Abstract
Paraneoplastic syndromes are rarely seen in gynecological tumors especially in endometrial cancer. Early identification of paraneoplastic syndromes plays a significant role in the treatment and prognosis of cancer. Here, we reported a rare case with endometrial cancer with a 2.7 cm × 2.2 cm × 3.4 cm lesion in the posterior cervix presenting leukemoid reaction and hypercalcemia as paraneoplastic syndromes simultaneously. During the progress of the endometrial cancer, her leukocyte level rose up to 60.7 × 109/L after anti-infection treatment. Meanwhile, the patient represented a series of severe clinical situation including hypercalcemia, hypokalemia, metabolic alkalosis. and respiratory failure. Finally, the patient died of respiratory circulatory failure 2 weeks later. In addition to symptomatic treatment, possible treatment targeted on the primary tumor as early as possible might help to improve the clinical prognosis.
Keywords: Endometrial cancer, paraneoplastic syndromes, leukemoid reaction, hypercalcemia
Introduction
Endometrial cancer (EC) is one of the most common gynecologic malignancy in female reproductive system. It has been reported that there were about 320,000 patients suffering from endometrial cancer and 76,000 died each year around the world. 1 Paraneoplastic syndromes (PNS), which caused by hormones, cytokines and autoimmune responses around the para-carcinoma cells are common in malignant tumors. PNS could influence multiple systems leading to severe life-threatening outcomes. 2 Leukemoid reaction and hypercalcemia are two of the paraneoplastic syndromes in malignant cancers.3–5 In 1989, Hiller described two patients with humoral hypercalcemia diagnosed endometrial carcinoma. 6 Early awareness of the PNS in certain gynecological malignancy types would benefit for earlier monitoring the progress of the disease. Here we reported a rare endometrial cancer case presenting the PNS of leukemoid reaction and hypercalcemia at the same time.
Case report
A 66-year-old women was admitted to our hospital on July 2nd, 2018 due to the intermittent minor vaginal bleeding for more than 2 years’ duration. She was previously diagnosed hypertension and well controlled with the administration of CCB. The patient had previously undergone cesarean section and transabdominal myomectomy. On physical examination: BMI 27.34 kg/m2, no swollen lymph nodes, no common Cushing syndrome was found, increased uterus as large as 8-week-pregnancy. Transvaginal ultrasonographic examination demonstrated the endometrium was thickened by 10 mm with multiple uterine fibroids. The routine blood test indicated: WBC: 9.7 × 109/L, neutrophils: 73.2%, lymphocytes: 19.6%, hemoglobin: 153 g/L, platelet: 180 × 109/L, normal blood clotting function. On July 6th, magnetic resonance imaging (MRI) revealed nonuniform signal in pelvic cavity indicating a 2.7 cm × 2.2 cm × 3.4 cm lesion in the posterior cervix (Figure 1). Dilation and curettage (D&C) HE morphology and immunohistochemical indicated: Negative CgA, ER, PR staining, and Ki-67 70% positive which suggested Grade 3 low-differentiated endometrial carcinoma (Figure 2). Multiple pelvic lymph nodes, para-aortic, and retroperitoneal lymph nodes were noted. Tumor makers analysis on July 23th indicated: SCCA: 12.6 ng/mL, CEA: 31.09 ng/mL, CA125: 46.11 U/mL, CA199: 746 U/mL.
Figure 1.
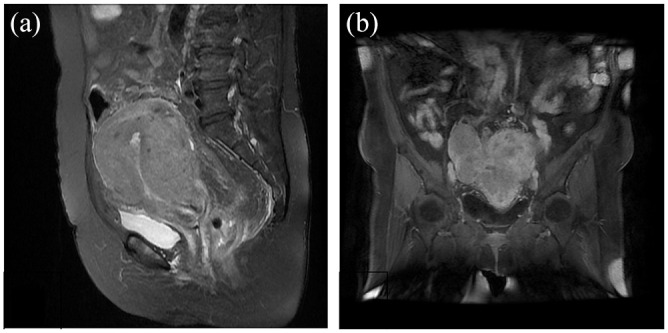
Magnetic resonance imaging of primary and recurrent cervical mass: (a) the arrow indicated a 2.7 cm × 2.2 cm × 3.4 cm lesion in posterior cervix accompanied by disappearance of the posterior fornix and (b) the arrow indicated a 68 mm × 63 mm × 58 mm mass in the vaginal cuff.
