Abstract
Sporting events were cancelled, and sports training was banned to prevent the spread of COVID-19. These changes during the COVID-19 pandemic decreased the physical activity levels, increased sedentary time, and also impaired the mental health of elite and sub-elite athletes. The impact on body composition and physical performance is not clear, however, especially considering a systematic review with meta-analysis. Thus, our objective was to conduct a review in accordance with the PRISMA Statement studies published in scientific journals (PubMed, Web of Science, or Scopus databases) that investigated the effect that social distancing during the COVID-19 pandemic had on the physical performance (muscle power, cardiorespiratory capacity, and sprint) or body composition (body weight, percentage of fat, fat mass, and fat-free mass) of athletes. Data from 24 studies indicate that, throughout the global lockdown, the athletes maintained muscle power, cardiorespiratory capacity, and sprint, and prevented significant changes in fat mass and fat-free mass. However, the total body weight (meta-analysis with 18 studies), showed a significant increase (p = 0.006), with a small ES = 0.12; 95% CI = 0.04 to 0.21. Furthermore, the time of follow-up, level of training, and the age of the athletes were possible moderators of these effects. The data reinforce the importance of general strength and endurance exercises sessions to maintain physical fitness during non-competitive periods or due to the mandatory lockdown.
Keywords: athletes, physical performance, lockdown, body composition
1. Introduction
The COVID-19 pandemic was caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2). In March 2020, the World Health Organization (WHO) published guidelines to prevent the spread, due to a high rate and number of progressing infections, which involved mainly social restriction; therefore, sports events were canceled, and sports training was banned [1]. A recent systematic review conducted by Jurecka et al. [2] demonstrated that athletes decreased their physical activity levels, increased sedentary time, and experienced impaired mental health during the COVID-19 pandemic. The authors suggested that the temporary restriction was associated with a decrease in overall physical fitness and the number of days and hours of training.
Likewise, other researchers identified that, during the social distancing period, the athletes decreased their total volume of training; however, most of them tried to maintain cardiorespiratory fitness and muscular strength, rather than exploiting sports-specific training [3]. These measures were taken to cope with the need to maintain cardiorespiratory fitness, velocity, power, and morphological functions, relevant factors to achieve successful results among highly trained athletes, independently of their sport. Furthermore, athletic performance improvement is often associated with a reduction in fat mass (FM) and increased fat-free mass (FFM) [4]. In accordance with this, Siders et al. found a positive relationship between sprint swimming performance and percentage of fat mass and a negative relationship with FFM in women [5]. Furthermore, athletes with lower FM showed higher aerobic capacity and more biochemical blood markers associated with an anabolic process, which may considerably affect the exercise capacity of athletes [6]. However, only 4 weeks of detraining is enough to reduce 6% to 20% of maximal oxygen uptake (O2peak) in highly trained athletes [7].
Spyrou et al. found that a short period of lockdown (9 weeks) decreased the sprint, countermovement jump (CMJ), rate of force development, peak power, velocity, and landing peak force of elite futsal players [8]. On the other hand, Fikenzer et al. and Grazioli et al. did not verify a significant difference in cardiorespiratory fitness after 8 weeks in handball and soccer players, respectively, although higher fat mass was found for the soccer players in Grazioli’s study [9,10]. Despite differing statements recommending the safe return to training and competition after the lockdown caused by the COVID-19 pandemic [11,12], the retraining period effect on these variables is not yet clear, particularly after a long detraining season. We highlight the study of Silva et al., which investigated the impact of long-period detraining due to the COVID-19 social restrictions (8 months and 1 year) on young badminton athletes. The authors showed that the athletes who stopped daily training routines for 1 year due to the COVID-19 social restrictions presented higher FM and lower FFM than the athletes who returned to regular daily training 4 months earlier; however, no significant differences were observed for O2peak and handgrip strength [13].
In regard to the previous information, it is possible to identify that there is no consensus about the effects of the COVID-19 pandemic on the physical performance and body composition of athletes; therefore, a clear understanding of the impact of COVID-19 on the physical fitness of athletes is essential to inform coaches, sports physiologists, and athletes when making decisions concerning the initial load and progressions during the return to training and matches. Furthermore, safe and healthy strategies can be developed to mitigate physiological and morphological responses for athletes to return to competition-level readiness.
For athletes, it is relatively easy to suffer a loss in body mass and FM due to higher energy expenditure during exhausting training routines and competition, however, it is hard for them to maintain the FFM required when performing muscular work [14]. On the other hand, long periods of discontinuing training might induce body mass and FM gain, decrease FFM, and impair performance throughout the season.
Thus, this is the first study to use both a meta-analysis and meta-regression to investigate the impact of the COVID-19 pandemic on the body composition (fat mass and fat-free mass) and physical fitness (muscle power, cardiorespiratory capacity, and sprint) of athletes, as well as to explore the possible moderators of these effects, such as the immediate impact of the initial lockdowns in 2020 and long periods of detraining caused by the COVID-19 social restrictions. Additionally, we investigated the potential influence that the level of training and age had on the athletes, since elite and non-elite athletes could show different morphological and physical responses during this period and across their life. We hypothesized that the COVID-19 pandemic would negatively affect the body composition, with higher fat mass and lower FFM, and would decrease cardiorespiratory fitness and muscle power.
2. Materials and Methods
This review has been reported in accordance with the PRISMA Statement (preferred reporting items for systematic reviews and meta-analyses) checklist [15].
2.1. Search Strategy
Searches were performed in PubMed, Web of Science, and Scopus using the following word combinations: (“COVID-19” OR “SARS-CoV-2” OR coronavirus OR pandemic OR “social isolation” OR quarantine OR epidemic) AND (Athletes OR “high-trained” OR players OR sportsmen OR “elite athletes” OR “amateur athletes” OR “young athletes”) AND (“Physical fitness” OR performance OR training OR exercise OR obesity OR “weight loss” OR body composition OR anthropometric OR “adipose tissue” OR adiposity OR “fat mass” OR “visceral fat” OR “lean body mass” OR “muscle mass” OR “fat free mass”).
The filter was applied to studies published from 2020 onwards, given that the COVID-19 infection only spread around the world in early 2020, and the confinements caused by the virus only started in February and March in most countries, according to the WHO [1].
2.2. Eligibility Criteria
Prospective and retrospective cohort articles, interrupted time series, case series, and clinical trials published in scientific journals, indexed in databases (PubMed, Web of Science, or Scopus), that investigated the effect social distancing during the COVID-19 pandemic on the physical performance or body composition of athletes were included. Articles were required to contain data before and after social distancing, or some period during the pandemic, in elite or sub-elite athletes (for the first population, we considered elite athletes who had competed in international or national sports events; whereas sub-elite athletes were considered those that competed regionally or recreationally). Athletes were required to be engaged in regular training during the week, with participation in national or international competitions. The exclusion criteria included articles with self-reported performance or composition and studies that included athletes with some type of disability (i.e., palsy or other diseases); moreover, athletes younger than 1 year old were not included, as well as studies that compared athletes who had COVID-19. Articles published in the form of abstracts, dissertations, and theses were not considered for this review.
