Abstract
The spectrum of canine muscular dystrophies has rapidly grown with the recent identification of several more affected breeds and associated mutations. Defects include those in genes and protein products associated with the sarcolemma (dystrophin deficient X-linked muscular dystrophy and sarcoglycan-deficient limb–girdle muscular dystrophy) and with the extracellular matrix (collagen 6, laminin α2, and α-dystroglycan-deficient congenital muscular dystrophies). With the increasing application of whole genome sequencing and whole exome sequencing, the clinical and pathological spectra associated with specific neuromuscular genetic defects are constantly evolving. In this report, we provide a brief overview of the current status of gene defects reported in canine muscular dystrophies. We also report the causative mutations for novel forms of X-linked muscular dystrophy in Brittany spaniels and in a French bulldog.
Keywords: dog, muscle, myopathy, whole genome sequencing, animal model
1. Introduction
The expansion of molecular testing in people and animals has led to the identification of many gene defects and protein products associated with neuromuscular disorders. With the increasing application of whole genome sequencing (WGS) and whole exome sequencing, the clinical and pathological spectra associated with established neuromuscular genetic defects are constantly evolving, while new variants, ranging from uncertain to likely functional significance, continue to be identified. In these latter cases, muscle pathology plays a key role in determining if a new variant is likely to be pathogenic. Genes encoding proteins throughout muscle fibers have now been shown to be responsible for neuromuscular disorders. These include proteins located in the extracellular matrix, the plasma membrane, the cytoskeleton, the Golgi, the internal membrane systems, nuclei, myofibrils, and the neuromuscular junction.
In people, the discovery in 1987 of the gene encoding dystrophin (DMD), responsible for X-linked Duchenne and Becker muscular dystrophy, paved the way for identifying and characterizing the role of many other sarcolemmal and extracellular matrix proteins, such as the sarcoglycans and laminin α2, and their own roles in other forms of muscular dystrophies [1]. Defects in other membrane proteins including dysferlin, caveolin 3, telethonin, as well as the enzyme calpain 3 also result in forms of limb–girdle and congenital muscular dystrophies [2]. Mutations in nuclear membrane proteins such as emerin, lamin A/C and nesprins are responsible for Emery–Dreifuss forms of muscular dystrophy [3]. Further, a growing number of genes and their protein products involved in post-translational glycosylation pathways, in particular of α-dystroglycan, are responsible for human neuromuscular disorders and various forms of congenital muscular dystrophy [4].
Muscular dystrophies in people are currently classified as the Duchenne and Becker muscular dystrophies associated with mutations in the DMD gene, the limb–girdle muscular dystrophies with either autosomal dominant or autosomal recessive modes of inheritance and associated with mutations in the sarcoglycans and other genes, the congenital muscular dystrophies associated with mutations in collagen 6, laminin α2 and others, Emery–Dreifuss muscular dystrophy, and facioscapulohumeral, myotonic and oculopharyngeal muscular dystrophies [5].
The identification and molecular characterization of muscular dystrophies is similarly growing in veterinary medicine (Table 1, [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]). The first mutation in the DMD gene encoding dystrophin and causing X-linked MD was identified in 1992 by Sharp et al. in the Golden Retriever [6]. Since that time, numerous DMD variants including deletions, insertions, nonsense or stop gain variants, and larger structural variants including inversions and tandem duplications have been described in several breeds of dogs (Table 1, [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]). The spectrum of muscular dystrophies in dogs has also expanded in recent years to include forms of limb–girdle muscular dystrophy associated with variants in sarcoglycan genes (SGCD and SGCA) and congenital muscular dystrophies with variants identified in the COL6, LAMA2, and LARGE1 genes [22,23,24,25,26,27,28,29]. The list is sure to grow, as molecular techniques are increasingly becoming available at a reasonable cost.
Table 1.
Classification of Muscular Dystrophies by Variant Identification.
| Dystrophin Deficient Muscular Dystrophy | |||||||
|---|---|---|---|---|---|---|---|
| Gene | Breed | Inherit. | Chr. | Genomic (g.) | Protein (p.) | Variant Phenotype Variant Phenotype |
References |
| DMD | Golden Retriever | X-linked | X | g.28,126,496T>C | p.P178* | splicing, out-of-frame (exon 7 skipping) |
Sharp et al., 1991, [6] |
| DMD | Rottweiler | X-linked | X | not reported nonsense mutation in exon 58 |
not reported | nonsense/stop-gain (exon 58) |
Winand et al., 1994, [7] |
| DMD | German Shorthaired Pointer | X-linked | X | deletion encompassing the entire DMD gene | Schatzberg et al., 1999, [8] | ||
| DMD | Labrador Retriever | X-linked | X | not reported 184 nucleotide [pseudoexon] insertion between exon 19 and exon 20 |
p.E795* | pseudoexon/stop-gain (intron 19) | Smith et al., 2007, [9]; Kornegay et al., 2012, [10] |
| DMD | Jack Russell Terrier | X-linked | X | g.27,787,968_28,181,867del | p.G33* | deletion, out-of-frame (exons 3–21) | Brunetti et al., 2010, [11] |
| DMD | Cavalier King Charles Spaniel | X-linked | X | g.27,122,234G>A | p.R2396* | splicing, out-of-frame (exon 50 skipping) (exon 50 skipping) |
Walmsley et al., 2010, [12] |
