Abstract
Danon disease is a rare x-linked dominant multisystemic disorder with a clinical triad of severe cardiomyopathy, skeletal myopathy, and intellectual disability. It is caused by defects in the lysosome-associated membrane protein-2 (LAMP2) gene. Numerous different mutations in the LAMP2 protein have been described. Danon disease is typically lethal by the mid-twenties in male patients due to cardiomyopathy and heart failure. Female patients usually present with milder and variable symptoms. This report describes a 42-year-old father and his 3-year-old daughter presenting with mild manifestations of the disease. The father has normal intellectual development and normal physical activity. At the age of 13, he was diagnosed with mild ventricular pre-excitation known as Wolf–Parkinson–White syndrome (WPWs), very mild and mostly asymptomatic cardiomyopathy and left ventricular hypertrophy, and at about the age of 25 presented with visual impairment due to cone–rod dystrophy. His daughter showed normal development and very mild asymptomatic electrocardiographic WPWs abnormalities with left mild ventricular hypertrophy. Genetic testing revealed an Xq24 microdeletion encompassing the entire LAMP2 gene. Relevant literature was reviewed as a reference for the etiology, diagnosis, treatment and case management.
Keywords: Danon disease, LAMP2-gene, genotype-phenotype correlation, penetrance, microdeletion, whole-exome-sequence
1. Introduction
Danon disease (OMIM: 300257) is an x-linked dominant lysosomal storage disease characterized by the triad of cardiomyopathy, skeletal myopathy, and intellectual disability [1]. This disease is caused by defects in the lysosome-associated membrane protein 2 (LAMP2) gene, encoding for LAMP2 protein, which plays important role in autophagosome–lysosome fusion in autophagy [2,3].
The pathogenicity of LAMP2 deficiency can be explained by the accumulation of autophagy materials [2,4], increased mitochondrial oxidative stress and increased apoptosis [5], altered metabolism, and impaired contractile function due to reduced calcium transients [2,4,5].
Typically, males are affected both earlier and more severely compared to females, with severe cardiomyopathy requiring heart transplantation; otherwise, the disease is lethal by their mid-twenties [6]. Additional clinical manifestations include retinal disease, hepatic disease, neurological manifestations, and pulmonary disease [6]. Currently, available treatments for Danon disease are limited to the prevention of sudden death and heart failure through a cardioverter defibrillator (implantable cardioverter-defibrillator, ICD) implantation and heart transplantation.
The most common clinical features in affected males include progressive hypertrophic cardiomyopathy (HCM), (in up to 96% of the patients) [6,7,8], accompanied by arrhythmias (in up to 80% of the patients), mild and slowly progressive proximal muscle weakness sometimes causing delayed motor developmental milestones (in up to 90% of the patients), progressive visual impairment due to retinopathy (in up to 20% of the patients) [9,10], and also mild intellectual disability (in up to 80% of the patients) [7]. Additional features may include elevated creatine kinase and hepatic enzymes, and a myopathic muscle biopsy [11]. Affected males usually develop heart failure, starting around 12 years old [12,13,14], and deteriorate to severe heart failure, almost certainly needing a transplantation, which occurs in the second and third decades of life [1,14]. Conduction abnormalities include complete heart block, atrial arrhythmias and life-threatening ventricular arrhythmias, pre-excitation, including Wolff–Parkinson–White syndrome (the most common finding on ECG), seen in approximately 48% of males at presentation [7,8,15]. Histologically, the myocard shows significant necrosis and fibrosis. HCM is a major determinant of clinical presentation and prognosis. The need for cardiac transplantation is inevitable for most males by the second to third decades of life [7,13,14,16]. Ventricular arrhythmias contribute significantly to sudden death in patients with Danon disease. Affected males, on average, live to age 19, while affected females live to an average age of 34 years [7,13,14,16].
Neuropsychiatric manifestations such as delayed speech, attention deficit disorder, behavioral problems, psychiatric disorders (e.g., severe depression, psychosis), autistic spectrum disorders, seizures, and axonal polyneuropathy with features of Charcot–Marie–Tooth disease have been described in many affected males (up to 69%) [7,17,18].
Some affected males may also exhibit hepatomegaly and elevated transaminases with preserved hepatic function, abdominal pain [7], diarrhea [7] and esophageal dysmotility in 77% of patients [7]. Respiratory disease is present in about half of affected males, with shortness of breath, chest tightness, coughing, and/or wheezing [7].
Females develop symptoms later than males, and usually have a cardiac-restricted phenotype presenting during adolescence or later by 19 years of age [12]. In affected females, either a hypertrophic (61–100% of female patients) or, less commonly, a dilated cardiomyopathy may develop. Up to 18% require cardiac transplantation, and arrhythmias occur in up to 100% of the patients. Atrioventricular block and sudden cardiac death due to arrhythmia have been reported in women. Relatively mild retinopathy has been reported in up to 20% of patients, mild non-progressive muscle weakness in up to 50% of patients, and mild intellectual disability in up to 10% of cases. Creatine kinase and hepatic enzymes may be normal or mildly elevated in affected females [12,13,14].
The treatment of Danon disease is mainly focused on the cardiac manifestations. A left ventricular assistance device may be implanted in order to provide sufficient cardiac output/bridge to transplantation and a cardioverted defibrillator in order to prevent sudden cardiac death from ventricular tachycardia [19]. Both male and female patients suffering from heart failure may benefit from heart transplantation. Five-year graft survival for male and female patients is relatively high, making heart transplantation an effective treatment option [19,20], although it appears that the long-term outcome in female patients is superior to that of males. Ablation of the arrhythmogenic focus may be considered in patients with cardiac pre-excitation and ventricular arrhythmia. There is no specific treatment for muscle weakness, but physiotherapy should be attempted and may become beneficial. Similarly, supportive interventions for developmental delay/intellectual disability, retinopathy, or neuropsychiatric manifestations should be offered. However, some preliminary evidence suggest that neuropsychiatric and intellectual function may improve after a successful heart transplantation [20,21]. A multidisciplinary team should follow these patients. Cardiac evaluation includes annual electrocardiography and echocardiography, a cardiac MRI every one to two years; ambulatory arrhythmia monitoring as needed based on symptoms; annual neurological assessment for muscle weakness and progression, monitoring of developmental progress, educational needs, and behavior at each visit with formal developmental assessments every three to five years during childhood; ophthalmologic evaluation at least every three to five years [1,13,22]. Genetic counseling to the patients and genetic testing of their relatives should be offered and undertaken [22].
As mentioned earlier, the underlying pathogenesis of Danon disease is the accumulation of lysosomal material secondary to LAMP2 gene variants that disrupt LAMP2 protein proper function and autophagosome–lysosome fusion in autophagy [2,3,4,5].
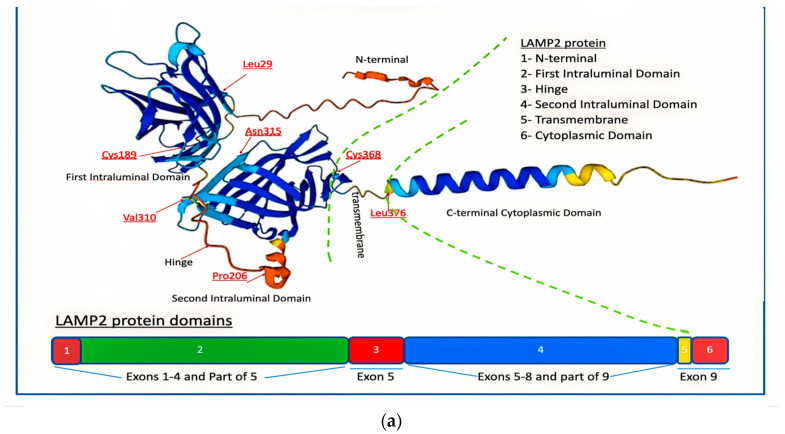
LAMP2 belongs to the LAMP protein family of highly homologous type I transmembrane proteins, each with a conserved glycosylated domain extending into the acidic lysosomal lumen. In humans, LAMP2 (CD107b) protein is derived from a single gene with nine coding exons and three alternate last exons, 9a, 9b, and 9c, generating three isoforms known as LAMP2A, LAMP2B, and LAMP2C, respectively, which differ primarily in the sequence of their transmembrane and cytosolic tail [16]. LAMP2A is a homodimer, with each monomer consisting of a large luminal domain, composed of two large domains (N-domain and C-domain separated by a hinge sequence), a transmembrane domain, and a C-terminal cytoplasmic domain [1] (Figure 1).