2.3. Selection Process
The entire study selection process, from titles, abstracts, and full text, was carried out by two independent reviewers (BVR and AOA). In cases of unresolved conflict, a third reviewer would help to decide (FER). The process of removing duplicates and selecting studies was performed using the Rayyan web application [16].
2.4. Data Collection Process
The data of interest were taken from the articles by 3 authors (BRV, AOA, and FER), where each author had an average of 20 articles for data extraction. Data were collected manually from studies and compiled in excel sheets. Studies or data not found were requested by email to the authors.
2.5. Data Items
For the pre- and post-confinement physical fitness outcomes, the following topics and data were extracted from each study: author and year, sport, level of training, number of subjects, gender, age, weeks of follow-up, performance or body composition measurement evaluation.
Additionally, the study design and training protocol during confinement were extracted (see Supplementary Materials).
Muscle power measured by counter movement jump (CMJ), cardiorespiratory fitness measured by O2peak (direct or indirect tests), and sprint (5 m, 10 m, and 30 m tests) were used for the meta-analysis of performance. Additionally, body mass (kg), percentage of fat mass (%), fat mass (kg), and fat-free mass (kg) were used for the meta-analysis of body composition.
In studies that presented data collection at different moments in time, data were used only from the moment closest to pre-pandemic (before COVID-19 pandemic) and closest to post-pandemic social restriction.
2.6. Study Risk of Bias Assessment
Two independent reviewers (BVR and FER) assessed the quality of the studies. Methodological quality was not an inclusion criterion. The risk of bias was assessed using the Joanna Briggs Institute (JBI) critical appraisal tool [17]. Each item was assigned a high, low, or unclear risk of material bias, and the quality of all studies was analyzed.
2.7. Data Synthesis
A random-effects model was used in all analyses, owing to an expectation of heterogeneity of data across studies. Standardized mean differences (SMD) in body composition and performance variables from pre- to post-COVID-19 pandemic social distancing were utilized to calculate the effect size (ES). We calculated the SMD for individual studies and then pooled the data using a random-effects meta-analysis. SMD was estimated from pre- to post-pandemic, with 0.7 correlations. The interpretation of the magnitude of standardized mean difference was as follows: 0 to <0.30 | small, | >0.30 | to | <0.8 | moderate, and | >0.80 | large [18].
Heterogeneity among studies was verified with the I2 statistic, with thresholds set at I2 = 25% (low), I2 = 50% (moderate), and I2 = 75% (high) [18,19,20,21]. Computations were carried out using Comprehensive Meta-Analysis Version 4 (Biostat Inc., Englewood, NJ, USA) [22].
We also performed sensitivity analyses to assess whether comparable effects were still observed following the removal of low-quality information (≤5 points on the JBI critical appraisal tool).
Meta-regression analysis of variables with ten or more studies was carried out. The moderators used were as follows: (a) age—years; (b) follow-up time—weeks; and (c) level of training—elite or non-elite. The significance level adopted was p ≤ 0.05.
3. Results
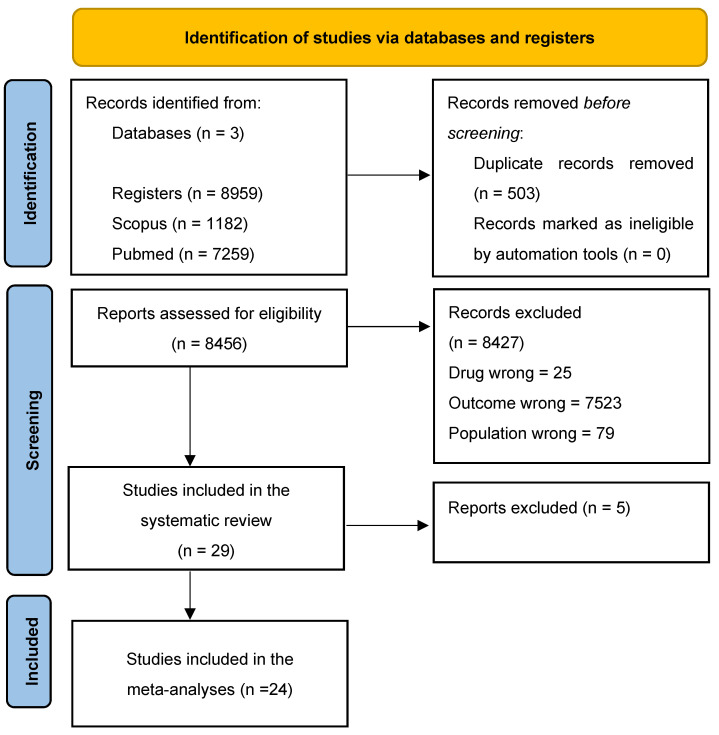
The initial search identified 8959 potentially relevant articles. After the removal of duplicates (503 studies), 8456 records remained. Of these, we removed 8427 studies based on their title and abstract (Drug wrong = 25; Outcome wrong = 7523; Population wrong = 79; Publication type = 62; Study design = 735; and Study duration = 3), and 29 studies were included in our systematic review. However, some articles did not provide pre- or post-pandemic data as mean and standard deviation (even after we requested them by email from the corresponding author), therefore, the meta-analysis considered 24 studies. The study selection process is detailed in Figure 1.
Figure 1.
The flowchart of study selection.
3.1. Characteristics of Included Trials and Study Quality
The 29 studies selected for systematic review [8,9,10,13,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] and the quality of all of the studies analyzed are presented in Table 1. The details of these studies are presented in Table 2. In summary, a total of 691 athletes were included from 23 studies with men (593 athletes), 2 studies with women (32 athletes), and 4 studies with men and women (66 athletes). In relation to modalities, nine different modalities were identified (badminton = 2, basketball = 1, cycling = 1, fencing = 2, handball = 2, indoor soccer = 1, kickboxing = 1, soccer = 18, and combat Sports = 1). The variables identified were body mass = 21, fat-free mass = 8, fat mass = 4, fat percentage = 10, CMJ = 14, and O2peak = 10.
Table 1.
