Abstract
The friction reduction mechanism of glycerol monooleate (GMO) was investigated under boundary lubrication with elevated temperature. Tribological performances were tested using reciprocating test rig by adding 5 wt.% GMO into Poly-alpha Olefin (PAO) base oil. Friction coefficient and wear were recorded during experiments. The used oil was evaluated by infrared detection after experiments. Results show that GMO could reduce friction coefficient at both low and high temperature. At elevated temperature, the friction coefficient of PAO-GMO blend climb up gradually, followed by a decrease tendency, and the wear increase gradually with temperature. The results of Quartz Crystal Microbalance show that the physical adsorption film plays the main role in friction reduction at low temperature. While at high temperature, the Infrared Spectrum and X-Ray Photoelectron Spectrum show that the GMO involves into the chemisorption with friction surface, producing Fe(OH)O and Fe3O4. The friction reduction mechanism of GMO transferred from physisorption to chemisorption, which reduced friction coefficient at both low and high temperature.
Keywords: Glycerol monooleate, friction modifier, tribological performance, physisorption, chemisorption
Introduction
Friction modifier additives have been extensively applied to lubricants to improve the friction properties of oil products.1,2 This process can form friction reduction film on the friction surface. Therefore, friction modifiers plays an important role in reducing friction coefficient and wear. MoDTC, MoDDP, and other molybdenum friction modifiers contain S and P elements, which are toxic to the environment. Thus, biodegradable and environment-friendly friction modifier has been investigated for environment-sensitive industries because of the increasing concern for environmental protection.3–6 Ester has long been used as a friction modifier additive. This compound can reduce the friction coefficients when added into base oil.7–9 Mixture of ester and ZDDP/MoDTP/MoDTC have also found to have effective friction reduction performance at Ta-C, DLC, CKS coatings, and cast iron surface.10–13 The friction reduction performance of ester is temperature dependent, high temperature will reduced the adsorbed friction reduction film of ester. Therefore, the investigation of ester lubrication performance at elevated temperature is important to determine the temperature that is the best suit for the application of ester friction modifier.
Glycerol monooleate (GMO) has linear molecular structures, which significantly improve the tribological performance. GMO has been widely used in lubricant oil as friction modifier, excellent lubricating performances were observed, such as the ultralow friction obtained between DLC and ta-C coatings with PAO base oil.14–16 The GMO was also found to have lower boundary friction coefficient than nitrogen-containing compounds under the same lubricating conditions. 17 Therefore, GMO was selected as the ester additive in this study. To clearly observe the influence of ester additive to lubrication properties, 5 wt.% GMO was added into PAO base oil, and the mixture is denoted as PAO-GMO. Engine cylinder liner and piston ring specimens were chosen to test the tribological performance of PAO-GMO. Friction coefficient and wear were recorded with test rig in elevated temperature, and the friction-reducing mechanism was discussed.
Materials and methods
Materials
Cast iron cylinder and Chrom-Keramik-Schicht (CKS) piston ring are selected as specimens, which are shown in Figure 1. The cast iron cylinder liner has an inner diameter of 110 mm, wall thickness of 8 mm, and length of 42 mm. Cylinder liner specimens were produced by cutting cylinder liner into 40 portions in circumferential direction. The width and length of every specimen is about 10 mm and 42 mm, respectively.
Figure 1.
The cut diagram of cylinder liner and piston ring.
The real piston ring is not a complete circular structure, and the radius in different positions may have different value. This characteristic may influence the contact area between piston ring and cylinder liner. Therefore, the CKS coated piston ring in this study is a custom made one. The ring has a circular structure with uniform outer radius. Piston ring outer diameter is 110 mm and the width is 7 mm. The outside surface of the piston ring is plated with a CKS layer. Piston ring specimen was produced by cutting the ring into 20 portions.