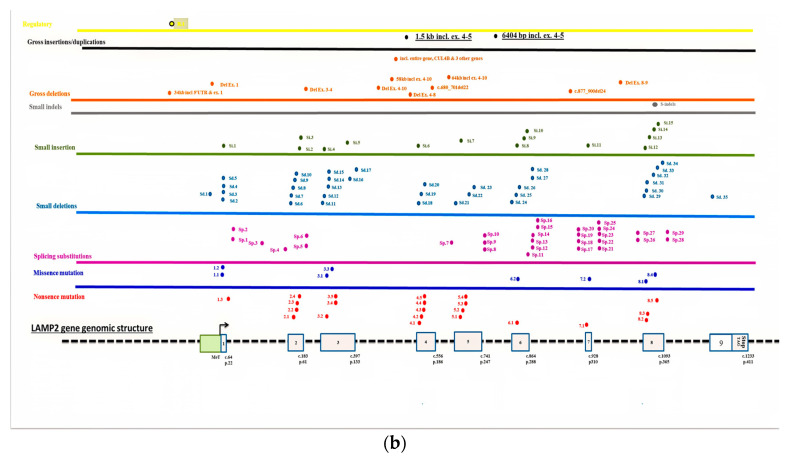
Figure 1.
(a) LAMP2 protein and domains. Schematic representation of LAMP2 protein’s predicted three-dimensional structure from AlphaFold, a courtesy of https://www.genecards.org accessible data. Some amino residues are depicted for illustration, and all others (except than 315Asn) are involved in Danon Disease. (b) Schematic illustration of HGMD LAMP2 mutations according to variant subtypes (nonsense, missense, frameshift, splice, deletions) and their LAMP2 exon location and distribution. The variant IDs in the specific location above exons are the key for the detailed molecular change as it appears in the Supplementary Materials (S. excel file-1).
Each domain has its own distinct function:
The N-terminal cytoplasmic domain of LAMP2A is composed of approximately 110 amino acids and contains several protein–protein interaction motifs, such as a WW domain, a PDZ-binding motif, and a dileucine motif. These motifs enable LAMP2A to interact with other proteins and participate in various cellular signaling pathways, including autophagy.
The transmembrane domain of LAMP2A consists of a single α-helix that is approximately 25 amino acids long. It spans the lysosomal membrane and anchors LAMP2A in place. The transmembrane domain is amphipathic, with hydrophobic residues on one side that interact with the lipid bilayer of the membrane [23].
The luminal domain of LAMP2A that faces the inside of the lysosome is composed of approximately 150 amino acids and contains several glycosylation sites. Glycosylation is the process by which sugar molecules are attached to proteins, and it plays a crucial role in proper folding, stability, and function of LAMP2A. The luminal domain also contains several conserved cysteine residues that form disulfide bonds, which help stabilize the protein [23].
The C-terminal cytoplasmic domain of LAMP2A faces the cytoplasm and is responsible for interactions with cytoskeletal proteins and lysosomal trafficking. It is composed of approximately 60 amino acids and contains a proline-rich motif and a dileucine motif. The proline-rich motif enables LAMP2A to interact with cytoskeletal proteins, such as actin, and participate in lysosomal trafficking. The dileucine motif is recognized by adaptor proteins that mediate the transport of LAMP2A from the trans-Golgi network to the lysosome [23].
Overall, the different domains of LAMP2A enable the protein to interact with other proteins, anchor itself in the lysosomal membrane, participate in cellular signaling pathways, and mediate lysosomal trafficking. These functions are essential for proper lysosomal function and autophagy.
The three active transcripts of LAMP2 gene encompass currently over 160 different published mutations [1,3] and more than 250 pathogenic and 28 likely pathogenic registered variants in well-acknowledged databases (LOVD (https://databases.lovd.nl/shared/genes/LAMP2, accessed on 15 May 2023), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar, accessed on 15 May 2023) and Human Gene Mutation Database (HGMD) (https://www.hgmd.cf.ac.uk/ac/index.php, accessed on 15 May 2023) (Figure 1b, Tables S1–S7).
The common variants in the LAMP2 gene that disrupt LAMP2 protein function include (1) missense mutations, the substitution of a single amino acid in the LAMP2 protein disrupts the formation of disulfide bonds and destabilizes the protein [7,24]; (2) nonsense, frameshift, splice, and deletion variants [7,24], which accordingly, create truncated or premature stop codons, non-functional proteins that are rapidly degraded [6,11,16,25].
Herein, two Danon patients are reported, a young male and his daughter, who both have an Xq24 microdeletion that includes the entire LAMP2 gene and present with a mild disease phenotype.
2. Diagnostic Assessment—Methods and Results
Patient Evaluation
This study was planned and executed by the Simon Winter Institute for human genetics’ team (Bnai-Zion Medical Center, Haifa, Israel). Informed consent was signed by Case 1 and by his wife for their affected child (Case 2). For initial evaluation, both parents and Case 1’s mother were interviewed. The medical records were reviewed, and a physical examination, chest radiography, electrocardiography (ECG), echocardiography, and molecular genetics tests were conducted. Left ventricular size and function were evaluated by M-mode and two-dimensional Doppler and color Doppler echocardiography, and cardiac arrhythmias were studied by 24-h Holter monitoring. In both the affected father and his wife and their daughter, a classical karyotype cytogenetics study was completed. In order to identify the specific responsible LAMP2 gene variants, primers were designed to amplify all 9 LAMP2 gene exons in Case 1. Then, a chromosomal microarray analysis (CMA) and whole-exome sequence (WES) were performed in both affected cases.
CMA Procedure and Interpretation of Results: Analysis of copy number variants (CNV) was performed as detailed elsewhere. An Affymetrix CytoScan750K CGH/SNP array was used to analyze DNA samples from the family according to the manufacturer’s protocol. DNA extracted from peripheral blood underwent digestion, ligation to adapters, amplification, and treatment with DNAse I. Fragments were end-labelled with a modified biotinylated base, hybridized to the array, and stained with a streptavidin-coupled dye and a biotinylated anti-streptavidin antibody. After scanning with a GeneChip Scanner, signal intensities were compared with reference reads using Chromosome Analysis Suite (ChAs V.4.0) software. Several public databases were routinely applied to characterize CNVs gains or losses, among them the UCSC Genome Browser (genome.ucsc.edu/), ClinVar (ncbi.nlm.nih.gov/clinvar/), the Database of Genomic Variants (dgv.tcag.ca/dgv/app/home), DECIPHER (decipher.sanger.ac.uk/), ISCA Consortium (iscaconsortium.org/), and ClinGen resource (clinicalgenome.org/). Following the American College of Medical Genetics and Genomics standards and guidelines for the interpretation and reporting of constitutional CNVs [26], CMA findings were classified as pathogenic, likely pathogenic, variants of unknown significance (VUSs), likely benign, and benign. Categorization was based on the original laboratory reports, which were reviewed according to the updated knowledge in the medical literature and the authors’ experience. Pathogenic and likely pathogenic findings (including both gross chromosomal aberrations and submicroscopic CNVs) were defined as clinically significant.