Risk of bias assessment assessed using the Joanna Briggs Institute (JBI) critical appraisal tool.
| Author and Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Aguilar et al. (2021) [23] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Alvurd et al. (2022) [24] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Ambrozy et al. (2021) [25] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Asimakidis et al. (2022) [26] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Campa et al. (2021) [27] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Cohen et al. (2020) [28] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Dauty et al. (2021) [29] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Fikenzer et al. (2021) [9] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Font et al. (2021) [30] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Freire et al. (2020) [43] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Grazioli et al. (2020) [10] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Junaidi et al. (2021) [46] | X | ✓ | ✓ | ✓ | X | X | X | ✓ |
| Kalinowski et al. (2021) [31] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Korkmaz et al. (2020) [22] | X | X | ✓ | ✓ | X | X | ✓ | ✓ |
| Kosova et al. (2021) [33] | X | ✓ | ✓ | ✓ | X | X | X | ✓ |
| Leo et al. (2021) [34] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Luna et al. (2021) [35] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| My Giulia et al. (2022) [36] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Paravlic et al. (2022) [37] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Parpa and Michaelides (2021) [38] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Pedersen et al. (2021) [39] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Pucsok et al. (2021) [40] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Scoz et al. (2022) [41] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Segalés-Gill et al. (2021) [42] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Silva et al. (2022) [13] | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ |
| Spyrou et al. (2021) [8] | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Valenzuela et al. (2021) [45] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Villaseca-Vicuña et al. (2022) [44] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
| Yasuda et al. (2021) [47] | X | ✓ | ✓ | ✓ | X | X | ✓ | ✓ |
Caption: The Joanna Briggs Institute (JBI) critical appraisal tool with 8 items. 1—Were the criteria for inclusion in the sample clearly defined?; 2—Were the study subjects and the setting described in detail?; 3—Was the exposure measured in a valid and reliable way?; 4—Were objective, standard criteria used for measurement of the condition?; 5—Were confounding factors identified?; 6—Were strategies to deal with confounding factors stated?; 7—Were the outcomes measured in a valid and reliable way?; 8—Was appropriate statistical analysis used? ✓ = Yes; X = No.
Table 2.
Characteristics of studies included in the systematic review.
| Author and Year | Sport | Level of Training | N | Gender | Age | Weeks | BMI | Body Mass | Fat-Free Mass | Fat Mass | Fat % | CMJ | O2peak |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aguilar et al. (2021) [23] | Soccer | Elite | 22 | M | 18 to 32 | 12 | X | X | X | ||||
| Alvurd et al. (2022) [24] | Soccer | Non-elite | 108 | M | 15 to 17 | 22 | X | X | X | ||||
| Ambrozy et al. (2021) [25] | Kickboxing | Elite | 20 | M | 25 ± 3 | 10 | X | X | X | ||||
| Asimakidis et al. (2022) [26] | Soccer | Non-elite | 29 | M | 13 ± 0 | 32 | X | X | |||||
| Campa et al. (2021) [27] | Soccer | Elite | 15 | M | 30 ± 4 | 9 | X | X | X | X | X | ||
| Cohen et al. (2020) [28] | Soccer | Elite | 16 | M | 24 ± 4 | 15 | X | X | |||||
| Dauty et al. (2021) [29] | Soccer | Elite | 19 | M | 14 | 9 | X | ||||||
| Fikenzer et al. (2021) [9] | Handball | Elite | 10 | M | 27 ± 3 | 44 | X | ||||||
| Font et al. (2021) [30] | Handball | Elite | 11 | M | 26 ± 4 | 16 | X | ||||||
| Freire et al. (2020) [43] | Soccer | Elite | 20 | M | 26 ± 4 | 6 | X | ||||||
| Grazioli et al. (2020) [10] | Soccer | Elite | 23 | M | 26 ± 6 | 21 | X | X | X | ||||
| Junaidi et al. (2021) [46] | Combat Sports | Non-elite | 100 | M | 21 ± 2 | 37 | X | X | |||||
| Kalinowski et al. (2021) [31] | Soccer | Elite | 24 | M | 15 ± 1 | 18 | X ** | ||||||
| Korkmaz et al. (2020) [22] | Soccer | Non-elite | 14 | M | 22 ± 3 | 12 | X | X | X | X | X | ||
| Kosova et al. (2021) [33] | Fencing | Non-elite | 15 | M&F | 16 ± 2 | 31 | X | X | |||||
| Leo et al. (2021) [34] | Cycling | Elite | 12 | M | 21 ± 1 | 30 | X | X | X | ||||
| Luna et al. (2021) [35] | Basketball | Elite | 9 | M | 17 ± 1 | 16 | X | X | |||||
| My Giulia et al. (2022) [36] | Soccer | Elite | 17 | M | 22 to 35 | 51 | X | X | X | X | X * | ||
| Paravlic et al. (2022) [37] | Soccer | Elite | 32 | M | 25 ± 5 | 8 | X | X | X | ||||
| Parpa and Michaelides (2021) [38] | Soccer | Elite | 19 | M | 28 ± 6 | 7 | X | X | X | X | |||
| Pedersen et al. (2021) [39] | Soccer | Elite | 13 | F | 19 ± 2 | 12 | X | ||||||
| Pucsok et al. (2021) [40] | Soccer | Elite | 11 | M | 17 ± 1 | 13 | X | ||||||
| Scoz et al. (2022) [41] | Soccer | Elite | 26 | M | 26 ± 5 | 6 | X | X | |||||
| Segalés-Gill et al. (2021) [42] | Soccer | Elite | 26 | M | 25 ± 5 | 11 | X | X | |||||
| Silva et al. (2022) [13] | Badminton | Elite | 23 | M&F | 19 ± 3 | 48 | X | X | X | X | |||
| Spyrou et al. (2021) [8] | Indoor soccer | Elite | 10 | M | 26 ± 3 | 10 | X | X | X | X | |||
| Valenzuela et al. (2021) [45] | Badminton | Elite | 7 | M&F | 21 ± 3 | 22 | X | ||||||
| Villaseca-Vicuña et al. (2022) [44] | Soccer | Elite | 19 | F | 27 ± 4 | 22 | X | X | X | X | |||
| Yasuda et al. (2021) [47] | Fencing | Elite | 21 | M&F | 26 ± 5 | 36 | X | X | X | X | X |
Caption: N = number of subjects; M = Male; F = Female; M&F = Male and female; Weeks = time between first and second assessments in weeks; Fat % = fat percentage; CMJ = Countermovement jump; Combat Sports = Karate, Taekwondo, and Pencak Silat (Indonesian martial arts). * = study not reported for this variable data in mean and standard deviation. ** = data of O2peak reported in meters. X = Variable reported in the current systematic review.