Figure 2.
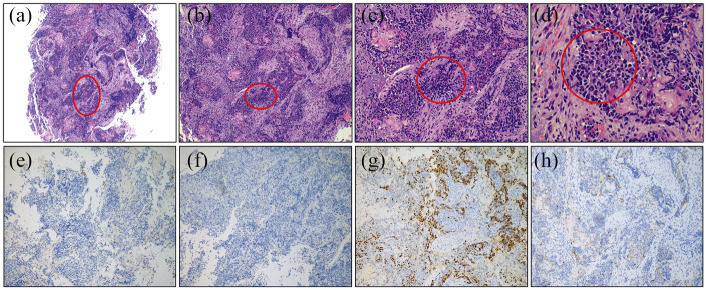
HE and immunohistochemical (ER, PR, Ki67, and CgA) staining of the patient’s endometrial tumor tissue slices: (a–d) irregular nests of neoplastic cells gathered in the uterine section (50×, 100×, 200×, 400×), (e) negative ER immunohistochemical staining 100×, (f) negative PR immunohistochemical staining 100×, (g) positive Ki67 immunohistochemical staining 100×, and (h) negative CgA immunohistochemical staining 100×.
Before the total abdominal hysterectomy, an infection was noted due to elevated leukocytes level combined with low-grade fever (37.4°C). The patient was first systemic treated with ceftriaxone and ornidazole. However, the level of WBC elevated to 34.2 × 109/L in 5 days (July 25th) and the temperature was fluctuated around 37.5°C. Five days later the antibiotics were changed to cefoperazone-sulbactam combined with fosfomycin. Meanwhile, oral potassium supplement treatment was ordered for hypokalemia (3.1 mmol/L). On July 28th, routine blood tests showed that the leukocytosis went up to 47.9 × 109/L and neutrophils rose up to 90.3%, lymphocytes went down to 6.3%. The peripheral blood smear indicated: neutrophilic segmented granulocyte: 83%, lymphocyte: 5% with myelocytes and metamyelocytes. After clinical consultation, hematologists considered there was a possibility that she might suffer from chronic granulocytic leukemia. She was treated with hydroxycarbamide and sodium bicarbonate tablets. Before the bone marrow aspiration on July 31th, the patient began to appear strong chest tightness, shortness of breath. The laboratory examinations suggested severe hypokalemia (1.6 mmol/L), combined with metabolic alkalosis (PH 7.66, HCO3− 36.4 mmol/L, BE 15.9 mmol/L), as well as severe hypoxemia (PaO2 52 mmHg, SaO2 93%). Later, the patient was transferred to ICU to receive further treatment. The result of laboratory examination before ICU was shown in Table 1.
Table 1.
Laboratory data before entering ICU.
| Normal values | Date |
|||||
|---|---|---|---|---|---|---|
| 07-06 | 07-20 | 07-23 | 07-26 | 07-31 | ||
| WBC | 4–10 × 109/L | 9.7 | 23.5 | 28.5 | 34.2 | 51.9 |
| Neutrophils (%) | 50%–70% | 73.2 | 90.1 | 87.9 | 92.1 | 89.8 |
| Nymphocyte (%) | 20%–40% | 19.6 | 6.5 | 7.7 | 4.1 | 6.9 |
| Potassium | 3.5–5.3 mmol/L | 3.5 | 3.1 | 1.6 | ||
| PCT | <0.25 ng/mL | 0.12 | 0.15 | |||
| CRP | 0–8 mg/L | 25 | 46 | 44 | 82 | |
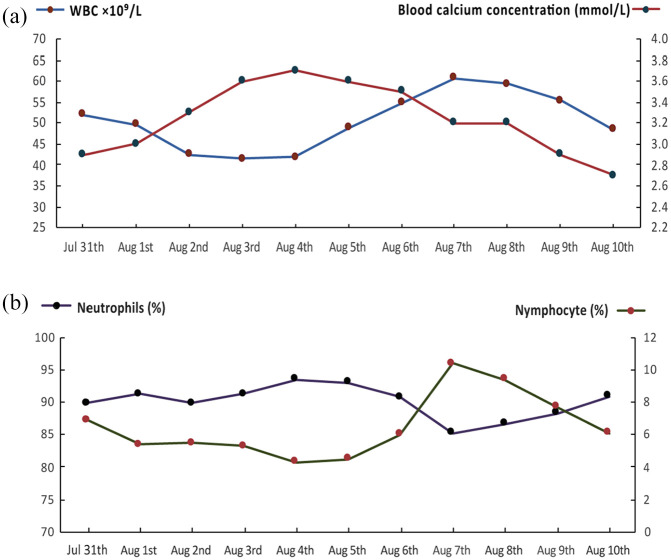
On the first day of admission to ICU, PaO2 was maintained at 50–75 mmHg after receiving NPPV. PaO2 elevated to 160 mmHg on the second day with HFNC and NPPV intermittent treatment. Blood biochemical examination still revealed severe hypercalcemia (2.9 mmol/L) and hypophosphatemia (0.3 mmol/L). The patient’s hypoxemia was improved after noninvasive positive pressure ventilation. However, all methods including diuresis, calcitonin intravenous injection, and oral diphosphonate were unable to rectify her blood calcium level. During the treatment, the calcium peak reached at 3.7 mmol/L accompanied with extremely high level of leukocyte, which rose to 60.7 × 109/L. Dynamic changes of serum calcium, leukocytes; neutrophils and lymphocytes ratios were shown in Figure 3. Results of bone marrow biopsy were shown in Table 2. All data above indicated that there was no possibility that the patient was suffering from chronic granulocytic leukemia.
