Abstract
The hypertension (HTN) and type 2 diabetes mellitus (T2DM) are a common multifactorial disease due to genetics and environmental factors. The alpha 2B adrenergic receptor (α2B-AR) has relationship with secretion of insulin and mediates the vasoconstriction that elevate blood pressure. This study aimed to determine the association between α2B-AR gene polymorphism with HTN and T2DM in Saudi cases. 200 cases and 100 healthy controls from Saudi population were recruited from the Internal Medicine clinic, Qassim University. The patients were grouped into: 72 HTN without T2DM; 62 HTN with T2DM and 66 T2DM only. Full medical history, examination and biochemical assays were performed for all participants. Genomic DNA was isolated from blood lymphocytes of all subjects for detection of α2B-AR gene polymorphism by using polymerase chain reaction (PCR). The results found a significant association between D carriers genotype and HTN with T2DM cases (p < 0.05) as well as with T2DM-only cases, (p < 0.05) compared to control. Regardless of HTN status, only cases with HTN and T2DM as well as those with T2DM were significantly associated with the recessive model DD versus II+ID (p < 0.05). So, D carriers genotype was significantly associated with total cases of HTN and T2DM (p < 0.05) compared to controls. Our results suggested that there is a relationship between the α2B-AR I/D gene polymorphism and the risk for T2DM with or without HTN, but no such comparable relationship is evident with HTN-only cases among Saudi population in Qassim region.
Keywords: Insertion/deletion polymorphism, α2B-AR gene, HTN, T2DM
Introduction
The worldwide economic burden of chronic diseases, particularly cardiovascular diseases, diabetes and obesity is significant. 1 Where diabetes mellitus occurs alongside hypertension, the result is vascular remodelling; and due to insulin resistance, which gives rise to hyperglycaemia and hyperinsulinemia, the volume of body fluids increases placing the peripheral arteries under increased pressure. 2 Between 90% and 95% of diabetes mellitus (DM) cases are type 2 (T2DM), which is described as being insulin resistant or the level of insulin is relatively low.3,4 T2DM is a multi-genic disease; according to the evidence obtained from a several studies, particular populations may carry genetic polymorphisms that depending upon the gene-variant, could variously promote or reduce the risk of developing T2DM.5–7 According to various epidemiological studies, environmental and genetic factors are also implicated in the multifactorial condition, HTN. 8 Several studies were conducted to determine the role of genetic factors in blood pressure and hypertension; however, there is lack consensus about the identity of the genes postulated as being involved.9,10 A theme that emerges in many patients of different ethnicities is a robust relationship between HTN and T2DM. Thus DM and HTN are prevalent diseases in a Saudi population among the study area (Qassim region). Continuously studies have evaluated the frequencies of genotypic variations of candidate genes might be play a role for development of HTN and T2DM in Saudi population. These genes include, beta-2-adrenergic receptors, angiotensin-converting enzyme, cytochrome P450, endothelial nitric oxide synthase and methylenetetrahydrofolate reductase.11–13
The cardiovascular system is regulated by the central nervous system, which releases catecholamines and activates alpha adrenergic receptors (AR). 14 There are three AR subtypes (α2A, α2B and α2C) that are implicated in the cardiovascular response to catecholamines (adrenaline and noradrenaline). However, these subtypes receptors are encoded by the αDRA2A, αDRA2B and αDRA2C genes. 15 The receptors’ pharmacological properties, prevalence in tissue, ability to be phosphorylated and desensitised are variable. 16 The clinical importance of the genetic variation of these subtypes remains unclear. 16 However, a positive correlation between α2A-AR polymorphism and both HTN, 17 and DM 18 have been identified. Furthermore, an association between congestive heart failure and the α2A-CR 322–325 I/D polymorphism was identified. 19 Whereas other study demonstrated that the α2B-AR polymorphism presents as an insertion/deletion mutation in the α2B-AR gene, mapped on (2q11.2), which results in 9 or 12 glutamic (Glu.) residues. This manifests as the absence or presence of three Glu. amino acids at sites 301–303 (I/D 301-303, rs29000568) in the third intracellular locus of the α2B-AR. 20 The presence of a relationship between different α2-AR subtype polymorphisms with vascular diseases, HTN and DM is disputed. Whilst the α2B-AR (301–303 I/D) polymorphism were confirmed to be related to HTN and coronary arterial disease (CAD)21,22 and vascular disease ischaemic stroke, 15 some of these findings have been contested in their study conducted in USA. Whereas, other study did not confirm any correlation between the α2B-AR (301–303 I/D) gene polymorphism and HTN. 23 On the other hand, studies conducted on Egyptian 24 and Malaysian cases 25 detected significant relationships between this polymorphism and HTN with or without T2DM. Also, a study of Saudi women reported the risk for gestational diabetes was increased in those with the α2B-AR polymorphism. 26 However, the study of young black adults did not discern a relation between this polymorphism and the predisposition to essential HTN. 20 This echoed the results of the study concluded that essential HTN was the product of various environmental and genetic factors. 23 The association between α2B-AR I/D gene polymorphism and diabetes is due to impairment of α2B-AR desensitisation which causes prolonged inhibition of insulin secretion. As insulin sensitivity decreases, the requirement for insulin secretion increases, so individuals with an impaired capacity to secrete insulin are predisposed to T2DM. 27 An alternative explanation for the association between α2-AR I/D polymorphism and early-onset diabetes is altered function of the autonomic nervous system that may influence glucose metabolism. 28 The possible mechanisms for the relation of DD genotype with hypertension was demonstrated in human in vivo studies includes decrease coronary blood flow, and enhance peripheral resistance on epinephrine infusion. 29 The aim of this study was to examine the relationship between the α2B-AR (301–303 I/D) gene polymorphism with HTN and T2DM diseases in Saudi population among Qassim region.