3.2. Meta-Analysis
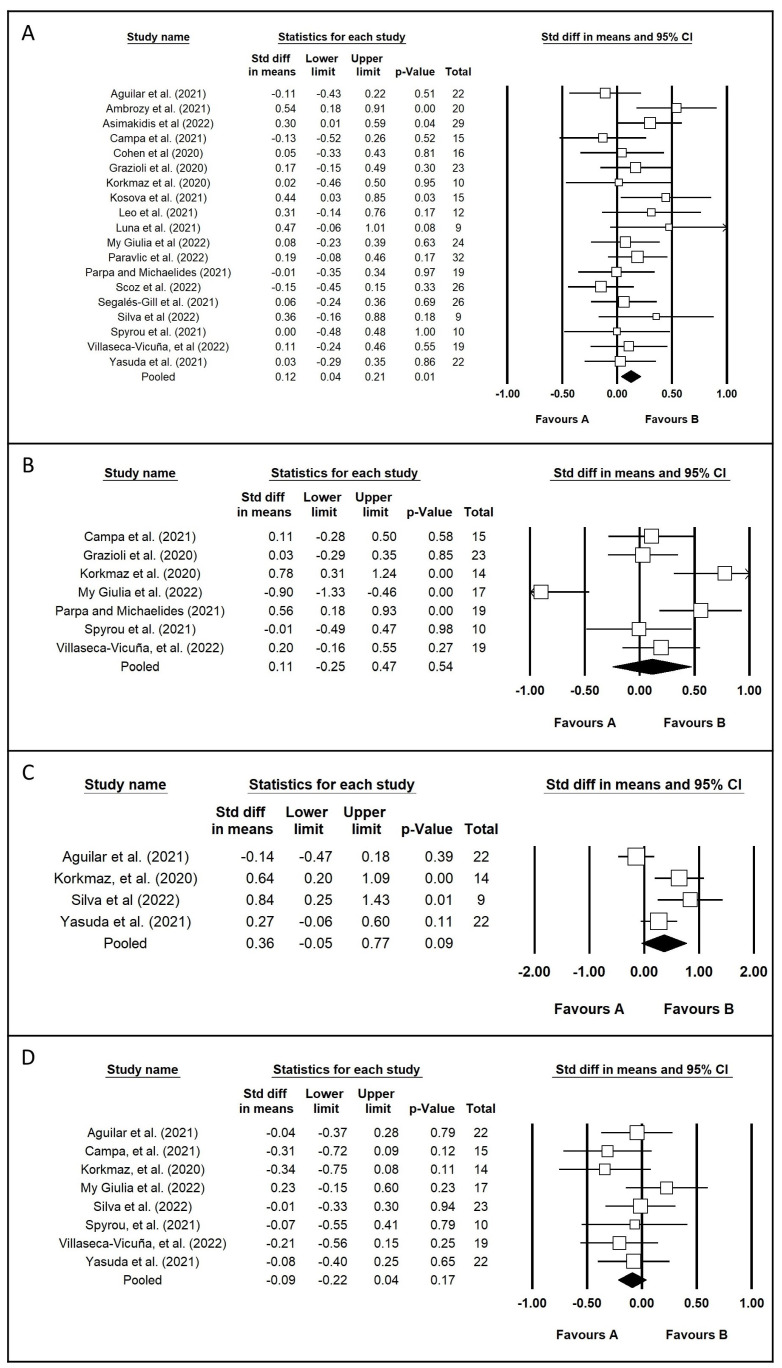
Of the 29 studies considered for the systematic review, 5 studies [24,31,43,45,46] were excluded. In summary, the motivation of exclusion was as follows: the number of subjects per group was not clear [24]; the data of O2peak was reported in other measurement units (meters) [31]; and no access to data in mean and standard deviation (even after email request) [43,45,46]. A total of 24 studies [8,9,10,13,23,25,26,27,28,29,30,32,33,34,35,36,37,38,39,40,41,42,44,47] and 447 athletes were included in this meta-analysis. This study resulted in six forest plots. In detail, we present (Figure 2) the following four forest plots of body composition:
Figure 2.
Forest plot of body composition variables: (A) = Body mass [8,10,13,23,25,26,27,28,32,33,34,35,36,37,38,41,42,44,47]; (B) = Percentage of fat [8,10,27,32,36,38,44]; (C) = Fat mass [13,23,32,47]; (D) = Fat-free mass [8,13,23,27,32,36,44,47]. Favor A = Decrease value of Variable; Favor B = Increase value of Variable.
Body mass variable [8,10,13,23,25,26,27,28,32,33,34,35,36,37,38,41,42,44,47].
Percentage of fat variable [8,10,27,32,36,38,44].
Fat mass variable [13,23,32,47].
Fat-free mass variable [8,13,23,27,32,36,44,47].
In summary, body weight analysis, included 18 studies and 336 participants, with a range from 9 to 32 participants. The mean of changes was 1.1 kg (range from −1.1 to 4.5 kg), or 1.7% (range from −1.4 to 7.4%). We found a significant (p = 0.006) change (increase in body mass) (ES = 0.12; 95% CI = 0.04 to 0.21, Q = 20.403, with 18 degrees of freedom, and p = 0.01, I2 = 17%).
Percentage of fat analysis included seven studies and 117 participants, with a range from 10 to 23 participants. The mean of changes was 0.5% (range from −1.5 to 2.5%). We found a nonsignificant (p = 0.54) change (ES = 0.11; 95% CI = −0.25 to 0.47, Q = 34.655, with 6 degrees of freedom, and p < 0.001, I2 = 83%).
Fat mass analysis included four studies and 67 participants, with a range from 9 to 22 participants. The mean of changes was 1.3 kg (range from −0.4 to 2.8 kg), or 11.6% (range from −4.0 to 22.0%). We found a nonsignificant (p = 0.087) change (ES = 0.36; 95% CI = −0.05 to 0.77, Q = 12.440, with 3 degrees of freedom, and p < 0.006, I2 = 76%).
Finally, fat-free mass analysis, included eight studies and 142 participants, with a range from 10 to 23 participants. The mean of changes was −0.3 kg (range from −1.2 to 1.3 kg), or −0.9% (range from −3.3 to 1.7%). We found a no significant (p = 0.17) change (ES = −0.09; 95% CI = −0.22 to 0.04, Q 6.050, with 6 degrees of freedom, and p < 0.534, I2 = 0%).
Additionally, we present in Figure 3 a total of three forest plots of performance variables, as follows:
Figure 3.
Forest plot of performance variables: (A) = Counter movement jump [8,10,26,28,30,37,38,39,40,44]; (B) = O2peak [9,13,25,27,29,34,38,42]; (C) = Sprint [8,10,22]. Favor A = Decrease value of Variable; Favor B = Increase value of Variable.
CMJ test [8,10,26,28,30,37,38,39,40,44].