Figure 3.
Dynamic changes of serum calcium, leukocytes, neutrophils, and lymphocytes ratios during the treatment process.
Table 2.
Result of bone marrow aspiration.
| Test | Results |
|---|---|
| Flow cytometric test | No increase of CD34 + primitive/immaturecells, normal in immunophenotyping analysis.Mature granulocytes account: 91.7%, normalin immunophenotyping analysis |
| Fusion gene test | P210BCR-ABL negative (−) |
| P190BCR-ABL negative (−) | |
| P230BCR-ABL negative (−) | |
| Chromosomalkaryotype analysis | No abnormal chromosome (number or structure)detected associated with tumor |
During ICU treatment, the patient’s uterus continuously enlarged to two fingers above umbilicus. Meanwhile, she was gradually developed deterioration of respiratory, circulation, and consciousness. Considering that all the symptoms of the patient were caused by terminal stage of endometrial cancer that couldn’t be reversed, her family decided to give up further treatment. The patient died of respiratory failure 2 weeks later after entering ICU unfortunately.
Discussion
Leukemoid reaction in malignant tumor
IR is one of the physiological paraneoplastic syndromes triggered by altered immune response to a neoplasm which is really rare in gynecologic malignant tumor. The occurrence of paraneoplastic leukemoid reaction was considered to be related with cytokines (such as G-CSF, GM-CSF, IL-1α, IL-6) secreted by tumor tissues.7,8 In addition to promoting the increase of white blood cells, cytokines also directly accelerate the growth of tumor tissues via paracrine. In our case, there were no significant increases in PCT and other infection indicators, no particular drugs were used during previous treatment. However, the level of leukocytes kept rising remarkably during anti-infection treatment. Meanwhile, the tumor grew progressively. No typical CML symptoms were observed in bone marrow tests. Taken together, we believed the abnormal changes in hemogram and myelogram were paraneoplastic leukemoid reactions caused by endometrial cancer. According to the report of Granger and Kontoyiannis, 9 patients diagnosed with PLR have a case-fatality rate up to 76% within 12 weeks, 89% within 1 year, the remaining 10% of the patients who survived more than 1 year have received aggressive chemotherapy or surgery for the tumor. Patients with hyperleukemia also showed a tendency to metastasize which might be related to the formation of tumor pre metastatic niches mediated by tumor secreted G-CSF and myeloid derived suppressor cells (MDSC). Clark et al. 10 reported a 65-year-old PLR symptom with recurrent endometrial cancer after surgical treatment appeared abnormal leukocyte elevation (51.4 × 109/L) and red blood cells/platelets myelosuppression during chemotherapy with elevated GCSF level. Eight months after PLR occurred, the patient died due to the intolerance to chemotherapy.
