Abstract
In this study, hydroxy benzoin ( 1-7 ), benzil ( 8-14 ), and benzoin/benzil-O-β-D-glucosides ( 15-25 ) were synthesized to investigate their biological activities. An efficient method for synthesizing hydroxy benzoin compounds ( 1 - 7 ) was prepared from four different benzaldehydes using an ultrasonic bath. Then, antioxidant (FRAP, CUPRAC, and DPPH), antimicrobial (3 Gram (-), 4/6 Gram (+), one tuberculosis and one fungus), and enzyme inhibition (acetylcholinesterase, butyrylcholine esterase, tyrosinase, α-amylase, and α- glucosidase) for the all synthesized compounds ( 1-25 ) were evaluated. And also, four most active compounds ( 4 , 12 , 18a+b , and 25 ) from each group were evaluated to the human cervical cancer cell line (HeLa) and anticancer screening tests against the human retinal normal cell line (RPE). Compound 4 showed HeLa and RPE cancer cell activities as much as cisplatin. The synthesized compounds were characterized by spectroscopic methods (NMR, FT-IR, UV, LC-QTOF-MS) and the ACD NMR program’s help.
Keywords: Hydroxy benzoin/benzil, benzoin/benzil-O-β-D-glucoside, antioxidant, antimicrobial, enzyme inhibition, cytotoxic activity
1. Introduction
It is known that drugs containing phenolic compounds are frequently used to treat diseases such as diabetes, Alzheimer’s, and cardiovascular diseases. One of the most abundant secondary metabolites in plants is phenolic compounds, such as simple phenols, phenolic acids, flavones, flavanones, and stilbenes. The main sources of phenolic compounds are fruits and vegetables, making up an important part of the human diet. According to the studies of the national health organization, due to the antioxidant effects of phenolic compounds found in herbal products, it has been revealed that rich fruit and vegetable consumption reduces the risks of diseases such as cancer, diabetes, Alzheimer’s, and cardiovascular diseases [1]. Some benzoin compounds with the phenolic structure are found in fruits and vegetables in nature.
The carbon-carbon bond formation is an important reaction in organic chemistry and studied extensively in the literature [2–5]. The benzoin condensation reaction is an important type of C-C bond formation reaction and is widely used to synthesize natural compounds and analogs. Symmetric and asymmetric benzoin derivative synthesis using different catalysts in benzoin condensation have been studied under milder reaction conditions [2–15]. However, the condensation of two different benzaldehydes may have a widely different character; only the more stable form of the isomeric mixed benzoins could be isolable in excess. When the carbonyl group is adjacent to the phenyl ring with the more electron-donating substituent, it is consistent with the reversibility of the reaction and the relative stability of the carbonyl groups in the possible products [16]. In the literature, the synthesis of mixed benzoin had been made by a variety of methods involving the generation of a “masked” acyl carbanion, which reacts with aromatic aldehydes [17], the addition of an excess of the Grignard reagent to a cyanohydrin or a protected cyanohydrin of an aromatic aldehyde [18], and reduction of unsymmetrical benzils [19]. Thus, all of the mixed benzoin synthesis involve masking or unmasking steps.
A literature search showed that various synthetic methods were reported for the benzil syntheses [20-22]. Diphenyl alkynes were oxidized efficiently to yield the corresponding benzil [23]. In another work, the selective addition of organomagnesium reagents to 2,4,6-trichlorophenyl isocyanide then following reactions leading to an efficient synthesis of benzil compounds [24]. Facile oxidation of benzylic alcohols and benzoin to give benzil compounds with various oxidation reagents had been reported [24–32].
Carbohydrates play important functional roles in numerous physiological processes, including various disease states [33–34]. Synthetic carbohydrates-based small molecule selective inhibitors are thereof being pursued as potential medicinal agents [35–38].
The significance of benzoin/benzil and carbohydrate-based agents caught our attention for the synthesis of benzoin/benzil-O- β -D-glucosides, and we decided to study their pharmacological activities. Due to the biological activities’ evaluation, we wish to report the synthesis of hydroxy benzoins ( 1-7 ) from hydroxy benzaldehydes, hydroxy benzils ( 8-14 ) from the oxidation of benzoins ( 1-7 ), and benzoin/benzil-O- β -D-glucosides ( 15-25 ) from the glycosylation of hydroxy benzoins/benzils ( 1-14 ). Then, their antioxidant, antimicrobial, enzyme inhibitions, and cytotoxic activity investigations were reported.
2. Material and methods
Solvents ( n -hexane, chloroform, ethyl acetate, acetone, methanol, and dimethyl sulfoxide), aldehyde compounds (benzaldehyde, 3-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, and 3,5-dihydroxybenzaldehyde), and any used reagent were purchased from by Sigma-Aldrich (Sigma-Aldrich Corp., St. Louis, MO, USA), Fluka, or Merck (Merck&Co., Inc., Kenilworth, NJ, USA) unless otherwise stated. 1H and 13C NMR spectra were obtained on a Bruker 400 MHz NMR spectrometer (400 MHz for 1H, 100 MHz for 13C), using tetramethylsilane (TMS) as an internal standard. CDCl3, CD3OD, and acetone-d6 were used as NMR solvents. 13C and APT spectra were adjusted according to deutero solvent peaks. Chemical shifts were expressed in δ (ppm), and coupling constants ( J ) were reported in hertz (Hz). ACD NMR program was used for the interpretation of spectra. Ultrasonic bath (340 W, WiseClean, VUC-A06H) was used for the benzoin synthesis. FT-IR spectra were taken using the Perkin-Elmer 1600 (ATR) (4000–400 cm–1) spectrophotometer (PerkinElmer, Inc., Waltham, MA USA). Melting points were determined using the Thermo-var apparatus fitted with a microscope. Normal phase silica gel (230–400 mesh) was used in vacuum column chromatography (VLC). TLC was carried out on silica gel 60 F254, and the spots were visualized by ultraviolet (UV) lamp (254 nm and 366 nm) or spraying with 20% H2SO4 and heating.
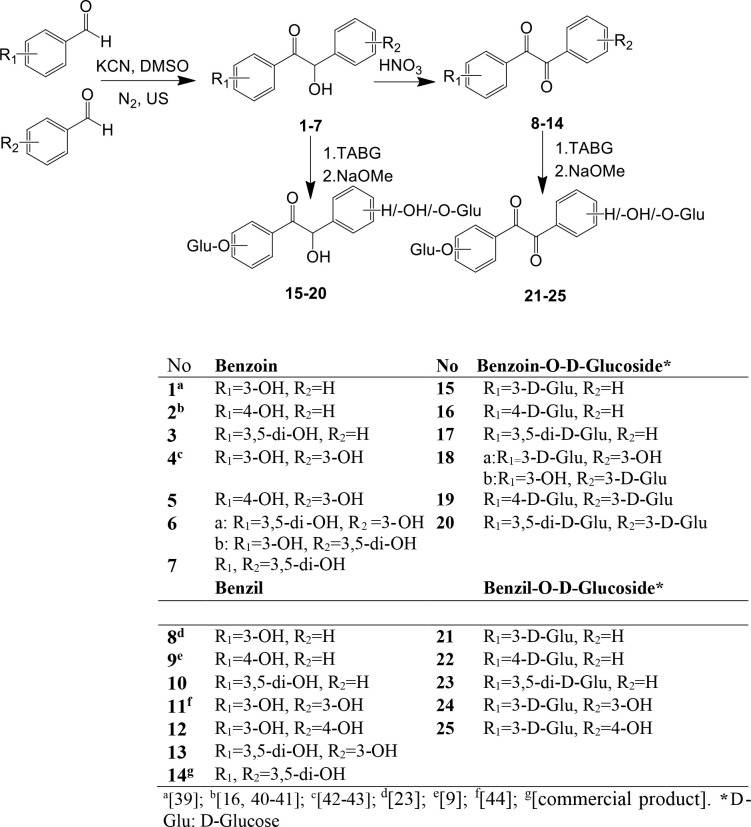
Synthesis of hydroxy benzoins (1-7): Hydroxy benzaldehydes (0.001 mol) in dry DMSO (10 mL) were reacted with KCN (0.001 mol) in an N2 environment using an ultrasonic bath (340 W, 120 min) at 70–85 °C. The reactions were terminated after the TLC control. Water (30 mL) was added to the flask, extracted with ethyl acetate (3×30 mL) to give a crude mixture then compounds 1-7 were purified as a racemic mixture with repeated vacuum liquid chromatography (VLC, Silica gel 230–400 mesh) using the increasing polarity of n -hexane, chloroform, ethyl acetate, and methanol solvent mixtures, and the fractions were checked by TLC (Figure, Table 1). The synthesis of compounds 1 [39], 2 [16, 40–41], and 4 [42–43] had been mentioned in the literature.
Table 1.
Experimental method for the synthesis of hydroxy benzoin compounds (1-7).
| Reagents (0.01mol each) | Method | Temp. | Time | Possible benzoin productsR1PhCOCH(OH)PhR2 | No | Yielda(%) |
|---|---|---|---|---|---|---|
| Benzaldehyde 3-HydroxybenzaldehydeKCN | US340 Watt85 oCDMSO (10 mL) N2 | 70–85 (oC) | 60min. | R1, R2=-HR1, R2=3-OHR1=-H, R2=3-OHR1=3-OH, R2=-H | 1 | 2408-45 |
| Benzaldehyde 4-HydroxybenzaldehydeKCN | R1, R2=-H R1, R2=4-OHR1=-H, R2=4-OHR1=4-OH, R2=-H | 2 | 32--48 | |||
| Benzaldehyde 3,5-Dihydroxybenzaldehyde KCN | R1, R2=-HR1, R2=3,5-diOHR1=-H, R2=3,5-diOHR1=3,5-diOH, R2=-H | 3 | 4511-40 | |||
| 3-HydroxybenzaldehydeKCN | R1, R2=3-OH | 4 | 68 | |||
| 3-Hydroxybenzaldehyde4-HydroxybenzaldehydeKCN | R1, R2=3-OHR1, R2=4-OHR1=3-OH, R2=4-OH R1=4-OH, R2=3-OH | 5 | 12--39 | |||
| 3-Hydroxybenzaldehyde3,5-Dihydroxybenzaldehyde KCN | R1, R2=3-OHR1, R2=3,5-di-OHR1/R2=3,5-diOH, R2/R1=3-OH | 6a+b | 171455 | |||
| 3,5-Dihydroxybenzaldehyde KCN | R1, R2=3,5-di-OH | 7 | 65 |
aStarting aldehydes were also observed
Figure.
Synthesis scheme for the hydroxy benzoin, benzil, and their D-glucoside derivatives (R1 and R2: -H, -OH, or D-Glucose).
Compound 1 (2-Hydroxy-1-(3-hydroxyphenyl)-2-phenylethanone): Yield: 45%; Rf = 0.5 (chloroform-ethyl acetate-acetic acid: 25:10:1); UV (MeOH) λ max nm (logɛ): 203(3,37); FT-IR (cm–1): 3198, 2924, 1682, 1597, 1584, 1485, 1450, 1285, 1240, 1068, 1014, 950, 787, 762, 700; 1H-NMR (400 MHz, CDCl3, d, ppm): 5.82 (s, 1H, H-2), 7.37–7.02 (m, 9H, H-2’/4’/5’/6’/2’’/3’’/4’’/5’’/6’’), 4.80 (bs, -OH); 13C-NMR (100 MHz, CDCl3, d, ppm): 198.86 (C-1), 76.16 (C-2), 130.34 (C-1’), 115.71 (C-2’), 156.74 (C-3’), 121.07 (C-4’), 134.64 (C-5’), 121.55 (C-6’), 138.60 (C-1’’), 127.79 (C-2’’), 129.13 (C-3’’), 119.28 (C-4’’), 129.13 (C-5’’), 128.67 (C-6’’).