| DMD | Pembroke Welsh Corgi | X-linked | X | g.27,894,093_27,894,094insN[(480)] | p.V536* | pseudoexon/stop-gain (intron 13) | Smith et al., 2011, [13] |
| DMD | Cocker Spaniel | X-linked | X | not reported 4 bp deletion in exon 65 |
not reported | frameshift/stop-gain (exon 65) | Kornegay et al., 2012, [10] |
| DMD | Tibetan Terrier | X-linked | X | not reported large deletion of exons 8–29 |
p.D217* | deletion/stop-gain (exons 8–29) |
Kornegay et al., 2012, [10] |
| DMD | Norfolk Terrier | X-linked | X | g.27,778,709del | p.G1029* | frameshift/stop-gain (exon 23) | Jenkins et al., 2015, [14] |
| DMD | Japanese Spitz | X-linked | X | g.27804717_33,211,801inv | p.E795* | ~5.4 Mb inversion (intron 19) |
Antencia-Fernandez et al., 2015, [15] |
| DMD | Cavalier King Charles Spaniel | X-linked | X | g.27,615,610_27,615,616del | p.N2021* | frameshift/stop-gain (exon 42) | Nghiem et al., 2016, [16] |
| DMD | Miniature Poodle | X-linked | X | >5 Mb deletion encompassing the entire DMD gene | Sanchez et al., 2018, [17] | ||
| DMD | Australian Labradoodle | X-linked | X | g.27,794,550G>A | p.R890* | nonsense/stop-gain (exon 21) |
Shrader et al., 2018, [18] |
| DMD | Border Collie | X-linked | X | g.27,799,217del | p.N852* | frameshift/stop-gain (exon 20) | Lopez et al., 2018, [19] |
| DMD | Labrador Retriever | X-linked | X | g.27,795,539_30,022,040inv | p.D876* | ~2.2 Mb inversion (intron 20) |
Barthelemy et al., 2020, [20] |
| DMD | Labrador Retriever | X-linked | X | g.28,052,927_28,453,564dup | p.E12_D217dup | ~400 kb duplication, in-frame (exons 2–7) | Shelton et al., 2022, [21] |
| DMD | Brittany Spaniel | X-linked | X | g.26,939,052G>A | p.Q2687* | nonsense/stop-gain (exon 55) |
Current paper |
| DMD | Brittany Spaniel | X-linked | X | g.27,796,380ins (RSPRY1 retrogene) |
unknown | Retrogene insertion (intron 20) |
Current paper |
| DMD | French Bulldog | X-linked | X | g.27,774,668C>CT | p.F1125* | frameshift/stop-gain (exon 25) |
Current paper |
| Limb Girdle Muscular Dystrophy | |||||||
| SGCD | Boston terrier | AR | 4 | g.54,241,112_54,241,113del | p.E178* | frameshift/stop-gain (exon 7) | Cox et al., 2017, [22] |
| SGCD | Boston terrier | AR | 4 | g.54,148,966-54,148,968delinsCC;54,148,978_54,168,381del] | p.R191* | ~19.4 kb deletion (exons 8–9) | Cox et al., 2017, [22] |
| SGCA | Miniature Dachshund | AR | 9 | g.26,117,203G>A | p.W75* | nonsense/stop-gain (exon 3) |
Mickelson et al., 2021, [23] |
| Congenital Muscular Dystrophy | |||||||
| COL6A1 | Landseer Newfoundland | AR | 31 | g.39,284,371G>T | p.E97* | nonsense/stop-gain (exon 3) |
Steffen et al., 2015, [24] |
| COL6A3 | Labrador retriever | AR | 25 | g.48,296,579G>A | p.R1576* | nonsense/stop-gain (exon 11) |
Bolduc et al., 2020, [25] |
| COL6A3 | Labrador retriever | AD | 25 | g.48,289,626C>T | p.G2053_P2070del | splicing (exon 17 skipping) |
Bolduc et al., 2020, [25] |
| COL6A3 | American Staffordshire Terrier | AR | 25 | g.48,287,602del | p.P2133* | frameshift/stop-gain (exon 21) | Jankelunas et al., 2023, [26] |
| LAMA2 | Italian Greyhound | AR | 1 | g.68,441,978G>A | p.W1046* | nonsense/stop-gain (exon 22) |
Christen et al., 2021, [27] |
| LAMA2 | Staffordshire Bull Terrier | AR | 1 | g.68,292,960_68,295,150 | p.I211_R270del | ~2.2 kb deletion, in-frame (exon 5) | Shelton et al., 2021, [28] |
| LARGE1 | Labrador retriever | AR | 10 | g.31,373,423C>T | p.R455* | nonsense/stop-gain (exon 11) |
Shelton et al., 2021, [29] |
All positions reported in UU_Cfam_GSD_1.0/canFam4. DMD: NM_001003343; COL6A1: XM_038581692.1; COL6A3: NM_001103215.1; LAMA2: XM_038526759.1; LARGE1: XM_038550745.1; SGCD: XM_038534931.1; SGCA: XM_038547640.1.
In addition to rapidly identifying new pathogenic gene candidates, next-generation sequencing in dogs is also widening the spectrum of clinical phenotypes associated with well-established disease-causing genes. This in turn is increasingly challenging the traditional classification of neuromuscular diseases based primarily on clinical features. There is often considerable clinical and pathological overlap between disorders, and it can now be the case that multiple variants can occur in the same gene in the same breed and result in variable clinical presentations. As such, a strong case can now be made that congenital neuromuscular diseases should be classified based on the protein or gene defect, for example, dystrophinopathy or sarcoglycanopathy, and according to the pathogenic mechanisms.
Dystrophin-deficient MD is currently the most common form of MD in dogs, although forms of LGMD [22,23] and CMD [24,25,26,27,28,29] have recently been recognized. It is difficult, if not impossible, to determine the specific form of canine MD (i.e., XLMD, LGMD or CMD, among others) based on clinical signs alone. A careful clinical assessment including measurement of CK activity and histological evaluation of muscle biopsies can point the clinician in the right direction for identification of a causative protein or gene defect. Here, we utilize this combined pathological approach coupled with WGS to identify and report the causative mutations for novel forms of X-linked MD in Brittany spaniels and in a French bulldog.
2. Materials and Methods
2.1. Animals
DNA was evaluated from two young, previously reported male Brittany spaniel dogs, one from the USA and one from Japan, with dystrophin-deficient muscular dystrophy based on clinical presentation, markedly elevated creatine kinase (CK) activity, histopathology and immunofluorescent staining of dystrophy-associated proteins [30]. An additional dog, a 3-month-old male French bulldog, was evaluated for dysphagia, dyspnea and macroglossia, with muscle biopsies submitted. Genomic DNA from all three dogs was isolated from archived frozen diagnostic muscle biopsy specimens using the Qiagen DNEasy kit according to package instructions.