Material and methods
Subjects
In this hospital based case-control study, 200 cases of both gender and 100 healthy controls that were available in the study period from May 2019 until January 2020 were recruited from the Internal Medicine clinic, Qassim University. All selecting participants were Saudi and their small number was a limitation of our study. Cases were allocated to three groups based on clinical evaluations: HTN without T2DM (n = 72), HTN and T2DM (n = 62) and T2DM only (n = 66). The cases were divided into two subgroups total HTN (n = 134) and total T2DM (n = 128). The control group were apparently healthy normotensive individuals who visited the hospital for routine check-up, have normal blood glucose and no relatives with either HTN or T2DM. The inclusion criteria were applied; for HTN cases, their systolic blood pressure needed to be ≥140 mmHg and diastolic ≥90 mmHg, or receiving antihypertensive treatment. For T2DM cases, their FBG had to be ≥126 mg/dl or being treated for T2DM. Patients who had renal, hepatic, respiratory diseases and a family history of hypercholesterolemia or congenital heart diseases were excluded. In addition any cases as well as healthy individuals who refused to participate/sign the informed consent of the study were excluded. Each participant underwent a general medical examination; full medical histories and written informed consent were obtained. The subcommittee of health research ethics, deanship of scientific research, Qassim University, KSA approved the research to be carried out (Code NO 01-05-2019).
Biochemical analysis
Five ml of peripheral blood samples were collected from each subjects in EDTA tubes. This process was performed in the clinical pathology laboratory. To separate the plasma, 3 ml from collected samples were centrifuged at 4000 rpm/20 min, were stored at −20°C until for biochemical assays. To analyse the total cholesterol, triglycerides and HDL, colorimetric methods were used in accordance with the protocol described by El-Moghazy et al. 30 Analysis kits were provided by the Cressent Company, KSA. To estimate LDL, the Friedewald formula was used. Glycated haemoglobin was measured using the Quo-labA1C analyser system.
Genotyping analysis
Genomic DNA was isolated from 2 ml blood lymphocytes of all subjects using an Accuprep genomic DNA extraction kit (K-3032) from the Bioneer Company (Republic of Korea). The concentration of DNA was estimated by UV spectrophotometer. The 20-µl reaction mixture contained 1 µl of template DNA, 2 µl (10 pmol/µl) of every primer, 10 µl (2× PCR master mix) and 5 μl of sterile water. The master mix contained 1 unit Taq DNA polymerase, 250 µM of each dNTP (dATP, dGTP, dTTP and dCTP) and 1.5 mM MgCl. PCR running was performed for DNA amplification by using the following protocol: initial pre-denaturation cycle of 5 min at 95°C for one time, 35 denaturation cycles at 95°C for 30 s, annealing at 57°C for 30 s, extension at 72°C for 45 s, and followed by one extension cycle of 5 min at 72°C. Finally stop the reaction at 4°C. PCR was run using the forward primer 5′AGGGTGTTTGTGGGGCATCT3′ and the reverse primer 5′CAAGCTGAGGCCGGAGACACT3′. PCR master mix and the primers were obtained from the Bioneer Company (Republic of Korea V2/2017-03-16).
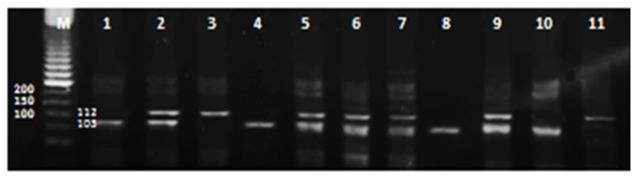
The α2B-AR gene subtypes II, I/D and DD were detected by running 2% agarose gel electrophoresis. The PCR amplified products were revealed by using ultraviolet beams after staining with ethidium bromide. 100-bp DNA ladder (Promega, US) was used for demonstration of the D allele fragments (with size 103 bp) and the I allele fragments (with size 112 bp) (Figure 1).
Figure 1.
Two percentage agarose gel electrophoresis of PCR products reveal that: lanes 2, 5, 6 and 7, show heterozygous ID genotypes (112/103 bp.); lane 3show homozygous II genotypes (112 bp) whereas lane 1,4 show homozygousDD genotype (103 bp).
M represents DNA ladder.
Statistical analysis
Data were analysed by using SPSS version 27. For comparing all demographic and clinical data of all participants, we used one way ANOVA test (post hoc Tukey’s test) for comparing all pair groups with each other. Hardy-Weinberg equilibrium (HWE) tests were conducted to establish the distribution of genotype in all subjects. Chi-square test (χ2), odds ratios (ORs) and confidence interval (95%CI) by using logistic regression test for genotyping and allelic frequencies among all subjects. 31 The threshold for statistical significance was set as p < 0.05.
Results
Demographic and clinical data of all participants
The mean age of the 200 patients of both gender in this study was (52.92 ± 9.34) years; ranged 30–68 years). They were matched against 100 healthy controls, ranging in age from 25–52 years (41.38 ± 7.76 years). The Clinical Laboratory, Medical City, Qassim University collected a total of 200 peripheral blood samples from cases that were clinically evaluated when they visited the Internal Medicine clinic during the study period. The patients were divided into three groups based in the clinical diagnosis; group 1 HTN-only 72 cases (42 male and 30 female); group 2 HTN + T2DM 62 cases (38 male and 24 female). The group 3 of 66 T2DM-only patients (34 males and 32 females). Blood samples were also obtained from the 100 unrelated healthy controls (58 males and 42 females).