3. Whole-Exome Sequence
In Case 2, next-generation sequencing (NGS) was performed for whole-exome sequencing (WES) using a NovaSeq 6000 platform, with an S1 Reagent Kit v1.5 (200 cycles) (Illumina, San Diego, CA, USA) and an IDT xGen Exome Research Panel v2 (Integrated DNA Technologies, Coralville, IA, USA) for library preparation. The bioinformatics analyses focused on protein-altering variants (missense, nonsense, frameshift, and splice-site). Alignment and detection of variants were performed with DRAGEN bioinformatics pipeline version 3.6, with GRCh37 as the human genome reference. All called variants were assigned a quality value for filtering. Based on validation studies, the pipeline showed precision and detection > 99% for SNVs in areas with coverage greater than 20x and high mapping quality. Variants were annotated with the following public and internal bioinformatics/genetics resources: gnomAD, Ensembl Variant Effect Predictor (VEP), dbNSFP, Emedgene© DB, SnpSift, SnpEff, ExAC, GWAS, GRC, HGMD, Clinvar, Breast Cancer Information Core (BIC), BRCA Exchange, The Greater Middle East (GME), Amish Coriell and Mennonite, dbSNP, GERP, 1000 Genomes, Online Mendelian Inheritance in Man® (OMIM), Clinical Genomic Database (CGD), Clinical Genomic Database (CGD), Orphanet, ClinVar, Emedgene© Gene-Disease Knowledge Graph.
Variants were also interpreted and classified using the ACMG/AMP/ClinVar standards and guidelines. Analysis for secondary findings was performed according to the current recommendations of the ACMG.
4. X Inactivation
An X inactivation pattern was determined at the Androgen Receptor (AR) gene locus using HhaI-sensitive methylation analysis: genomic DNA was PCR-amplified with primers flanking the polymorphic CAG repeat at the 5’ end of the AR gene after digestion with an HhaI methylation-sensitive restriction enzyme. PCR products were run on an ABI genetic analyzer and analyzed with GeneMapper software.
5. Results
Case Description
Case 1
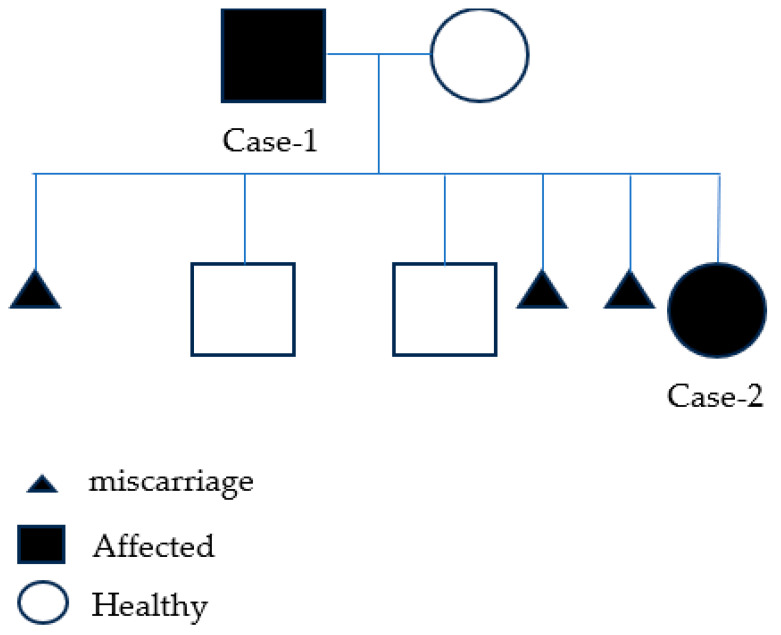
Initially, the healthy spouse of Case 1 was referred for genetic counselling in her sixth pregnancy with Case 2. She has healthy 7- and 5-year-old male children and a history of three spontaneous miscarriages, all of them occurring at the first trimester of spontaneous pregnancies. The family pedigree is presented below, depicting both affected and unaffected family members (Figure 2).
Figure 2.
Family pedigree presenting the affected (filled) and the unaffected (no fill) family members, with three miscarriages.
Her spouse complained of visual impairment secondary to retinitis pigmentosa as determined by ophthalmologic assessment, as described below.
For recurrent abortions study, peripheral blood chromosome analysis was performed in the couple, and a normal karyotype was found.
Case 1 was invited for further clinical and genetic evaluation, and a more complex clinical picture emerged. Today he is 42 years old, maintaining a full-time job as a storekeeper at a local industrial factory. Since the age of 25, he has reported progressive visual impairment with increased severity during the dark hours. Ophthalmic examination including visual evoked potentials (VEP) and an electroretinogram (ERG) studies revealed rods and cones dystrophy.
His healthy mother, who herself refused to undergo any genetic investigation, had reported that Case 1 complained of heart palpitations at the age of 13 years. According to his electrocardiogram (ECG) obtained then, an anomally appropriate with Wolf–Parkinson–White (WPW) pattern was described. However, his first echocardiography showed normal contraction of both ventricles and no signs of cardiomyopathy. Moreover, 24-h Holter ECG monitoring showed a short PR interval, multiple wide QRS complexes compatible with ventricular premature beats, and non-sustained ventricular tachycardia (VT) or supraventricular tachycardia (SVT) with an aberrant conduction, for which he was treated by amiodarone for several years. Basic metabolic evaluation (urine organic acids, plasma amino acids) was normal. Danon disease was considered by the pediatric team but further evaluation was not accomplished.
Of note, he has normal intelligence and no psychiatric, gastrointestinal, or pulmonary complaints. At the age of 18, despite the demonstration of increased echogenicity and borderline increase in antero-septal wall thickness which suggested a possible cardiomyopathy, he was drafted and was able to perform full combat warrant military service between 18 and 21 years of age without complications or physical limitations.
At the age of 27, a dual-chamber VDD (SENIA) cardiac pacemaker was implanted due to complete heart block. On recent evaluation, he has had atrial fibrillation and does not take medications. He reports good functional capacity, denies orthopnea, postural nocturnal dyspnea, palpitations, or syncope. However, he describes physical limitation only while running.
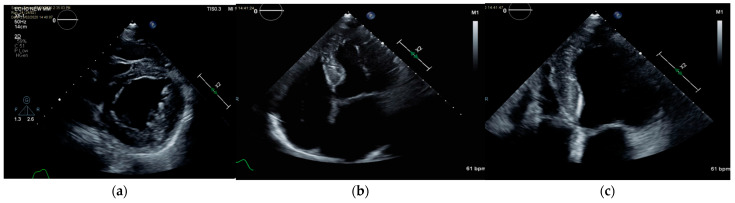
On physical examination, he has no signs of heart failure. His echocardiography (Figure 3) at age 39 showed normal ventricular size, moderate hypertrophy of the left ventricular wall, more prominent at the posterolateral segment than at the septum. Increased echogenicity of the myocardium with speckling was demonstrated, with a mildly thick aortic valve with minimal aortic regurgitation and mild mitral regurgitation. The left atrium is mildly dilated (28.3 cm2). Abnormal E/E’ was measured, supporting diastolic dysfunction. Representative echo mp4 video records can be found in Supplementary Materials (S. mp4 Case-1). He presented with a paced rhythm of 60 beats per minute with a wide QRS and atrial fibrillation (no A wave), and a pacing electrode seen in the right ventricle. However, in the original ECG recorded at 15 years old, a WPW pattern was demonstrated (Figure S1).
Figure 3.
(a) Echocardiography of Case 1, short-axis view of the left ventricle showing mild hypertrophy, increased echogenicity, and normal contraction. (b) Four-chambers view (4cv) showing left ventricular (LV) hypertrophy with lateral wall involvement > septum, normal contraction, increased echogenicity, left atrial dilatation. (c) 4cv zoom of the left ventricle.
