Abstract
The early identification of women with an increased risk of preeclampsia (PE) is desirable, but apart from soluble fms-like tyrosine kinase-1 (sFlt-1), few biomarkers have previously been identified as relevant for predicting preeclampsia. Since kinases and phosphatases regulate critical biological processes and previous evidence suggests a potential role of these molecules in preeclampsia, we performed this systematic review and metanalysis. The objective was to determine if there are kinases and phosphatases whose serum levels are different between women with and without PE, being relevant biomarkers of PE. We followed the recommendations of Cochrane and the Preferred Reported Items for Systematic Reviews and Metanalysis (PRISMA) to perform this study. The MESH terms preeclampsia, kinases, phosphatases, angiopoietins, soluble tyrosine protein kinase receptor (sTIE2), and cellular-mesenchymal-epithelial transition factor (c-MET) were combined to find relevant articles in the PubMed, PROSPERO, and Cochrane databases. Then, a qualitative and quantitative analysis was performed in R Studio software. From 580 abstracts identified, 37 were included in the final analysis, which comprised 24,211 pregnant women (2879 with PE and 21,332 women without PE [HP]. The pooled analysis showed that serum creatine kinase (CK) (SMD: 2.43, CI 95% 0.25–4.62) was significantly higher in PE, whereas sTIE2 and anti-angiogenic factor soluble c-Met (sMet)were significantly lower in PE than in HP (SMD: −0.23, CI95% −0.37 to −0.09; and SMD:0.24, CI95% 0.01–0.47, respectively). Adenosine monophosphate-activated protein kinase (AMPK), angiopoietin-1 (ANG-1), angiopoietin-2 (ANG-2), the ratio angiopoietin-1/angiopoietin-2, acid phosphatase, and alkaline phosphatase were not different between women with PE and HP. In summary CK, sTIE2, and c-MET are relevant biomarkers of PE. It is desirable to incorporate them into current models for PE prediction to evaluate their utility as biomarkers.
Keywords: biomarkers, preeclampsia, serum kinases, sTIE2, c-MET, CK
1. Introduction
Preeclampsia (PE) is a multisystemic syndrome affecting 3–5% of all pregnant women, and is characterized by new-onset hypertension associated with organ dysfunction after 20 weeks of gestation, which remains a significant cause of maternal morbidity and mortality [1,2].
Although the etiology of preeclampsia remains incompletely uncovered, some maternal and placental factors are involved in its pathogenesis [3,4]. Under normal conditions, the syncytiotrophoblast releases molecules like vascular endothelial growth factor (VEGF), placental growth factor (PIGF), transforming growth factor-beta (TGF-β), and insulin-like growth factor-1 (IGF-1) that regulate vascular function and help to maintain adequate blood flow to the placenta [4,5]. However, factors inducing oxidative stress, endothelial dysfunction, and inflammation can cause damage to the syncytiotrophoblast, causing a disbalance in the release of growth factors, cytokines, and their soluble forms, including soluble placental growth factor 1 (sFlt-1), VEGF, and PIGF [6]. Consequently, there is a reduction in new blood vessel formation, abnormal placentation, and increased blood pressure, resulting in preeclampsia [7].
sFlt-1 is a circulating antiangiogenic protein that binds to VEGF and PlGF. This interaction disrupts the VEGF pathway, leading to disturbances in endothelial and cellular homeostasis [8]. Preeclampsia is characterized by an imbalance between pro-angiogenic (VEGF or PlGF) and antiangiogenic (sFlt-1) factors in the placenta, resulting in reduced blood flow [9,10]. In pregnant women with preeclampsia, circulating serum levels of sFlt-1 are increased, while PlGF serum concentrations are decreased. The sFlt-1/PlGF ratio is utilized to assess this imbalance and assist in the prediction of preeclampsia [9,10].
Although sFlt-1 is a well-recognized biomarker of PE [9,10,11], we still need to understand the pathophysiology of the disease fully and know all of the signaling pathways and molecular mechanisms involved [7].
Preclinical studies have shown that kinases and phosphatases are important regulators of angiogenesis, vascular stabilization, and endothelial function [12,13,14]. However, while the utility of sFlt-1 as a crucial kinase in preeclampsia has been demonstrated [15,16], it remains unclear whether other serum kinases and phosphatases, routinely measured in patients, could serve as promising biomarkers of the disease, or are implicated in its pathogenesis [17,18].
As a consequence, identifying emerging biomarkers could help us to improve our understanding of the disease’s pathophysiology and the performance of the current models of preeclampsia prediction that already include sFlt-1 and PIGF, or to monitor the efficacy of prophylactic interventions [17,19]. Since systematic reviews and meta-analyses are potent methods to combine and analyze all of the data available in the literature, their use is highly demanded to summarize the existing evidence of new biomarkers for diverse pathologies such as preeclampsia [20,21].
This study aims to identify kinases and phosphatases whose serum levels are different between women, with and without PE, being relevant biomarkers in PE.
2. Materials and Methods
2.1. Protocol Registration
This study was registered at the prospective international register of systematic reviews (PROSPERO: CDR439182), but no approval from the ethics committee was required to perform this systematic review and meta-analysis.
2.2. Information Sources and Search Strategy
A search in the PubMed, Cochrane Library, and PROSPERO databases was performed, limited to humans, in order to find relevant papers related to our objective, including the following keywords: preeclampsia, kinases, phosphatases, angiopoietins, sTIE2, and cMET. The first search was run on March 2023 and updated on 1 May 2023. We strictly adhered to recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for observational studies.
2.3. Eligibility Criteria
Observational studies that reported the serum levels of any kinase or phosphatase (excluding s-FLT1) in at least two groups (preeclampsia versus non-preeclampsia pregnancy) were eligible to be included in the present study. Studies were excluded if not any kinase, phosphatase, or any ratio was estimated from these biomarkers.
2.4. Study Selection
Two independent researchers (K.M. and J.R.V.B.) reviewed the abstracts, and were blinded to the authorship, authors’ affiliations, and study results. Data extraction was performed using a standardized form, including study characteristics (author, year, country, study design), participant characteristics, details of serum kinase and phosphatase measurements, and outcome measures. Any discrepancies were resolved through discussion and consensus. If the papers contained information of interest, the full texts were obtained to extract the information of interest. If serum values of a biomarker of interest were only available in graphs, we employed the R software package “digitize” to estimate the levels of such biomarkers accurately. If any disagreement existed between researchers, a third or fourth investigator resolved it. When authors did not provide the interest biomarker’s mean and SD, they were contacted via e-mail. The details of the search syntaxes are presented in Supplementary Materials Table S1.
2.5. Assessment of Risk of Bias
The Joanna Briggs Risk of Bias Case Control Tool was used to evaluate the quality of observational studies by two independent reviewers (J.T.T. and I.P.G.G.). The third and fourth evaluators resolved any reviewer disagreements (R.J.M.P. and S.E.S.). The quality of the studies was judged based on three dimensions: the selection of the study groups, the comparability of the groups, and the ascertainment of the exposure.