Compound 2 (2-Hydroxy-1-(4-hydroxyphenyl)-2-phenylethanone) : Yield: 48%; Rf = 0.48 (chloroform-ethyl acetate-acetic acid: 25:10:1); FT-IR (cm–1): 3371, 3040, 2920, 1661, 1584, 1514, 1455, 1388, 1260, 1065, 971, 836, 763, 701; 1H-NMR (400 MHz, CD3OD, d, ppm): 6.05 (s, 1H, H-2), 7.90 (d, J = 8.0 Hz, 2H, H-2’/6’), 6.78 (d, J = 8.0 Hz, 2H, H-3’/5’), 7.43 (d, J = 8.0 Hz, 2H, H-2’’/6’’), 7.34 (t, J = 8.0 Hz, 2H, H-3’’/5’’), 7.26 (d, J = 8.0 Hz, 1H, H-4’’), 8.52 (bs, Ar - OH); 5.02 (bs, 1H, - OH); 13C-NMR (100 MHz, CD3OD, d, ppm): 197.34 (C-1), 75.45 (C-2), 125.90 (C-1’), 131.35 (C-2’), 114.83 (C-3’), 162.65 (C-4’), 114.83 (C-5’), 131.35 (C-6’), 139.74 (C-1’’), 127.42 (C-2’’), 128.45 (C-3’’), 127.88 (C-4’’), 128.45 (C-5’’), 127.42 (C-6’’).
Compound 3 (2-Hydroxy-1-(3,5-dihydroxyphenyl)-2-phenylethanone): Yield: 40%; Rf = 0.48 (chloroform-ethyl acetate-acetic acid: 25:10:1); brown oil; UV (MeOH) λ max nm (logɛ): 213 (2,57); FT-IR (cm–1): 3367, 3028, 2960, 1681, 1598, 1452, 1341, 1304, 1164, 1082, 1036, 1004, 699; 1H-NMR (400 MHz, CDCl3/CD3OD, d, ppm): 5.80 (s, 1H, H-2), 6.80 (d, J = 3.0 Hz, 2H, H-2’/6’), 6.40 (t, J = 3.0 Hz, 1H, H-4’), 7.22-7.15 (m, 5H, H-2’’/3’’/4’’/5’’/6’’), 8.79 (bs, -OH), 5.80 (bs, 1H, -OH); 13C-NMR (100 MHz, CDCl3/CD3OD, d, ppm): 199.26 (C-1), 75.92 (C-2), 135.31 (C-1’), 107.91 (C-2’), 157.95 (C-3’), 108.54 (C-4’), 157.95 (C-5’), 107.91 (C-6’), 138.53 (C-1’’), 127.66 (C-2’’), 129.05 (C-3’’), 128.59 (C-4’’), 129.05 (C-5’’), 127.66 (C-6’’); Positive LC-QTOF-MS m/z (%): [M+K-H]+ 282.2693(67), calc. 282.2670.
Compound 4 (1,2-Bis(3-hydroxyphenyl)-2-hydroxyethanone): Yield: 68%; Rf = 0.45 (chloroform-ethyl acetate-acetic acid: 25:10:1); FT-IR (cm–1): 3320, 2946, 1678, 1586, 1486, 1452, 1277, 1234, 1069, 1016, 996, 876, 779; 1H-NMR (400 MHz, CDCl3/CD3OD, d, ppm): 5.84 (s, 1H, H-2), 6.79 (s, 1H, H-2’), 6.70-6.67 (m, 1H, H-4’), 7.36-7.31 (m, 2H, H-5’/5’’), 7.14 (d, 1H, J = 8.0 Hz, H-6’), 6.77 (s, 1H, H-2’’), 6.96-6.94 (m, 1H, H-4’’), 7.05 (d, J = 8.0 Hz, 1H, H-6’’), 5.07 (bs, 2-OH); 13C-NMR (100 MHz, CDCl3/CD3OD, d, ppm): 199.15 (C-1), 75.89 (C-2), 134.66 (C-1’), 115.87 (C-2’), 156.92 (C-3’), 115.40 (C-4’), 130.31 (C-5’), 121.47 (C-6’), 139.83 (C-1’’), 114.61 (C-2’’), 156.83 (C-3’’), 119.28 (C-4’’), 129.83 (C-5’’), 120.77 (C-6’’).
Compound 5 (2-Hydroxy-2-(3-hydroxyphenyl)-1-(4-hydroxyphenyl)ethanone): Yield: 39%; Rf = 0.46 (chloroform-ethyl acetate-acetic acid: 25:10:1); light yellow oil; FT-IR (cm–1): 3292, 3045, 2211, 1740, 1668, 1590, 1514, 1453, 1239, 1171, 982; 1H-NMR (400 MHz, CD3OD, d, ppm): 5.98 (s, 1H, H-2), 7.89 (d, J = 8.0 Hz, 2H, H-2’/6’), 6.80 (d, J = 8.0 Hz, 2H, H-3’/5’), 6.89 (s, 1H, H-2’’), 6.73 (d, J = 8.0 Hz, 1H, H-4’’), 7.14 (t, J = 8.0 Hz, 1H, H-5’’), 6.90 (d, J = 8.0 Hz, 1H, H-6’’), 5.21 (bs, -OH); 13C-NMR (100 MHz, CD3OD, ppm): 197.43 (C-1), 75.42 (C-2), 125.89 (C-1’), 131.46 (C-2’), 115.00 (C-3’), 162.60 (C-4’), 115.00 (C-5’), 131.46 (C-6’), 140.96 (C-1’’), 114.20 (C-2’’), 157.41 (C-3’’), 115.18 (C-4’’), 129.75 (C-5’’), 118.90 (C-6’’); Positive LC-QTOF-MS m/z (%): [M+K+Na+CH3OH-H]+ 337.2229(85), calc.337.2214.
Compounds 6a and 6b (2-Hydroxy-1-(3,5-dihydroxyphenyl)-2-(3-hydroxyphenyl)ethanone) and (2-Hydroxy-1-(3-hydroxyphenyl)-2-(3,5-dihydroxyphenyl)ethanone): Yield: 55%; Rf = 0.45 (chloroform-ethyl acetate-acetic acid: 25:10:1). Mix. m.p. (oC): 110-112; UV (MeOH) λ max nm (logɛ):210 (4,28); FT-IR (cm–1): 3363, 2915, 1682, 1600, 1457, 1339, 1283, 1165, 999, 722; 1H-NMR (400 MHz, CDCl3/(CD3)2CO, d, ppm): 7.89-6.70 (m, 14H, Ar-H), 6.10, 5.98 (s, s 1H/1H, 2x H-2); 9.60 (bs, Ar-OH); 13C-NMR (100 MHz, CDCl3/(CD3)2CO, d, ppm): 198.73, 193.62 (C=O), 77.78, 75.76 (C-2), 165.77, 158.51, 158.25, 158.19, 157.40, 156,94 136.26, 135.72, 135.40, 134.88, 130.80, 130.41 (Ar-C), 130.01, 129.67, 129.44, 121.10, 120.97, 120.74, 120.61, 120.14, 119.78, 116.39, 108.33, 107.56, 107.28, 103.79 (Ar-CH); Positive LC-QTOF-MS m/z (%): [M-H2O+CH3OH]+ 274.2644(100), calc. 274.2647.
Compound 7 (1,2-Bis(3,5-dihydroxyphenyl)-2-hydroxyethanone): Yield: 65%; Rf = 0.35 (chloroform-ethyl acetate-acetic acid: 25:10:1); light brown oil; UV (MeOH) λ max nm (logɛ):220(3,40); FT-IR (cm-1): 3360, 3160, 3037, 2917, 1687, 1594, 1453, 1343, 1306, 1166, 1006, 951, 707.; 1H-NMR (400 MHz, CDCl3/CD3OD, d, ppm): 5.75 (s, 1H, H-2), 6.80 (d, J = 3.0 Hz, 2H, H-2’/6’), 6.40 (bs, 1H, H-4’), 6.31 (d, J = 3.0 Hz, 2H, H-2’’/6’’), 6.22 (bs, 1H, H-4’’); 13C-NMR (100 MHz, CDCl3/CD3OD, d, ppm): 199.02 (C-1), 75.87 (C-2), 136.08 (C-1’), 107.49 (C-2’), 158.62 (C-3’), 108.29 (C-4’), 158.62 (C-5’), 107.49 (C-6’), 141.33 (C-1’’), 106.44 (C-2’’), 158.50 (C-3’’), 102.90 (C-4’’), 158.50 (C-5’’), 106.44 (C-6’’); Positive LC-QTOF-MS m/z (%): [M+CO2-H2O+2H]+ 304.2571(85), calc. 304.2500, [M+CO]+ 304.2526(80), calc. 304.2500.
Synthesis of hydroxy benzils (8-14): Hydroxy benzoins (100–400 mg) were dissolved in acetone (5 mL) and conc. HNO3 (2-3 mL) was added, and the reactions were stirred at 50–70 °C for 30–120 min [31]. The reactions were terminated after the TLC control. Acetone was evaporated, then water (30 mL) was added to the flask, extracted with ethyl acetate (3×30 mL) to give crude mixture then compounds 8-15 were purified with repeated VLC (Silica gel 230–400 mesh) using the increasing polarity of n -hexane, chloroform, ethyl acetate, and methanol solvent mixtures, and the fractions were checked by TLC (Figure). The synthesis of compounds 8 [23], 9 [9], 11 [44], and 15 [commercial product] had been mentioned in the literature.
Compound 8 (1-(3-Hydroxyphenyl)-2-phenylethane-1,2-dione): Yield: 25%;Rf = 0.55 (chloroform-ethyl acetate-acetic acid: 25:10:1); light yellow oil; FT-IR (cm–1): 3396, 2933, 1671, 1597, 1450, 1303, 1263, 1176, 942, 840, 780, 749, 635; 1H-NMR (400 MHz, CD3OD, d, ppm): 7.55 (d, J = 7.8 Hz, 1H, H-6’), 7.35 (m, 1H, H-5’), 7.28 (s, 1H, H-2’), 7.13 (m, 1H, H-4’); 7.91 (d, J = 8.0 Hz, 2H, H-2’’/6’’), 7.54 (t, J = 8.0 Hz, 2H, H-3’’/5’’), 7.69 (t, J = 8.0 Hz, 1H, H-4’’); 13C-NMR (100 MHz, CD3OD, d, ppm): 196.58 (C-1), 202.50 (C-2), 134.40 (C-1’), 116.36 (C-2’), 159.60 (C-3’), 122.42 (C-4’), 131.53 (C-5’), 123.62 (C-6’), 135.62 (C-1’’), 130.81 (C-2’’), 130.40 (C-3’’), 136.30 (C-4’’), 130.40 (C-5’’), 130.81 (C-6’’).
Compound 9 (1-(4-Hydroxyphenyl)-2-phenylethane-1,2-dione): Yield: 16%; Rf = 0.53 (chloroform-ethyl acetate-acetic acid: 25:10:1); light yellow oil; FT-IR (cm-1): 3368, 3027, 2927, 2856, 1740, 1678, 1599, 1582, 1448, 1369, 1267, 1213, 1164, 1043, 879, 719, 611; 1H-NMR (400 MHz, CDCl3/(CD3)2CO, d, ppm): 7.83 (d, J = 8.0 Hz, 2H, H-2’/6’), 6.90 (d, J = 8.0 Hz, 2H, H-3’/5’), 7.94 (d, J = 8.0 Hz, 2H, H-2’’/6’’), 7.47 (t, J = 8.0 Hz, 2H, H-3’’/5’’), 7.62 (t, J = 8.0 Hz, 1H, H-4’’); 13C-NMR (100 MHz, CDCl3/(CD3)2CO, d, ppm): 193.49 (C-1), 195.35 (C-2), 125.15 (C-1’), 132.72 (C-2’), 116.15 (C-3’), 163.40 (C-4’), 116.15 (C-5’), 132.72 (C-6’), 133.12 (C-1’’), 129.88 (C-2’’), 128.98 (C-3’’), 134.83 (C-4’’), 128.98 (C-5’’), 129.88 (C-6’’).