2.2. Light Microscopy and Immunofluorescent Staining
Histopathological and immunofluorescent characterizations of muscle biopsies from both Brittany spaniels were previously described [30]. Unfixed, chilled and formalin-fixed diagnostic muscle biopsy specimens were collected post-mortem from the biceps femoris, diaphragm and tongue muscles of the French bulldog and shipped by an overnight express service under refrigeration to the Comparative Neuromuscular Laboratory, University of California San Diego. Upon receipt, the unfixed muscle specimens were snap frozen in isopentane, pre-cooled in liquid nitrogen, and stored at −80 °C until further processed. Cryosections were evaluated using a standard panel of histochemical stains and reactions [31]. Additional cryosections were cut and stained for indirect immunofluorescence as previously described [32], using monoclonal antibodies against the rod (1:100, NCL-DYS1) and carboxy-terminus (1:100, NCL-DYS2) of dystrophin, utrophin (1:20, NCL-DRP2), and developmental myosin heavy chain for regenerating fibers (1:20, NCL-dMHC), all from Novocastra Laboratories, Newcastle, UK; a monoclonal antibody against caveolin 3 (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and polyclonal antibodies against laminin α2 (1:200), α-sarcoglycan (1:200), and collagen VI (direct apply, monoclonal antibody 3G7), all gifts of Professor Eva Engvall [33,34].
2.3. Whole Genome Sequencing and Variant Analysis
A PCR-free library was prepared from all three cases, and 150 bp paired-end reads were generated on an Illumina HiSeq 4000 sequencer by GeneWiz (South Plainfield, NJ, USA). For one Brittany spaniel, 767.6 million paired-end reads were generated, corresponding to a mean 45.1 fold genome-wide coverage; for the second Brittany spaniel, 382.5 million paired-end reads were generated, corresponding to a mean 22.5 fold genome-wide coverage; and for the French bulldog, 314.1 million paired-end reads were generated, corresponding to a mean 18.4 fold genome-wide coverage. Reads were mapped against the dog reference genome assembly (CanFam4) and processed using the OnlyWAG pipeline as described [35]. Raw sequence reads are available in NCBI’s Short Read Archive at BioProject PRJNA982829.
WGS variants from the affected dogs were compared to those of control genomes from an internal WGS database developed at the University of Minnesota and containing 671 dogs of 63 diverse breeds (including 3 additional Brittany spaniels and 22 French bulldogs from unrelated projects). The WGS data from these 671 dogs were processed using the same bioinformatics pipeline referenced above, and included dogs from the following breeds: Golden Retriever (n = 46), Yorkshire Terrier (n = 42), Miniature Schnauzer (n = 38), Boxer (n = 37), English Bulldog (n = 34), Standard Poodle (n = 28), German Shepherd (n = 27), Portuguese Water Dog (n = 25), Great Dane (n = 25), Cavalier King Charles Spaniel (n = 23), Labrador Retriever (n = 22), French Bulldog (n = 21), Irish Wolfhound (n = 20), Bull Mastiff (n = 19), Rottweiler (n = 17), Siberian Husky (n = 16), Whippet (n = 15), Newfoundland (n = 15), Dachshund (n = 15), Pomeranian (n = 13), Scottish Deerhound (n = 10), Miniature Poodle (n = 10), Bouvier (n = 10), Border Collie (n = 10), and 133 dogs of 39 additional breeds, each with less than 10 dogs per breed. Variants unique to the affected dogs that were within or in close proximity to coding exons were prioritized as high (frame shift, loss or gain of stop or start codon, affecting a splice junction), moderate (missense), or low (synonymous, near splice junction) for further evaluation. A list of the unique coding variants of each dog is provided as Table S1.
2.4. Sanger Sequencing
Sanger sequencing of a PCR amplicon was employed to genotype a relative of Brittany spaniel case 1 for an identified DMD point mutation. This PCR utilized standard conditions, with primers DMD exon 55F (CCCTCTGCCTCTTTCCTTCT) and DMD exon 55R (AGCAACAACCCATACCCTTG) producing a 476 bp product.
Sanger sequencing was also employed on two PCR amplicons to confirm the boundaries of a hypothesized insertion in the DMD gene in Brittany spaniel case 2. PCR utilized standard conditions, with primers for the first amplicon being DMD intron 20F (TGACCCACCTCTCTCATTCTG) and RSPRY1 exon 3R (TTCTGCCATTTCGTGCAA), producing a 701 bp product, and for the second amplicon being RSPRY1 3′UTR F (GTACCTCAGCAGCTGCCTTT) and DMD intron 20R (AGCCCCCTAAACAGGAAGAA), producing a 430 bp product.
Additionally, Sanger sequencing was employed to genotype an archived French bulldog with a dystrophic phenotype for the DMD 1 bp insertion (p.F1125*) variant identified in the French bulldog case presented in this manuscript. PCR utilized standard conditions, with primers DMD exon 25F (AGCCTTCAAGGAGGTCGTTT) and DMD exon 25R (CAATTTTTGCAAGGTTGAGACA) producing a 595 bp product.
3. Results
3.1. Animals
The history, clinical presentation, and laboratory findings in both young male Brittany spaniels were previously reported [30]. Dystrophin-deficient MD was confirmed in both cases by the undetectable labeling of the rod and carboxy terminus of dystrophin using immunofluorescent staining. A 3-month-old male intact French bulldog was evaluated for dysphagia and dyspnea from severe macroglossia, low head carriage and tetraparesis. Progressive muscle contracture resulted in an inability to open the jaw or stand. The CK activity was reported as elevated. The dog was euthanized, and muscles collected for further evaluation.