2.6. Data Collection and Analysis
The data of interest were collected on datasheet templates that included the following information: author, year, country where the study was conducted, the kinase or phosphatase measured, inclusion and exclusion criteria, the total number of patients and by group, the trimester of biomarker measurement, and the preeclampsia type. In addition, we obtained the means and standard deviations of serum biomarkers measured.
The serum levels of each biomarker measured in at least two studies were pooled in the meta-analysis, expressing the effect size as a standardized mean difference (SMD) using random-effect model (REM) weighting by inverse of variance, and expressed graphically by means of forest plots. The heterogeneity between studies was calculated through the τ2, Cochran’s Q, and I2 statistics. If more than five studies were found for an effect size, a Baujat analysis was performed to evaluate the heterogeneity contribution of each study to the overall effect size. A funnel plot was constructed to visually detect bias and systematic heterogeneity. A subgroup analysis was executed to detect differences in biomarkers when enough studies existed to detect differences in SMD by the trimester of gestation.
The statistical analysis was run using the meta, metafor, and metasens packages in R studio v4.2.1 (The R Foundation for Statistical Computing, Indianapolis, IN, USA).
3. Results and Discussion
3.1. Study Selection and Study Characteristics
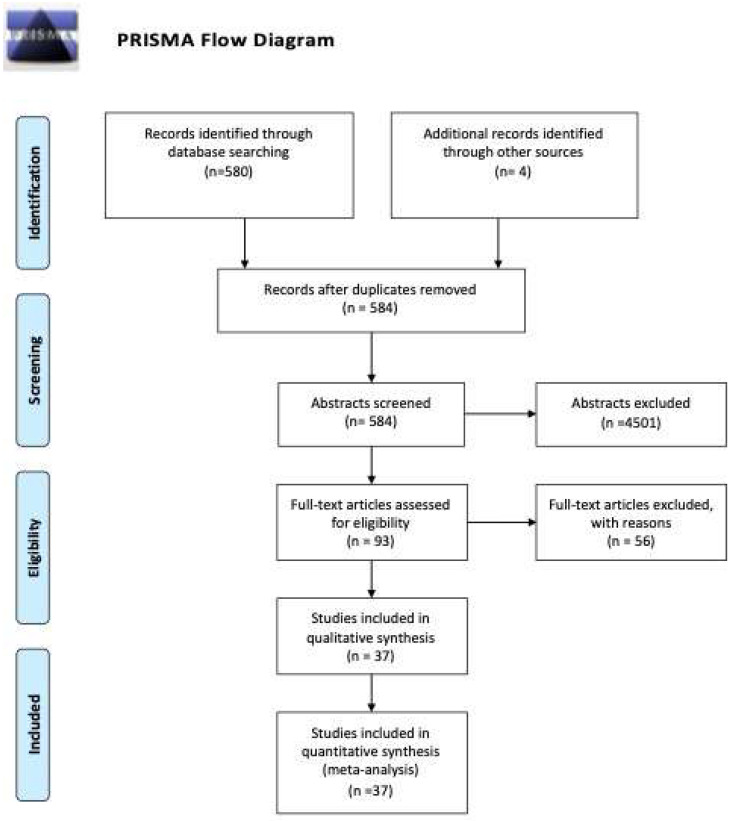
We identified a total of 595 studies through database searching, and 3 manually. After removing the duplicates, 584 abstracts were screened, and 93 studies were eligible for full-text review. Thirty-seven studies were retained for systematic review and meta-analysis. Studies were excluded if they were not case-control or cohort studies, did not provide sufficient data to calculate effect sizes, were review articles, editorials, case reports, or conference abstracts. Supplementary Materials Table S2 contains the reasons for excluding 56 studies. The PRISMA flow diagram is presented in Figure 1. The main characteristics of the included studies are shown in Table 1.
Figure 1.
PRISMA flow diagram.
Table 1.
General characteristics of the included studies.
| Author, Year | Country | Biomolecule | Total Number of Patients/PE Group/Control Group | Trimester of Measurement | Preeclampsia Type |
|---|---|---|---|---|---|
| Akolekar, 2009 [22] | UK | ANG-2 (angiopoietin-2) | 324/116/208 | First | Unspecified |
| Aoba, 1967 [23] | Japan | ALP (Alkaline Phosphatase) | 162/11/151 | Second | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | ALP (Alkaline Phosphatase) | 162/11/151 | Third | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HSAP (Heat stable alkaline phos- phatase) | 162/11/151 | Second | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HSAP (Heat stable alkaline phos- phatase) | 162/11/151 | Third | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HLP (Heat-Labile Alkaline Phosphatase) | 162/11/151 | second | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HLP (Heat-Labile Alkaline Phosphatase) | 162/11/151 | Third | Severe preeclampsia |
| Bagga, 1969 [24] | India | ALP (Alkaline Phosphatase) | 100/45/55 | Third | Unspecified |
| Bolin, 2009 [25] | Sweden | Ang1/Ang2 ratio | 62/19/43 | First | Unspecified |
| Bolin, 2009 [25] | Sweden | Ang1/Ang2 ratio | 62/19/43 | Second | Unspecified |
| Bolin, 2009 [25] | Sweden | ANG-2 (angiopoietin-2) | 62/19/43 | First | Unspecified |
| Bolin, 2009 [25] | Sweden | ANG-2 (angiopoietin-2) | 62/19/43 | Second | Unspecified |
| Bolin, 2009 [25] | Sweden | ANG-2 (angiopoietin-2) | 62/19/43 | Third | Unspecified |
| Chen, 2021 [26] | China | ALP (Alkaline Phosphatase) | 1012/31/981 | First, Second and Third | Unspecified |
| Gotsch, 2008 [27] | USA | (sTie-2) | 247/112/135 | Second and Third | Mild, severe, early and late preeclampsia |
| Han, 2012 [28] | Korea | ANG-2 (angiopoietin-2) | 45/16/29 | Third | Severe preeclampsia |
| Hirokoshi, 2005 [29] | Japan | ANG-2 (angiopoietin-2) | 55/26/29 | Second and Third | Mild and severe preeclampsia |
| Hirokoshi, 2007 [30] | Japan | ANG-2 (angiopoietin-2) | 65/36/29 | Second and Third | Mild and severe preeclampsia |
| Horjus, 2019 [31] | Netherlands | Creatine kinase (CK) | 3215/127/3088 | First and second | Early preeclampsia |
| Kamal, 2011 [32] | Egypt | ANG-2 (angiopoietin-2) | 103/68/35 | Not specified | Unspecified |
| Karakus, 2015 [33] | Germany | Ang1/Ang2 ratio | 62/25/37 | Third | Unspecified |
| Karakus, 2015 [33] | Germany | ANG-2 (angiopoietin-2) | 51/17/34 | Second and Third | Unspecified |
| Khalil, 2014 [34] | UK | ANG-2 (angiopoietin-2) | 106/22/84 | First, Second and Third | Preterm preeclampsia and term preeclampsia |
| Koroglu, 2018 [35] | Finland | Adenosine AMP-activated protein kinase (AMPK) | 80/50/30 | Third | Mild and severe preeclampsia |
| Kumar, 2011 [36] | India | sBAP (serum bone alkaline phosphatase) | 120/22/98 | Second | Unspecified |
| Leijnse, 2018 [37] | Netherlands | Ang1/Ang2 ratio | 57/6/51 | First | Late onset preeclampsia |
| Leijnse, 2018 [37] | Netherlands | ANG-2 (angiopoietin-2) | 57/6/51 | First | Late onset preeclampsia |
| Leinonen, 2009 [38] | Finland | Ang1/Ang2 ratio | 91/50/41 | Second | Mild and severe preeclampsia |
| Leinonen, 2009 [38] | Finland | Specific tyrosine kinase receptor (sTie2) | 108/49/59 | Second | Mild and severe preeclampsia |
| Leinonen, 2009 [38] | Finland | ANG-2 (angiopoietin-2) | 108/49/59 | Second | Mild and severe preeclampsia |
| Machado, 2019 [39] | Brazil | Ang1/Ang2 ratio | 120/30/90 | Second | Unspecified |
| Machado, 2019 [39] | Brazil | ANG-2 (angiopoietin-2) | 120/30/90 | Second | Unspecified |
| Martinez, 2018 [40] | Mexico | ANG-2 (angiopoietin-2) | 36/16/20 | Second | Early, late and severe preeclampsia |
| Mazibuko, 2019 [41] | South Africa | Specific tyrosine kinase receptor (sTie2) | 40/20/20 | Not specified | Unspecified |
| Morrison, 1971 [42] | USA | Creatine phosphokinase | 65/35/30 | Third | Severe preeclampsia |