The results revealed that the significant differences in age, BMI in all three cases groups compared to controls (p < 0.0001, p < 0.05) respectively. The SBP, DBP, total cholesterol and LDL were significantly higher in HTN + T2DM as well as HTN only cases in comparison with controls and T2DM groups (p < 0.0001). In addition, HBA1C and TG, were significantly higher in cases with HTN + T2DM group and T2DM cases compared to controls and HTN only cases (p < 0.0001). However, there was no difference in SBP and DBP between T2DM-only cases and controls, and HBA1C did not differ between HTN-only cases and controls. Also, the cases groups and controls were homogenous in terms of gender and smoking status groups (p > 0.05) (Table 1).
Table 1.
Demographic and clinical data of all participants.
| HTN without T2DM |
HTN with T2DM |
T2DM without HTN |
Controls |
p-Value | |
|---|---|---|---|---|---|
| N = 72 (mean ± SD) | N = 62 (mean ± SD) | N = 66 (mean ± SD) | N = 100 (mean ± SD) | ||
| Age | 52.32 ± 10.08 a | 53.03 ± 8.43 a | 53.45 ± 9.43 a | 41.38 ± 7.76 | <0.0001*** |
| Gender: | |||||
| Male (%) | 42 (58.3) | 38 (61.3) | 34 (51.5) | 58 (58) | (NS) |
| Female (%) | 30 (41.7) | 24 (38.7) | 32 (49.5) | 42 (42) | |
| Body mass index (BMI) | 29.71 ± 6.29 a | 30.59 ± 5.94 a | 29.22 ± 6.76 a | 24.03 ± 3.27 | <0.05* |
| Smokers (%) | 12 (16.7) | 15 (24.2) | 9 (13.6) | 11 (11) | (NS) |
| Exsmokers (%) | 3 (4.2) | 2 (3.2) | 3 (4.5) | 3 (3) | (NS) |
| Non-Smokers (%) | 57 (79.1) | 45 (72.6) | 54 (81.9) | 86 (86) | (NS) |
| Systolic blood pressure (SBP) | 149.4 ± 25.20a,c | 159.6 ± 21.10a,b,c | 123.3 ± 10.11 b | 119.1 ± 11.75 | <0.0001*** |
| Diastolic blood pressure (DBP) | 92.51 ± 11.70a,c | 96.29 ± 10.09a,c | 79.11 ± 6.65 b | 77.94 ± 6.82 | <0.0001*** |
| HBA1C | 5.18 ± 0.54 c | 7.18 ± 1.06a,b,c | 6.56 ± 0.96a,b | 5.04 ± 0.40 | <0.0001*** |
| Total cholesterol | 211.3 ± 55.23a,c | 217.5 ± 37.34a,c | 177.5 ± 28.51a,b | 163.1 ± 34.54 | <0.0001*** |
| Triglycerides | 188.8 ± 83.89a,c | 260.4 ± 82.72a,b | 241.1 ± 75.58a,b | 111.7 ± 24.72 | <0.0001*** |
| High density lipoprotein (HDL) | 39.85 ± 7.12 a | 37.65 ± 7.08 a | 40.09 ± 2.37 a | 49.57 ± 4.682 | <0.0001*** |
| Low density lipoprotein (LDL) | 124.6 ± 28.68a,c | 127.0 ± 25.55a,c | 104.9 ± 23.40a,b | 84.47 ± 12.73 | <0.0001*** |
One way a nova with post hoc Tukey’s test and Chi-Square for comparing all pair groups with each other.
HBA1C: glycated haemoglobin; HTN: hypertension; NS: non-significant; SD: standard deviation; T2DM: type 2 diabetes mellitus.
Significant compared to controls.
Significant to HTN.
Significant to DM.
p Value is significant if <0.05.
Genetic polymorphisms of α2B-AR I/D among all subjects
To monitor the genotypic distributions of α2B-AR I/D gene, HWE test showed significant divergence between the observed and expected frequencies in all cases groups, whereas no deviation was detected in the control group. These findings are attributed to small scale of study participants with eligible inclusion as well as exclusion criteria who visited the Internal Medicine Clinic, Qassim University Hospital during the study period from May 2019 until January 2020 (Table 2).
Table 2.
Hardy-Weinberg equilibrium tests for the investigated α2B-AR I/D polymorphisms among all cases groups and control.
| Genotype | Observed | Expected | χ2 | p Value |
|---|---|---|---|---|
| HTN without T2DM | ||||
| II | 53 | 48.3 | 13.7 | p*** = 0.0005 |
| ID | 12 | 21.3 | ||
| DD | 7 | 2.3 | ||
| HTN with T2DM | ||||
| II | 43 | 37.9 | 14.2 | p*** = 0.0005 |
| ID | 11 | 21.4 | ||
| DD | 8 | 2.9 | ||
| T2DM only | ||||
| II | 46 | 23.6 | 21.3 | p*** = 0.00001 |
| ID | 10 | 31.7 | ||
| DD | 10 | 10.6 | ||
| Controls | ||||
| II | 77 | 75.69 | 1.3 | p = 0.13 |
| ID | 20 | 22.62 | ||
| DD | 3 | 1.69 | ||
D: deletion; HTN: hypertension; I: insertion; p Value: non-significant; p***: very high significant; T2DM: type 2 diabetes mellitus; χ2: Chi-Square.