Compound 10 (1-(3,5-Dihydroxyphenyl)-2-phenylethane-1,2-dione): Yield: 35%; Rf = 0.48 (chloroform-ethyl acetate-acetic acid: 25:10:1); light brown oil; FT-IR (cm-1): 3434, 2964, 1747, 1598, 1450, 1368, 1227, 1166, 1035; UV (MeOH) λ max nm (logɛ): 220(3,40); 1H-NMR (400 MHz, CDCl3/ (CD3)2CO, d, ppm): 6.94 (d, J = 3.0 Hz, 2H, H-2’/6’), 7.35 (bs, 1H, H-4’); 7.92 (d, J = 8.0 Hz, 2H, H-2’’/6’’), 7.50 (t, J = 8.0 Hz, 2H, H-3’’/5’’), 7.65 (t, J = 8.0 Hz, 1H, H-4’’), 9.08 (bs, -OH); 13C-NMR (100 MHz, CDCl3/ (CD3)2CO, d, ppm): 195.04 (C-1), 195.15 (C-2), 132.96 (C-1’), 108.30 (C-2’), 158.88 (C-3’), 110.04 (C-4’), 158.88 (C-5’), 108.30 (C-6’), 134.42 (C-1’’), 129.75 (C-2’’), 128.95 (C-3’’), 134.77 (C-4’’), 128.95 (C-5’’), 129.75 (C-6’’); Positive LC-QTOF-MS m/z (%): [M+Na+K]+ 304.2539(100), calc. 304.2580; [M+K+H]+ 282.2722(100), calc. 282.2753.
Compound 11 (1,2-Bis(3-hydroxyphenyl)ethane-1,2-dione): Yield : 45%; Rf = 0.45 (chloroform-ethyl acetate-acetic acid: 25:10:1); FT-IR (cm–1): 3380, 2960, 2931, 2874, 1736, 1646, 1618, 1582, 1452, 1350, 1225, 1194, 1108, 983, 865, 785, 684; 1H-NMR (400 MHz, (CD3)2CO, d, ppm): 7.72 (m, 6H-4’/4’’/5’/5’’/6’/6’’), 7.25 (m, 2H, H-2’/2’’), 9.00 (bs, 2H, -OH); 13C-NMR (100 MHz, (CD3)2CO, d ppm): 194.95 (C-1/2) 134.32 (C-1’/1’’), 115.03 (C-2’/2’’), 158.10 (3’/3’’), 121.25 (4’/4’’), 130.55 (5’/5’’), 122.41 (6’/6’’).
Compound 12 (1-(4-Hydroxyphenyl)-2-(3-hydroxyphenyl)ethane-1,2-dione: Yield: 23%;Rf = 0.40 (chloroform-ethyl acetate-acetic acid: 25:10:1); m.p. (oC): 60–62; UV (MeOH) λ max nm (logɛ): 203(4,03); FT-IR (cm-1): 3436, 2947, 1751, 1598, 1450, 1369, 1232, 1166, 1034; 1H-NMR (400 MHz, (CD3)2CO, d, ppm): 7.05 (s, 1H, H-2’), 7.38–7.24 (m, 2H, H-4’, 6’), 7.22 (t, 1H, J = 7.8 Hz, H-5’), 7.55/7.51 (s, s, 1H/1H, H-2’’, 6’’), 6.70/6.66 (s, s, 1H/1H, H-3’’, 5’’), 5.14 (bs, -OH); 13C-NMR (100 MHz, (CD3)2CO, d, ppm): 200.15 (C-1/2), 163.74, 157.64, 135.73, 129.73 (Ar-C), 149.93, 144.61, 132.15, 129.73, 126.36, 119.89, 117.64, 115.54, 114.19 (Ar-CH), 191.58 (-CHO); Positive LC-QTOF-MS m/z (%): [M+CH3OH] + 274.2679(90), calc. 274.2695.
Compound 13 (1-(3,5-Dihydroxyphenyl)-2-(3-hydroxyphenyl)ethane-1,2-dione): Yield: 18%; Rf = 0.42 (chloroform-ethyl acetate-acetic acid: 25:10:1); light brown oil; UV (MeOH) λ max nm (logɛ): 211(4,34); FT-IR (cm-1): 3372, 2957, 1675, 1603, 1453, 1279, 1245, 1171; 1H-NMR (400 MHz, CDCl3/ (CD3)2CO), d, ppm): 7.15 (d, J = 3.0 Hz, 2H, H-2’, 6’), 6.93 (dd, J = 3.0/3.0Hz, 1H, H-4’), 7.68-7.25 (m, 4H, H-2’’,4’’,5’’,6’’), 8.86/8.75 (bs, 3x Ar-OH); 13C-NMR (100 MHz, CDCl3/ (CD3)2CO), d, ppm): 191.67 (C-1), 191.95 (C-2), 133.90 (C-1’), 107.97 (C-2’), 158.32 (C-3’), 109.31 (C-4’), 158.32 (C-5’), 107.97 (C-6’), 134.35 (C-1’’), 115.26 (C-2’’), 157.22 (C-3’’), 121.19 (C-4’’), 129.75 (C-5’’), 122.00 (C-6’’); Positive LC-QTOF-MS m/z (%): [M-H2O+CH3OH+2H]+ 274.2711(100), calc. 274.2720.
Compound 14 (1,2-Bis(3,5-dihydroxyphenyl)ethane-1,2-dione): Yield: 28%;Rf = 0.38 (chloroform-ethyl acetate-acetic acid: 25:10:1); FT-IR (cm-1): 3369, 2938, 1726, 1602, 1366, 1267, 1221, 1165, 1034; 1H-NMR (400 MHz, CDCl3/ (CD3)2CO, d, ppm): 6.85 (s, 4H, H-2’/6’/2’’/6’’), 6.69 (s, 2H, H-4’/4’’); 13C-NMR (100 MHz, CDCl3/ (CD3)2CO, d, ppm): 195.48 (C-1/2), 134.79 (C-1’/1’’), 107.45 (C-2’/6’/2’’/6’’), 159.58 (C-3’/5’/3’’/5’’), 109.39 (C-4’/4’’).
Synthesis of benzoin/benzil-D-glucosides (15-25): Hydroxy benzoins (100-150 mg each, 1-7 ) or benzils (100-200 mg each, 8-14 ) were dissolved in anhydrous methanol (10 mL) under the inert nitrogen atmosphere. KOH (2-4 equiv.) dissolved in methanol (5 mL) and added to the reaction mixtures, which were stirred in an ice bath for half an hour. Then, tetra-O-acetyl- α -D-bromoglucose (TABG, 4 equiv.) in acetone was added to the reaction medium and stirred at room temperature for 12 h [36–38]. As a result of the TLC control of the reactions, NaOMe (5 equiv.) was added to the medium, and the reactions were terminated after 12–24 h with the control of TLC. Excess of NaOMe was killed by the addition of MeOH. The solvent was evaporated, then water (15 mL) was added to the flask, extracted with ethyl acetate (3×20 mL) to give crude mixture then compounds 15-25 were purified with repeated VLC (Silica gel 230–400 mesh) using the increasing polarity of n -hexane, chloroform, ethyl acetate, and methanol solvent mixtures, and the fractions were checked by TLC (Figure).
Compound 15 (2-Hydroxy-1-(3-O- β -D-glucopyranosylphenyl)-2-phenylethanone): Yield: 15%; diastereomer; Rf = 0.5 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 210(4,56); FT-IR (cm–1): 3342, 3020, 2924, 1676, 1641, 1596, 1448, 1400, 1256, 1072, 1040, 892; 1H-NMR (400 MHz, (CD3)2CO, d, ppm): 7.67–7.21 (m, 18H, Ar-H), 6.13, 6.12 (m, 2H, H-2/H-2), 5.09 (d, J = 7.6 Hz, 1H, Glu H-1), 5.01(d, J = 7.6 Hz, 1H, Glu H-1), 4.75-3.22 (m, 12H, glucose H2-H6); 13C-NMR (100 MHz, (CD3)2CO, d, ppm): 191.83 (C = O), 163.53, 144.58, 133,81, 133,49 (Ar-C), 131.43, 130.21, 129,64, 128.65, 123.29, 122.87, 122.53, 121.02, 117.55, 116.58 (Ar-CH), 101.23, 101.03 (anomeric CH), 76.95, 76.92 (C-2), 77.0, 73.80, 73.76, 70.73 (glucose CH), 61.67 (glucose CH2). Positive LC-QTOF-MS m/z (%): [M+Na]+ 413.2563(20), calc. 413.2549.
Compound 16 (2-Hydroxy-1-(4-O- β -D-glucopyranosylphenyl)-2-phenylethanone): Yield: 17%; diastereomer; Rf = 0.68 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 210(5,43); FT-IR (cm–1): 3374 3018, 2927, 1582, 1410, 1348, 1313,1160, 1078, 1048, 610; 1H-NMR (400 MHz, CD3OD, d, ppm): 7.64 (bd, 4H, H-2’/H-6’), 6.40 (bd, 4H, H-3’/H-5’), .7.42–7.10 (m, 10H, H-2’’-6’’), 4.80 (anomeric CH remained within the water peak), 4.62-3.12 (m, 12H, glucose H2-H6); 13C-NMR (100 MHz, CD3OD, d, ppm): 196.29 (C=O), 165.07, 140.89, 119.28 (Ar-C), 131.98, 128.35, 127.63, 127.39, 118.76 (Ar-CH), 103.99 (anomeric CH), 76.53 (benzoin CH), 76.41, 74.41, 73.60, 69.99 (glucose CH), 60.69 (glucose CH2). Positive LC-QTOF-MS m/z (%): [M+Na]+ 413.1121(9), calc. 413.1141; [M+Na-H]+ 412.1015(23), calc. 412.1063.
Compound 17 (2-Hydroxy-1-(3,5-Di-O- β -D-glucopyranosylphenyl)-2-phenylethanone): Yield: 14%; diastereomer (2:1); Rf = 0.74 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 211(3,52); FT-IR (cm–1): 3367, 2972, 2270, 1720, 1269, 1057; 1H-NMR (400 MHz, (CD3)2CO, d, ppm): 8.05-6.48 (m, 16H, Ar-H), 6.06/6.00 (s, s, 1H, 1H, H-2/H-2), 5.03 (d, J = 7.6 Hz, 1H, Glu H-1), 4.98 (d, J = 7.6 Hz, 1H, Glu H-1), 4.46–3.32 (m, 24H, glucose H2-H6); 13C-NMR (100 MHz, (CD3)2CO, d, ppm): 199.00 (C=O), 159.02, 158.57, 141.90, 135.09 (Ar-C), 129.46, 129.02, 128.72, 128.65, 127.64, 127.52, 110.16, 109.95, 108.21, 108.02 (Ar-CH), 100.89/100.83 (anomeric CH), 76.90, 76.84, 76.06, 74.12, 73.84, 73.65, 70.38, 70.17 (benzoin CH and glucose CH), 63.56/63.38 (glucose CH2); Positive LC-QTOF-MS m/z (%): [M+K-CH3OH-2H]+ 573.1286(100), calc. 573.1249.