| Nadar, 2005 [43] | UK | Ang1/Ang2 ratio | 99/35/64 | Third | Unspecified |
| Nadar, 2005 [43] | UK | ANG-2 (angiopoietin-2) | 99/35/64 | Third | Unspecified |
| Naghshvar, 2013 [44] | Iran | s-Met (soluble mesenchymal-epithelial transition factor) | 95/44/51 | First and second | Mild, severe, early and late preeclampsia |
| Nayel, 1982 [45] | Egypt | ALP (Alkaline Phosphatase) | 30/20/10 | Third | Severe preeclampsia |
| Puttapitakpong, 2015 [46] | Japan | ANG-2 (angiopoietin-2) | 366/25/341 | Second | Early preeclampsia |
| Aref, 2013 [47] | India | ANG-1 (angiopoietin-1) and Soluble Tie-2 receptor (sTie2) | 238/150/88 | Not specified | Mild, severe, early and late preeclampsia |
| Salgó, 1989 [48] | Hungary | Alkaline phosphatase, acid phosphatase and creatine kinase | 184/172/12 | Second and Third | Unspecified |
| Sammour, 1974 [49] | Egypt | Creatine phospho-kinase | 30/20/10 | Third | Mild and severe preeclampsia |
| Sammour, 1975 [50] | Egypt | HSP (Heat-stable alkaline phosphatase) | 30/20/10 | Third | Unspecified |
| Schneuer, 2013 [51] | Australia | ANG-2 (angiopoietin-2) | 3893/163/3730 | First | Early preeclampsia |
| Shim, 2015 [52] | Korea | Ang1/Ang2 ratio | 74/37/37 | Second | Mild and severe preeclampsia |
| Shim, 2015 [52] | Korea | ANG-2 (angiopoietin-2) | 74/37/37 | Second | Mild and severe preeclampsia |
| Shin, 2013 [53] | Seoul | sMet | 331/115/216 | Second and Third | Unspecified |
| Sung, 2011 [54] | USA | Specific tyrosine kinase receptor (sTie2) | 55/24/31 | First, Second and Third | Unspecified |
| Wang, 2011 [55] | China | ANG-2 (Angiopoietin-2) | 92/62/30 | Not specified | Moderate and severe preeclampsia |
| Watson, 1965 [56] | Australia | ALP (Alkaline Phosphatase) | 28/3/25 | Third | Unspecified |
| Watson, 1965 [56] | Australia | HSP (Heat-stable alkaline phosphatase) | 28/3/25 | Third | Unspecified |
3.2. Risk of Bias in the Included Studies
The results of the risk of bias evaluation using the Joanna Briggs Risk of Bias Case Control Tool are shown in Table 2. Most of studies had a moderate risk of bias due to some limitations in the methodology and reporting.
Table 2.
Evaluation of risk of bias.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akolekar et al., 2009 [22] | YES | YES | YES | YES | YES | YES | UNC | UNC | YES | YES | 8 |
| Aoba et al., 1967 [23] | NO | NO | UNC | NO | NO | NO | NO | NO | YES | NO | 1 |
| Bagga et al., 1969 [24] | YES | NO | NO | NO | NO | NO | NO | NO | UNC | UNC | 1 |
| Bolin et al., 2009 [25] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Chen et al., 2021 [26] | UNC | YES | YES | YES | YES | YES | YES | YES | YES | YES | 9 |
| Gotsch et al., 2008 [27] | YES | YES | YES | YES | YES | UNC | YES | YES | YES | YES | 9 |
| Han et al., 2012 [28] | YES | YES | YES | YES | YES | UNC | YES | YES | NO | YES | 8 |
| Hirokoshi et al., 2005 [29] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Hirokoshi et al., 2007 [30] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Horjus et al., 2019 [31] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10 |
| Kamal et al., 2011 [32] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Karakus et al., 2015 [33] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Khalil et al., 2014 [34] | YES | YES | YES | YES | YES | YES | YES | YES | UNC | YES | 9 |
| Koroglu et al., 2018 [35] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Kumar et al., 2011 [36] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Leinonen et al., 2009 [38] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Leijnse et al., 2018 [37] | YES | YES | YES | YES | YES | YES | NO | YES | YES | YES | 9 |
| Machado et al., 2019 [39] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Martínez et al., 2018 [40] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Mazibuko et al., 2019 [41] | YES | UNC | YES | YES | YES | NO | NO | YES | YES | UNC | 6 |
| Morrison et al., 1971 [42] | NO | NO | UNC | YES | UNC | NO | NO | UNC | YES | NO | 2 |
| Nadar et al., 2005 [43] | YES | YES | YES | YES | YES | UNC | UNC | YES | YES | YES | 8 |
| Naghshvar et al., 2013 [44] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Nayel et al., 1982 [45] | YES | UNC | UNC | NO | UNC | NO | NO | NO | YES | NO | 2 |
| Puttapitakpong et al., 2015 [46] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Aref et al., 2013 [47] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Salgo et al., 1989 [48] | NO | NO | NO | NO | UNC | NO | NO | NO | UNC | UNC | 0 |
| Sammour et al., 1974 [49] | YES | YES | UNC | YES | YES | NO | NO | YES | UNC | YES | 6 |
| Sammour et al., 1975 [50] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Schneuer et al., 2013 [51] | YES | NO | YES | UNC | UNC | NO | NO | YES | YES | YES | 5 |
| Shim et al., 2015 [52] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Sung et al., 2011 [54] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Wang et al., 2011 [55] | YES | YES | YES | YES | YES | NO | NO | YES | UNC | YES | 7 |
| Watson et al., 1965 [56] | NO | NO | YES | UNC | UNC | NO | NO | UNC | YES | UNC | 2 |
| Young et al., 1968 [57] | NO | YES | YES | YES | YES | NO | NO | YES | YES | YES | 7 |
| Kim et al., 2013 [53] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10 |
| Zeng et al., 2009 [58] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Q1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? Q2. Were cases and controls matched appropriately? Q3. Were the same criteria used for the identification of cases and controls? Q4. Was exposure measured in a standard, valid, and reliable way? Q5. Was exposure measured in the same way for cases and controls? Q6. Were confounding factors identified? Q7. Were strategies to deal with confounding factors stated? Q8. Were outcomes assessed in a standard, valid, and reliable way for cases and controls? Q9. Was the exposure period of interest long enough to be meaningful? Q10. Was appropriate statistical analysis used? | |||||||||||
Green—Low risk of bias. Yellow—Unclear risk of bias. Red—High risk of bias.