Our results showed the frequency of I/D genotypes and allele of the α2B-AR gene in the different groups and subgroups. No significant association was found in the heterozygote ID genotype with all cases groups (HTN-only, p = 0.7; HTN with T2DM, p = 0.9; and T2DM-only, p = 0.7) and subgroups (total cases of HTN, p = 0.8; and total cases of T2DM, p = 0.7) compared to controls. However, There is a significant association was detected between the homozygote DD genotype in HTN with T2DM group (p = 0.03) as well as T2DM-only group (p = 0.01) in comparison with controls. Whereas such association was not found for the DD genotype in the HTN-only group (p = 0.09). In addition there were significant association between the homozygote DD genotype with total HTN and total T2DM cases (subgroups) (p = 0.03, p = 0.01) respectively in comparison with controls. In relation to the frequency of the D allele, the association was significant for the HTN with T2DM group (p = 0.04) and the T2DM-only group (p = 0.02). On the other hand, compared to controls, there was no significant association between D allele and the HTN-only group (p = 0.2).While there were significant association between D allele with total HTN and total T2DM cases (subgroups) (p = 0.05, p = 0.01) respectively in comparison with controls (Figure 1) and (Tables 3 and 4).
Table 3.
Frequency of α2B-AR I/D genotypes and alleles among all cases groups compared to control.
| Genotype | HTN without T2DM |
Control |
p [OR (95% CI)] |
|---|---|---|---|
| N (%) 72 | N (%) 100 | ||
| II | 53 (73.6) | 77 (77) | Reference 1.0 |
| ID | 12 (16.7) | 20 (20) | p = 0.7 [ 0.9 (0.4–1.9)] |
| DD | 7 (9.7) | 3 (3) | p = 0.09 [3.3 (0.8–13.7)] |
| Allele | |||
| I | 118 (81.9) | 174 (88.0) | Reference 1.0 |
| D | 26 (18.1) | 26 (12.0) | p = 0. 2 [1.4 (0.8–2.7)] |
| Statistics | |||
| II vs DD + ID (dominant model) | 53 vs 19 | 77 vs 23 | p = 0.6 [0.8 (0.4–1.6)] |
| II + ID vs DD (recessive model) | 65 vs 7 | 97 vs 3 | p = 0.07 [0.3 (0.1–1.1)] |
| Genotype | HTN with T2DM |
Control |
p [OR (95% CI)] |
| N (%) 62 | N (%) 100 | ||
| II | 43 (69.4) | 77 (77) | Reference 1.0 |
| ID | 11 (17.7) | 20 (20) | p = 0.9 [1.0 (0.4–2.0)] |
| DD | 8 (12.9) | 3 (3) | p* = 0.03 [4.8 (1.2–18.9)] |
| Allele | |||
| I | 97 (78.0) | 174 (88.0) | Reference 1.0 |
| D | 27 (22.0) | 26 (12.0) | p* = 0.04 [1.8 (1.0–3.4)] |
| Statistics | |||
| II vs DD + ID (dominant model) | 43 vs 19 | 77 vs 23 | p = 0.3 [0.7 (0.3–1.3)] |
| II + ID vs DD (recessive model) | 54 vs 8 | 97 vs 3 | p* = 0.02 [0.2 (0.1–0.8)] |
| Genotype | T2DM without HTN |
Control |
p [OR (95% CI)] |
| N (%) 66 | N (%) 100 | ||
| II | 46 (69.8) | 77 (77) | Reference 1.0 |
| ID | 10 (15.1) | 20 (20) | p = 0.7 [0.8 (0.4–1.9)] |
| DD | 10 (15.1) | 3 (3) | p** = 0.01 [5.5 (1.5–21.3)] |
| Allele | |||
| I | 102 (77.3) | 174 (88.0) | Reference 1.0 |
| D | 30 (22.7) | 26 (12.0) | p* = 0.02 [2.0 (1.1–3.5)] |
| Statistics | |||
| II vs DD + ID (dominant model) | 46 vs 20 | 77 vs 23 | p = 0.3 [0.6 (0.3–1.4)] |
| II + ID vs DD (recessive model) | 56 vs 10 | 97 vs 3 | p** = 0.01 [0.2 (0.1–0.7)] |
95% CI: confidence interval; D: deletion; HTN: hypertension; I: insertion; OR: odds ratio; T2DM: type 2 diabetes mellitus; p Value: non-significant.
p* significant <0.05. p** high significant.
Table 4.
Frequency of α2B-AR I/D genotypes and alleles among cases subgroups compared to control.
| Genotype | Total HTN |
Control |
p [OR (95% CI)] |
|---|---|---|---|
| N (%) 134 | N (%) 100 | ||
| II | 96 (71.7) | 77 (77) | Reference 1.0 |
| ID | 23 (17.1) | 20 (20) | p = 0.8 [0.9 (0.4–1.8)] |
| DD | 15 (11.2) | 3 (3) | p* = 0.03 [4.0 (1.1–14.3)] |
| Allele | |||
| I | 215 (80) | 174 (88.0) | Reference 1.0 |
| D | 53 (20) | 26 (12.0) | p* = 0.05 [1.6 (1.0–2.7)] |
| Statistics | |||
| II vs ID + DD (dominant model) | 96 vs 38 | 77 vs 23 | p = 0.3 [0.7 (0.4–1.3)] |
| II + ID vs DD (recessive model) | 119 vs 15 | 97 vs 3 | p* = 0.03 [0.2 (0.1–0.8)] |
| Genotype | Total T2DM |
Control |
p [OR (95% CI)] |
| N (%) 128 | N (%) 100 | ||
| II | 89 (69.5) | 77 (77) | Reference 1.0 |
| ID | 21 (16.4) | 20 (20) | p = 0.7 [0.9 (0.4–1.8)] |
| DD | 18 (14.1) | 3 (3) | p** = 0.01 [5.1 (1.4–18.2)] |
| Allele | |||
| I | 199 (77.7) | 174 (88.0) | Reference 1.0 |
| D | 57 (22.3) | 26 (12.0) | p** = 0.01 [1.9 (1.1–3.1)] |
| Statistics | |||
| II vs ID + DD (dominant model) | 89 vs 39 | 77 vs 23 | p = 0.2 [0.6 (0.3–1.2)] |
| II + ID vs DD (recessive model) | 110 vs 18 | 97 vs 3 | p** = 0.009 [0.1 (0.05–0.6)] |
95%CI: confidence interval; D: deletion; I: insertion; HTN: hypertension; OR: odds ratio; T2DM: type 2 diabetes mellitus; p Value: non-significant.
p* significant <0.05.
p** high significant.