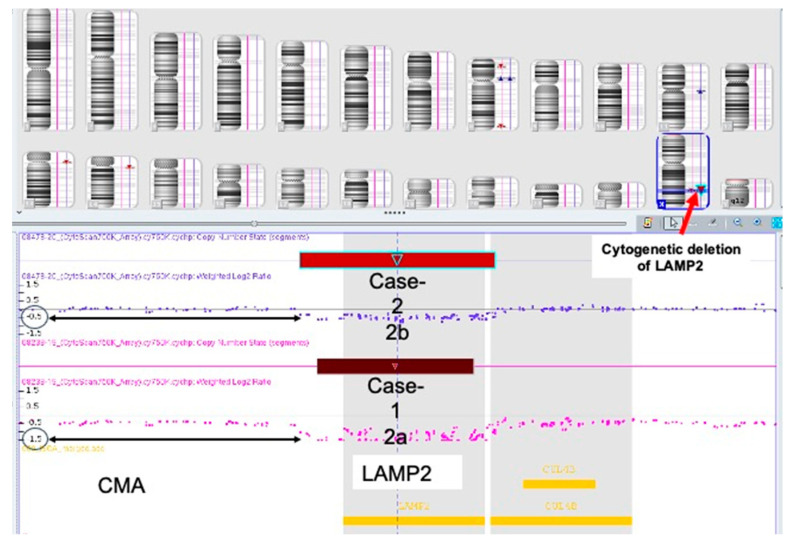
After his marriage in 2014, Case 1 participated in a research program aimed to investigate retinal disorders by NGS technology and WES analysis (PI Tamar Ben Yousef). The analysis was negative, and no pathogenic/likely pathogenic variants were found. The coverage was around 97%, and reading depth was X20. However, it is important to emphasize that the familiar WES platform and the bioinformatic tools used then (9 years ago) did not include CNVs detection in its routine analysis report. Taking into account this and his medical history (the combination of early cardiomyopathy, arrhythmia and conduction abnormalities, and ophthalmic involvement) that implied Danon disease (caused by LAMP2 gene mutation) as the most appropriate diagnosis, we decided to focus on the LAMP2 gene. We used the published primers [3] for direct Sanger sequencing. Compared to healthy control’s DNA sample (extracted from a peripheral blood sample), several LAMP2 PCR analysis attempts of Case 1 DNA failed to retrieve any PCR products in all nine LAMP2 exons. Reasonably, a suspicion of entire LAMP2 gene’s deletion was raised. This assumption was confirmed by CMA analysis, indicating Danon disease (Figure 4). The mode of inheritance was discussed with his mother and his young sister. The sister underwent a CMA test, which was normal (two LAMP2 alleles were seen), while his mother refused to be tested.
Figure 4.
CMA analysis of Case 1 (a) and Case 2 (b), lower and upper blots, respectively. CMA of Case 1 identified deletion of 110.5 Kb on Xq24. (GRCh37)(Xq24(119513013_119623579)x0 (a). The same deletion was seen in a heterozygous state in his daughter (Case2) (b).
Case 2
The daughter of Case 1 was subsequently evaluated. Today, she is three years old, born after uneventful spontaneous pregnancy and delivery. During the pregnancy, the mother received two consequential genetic counselling sessions due to her history of recurrent miscarriages and her husband’s health condition. The first meeting was before and the second meeting was after the identification of LAMP2 gene deletion in Case 1, supporting the diagnosis of Danon disease. The mother declined an amniocentesis procedure since; she intended to deliver the baby under any circumstances.
The family described a healthy and happy child with normal developmental progress. At the age of 14 months, she was examined by us in the genetic clinic as a routine follow-up and underwent karyotype and CMA analysis. As expected, she had inherited a normal maternal X-chromosome and a mutated paternal X-chromosome (harboring the LAMP2 gene deletion). Therefore, she was a heterozygous carrier of a LAMP2 deletion (Figure 4b). In the karyotype study, there was no evidence for chromosomal translocations or intra X-chromosome rearrangements.
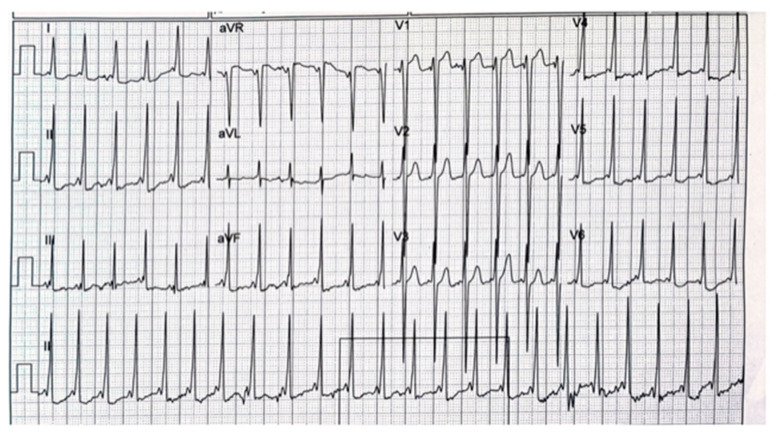
She was referred to a pediatric cardiologist at 18 months old and was diagnosed with heart murmur and asymptomatic WPW on baseline ECG (Figure 5).
Figure 5.
Case 2’s ECG results at the age of 18 months old, showing a WPW pattern.
She is thriving and is well developed with no heart-related symptoms. Her parents deny fatigue, syncope, or behavioral changes, and arrhythmia was not documented.
On recent clinical examination, she has no signs of congestive heart failure. A 1/6 systolic murmur was auscultated at the left sternal border. In the ECG, there was a normal sinus rhythm 139/min with high precordial voltage, a WPW pattern with a slightly widened QRS of 100 milliseconds with mild ST segment depression at the inferior and left lateral leads (Figure 5). Holter ECG showed a sinus rhythm of 85–167/min (average 119/min) with sparse atrial and ventricular ectopy. Some complexes were conducted with a narrow QRS and a different repolarization pattern and could represent competition between the accessory pathway and normal conduction.
The echocardiography showed normal ventricular size and normal contraction without wall motion abnormality. Increased echogenicity and hypertrophy involving the papillary muscles and posterolateral and apical segments was demonstrated. Valve morphology and estimated pulmonary pressure were normal, without pericardial effusion. The rest of the study was normal. Representative echo mp4 video records can be found in the Supplementary Materials (S. mp4 case-2).
In summary: both cases have Danon disease due to LAMP2 gene microdeletion (confirmed by PCR testing (in the father) and CMA studies (in both)). The father is hemizygous (Figure 4a) and his daughter is heterozygous for a 110.5Kb Xq24 microdeletion that includes the entire LAMP2 gene (Figure 4b).
6. X-Inactivation Study
Although Case 2 presented with a mild phenotype, the early cardiac manifestation is uncommon in a female patient, and X-inactivation was tested, which may contribute to the understanding of Case 2’s cardiological findings. X-inactivation was tested by a restriction enzyme, sensitive for methylation of 5-prime of the Androgen Receptor (AR) gene [27]. The paternal/maternal alleles’ expression measured ratio of 35/65 (defined as normal) was compared with the 10/90 for X-inactivation ratio (the cutoff ratio for X-inactivation as defined by the by referral Lab of the Department of Medical Genetics, Hadassah Medical Organization).
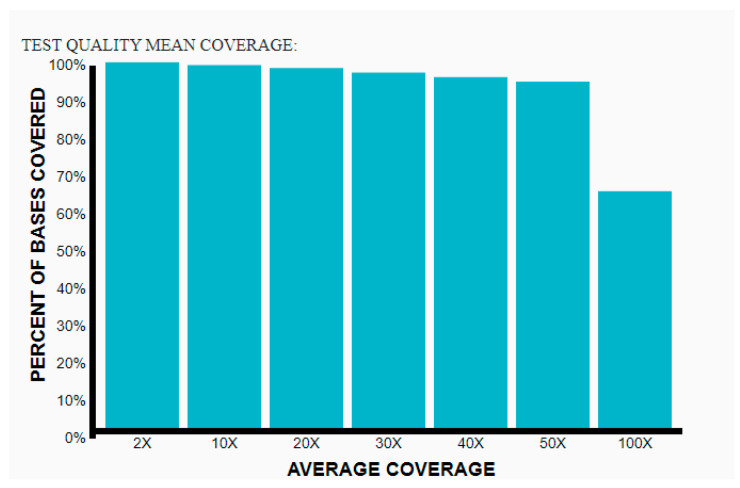
Whole-Exome Sequence (WES): As described earlier, Case 2 was diagnosed with WPWS and mild hypertrophic cardiomyopathy. In order to investigate the early presentation independently, we decided to look for other genes involved in cardiomyopathy and arrythmia by WES analysis. An exome sequence is a technique used to analyze a person’s DNA sequence by sequencing only the protein-coding regions, comprising approximately 1% of the human genome, which contains ~180,000 exons split among ~22,000 different genes. The majority of known disease-causing mutations reside in these regions. To capture the exomic regions of DNA, a targeted capture method is used, followed by high-throughput sequencing using next-generation sequencing technologies. As described in the Methods section, a solo WES for Case 2 was run, and for analysis, the human phenotype ontology (HPO) “WPW syndrome” and “cardiomyopathy” was used following the ACMG standards and guidelines for the interpretation of sequence variants [26,28]. The sample quality pass was sufficient for clinical grade and precision and detection of >99% for SNVs in areas with coverage greater than 20× and high mapping quality as demonstrated in Figure 6.