In Figure 2, the topography and composition of cylinder liner surface was analyzed by laser scanning confocal microscope (LSCM) and EDS, respectively. Cylinder liner surface was honed with micro groove, which could retain lube oil in the surface. The angle of honing groove was 56°. The average surface roughness and hardness of cast iron cylinder were 3.104 μm and HV0.1 381 respectively. The EDS of cylinder liner show the specimen were mainly composed of Fe, P, Cr, and Si. The existence of phosphorus form iron phosphide eutectic in cast iron cylinder liner, which improve the anti-wear performance of cylinder liner.
Figure 2.
Topography and composition of cylinder liner: (a) topography obtained by LSCM, (b) surface obtained by SEM, and (c) chemical composition obtained by EDS.
Figure 3 shows the surface and cross section of CKS piston ring. The black lines in the SEM image of piston ring is the Al2O3 hard particles. CKS coating introduce Al2O3 particles into the chrome plating solution, which increased the anti-wear performance of piston ring. The SEM cross-section image shows that piston ring was coated with a CKS layer of 60 μm. The surface roughness and hardness of piston ring specimen were 1.027 μm and HV0.1 761 respectively.
Figure 3.
The surface and cross section of CKS piston ring: (a) surface of piston ring obtained by SEM, (b) cross section of piston ring obtained by SEM, and (c) chemical composition obtained by EDS.
PAO was used as base oil. Table 1 shows the viscosity and viscosity index of PAO and its blend with 5 wt.% GMO. The GMO molecular structure is shown in Figure 4.
Table 1.
Viscosity and viscosity index of PAO and the blend PAO-GMO.
| Parameter | PAO | PAO-GMO |
|---|---|---|
| Viscosity (40°C cst) | 24.62 | 26.06 |
| Viscosity (100°C cst) | 5.17 | 5.48 |
| Viscosity index | 146 | 154 |
Figure 4.

The molecular structure of GMO.
Methods
Tribological experiments were performed on a custom-made reciprocating tribological test rig, which is shown in Figure 5. The revolution of driving motor is 200 r/min, which gives a maximum reciprocating speed of 315 mm/s with stroke of 30 mm. The piston ring was fixed in the jag and kept stationary during experiment. The cylinder liner sample performs a reciprocating motion. Friction coefficients were detected by the transducer in the test rig. Lubrication oil was circulated with 0.1 ml/min through the oil filler hole to the friction interface by a peristaltic pump. Temperature sensor was installed to control the electric heater and maintain the target temperature. The temperature was controlled from 70°C to 150°C. Normal load was applied to the friction interface by a loading device. 5 MPa to 20 MPa load between cylinder liner and piston ring was applied. The testing pressure range is close to the pressure within the combustion chamber of diesel engine. A 60-min low-load running-in period was first conducted with 3 MPa load prior to testing. Then, the test was performed for 10 h with target load.
Figure 5.
Tribological test rig.
After experiment, 10 ml used oil was collected and analysis by infrared spectrometry (IR). The piston ring and cylinder liner specimens were cleaned with petroleum ether and ethyl alcohol in ultrasonic bath, which remove the adsorbed base oil and GMO on friction surface. Wear of piston ring and cylinder liner were measured by Mettler AL204-IC analytical balance, with resolution of 0.1 mg. The topography of cylinder liner surface after friction was analyzed by OLYMPUS OLS4000 LSCM and ZEISS SUPRA 55 scanning electron microscopy (SEM) in conjunction with energy-dispersive X-ray spectrometry (EDS). The physisorption properties of PAO-GMO was tested with QCM-200 quartz crystal microbalance. The QCM200 uses a 5 MHz, 1″ diameter, AT-cut quartz crystal wafer with circular electrodes, the digital controller contains a frequency counter with 0.01 Hz resolution, and a resistance meter with five digits of resolution covering a range of 0 to 5000 Ω.