The most common sources of bias were related to the selection of controls, the ascertainment of exposure, and the comparability of cases and controls. Some studies did not adequately match cases and controls, and the criteria for selecting controls were unclear. Additionally, there was variation in how the exposure to serum kinases and phosphatases was measured, which could lead to misclassification of exposure. The outcome assessment showed a low risk of bias in most studies, as the diagnosis of preeclampsia was generally based on established criteria. However, the blinding of outcome assessments were not consistently reported, and some studies lacked blinding, which may introduce bias. The handling of confounding factors and statistical analysis were generally well-addressed in the included studies, with appropriate adjustments for confounders. Withdrawals and dropouts were also adequately addressed in most of the studies.
Based on the assessment of the risk of bias using the Joanna Briggs Risk of Bias Case Control Tool, the included studies demonstrated an overall moderate risk of bias.
3.3. Synthesis of Results
In total, 37 studies were included in this meta-analysis comprising 24,211 pregnant women (2879 with preeclampsia and 21,332 controls). Seven studies were performed in early PE, five in late PE, and one in term PE, whereas twenty-nine studies did not specify the PE subtype.
3.3.1. Kinases Significantly Related to Preeclampsia
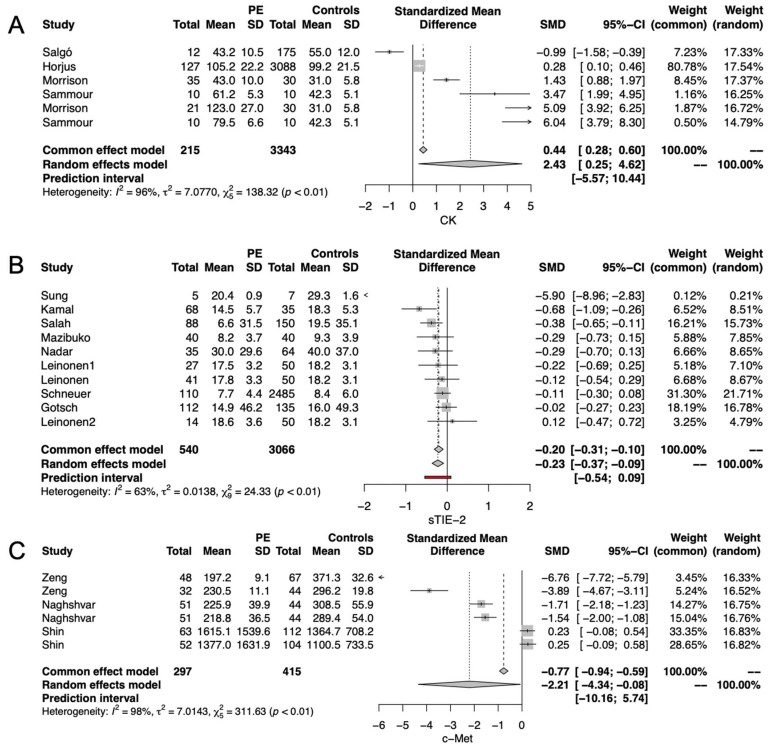
The pooled analysis of six studies demonstrated that serum creatine kinase (CK) was significantly higher in PE than in healthy pregnancies (HP) (SMD:2.43, CI 95% 0.25–4.62, p < 0.01) (Figure 2A). On the contrary, the metanalysis of eight studies proved that the soluble tyrosine protein kinase receptor (sTIE2) was significantly lower in the serum of PE than in HP (SMD: −0.23, CI 95% −0.37 to −0.09, p < 0.001) (Figure 2B). Moreover, the pooled results of three studies revealed lower c-MET serum levels in PE than in HP (SMD: −2.21, CI 95% −4.34 to −0.08, p < 0.001) (Figure 2C).
Figure 2.
Kinases significantly related to preeclampsia; (A) serum creatine kinase (CK); (B) soluble tyrosine-protein kinase receptor (sTIE2); (C) serum cellular- mesenchymal-epithelial transition factor (c-MET).
3.3.2. Kinases Non-Significantly Related to Preeclampsia
We found no significant differences in serum levels of AMPK, angiopoietin-1, angiopoietin-2, and the ratio Ang1/Ang2 between PE and HP (Supplementary Materials Figure S1).
3.3.3. Phosphatases and Preeclampsia
Alkaline phosphatase (ALP), acid phosphatase, and heat-stable alkaline phosphatase were not significantly related to preeclampsia (Supplementary Materials Figure S2).
3.3.4. Meta-Regression and Publication Bias
To explain the high I2 in CK and c-MET and the moderate I2 in the sTie2 estimates, we performed meta-regressions that introduced as covariates gestational age at measurement, maternal age, and pregestational BMI when available. The results indicate that maternal age explained 71.7% of heterogeneity in CK SMD (p = 0.0240) (Table 3). In comparison, the factor explaining the high I2 of c-MET was pregestational BMI, which accounts for 84.72% of the heterogeneity (p < 0.0001) (Table 4). None of the covariates explained the heterogeneity in the sTie2 estimates (Table 5). The funnel plot and Copas analysis revealed evidence of publication bias, and reflected heterogeneity among the studies measuring CK, c-MET, and sTie2 (Supplementary Materials Figure S3).
Table 3.
Meta-regression analysis of heterogeneity modulators in CK.
| Covariate/Modulator | Estimate | 95% CI | p-Value | R2 (%) | |
|---|---|---|---|---|---|
| Gestational Age | 0.01794 | −0.0316 | 0.3903 | 0.0956 | 26.59 |
| Maternal Age | −1.4117 | −2.6379 | −0.1856 | 0.0240 | 71.70 |
| Pregestational BMI | - | - | - | - | - |
Table 4.