O2peak variable [9,13,25,27,29,34,38,42].
In summary, CMJ analysis, included 10 studies, with 183 participants, with a range from 10 to 32 participants. The mean of changes was 0.3 cm (range from −1.7 to 6.1 cm), or 0.8% (range from −4.7 to 15.4%). We found a nonsignificant (0.99) change (ES = 0.00; 95% CI = −0.25 to 0.25, Q 39.334, with 9 degrees of freedom, and p < 0.001, I2 = 77%).
O2peak analysis, included eight studies, with 144 participants, with a range from 10 to 26 participants. We found a nonsignificant (p = 0.14) change (ES = −0.38; 95% CI = −0.90 to 0.13, Q 88.842, with 7 degrees of freedom, and p < 0.001, I2 = 92%).
Finally, data of sprint included from three studies, with 31 participants, with a range from 9 to 11 participants. We found a nonsignificant (p = 0.22) change (ES = 0.36; 95% CI= −0.21 to 0.94, Q 146.729, with 2 degrees of freedom, and p < 0.001, I2 = 99%).
3.3. Sensibility Analysis
A sensibility analysis was performed excluding studies with low quality (≤5 points on the JBI critical appraisal tool—see Table 1). There was a total of 13 studies (n = 348) that were included in this analysis [8,9,10,13,23,24,27,29,31,36,37,38,41].
In relation to the body composition variables, we did not find significant ES—Body mass variable (nine studies, n = 180) [8,10,13,23,27,36,37,38,41], the effect size was SMD = 0.01 (95% CI = −0.09–0.10, p = 0.91), and heterogeneity I2 = 95% (Q = 166.75, p < 0.00); Percentage of fat variable (five studies, n = 82) [8,10,27,36,38], the effect size was SMD = 0.03 (95% CI = −0.31–0.39 p = 0.828), and heterogeneity I2 = 99% (Q = 490.55, p = 0.00); Fat mass variable (two studies, n = 31) [13,23], the effect size was SMD = 0.11 (95% CI = −0.38–0.60, p = 0.663), and heterogeneity I2 = 99% (Q = 77.41, p < 0.00); Fat-free mass variable (five studies, n = 84) [8,13,23,27,36], the effect size was SMD = −0.01 (95% CI = −0.01–0.01, p = 0.718), and heterogeneity I2 = 86% (Q = 28.26, p < 0.00).
As for the body composition variables, in relation to the performance variables, we did not find significant ES–CMJ test (four studies, n = 84) [8,10,37,38], the effect size was SMD = 0.12 (95% CI = −0.45–0.69, p = 0.69), and heterogeneity I2 = 90% (Q = 28.97, p < 0.00); O2peak variable (five studies, n = 86) [9,10,13,27,29,38], the effect size was SMD = −0.21 (95% CI = −0.85–0.43, p = 0.52), and heterogeneity I2 = 92% (Q = 47.94, p < 0.00). Due to the low number of studies on the sprint variable, sub-analyses/sensibility analysis were not performed.
3.4. Meta-Regression
Table 3 presents the meta-regression of moderators for body weight and CMJ variables. Considering body weight, a significant moderator was observed in age—years (β = −0.023, 95% CI = −0.027 to −0.020, p < 0.001), follow-up time—weeks (β = −0.006, 95% CI = −0.005 to −0.008, p < 0.001), and level—elite or non-elite (β = −0.169, 95% CI = −0.200 to −0.128, p < 0.001). Considering the CMJ, a significant moderator was observed in age (β = −0.008, 95% CI = −0.009 to −0.006, p < 0.001), follow-up time (weeks) (β = −0.065, 95% CI = −0.074 to −0.057, p < 0.001), and level (elite or non-elite) (β = 0.076, 95% CI = 0.017 to 0.135, p < 0.001).
Table 3.
Meta-regression of moderators for the change in body weight or CMJ variables during COVID-19 lockdown in athletes.
| Moderator | Number of Studies | β Coefficient | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|
| Body weight | Age (years) | 15 | −0.023 | −0.027 | −0.020 | <0.001 |
| Follow-up time (weeks) | 17 | −0.006 | −0.005 | −0.008 | <0.001 | |
| Level (Elite or Non-elite) | 17 | −0.169 | −0.200 | −0.128 | <0.001 | |
| CMJ | Age (years) | 7 | −0.008 | −0.009 | −0.006 | <0.001 |
| Follow-up time (weeks) | 7 | −0.065 | −0.074 | −0.057 | <0.001 | |
| Level (Elite or Non-elite) | 7 | 0.076 | 0.017 | 0.135 | 0.012 | |
CI = confidence interval; CMJ = Countermovement Jump.
4. Discussion
The current systematic review and meta-analysis revealed that athletes maintained their physical performance and prevented fat mass gain and fat-free mass loss during the COVID-19 pandemic; however, they gained total body mass. This main finding is important, since most of them trained alone (80%) and intended to maintain their physical capacity, with a focus on body mass (65%) and cardiovascular (59%) capacities, while sports events and regular training routines were banned, and less than the 40% of athletes worldwide were able to maintain their sport-specific training [3].
Christensen et al. [48] demonstrated that a high-intensity training program is a feasible intervention to maintain physical fitness after two weeks in football players; however, the participants who interrupted their training completely showed a severe reduction in their physical fitness. Our meta-analysis demonstrated that the COVID-19 pandemic did not negatively affect the fitness level. However, it is important to mention that, during this period, most of the athletes that were analyzed performed home-based training programs. Our results support the recommendations previously mentioned by other authors [12]; specifically, the authors emphasized that the regular practice of exercise training at home during the social distancing period was the best way to attenuate the loss of function in athletes. However, during the regular season, athletes need to focus both on physical fitness and technical-tactical skills. On the other hand, during the COVID-19 pandemic, their training activities were mainly focused on physical conditioning, which, in turn, probably induced higher positive adaptations.
Mujika and Padilla [7] found a reduction of 6% to 20% O2max after a 4-week detraining period in highly trained athletes. However, the findings obtained in our work showed that home-based training was a practicable intervention to maintain cardiorespiratory fitness during lockdown. Nevertheless, it was noticeable that there were only eight studies verifying this variable; furthermore, by using different ergometers or tests, such as a treadmill and shuttle-run test, the heterogeneity in the procedures can be a risk of bias in the results obtained [49]. Therefore, caution should be taken when interpreting that data, and further studies should be conducted to confirm our findings.