Hypercalcemia in malignant tumor
Hypercalcemia or humoral hypercalcemia of malignancy (HHM) is a common paraneoplastic syndrome, with the incidence of 10% in cancer patients. 11 Symptoms could range from mild thirst and nausea to life-threatening conditions such as kidney failure and coma. Laboratory tests reveal elevated blood calcium levels, low level of PTH, and elevated PTHrP levels accounted for approximately 80% of all tumor-associated hypercalcemia. 12 The prognosis of tumor-associated hypercalcemia is often poor, with a 30-day mortality rate up to 50%. HHM is commonly seen in solid tumors such as lung cancer, breast cancer, and bladder cancer but it is fairly rare in female malignant tumors. According the literature, approximately two-third of the ovarian small cell carcinoma would co-occur with hypercalcemia. 13 In endometrial cancer, the occurrence of hypercalcemia is very rare, only few cases combined with HHM have been reported14–17(Table 3). In our case, although the serum calcium level dropped after receiving medications, her condition was getting worse since the primary tumor was incapable to treat effectively. After all possible treatments failed, hypocalcemia treatment termination (hypercalcemia would lead to coma to death) might be an appropriate and more humane approach.
Table 3.
Pathoclinical features in the cases of endotremial cancer with hypercalcemia.
| Author | Pathology | Serum Ca2+ level | Treatment accepted | Outcome |
|---|---|---|---|---|
| Visnyei et al. 14 | ECΩ | 3 mmol/L | Bisphosphonate | Discharged |
| Nehru et al. 15 | SS& | 4.8 mmol/L | Chemotherapy+radiation | DOD# |
| Sachmech et al. 16 | PSCΔ | 3.2 mmol/L | Surgery+chemotherapy | Discharged |
| Mitchell et al. 17 | EEA$ | 3.7 mmol/L | Bisphosphonate+calcitonin | DOD |
| Current report | NCФ | 3.7 mmol/L | Bisphosphonate+calcitonin | DOD |
ECΩ: endometrioid carcinoma; SS&: stromal sarcoma; DOD#: dead of disease; PSCΔ: papillary serous carcinoma; EEA$: endometrioid endometrial adenocarcinoma; NCФ: neuroendocrine carcinoma.
Conclusion
In conclusion, we reported a rare case of a patient who had endometrial cancer co-occurred with two paraneoplastic syndromes (leukemoid reaction and hypercalcemia). For this kind of patients, the prognosis would be extremely poor without effectively anti-tumor therapy.
Author biographies
Weimin Tao received his MS in surgery from Tongji University, School of Medicine (China) in 2016. He is an attending physician of the department of anesthesiology & ICU of Shanghai First Maternity and Infant Hospital. His research interest are perioperative management of surgical patients and critical care in obstetric.
Qin Yan received his Ph.D in obstetrics and gynecology from Shanghai Jiaotong University School of Medicine(China) in 2016. Now he is an Associate Professor of Tongji University School of Medicine and is working in the department of gynecology of Shanghai First Maternity and Infant Hospital.
Yao Zhou received his BS in medcine from Xuzhou Medical University (China) in 2011. Now she is a resident physician of the department of anesthesiology & ICU of Shanghai First Maternity and Infant Hospital.
Yanli Wang received his MS in nursing from the Second Military Medical University (China) in 2020. Now she is the chief nurse of ICU of Shanghai First Maternity and Infant Hospital.
Zhiqiang Liu received his Ph.D in anesthesiology from the Second Military Medical University (China) in 2012. Now he is an Professor of Tongji University School of Medicine and is working in the department of anesthesiology & ICU of Shanghai First Maternity and Infant Hospital.
Zhendong Xu received his MS in anesthesiology in 2005 and the Ph.D in anesthesiology from the Second Military Medical University (China) in 2018. Now he is an Associate Professor of Tongji University School of Medicine and is working in Shanghai First Maternity and Infant Hospital. Currently he is the head of ICU. Research on obstetric anesthesia and critical care in obstetric. He had several papers in international journals.