3.2. Light Microscopy and Immunofluorescent Staining
A degenerative (pale staining myofibers consistent with myonecrosis) and regenerative (regenerating fibers confirmed with the antibody against dMHC) myopathy was identified in the muscles of all three cases consistent with a dystrophic phenotype (H&E stained muscle cryosections of the Brittany spaniel Cases 1 and 2 are shown in Figure 1A and reference [30], and H&E stained cryosections from the biceps muscle and diaphragm of the French bulldog are shown in Figure 2A). Scattered calcific deposits were evident in Cases 1 and 2 but not in the French Bulldog. Compared to archived control muscle, immunofluorescent staining was absent for the rod- and carboxy-terminus of dystrophin in all three cases (Figure 1B and Figure 2B), with upregulation of utrophin consistent with a diagnosis of a dystrophin-deficient muscular dystrophy.
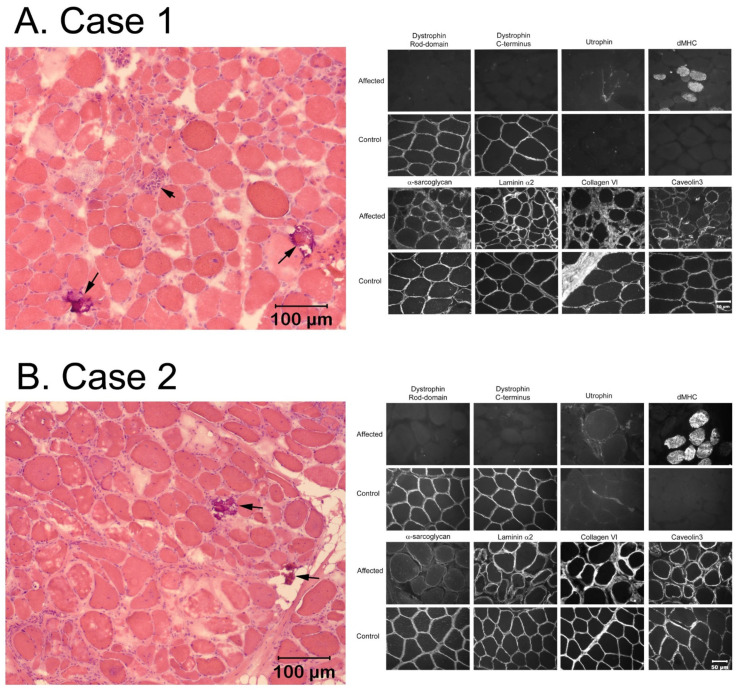
Figure 1.
Histopathology and immunofluorescent staining of muscle cryosections from two Brittany spaniels with dystrophin-deficient muscular dystrophy. (A) is Case 1, a 6-month-old intact male Brittany from the USA, and (B) is Case 2, a 7-month-old intact male Brittany from Japan. In (A), H&E stained cryosections (left) show variability in myofiber size, myonecrosis (arrowhead) and calcific deposits (arrows) consistent with a degenerative myopathy. Immunofluorescence staining for dystrophy-associated proteins (right) shows absent or markedly decreased staining for the rod and carboxy terminus of dystrophin, upregulation of utrophin, and clusters of regenerating fibers using the dMHC antibody for regenerating fibers. In (B), H&E stained cryosections (left) show variability in myofiber size and calcific deposits (arrows). Immunofluorescent staining for dystrophy-associated proteins showed a staining pattern similar to that of Case 1, with undetectable staining for the rod and carboxy terminus of dystrophin, upregulation of utrophin, and clusters of regenerating fibers (dMHC antibody). Staining for other dystrophy-associated proteins, including those for laminin α2, collagen 6, and caveolin 3, was similar to control in both cases.
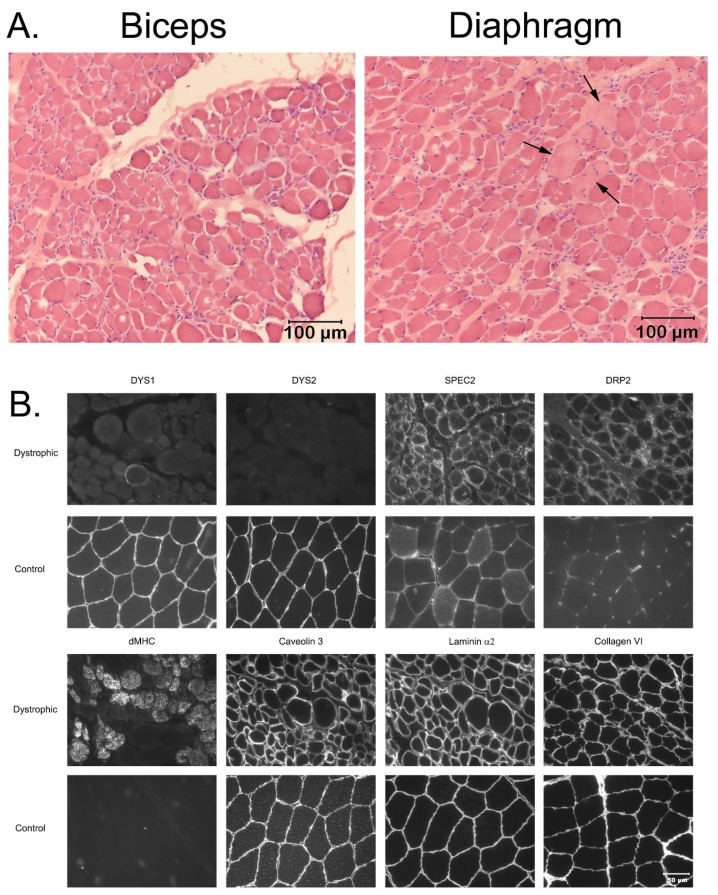
Figure 2.