Meta-regression analysis of heterogeneity modulators in c-MET.
| Covariate/Modulator | Estimate | 95% CI | p-Value | R2 (%) | |
|---|---|---|---|---|---|
| Gestational Age | −0.2335 | −0.4834 | 0.0163 | 0.0670 | 32.63 |
| Maternal Age | 0.1996 | −0.5839 | 0.9831 | 0.6176 | 0.00 |
| Pregestational BMI | −24.0521 | −35.7118 | −12.3924 | <0.001 | 84.72 |
Table 5.
Meta-regression analysis of heterogeneity modulators in sTie2.
| Covariate/Modulator | Estimate | 95% CI | p-Value | R2 (%) | |
|---|---|---|---|---|---|
| Gestational Age | −0.0078 | −0.0226 | 0.0069 | 0.2964 | 0.000 |
| Maternal Age | −0.0214 | −0.0646 | 0.0217 | 0.3295 | 0.000 |
| Pregestational BMI | −0.1046 | −0.3795 | 0.1703 | 0.9042 | 0.000 |
3.4. Main Findings
This study allowed us to identify kinases that were distinct from sFLT-1 and altered in women with preeclampsia compared to women with healthy pregnancies. These biomarkers (CK, sTIE2, and sMET) consistently show differences in women with preeclampsia, and are believed to play a plausible biological role in the development of preeclampsia. It is recommended that these biomarkers be tested in current first-trimester models of preeclampsia to assess their potential for improving the prediction of preeclampsia, and their utility in clinical practice for monitoring the effectiveness of prophylactic interventions, such as aspirin. We found no serum-relevant phosphatases in preeclampsia.
3.5. Comparison with Existing Literature and Biological Plausibility of the Findings
Despite there being no previous systematic review and metanalysis performed to identify kinases different from s-Flt-1 and phosphatases as emerging biomarkers of preeclampsia, there is biological plausibility of our findings, and our approach allows us to identify serum biomarkers consistently related to preeclampsia, as we discussed. During normal pregnancy, VEGF, PlGF, and angiopoietins (Ang) help to maintain angiogenesis and endothelial health by interacting with their endogenous endothelial receptors including the vascular endothelial growth factor receptor-1 (VEGFR-1) also called FLT1, the vascular endothelial growth factor receptor 2 (VEGFR-2) also called Kinase insert Domain Receptor (KDR), the Tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE1), and the tyrosine kinase receptor TIE2 [59,60]. However, in PE, an excessive placental secretion of sFlt1 inhibits VEGF signaling in the vasculature, resulting in endothelial cell dysfunction that contributes to PE development [61]. Based on the literature, the lower serum levels of sTIE2 in women with PE found in this systematic review and meta-analysis may be related to the decrease in VEGF signaling induced by the elevation of sFLT-1 [54,62]. Usually, VEGF induces the proteolytic cleavage and shedding of Tie2 [62]. However, we hypothesize that the increase in sFLT1, which occurs in preeclampsia, causes a decrease in VEGF, reducing the proteolytic cleavage and shedding of Tie2. In this sense, a previous study by Findley and colleagues found that decreased circulating soluble Tie2 levels in preeclampsia may result from inhibiting vascular endothelial growth factor (VEGF) signaling [54]. Nevertheless, further research is warranted to comprehensively understand sTIE2′s role in regulating angiogenesis and its impact on the pathophysiology of preeclampsia [54,62]. In another way, MET is primarily found in endothelial and epithelial cells, and participates in angiogenesis [63] during the first and second trimesters of pregnancy [64]. Thus, the pooled lower levels of sMET found in women with preeclampsia are plausible, because this may produce an underdeveloped placental vasculature, facilitating the subsequent progression to preeclampsia. Furthermore, future research involving c-MET in preeclampsia should be complemented with the quantification of liver function tests, because this biomarker is associated with liver function and is elevated in HELLP syndrome [44,58].
The findings of this systematic review and meta-analysis let us recognize potential pathways involved in preeclampsia pathogenesis (the pathways TIE-2 and c-MET), whose components, measured in sera/plasma, may be suitable as emerging biomarkers of preeclampsia. TIE-2 and c-MET participate in two independent pathways that modulate common biological processes, including angiogenesis and macrophage infiltration [65]. In another way, the elevation of CK is consistent with previous observations of single studies where CK was higher in mild and severe preeclampsia than in normal pregnancies [31]. Previous studies also found that plasma CK activity measured in early pregnancy is associated with blood pressure during pregnancy, and is related to severe gestational hypertension [31,48]. Although we cannot explain the biological plausibility of this finding, current evidence suggests that CK could be a potential biomarker in preeclampsia.
3.6. Clinical Implications
Identifying biomarkers that accurately predict the development and progression of preeclampsia is crucial for timely interventions and improving maternal and fetal outcomes. In this context, this systematic review and meta-analysis that examined influential serum kinases (non-s-Flt-1) has the following significant clinical implications:
I. Identification of Potential Biomarkers: Our findings provide valuable insights into the role of serum kinases (non-s-Flt-1) that are applicable as potential biomarkers for preeclampsia, since they are clinically different between PE and HP.
II. Diagnostic Accuracy: Our results identified CK, sTIE2, and sMET as kinases significantly related to preeclampsia, and placed them as potential biomarkers to improve the performance of current PE prediction models and enable future timely interventions.
III. Prognostic Value: Understanding the prognostic value of influential serum kinases (non-s-Flt-1) and phosphatases in preeclampsia is crucial for predicting the severity and progression of the disease. The systematic review and meta-analysis provide evidence regarding the associations between these biomarkers and adverse maternal and fetal outcomes. This knowledge enables healthcare providers to identify high-risk cases and implement appropriate monitoring and intervention strategies to mitigate potential complications.
IV. Targeted Therapies and Personalized Medicine: Since our findings identified c-MET and sTIE2 as relevant molecules in PE, targeting these molecular pathways should be tested in the future to determine if their modulation may be preventive or therapeutic targets for this disorder. Our findings contribute to the growing field of personalized medicine by identifying influential serum kinases (non-s-Flt-1) that may help to individualize patient care, as well as interventions to improve outcomes and reduce the burden of PE for the mother and the fetus.
3.7. Limitations of the Study
This study has several limitations, including the intrinsic characteristics of preeclampsia, a complex disorder influenced by various genetic, environmental, and clinical factors. In addition, through our systematic review and meta-analysis, we cannot adjust for all potential confounding factors that could influence the serum kinases and phosphatases since we do not have the complete data of each study, including liver function tests. Furthermore, the primary studies are heterogeneous in terms of their design, patient populations, methodologies, and outcome measures. While these limitations do not invalidate the findings, they highlight areas for further research and underscore the need for cautious interpretation and consideration of the broader context. Improvements in the selection of controls, standardization of exposure ascertainment, and blinding of outcome assessment would enhance the validity of future research in this area. Further studies with rigorous methodologies are needed to strengthen the evidence on the associations between serum kinases (different from s-Flt-1) or phosphatases and preeclampsia.