Compounds 18a+b (2-Hydroxy-1-(3-O- β -D-glucopyranosylphenyl)-2-(3-hydroxyphenyl)-ethanone; 2-Hydroxy-1-(3-hydroxyphenyl)-2-(3-O- β -D-glucopyranosylphenyl)ethanone): Yield: 11%; diastereomer (2:1); Rf = 0.80 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 220(3,45); FT-IR (cm–1): 3343, 3030, 2923, 1636, 1586, 1447, 1397, 1251, 1067, 1033, 1014, 892, 786; 1H-NMR (400 MHz, CD3OD, d, ppm): 7.48–6.55 (m, 32H, Ar-H), 6.00, 5.95 (m, 4H, H-2/H-2), 4.84–4.74 (anomeric CH remained within the water peak), 4.43-3.17 (m, 24H, glucose H2-H6); 13C-NMR (100 MHz, CD3OD, d, ppm): 198.97, 198.94, 198.73, 198.50 (C=O), 157.80, 157.59, 157.54, 157.44, 140.64, 140.59, 140.37, 140.32, 135.97, 135.86, 135.84, 135.74 (Ar-C), 129.75-114.29 (Ar-CH), 103.93, 103.78, 103.1, 100.64 (anomeric -CH), 76.45, 76.37, 76.25, 76.13, 73.98, 73.63, 73.65, 73.56, 70.65, 70.13 (benzoin CH and Glucose CH (C-2-5)), 63.45, 63.38 (Glucose -CH2OH); Positive LC-QTOF-MS m/z (%): [M+C6H12O6-CH3OH-H]+ 563.5404(100), calc. 563.5404.
Compound 19 (2-Hydroxy-1-(4-O- β -D-glucopyranosylphenyl)-2-(3-O- β -D-glucopyranosyl-phenyl)ethanone): Yield: 18%; diastereomer (1:2); Rf = 0.45 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 213(4,58); FT-IR (cm-1): 3380, 3032, 2924, 1734, 1596, 1450, 1376, 1250, 1053; 1H-NMR (400 MHz, CD3OD, d, ppm): 8.02 (d, J = 7.8 Hz, 8H, H-2’/6’), 7.34 (d, J = 7.8 Hz, 8H, H-3’/5’), 7.80-7.45 (m, 16H, H-2’’,4’’,5’’,6’’), 6.11, 6.08 (s, s, 1H, 1H, H-2/H-2), 5.13–5.08 (anomeric CH beside the water peak), 4.52–3.38 (m, 48H glucose CH and CH2); 13C-NMR (100 MHz, CD3OD, d, ppm): 193.74, 192.80 (C=O), 76.50, 76.03 (C-2), 164.01, 159.30, 139.28, 114.59 (Ar-C), 130.28, 129.8, 122.25, 122.15, 122.06, 119.88, 116.14 (Ar-CH), 102.05, 101.36 (anomeric -CH), 77.90, 74.63, 74,55, 73.45, 73.33, 73.21, 69.89, 69.67, 69.54, 68.52 (Glucose C2-C5), 62.92, 60.26 (Glucose -CH2); Positive LC-QTOF-MS m/z (%): [M+Na]+ 575.2733(75), calc. 575.2740.
Compound 20 (2-Hydroxy-1-(3,5-di-O- β -D-glucopyranosylphenyl)-2-(3-O- β -D-glucopyranosylphenyl)ethanone): Yield: 12%; diastereomer; Rf = 0.5 (chloroform-methanol: 8:2); light yellow oily; UV (MeOH) λ max nm (logɛ): 213(4,03); FT-IR (cm–1): 3385, 3028, 2923, 2568, 1688, 1597, 1456, 1287, 1075, 1034; 1H-NMR (400 MHz, CD3OD, d, ppm): 7.74-6.87 (m, 16H, Ar-H), 5.84, 5.71 (s, s, benzoin -CH), 5.04–4.8 (anomeric CH beside the water peak), 4.65-3.30 (m, 36H, glucose CH and CH2); 13C-NMR (100 MHz, CD3OD, d, ppm): 198.73, 194.24 (C=O), 76.74 (C-2), 166.90, 160.50, 158.48, 158.27, 158.20, 157.89, 157.84 139.25, 138.64, 134.41, 134.28, 131.85, 131.22 (Ar-C), 131.22, 129.88, 129.63, 124.10, 123.65, 120.32, 120.11, 120.08, 119.87, 116.59, 116.20, 116.06, 109.33, 109.16, 108.78 (Ar-CH), 103.97, 100.72 (anomeric CH), 76.48, 76.16, 74.98, 72.56, 69.81 (Glucose CH (C-2-5)), 61.31, 61.01 (glucose CH2); Positive LC-QTOF-MS m/z (%): [M+Na-H2O-H]+ 750.5818(74), calc. 750.5836; [M+Na-CH3OH-H]+ 736.5696(100), calc. 736.5600.
Compound 21 (1-(3-O- β -D-Glucopyranosylphenyl)-2-phenylethane-1,2-dione): Yield: 18%; Rf = 0.77 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 205(4,61);FT-IR (cm–1): 3364, 2938, 1739, 1665, 1445, 1227, 1074; 1H-NMR (400 MHz, (CD3OD, d, ppm): 7.96-7.31 (m, 9H, Ar-H), 4.99 (d, J = 7.6 Hz, anomeric CH), 4.49-3.33 (m, 6H, glucose CH and CH2); 13C-NMR (100 MHz, (CD3OD, d, ppm): 194.51, 192.42 (C=O), 158.10, 134.94, 132.82 (Ar-C), 134.16, 130.07, 129.39, 128.98, 124.20, 123.60, 115.90 (Ar-CH), 100.68 (anomeric CH), 76.26, 74.12, 73.32, 70.21 (glucose CH), 63.42 (glucose CH2); Positive LC-QTOF-MS m/z (%): [M-2CH3OH+H]+ 325.2283(100), calc. 325.2280.
Compound 22 (1-(4-O- β -D-Glucopyranosylphenyl)-2-phenylethane-1,2-dione): Yield: 12%;Rf = 0.77 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 210(3,92); FT-IR (cm-1): 3385, 2972, 1710, 1603, 1445, 1270, 1058; 1H-NMR (400 MHz, ((CD3)2CO, d, ppm): 7.94 (m, 4H, H-2’,6’, H-2’’,6’’), 7.63 (t, J = 7.6 Hz, 2H, H-3’’, 5’’), 7.74 (t, J = 7.7 Hz, 1H, H4’’), 7.22 (d, J = 7.8 Hz, 2H, H-3’, 5’), 5.20 (d, J = 7.6 Hz, 1H, Glu H-1), 4.43-3.43 (m, 6H, glucose H2-H6); 13C-NMR (100 MHz, ((CD3)2CO, d ppm): 198.00, 194.58 (C=O), 161.59, 133.36, 126.93 (Ar-C), 134.98, 131,88, 129.57, 129.24, 116.72 (Ar-CH), 100.11 (anomeric CH), 76.80, 74.26, 73.58, 70.15 (glucose CH), 63.24 (glucose CH2); Positive LC-QTOF-MS m/z (%): [M+K+Na+3H]+ 453.1011(100), calc. 453.1016.
Compound 23 (1-(3,5-Di-O- β -D-glucopyranosylphenyl)-2-phenylethane-1,2-dione): Yield: 9%; Rf = 0.75 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 215(4,03); FT-IR (cm–1): 3627, 2975, 2256, 1713, 1524, 1386, 1058; 1H-NMR (400 MHz, (CD3)2CO, d, ppm): 7.80 (d, J = 7.8 Hz, 2H, H-2’’, 6’’), 7.80-7.40 (m, 3H, H-3’’, 4’’, 5’’), 7.08 (bs, 2H, H-2’, 6’), 6.95 (bs, 1H, H4’), 4.98 (d, J = 7.6 Hz , Glu H-1), 4.30-3.44 (m, 6H, glucose H2-H6); 13C-NMR (100 MHz, (CD3)2CO, d, ppm): 194.98, 193.68 (C=O), 159.27, 134.73, 133.96 (Ar-C), 135.21, 129.55, 129.45, 129.33, 124.71, 110.25, 109.18 (Ar-CH), 101.05 (anomeric CH), 77.08, 73.67, 70.21 (glucose CH), 61.57 (glucose CH2); Positive LC-QTOF-MS m/z (%): [M-CH3OH-CO2-3H]+ 325.2162(100), calc. 325.2162.
Compound 24 (1-(3-O- β -D-Glucopyranosylphenyl)-2-(3-hydroxyphenyl)ethane-1,2-dione): Yield: 42%; Rf = 0.75 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 210(4,67); FT-IR (cm–1): 3364, 2938, 1739, 1665, 1445, 1227, 1075; 1H-NMR (400 MHz, (CD3OD, d, ppm): 7.78-6.77 (m, 8H, H-2’,4’,5’,6’, H-2’’,4’’,5’’,6’’), 4.97 (d, J = 7.8 Hz , anomeric CH), 3.96-3.17 (m, 6H, glucose CH and CH2); 13C-NMR (100 MHz, (CD3OD, d, ppm): 193.42, 193.35, (C=O), 156.56, 135.01, 134.50, 132.58 (Ar-C), 128.54, 128.60, 126.72, 123.45, 125.82, 121.65, 120.91, 114.28 (Ar-CH), 102.43 (anomeric -CH), 75.20, 74.96, 72.10, 68.68 (glucose CH), 59.77 (glucose CH2); Positive LC-QTOF-MS m/z (%) : [M+Na-H]+ 588.4345(100), calc. 588.4387.
Compound 25 (1-(3-O- β -D-Glucopyranosylphenyl)-2-(4-hydroxyphenyl)ethane-1,2-dione):
Yield: 12%; Rf = 0.60 (chloroform-methanol: 8:2); light yellow oil; UV (MeOH) λ max nm (logɛ): 206(4,97); FT-IR (cm-1): 3748, 3620, 2973, 2302, 1732, 1386, 1228, 1057; 1H-NMR (400 MHz, (CD3OD, d, ppm): 7.42 (s, 1H, H-2’), 7.38–7.24 (m, 2H, H-4’, 5’), 7.18 (d, 1H, J = 7.8 Hz, H-6’), 7.66/7.64 (s, s, 1H/1H, H-2’’, 6’’), 6.83/6.79 (s, s, 1H/1H, H-3’’, 5’’), 4.96 (d, J = 7.6 Hz , 1H, Glu H-1), 4.31–3.37 (m, 6H, glucose H2-H6); 13C-NMR (100 MHz, (CD3OD, d, ppm): 192.34, 191.98 (C=O), 158.20, 143.92, 135.94, 126.86 (Ar-C), 129.67, 129.18, 126.33, 122.95, 119.40, 117.31, 115.17, 114.28 (Ar-CH), 100.95 (anomeric CH), 76.90, 76.57, 73.51, 70.04 (glucose CH), 61.14 (glucose CH2); Positive LC-QTOF-MS m/z (%): [M+H+C6H12O6]+ 585.5184(15), calc. 585.5156.
2.1. Biological activities
2.1.1. Antioxidant activity
Antioxidant activities of the synthetic compounds 1-25 were tested against iron (III) / ferric reducing antioxidant power (FRAP), Cu (II) reducing antioxidant capacity (CUPRAC), and 2,2-Diphenyl-1-picrylhydrazyl radical quenching capacity (DPPH) methods according to the literature [45–50] (Table 2). Butylated hydroxytoluene for DPPH and Trolox for CUPRAC and FRAP was used as standard.
Table 2.