Figure 6.
All called variants are assigned a quality value for filtering. Based on validation studies, the pipeline showed precision and detection > 99% for SNVs in areas with coverage greater than 20× and high mapping quality.
The only pathogenic relevant variant found was a heterozygous deletion of 98.456 Kbp length in chromosome X (chrX:119504569—119603024(GRCh37/hg19) that included ATP1B4 and LAMP2 genes. In addition, one heterozygous missense variant of unknown significance (VUS) in the TSC1 gene (chr9: 135786463G>T, TSC1, NM_000368.5: c.1067C>A, NP_000359.1: p.Thr356Asn) was identified. In silico tools predict this variant to be neutral. The conservation at this position is high, and splice prediction at this position is low. This variant has not been seen previously in our laboratory. The variant is present in ClinVar and absent from HGMD. This variant is reported in gnomAD (MAF 0). This variant is not present in the homozygous state in gnomAD. According to the ACMG guidelines, the variant is classified as uncertain significance. On physical examination, no signs of tuberous sclerosis were found, and a brain MRI is expected to provide more relevant findings. Another heterozygous VOUS incidental finding (chr8:145623065G>T, CPSF1, NM_013291.3:c.2103C>A, NP_037423.2:p.Tyr701Ter, Het) was found. The CPSF1 gene is responsible for autosomal dominant Myopia-27 (MYP27), characterized by early-onset high myopia with increased axial lengths. An ophthalmic evaluation performed by a pediatric ophthalmologist determined mild myopia, and a routine follow-up was recommended
7. Discussion
Danon disease is an X-linked multisystem condition with predominant involvement of the heart, skeletal muscles, and retina, with overlying cognitive dysfunction [1]. It is caused by genetic mutations in the lysosome-associated membrane protein 2 (LAMP2) gene, resulting in LAMP2 protein dysfunction [2,3]. Thus, the genetic defect disrupts cytoplasmic trafficking and leads to the accumulation of autophagic material and glycogen in skeletal and cardiac myocytes [2,3].
The vast majority of mutations in LAMP2 are point mutations, of which NM_002294.3(LAMP2):c.928G>A (p.Val310Ile) is the most frequently encountered (described in five patients) (Figure 1a) [11].
This study reveals that an Xq24 microdeletion encompassing the entire LAMP2 gene is responsible for the Danon disease. However, multiple Xq24 microdeletions have been previously associated with Danon disease, hence reducing the novelty of this paper, as indicated by the following references [25,29,30]. Nevertheless, the unique clinical picture presented in this study opens excitedly the discussion about Danon phenotype expressivity. In 119 out of 126 pathogenic microdeletion cases reported in Clinvar in the LAMP2 site (Figure 1b, Tables S3 and S4), the deletions include LAMP2 and several other morbid genes. However, in the remaining seven reports, the deletions are relatively small and include the entire or part of the LAMP2 gene [25,29]. The microdeletion mechanism appears to involve Alu-mediated unequal recombination and chromosomal breakage points involving TA-rich repeat sequences [30].
Although LAMP2 variants with partial protein activity may be associated with a milder phenotype, there is no clear and consistent correlation between mutation type and phenotype. For example, the c.928G>A mutation, a highly variable phenotype described in the reported cases inclues learning and movement difficulties in infancy with a diagnosis of hypertrophic cardiomyopathy (HCM) at the age of 13; a typical triad of Danon disease; an unusual presentation of autism, and normal cardiac evaluation [11]. Further, Bottillo et al. [30] investigated the association between mutation types and clinical symptoms. They described that missense mutations were associated with a lower incidence of cardiomyopathy; truncation mutations had the earliest onset, followed by splicing mutations, and missense mutations had the latest onset, which is consistent with the findings of D’souza et al. [7]. Similarly, Fu L et al. [31] suggested that truncating mutations may lead to an earlier onset and more severe phenotypes [7,11,30,31].
The underlying mechanism for a pre-excitation abnormality may be linked to abnormal autophagy [15]. Danon-disease-patient-specific iPSC-based generated models have demonstrated that failure to clear the autophagic residue, rather than over-activation of the autophagy signaling pathway, is responsible for the accumulation of autophagy materials inside the affected cardiomyocytes [4,15]. Preliminary studies show that the correction of the autophagy defect ameliorates pathogenic pathways involved in Danon disease [4,32].
Currently, there are no available genetic nor biological therapies in Danon disease. Since the underlying mechanism of Danon disease is the blockade of autophagy, new drugs associated with autophagy, including autophagy activators and inhibitors, may be used in the near future to design new intervention strategies [15,32,33]. Moreover, in a recent study, Manso AM et al. [33] demonstrated the efficacy of a human LAMP2B gene transfer using a recombinant adeno-associated virus 9 carrying human LAMP2B (AAV9.LAMP2B) in a LAMP2 knockout mouse, a Danon disease model. Hence, an intravenous AAV9 LAMP2B injection demonstrated a dose-dependent restoration of human LAMP2B protein in the heart, liver, and skeletal muscle tissue. Consequently, correction of the impaired autophagic flux (evidenced by increased LC3-II) was induced by a LAMP2B gene transfer in all tissues [33]. Future studies are needed to shed the light on the relevance of this strategy and its applicability in humans.
The mild phenotype in our affected 42-year-old male patient with entire LAMP2 gene deletion is unexpected. Specifically, our male patient ended his fourth decade without any signs of heart failure and with mainly an ophthalmic phenotype of visual impairment mainly at night and dark spaces, explained by VEP and ERG studies, which revealed rods and cones dystrophy. To the best of our knowledge, this is the first such unique case. On the contrary, his daughter had her first cardiac evaluation at 18 months, and a pattern of WPWS in the ECG was found (Figure 5), accompanied with a normal cardiac echo study. Moreover, at 3 years of age, her cardiac echo study demonstrated increased echogenicity and hypertrophy involving the papillary muscles and posterolateral and apical segments ( mp4-Case-2). Of note, her WES analysis revealed a LAMP2 deletion with no evidence for other pathogenic/likely pathogenic variants. If the TSC1 gene VUS variant found will be classified as pathogenic in future, it may explain WPWS [34], but it cannot explain the papillary muscle and posterolateral and apical segments’ hypertrophy. Moreover, there was no evidence for cardiac rhabdomyoma generally found in tuberous sclerosis or any other typical disease features. Future studies are needed to clarify and elucidate this unpredicted clinical presentation in both.
The clinical heterogeneity in female patients could be attributed to the extent of an X-chromosome inactivation pattern (XCI), which occurs in female cells. When a random XCI occurs, the overlap of nuclear domains can rescue LAMP2 expression in skeletal muscle fibers but not in cardiomyocytes (which do not regenerate), thus explaining why the majority of female patients developed cardiomyopathy but not skeletal myopathy. A random XCI pattern has been found in a variety of tissues from different patients [12,35] and different lysosomal storage diseases, including MPS-II and Fabry [33,36]. Conversely, when a skewed XCI favoring the mutant allele occurs, the majority of cardiomyocytes show LAMP2 deficiency, and the overlap of nuclear domains in skeletal muscle is insufficient, resulting in early onset and severe cardiomyopathy and myopathy. A detrimental heterogeneous distribution of LAMP2 (rather than an overall protein reduction) is crucial for the development of cardiomyopathy [35]. Eventually, our normal Case-2 XCI methylation analysis had excluded skewed XCI (meaning LAMP2 alleles are supposed to be expressed equally). However, accurate preferential LAMP2 gene expression can be evaluated in a muscle biopsy. It may be reasonable to think that despite a normal XCI study, the mutated allele was mainly expressed, and, accordingly, a diminished LAMP2 gene expression predominates.