4. Conclusions
We identified serum CK, sTIE2, and c-MET as potentially relevant biomarkers for preeclampsia. Our findings suggest that the TIE-2 and the c-MET pathways may influence preeclampsia. Therefore, it is crucial to validate these findings through cohort studies to assess their potential for predicting preeclampsia and improve current first-trimester prediction models. It would also be desirable to investigate the impact of modulating the TIE-2 and c-MET pathways for the prevention and treatment of preeclampsia. However, it is important to interpret these findings cautiously due to limitations of the included studies, such as potential biases. Further prospective studies with larger sample sizes and standardized measurement methods are warranted to address these limitations. These studies would validate the identified biomarkers and explore their clinical utility in predicting and managing preeclampsia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241612842/s1, https://drive.google.com/drive/folders/13rBqSM_TZTSvTE-w5fzhEjDG4moSKzhW?usp=drive_link. Just send an e-mail to jvillafan@inmegen.edu.mx or torresmmf@gmail.com and a password will be provided.
Author Contributions
Conceptualization, K.C.M.-G., J.T.-T. and J.R.V.-B.; methodology, J.R.V.-B. and J.T.-T.; formal analysis, J.R.V.-B.; investigation, K.C.M.-G., I.P.G.-G. and R.J.M.-P.; writing—original draft preparation, K.C.M.-G., J.T.-T. and J.R.V.-B.; writing—review and editing, K.C.M.-G., J.T.-T., S.E.-y.-S., G.E.-G. and J.R.V.-B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data employed for conducting this metanalysis are available if requested to the following e-mails: jvillafan@inmegen.edu.mx; torresmmf@gmail.com.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Magee L.A., Nicolaides K.H., von Dadelszen P. Preeclampsia. N. Engl. J. Med. 2022;386:1817–1832. doi: 10.1056/NEJMra2109523. [DOI] [PubMed] [Google Scholar]
- 2.Espinoza J., Vidaeff A., Pettker C.M., Simhan H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020;135:e237–e260. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 3.Jung E., Romero R., Yeo L., Gomez-Lopez N., Chaemsaithong P., Jaovisidha A., Gotsch F., Erez O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022;226:S844–S866. doi: 10.1016/j.ajog.2021.11.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phipps E., Prasanna D., Brima W., Jim B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016;11:1102–1113. doi: 10.2215/CJN.12081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton G.J., Redman C.W., Roberts J.M., Moffett A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 6.Redman C.W., Staff A.C., Roberts J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2021;226:S907–S927. doi: 10.1016/j.ajog.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Dimitriadis E., Rolnik D.L., Zhou W., Estrada-Gutierrez G., Koga K., Francisco R.P.V., Whitehead C., Hyett J., Costa F.d.S., Nicolaides K., et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 2023;9:1–22. doi: 10.1038/s41572-023-00417-6. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis. 2006;9:225–230. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 9.Tanner M.S., Davey M.-A., Mol B.W., Rolnik D.L. The evolution of the diagnostic criteria of preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 2022;226:S835–S843. doi: 10.1016/j.ajog.2021.11.1371. [DOI] [PubMed] [Google Scholar]
- 10.Rana S., Burke S.D., Karumanchi S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022;226:S1019–S1034. doi: 10.1016/j.ajog.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foidart J., Schaaps J., Chantraine F., Munaut C., Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia—a step forward but not the definitive answer. J. Reprod. Immunol. 2009;82:106–111. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Drexler H.C.A., Vockel M., Polaschegg C., Frye M., Peters K., Vestweber D. Vascular Endothelial Receptor Tyrosine Phosphatase: Identification of Novel Substrates Related to Junctions and a Ternary Complex with EPHB4 and TIE2* [S] Mol. Cell. Proteom. 2019;18:2058–2077. doi: 10.1074/mcp.RA119.001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobierzewska A., Palominos M., Sanchez M., Dyhr M., Helgert K., Venegas-Araneda P., Tong S., Illanes S.E. Impairment of Angiogenic Sphingosine Kinase-1/Sphingosine-1-Phosphate Receptors Pathway in Preeclampsia. PLOS ONE. 2016;11:e0157221. doi: 10.1371/journal.pone.0157221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czikk M., Drewlo S., Baczyk D., Adamson S., Kingdom J. Dual specificity phosphatase 9 (DUSP9) expression is down-regulated in the severe pre-eclamptic placenta. Placenta. 2013;34:174–181. doi: 10.1016/j.placenta.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Dathan-Stumpf A., Rieger A., Verlohren S., Wolf C., Stepan H. sFlt-1/PlGF ratio for prediction of preeclampsia in clinical routine: A pragmatic real-world analysis of healthcare resource utilisation. PLOS ONE. 2022;17:e0263443. doi: 10.1371/journal.pone.0263443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeisler H., Llurba E., Chantraine F., Vatish M., Staff A.C., Sennström M., Olovsson M., Brennecke S.P., Stepan H., Allegranza D., et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 17.Han L., Holland O.J., Costa F.D.S., Perkins A.V. Potential biomarkers for late-onset and term preeclampsia: A scoping review. Front. Physiol. 2023;14:1143543. doi: 10.3389/fphys.2023.1143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielli M., Thomas R.C., Gillies C.L., Hu J., Khunti K., Tan B.K. Blood biomarkers to predict the onset of pre-eclampsia: A systematic review and meta-analysis. Heliyon. 2022;8:e11226. doi: 10.1016/j.heliyon.2022.e11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayeux R. Biomarkers: Potential uses and limitations. NeuroRx. 2004;1:182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahid R., Bari M.F., Hussain M. Serum biomarkers for the prediction and diagnosis of preeclampsia: A meta-analysis. J. Taibah Univ. Med Sci. 2022;17:14–27. doi: 10.1016/j.jtumed.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn E., Kang H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018;71:103–112. doi: 10.4097/kjae.2018.71.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akolekar R., Casagrandi D., Skyfta E., Ahmed A.A., Nicolaides K.H. Maternal serum angiopoietin-2 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat. Diagn. 2009;29:847–851. doi: 10.1002/pd.2307. [DOI] [PubMed] [Google Scholar]