Antioxidant (FRAP, CUPRAC, and DPPH) activities of compounds 1-25.
| Hydroxy Benzoin | |||
|---|---|---|---|
| No | FRAPa | CUPRACb | DPPHc |
| 1 | 1238 ± 34.7 | 738.33 ± 12.5 | 15.21 ± 2.1 |
| 2 | 1881 ± 75.5 | 140.00 ± 11.5 | 13.78 ± 1.3 |
| 3 | 1111 ± 47.9 | 90.00 ± 6.8 | 8.72 ± 0.26 |
| 4 | 1534 ± 75.0 | 398.33 ± 22.1 | 8.16 ± 0.3 |
| 5 | 2090 ± 101.4 | 1113.33 ± 64.9 | 8.12 ± 1.2 |
| 6a+b | 2237 ± 58.3 | 506.67 ± 17.3 | 9.48 ± 0.3 |
| 7 | 1715 ± 96.8 | 95.00 ± 2.4 | 10.85 ± 0.7 |
| Hydroxy Benzil | |||
| 8 | 1678 ± 64.6 | 1095.00 ± 18.1 | 13.56 ± 1.2 |
| 9 | 1946 ± 83.7 | 48.33 ± 5.5 | 8.64 ± 0.5 |
| 10 | 1830 ± 44.8 | 455.00 ± 10.1 | 52.10 ± 0.4 |
| 11 | 1340 ± 37.9 | 175.00 ± 3.5 | 10.03 ± 0.8 |
| 12 | 1555 ± 34.1 | 155.00 ± 1.0 | 12.87 ± 0.9 |
| 13 | 1844 ± 56.2 | 143.33 ± 7.6 | 9.42 ± 0.8 |
| 14 | 1974 ± 76.9 | 133.33 ± 10.9 | 7.38 ± 1.0 |
| Benzoin-O-β-D-Glucoside | |||
| 15 | 2311.04 ± 31.21 | 141.74 ±28.35 | 107.60 ± 9.09 |
| 16 | 1956.25 ± 48.13 | 73.54 ± 17.36 | 44.75 ± 3.04 |
| 17 | 2647.92 ± 31.92 | 287.40 ± 9.81 | 8.22 ± 1.08 |
| 18a+b | 2743.75 ± 25.20 | 385.80 ± 37.12 | 12.11 ± 0.43 |
| 19 | 2612.50 ± 37.04 | 421.74 ± 24.39 | 8.86 ± 0.37 |
| 20 | 2918.75 ± 36.14 | 775.58 ± 12.34 | 7.78 ± 0.12 |
| Benzil-O-β-D-Glucoside | |||
| 21 | 1981.25 ± 28.33 | 109.94 ± 19.42 | 36.94 ± 1.71 |
| 22 | 1845.42 ± 41.36 | 248.97 ± 34.06 | 47.54 ± 1.05 |
| 23 | 2267.50 ± 24.14 | 273.67 ± 27.23 | 17.74 ± 0.35 |
| 24a,b | 2401.25 ± 56.34 | 251.46 ± 43.08 | 25.40 ± 0.19 |
| 25 | 2570.42 ± 25.01 | 147.54 ± 25.04 | 26.33 ± 0.39 |
| BHT | - | - | 6.47 ± 0.12 |
aFRAP, the iron reducing antioxidant power (μg/mL trolox/gram DW), bCUPRAC, copper reducing antioxidant power (μg/mL trolox/gram DW), cDPPH, 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity (mg/mL), BHT: di-t-butylhydroxytoluene.
Ferric reducing antioxidant power (FRAP) assay: The method was carried out based on the determination of the iron ions reducing the samples’ power. First, 2,4,6-tripyridyl-s-triazine (31.2 mg, TPTz) was dissolved in a mixture of hydrochloric acid (50 μL) and distilled water (10 mL). Then, FeCl3 (32 mg) was dissolved in distilled water (10 mL). Finally, distilled water (250 mL) was added to acetic acid (4.1 mL, 80%), and sodium acetate (0.66 g) was completely dissolved in this solution. Buffer, TPTz, and FeCl3 were mixed at 10/1/1 ratios, and 2 mL of this mixture was mixed with 0.1 mL of compounds 1-25 (2 mg/mL) and incubated at 30oC for 30 min. As a standard, different concentrations of Trolox solution (15.63, 31.25, 62.5, 125, and 250 μg/mL) were used instead of the sample. At the end of the incubation, the samples’ absorbance was read at 595 nm, and the results are given as Trolox equivalents. Results were expressed as μmol Trolox/g dry weight of compounds 1-25 (μg/mL Trolox/g DW) [48,50] (Table 2).
Copper ions reducing activity (CUPRAC): In a test tube, ammonium acetate (1 mL, 1 M), CuCl2 (1 mL, 10 mM), and neocuproin (1 mL, 7.5 mM) solutions were taken, and 0.5 mL of compounds 1 - 25 and standards (Trolox) at different concentrations (15.63, 31.25, 62.5, 125 and 250 μg/mL) were mixed, and 1 mL of distilled water was added to each tube. After 30 min in a dark environment at room temperature, it was read against blank at 450 nm using Shimadzu UV-1600 spectrophotometer [49], and results are given in Table 2.
DPPH radical scavenging activity: In vitro antioxidant properties of compounds, 1-25 were tested using 2,2-diphenyl-1-picrylhydrazyl scavenging (DPPH). 0.75 mL of compounds 1 - 25 and standard (BHT) at varying concentrations (mg/mL) and 0.75 mL of 0.1 mM DPPH solution were mixed. All tubes were left in the dark for 50 min at room temperature. The absorbance was read 517 nm using Shimadzu UV-1600 spectrophotometer, and results are given as SC50 value (mg/mL) in Table 2 [45–47].
2.1.2. Microorganisms used for antimicrobial activity
The test microorganisms used in the study were obtained from Refik Saydam Hıfzısıhha Institute (Ankara, Turkey) and are as follows. Escherichia coli ATCC 25922 (Ec), Yersinia pseudotuberculosis ATCC911 (Yp), Pseudomonas aeruginosa ATCC27853 (Pa), Staphylococcus aureus ATCC25923 (Sa), Streptococcus mutans RSKK07038 (Sm), Enterococcus faecalis ATCC29212 (Ef), Paenibacillus larvae DSM7030 (PSP), Bacillus cereus Roma709 (Bc), Bacillus subtilis ATCC1266 (Bs), Mycobacterium smegmatis ATCC607 (Ms), Candida albicans ATCC60193 (Ca). Inhibition diameters were measured by the agar well diffusion method [51–53], and the MIC value was determined as microgram-milliliter (µg / mL) to the microdilution technics (Table 3).
Table 3.
Antimicrobial minimum inhibition concentrations of compounds 1-25.
| No | Stock sol.µg/mL | Microorganism and minimum inhibition concentration (MIC, µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram (-) | Gram (+) | Tub. | Mush. | |||||||
| Ec | Yp | Pa | Sa | Sm | Ef | Bc | Ms | Ca | ||
| Hydroxy Benzoin | ||||||||||
| 1 | 26400 | 330 | 1320 | - | - | - | - | 1320 | 165 | 330 |
| 2 | 41300 | 1032 | 1032 | 2065 | 1032 | 1032 | - | 1032 | 129 | 516 |
| 3 | 66300 | 820 | 1657 | 207 | 207 | 414 | 820 | 207 | 103 | 414 |
| 4 | 51200 | 320 | 640 | 320 | 640 | - | - | 160 | 160 | 1280 |
| 5 | 62000 | 387 | 775 | 96 | 96 | 48 | 1550 | 48 | 48 | - |
| 6a+b | 8300 | 103 | 103 | 52 | 12 | 12 | 24 | 24 | 12 | 103 |
| 7 | 22700 | 141 | 1135 | 283 | 283 | - | 141 | 141 | 70 | 283 |
| Hydroxy Benzil | ||||||||||
| 8 | 13400 | 167 | 167 | 11 | 11 | 21 | 21 | 11 | 11 | 41 |
| 9 | 17400 | 108 | 217 | 108 | 108 | - | 108 | 108 | 108 | 108 |
| 10 | 1300 | 17 | 65 | 32 | 65 | - | - | 17 | 17 | 17 |
| 11 | 5800 | 36 | 145 | 18 | 9 | 18 | - | 18 | 9 | 36 |
| 12 | 1500 | 75 | 38 | 75 | 38 | - | 18 | 75 | 18 | 18 |
| 13 | 13400 | 167 | 167 | 11 | 11 | 21 | 21 | 11 | 11 | 41 |
| 14 | 4100 | 102 | 102 | 51 | - | - | 51 | 102 | 51 | 51 |
| Amp. | 10 | 10 | 18 | >128 | 35 | NT | 10 | NT | - | - |
| Strep. | 10 | - | - | - | - | - | - | - | 4 | - |
| Flu. | 5 | - | - | - | - | - | - | - | - | <8 |
Ec: E. coli, Yp: Y. pseudotuberculosis, Pa: P. aeruginosa, Sa: S. aureus, Sm: S. mutans, Ef: E. faecalis, Psp: P. larvae, Bc: B. cereus, Bs: B. suptilis, Ms: M. smegmatis, Ca: C. albicans, (-): no result. Amp.: Ampicillin, Str.: Streptomycin, Flu.: Fluconazole, NT; not tested. Tub.: Tuberculosis
Antimicrobial activity assessment (agar-well diffusion method): The antimicrobial screening test using the agar-well diffusion method as adapted was used earlier [53–54]. Each microorganism was suspended in Mueller-Hinton broth (Difco, Detroit, MI) and diluted approximately 106 colony-forming units (CFU) per mL. They were “flood-inoculated” onto the surface of Mueller–Hinton agar, brain heart infusion agar, and potato dextrose agar (PDA) (Difco, Detriot, MI) and then dried. Brain heart infusion agar was used for M. smegmatis and S. mutans . For C. albicans, PDA was used. Five-millimeter diameter wells were cut from the agar using a sterile cork-borer, and 50 μL of the compound substances were delivered into the wells. The plates were incubated for 24–48 h at 36 °C. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test organism. Compound stock solutions were prepared at different concentrations (1.100–80.200 μg/mL) according to the amount of material obtained. The 1/10 dilution of each solvent was used as a control.
Minimal inhibition concentration (MIC) assay: The antimicrobial properties of compounds 1-25 were investigated quantitatively in respective broth media by using the microdilution method, and the minimal inhibition concentration (MIC) values (μg/mL) were examined [53]. The antibacterial activity assays were carried out in Mueller–Hinton broth (MHB) at pH = 7.0±0.2 and 18–24 h at 36 °C incubated. For the antifungal activity test were used yeast extract peptone dextrose (YEPD) broth (pH = 6.5 ± 0.2) and 48 h at 36 °C incubated. Brain heart infusion broth (BHI) (Difco, Detriot, MI) was used for M. smegmatis and S. mutans and incubated for 72 h at 36 °C. The minimal inhibition concentration value was defined as the lowest concentration that showed no growth. Ampicillin (10 mg/mL), streptomycin (10 mg/mL) and fluconazole (5 mg/mL) were used as standard antibacterial and antifungal drugs, respectively (Table 3). The 1/10 dilution of each solvent was used as a control.
2.1.3. Enzyme inhibitions
Acetylcholinesterase (AChE) inhibition: The acetylcholinesterase method is based on the principle that thiocholine released by a chromogenic reagent 5,5-dithio-bis-(2-nitrobenzoic acid) gives a colored product. The sample solution (10 μL) and acetylcholinesterase solution (20 μL) were mixed in Tris-HCl buffer (130 μL, pH 8.0). It was then incubated at 25 °C for 10 min in a 96-well microplate. Then, DTNB (20 μL) and acetylthiocholine iodide (20 μL) were mixed. Similarly, an enzyme-free tube was prepared blindly. Sample and blank absorbances were read after 10 min of incubation at 25 °C at 405 nm. Acetylcholinesterase inhibitory activity was given equivalent to galantamine [55], and the results were given in Table 4.
Table 4.