Results of friction and wear
Figure 6 shows the friction coefficient versus testing temperature. Figure 6(a) illustrates the friction coefficient versus test time at 20 MPa. During the running in period, the friction coefficient keep stable in a low value. After loading to testing pressure, the friction coefficient increased sharply, then decreased to stable period. Figure 6(b) illustrates the average stable friction coefficient at different temperature and load, the data was obtained at 400–600 min during test. Generally, the PAO-GMO blend oil shows lower friction coefficient than PAO base oil, which means the GMO has obvious friction reduction performance. With the rise of load, the friction coefficient of PAO-GMO increased gradually. With the rise of temperature, friction coefficient of PAO base oil increase sharply. However, PAO-GMO blend oil shows different performance. The friction coefficient increase gradually from 70°C to 110°C, then followed with a decrease tendency.
Figure 6.
Friction coefficient verse testing temperature: (a) friction coefficient versus test time at 20 MPa and (b) the average stable friction coefficient at different temperature and load.
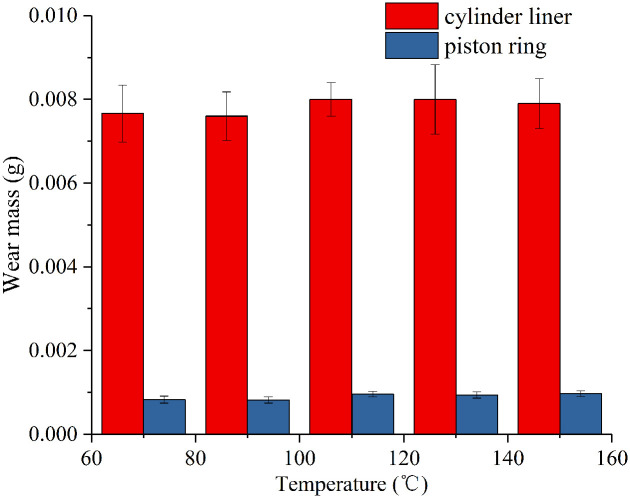
Figure 7 shows the wear verse temperature at 20 MPa. Generally, the wear of cylinder liner is much higher than piston ring, as the hardness of cylinder liner is lower than piston ring. The wear of cylinder liner increase slightly with temperature, which increase from 0.0076 g to 0.0082 g. While the temperature shows little influence to the wear of piston ring. The wear mass of piston ring is around 0.0008 g.
Figure 7.
Wear of cylinder liner and piston ring versus temperature at 20 MPa.
Discussion
GMO is a typical organic friction modifier, which form physisorption film on the friction surface and reduce friction coefficient. The physisorption will decrease with the increase of temperature. 18 Therefore, there should be an increase of friction coefficient with elevated temperature. However, test results in Figure 6 show different tendency compared with traditional prediction. In order to investigate the friction reduction mechanism, the physisorption properties, infrared spectrum (IR) of testing oil, topography of cylinder liner surface after friction was analyzed by LSCM, and the X-ray photoelectron spectrum (XPS) of the surface were analyzed.
(1) Adsorption film at elevated temperature
The physisorption properties of PAO-GMO are shown in Figure 8. It is clear that the adsorption mass of PAO-GMO decrease sharply from 70°C to 130°C. Despite the reduced friction coefficient from 110°C to 150°C, the adsorption film reduced sharply. This suppose that friction reduction substances, rather than the adsorption film, is the key to reduce friction coefficient at high temperature. Based on this assumption, the following analysis was focused on the used oil and friction surface.
Figure 8.
Adsorption mass of PAO-GMO.
(2) Infrared spectroscopy of used oil sample
Figure 9 is the infrared spectroscopy of oil samples, (a) is the infrared spectroscopy of PAO and PAO-GMO before test, (b) is used PAO after tribological experiment, (c) is used PAO-GMO after tribological experiment. In order to verify whether the friction reduction product comes from the thermal cracking or the chemisorption of ester, Figure 9(d) compares the used PAO-GMO after 150°C test and PAO-GMO after 150°C baking for 4 h.