- 23.Aoba H., Hariu Y., Yamaguchi R. Serum Heat-stable Alkaline Phosphatase in Normal and Abnormal Pregnancy. Tohoku J. Exp. Med. 1967;91:201–207. doi: 10.1620/tjem.91.201. [DOI] [PubMed] [Google Scholar]
- 24.Bagga O., Mullick V., Madan P., Dewan S. Total serum alkaline phosphate and its isoenzymes in normal and toxemic pregnancies. Am. J. Obstet. Gynecol. 1969;104:850–855. doi: 10.1016/0002-9378(69)90636-X. [DOI] [PubMed] [Google Scholar]
- 25.Bolin M., Wiberg-Itzel E., Wikström A.-K., Goop M., Larsson A., Olovsson M., Åkerud H. Angiopoietin-1/Angiopoietin-2 Ratio for Prediction of Preeclampsia. Am. J. Hypertens. 2009;22:891–895. doi: 10.1038/ajh.2009.97. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Ou W., Lin D., Lin M., Huang X., Ni S., Chen S., Yong J., O’Gara M.C., Tan X., et al. Increased Uric Acid, Gamma-Glutamyl Transpeptidase and Alkaline Phosphatase in Early-Pregnancy Associated With the Development of Gestational Hypertension and Preeclampsia. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.756140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotsch F., Romero R., Kusanovic J.P., Chaiworapongsa T., Dombrowski M., Erez O., Than N.G., Mazaki-Tovi S., Mittal P., Espinoza J., et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: Soluble Tie-2. J. Matern. Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S.Y., Jun J.K., Lee C.-H., Park J.S., Syn H.C. Angiopoietin-2: A Promising Indicator for the Occurrence of Severe Preeclampsia. Hypertens. Pregnancy. 2010;31:189–199. doi: 10.3109/10641955.2010.507844. [DOI] [PubMed] [Google Scholar]
- 29.Hirokoshi K., Maeshima Y., Kobayashi K., Matsuura E., Sugiyama H., Yamasaki Y., Masuyama H., Hiramatsu Y., Makino H. Increase of Serum Angiopoietin-2 During Pregnancy Is Suppressed in Women With Preeclampsia. Am. J. Hypertens. 2005;18:1181–1188. doi: 10.1016/j.amjhyper.2005.03.745. [DOI] [PubMed] [Google Scholar]
- 30.Hirokoshi K., Maeshima Y., Kobayashi K., Matsuura E., Sugiyama H., Yamasaki Y., Masuyama H., Hiramatsu Y., Makino H. Elevated Serum sFlt-1/Ang-2 Ratio in Women with Preeclampsia. Nephron Clin. Pr. 2007;106:c43–c50. doi: 10.1159/000101483. [DOI] [PubMed] [Google Scholar]
- 31.Horjus D.L., Bokslag A., Hooijberg F., Hutten B.A., Middeldorp S., De Groot C.J. Creatine kinase and blood pressure in women with a history of early-onset preeclampsia. Pregnancy Hypertens. 2018;15:118–122. doi: 10.1016/j.preghy.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Kamal M., El-Khayat W. Do Serum Angiopoietin-1, Angiopoietin-2, and Their Receptor Tie-2 and 4G/5G Variant of PAI-1 Gene Have a Role in the Pathogenesis of Preeclampsia? J. Investig. Med. 2011;59:1147–1150. doi: 10.2310/JIM.0b013e31822c5bdf. [DOI] [PubMed] [Google Scholar]
- 33.Karakus S., Akkar O.B., Yildiz C., Sancakdar E., Cetin M., Cetin A. Serum levels of ET-1, M30, and angiopoietins-1 and -2 in HELLP syndrome and preeclampsia compared to controls. Arch. Gynecol. Obstet. 2015;293:351–359. doi: 10.1007/s00404-015-3803-1. [DOI] [PubMed] [Google Scholar]
- 34.Khalil A., Maiz N., Garcia-Mandujano R., Elkhouli M., Nicolaides K.H. Longitudinal changes in maternal soluble endoglin and angiopoietin-2 in women at risk for pre-eclampsia. Ultrasound Obstet. Gynecol. 2014;44:402–410. doi: 10.1002/uog.13439. [DOI] [PubMed] [Google Scholar]
- 35.Koroglu N., Tola E., Yuksel I.T., Cetin B.A., Turhan U., Topcu G., Dag I. Maternal serum AMP-activated protein kinase levels in mild and severe preeclampsia. J. Matern. Neonatal Med. 2018;32:2735–2740. doi: 10.1080/14767058.2018.1448774. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A., Devi S.G., Prasad S., Kapoor S., Sharma S. Bone turnover in preeclampsia-complicated pregnancy in North Indian women. J. Obstet. Gynaecol. Res. 2011;38:172–179. doi: 10.1111/j.1447-0756.2011.01664.x. [DOI] [PubMed] [Google Scholar]
- 37.Leijnse J.E., de Heus R., de Jager W., Rodenburg W., Peeters L.L., Franx A., Eijkelkamp N. First trimester placental vascularization and angiogenetic factors are associated with adverse pregnancy outcome. Pregnancy Hypertens. 2018;13:87–94. doi: 10.1016/j.preghy.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Leinonen E., Wathén K.-A., Alfthan H., Ylikorkala O., Andersson S., Stenman U.-H., Vuorela P. Maternal Serum Angiopoietin-1 and -2 and Tie-2 in Early Pregnancy Ending in Preeclampsia or Intrauterine Growth Retardation. J. Clin. Endocrinol. Metab. 2010;95:126–133. doi: 10.1210/jc.2009-0715. [DOI] [PubMed] [Google Scholar]
- 39.Machado J., Bertagnolli T., Martins L., Freitas S., Ovidio P., Sandrim V., Cardoso V., Bettiol H., Barbieri M., Cavalli R. Role of plasma PlGF, PDGF-AA, ANG-1, ANG-2, and the ANG-1/ANG-2 ratio as predictors of preeclampsia in a cohort of pregnant women. Pregnancy Hypertens. 2019;16:105–111. doi: 10.1016/j.preghy.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Fierro M.L., Castruita-De La Rosa C., Garza-Veloz I., Cardiel-Hernandez R.M., Espinoza-Juarez M.A., Delgado-Enciso I., Castañeda-Lopez M.E., Cardenas-Vargas E., Trejo-Vázquez F., Sotelo-Ham E.I., et al. Early pregnancy protein multiplex screening reflects circulating and urinary divergences associated with the development of preeclampsia. Hypertens. Pregnancy. 2018;37:37–50. doi: 10.1080/10641955.2017.1411946. [DOI] [PubMed] [Google Scholar]
- 41.Mazibuko M., Moodley J., Naicker T. Dysregulation of circulating sTie2 and sHER2 in HIV-infected women with preeclampsia. Hypertens. Pregnancy. 2019;38:89–95. doi: 10.1080/10641955.2019.1584211. [DOI] [PubMed] [Google Scholar]
- 42.Morrison J.C., Whybrew D., Wiser W.L., Bucovaz E., Fish S.A. Enzyme levels in the serum and cerebrospinal fluid in eclampsia. Am. J. Obstet. Gynecol. 1971;110:619–624. doi: 10.1016/0002-9378(71)90240-7. [DOI] [PubMed] [Google Scholar]