Histology and Immunofluorescent staining of muscle cryosections from a French Bulldog. (A) Cryosections from the dystrophic French bulldog biceps femoris muscle and diaphragm showing degenerative and regenerative changes consistent with a form of muscular dystrophy. Variability in myofiber size was present in both muscles, with scattered and pale staining necrotic fibers (arrows) present in the diaphragm. H&E stain, bar = 100 µm for both images. (B) Immunofluorescent staining of muscle cryosections from the dystrophic French bulldog and an archived non-age matched control muscle using antibodies against dystrophy-associated proteins. Staining was absent for the rod (DYS1) and carboxy-terminus (DYS2) of dystrophin, with upregulation of utrophin (DRP2) consistent with a diagnosis of a dystrophin-deficient muscular dystrophy. Regenerating muscle fibers are highlighted with the antibody against developmental myosin heavy chain (dMCH). Results of staining for caveolin 3, laminin α2 and collagen VI are similar to those for control muscle. Bar in the lower right corner = 50 µm for all images.
3.3. Two Novel DMD Variants Identified in Dystrophin-Deficient Brittany Spaniels
Case 1 (USA Brittany spaniel) reported in 2022 [30]. Examination of this variant call format (VCF) for unique coding variants revealed a nonsense variant in the juvenile USA Brittany spaniel (stop gained, DMD p.Q2687*). Integrative Genomics Viewer (IGV) view provided in Figure 3.
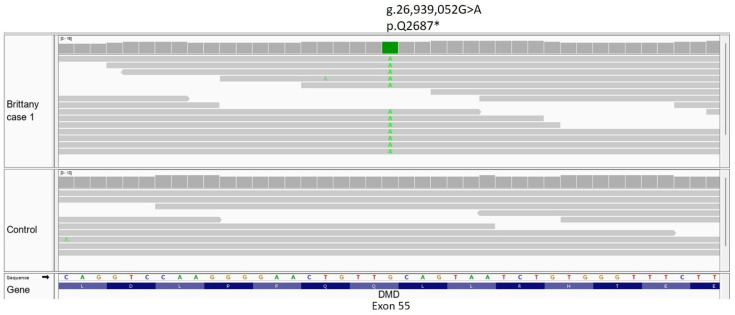
Figure 3.
IGV view of a DMD point mutation in exon 55 of Brittany spaniel Case 1. The green base identified in all reads covering this base in the case is the replacement of a G at genomic position 26,939,052 with an A, resulting in a p.Q2687* mutation in the DMD protein.
Case 2 (Japanese Brittany spaniel also reported in 2022 [30]). This dog was clear for the above stop gained p.Q2687* variant and was presumed to have a different DMD variant. No DMD coding variants were found in the VCF, but visual inspection of the WGS read alignments of the DMD gene revealed evidence for a retrogene insertion in intron 20 (Figure 4).
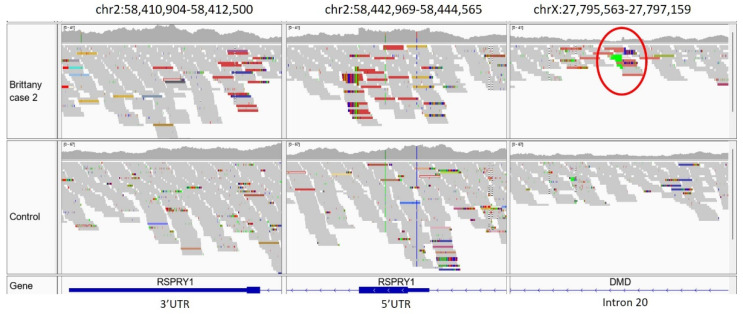
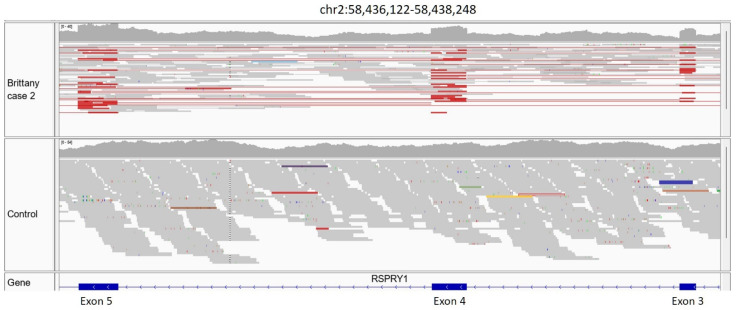
Figure 4.
WGS reads from Brittany spaniel Case 2 aligned to dog chromosome 2 (left and center) and chromosome X (right) in the vicinities of the Ring Finger and SPRY Domain Containing 1 (RSPRY1) and DMD genes, respectively. The case is the top dog in all three panels. The colored bars circled in the case dog on the right represent reads that lie in intron 20 of DMD in which their mates map to the 3′ UTR or 5′ UTR (clusters of colored reads) of RSPRY1. WGS read mate pairs aligning to two different chromosomes can be indicative of a structural variant.
Sanger sequencing of a PCR amplicon derived from a forward primer from DMD intron 20 and a reverse primer from RSPRY1 exon 3 identified a contiguous exonic sequence from the 5′ UTR of RSPYR1 to exon 3 (Sup Figure 1). In addition, sequencing of a PCR amplicon derived from a forward primer from RSPYR1 3′ UTR sequence and a reverse primer from DMD intron 20 revealed RSPYR1 3‴-UTR sequence and a poly A tail. The presence of target site duplication (TSD) sequences in both amplicons confirms their origin as a retrogene insertion (Figure 5).
Figure 5.
Evidence for the presence of a RSPYR1 retrogene in Brittany spaniel Case 2. The red mate pairs aligned over consecutive exons of RSPYR1 (skipping over intronic sequence but connecting red lines indicating their mates) are expected from reads derived from a retrogene-mediated RSPYR1 cDNA insertion elsewhere in the genome. A similar result is not observed in the control dog.