Enzyme inhibition of compounds 1-25, IC50 (μg/mL).
| Hydroxy Benzoin | |||||
|---|---|---|---|---|---|
| No | AChE | BChE | Tyrosinase | α-Amylase | α-Glucosidase |
| 1 | >1000 | 431.18 | 46.99 | >1000 | 58.16 |
| 2 | 244.54 | 73.62 | >1000 | >1000 | 547.97 |
| 3 | 397.36 | 144.49 | 83.14 | 206.25 | 812.10 |
| 4 | 110.96 | 112.86 | >1000 | 239.82 | >1000 |
| 5 | 787.84 | >1000 | 163.82 | 104.99 | >1000 |
| 6a+b | >1000 | 592.92 | 25.45 | 230.76 | 76.53 |
| 7 | 122.40 | >1000 | 130.44 | 97.51 | >1000 |
| Hydroxy Benzil | |||||
| 8 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 9 | 144.01 | >1000 | >1000 | 219.82 | 47.78 |
| 10 | 38.90 | >1000 | 54.44 | >1000 | 100.08 |
| 11 | 62.16 | 339.49 | >1000 | 349.71 | 66.700 |
| 12 | 501.53 | 462.11 | >1000 | >1000 | 54.69 |
| 13 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 14 | 70.39 | >1000 | 634.91 | >1000 | 87.10 |
| Galantamine | 9.27 | 33.73 | - | - | - |
| Kojic acid | - | - | 12.78 | - | - |
| Acarbose | - | - | - | 93.12 | 36.65 |
| Benzoin-O-β-D-Glucoside | |||||
| 15 | 11.97 | >1000 | >1000 | 251.48 | >1000 |
| 16 | 155.85 | 188.83 | >1000 | >1000 | >1000 |
| 17 | >1000 | >1000 | >1000 | >1000 | 193.40 |
| 18a+b | >1000 | 462.26 | >1000 | >1000 | >1000 |
| 19 | 10.52 | >1000 | 31.22 | 139.78 | 124.94 |
| 20 | >1000 | 189.56 | 71.81 | >1000 | 48.25 |
| Benzil-O-β-D-Glucoside | |||||
| 21 | >1000 | >1000 | 89.93 | 282.98 | >1000 |
| 22 | 132.83 | 527.25 | 727.98 | >1000 | 212.24 |
| 23 | 70.47 | >1000 | 208.42 | 101.97 | >1000 |
| 24 | 281.66 | 212.79 | 148.48 | 176.99 | 58.90 |
| 25 | 227.19 | 133.86 | 416.31 | >1000 | >1000 |
| Galantamine | 7.71 | 33.71 | - | - | - |
| Kojic acid | - | - | 15.68 | - | - |
| Acarbose | - | - | - | 85.83 | 36.87 |
Butyryl cholinesterase (BChE) inhibition: Butyrylcholinesterase inhibition is based on acetylcholine’s hydrolysis by cholinesterase to 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) into yellow colored 5-thio-2-nitrobenzoic acid. The sample solution (10 μL) and butyrylcholinesterase solution (20 μL) were mixed in Tris-HCl buffer (130 μL, pH = 8.0). It was then incubated at 25 °C for 10 min in a 96-well. Similarly, an enzyme-free tube was prepared blindly. Sample and blank absorbances were read after 10 min of incubation at 25 °C at 405 nm. Butyrylcholinesterase inhibitory activity was given equivalent to galantamine [55], and results were given in Table 4.
Tyrosinase inhibition: Tyrosinase inhibitor activity was performed by the dopachrome method using L-DOPA as a substrate. The sample solution (25 μL) was mixed with tyrosinase solution (40 μL) and phosphate buffer (100 μL, pH 6.8) in a 96-well microplate and incubated at 25 °C for 15 min. The reaction was initiated by the addition of L-DOPA (40 μL). Similarly, the enzyme-free blank solution was prepared, and the sample and blank absorbance were read at 492 nm after incubating at 25 °C for 10 min. Tyrosinase inhibitory activity results were given as equivalent to kojic acid [56], and results were given in Table 4.
α-Amylase inhibition: α-Amylase inhibitor activity was applied using the Caraway-Somogyi iodine/potassium iodide (I2/KI) method. Sample solutions (25 μL) were mixed with the α-amylase solution (50 μL) in phosphate buffer (pH = 6.9, 6 mM sodium chloride) in a 96-well microplate. The mixture was incubated at 37 °C for 10 min. After pre-incubation, the reaction was initiated when the starch solution (50 μL, 0.05%) was added. Similarly, the enzyme-free blank solution was prepared. The reaction mixture was incubated for 10 min at 37 °C, and the reaction was stopped by adding HCl (25 μL, 1 M). Following this, iodine -potassium iodide solution (100 μL) was added. Sample and blank absorbance were read at 630 nm, and α-amylase inhibitor activity results were given as acarbose equivalent [57–58], and results were given in Table 4.
α-Glucosidase inhibition: α-Glucosidase inhibitor activity was applied according to the method of Palanisamy et al. Sample solution (50 μL), glutathione (50 μL), α-glucosidase solution (50 μL) phosphate buffer (pH = 6.8) and PNPG (4-Nitrophenyl β -D-glucuronide) (50 μL) solution mixed in a 96-well microplate. It was incubated at 37 °C for 15 min. Similarly, an enzyme-free blank was prepared. The reaction was stopped when sodium carbonate (50 μL, 0.2 M) was added. Sample and blank absorbance were read at 400 nm. α-Glucosidase inhibitor activity was given as acarbose equivalent [57–58], and results were given in Table 4.
2.1.4. Anticancer activity
Anticancer cell lines and cell culture: The human cervical cancer cell line (HeLa) and human retinal normal cell line (RPE) were used to determine the anticancer activities of molecules synthesized within the scope of the project [59–61]. All cell preparation processes were carried out in a sterile environment in a laminar cabinet. HeLa cell line was used in DMEM medium supplemented with 10% FBS (Fetal Bovine Serum) and 2% PenStrep (Penicillin-Streptomycin) solution at 37 °C, 5% CO2 conditions, after achieving sufficient concentration (confluent). DMEM/F12 medium was used for RPE cell lines. Inoculation was carried out on the measurement plates with 10,000 cells per well. After approximately 16 h of pre-incubation, test molecules were added, and measurements were made after 24 h of incubation, and results were given in Table 5.
Cell proliferation measurement, determination of GI 50 and IC 50 values: MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] test was used to measure the effects of synthesized test compounds 4 , 12 , 18a+b , and 25 on cell proliferation (IC50 and GI50 values). This test protocol was applied after the cancer cell lines were incubated for 24 h with test substances. The results were reported as % cell inhibition, with the solvent’s optical density (DMSO) treated cells considered to be 100%. Accordingly, the % inhibition was calculated according to the formula [1-(test substance A/solvent control A)×100. MTT on cells of increasing concentrations (1, 2, 4, 8, 16, 32, 64, and 128 µg / mL) of each test substance over a certain range to determine the IC50 concentrations of test substances (concentration that inhibits the proliferation of 50% of cells in the medium) were prepared. Results were analyzed using a logarithmic function with the help of the Excel program over the logarithmic curve prepared from the absorbance values obtained after the test (Table 5). The following formula is used for the GI50 value calculation as cell proliferation: [(Ti-Tz)/(C-Tz)]x100 if Ti>/=Tz (cytostatic effect) or [(Ti-Tz)/Tz]x100 if Ti<Tz (cytotoxic or cytotoxic effect) (Tz; zero points, C; control growth, Ti; inhibition caused by test substance). GI50: Concentration value that reduces growth by 50% ([(Ti-Tz)/(C-Tz)]×100 = 50). The following formula is used for the IC50 value calculation. Accordingly, the % inhibition was calculated according to the formula [1-(A test substance/A solvent control)×100 [59-61].
Table 5.
Antiproliferative effects of compounds 4, 12, 18a+b, and 25 on HeLa and RPE normal cell lines, µg/mL.
| Compounds | 4 | 12 | 18a+b | 25 | Cisplatin |
|---|---|---|---|---|---|
| HeLa cell line | |||||
| GI50 | 1.12 | 2.04 | 2.23 | 2.21 | 1.13 |
| IC50 | 42.67 | 85.17 | 104.49 | 81.49 | 42.76 |
| RPE normal cell line | |||||
| GI50 | 1.15 | 1.92 | 1.90 | 3.38 | 1.56 |
| IC50 | 30.27 | 105.22 | 83.81 | 101.96 | 58.77 |
3. Results and discussion
In this study, self or cross-benzoin reaction of benzaldehyde, 3-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, and 3,5-dihydroxy benzaldehyde was carried out by using different methods. Substituted groups are very effective for the synthesis of benzoin when the electron donating groups are present on benzaldehyde; it is difficult to synthesize the benzoin as known in the literature [5,11,14–16,40–42]. For this purpose, MW, US, and reflux methods were investigated to find out the method to synthesize electron donating substituted group contains asymmetric or symmetric benzoin according to known methods. MW and reflux methods gave low yield or no reactions, and HCN gas evolution was observed. Dozens of trials have been made. However, for the most part, hydroxy substituted benzoin compounds could not be difficult to be synthesized. The US method was the best method to synthesize some of the hydroxy substituted benzoin even in low yield. In the case of using different aldehyde compounds, it is possible to form four alternative benzoins (Table 1). After chromatographic purification, compounds 1-7 ( 39-68% yields, respectively) were obtained as a racemic mixture (Figure) [4,2–71].
1H NMR spectra of benzoin compounds gave benzylic -CH(OH) at δ 5.8-6.4 (H-2, bs) ppm and 13C NMR spectra revealed peaks at δ 195-198 (C = O) for C-1 and δ 75-78 ppm for C-2, which indicates benzoin structures (Figure).
Hydroxy benzil compounds ( 8-14 ) were synthesized from hydroxy benzoins’ oxidation ( 1-7 ) [72–74] with conc. nitric acid, PCC, and Fehling reagent. It was observed that during the oxidation of benzoin compounds, they decomposed to aldehydes or benzil compounds rearranged to benzylic acids. It has been known that substituted benzoin/benzil compounds decompose during the oxidation [12,13,32, 62,67]. Therefore, the yields of benzil reactions were found in the range of 18%–45%. The disappearance of the benzylic proton peak at δ 5.8–6.4 ppm and benzylic carbon peak at δ 75–78 ppm for the hydroxy benzoin compounds, and the appearance of 1,2-dione carbon peaks at δ 192-195 ppm (C = O) for C-1 and C-2 indicated the hydroxy benzil structures [62, 75] (Figure).
The reaction of hydroxy benzoins ( 1-7 ) and benzils ( 8-14 ) with TABG then hydrolysis of acetyl group yielded benzoin-D-glucosides ( 15-20 ) and benzil-D-glucosides ( 22-25 ), respectively [21-22]. Observation of the anomeric proton coupling constant value around J = 7-8 Hz in the 1H NMR spectrum of the synthesized benzoin/benzil-O- β -D-glucosides shows that the D-glucose unit was in the β form. 13C NMR spectra of benzoin/benzil-O- β -D-glucoside compounds indicate anomeric carbon peaks at δ 100–105 ppm, which showed that one or more D-glucose units were attached to benzoin/benzil compounds (Figure). Benzoin-O- β -D-glucoside compounds were observed as diastereomeric mixtures, as seen in NMR spectra. Compounds 6 and 18 were observed as an isomeric mixture. In total, 7 benzoin, 7 benzil, and 6/5 benzoin/benzil-D-glucoside compounds were synthesized, respectively. In the synthesis of the D-glucoside derivative of compounds 7 , 13, and 14 , highly mixed products were obtained and could not be purified. All the synthetic compounds were characterized by NMR (1D and 2D) and ACD NMR program. According to our literature survey, compounds 3 , 5-7 , 10 , 12-13 , and 15-25 have not been found in the literature.
Antioxidant properties of synthesized compounds 1 - 25 were made according to FRAP, CUPRAC, and DPPH methods [49–52], as seen in Table 2. The highest FRAP and CUPRAC values of hydroxy benzoin compounds ( 1-7) were 2237±58.3 and 1113.33±64 (μg/mL Trolox/gram DW) in compounds 6 and 5 , and the lowest DPPH values for compounds 5 and 4 were found to be 8.12 ± 1.2 mg/mL and 8.16 ± 0.3 mg/mL, respectively. Among the benzil compounds ( 8-14 ), compound 14 (1974 ± 76.9 μg/mL Trolox/gram DW) to FRAP, compounds 8 and 13 (1095.00 ± 18.1 μg/mL Trolox/gram DW) to CUPRAC, and compound 14 (7.38 ± 1.0 mg/mL) to DPPH methods, were found to be the most active compounds. In the benzoin-D-glucoside compounds (15-20 ), the highest FRAP, CUPRAC, and lowest DPPH values for benzoin-D-glucoside were 2918.75±36.14, 775.58±12.34 (μg/mL Trolox/gram DW), and 7.78±0.12 mg/mL for compound 20 , respectively. When the activities of all compounds according to FRAP, CUPRAC and DPPH were examined, it was seen that compound 20 for FRAP, compound 5 for CUPRAC, and compound 14 for DPPH were the most effective antioxidant compounds. The antioxidant activities for the benzil-O- β -D-glucoside ( 21-25 ) showed that compound 25 was the most effective for FRAP (2570.42 ± 25.01 μg/mL Trolox/gram DW) and the compound 23 was the most active to CUPRAC (273.67 ± 27.23, μg.mL–1 Trolox/gram DW) and DPPH (17.74 ± 0.35, mg/mL) methods. When looking at the substitution positions of these compounds, it has been found that they are generally more effective when they are substituted at the 3-position.