As mentioned earlier, the vast majority of Danon disease cases are due to point mutations in LAMP2 gene. There are only a few reports of Xq24 microdeletions encompassing the LAMP2 gene being the causative factor of Danon disease [29,36]. The penetrance in Danon disease is age-dependent and has not been well studied in the literature. Given the severity of the cardiac phenotype, the penetrance is estimated as high or nearly complete in males by the second decade [7].
In this study, the clinical picture of two related patients with an Xq24 microdeletion spanning the entire LAMP2 gene was described. The male patient presented with a milder phenotypes of Danon disease, and his daughter with anearly onset phenotype. Together with the previous reports, they demonstrated that disease severity is more variable than was previously described. Thus, the Xq24 microdeletions can result in a milder clinical phenotype of Danon disease. These findings widen the understanding of the different mutation types leading to Danon disease and highlight the important value of the genetic testing in characterizing the phenotype and subsequent prognosis in patients with Danon disease.
Study limitations: Despite the comprehensive genetic evaluation, the exact underlying mechanism of this unique clinical picture of our Danon patients is still not fully understood. In the next paragraph, important points are raised in order to elaborate this unresolved issue.
In Danon disease, like in many other genetic disorders, disease penetrance and phenotype expressivity are variable [7,11,31,35,37]. This can be reflected by variable clinical presentation in individuals and familial cases having the same genetic variant [35,38,39,40,41,42,43]. Moreover, the genotype–phenotype correlation is well established in some genetic disorders, but no such correlation exists in others [38,39,40,41,42,43]. In Danon disease, there is controversy regarding the genotype–phenotype correlation [7,11,31,37].
It is reasonable to think that genetic/genomic variants (including coding and no-coding genes and intra- and intergenic alterations), epigenetics, environmental factors, and lifestyle [35] determine the gene expression profile which influences and determine protein–protein interactions and, consequently, contributes to the clinical picture and phenotypic heterogeneity and penetrance [35].
Moreover, in few genetic disorders the pseudogenes have an impact on phenotype severity. Spinal muscular atrophy (SMA) as a prototype is an excellent example, where, the majority of SMA patients are homozygous for SMN1-exons 7-8 deletion; however, phenotype severity is directly influenced by SMN2 (SMN1 pseudogene) copies that determine SMA subtype and can ameliorate the SMA phenotype [38]. Three paralogs, LAMP1, LAMP2, and LAMP3, are known. On the one hand, there is a pseudogene for LAMP1 that was mapped to 12p13.3 [44], but, in contrast, there is no registered pseudogene for either LAMP2 or LAMP3. Thus, the speculation that LAMP2 deletion may be compensated by pseudogene expression remains theoretical. Moreover, the probability of compensation for LAMP2 ablation by its paralogs is low. Hence, in a recent publication [44], the authors demonstrated that the expression of LAMP1 and LAMP2 is often associated with protection from lysosomal membrane permeabilization [44]. Meanwhile, LAMP3 expression is associated with degradation of LAMP1, thus increasing lysosomal membrane permeabilization, inhibiting autophagy, and contributing to cell death; no such correlation between LAMP2 and LAMP3 was found [44].
Finally, the epigentics should be considered. In a Ng, K.M 2016 publication [4], it was demonstrated that downregulation of DNA methylation can ameliorate autophagy failure seen in Danon disease [4]. These findings encourage us to raise a question about the genomic DNA methylation (known as genomic episignature) profile of Case 1 patient compared with that of other Danon patients. We assume that his genomic episignature is be different and comparable to that found in mild patients rather than in severe Danon patients. This conjecture can be supported by plenty of publications in different genetic disorders [45,46,47].
The clinical phenotype was directly influenced by the episignature profile, and unique diagnostic episignature patterns were found in several entities, including neurodevelopmental disorders [45,46,47,48,49]. In conclusion, the phenotypic spectrum and genotype–phenotype correlations are strongly linked with the whole-genome methylation signature [48,49,50]. Future analyses with whole-genome sequencing, genomic epsignature, and gene expression profile in our Danon patients may contribute to further understanding.
Acknowledgments
The authors are grateful to the patients for participating in the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes14081539/s1, Figure S1: The Case’s 1 ECG results at the age of 15 years old, showing the Wolf Parkinson white (WPW) pattern; Table S1: HGMD LAMP2 mutation list as described in professional HGMD; Table S2: ClinVar 1 LAMP2 pathogenic variants; Table S3: ClinVar 2 LAMP2 likely pathogenic variants; Table S4: ClinVar 3 LAMP2 missense variants; Table S5: ClinVar 4 LAMP2 nonsense variants; Table S6: ClinVar 5 LAMP2 frameshift variants; Table S7: ClinVar 6 LAMP2 splice variants; Video S1: Case 1 mp4-a: Patient 1, Echocardiography, short axis view of the left ventricle showing mild hypertrophy, increased echogenicity, and normal contraction; Case 1—mp4-b: Four-chambers view (4cv) showing left ventricular (LV) hypertrophy with lateral wall involvement; septum, normal contraction, increased echogenicity, left atrial dilatation; Video S2: 4cv zoom of the LV; Case 2—Echocardiography of Case 2; mp4-a: Four-chambers view: mild LV hypertrophy with lateral wall and papillary muscle involvement; mp4-b: Short-axis view of LV: papillary muscle hypertrophy, normal systolic contraction.
Author Contributions
Conceptualization and formal analysis, A.S., T.B.-Y., Y.H., M.M., L.Y., A.L., E.R. and J.G.; resources, A.S. and M.B.-S.; methodology: A.S., Y.H., M.M. and T.B.-Y.; writing—original draft, review and editing: A.S., M.B.-S. and L.Y.; mutation files’ preparation and figure construction and editing: M.M., Z.E.S. and H.M.; supervision, project administration, and funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All described data are provided upon request.