From the analysis of the dominant and recessive models, no significant association was detected for II versus DD+ID (dominant model) between the patients and control groups HTN-only (p = 0.6); HTN with T2DM (p = 0.3) and T2DM-only (p = 0.3) as well as total HTN (p = 0.3) and total T2DM (p = 0.2). However, there were a significant association between II + ID versus DD (recessive model) in the HTN with T2DM group (p = 0.02) and T2DM-only group (p = 0.01) as well as total HTN (p = 0.03) and total T2DM (p = 0.009) compared to the controls. No such association was found for the HTN-only group (p = 0.07) (Tables 3 and 4).
As regard the genotype and allele internal comparison of cases groups with each other, no significant difference was demonstrated in both genotype and allele frequencies between HTN versus HTN with T2DM cases (p = 0.81, p = 0.4) respectively. Also, no significant difference was detected in both genotype and allele frequencies between T2DM versus HTN with T2DM cases (p = 0.9, p = 0.85) respectively (Table 5).
Table 5.
Genotype and allele internal comparison among the three cases groups (HTN vs HTN with T2DM) and (T2DM vs HTN with T2DM).
| Genotype | HTN without T2DM |
T2DM without HTN |
HTN with T2DM |
|---|---|---|---|
| N (%) 72 | N (%) 66 | N (%) 62 | |
| II | 53 (73.6) | 46 (69.8) | 43 (69.4) |
| ID | 12 (16.7) | 10 (15.1) | 11 (17.7) |
| DD | 7 (9.7) | 10 (15.1) | 8 (12.9) |
| χ2; p Value for genotype | χ2 = 0.4, p = 0.81 | χ2 = 0.2, p = 0.9 | Reference group |
| Allele | |||
| I | 118 (81.9) | 102 (77.3) | 97 (78.0) |
| D | 26 (18.1) | 30 (22.7) | 27 (22.0) |
| χ2; p Value for allele | χ2 = 0.5, p = 0.4 | χ2 = 0.03, p = 0.85 | Reference group |
D: deletion; I: insertion; HTN: hypertension; T2DM: type 2 diabetes mellitus; χ2: Pearson-chi-square test.
p > 0.05: non-significant.
Discussion
The factors responsible for causing the chronic non-infectious diseases, HTN and T2DM, are multiple and of genetic and environmental origins. The prevalence of these diseases in developing and developed nations is high, particularly among older people. 32 There is abundant evidence that indicates the presence of a relationship between HTN and T2DM. The WHO estimates that 10% of the world’s adult population has diabetes. In Saudi population the percentage of cases affected by DM is reported to be 25%, 33 potentially even as high as 30%.32,34 This high prevalence emphasises the importance of determining the influence and prevalence of the polymorphic alpha-2 beta-adrenergic receptor gene in cases with HTN and T2DM among Saudi population in Qassim region. The results of the current study may help to identify the predisposing risk factor for HTN and T2DM diseases and effective management strategies.
The results of this study demonstrated a significant difference among the cases and control groups in relation to age, BMI. SBP, DBP and lipid profiles. Furthermore, the SBP, DBP, total cholesterol and LDL were significantly higher in HTN + T2DM group and HTN cases in comparison with T2DM group. HBA1C and TG, were significantly elevated in HTN + T2DM and T2DM cases compared to HTN cases.
There were two exceptions, being the level of DBP in the T2DM-only group, and the level of HBA1C in the HTN-only groups. In both cases the levels did not differ significantly compared to controls. Similarly, in relation to gender and smoking habits, no significant differences were found between the groups. These results come in accordance with previous studies reported that, HTN was more common in older age regardless of geographical, cultural, social and economic factors, and gender.35,36 Moreover, other studies reported that, the prevalence of HTN was found to be higher in poorly educated workers and unskilled retirees. 37
Primary HTN is a complex disease due to environmental and genetic factors. 38 The rich expression of α2 adrenergic receptor gene in the central and peripheral nervous systems is demonstrated. The different categories of α2 adrenergic receptor work antagonistically to modulate blood pressure. Hypertension is induced by the stimulation of α2B-AR by the sympathetic nervous system, which inhibits α2A-AR; whereas hypotension arises from the stimulation of α2-AR inhibiting the sympathetic outflow from the central nervous system. 24
The current study showed no significant association was found in the heterozygote ID genotype with all cases groups (HTN-only; HTN with T2DM and T2DM-only) and subgroups (total HTN and total T2DM cases) compared to controls. However, There is a significant association was detected between the homozygote DD genotype in HTN with T2DM group as well as T2DM only group in comparison with controls. Whereas such association was not detected for the DD genotype in HTN only group. In addition there were significant association between the homozygote DD genotype with total HTN and total T2DM cases (subgroups) in comparison with controls.
In relation to the frequency of the D allele, significant association was detected for the HTN with T2DM and the T2DM-only groups compared to controls. On the other hand, compared to controls, there was no significant association found between D allele and the HTN only group. While there were significant association between D allele with total HTN and total T2DM cases (subgroups) in comparison with controls.