Figure 9.
Infrared spectroscopy of tested oil: (a) PAO and PAO-GMO before test, (b) PAO after test, (c) PAO-GMO after test, and (d) used PAO-GMO after 150°C test and PAO-GMO after 150°C
Figure 9(a) illustrates that the primary difference between PAO and PAO-GMO is the functional group carbonyl, with wave number between 1800 cm−1 and 1600 cm−1. 19 The functional groups facilitate the adsorption of the ester on the friction surface. IR results in Figure 9(b) illustrate that no obvious difference was found among the PAO samples in different test temperature. Figure 9(c) shows that the carbonyl peak of used blend oil at 130°C and 150°C were weaker than that in other temperatures. Therefore, chemisorption is deduced to occur between GMO and cylinder liner at high temperature, which results in the reduced friction coefficient in 130°C and 150°C (Figure 6). Figure 9(d) shows that the carbonyl peak after baking at 150°C is stronger than that after testing, which indicates that the thermal cracking effect was not clear at 150°C. On friction surface, it is undeniable that the temperature at the spots where micro-bulges contact may be higher than the controlled temperature (150°C) due to the friction. The GMO may undergoing severe shear and high temperature in the friction surface, which may lead to the decomposition of GMO. However, the real temperature at the friction interface is very hard to detect, and no other organo-functional groups were found in the peaks of lubricant after friction. Therefore, the results in Figure 9 indicate that the friction reduction product comes from the chemisorption, rather than thermal cracking of ester.
(3) Topography and XPS analysis of worn cylinder liner surface
Figure 10 shows the topography of cylinder liner surface before and after friction. The cylinder liner before friction show bright surface and clear honing groove. After friction, the surface were covered with brown friction layer, and the honing groove become unclear. Surface roughness detected by LSCM show the roughness decrease from 3.104 μm to 1.635 μm, which was caused by the diminish of honing groove.
Figure 10.
The topography of cylinder liner surface: (a) before and (b) after friction.
In order to investigate the friction reduction substance in the friction surface, the XPS spectra of worn cylinder surface was analyzed. With the deconvolution of overlap peaks in Fe 2p and O 1s, the tribo-chemical products on friction surface were analyzed.
Figure 11 illustrates the Fe 2p XPS spectrum of friction surface. The friction products presented in each peak were check with NIST XPS data base. 20 As for the friction products at 70°C in Figure 11(a), the products are mainly composed of Fe2O3 (709.9 eV, 711.6 eV), Fe3O4 (710.8 eV), and Fe hydrocarbons (712.6 eV). In Figure 11(b), the friction products at 150°C are mainly composed of Fe2O3 (711.1 eV), Fe3O4 (710.2 eV), Fe(OH)O (711.8 eV) and other Fe oxidants (713.7 eV).
Figure 11.
Fe 2p XPS spectrum of cylinder liner surface: (a) 70°C and (b) 150°C.
The peak around 707.0 in Figure 11(b) corresponding to Fe76Cr24. 20 The existence of Fe76Cr24 peak indicates the transfer of Cr from piston ring to cylinder liner due to friction. The peak of Fe76Cr24 become more obvious in 150°C than 70°C, which means the transfer rate of Cr increase with elevated temperature.
Figure 12 illustrates the O 1s XPS spectrum of friction surface. In Figure 12(a), the peaks at 70°C show Fe–O–Fe bond (530.4 eV) and Fe–O-–C bond (531.8 eV). 21 This means the friction surface was covered with Fe-oxide, which is coincide with the results in Figure 11(a). In Figure 12(b), the peaks at 150°C show Fe–O–Fe bond (530.4 eV), Fe–O–H bond (531.2 eV) and –OH bond (532 eV), 22 which means the friction surface was covered with Fe-oxide and Fe(OH)O, which is coincide with the results in Figure 11(b).
Figure 12.
O 1 s XPS spectrum of cylinder liner surface: (a) 70°C and (b) 150°C.