Conflicts of Interest
The authors declare that the case report was written in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Funding Statement
The project was funded by Ginatuna, a non-profit organization in Sakhnin City, Israel.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cenacchi G., Papa V., Pegoraro V., Marozzo R., Fanin M., Angelini C. Review: Danon Disease: Review of Natural History and Recent Advances. Neuropathol. Appl. Neurobiol. 2020;46:303–322. doi: 10.1111/nan.12587. [DOI] [PubMed] [Google Scholar]
- 2.Chi C., Leonard A., Knight W., Beussman K., Zhao Y., Cao Y., Londono P., Aune E., Trembley M., Smal E., et al. Lamp-2b Regulates Human Cardiomyocyte Function by Mediating Autophagosome-Lysosome Fusion. Proc. Natl. Acad. Sci. USA. 2019;116:556–565. doi: 10.1073/pnas.1808618116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino I., Fu J., Tanji K., Yamada T., Shimojo S., Koori T., Mora M., Riggs J.E., Oh S.J., Koga Y., et al. Primary LAMP-2 Deficiency Causes X-Linked Vacuolar Cardiomyopathy and Myopathy (Danon Disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 4.Ng K.M., Mok P.Y., Butler A.W., Ho J.C.Y., Choi S.W., Lee Y.K., Lai W.H., Au K.W., Lau Y.M., Wong L.Y., et al. Amelioration of X-Linked Related Autophagy Failure in Danon Disease with DNA Methylation Inhibitor. Circulation. 2016;134:1373–1389. doi: 10.1161/CIRCULATIONAHA.115.019847. [DOI] [PubMed] [Google Scholar]
- 5.Hashem S.I., Perry C.N., Bauer M., Han S., Clegg S.D., Ouyang K., Deacon D.C., Spinharney M., Panopoulos A.D., Izpisua Belmonte J.C., et al. Brief Report: Oxidative Stress Mediates Cardiomyocyte Apoptosis in a Human Model of Danon Disease and Heart Failure. Stem Cells. 2015;33:2343–2350. doi: 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z., McMahon C.J., Smith L.R., Bersola J., Adesina A.M., Breinholt J.P., Kearney D.L., Dreyer W.J., Denfield S.W., Price J.F., et al. Danon Disease as an Underrecognized Cause of Hypertrophic Cardiomyopathy in Children. Circulation. 2005;112:1612–1617. doi: 10.1161/CIRCULATIONAHA.105.546481. [DOI] [PubMed] [Google Scholar]
- 7.D’souza R.S., Levandowski C., Slavov D., Graw S.L., Allen L.A., Adler E., Mestroni L., Taylor M.R.G. Danon Disease Clinical Features, Evaluation, and Management. Circ. Heart Fail. 2014;7:843–849. doi: 10.1161/CIRCHEARTFAILURE.114.001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Chen X., Wang F., Liang Y., Deng H., Liao H., Zhang Q., Zhang B., Zhan X., Fang X., et al. Prevalence and Clinical Characteristics of Danon Disease among Patients with Left Ventricular Hypertrophy and Concomitant Electrocardiographic Preexcitation. Mol. Genet. Genom. Med. 2019;7:e638. doi: 10.1002/mgg3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schorderet D.F., Cottet S., Lobrinus J.A., Borruat F.X., Balmer A., Munier F.L. Retinopathy in Danon Disease. Arch. Ophthalmol. 2007;125:231–236. doi: 10.1001/archopht.125.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Prall F.R., Drack A., Taylor M., Ku L., Olson J.L., Gregory D., Mestroni L., Mandava N. Ophthalmic Manifestations of Danon Disease. Ophthalmology. 2006;113:1010–1013. doi: 10.1016/j.ophtha.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Xu J., Li Z., Liu Y., Zhang X., Niu F., Zheng H., Wang L., Kang L., Wang K., Xu B. Danon Disease: A Case Report and Literature Review. Diagn. Pathol. 2021;16:39. doi: 10.1186/s13000-021-01100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brambatti M., Caspi O., Maolo A., Koshi E., Greenberg B., Taylor M.R.G., Adler E.D. Danon Disease: Gender Differences in Presentation and Outcomes. Int. J. Cardiol. 2019;286:92–98. doi: 10.1016/j.ijcard.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Sugie K., Komaki H., Onoue K., Eura N., Shiota T., Tsukaguchi H., Namatame S., Koito H., Kiriyama T., Saito Y., et al. Clinicopathological Features and Management of Danon Disease in Japan: A Nationwide Survey. Neuromuscul. Disord. 2017;27:S205. doi: 10.1016/j.nmd.2017.06.403. [DOI] [Google Scholar]
- 14.Boucek D., Jirikowic J., Taylor M. Natural History of Danon Disease. Genet. Med. 2011;13:563–568. doi: 10.1097/GIM.0b013e31820ad795. [DOI] [PubMed] [Google Scholar]
- 15.Rowland T.J., Sweet M.E., Mestroni L., Taylor M.R.G. Danon Disease—Dysregulation of Autophagy in a Multisystem Disorder with Cardiomyopathy. J. Cell Sci. 2016;129:2135–2143. doi: 10.1242/jcs.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugie K., Nishino I. Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease: Fifth Edition. Academic Press; Cambridge, MA, USA: 2014. Lysosomal Membrane Disorders: LAMP-2 Deficiency. [Google Scholar]
- 17.Yardeni M., Weisman O., Mandel H., Weinberger R., Quarta G., Salazar-Mendiguchía J., Garcia-Pavia P., Lobato-Rodríguez M.J., Simon L.F., Dov F., et al. Psychiatric and Cognitive Characteristics of Individuals with Danon Disease (LAMP2 Gene Mutation) Am. J. Med. Genet. A. 2017;173:2461–2466. doi: 10.1002/ajmg.a.38320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuneu C.M., Soria V., Gascón-Bayarri J. Psychotic Symptoms in Danon Disease: A Clinical Case Report. Eur. Psychiatry. 2021;64:S736. doi: 10.1192/j.eurpsy.2021.1950. [DOI] [Google Scholar]
- 19.Miani D., Fresco C., Muser D., Driussi M., Nalli C., Tursi V., Livi U., Proclemer A. Long Term Follow up after Heart Transplantation in Danon Disease. Eur. J. Heart Fail. 2015;17:244–245. [Google Scholar]
- 20.Di Nora C., Miani D., D’Elia A.V., Poli S., Iascone M., Nucifora G., Finato N., Sponga S., Proclemer A., Livi U. Heart Transplantation in Danon Disease: Long Term Single Centre Experience and Review of the Literature. Eur. J. Med. Genet. 2020;63:103645. doi: 10.1016/j.ejmg.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Salisbury D., Meredith K. Neuropsychological Functioning Following Cardiac Transplant in Danon Disease. Dev. Neurorehabilit. 2019;22:67–70. doi: 10.1080/17518423.2017.1326184. [DOI] [PubMed] [Google Scholar]
- 22.Taylor M.R.G., Adler E.D. GeneReviews®. University of Washington; Seattle, WA, USA: 2020. Danon Disease—GeneReviewsTM—NCBI Bookshelf. [PubMed] [Google Scholar]
- 23.Ikami Y., Terasawa K., Watabe T., Yokoyama S., Hara-Yokoyama M. The Two-Domain Architecture of LAMP2A within the Lysosomal Lumen Regulates Its Interaction with HSPA8/Hsc70. Autophagy Rep. 2022;1:205–209. doi: 10.1080/27694127.2022.2069968. [DOI] [PubMed] [Google Scholar]
- 24.Endo Y., Furuta A., Nishino I. Danon Disease: A Phenotypic Expression of LAMP-2 Deficiency. Acta Neuropathol. 2015;129:391–398. doi: 10.1007/s00401-015-1385-4. [DOI] [PubMed] [Google Scholar]
- 25.Vatta M., Yang Z., Funke B.H., Cripe L.H., Vick G.W., Mancini-Dinardo D., Peña L.S., Kanter R.J., Wong B., Westerfield B.H., et al. LAMP2 Microdeletions in Patients With Danon Disease. Circ. Cardiovasc. Genet. 2010;3:129–137. doi: 10.1161/CIRCGENETICS.109.901785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen R.C., Zoghbi H.Y., Moseley A.B., Rosenblatt H.M., Belmont J.W. Methylation of HpaII and HhaI Sites near the Polymorphic CAG Repeat in the Human Androgen-Receptor Gene Correlates with X Chromosome Inactivation. Am. J. Hum. Genet. 1992;51:1229. [PMC free article] [PubMed] [Google Scholar]