A correlation has been demonstrated between the SNP in the 3’ UTR of ADRA2A and the increased expression of α2A-AR in mRNA and proteins. 18 This same correlation also appears to be responsible for aberrant insulin synthesis when stimulated by glucose, a decline in fasting insulin and increasing the risk of T2DM. In vitro study of the risk allele found that it disrupted the attachment of insulin granules to the beta cell membrane and exocytosis of insulin at a site distal to modulation of calcium ion levels within cells. Other adverse metabolic and vascular effects associated with the deletion allele are the elevated risk of coronary ischaemia, impaired insulin synthesis prior to the onset of diabetes, a decline in the basal metabolic rate and obesity. 27
In its statistical analysis of dominant and recessive models, this study did not detect any significant relationship between II versus DD+ID (dominant model) in the cases groups in comparison with controls. However, it did find a significant association between II + ID versus DD (recessive model) in the HTN with T2DM and T2DM-only groups as well as total HTN and total T2DM compared to controls. This recessive model-association was not evident in the HTN-only. As regard the genotype and allele comparison of cases groups with each other, no significant difference was detected in both genotype and allele frequencies among cases groups compared to each other. These results echo those of Vasudevan et al., 25 who found the D allele variant of the α2B-AR gene in Malaysian cases with essential HTN with and without T2DM. Moreover, other study demonstrated a strong association between the DD genotype, the D allele with HTN disease and that this association is more evident in hypertensive patients complicated by diabetes in Egyptian population. 24 Also, Siitonen et al., 39 reported that the function of the α2B-AR receptor appears to be adversely affected by the I/D 12Glu9 polymorphism of α2B-AR that leads to impairment of first stage insulin secretion and increase the risk of Type 2 diabetes in Finnish patient.
Moreover, the polymorphism affects the agonist-mediated phosphorylation and desensitisation of the receptor. The association between α2B-AR I/D gene polymorphism and diabetes results from impairment of α2B-AR desensitisation due to the allelic variant that inhibits insulin secretion. As insulin sensitivity decreases, the requirement for insulin increases, so individuals with an impaired capacity to secrete insulin are predisposed to T2DM. 27 An alternative explanation for the association between α2-AR I/D polymorphism and early-onset diabetes is altered function of the autonomic nervous system that may influence glucose metabolism. 28
This study did not detect any significant relationship between HTN disease not complicated with DM and the α2B-AR (301–303 I/D) gene of cases in the Qassim region; this might be a product of the diverse genetics and environmental factors involved in essential hypertension. Our results were similar to the study conducted in Sweden population demonstrated a weak association between the DD genotype and nondiabetic primary hypertension and a stronger association with early-onset hypertension and the diabetic phenotype seems to add complexity to the phenotype of primary hypertension. 38 The possible mechanism for the relation of DD genotype with hypertension was demonstrated in human in vivo studies includes decrease coronary blood flow, and enhance peripheral resistance on epinephrine infusion. 29
Meta-analyses of genome-wide association studies have improved our understanding of the genetic foundations of a number of diseases, including diabetes. These SNPs have often been validated through re-sequencing efforts as not just tag SNPs, but as causative SNPs, and so must play a role in disease development or progression. 40 More than 120 published reports have described associations between SNPs and T2DM. This association involved markers in or around a candidate gene or linkage region that have a potential role in diabetes-related metabolic pathways. 41
Moreover other study revealed a significant association between rs7903146, rs2283228, rs13266634, rs179881 and rs5210 SNPs and gestational diabetes mellitus (GDM) referred to meta-analysis conducted on T2DM among Indian population. 31 On the other hands, similar meta-analysis was performed in Japanese population confirmed the linkage between the risk for hypertension and several common SNPs of ATP2B1, CYP17A1, CSK and FGF5 genes. 42
Strengths and constraints of the current study
Although the current study is relatively small scale among Qassim region which is a tribalism area, the results confirmed that the DD polymorphism of the α2B-AR genotype have a significant association with risk for T2DM for the first time in Saudi population in Qassim region. However there are limitation in our study included small number of participants with eligible inclusion as well as exclusion criteria visited the Internal Medicine Clinic, Qassim University Hospital during the study period from May 2019 until January 2020. However, further study with large scale should be covered the most different regions to determine the relationship between the I/D α2B-AR gene polymorphism with the risk for HTN or T2DM diseases in Saudi population.
Conclusion
This study showed, there was a significant association between the α2B-AR I/D gene polymorphism and D allele with the risk for T2DM with or without HTN disease among Saudi population in Qassim region. However, no significant association was detected between the same polymorphism and allele with the risk for HTN not complicated by T2DM disease. In future, large scale study involving different regions among Saudi population are recommended to confirm the role of α2B-AR (301–303 I/D) gene polymorphism or D allele for development of T2DM and HTN diseases.
Author biographies
Hussein Mohammad Eldeeb is Assistant Professor of Biochemistry and Molecular Biology, College of Medicine, Qassim University, KSA until now. The author has published 11 articles in field of Biochemistry, molecular biology and natural product. The author has participated in many conferences in the field of biotechnology, genetics and molecular biology. He is a member of many societies as Doctors Syndicate. He is a reviewer in many journals (e.g., Reviewer of the International Journal of Health Science (IJHS), which is a peer reviewed international journal belong to Qassim University.
