. doi: 10.1590/0102-672020190001e1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y., Chen X., Lu Y., Xia L., Fan S., Huang Q., Liu X., Peng X. Glutamine Mitigates Murine Burn Sepsis by Supporting Macrophage M2 Polarization Through Repressing the SIRT5-Mediated Desuccinylation of Pyruvate Dehydrogenase. Burns Trauma. 2022;10:tkac041. doi: 10.1093/burnst/tkac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koksal G.M., Erbabacan E., Tunali Y., Karaoren G., Vehid S., Oz H. The Effects of Intravenous, Enteral and Combined Administration of Glutamine on Malnutrition in Sepsis: A Randomized Clinical Trial. Asia Pac. J. Clin. Nutr. 2014;23:34–40. doi: 10.6133/apjcn.2014.23.1.11. [DOI] [PubMed] [Google Scholar]