Based on the experimental results, it is suggested that chemisorption occurs on cylinder liner surface, and the boundary film composed of Fe(OH)O and Fe3O4 reduced the friction coefficient at high temperature. 23
Conclusion
GMO has linear molecular structure, in which the straight carbon chain and the functional groups are located at the end of carbon chain. This structure facilitates the orderly and close absorption of GMO on the iron surface. Therefore, physisorption play important role in reducing friction at low temperatures. However, physisorption is related with temperature and is reversible. Desorption happens at high temperatures, and the chemisorption films play the important role in improving the tribological performance when temperature is higher than 110°C.
Friction coefficient of GMO-PAO blend oil increase with the rise of temperature, the highest friction coefficient appears at 110°C, followed by a decrease tendency. Test temperature has little influence to the anti-wear performance of GMO-PAO.
The test results of QCM show that physical adsorption film decrease sharply from 70°C to 130°C, which indicates that the reduced friction coefficient at low temperature was caused by the physical adsorption film of GMO, while the friction reduction performance at high temperature was caused by the chemisorption products.
The weakened IR peak of carbonyl in used oil indicates that carbonyl in GMO involves into the chemisorption with friction surface. XPS peaks indicate the Fe(OH)O and Fe3O4 were produced during high temperature friction, which reduced the friction coefficient obviously.
Author biographies
Weiwei Wang is Lecturer of Ocean School at Yantai University, China. His research includes engine lubrication, lubricant additives and anti-wear coatings. Recently, his research primarily focuses on the organic friction modifier and the auto-restore additives.
Bo Shen is an engineer in China Merchants Group. He mainly engaged in strategic planning, investment management and management of marine propulsion system.
Yang Li is an Associate Professor in College of Nuclear Equipment and Nuclear Engineering at Yantai University, China. His research primarily focuses on the engine lubrication and anti-wear coatings. He has published more than 10 papers about friction research.
Qiang Ni is an Engineer in CRRC Changchun Railway Vehicles Co., Ltd, China. He mainly engaged in Railway equipment management and maintenance.
Li Zhou is Professor of School of Electromechanical and Automotive Engineering at Yantai University, China. Her research field includes material machining and simulation. Recently, her research primarily focuses on the material mechanical. She has published 40 papers about material mechanical and simulation.
Fengming Du is an Associate Professor of Marine engineering college at Dalian Maritime University, China. His research includes friction and wear of diesel engine. Recently, his research primarily focuses on the friction of bearing bush. He has published more than 10 papers about friction research.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was support by the Natural Science Foundation of Shandong Province [Grant number ZR2019BEE073, ZR2019MEE074]; China Postdoctoral Science Foundation [Grant number 2017M611209]; and Natural Science Foundation of Liaoning Province, [Grant number 20170540083].