The results shown in Figure 3 indicate that the muscle power and sprint were not significantly affected by the COVID-19 lockdown. Koundourakis et al. verified a decrease in SJ and CMJ performance after six weeks of detraining in both elite and sub-elite soccer players [50]; however, no differences were identified in recreationally strength-trained men [49]. The data suggested that athletes with high initial strength levels may suffer a reduction in power, while the jump ability of individuals with lower strength levels may not be influenced by a period of inactivity. The I2 value for those variables was high, indicating a considerable heterogeneity; therefore, we did not disregard the fact that the participants analyzed in this meta-analysis had different levels of lower-body strength (in accordance with their sports demands), and this situation could be a partial explanation for the null statistical effects found.
In addition, a detraining period can reduce the fast-twitch fibers that are associated with maximal strength, explosive power, and velocity [51], while a retraining period may induce supercompensation, which also can be a reason to maintain FFM. Chronic muscle contraction induces a variety of metabolic and morphological adaptations in contracted skeletal muscles to maintain homeostasis and minimize FFM loss, thus, the sufficient stimulation performed by athletes with greater volume and frequency attenuated FFM and would become sensitive again after a short detraining, or non-training, period [52].
Regarding body fat, there were no significant changes in fat mass or percentage of fat, although athletes increased their total body mass. In non-athletes, Javadi Arjmand et al. conducted a cohort study with 24,968 participants and found that high psychological distress was strongly associated with higher levels of emotional eating and high-sugar food intake [53]. In fact, the consumption of high caloric foods, due to impulse or anxiety, as well as lower levels of healthy eating habits were found, mainly at the start of the pandemic, which can maybe explain the changes in total body mass. This has also been a challenge for athletes during the home confinement caused by the COVID-19 pandemic [54]. However, further studies should be conducted on athletes to verify this hypothesis.
Furthermore, it is worth mentioning that most of the studies analyzed here were conducted on men. Concretely, from the 29 studies studied in the current meta-analysis, only 5 works involved women (2 of which were exclusive to women). Despite the controversy regarding the difference between the genders on physical performance, Ivey et al. reported that men retain the benefits of exercise training on muscle volume (despite 31 weeks of detraining), whereas women did not show the same ability [55]. On other hand, another study reported that women did retain the strength gained (as consequence of 9 weeks of strength training) after a period of 31 weeks, whereas, in men, the loss of muscle strength was significant after 31 weeks of detraining [56]. Those studies lead us to take care of the findings, mainly considering that gender is a variable that may regulate muscle quality (mass and muscle strength) in a detraining intervention. Therefore, future studies are needed in order to achieve a clear understanding about the gender effect on the physical performance of athletes during social distancing (i.e., the COVID-19 lockdown).
5. Conclusions
In summary, the athletes maintained muscle power, cardiorespiratory capacity, and sprint velocity, and prevented significant changes in their fat mass and fat-free mass during the COVID-19 pandemic; however, they increased their total body mass. Furthermore, the time of follow-up, the level of training, and the age of the athletes were possible moderators of these effects. Therefore, these data reinforce the importance of general strength and endurance exercise sessions to maintain physical fitness during non-competitive periods, or due to a mandatory lockdown. However, strategies such as combining training with diet to prevent body fat gain should be used, mainly if a long period of detraining is necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare11162319/s1. Supplementary data has data of pre- and post-confinement physical fitness outcomes, and the following topics and data were extracted from each study: title, author, year, study design, participant characteristics, performance or body composition measurement, evaluation protocol and instrument, follow-up time, and training protocol during confinement (Table S1).
Author Contributions
Conceptualization, F.E.R. and L.M.N.; methodology, B.V.R. and A.O.d.A.; formal analysis, L.M.N.; writing—original draft preparation, B.V.R., A.J.M., A.O.d.A., L.M.N. and F.E.R.; writing—review and editing, B.V.R., A.J.M., A.O.d.A., L.M.N. and F.E.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (F.E.R.) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
L.M.N. received two grants from the Fundação de Amparo à Pesquisa do Estado de So Paulo—FAPESP (2022/09863-0; 2021/11351-5). F.E.R. is a researcher supported by CNPq (PQ Level 2, data: 2023-2026).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sachs J.D., Karim S.A., Aknin L., Allen J., Brosbøl K., Barron G.C., Daszak P., Espinosa M.F., Gaspar V., Gaviria A. COVID-19 Commission Statement on the occasion of the 75th session of the UN General Assembly. Lancet. 2020;396:1102–1124. doi: 10.1016/S0140-6736(20)31927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurecka A., Skucinska P., Gadek A. Impact of the SARS-CoV-2 Coronavirus Pandemic on Physical Activity, Mental Health and Quality of Life in Professional Athletes—A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18:9423. doi: 10.3390/ijerph18179423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Washif J.A., Farooq A., Krug I., Pyne D.B., Verhagen E., Taylor L., Wong D.P., Mujika I., Cortis C., Haddad M. Training during the COVID-19 lockdown: Knowledge, beliefs, and practices of 12,526 athletes from 142 countries and six continents. Sports Med. 2022;52:933–948. doi: 10.1007/s40279-021-01573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez N.R., DiMarco N.M., Langley S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Am. Diet. Assoc. 2009;109:509–527. doi: 10.1016/j.jada.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Siders W.A., Lukaski H.C., Bolonchuk W.W. Relationships among swimming performance, body composition and somatotype in competitive collegiate swimmers. J. Sports Med. Phys. Fit. 1993;33:166–171. [PubMed] [Google Scholar]
- 6.Durkalec-Michalski K., Podgorski T., Sokolowski M., Jeszka J. Relationship between body composition indicators and physical capacity of the combat sports athletes. Arch Budo. 2016;12:247–256. [Google Scholar]