- 43.Nadar S., Karalis I., Al Yemeni E., Blann A., Lip G. Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb. Haemost. 2005;94:1071–1076. doi: 10.1160/TH05-03-0167. [DOI] [PubMed] [Google Scholar]
- 44.Naghshvar F., Torabizadeh Z., Zadeh N.M., Mirbaha H., Gheshlaghi P. Investigating the Relationship between Serum Level of s-Met (Soluble Hepatic Growth Factor Receptor) and Preeclampsia in the First and Second Trimesters of Pregnancy. ISRN Obstet. Gynecol. 2013;2013:1–5. doi: 10.1155/2013/925062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nayel S.A., Enin M.A., El Hefni S.E., Khalil S.A. Serum Alkaline Phosphatase in EPH Gestosis. Asia-Oceania J. Obstet. Gynaecol. 2010;8:279–282. doi: 10.1111/j.1447-0756.1982.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 46.Puttapitakpong P., Phupong V. Combination of serum angiopoietin-2 and uterine artery Doppler for prediction of preeclampsia. Hypertens. Res. 2015;39:95–99. doi: 10.1038/hr.2015.113. [DOI] [PubMed] [Google Scholar]
- 47.Aref S., Goda H., Abdelaal E. Circulating Vascular Growth Factor (VEGF) Angiopoietin-1 (Angi-1) and Soluble Tie-2 Receptor in Pregnancy Complicated with Pre-eclampsia: A Prospective Study. J. Obstet. Gynecol. India. 2013;63:316–320. doi: 10.1007/s13224-013-0388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salgó L., Pál A. Variation in Some Enzymes in Amniotic Fluid and Maternal Serum during Pregnancy. Enzyme. 1989;41:101–107. doi: 10.1159/000469060. [DOI] [PubMed] [Google Scholar]
- 49.Sammour M.B., Fattah M.M., Ibrahim F.K., Ramadan M.A. Creatine phosphokinase activity in maternal, cord blood and placenta of normal pregnancy, and in EPH-gestosis. Biochem. Med. 1974;11:205–209. doi: 10.1016/0006-2944(74)90116-1. [DOI] [PubMed] [Google Scholar]
- 50.Sammour M.B., Ramadan M.A., Khalil F.K., Abd-El-Fattah M.M. Serum and Placental Lactic Dehydrogenase and Alkaline Phosphatase Isoenzymes in Normal Pregnancy and in Pre-Eclampsia. Acta Obstet. et Gynecol. Scand. 1975;54:393–400. doi: 10.3109/00016347509157100. [DOI] [PubMed] [Google Scholar]
- 51.Schneuer F.J., Roberts C.L., Ashton A.W., Guilbert C., Tasevski V., Morris J.M., Nassar N. Angiopoietin 1 and 2 serum concentrations in first trimester of pregnancy as biomarkers of adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 2014;210:345.e1–345.e9. doi: 10.1016/j.ajog.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Shim S.-S., Lee C.H., Jun J.K. Midtrimester maternal plasma concentrations of angiopoietin 1, angiopoietin 2, and placental growth factor in pregnant women who subsequently develop preeclampsia. Obstet. Gynecol. Sci. 2015;58:10–16. doi: 10.5468/ogs.2015.58.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S.Y., Park S.Y., Kim M.J., Lee B.Y., Han J.Y., Ryu H.M. Preeclampsia is associated with an elevation of plasma sMet concentrations in the second trimester. J. Matern. Neonatal Med. 2013;26:860–865. doi: 10.3109/14767058.2013.769952. [DOI] [PubMed] [Google Scholar]
- 54.Sung J.F., Fan X., Dhal S., Dwyer B.K., Jafari A., El-Sayed Y.Y., Druzin M.L., Nayak N.R. Decreased Circulating Soluble Tie2 Levels in Preeclampsia May Result from Inhibition of Vascular Endothelial Growth Factor (VEGF) Signaling. J. Clin. Endocrinol. Metab. 2011;96:E1148–E1152. doi: 10.1210/jc.2011-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L.-J., Chen W.-P., Peng W., Xu L., Sui A.-H., Ye Y.-H. Correlation of angiopoietin-2 and angiopoietin-2 receptor expressions in serum and placenta with preeclampsia. Zhonghua Fu Chan Ke Za Zhi. 2011;46 [PubMed] [Google Scholar]
- 56.Watson D., Weston W., Porter R. Plasma Alkaline Phosphatases in Normal and Abnormal Terminal Pregnancy. Enzym. Biol. et Clin. 1965;5:25–28. doi: 10.1159/000457971. [DOI] [PubMed] [Google Scholar]
- 57.Young B.K., Beller F.K. Plasma acid phosphatase in normal and pre-eclamptic pregnancy. Am. J. Obstet. Gynecol. 1968;101:1068–1072. doi: 10.1016/0002-9378(68)90350-5. [DOI] [PubMed] [Google Scholar]
- 58.Zeng X., Sun Y., Yang H.-X., Li D., Li Y.-X., Liao Q.-P., Wang Y.-L. Plasma level of soluble c-Met is tightly associated with the clinical risk of preeclampsia. Am. J. Obstet. Gynecol. 2009;201:618.e1–618.e7. doi: 10.1016/j.ajog.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 59.Furuya M., Kurasawa K., Nagahama K., Kawachi K., Nozawa A., Takahashi T., Aoki I. Disrupted Balance of Angiogenic and Antiangiogenic Signalings in Preeclampsia. J. Pregnancy. 2011;2011:1–10. doi: 10.1155/2011/123717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helske S., Vuorela P., Carpén O., Hornig C., Weich H., Halmesmäki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol. Hum. Reprod. 2001;7:205–210. doi: 10.1093/molehr/7.2.205. [DOI] [PubMed] [Google Scholar]
- 61.Maynard S.E., Min J.-Y., Merchan J., Lim K.-H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Findley C.M., Cudmore M.J., Ahmed A., Kontos C.D. VEGF Induces Tie2 Shedding via a Phosphoinositide 3-Kinase/Akt–Dependent Pathway to Modulate Tie2 Signaling. Arter. Thromb. Vasc. Biol. 2007;27:2619–2626. doi: 10.1161/ATVBAHA.107.150482. [DOI] [PubMed] [Google Scholar]
- 63.Kauma S., Hayes N., Weatherford S. The Differential Expression of Hepatocyte Growth Factor and Met in Human Placenta*. J. Clin. Endocrinol. Metab. 1997;82:949–954. doi: 10.1210/jcem.82.3.3806. [DOI] [PubMed] [Google Scholar]
- 64.Somerset D.A., Li X.-F., Afford S., Strain A.J., Ahmed A., Sangha R.K., Whittle M.J., Kilby M.D. Ontogeny of Hepatocyte Growth Factor (HGF) and Its Receptor (c-met) in Human Placenta: Reduced HGF Expression in Intrauterine Growth Restriction. Am. J. Pathol. 1998;153:1139–1147. doi: 10.1016/S0002-9440(10)65658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elbanna M., Orillion A.R., Damayanti N.P., Adelaiye-Ogala R., Shen L., Miles K.M., Chintala S., Ciamporcero E., Ramakrishnan S., Ku S.-Y., et al. Dual Inhibition of Angiopoietin-TIE2 and MET Alters the Tumor Microenvironment and Prolongs Survival in a Metastatic Model of Renal Cell Carcinoma. Mol. Cancer Ther. 2020;19:147–156. doi: 10.1158/1535-7163.MCT-18-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data employed for conducting this metanalysis are available if requested to the following e-mails: jvillafan@inmegen.edu.mx; torresmmf@gmail.com.