3.4. Novel DMD Variant Identified in a 3-Month-Old Male Intact French Bulldog
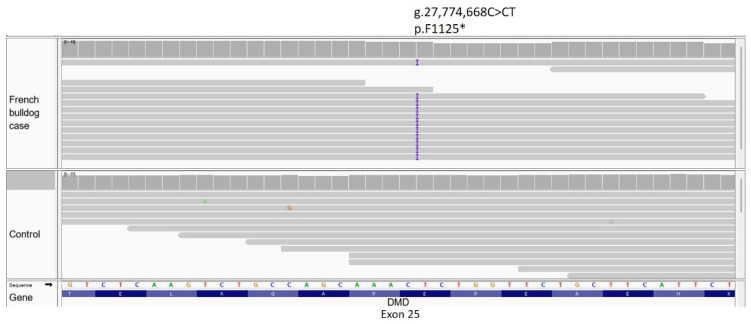
Examination of this VCF for unique coding variants revealed a 1 bp insertion frameshift variant in DMD, resulting in a premature stop codon (DMD, p.F1125*, IGV presented in Figure 6).
Figure 6.
IGV view of a DMD 1 bp insertion mutation in exon 25 of a French bulldog with dystrophin-deficient MD. The purple “I” identified in all but one aligned read in the case is the insertion of a T at genomic DNA position 27,774,668, resulting in a p.F1125* mutation in the DMD protein.
4. Discussion
In this report, we updated all previously reported DMD variants along with their complete descriptions to CanFam4 (Table 1) and added our newly identified DMD variants in Brittany spaniels and the French bulldog. Table 1 also contains the current list of all other known mutations resulting in other forms of canine MD. Thus, in addition to the many known forms of dystrophin-deficient MD across many breeds, the spectrum of known canine MDs also includes forms of limb–girdle MDs in the Boston terrier [22] and miniature dachshund [23], and congenital MDs associated with variants in COL6 in Landseer Newfoundland dogs [24], Labrador retrievers [25], and American Staffordshire terriers [26], in LAMA2 in the Italian greyhound [27] and Staffordshire bull terrier [28], and in LARGE1 in Labrador retrievers [29]. This list will no doubt continue to grow as muscle biopsies are pathologically characterized and new variants identified and correlated with the clinical presentations.
The original clinical report of dystrophin-deficient MD in Brittany spaniels [30] did not attempt to identify a causative mutation. In this report, we analyzed WGS from the Brittany spaniel originating in the United States (Case 1) and identified a nonsense mutation in DMD exon 55 resulting in a premature stop codon. Since the original case report, an additional 6-month-old male Brittany spaniel related to Case 1 was evaluated for progressive exercise intolerance, dysphagia first noted when the dog was 8 weeks of age, and muscle atrophy. A congenital muscle disease was suspected, muscle biopsies evaluated, and genotyping performed for the variant identified in Case 1. This second dog had the same stop gained DMD p.Q2687* variant as Case 1. Genetic testing of Brittany spaniels used for breeding should help eliminate this variant.
The Japanese Brittany spaniel (Case 2) in our original report [30] was clear for the variant affecting Case 1. However, further inspection of the WGS read alignments within the DMD gene was able to identify evidence of a retrogenic insertion of RSPYRI cDNA within intron 20. We suspect that the RSPYR1 retrogene insertion within the DMD gene of Brittany spaniel case 2 is capable of disrupting DMD gene splicing and expression due to incorporation of the retrogene sequence into the initial transcript [36].
The discovery of a 1 bp insertion frameshift variant in DMD resulting in a premature stop codon in a young male French bulldog was also provided in this report. This novel variant was not found in any of the 22 other French bulldogs used in creating the VCF file. Although we cannot comment on the possibility of its presence in the dog population at large, it is possible that, like many/most DMD mutations discovered thus far, it is a variant isolated to this case. However, in 2009, an abstract was presented describing an 8-month-old male French bulldog in Brazil with clinical signs of dysphagia, dyspnea and weakness, with markedly elevated CK activity. Immunohistochemical staining and immunoblotting confirmed dystrophin deficiency [37]. Further, in 2012, a 5-month-old male French bulldog was reported with a 3-month history of dysphagia, regurgitation, exercise intolerance and delayed growth, and markedly elevated CK activity. Pathological diagnosis was of a dystrophic phenotype, and immunofluorescence staining and immunoblotting confirmed dystrophin deficiency [38]. At the time of those reports, WGS was not available for evaluation of DMD variants in these dogs. Recently, archived frozen muscle from the dog of the 2012 report was located, DNA extracted, and tested for the DMD genotype in the French bulldog reported here. That dog did not have the same 1 bp insertion frameshift variant as the French bulldog in this report. It is possible that other variants occur in French bulldogs, as more than one DMD variant has been identified in dystrophic Cavalier King Charles spaniels [12,16] and in Labrador retrievers [9,10,20,21]. Since French bulldogs have now become the #1 breed registered by the American Kennel Club (https://www.akc.org/expert-advice/dog-breeds/most-popular-dog-breeds (accessed on 1 July 2023)). Identification of muscle diseases and development of genetic testing to eliminate at-risk breeding animals is crucial.
5. Conclusions
The list of various forms of canine MD and affected breeds is certain to grow in the coming years with the advances in gene sequencing at affordable prices. Most canine inherited muscle diseases with variants identified to date have been associated with single genes and amenable to reduction or elimination by altered breeding practices. Complete clinical evaluations, pathological characterization of muscle biopsies and continued collaboration with geneticists are essential moving forward.
Acknowledgments
The authors thank Rebecca Stevens, Shinichi Kanazono, and Melanie Puchot for submitting clinical information and diagnostic muscle biopsies on the dystrophic Brittany spaniels and French bulldog.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081557/s1, Figure S1: Sanger sequencing results for PCR amplicons designed to detect the retrogene-mediated insertion of a RSPRY1 cDNA within intron 20 of the DMD gene. Table S1: A list of the unique coding variants of each dog.