- 7.Mujika I., Padilla S. Detraining: Loss of training-induced physiological and performance adaptations. Part I: Short term insufficient training stimulus. Sports Med. 2000;30:79–87. doi: 10.2165/00007256-200030020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Spyrou K., Alcaraz P.E., Marin-Cascales E., Herrero-Carrasco R., Cohen D.D., Calleja-Gonzalez J., Pereira L.A., Loturco I., Freitas T.T. Effects of the COVID-19 Lockdown on Neuromuscular Performance and Body Composition in Elite Futsal Players. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2021;35:2309–2315. doi: 10.1519/JSC.0000000000004028. [DOI] [PubMed] [Google Scholar]
- 9.Fikenzer S., Fikenzer K., Laufs U., Falz R., Pietrek H., Hepp P. Impact of COVID-19 lockdown on endurance capacity of elite handball players. J. Sports Med. Phys. Fit. 2021;61:977–982. doi: 10.23736/S0022-4707.20.11501-9. [DOI] [PubMed] [Google Scholar]
- 10.Grazioli R., Loturco I., Baroni B.M., Oliveira G.S., Saciura V., Vanoni E., Dias R., Veeck F., Pinto R.S., Cadore E.L. Coronavirus disease-19 quarantine is more detrimental than traditional off-season on physical conditioning of professional soccer players. J. Strength Cond. Res. 2020;34:3316–3320. doi: 10.1519/JSC.0000000000003890. [DOI] [PubMed] [Google Scholar]
- 11.Wilson M.G., Hull J.H., Rogers J., Pollock N., Dodd M., Haines J., Harris S., Loosemore M., Malhotra A., Pieles G. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020;54:1157–1161. doi: 10.1136/bjsports-2020-102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokes K.A., Jones B., Bennett M., Close G.L., Gill N., Hull J.H., Kasper A.M., Kemp S.P., Mellalieu S.D., Peirce N. Returning to play after prolonged training restrictions in professional collision sports. Int. J. Sports Med. 2020;41:895–911. doi: 10.1055/a-1180-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva I.A., da Silva Santos A.M., Maldonado A.J., de Moura H., Rossi P.A.Q., Neves L.M., Dos Santos M.A.P., Machado D.C.D., Ribeiro S.L.G., Rossi F.E. Detraining and retraining in badminton athletes following 1-year COVID-19 pandemic on psychological and physiological response. Sport Sci. Health. 2022;18:1427–1437. doi: 10.1007/s11332-022-00939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esco M.R., Fedewa M.V., Cicone Z.S., Sinelnikov O.A., Sekulic D., Holmes C.J. Field-Based Performance Tests Are Related to Body Fat Percentage and Fat-Free Mass, But Not Body Mass Index, in Youth Soccer Players. Sports. 2018;6:105. doi: 10.3390/sports6040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute J.B. Joanna Briggs Institute Reviewers’ Manual. 2014. The Joanna Briggs Institute; Adelaide, Australia: 2014. pp. 88–91. [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press; Cambridge, MA, USA: 2013. [Google Scholar]
- 19.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 21.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M. Comprehensive Meta-Analysis Software. Systematic Reviews in Health Research: Meta-Analysis in Context. John Wiley & Sons; Hoboken, NJ, USA: 2022. [Google Scholar]
- 23.Aguilar A.J., Mendez-Rebolledo G., Rojano-Ortega D., Moya-Amaya H., Molina-López A., Berral-de-la-Rosa F.J. Valoración del Impacto del Confinamiento por SARS-CoV-2 sobre la Composición Corporal de una Población de Futbolistas de Élite. Int. J. Morphol. 2021;39:1088–1095. doi: 10.4067/S0717-95022021000401088. [DOI] [Google Scholar]
- 24.Alvurdu S., Baykal C., Akyildiz Z., Şenel Ö., Silva A.F., Conte D., Clemente F.M. Impact of prolonged absence of organized training on body composition, neuromuscular performance, and aerobic capacity: A study in youth male soccer players exposed to COVID-19 lockdown. Int. J. Environ. Res. Public Health. 2022;19:1148. doi: 10.3390/ijerph19031148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambroży T., Rydzik Ł., Obmiński Z., Klimek A.T., Serafin N., Litwiniuk A., Czaja R., Czarny W. The impact of reduced training activity of elite kickboxers on physical fitness, body build, and performance during competitions. Int. J. Environ. Res. Public Health. 2021;18:4342. doi: 10.3390/ijerph18084342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asimakidis N.D., Vasileiou S.S., Dalamitros A.A., Nikolaidis P.T., Manou V. Effect of the COVID-19 Confinement Period on Selected Neuromuscular Performance Indicators in Young Male Soccer Players: Can the Maturation Process Counter the Negative Effect of Detraining? Int. J. Environ. Res. Public Health. 2022;19:4935. doi: 10.3390/ijerph19094935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campa F., Bongiovanni T., Trecroci A., Rossi A., Greco G., Pasta G., Coratella G. Effects of the COVID-19 lockdown on body composition and bioelectrical phase angle in Serie A soccer players: A comparison of two consecutive seasons. Biology. 2021;10:1175. doi: 10.3390/biology10111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen D.D., Restrepo A., Richter C., Harry J.R., Franchi M.V., Restrepo C., Poletto R., Taberner M. Detraining of specific neuromuscular qualities in elite footballers during COVID-19 quarantine. Sci. Med. Footb. 2021;5:26–31. doi: 10.1080/24733938.2020.1834123. [DOI] [PubMed] [Google Scholar]
- 29.Dauty M., Menu P., Fouasson-Chailloux A. Effects of the COVID-19 confinement period on physical conditions in young elite soccer players. J. Sports Med. Phys. Fit. 2020;61:1252–1257. doi: 10.23736/S0022-4707.20.11669-4. [DOI] [PubMed] [Google Scholar]
- 30.Font R., Irurtia A., Gutierrez J.A., Salas S., Vila E., Carmona G. The effects of COVID-19 lockdown on jumping performance and aerobic capacity in elite handball players. Biol. Sport. 2021;38:753–759. doi: 10.5114/biolsport.2021.109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinowski P., Myszkowski J., Marynowicz J. Effect of Online Training during the COVID-19 Quarantine on the Aerobic Capacity of Youth Soccer Players. Int. J. Environ. Res. Public Health. 2021;18:6195. doi: 10.3390/ijerph18126195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korkmaz S., Aslan C.S., Eyuboğlu E., Çelebi M., Kır R., Karakulak İ., Akyüz Ö., Özer U., Geri S. Impact of detraining process experienced during the COVID-19 pandemic on the selected physical and motor features of football players. Prog. Nutr. 2020;22:e2020029. [Google Scholar]
- 33.Kosova S., Kosova M.K. The effect of the detraining period caused by the COVID-19 pandemic on the change of direction performance of fencers. Phys. Educ. Stud. 2021;25:4–9. doi: 10.15561/20755279.2021.0101. [DOI] [Google Scholar]