Rehab M. Elgharabawy is Associate Professor of Pharmacology and Toxicology, College of Pharmacy, Qassim University, KSA until now. The author has participated in many conferences in the field of pharmacology, toxicology and medicine. She has award of excellence from 3rd International Conference on Legal Medicine, Medical Negligence and Litigation in Medical Practice, 2012 in Jaipur, Rajasthan, India. She is the author of 55 publications in the field of Pharmacology and toxicology. She is a member of many societies e.g., Egyptian Pharmaceutical Association. She is a reviewer in many journals (e.g., immunobiology, immunotoxicology, research in pharmaceutical biotechnology journals).
Alaa E Abd Elmoniem is Professor and Head of Department of Medicine and Cardiology, College of Medicine, Qassim University, KSA till June, 2018 and now College of Medicine, Assiut University. Member of the permanent committee to upgrade Professors which belongs to the Supreme Council of Universities (SCU) from 2019 to 2022. The author has published 50 publications in the field of Medicine and Cardiology. He is a member of many societies as Egyptian Society of Cardiology, European Society of Cardiology. He is a reviewer in many journals e.g., Reviewer of the International Journal of Health Science (IJHS), European Respiratory Journal (ERS).
Ahmed Ali Ahmed is Professor of Molecular Biology, College of Medicine, Qassim University, KSA until now. The author has published 60 articles in field of genetics and molecular biology. The author has participated in many conferences in the field of biotechnology, cell biology, genetics and molecular biology. He is a reviewer in several international journals (e.g., International Journal of Health Sciences, Genetic testing and molecular biomarker, journal of the Renin-Angiotensin-Aldosterone System (JRAAS), International journal of diabetes in developing countries and Annals of Saudi Medicine).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Researchers would like to thank the Deanship of Scientific Research, Qassim University for its funding this research project with No (Sw-3801).
Ethics approval: Ethical approval for this study entitled ‘Alpha-2 beta-adrenergic receptor (301–303 I/D) gene polymorphism in hypertension and type 2 diabetes mellitus diseases among Saudi cases in the Qassim region of Saudi Arabia’ was obtained from *The Subcommittee of Health Research Ethics, Deanship of Scientific Research, Qassim University, KSA (APPROVAL NUMBER/ID (01-05- .*(2019).
Informed consent: Informed consent Written informed consent was obtained from all subjects before the study.
ORCID iD: Rehab M Elgharabawy  https://orcid.org/0000-0002-4399-8195
https://orcid.org/0000-0002-4399-8195
References
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365(9455): 217–223. [DOI] [PubMed] [Google Scholar]
- 2.Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res 2018; 41(6): 389–393. [DOI] [PubMed] [Google Scholar]
- 3.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(Supplement 1): S81–S90. [DOI] [PubMed] [Google Scholar]
- 4.Facundo ACDS. Análise hematológica por hemograma, creatinina, triglicérides e colesterol em ratos diabéticos portadores de infecção pulpar e/ou periodontal. 2013. [Google Scholar]
- 5.Scheen AJ, Paquot N. Type 2 diabetes: journey in the heart of a complex disease. Rev Med Liege 2012; 67(5–6): 326–331. [PubMed] [Google Scholar]
- 6.Marchetti P, Syed F, Suleiman M, et al. From genotype to human β cell phenotype and beyond. Islets 2012; 4(5): 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meybodi HRA, Hasanzad M, Larijani B. Path to personalized medicine for type 2 diabetes mellitus: reality and hope. Acta Med Iran 2017; 55(3): 166–174. [PubMed] [Google Scholar]
- 8.Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med 2011; 9(1): 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med 2003; 348(12): 1170–1175. [DOI] [PubMed] [Google Scholar]
- 10.Mountain JL, Risch N. Assessing genetic contributions to phenotypic differences among’racial’and’ethnic’groups. Nat Genet 2004; 36(11): S48–S53. [DOI] [PubMed] [Google Scholar]
- 11.Al-Saikhan FI, Abd-Elaziz MA, Ashour RH. Association between risk of type 2 diabetes mellitus and angiotensin-converting enzyme insertion/deletion gene polymorphisms in a Saudi Arabian population. Biomed Rep 2017; 7(1): 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali A, Alghasham A, Ismail H, et al. ACE I/D and eNOS E298D gene polymorphisms in Saudi subjects with hypertension. J Renin Angiotensin Aldosterone Syst 2013; 14(4): 348–353. [DOI] [PubMed] [Google Scholar]
- 13.Alghasham A, Ali A, Ismail H, et al. CYP2J2− 50 G/T and ADRB2 G46A gene polymorphisms in Saudi subjects with hypertension. Genet Test Mol Biomarkers 2012; 16(9): 1027–1031. [DOI] [PubMed] [Google Scholar]
- 14.Guimaraes S, Moura D. Vasc adrenoceptors: an update. Pharmacol Rev 2001; 53: 319–356. [PubMed] [Google Scholar]
- 15.Oh S-H, Min K-T, Jeon Y-J, et al. Association between common genetic variants of α2A-, α2B-, and α2C-adrenergic receptors and ischemic stroke. Clin Neurol Neurosurg 2013; 115(1): 26–31. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald E, Kobilka BK, Scheinin M. Gene targeting—homing in on α2-adrenoceptor-subtype function. Trends Pharmacol Sci 1997; 18(4): 211–219. [DOI] [PubMed] [Google Scholar]
- 17.Lockette W, Ghosh S, Farrow S, et al. α2-Adrenergic receptor gene polymorphism and hypertension in blacks. Am J Hypertens 1995; 8(4_Pt_1): 390–394. [DOI] [PubMed] [Google Scholar]