Author Contributions
Conceptualization, G.D.S., K.M.M., J.R.M.; Data curation, G.D.S., K.M.M., S.G.F., J.N.C.; Investigation, G.D.S., L.T.G., K.M.M.; Methodology, G.D.S., K.M.M., J.N.C., L.T.G.; Project administration, G.D.S., J.R.M.; Supervision, G.D.S., J.R.M.; Visualization, G.D.S., L.T.G., K.M.M.; Writing—original draft, G.D.S., J.R.M.; Writing—review and editing, G.D.S., L.T.G., K.M.M., J.R.M., S.G.F., J.N.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Owner approval for all diagnostic evaluations was obtained, and IACUC approval was not required for evaluating diagnostic tissue specimens. Muscle or venous blood samples for DNA isolation from dogs were obtained under the University of Minnesota IACUC protocol 1903–36865A.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw sequence reads are available in NCBI’s Short Read Archive at BioProject PRJNA982829.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
S.F. is supported in part by an NIH Special Emphasis Research Career Award (1 K01 OD027058) in Pathology and Comparative Medicine sponsored by the Division of Comparative Medicine, Office of Research Infrastructure Programs.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Johnson N.E., Statland J.M. The Limb-Girdle Muscular Dystrophies. Contin. Lifelong Learn. Neurol. 2022;28:1698–1714. doi: 10.1212/CON.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 3.Heller S.A., Shih R., Kalra R., Kang P.B. Emery-Dreifuss muscular dystrophy. Muscle Nerve. 2020;61:436–448. doi: 10.1002/mus.26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambon A.A., Muntoni F. Congenital muscular dystrophies: What is new? Neuromuscul. Disord. 2021;31:931–942. doi: 10.1016/j.nmd.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Dubowitz V., Sewry C.A., Oldfors A. Muscle Biopsy: A Practical Approach. 5th ed. Elsevier; Amsterdam, The Netherlands: 2021. Pathological Muscle: Individual diseases. [Google Scholar]
- 6.Sharp N.J., Kornegay J.N., Van Camp S.D., Herbstreith M.H., Secore S.L., Kettle S., Hung W.Y., Constantinou C.D., Dykstra M.J., Roses A.D., et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-J. [DOI] [PubMed] [Google Scholar]
- 7.Winand N., Pradham D., Cooper B. Molecular Mechanism of Neuromuscular Disease. Muscular Dystrophy Association; Tucson, AZ, USA: 1994. Molecular characterization of severe Duchenne-type muscular dystrophy in a family of Rottweiler dogs. [Google Scholar]
- 8.Schatzberg S.J., Olby N.J., Breen M., Anderson L.V., Langford C.F., Dickens H.F., Wilton S.D., Zeiss C.J., Binns M.M., Kornegay J.N., et al. Molecular analysis of a spontaneous dystrophin “knockout” dog. Neuromuscul. Disord. 1999;9:289–295. doi: 10.1016/S0960-8966(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith B.F., Kornegay J.N., Duan D. Independent canine models of Duchenne muscular dystrophy to intronic insertions of repetitive DNA. Mol. Ther. 2007;15:S51. [Google Scholar]
- 10.Kornegay J.N., Bogan J.R., Bogan D.J., Childers M.K., Li J., Nghiem P., Detwiler D.A., Larsen C.A., Grange R.W., Bhavaraju-Sanka R.K., et al. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm. Genome. 2012;23:85–108. doi: 10.1007/s00335-011-9382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunetti B., Muscatello L.V., Letko A., Papa V., Cenacchi G., Grillini M., Murgiano L., Jagannathan V., Drögemüller C. X-Linked Duchenne-Type Muscular Dystrophy in Jack Russell Terrier Associated with a Partial Deletion of the Canine DMD Gene. Genes. 2020;11:1175. doi: 10.3390/genes11101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walmsley G.L., Arechavala-Gomeza V., Fernandez-Fuente M., Burke M.M., Nagel N., Holder A., Stanley R., Chandler K., Marks S.L., Muntoni F., et al. A Duchenne muscular dystrophy gene hot spot mutation in dystrophin-deficient cavalier king Charles spaniels is amenable to exon 51 skipping. PLoS ONE. 2010;5:e8647. doi: 10.1371/journal.pone.0008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith B.F., Yue Y., Woods P.R., Kornegay J.N., Shin J.H., Williams R.R., Duan D. An intronic LINE-1 element insertion in the dystrophin gene aborts dystrophin expression and results in Duchenne-like muscular dystrophy in the corgi breed. Lab. Investig. 2011;91:216–231. doi: 10.1038/labinvest.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins C.A., Forman O.P. Identification of a novel frameshift mutation in the DMD gene as the cause of muscular dystrophy in a Norfolk terrier dog. Canine Genet. Epidemiol. 2015;2:7. doi: 10.1186/s40575-015-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atencia-Fernandez S., Shiel R.E., Mooney C.T., Nolan C.M. Muscular dystrophy in the Japanese Spitz: An inversion disrupts the DMD and RPGR genes. Anim. Genet. 2015;46:175–184. doi: 10.1111/age.12266. [DOI] [PubMed] [Google Scholar]
- 16.Nghiem P.P., Bello L., Balog-Alvarez C., López S.M., Bettis A., Barnett H., Hernandez B., Schatzberg S., Piercy R.J., Kornegay J.N. Whole genome sequencing reveals a 7 base-pair deletion in DMD exon 42 in a dog with muscular dystrophy. Mamm. Genome. 2017;28:106–113. doi: 10.1007/s00335-016-9675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez L., Beltrán E., de Stefani A., Guo L.T., Shea A., Shelton G.D., De Risio L., Burmeister L.M. Clinical and genetic characterization of dystrophin-deficient muscular dystrophy in a family of Miniature Poodle dogs. PLoS ONE. 2018;13:e0193372. doi: 10.1371/journal.pone.0193372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrader S.M., Jung S., Denney T.S., Smith B.F. Characterization of Australian Labradoodle dystrophinopathy. Neuromuscul. Disord. 2018;28:927–937. doi: 10.1016/j.nmd.