- 28.Kalia S.S., Adelman K., Bale S.J., Chung W.K., Eng C., Evans J.P., Herman G.E., Hufnagel S.B., Klein T.E., Korf B.R., et al. Corrigendum: Recommendations for Reporting of Secondary Findings in Clinical Exome and Genome Sequencing, 2016 Update (ACMG SF v2.0): A Policy Statement of the American College of Medical Genetics and Genomics. Genet. Med. 2017;19:484–485. doi: 10.1038/gim.2017.17. [DOI] [PubMed] [Google Scholar]
- 29.Majer F., Piherova L., Reboun M., Stara V., Pelak O., Norambuena P., Stranecky V., Krebsova A., Vlaskova H., Dvorakova L., et al. LAMP2 Exon-Copy Number Variations in Danon Disease Heterozygote Female Probands: Infrequent or Underdetected? Am. J. Med. Genet. A. 2018;176:2430–2434. doi: 10.1002/ajmg.a.40430. [DOI] [PubMed] [Google Scholar]
- 30.Bottillo I., Giordano C., Cerbelli B., D’Angelantonio D., Lipari M., Polidori T., Majore S., Bertini E., D’Amico A., Giannarelli D., et al. A Novel LAMP2 Mutation Associated with Severe Cardiac Hypertrophy and Microvascular Remodeling in a Female with Danon Disease: A Case Report and Literature Review. Cardiovasc. Pathol. 2016;25:423–431. doi: 10.1016/j.carpath.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Fu L., Luo S., Cai S., Hong W., Guo Y., Wu J., Liu T., Zhao C., Li F., Huang H., et al. Identification of LAMP2 Mutations in Early-Onset Danon Disease With Hypertrophic Cardiomyopathy by Targeted Next-Generation Sequencing. Am. J. Cardiol. 2016;118:888–894. doi: 10.1016/j.amjcard.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Dvornikov A.V., Wang M., Yang J., Zhu P., Le T., Lin X., Cao H., Xu X. Phenotyping an Adult Zebrafish Lamp2 Cardiomyopathy Model Identifies MTOR Inhibition as a Candidate Therapy. J. Mol. Cell. Cardiol. 2019;133:199–208. doi: 10.1016/j.yjmcc.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manso A.M., Hashem S.I., Nelson B.C., Gault E., Soto-Hermida A., Villarruel E., Brambatti M., Bogomolovas J., Bushway P.J., Chen C., et al. Systemic AAV9.LAMP2B Injection Reverses Metabolic and Physiologic Multiorgan Dysfunction in a Murine Model of Danon Disease. Sci. Transl. Med. 2020;12:eaax1744. doi: 10.1126/scitranslmed.aax1744. [DOI] [PubMed] [Google Scholar]
- 34.Řeboun M., Rybová J., Dobrovolný R., Včelák J., Veselková T., Štorkánová G., Mušálková D., Hřebíček M., Ledvinová J., Magner M., et al. X-Chromosome Inactivation Analysis in Different Cell Types and Induced Pluripotent Stem Cells Elucidates the Disease Mechanism in a Rare Case of Mucopolysaccharidosis Type Ll in a Female. Folia Biol. Czech Repub. 2016;62:82. doi: 10.14712/fb2016062020082. [DOI] [PubMed] [Google Scholar]
- 35.Kingdom R., Wright C.F. Incomplete Penetrance and Variable Expressivity: From Clinical Studies to Population Cohorts. Front. Genet. 2022;13:920390. doi: 10.3389/fgene.2022.920390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majer F., Kousal B., Dusek P., Piherova L., Reboun M., Mihalova R., Gurka J., Krebsova A., Vlaskova H., Dvorakova L., et al. Alu-Mediated Xq24 Deletion Encompassing CUL4B, LAMP2, ATP1B4, TMEM255A, and ZBTB33 Genes Causes Danon Disease in a Female Patient. Am. J. Med. Genet. A. 2020;182:219–223. doi: 10.1002/ajmg.a.61416. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Chou O.H.I., Tse Y.L., Ng K.M., Tse H.F. Application of Patient-Specific Ipscs for Modelling and Treatment of x-Linked Cardiomyopathies. Int. J. Mol. Sci. 2021;22:8132. doi: 10.3390/ijms22158132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Harting J., Farrow E., Thiffault I., Kasperaviciute D., Hoischen A., Gilissen C., Pastinen T., Eberle M.A. Comprehensive SMN1 and SMN2 Profiling for Spinal Muscular Atrophy Analysis Using Long-Read PacBio HiFi Sequencing. Am. J. Hum. Genet. 2023;110:240–250. doi: 10.1016/j.ajhg.2023.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Jimeno C., Blanco-Kelly F., López-Grondona F., Losada-Del Pozo R., Moreno B., Rodrigo-Moreno M., Martinez-Cayuelas E., Riveiro-Alvarez R., Fenollar-Cortés M., Ayuso C., et al. Attention Deficit Hyperactivity and Autism Spectrum Disorders as the Core Symptoms of AUTS2 Syndrome: Description of Five New Patients and Update of the Frequency of Manifestations and Genotype-Phenotype Correlation. Genes. 2021;12:1360. doi: 10.3390/genes12091360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaygusuz S.B., Alavanda C., Kirkgoz T., Eltan M., Yavas Abali Z., Helvacioglu D., Guran T., Ata P., Bereket A., Turan S. Does Genotype–Phenotype Correlation Exist in Vitamin D-Dependent Rickets Type IA: Report of 13 New Cases and Review of the Literature. Calcif. Tissue Int. 2021;108:576–586. doi: 10.1007/s00223-020-00784-2. [DOI] [PubMed] [Google Scholar]
- 41.Pascual-Alonso A., Martínez-Monseny A.F., Xiol C., Armstrong J. MECP2-Related Disorders in Males. Int. J. Mol. Sci. 2021;22:9610. doi: 10.3390/ijms22179610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutiérrez-Cerrajero C., Sprecher E., Paller A.S., Akiyama M., Mazereeuw-Hautier J., Hernández-Martín A., González-Sarmiento R. Ichthyosis. Nat. Rev. Dis. Primer. 2023;9:2. doi: 10.1038/s41572-022-00412-3. [DOI] [PubMed] [Google Scholar]
- 43.Fan Y., Xu Y., Shi C. NOTCH2NLC-Related Disorders: The Widening Spectrum and Genotype–Phenotype Correlation. J. Med. Genet. 2022;59:1–9. doi: 10.1136/jmedgenet-2021-107883. [DOI] [PubMed] [Google Scholar]
- 44.Mattei M.G., Matterson J., Chen J.W., Williams M.A., Fukuda M. Two Human Lysosomal Membrane Glycoproteins, h-Lamp-1 and h-Lamp-2, Are Encoded by Genes Localized to Chromosome 13q34 and Chromosome Xq24-25, Respectively. J. Biol. Chem. 1990;265:7548–7551. doi: 10.1016/S0021-9258(19)39148-3. [DOI] [PubMed] [Google Scholar]
- 45.Sadikovic B., Aref-Eshghi E., Levy M.A., Rodenhiser D. DNA Methylation Signatures in Mendelian Developmental Disorders as a Diagnostic Bridge between Genotype and Phenotype. Epigenomics. 2019;11:563–575. doi: 10.2217/epi-2018-0192. [DOI] [PubMed] [Google Scholar]
- 46.Lee S., Menzies L., Hay E., Ochoa E., Docquier F., Rodger F., Deshpande C., Foulds N.C., Jacquemont S., Jizi K., et al. Epigenotype-Genotype–Phenotype Correlations in SETD1A and SETD2 Chromatin Disorders. Hum. Mol. Genet. 2023:ddad079. doi: 10.1093/hmg/ddad079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aref-Eshghi E., Kerkhof J., Pedro V.P., Barat-Houari M., Ruiz-Pallares N., Andrau J.-C., Lacombe D., Van-Gils J., Fergelot P., Dubourg C., et al. Evaluation of DNA Methylation Episignatures for Diagnosis and Phenotype Correlations in 42 Mendelian Neurodevelopmental Disorders. Am. J. Hum. Genet. 2020;106:356–370. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dingemans A.J.M., Truijen K.M.G., van de Ven S., Bernier R., Bongers E.M.H.F., Bouman A., de Graaff—Herder L., Eichler E.E., Gerkes E.H., De Geus C.M., et al. The Phenotypic Spectrum and Genotype-Phenotype Correlations in 106 Patients with Variants in Major Autism Gene CHD8. Transl. Psychiatry. 2022;12:421. doi: 10.1038/s41398-022-02189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foroutan A., Haghshenas S., Bhai P., Levy M.A., Kerkhof J., McConkey H., Niceta M., Ciolfi A., Pedace L., Miele E., et al. Clinical Utility of a Unique Genome-Wide DNA Methylation Signature for KMT2A-Related Syndrome. Int. J. Mol. Sci. 2022;23:1815. doi: 10.3390/ijms23031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Laan L., Rooney K., Trooster T.M., Mannens M.M., Sadikovic B., Henneman P. DNA Methylation Episignatures: Insight into Copy Number Variation. Epigenomics. 2022;14:1373–1388. doi: 10.2217/epi-2022-0287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All described data are provided upon request.