- 34.Leo P., Mujika I., Lawley J. Influence of COVID-19 Restrictions on Training and Physiological Characteristics in U23 Elite Cyclists. J. Funct. Morphol. Kinesiol. 2021;7:1. doi: 10.3390/jfmk7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna B., Chiner P.M., Puchades V.P., Marzal A.C., Aliaga A.R., Lafarga C.B. Cambios en fuerza explosiva y agilidad tras un entrenamiento online en jóvenes jugadores de baloncesto confinados por COVID-19. Retos Nuevas Tend. Educ. Física Deporte Recreación. 2021;41:256–264. [Google Scholar]
- 36.My G., Marsigliante S., Bianco A., Zangla D., Silva C.M.D., Muscella A. Biological, Psychological, and Physical Performance Variations in Football Players during the COVID-19 Lockdown: A Prospective Cohort Study. Int. J. Environ. Res. Public Health. 2022;19:2739. doi: 10.3390/ijerph19052739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paravlic A.H., Simunic B., Pisot S., Kleva M., Teraz K., Vogrin M., Marusic U., Pisot R. Lower-Limb Muscle Contractile Properties, Explosive Power and the Subjective Response of Elite Soccer Players to the COVID-19 Lockdown. Int. J. Environ. Res. Public Health. 2022;19:474. doi: 10.3390/ijerph19010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parpa K., Michaelides M. The impact of COVID-19 lockdown on professional soccer players’ body composition and physical fitness. Biol. Sport. 2021;38:733–740. doi: 10.5114/biolsport.2021.109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen S., Johansen D., Casolo A., Randers M.B., Sagelv E.H., Welde B., Winther A.K., Pettersen S.A. Maximal Strength, Sprint, and Jump Performance in High-Level Female Football Players Are Maintained With a Customized Training Program During the COVID-19 Lockdown. Front. Physiol. 2021;12:623885. doi: 10.3389/fphys.2021.623885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pucsok J.M., Kovacs M., Rathonyi G., Pocsai B., Balogh L. The Impact of COVID-19 Lockdown on Agility, Explosive Power, and Speed-Endurance Capacity in Youth Soccer Players. Int. J. Environ. Res. Public Health. 2021;18:9604. doi: 10.3390/ijerph18189604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scoz R.D., Burigo R.L., Ferreira I.C., Hespanhol L., Silveira Ramos A.P., Schlosser A., Baltazar Mendes J.J., Alves Ferreira L.M., Amorim C.F. Strength Level of Professional Elite Soccer Players after the COVID-19 Lockdown Period: A Retrospective Double-Arm Cohort Study. J. Sports Med. 2022;2022:8242210. doi: 10.1155/2022/8242210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segalés-Gill D., Cofré-Bolados C., Beas-Jimenez J., Valdivia-Moral P., DeMoraes G. Reducción del consumo máximo de oxígeno después de once semanas de desentrenamiento en futbolistas profesionales. J. Sport Health Res. 2021;13:121–130. [Google Scholar]
- 43.Freire L.d., Tannure M., Sampaio M., Slimani M., Znazen H., Bragazzi N.L., Aedo-Muñoz E., Sobarzo Soto D.A., Brito C.J., Miarka B. COVID-19-related restrictions and quarantine COVID-19: Effects on cardiovascular and yo-yo test performance in professional soccer players. Front. Psychol. 2020;11:589543. doi: 10.3389/fpsyg.2020.589543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villaseca-Vicuña R., Pérez-Contreras J., Merino-Muñoz P., Aedo-Muñoz E., Jurado J.A.G., Zabaloy S. Effects of COVID-19 Lockdown on Body Composition and Physical Performance of Elite Female Football Players. Women Sport Phys. Act. J. 2022;30:44–52. doi: 10.1123/wspaj.2022-0002. [DOI] [Google Scholar]
- 45.Valenzuela P.L., Rivas F., Sánchez-Martínez G. Effects of COVID-19 lockdown and a subsequent retraining period on elite athletes’ workload, performance, and autonomic responses: A case series. Int. J. Sports Physiol. Perform. 2021;16:1707–1711. doi: 10.1123/ijspp.2020-0735. [DOI] [PubMed] [Google Scholar]
- 46.Junaidi J., Apriyanto T., Winata B., Inarota L. Monitoring body mass status during the COVID-19 quarantine in combat and aesthetic sports. Postep. Rehabil. 2021;35:1–7. doi: 10.5114/areh.2021.107787. [DOI] [Google Scholar]
- 47.Yasuda J., Kondo E., Takai E., Eda N., Azuma Y., Motonaga K., Dohi M., Kamei A. The Effects of the COVID-19 Environments on Changes in Body Composition in Japanese Elite Fencing Athlete. Sports. 2021;9:95. doi: 10.3390/sports9070095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christensen P.M., Krustrup P., Gunnarsson T.P., Kiilerich K., Nybo L., Bangsbo J. VO2 kinetics and performance in soccer players after intense training and inactivity. Med Sci Sports Exerc. 2011;43:1716–1724. doi: 10.1249/MSS.0b013e318211c01a. [DOI] [PubMed] [Google Scholar]
- 49.Kraemer W.J., Koziris L.P., Ratamess N.A., Hakkinen K., Triplett-Mcbride N.T., Fry A.C., Gordon S.E., Volek J.S., French D.N., Rubin M.R., et al. Detraining produces minimal changes in physical performance and hormonal variables in recreationally strength-trained men. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2002;16:373–382. [PubMed] [Google Scholar]
- 50.Koundourakis N.E., Androulakis N.E., Malliaraki N., Tsatsanis C., Venihaki M., Margioris A.N. Discrepancy between exercise performance, body composition, and sex steroid response after a six-week detraining period in professional soccer players. PLoS ONE. 2014;9:e87803. doi: 10.1371/journal.pone.0087803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorstensson A. Muscle strength, fibre types and enzyme activities in man. Acta Physiol. Scand. Suppl. 1976;443:1–45. [PubMed] [Google Scholar]
- 52.Ogasawara R., Yasuda T., Ishii N., Abe T. Comparison of muscle hypertrophy following 6-month of continuous and periodic strength training. Eur. J. Appl. Physiol. 2013;113:975–985. doi: 10.1007/s00421-012-2511-9. [DOI] [PubMed] [Google Scholar]
- 53.Javadi Arjmand E., Bemanian M., Vold J.H., Skogen J.C., Sandal G.M., Arnesen E.K., Maeland S., Fadnes L.T. Emotional Eating and Changes in High-Sugar Food and Drink Consumption Linked to Psychological Distress and Worries: A Cohort Study from Norway. Nutrients. 2023;15:778. doi: 10.3390/nu15030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreato L.V., Coimbra D.R., Andrade A. Challenges to athletes during the home confinement caused by the COVID-19 pandemic. Strength Cond. J. 2020;42:1–5. doi: 10.1519/SSC.0000000000000563. [DOI] [Google Scholar]
- 55.Ivey F.M., Roth S.M., Ferrell R.E., Tracy B.L., Lemmer J.T., Hurlbut D.E., Martel G.F., Siegel E.L., Fozard J.L., Jeffrey Metter E., et al. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000;55:M641–M648. doi: 10.1093/gerona/55.11.M641. [DOI] [PubMed] [Google Scholar]
- 56.Lemmer J.T., Hurlbut D.E., Martel G.F., Tracy B.L., Ivey F.M., Metter E.J., Fozard J.L., Fleg J.L., Hurley B.F. Age and gender responses to strength training and detraining. Med. Sci. Sports Exerc. 2000;32:1505–1512. doi: 10.1097/00005768-200008000-00021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (F.E.R.) upon reasonable request.