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Mata López S., Hammond J.J., Rigsby M.B., Balog-Alvarez C.J., Kornegay J.N., Nghiem P.P. A novel canine model for Duchenne muscular dystrophy (DMD): Single nucleotide deletion in DMD gene exon 20. Skelet. Muscle. 2018;8:16. doi: 10.1186/s13395-018-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barthélémy I., Calmels N., Weiss R.B., Tiret L., Vulin A., Wein N., Peccate C., Drougard C., Beroud C., Deburgrave N., et al. X-linked muscular dystrophy in a Labrador Retriever strain: Phenotypic and molecular characterization. Skelet. Muscle. 2020;10:23. doi: 10.1186/s13395-020-00239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shelton G.D., Minor K.M., Vieira N.M., Kunkel L.M., Friedenberg S.G., Cullen J.N., Guo L.T., Zatz M., Mickelson J.R. Tandem duplication within the DMD gene in Labrador retrievers with a mild clinical phenotype. Neuromuscul. Disord. 2022;32:836–841. doi: 10.1016/j.nmd.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox M.L., Evans J.M., Davis A.G., Guo L.T., Levy J.R., Starr-Moss A.N., Salmela E., Hytönen M.K., Lohi H., Campbell K.P., et al. Exome sequencing reveals independent SGCD deletions causing limb girdle muscular dystrophy in Boston terriers. Skelet. Muscle. 2017;7:15. doi: 10.1186/s13395-017-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mickelson J.R., Minor K.M., Guo L.T., Friedenberg S.G., Cullen J.N., Ciavarella A., Hambrook L.E., Brenner K.M., Helmond S.E., Marks S.L., et al. Sarcoglycan A mutation in miniature dachshund dogs causes limb-girdle muscular dystrophy 2D. Skelet. Muscle. 2021;11:2. doi: 10.1186/s13395-020-00257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffen F., Bilzer T., Brands J., Golini L., Jagannathan V., Wiedmer M., Drögemüller M., Drögemüller C., Leeb T. A Nonsense Variant in COL6A1 in Landseer Dogs with Muscular Dystrophy. G3 Genes Genomes Genet. 2015;5:2611–2617. doi: 10.1534/g3.115.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolduc V., Minor K.M., Hu Y., Kaur R., Friedenberg S.G., Van Buren S., Guo L.T., Glennon J.C., Marioni-Henry K., Mickelson J.R., et al. Pathogenic variants in COL6A3 cause Ullrich-like congenital muscular dystrophy in young Labrador Retriever dogs. Neuromuscul. Disord. 2020;30:360–367. doi: 10.1016/j.nmd.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankelunas L., Murthy V.D., Chen A.V., Minor K.M., Friedenberg S.G., Cullen J.N., Guo L.T., Mickelson J.R., Shelton G.D. Novel COL6A3 Frameshift mutation causes Ullrich-like congenital muscular dystrophy in American Staffordshire dogs. J. Vet. Intern. Med. 2003. submitted . [DOI] [PMC free article] [PubMed]
- 27.Christen M., Indzhova V., Guo L.T., Jagannathan V., Leeb T., Shelton G.D., Brocal J. LAMA2 Nonsense Variant in an Italian Greyhound with Congenital Muscular Dystrophy. Genes. 2021;12:1823. doi: 10.3390/genes12111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelton G.D., Minor K.M., Thomovsky S., Guo L.T., Friedenberg S.G., Cullen J.N., Mickelson J.R. Congenital muscular dystrophy in a dog with a LAMA2 gene deletion. J. Vet. Intern. Med. 2022;36:279–284. doi: 10.1111/jvim.16330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shelton G.D., Minor K.M., Guo L.T., Friedenberg S.G., Cullen J.N., Hord J.M., Venzke D., Anderson M.E., Devereaux M., Prouty S.J., et al. Muscular dystrophy-dystroglycanopathy in a family of Labrador retrievers with a LARGE1 mutation. Neuromuscul. Disord. 2021;31:1169–1178. doi: 10.1016/j.nmd.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens R., Kanazono S., Petesch S., Guo L.T., Shelton G.D. Dystrophin deficient muscular dystrophy in two male juvenile Brittany dogs. J. Am. Anim. Hosp. Assoc. 2022;58:292–296. doi: 10.5326/JAAHA-MS-7255. [DOI] [PubMed] [Google Scholar]
- 31.Dubowitz V., Sewry C.A., Oldfors A. Muscle Biopsy: A Practical Approach. 5th ed. Elsevier; Amsterdam, The Netherlands: 2021. Histological and histochemical stains and reactions. [Google Scholar]
- 32.Guo L.T., Moore S.A., Forcales S., Engvall E., Shelton G.D. Evaluation of commercial dysferlin antibodies on canine, mouse, and human skeletal muscle. Neuromuscul. Disord. 2010;20:820–825. doi: 10.1016/j.nmd.2010.07.278. [DOI] [PubMed] [Google Scholar]
- 33.Leivo I., Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc. Natl. Acad. Sci. USA. 1988;85:1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hessle H., Engvall E. Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J. Biol. Chem. 1984;259:3955–3961. doi: 10.1016/S0021-9258(17)43189-9. [DOI] [PubMed] [Google Scholar]
- 35.Cullen J.N., Friedenberg S.G. WAGS: User-friendly, rapid, containerized pipelines for processing, variant discovery, and annotation of short read whole genome sequencing data. G3 Genes Genomes Genet. 2023:jkad117. doi: 10.1093/g3journal/jkad117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batcher K., Varney S., York D., Blacksmith M., Kidd J.M., Rebhun R., Dickinson P., Bannasch D. Recent, full-length gene retrocopies are common in canids. Genome Res. 2022;32:1602–1611. doi: 10.1101/gr.276828.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gama Filho H.A.N., Oliveira C.M., Dias C.T.S., Santos A.L.F., Ambrosio C.E., Vainzof M., Gioso M.A., Maiorka P.C. Muscular dystrophy in a French Bulldog: First case report; Proceedings of the 34th World Veterinary Association Congress; São Paulo, Brazil. 24 July 2009. [Google Scholar]
- 38.de la Fuente C., Shelton G.D., Molín J., Pumarola M., Guo L.T., Añor S. Pathology in practice. J. Am. Vet. Med. Assoc. 2012;240:1423–1425. doi: 10.2460/javma.240.12.1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence reads are available in NCBI’s Short Read Archive at BioProject PRJNA982829.