The antimicrobial activities of the synthesized compounds 1-14 against eight bacteria and one yeast, and compounds 16-25 against ten bacteria and one yeast were evaluated. After the inhibition diameters were observed in mm (data are not shown), the MIC values (µg/mL) were calculated [53–54] (Table 3). The best MIC values for hydroxy benzoin/benzil compounds 6a+b , 8 , 10, and 13 were found within the range of 9–52 µg/mL against bacteria Y. pseudotuberculosis, P. aeruginosa, S. aureus, S. mutans, E. faecalis, and B. cere us. Compounds 6a+b , 8 , 10 , 11 , 12, and 13 showed the best antituberculosis activity with the MIC value in the range of 9–18 µg/mL against M. smegmatis . Considering the antifungal activity results, compounds 8 and 10-15 showed closer activity against C. albicans with 17-51 µg/mL MIC values. The antibacterial activity results of compounds 15-25 (MIC values, 6-250 µg/mL) showed that compounds ( 15-25 ) were generally the most effective against Gram (+) nonpathogenic spore forming bacteria P. larvae and B. suptilis. None of the tested compounds 15-25 g ave any activity against Y. pseudotuberculosis , P. aeruginosa , and S. mutans . Compound 25 showed MIC values in the range of 8–15 µg/mL against bacteria ( E. coli, M. smegmatis, and P. larvae ). Among the glycoside compounds, compounds 21 and 22 were only active against nonpathogenic B. subtilis and P. larvae with 12–27 µg/mL MIC value. Only compound 15 was found to be active against C. albicans with 25 µg/mL MIC value among the glycoside compounds. It has been observed that compounds carrying –OH/-H as the -R group in the meta position compared to the carbonyl in their structure and the meta position in the other phenyl ring are more active.
Acetylcholinesterase [55], butyrylcholine esterase [55], tyrosinase [56], α-amylase [58], α-glucosidase inhibition [58] activity results were made according to spectrophotometric methods. IC50 values were calculated and given in Table 4. The result of enzyme inhibition (ACh, BCh, tyrosinase, α-amylase, and α-glucosidase) for hydroxy benzoin/benzil ( 1-14 ) gave that compound 4/10 , 2 , 6a+b /10 , 7/9 , and 1/9 were the most active. Their IC50 values were within the range of 25.45–110.96 µg/mL, and 38.90–219.82 µg/mL, respectively. The five different enzyme inhibition (ACh, BCh, tyrosinase, α-amylase, and α-glucosidase) of benzoin/benzil-O- β -D-glucosides ( 15-25 ) resulted that compounds 19/23 , 16/25 , 19/21 , 19/23 , and 20/24 were the most active. Their IC50 values were within the range of 10.52 –188.93 µg/mL, and 58.90–133.86 µg/mL, respectively. In the enzyme inhibition study, compounds 19 and 2 against ACh and BCh, compounds 7 and 12 against α-amylase and α-glucosidase, and compound 19 against tyrosinase showed activity as much as galantamine, acarbose, and kojic acid standards used, respectively. When these results are compared with the used standards, compounds 19 , 2 , 7 , 12 , and 19 could be used as ACh, BCh, tyrosinase, α-amylase, and α-glucosidase inhibitors, respectively. The highest activities were seen by the compounds bearing the 4-OH, 3-OH substituent on the phenyl rings considering the structure-activity relationship. It is known in the literature that many different hydroxy phenolic compounds show biological activity in a wide spectrum range [1,71].
HeLa test results of compounds 4 , 12 , 18a+b , and 25 showed that only compound 4 (IC50 42.67 µg/mL) had a strong antiproliferative effect on cancer cells (Table 5). However, the toxicity caused by compound 4 on the normal cell line was examined (IC50 30.27 µg/mL), it was found to be toxic. The inhibitor concentrations (IC50) of the compounds 4 , 12 , 18a+b , and 25 were compared on the HeLa cancer cell, compound 4 (IC50 42.67 µg/mL) had a strong antiproliferative effect as cisplatin (IC50 42.76 µg/mL). The growth inhibition (GI50) of compound 4 (GI50 1.12 µg/mL) on the HeLa cancer cell was as the control compound cisplatin (GI50 1.13 µg/mL) [59–61]. The inhibitor concentrations (IC50) of the compounds 4 , 12 , 18a+b , and 25 on the RPE normal cell line gave that compound 4 (IC50 30.27 µg/mL) had better antiproliferative activity than control compound cisplatin (IC50 58.77 µg/mL). Comparison of growth inhibition (GI50) on the RPE cancer cells, compound 4 (GI50 1.15 µg/mL) showed growth inhibition as much as cisplatin (GI50 1.56 µg/mL).
4. Conclusion
In this study, benzoin reaction was carried out in a modified ultrasonic bath. Antioxidant activities of all compounds were compared according to FRAP, CUPRAC, and DPPH methods. It was found that compound 21 (2918.75±36.14 μg/mL Trolox/gram DW) for FRAP, compound 5 (1113.33 ± 64.9 μg/mL Trolox/gram DW) for CUPRAC, and compound 15 (7.38±1.0 mg/mL) for DPPH were the most effective antioxidant compounds. Hydroxy benzoin/benzil compounds 1-14 were more effective against test microorganisms in the antimicrobial activity among the synthesized compounds 1-25 . Compound 24 showed the only antitubercular activity with the MIC value of 15 µg/mL against M. smegmatis, and compound 15 was found to be active against only C. albicans with 25 µg/mL MIC value. Thus, compounds 24 and 15 could be used as the standard for M. smegmatis and C. albicans , respectively. Enzyme inhibition study showed that compounds 19 and 2 against ACh and BCh, compounds 7 and 12 against α-amylase and α-glucosidase, and compound 19 against tyrosinase gave the activity as much as galantamine, acarbose, and kojic acid standards used, respectively. However, it has been observed that all compounds synthesized show different levels of activity against tested enzymes. HeLa and RPE cancer cell activities of compound 4 were observed as much as cisplatin. Biological activity studies of compounds 1-25 showed benzoin and benzil analogs were more active when the substituents in the meta positions. Compounds 3 , 5-7 , 10 , 12-13 , and 15-25 are new, and their biological evaluation was made first time in this work.
Acknowledgments
This work is supported by the TÜBİTAK (Project No: 117R048). And also, thanks to Karadeniz Technical University, Faculty of Pharmacy research facilities.
References
- 2006.
- Chen Y T Barletta G L Haghjoo K Jordan F Reactions of benzaldehyde with thiazolium salts in Me2SO: evidence for initial formation of 2-(a-hydroxybenzyl)thiazolium by nucleophilic addition and for dramatic solvent effects on benzoin formation. Journal of Organic Chemistry . 1994;59:7714–7722. [Google Scholar]
- Sawada H Okazaki M Morita D Kuroda T Matsuno K Riccardin C derivatives as anti-MRSA agents: structure-activity relationship of a series of hydroxylated bis (bibenzyl)s. Bioorganic & Medicinal Chemistry Letters . 2012;22:7444–7447. doi: 10.1016/j.bmcl.2012.10.052. [DOI] [PubMed] [Google Scholar]
- Bilir G Demir A S Ozcubukcu S Enzyme-catalyzed trans-benzoin condensation. Journal of the Turkish Chemical Society, Section A: Chemistry . 2018;5:737–750. [Google Scholar]
- Sathyanarayana A Prabusankar G ±)Methanodibenzodiazocine tethered [C-H]δ+ functional site: study towards benzoin condensation and Baylis-Hillman reactions. Journal of Chemical Sciences . 2015;127:821–831. [Google Scholar]
- Suh Y Lee J Kim S Rieke R D. Direct preparation of benzylic manganese reagents from benzyl halides, sulfonates, and phosphates and their reactions: applications in organic synthesis. Journal of Organometallic Chemistry . 2003;684:20–36. [Google Scholar]
- Skonieczny K Jazwinski J Gryko D T. -phenanthridines via Intramolecular Oxidative Aromatic Coupling. Phenanthro[9’,10’:4,5]imidazo[1 . 2017;1:2–2. [Google Scholar]
- Sun T Zhang Y Qiu B Wang Y Qin Y -(I -catalyzed carboacylation aromatization cascade initiated by regioselective C-C activation of benzocyclobutenones. Angewandte Chemie International Edition . 2018;57:2859–2863. doi: 10.1002/anie.201713179. [DOI] [PubMed] [Google Scholar]
- Schwaerzer K Bellan A Zoeschg M Karaghiosoff K P. Magnesium aldimines prepared by addition of organomagnesium halides to 2,4,6-trichlorophenyl isocyanide: synthesis of 1,2-dicarbonyl derivatives. Chemistry A European Journal . 2019;25:9415–9418. doi: 10.1002/chem.201900903. [DOI] [PubMed] [Google Scholar]
- Lapworth A. CXXII. Reactions involving the addition of hydrogen cyanide to carbon compounds. Part II. Cyanohydrins regarded as complex acids. Journal of the Chemical Society . Transactions 1904;85:1206–1214. [Google Scholar]
- Roger A Marvel C S. Benzoin Organic Syntheses . 1921;1:33–33. [Google Scholar]
- Clarke H T Dreger Benzil Organic Syntheses . 1941;1:87–87. [Google Scholar]
- Skobridis K Theodorou V Weber E. A very simple and chemoselective air oxidation of benzoins to benzils using alumina. Arkivoc . 2006;10:102–106. [Google Scholar]
- Enders D Niemeier O Henseler A Organocatalysis by N-heterocyclic carbenes. Chemical Reviews . 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]
- Menon R S Biju Nair V Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions. Beilstein Journal of Organic Chemistry . 2016;12:444–461. doi: 10.3762/bjoc.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici A Porta O. Reductive coupling of benzoyl cyanide and carbonyl compounds by aqueous titanium (III) ions. A new convenient and selective access to the less stable mixed benzoins. The Journal of Organic Chemistry . 1993;58:2889–2893. [Google Scholar]
- Koenigkramer R E α-Heterosubstituted phosphonate carbanions IX: ethyl 1-phenyl-1-trimethylsiloxymethane phosphonate as an acyl anion equivalent; A novel method for the preparation of α-hydroxyketones. Tetrahedron Letters . 1980;21:1017–1020. [Google Scholar]
- Krepski L R Heilmann S M Rasmussen J K. Addition of Grignard reagents to O-trimethylsulylated cyanohydrins: Synthesis of acyloins. Tetrahedron Letters . 1983;24:4075–4078. [Google Scholar]
- Heilmann S M Rasmussen J K of unsymmetrical benzils using sodium dithionite. The Journal of Organic Chemistry . 1983;48:987–992. [Google Scholar]
- Bi X Wu L Yan C Jing X Zhu H One-pot synthesis benzils from aldehydes via NHC-catalyzed benzoin dimerization under metal-free conditions in water. Journal of the Chilean Chemical Society . 2011;56:663–664. [Google Scholar]
- Shimakawa Y Morikawa T Sakaguchi S. Facile route to benzils from aldehydes via NHC-catalyzed benzoin condensation under metal-free conditions. Tetrahedron Letters . 2010;51:1786–1789. [Google Scholar]
- Huang L-H Lou Y-C Wang Zhang C. Ma1. Adsorption Science & Technology . 2011;29:871–874. [Google Scholar]
- Chandrasekhar S Reddy N K Kumar V P Oxidation of alkynes using PdCl2/CuCl2 in PEG as a recyclable catalytic system: one-pot synthesis of quinoxalines. Tetrahedron Letters . 2010;51:3623–3625. [Google Scholar]