ORCID iD: Weiwei Wang  https://orcid.org/0000-0001-8067-8337
https://orcid.org/0000-0001-8067-8337
References
- 1.Spikes H.Friction modifier additives. Tribol Lett 2015; 60: 1–26. [Google Scholar]
- 2.Tang Z, Li S.A review of recent developments of friction modifiers for liquid lubricants (2007-present). Curr Opin Solid State Mater Sci 2014; 18: 119–139. [Google Scholar]
- 3.Randles SJ.Environmentally considerate ester lubricants for the automotive and engineering industries. J Synth Lubr 1992; 9: 145–161. [Google Scholar]
- 4.Sheng C, Chen H, Zhang J, et al. Friction and wear behaviour of 1-octyl-3-methylimidazolium lactate ionic liquid as lubricant in steel-steel contacts. Tribol Trans 2019; 62: 955–961. [Google Scholar]
- 5.Chan CH, Tang SW, Mohd NK, et al. Tribological behavior of biolubricant base stocks and additives. Renew Sust Energ Rev 2018; 93: 145–157. [Google Scholar]
- 6.Kim KS, Lee MS.Biodegradable grease composition using distillation residue generated in production of biodiesel. Patent US8481466 B2, USA, 2013. [Google Scholar]
- 7.Choo JH, Forrest AK, Spikes HA.Influence of organic friction modifier on liquid slip: a new mechanism of organic friction modifier action. Tribol Lett 2007; 27: 239–244. [Google Scholar]
- 8.Vilics T, Ciorbagiu F, Ciucu B.Ester base stocks for synthetic lubricants. J Synth Lubr 1996; 13: 289–296. [Google Scholar]
- 9.Khalkar S, Bhowmick D, Pratap A.Effect of wax esters as friction modifiers in petroleum base stock. J Oleo Sci 2012; 61: 723–728. [DOI] [PubMed] [Google Scholar]
- 10.Okubo H, Tadokoro C, Sasaki S.Tribological properties of a tetrahedral amorphous carbon (ta-C) film under boundary lubrication in the presence of organic friction modifiers and zinc dialkyldithiophosphate (ZDDP). Wear 2015; 332: 1293–1302. [Google Scholar]
- 11.Ng E, Kumar Sinha S.Effects of antiwear additives in the base oil on the tribological performance of hydrogen-free DLC coating. Ind Lubr Tribol 2014; 66: 633–639. [Google Scholar]
- 12.Wang W, Li C, Yang J, et al. Friction performance of MoDTP and ester-containing lubricants between CKS piston ring and cast iron cylinder liner. Lubr Sci 2018; 30: 33–43. [Google Scholar]
- 13.Trindade ED, Zuleta Durango A, Sinatora A.Friction and wear performance of MODTC-containing and ester-containing lubricants over steel surfaces under reciprocating conditions. Lubr Sci 2015; 27: 217–229. [Google Scholar]
- 14.Kano M, Yasuda Y, Okamoto Y, et al. Ultralow friction of DLC in presence of glycerol mono-oleate (GNO). Tribo Lett 2005; 18: 245–251. [Google Scholar]
- 15.Tasdemir HA, Wakayama M, Tokoroyama T, et al. Ultra-low friction of tetrahedral amorphous diamond-like carbon (ta-C DLC) under boundary lubrication in poly alpha-olefin (PAO) with additives. Tribo Int 2013; 65: 286–294. [Google Scholar]
- 16.Kano M.Super low friction of DLC applied to engine cam follower lubricated with ester-containing oil. Tribo Int 2006; 39: 1682–1685. [Google Scholar]
- 17.Castle RC, Bovington CH.The behaviour of friction modifiers under boundary and mixed EHD conditions. Lub Sci 2003; 15: 253–263. [Google Scholar]
- 18.Rudnick LR.Lubricant additives: chemistry and applications. 3nd ed. Boca Raton: CRC, 2017. [Google Scholar]
- 19.NIST standard reference database number 69, http://webbook.nist.gov/chemistry/ (2018, accessed 16 January 2020).
- 20.NIST X-ray photoelectron spectroscopy database, https://srdata.nist.gov/xps/selEnergyType.aspx (2012, accessed 11 October 2020).
- 21.Yang F, Zhang S, Li H, et al. Corn straw-derived biochar impregnated with α-FeOOH nanorods for highly effective copper removal. Chem Eng J 2018; 348: 191–201. [Google Scholar]
- 22.Gu Y, Li C, Gong Z, et al. Photocatalytic decontamination of tetracycline and Cr(VI) by a novel α-FeOOH/FeS2 photocatalyst: one-pot hydrothermal synthesis and Z-scheme reaction mechanism insight. J Hazard Mater 2020; 397: 122580. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Zhao Q, Zhang M, et al. Tribological properties of castor oil tris (diphenyl phosphate) as a high-performance antiwear additive in lubricating greases for steel/steel contacts at elevated temperature. RSC Adv 2014; 4: 54760–54768. [Google Scholar]