- 18.Rosengren AH, Jokubka R, Tojjar D, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science (80- ) 2010; 327(5962): 217–220. [DOI] [PubMed] [Google Scholar]
- 19.Small KM, Wagoner LE, Levin AM, et al. Synergistic polymorphisms of β1-and α2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347(15): 1135–1142. [DOI] [PubMed] [Google Scholar]
- 20.Kelsey RM, Alpert BS, Dahmer MK, et al. Alpha-adrenergic receptor gene polymorphisms and cardiovascular reactivity to stress in Black adolescents and young adults. Psychophysiology 2012; 49(3): 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snapir A, Heinonen P, Tuomainen T-P, et al. An insertion/deletion polymorphism in the α2B-adrenergic receptor gene is a novel genetic risk factor for acute coronary events. J Am Coll Cardiol 2001; 37(6): 1516–1522. [DOI] [PubMed] [Google Scholar]
- 22.Snapir A, Mikkelsson J, Perola M, et al. Variation in the alpha2B-adrenoceptorgene as a risk factor for prehospitalfatal myocardial infarction and sudden cardiac death. J Am Coll Cardiol 2003; 41(2): 190–194. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin CT, Schwartz F, Baima J, et al. Identification of a polymorphic glutamic acid stretch in the α2B-adrenergic receptor and lack of linkage with essential hypertension. Am J Hypertens 1999; 12(9): 853–857. [DOI] [PubMed] [Google Scholar]
- 24.Tayel SI, Khader HF, El-Helbawy NG, et al. Association of deletion allele of insertion/deletion polymorphism in α2B adrenoceptor gene and hypertension with or without type 2 diabetes mellitus. Appl Clin Genet 2012; 5: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasudevan R, Ismail P, Stanslas J, et al. Association of insertion/deletion polymorphism of alpha-adrenoceptor gene in essential hypertension with or without type 2 diabetes mellitus in Malaysian subjects. Int J Biol Sci 2008; 4(6): 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hakeem MM, Abotalib Z, Alharbi KK, et al. Insertion and deletion polymorphism in the alpha-2B adrenoceptor gene in pregnant women ripens gestational diabetes mellitus. Saudi J Biol Sci 2016; 23(1): 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papazoglou D, Papanas N, Papatheodorou K, et al. An insertion/deletion polymorphism in the alpha2B adrenoceptor gene is associated with age at onset of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2006; 114(08): 424–427. [DOI] [PubMed] [Google Scholar]
- 28.Clerk LH, Vincent MA, Lindner JR, et al. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev 2004; 20(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 29.Snapir A, Koskenvuo J, Toikka J, et al. Effects of common polymorphisms in the α1A-, α2B-, β1-and β2-adrenoreceptors on haemodynamic responses to adrenaline. Clin Sci 2003; 104(5): 509–520. [DOI] [PubMed] [Google Scholar]
- 30.El-Moghazy M, Zedan NS, El-Atrsh AM, et al. The possible effect of diets containing fish oil (omega-3) on hematological, biochemical and histopathogical alterations of rabbit liver and kidney. Biomed Prev Nutr 2014; 4(3): 371–377. [Google Scholar]
- 31.Khan IA, Jahan P, Hasan Q, et al. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab Syndr Clin Res Rev 2019; 13(1): 688–694. [DOI] [PubMed] [Google Scholar]
- 32.Aljabri K, Bokhari SA, Aljabri BK. Hypertension in Saudi adults with type 2 diabetes. Interv Obes C Diabetes 2018; 1(4): 1–5. [Google Scholar]
- 33.Al-Kayyal MA, Halawani SY, Al-Ghalayini RM, et al. Dentists may play a pivotal role in the screening of diabetes and hypertension. Forum 2013; 1: 44–53. [Google Scholar]
- 34.Al-Daghri NM, Al-Attas OS, Alokail MS, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med 2011; 9(1): 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnaout MS, Almahmeed W, Ibrahim M, et al. Hypertension and its management in countries in Africa and the Middle East, with special reference to the place of β-blockade. Curr Med Res Opin 2011; 27(6): 1223–1236. [DOI] [PubMed] [Google Scholar]
- 36.Son PT, Quang NN, Viet NL, et al. Prevalence, awareness, treatment and control of hypertension in Vietnam—results from a national survey. J Hum Hypertens 2012; 26(4): 268–280. [DOI] [PubMed] [Google Scholar]
- 37.Van Minh H, Byass P, Chuc NTK, et al. Gender differences in prevalence and socioeconomic determinants of hypertension: findings from the WHO STEPs survey in a rural community of Vietnam. J Hum Hypertens 2006; 20(2): 109–115. [DOI] [PubMed] [Google Scholar]
- 38.von Wowern F, Bengtsson K, Lindblad U, et al. Functional variant in the α2B adrenoceptor gene, a positional candidate on chromosome 2, associates with hypertension. Hypertension 2004; 43(3): 592–597. [DOI] [PubMed] [Google Scholar]
- 39.Siitonen N, Lindström J, Eriksson J, et al. Association between a deletion/insertion polymorphism in the α2B-adrenergic receptor gene and insulin secretion and type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia 2004; 47(8): 1416–1424. [DOI] [PubMed] [Google Scholar]
- 40.Schierding W, O’Sullivan JM. Connecting SNPs in diabetes: a spatial analysis of meta-GWAS loci. Front Endocrinol (Lausanne) 2015; 6: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barroso I, Middelberg RPS, Harding A-H, et al. Candidate gene association study in type 2 diabetes indicates a role for genes involved in β-cell function as well as insulin action. PLoS Biol 2003; 1(1): e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabara Y, Kohara K, Kita Y, et al. Common variants in the ATP2B1 gene are associated with susceptibility to hypertension: the Japanese Millennium Genome Project. Hypertension 2010; 56(5): 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]