- Gasparrini F Giovannoli M Misiti D Natile G Palmieri G Nitric acid facile oxidation of mono and diarylcarbinols to carbonyl compounds in a biphasic system. Synthetic Communications . 1988;18:69–75. [Google Scholar]
- Barrett A G M Braddock McKinnell R M Waller F J. Ytterbium(III) triflate as a recyclable catalyst for the selective atom economic oxidation of benzyl alcohols to benzaldehydes. Synlett . 1999;9:1489–1490. [Google Scholar]
- Hajipour A R Mallakpour S E Khoee S. An efficient, fast and selective oxidation of aliphatic and benzylic alcohols to the corresponding carbonyl compounds under microwave irradiation. Synlett . 2000;5:740–742. [Google Scholar]
- Lidström P Tierney J Wathey B Westman J Microwave assisted organic synthesis-a review. Tetrahedron Letters . 2001;57:9225–9283. [Google Scholar]
- Varma R S. Solvent-free accelerated organic syntheses using microwaves. Pure and Applied Chemistry . 2001;73:193–198. [Google Scholar]
- Strazzolini P Runcio A Oxidation of benzylic alcohols and ethers to carbonyl derivatives by nitric acid in dichloromethane. European Journal of Organic Chemistry . 2003;3:526–536. [Google Scholar]
- Surendra K Krishnaveni N S Reddy M A Nageswar Y V Rao K R Mild oxidation of alcohols with o-iodoxybenzoic acid (IBX) in water/acetone mixture in the presence of beta-cyclodextrin. Journal of Organic Chemistry . 2003;68:2058–2059. doi: 10.1021/jo026751w. [DOI] [PubMed] [Google Scholar]
- Lee J-H Lee J-Y Lee J-M. Facile Oxidation of benzyl alcohols with sodium nitrate/p-TsOH under microwave irradiation. Bulletin of the Korean Chemical Society . 2005;26:1300–1302. [Google Scholar]
- Bhosale S M Momin Gawade R L Puranik V G Kusurkar R S. A new synthetic route for 1,2-diketo compounds using unexpected C–C bond cleavage by PCC. Tetrahedron Letters . 2012;53:5327–5330. [Google Scholar]
- Varki A Biological roles of oligosaccharides: all of the theories are correct. Glycobiology . 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek R A. Glycobiology : toward understanding the function of sugars. Chemical Reviews . 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- Simanek E McGarvey G J Jablonowskij J A Wong C H Selectin-carbohydrate interactions: from natural ligands to designed mimics. Chemical Reviews . 1998;98:833–862. doi: 10.1021/cr940226i. [DOI] [PubMed] [Google Scholar]
- Esaki S Goda T Takase S Sugiyama N Kamiya S. Synthesis of phloretin 2′-O-β-l-glycosides and their inhibitory action against sugar transport in rat small intestine. Agricultural and Biological Chemistry . 1991;55:2855–2860. [Google Scholar]
- Sheikh J Parvez A Juneja H Ingle V Chohan Z Synthesis, biopharmaceutical characterization, antimicrobial and antioxidant activities of 1-(4’- European Journal of Medicinal Chemistry . 2011;1:3–diones. doi: 10.1016/j.ejmech.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Parker M Osidacz P Baldock G A Hayasaka Y Black C A Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. Journal of Agricultural and Food Chemistry . 2012;60:2929–2637. doi: 10.1021/jf2040548. [DOI] [PubMed] [Google Scholar]
- Englert C Nischang I Bader C Borchers P Alex J Photocontrolled release of chemicals from nano- and microparticle containers. Angewandte Chemie . 2018;57:2479–2482. doi: 10.1002/anie.201710756. [DOI] [PubMed] [Google Scholar]
- Xu S Du J Li H Tang J Pd/Al2O3 core–shell catalyst for efficient hydrodeoxygenation of phenolic biomolecules. 2018;57:14088–14095. [Google Scholar]
- Kothapalli R B Niddana Balamurugan R Synthesis of chiral α-diarylacetic esters by stereospecific 1,2-aryl migration promoted by in situ generated acetals from benzoins. Organic Letters . 2014;16:1278–1281. doi: 10.1021/ol500292c. [DOI] [PubMed] [Google Scholar]
- Hernandez K Parella T Joglar J Bujons J Pohl M Expedient synthesis of C-Aryl carbohydrates by consecutive biocatalytic benzoin and aldol reactions. Chemistry A European Journal . 2015;21:3335–3346. doi: 10.1002/chem.201406156. [DOI] [PubMed] [Google Scholar]
- Demir A S Sesenoglu O Eren E Hosrik B POhl M Enantioselective synthesis of α-hydroxy ketones via benzaldehyde lyase-catalyzed C-C bond formation reaction. Advanced Synthesis & Catalysis . 2002;344:96–103. [Google Scholar]
- You L Cho E J Leavitt J Ma L-C Synthesis and evaluation of quinoxaline derivatives as potential influenza NS1A protein inhibitors. Bioorganic & Medicinal Chemistry Letters . 2011;21:3007–3011. doi: 10.1016/j.bmcl.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muğlu H Yakan H Bakir T K Synthesis, spectroscopic studies, and antioxidant activities of novel thio/carbohydrazones and bis-isatin derivatives from terephthalaldehyde. Turkish Journal of Chemistry . 2020;44:237–248. doi: 10.3906/kim-1910-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp A S Kılcıgil G Özdamar E D Çoban T Eke B Synthesis and evaluation of antioxidant activities of novel 1,3,4-oxadiazole and imine containing 1H-benzimidazoles. Turkish Journal of Chemistry . 2015;39:42–53. [Google Scholar]
- Kirby A J Schmidt R J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs. Journal of Ethnopharmacology . 1997;56:103–108. doi: 10.1016/s0378-8741(97)01510-9. [DOI] [PubMed] [Google Scholar]
- Benzie I F F Strain J J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Analytical Biochemistry . 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Apak R Güclü K Ozyürek M Karademir S E Ercag E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. International Journal of Food Sciences and Nutrition . 2006;57:292–304. doi: 10.1080/09637480600798132. [DOI] [PubMed] [Google Scholar]
- Ertaş A Boga M Haşimi N Yeşil Y Gören A C Antioxidant, anticholinesterase, and antimicrobial activities and fatty acid constituents of Achillea cappadocica Hausskn. Turkish Journal of Chemistry . 2014;38:592–599. [Google Scholar]
- Perez C Pauli M Bazerque P. An antibiotic assay by the well agar method. Acta Biologia et Medicine Experimentalis . 1990;15:113–115. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Third Edition . 1993;13:25–25. [Google Scholar]
- Woods G L Brown-Elliott B A Desmond E P Hall G S Heifets L Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. Approved Standard NCCLS document M24-A . 2003;23:18–18. [PubMed] [Google Scholar]
- Abbas I Gomha S Elneairy M Elaasser M Mabrouk B Synthesis and biological evaluation of novel fused triazolo[4,3-a] pyrimidinones. Turkish Journal of Chemistry . 2015;39:510–531. [Google Scholar]
- Tuğrak M Gül H I Anıl B Gülçin İ Synthesis and pharmacological effects of novel benzenesulfonamides carrying benzamide moiety as carbonic anhydrase and acetylcholinesterase inhibitors. Turkish Journal of Chemistry . 2020;44:1601–1609. doi: 10.3906/kim-2007-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T Yamashita D Takeda Y Yonemori S Screening for tyrosinase inhibitors among extracts of sea shore plants and identification of potent inhibitors from Garcinias elliptica. Bioscience, Biotechnology and Biochemistry . 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- Palanisamy U D Manaharan T Appleton D Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chemistry . 2011;127:21–27. [Google Scholar]
- Yang X W Huang M Z Jin Y S Sun L N Song Y Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia . 2012;83:1169–1175. doi: 10.1016/j.fitote.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Porstmann T Ternynck T Avrameas S. Quantitation of 5-bromo-2-deoxyuridine incorporation into DNA: an enzyme immunoassay for the assessment of the lymphoid cell proliferative response. Journal of Immunological Methods . 1985;82:169–179. doi: 10.1016/0022-1759(85)90236-4. [DOI] [PubMed] [Google Scholar]
- Decker T Lohmann-Matthes M L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of Immunological Methods . 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Gong J Traganos F Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Analytical Biochemistry . 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- Mousavi M Seyfi H One-pot synthesis of benzil derivatives from aromatic aldehydes. The Journal of Organic Chemistry . 2014;3:28–33. [Google Scholar]
- The Species of Fehling’s Solution. European Journal of Inorganic Chemistry . 2016. pp. 1798–1807.
- Estager J Leveque J M Turgis Draye M. Solventless and swift benzoin condensation catalyzed by 1-alkyl-3-methylimidazolium ionic liquids under microwave irradiation. Journal of Molecular Catalysis A: Chemical . 2006;256:261–264. [Google Scholar]
- Aupoix A Pegot B Vo-Thanh G Synthesis of imidazolium and pyridinium-based ionic liquids and application of 1-alkyl-3-methylimidazolium salts as pre-catalysts for the benzoin condensation using solvent-free and microwave activation. Tetrahedron Letters . 2010;66:1352–1356. [Google Scholar]
- Mavis M E Yolacan C Aydogan F An investigation of the catalytic potential of mono- and dicationic imidazolium N-heterocyclic carbenes in the benzoin condensation. Tetrahedron Letters . 2010;51:4509–4511. [Google Scholar]
- The Synthesis of Benzoins 2011.
- Sayyahi S. Preparation and application of 1,1’-bis-methyl-3, 3’-methylene-bisimidazolium dicyanide as a task-specific ionic liquid: an efficient catalyst in benzoin condensations. Chemical Science Transactions . 2012;1:9–12. [Google Scholar]
- Kim Y J Kim N Y Cheon C H. Beyond benzoin condensation: trimerization of aldehydes via metal-free aerobic oxidative esterification of aldehydes with benzoin products in the presence of cyanide. Organic Letters . 2014;16:2514–2517. doi: 10.1021/ol5008845. [DOI] [PubMed] [Google Scholar]
- Acharjee J Ghoshal A Ghosh S K. Microwave -assisted synthesis: need of the hour. World Journal of Pharmacy and Pharmaceutical Sciences . 2015;4:1741–1749. [Google Scholar]
- Safari J Zarnegar Z Ahmadi M Seyyedi S. An investigation of the catalytic potential of potassium cyanide and imidazolium salts for ultrasound-assisted synthesis of benzoin derivatives. Journal of Saudi Chemical Society . 2015;19:628–633. [Google Scholar]
- Perkins F McBay H C. J Hydrogenation reactions of some spool-S haped acetylenes. Journal of Organic Chemistry . 1981;46:4695–4700. [Google Scholar]
- Depreux P Bethegnies G Marcincal-Lefebvre A Synthesis of benzil from benzoin with copper(II) acetate. Journal of Chemical Education . 1988;65:553–553. [Google Scholar]
- Ashnagar A Gharib N N Amini M. Synthesis of 5,5-diphenyl-2,4-imidazolidinedione (Phenytoin) from almond. International Journal of ChemTech Research . 2009;1:47–52. [Google Scholar]
- Matsuda T Oyama S. Synthesis of unsymmetrical benzils via palladium-catalysed α-arylation-oxidation of 2-hydroxyacetophenones with aryl bromides. Organic & Biomolecular Chemistry . 2020;18:3679–3683. doi: 10.1039/d0ob00575d. [DOI] [PubMed] [Google Scholar]