Footnotes
Authors’ contribution: Zhendong Xu is in charge of the project and the writing of the thesis. Weimin Tao completed the collection and analysis of relevant literature, as well as writing the paper. Qin Yan, Yao Zhou, Yanli Wang and Zhiqiang Liu are the main participants in the treatment process, and involved in the data collection process.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval to report this case was obtained from *Shanghai First Maternity and Infant Hospital (KS20203)*.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Zhendong Xu  https://orcid.org/0000-0003-4343-8080
https://orcid.org/0000-0003-4343-8080
References
- 1.Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer 2019; 19(9): 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashour AA, Verschraegen CF, Kudelka AP, et al. Paraneoplastic syndromes of gynecologic neoplasms. J Clin Oncol 1997; 15(3): 1272–1282. [DOI] [PubMed] [Google Scholar]
- 3.Chapireau D, Adlam D, Cameron M, et al. Paraneoplastic syndromes in patients with primary oral cancers: a systematic review. Br J Oral Maxillofac Surg 2010; 48(5): 338–344. [DOI] [PubMed] [Google Scholar]
- 4.Pascual Samaniego M, Torrecilla Garcia-Ripoll JR, Calleja Escudero J, et al. [Hypercalcemia, leukemoid reaction, and thrombocytosis as paraneoplastic presentation of transitional cell carcinoma of the kidney]. Actas Urol Esp. 2001; 25(5): 400–403. [DOI] [PubMed] [Google Scholar]
- 5.Cvitkovic E, Bachouchi M, Boussen H, et al. Leukemoid reaction, bone marrow invasion, fever of unknown origin, and metastatic pattern in the natural history of advanced undifferentiated carcinoma of nasopharyngeal type: a review of 255 consecutive cases. J Clin Oncol 1993; 11(12): 2434–2442. [DOI] [PubMed] [Google Scholar]
- 6.Hiller N, Sonnenblick M, Hershko C. Paraneoplastic hypercalcemia in endometrial carcinoma. Oncology 1989; 46(1): 45–48. [DOI] [PubMed] [Google Scholar]
- 7.Wetzler M, Estrov Z, Talpaz M, et al. Granulocyte-macrophage colony-stimulating factor as a cause of paraneoplastic leukaemoid reaction in advanced transitional cell carcinoma. J Intern Med 1993; 234(4): 417–420. [DOI] [PubMed] [Google Scholar]
- 8.Hocking W, Goodman J, Golde D. Granulocytosis associated with tumor cell production of colony-stimulating activity. Blood 1983; 61(3): 600–603. [PubMed] [Google Scholar]
- 9.Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer 2009; 115(17): 3919–3923. [DOI] [PubMed] [Google Scholar]
- 10.Clark LH, Moll S, Houghton D, et al. Leukocytosis due to markedly elevated granulocyte-colony stimulating factor levels in a patient with endometrial cancer: case report and literature review. Gynecol Oncol Rep 2017; 20: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumachi F, Brunello A, Roma A, et al. Medical treatment of malignancy-associated hypercalcemia. Curr Med Chem 2008; 15(4): 415–421. [DOI] [PubMed] [Google Scholar]
- 12.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. New Engl J Med 2005; 352(4): 373–379. [DOI] [PubMed] [Google Scholar]
- 13.Estel R, Hackethal A, Kalder M, et al. Small cell carcinoma of the ovary of the hypercalcaemic type: an analysis of clinical and prognostic aspects of a rare disease on the basis of cases published in the literature. Arch Gynecol Obstet 2011; 284(5): 1277–1282. [DOI] [PubMed] [Google Scholar]
- 14.Visnyei K, Shahrokni A, Hashmi S, et al. A case of groans, moans and stones with malignant undertones: endometrioid carcinoma-associated hypercalcemia. Oncol Lett 2012; 3(2): 335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motilal Nehru V, Garcia G, Ding J, et al. Humoral hypercalcemia in uterine cancers: a case report and literature review. Am J Case Rep 2017; 18: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachmechi I, Kalra J, Molho L, et al. Paraneoplastic hypercalcemia associated with uterine papillary serous carcinoma. Gynecol Oncol 1995; 58(3): 378–382. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell E, Ciccone M, Zhang B, et al. Paraneoplastic Cushing’s syndrome and hypercalcemia arising from metastatic endometrioid endometrial adenocarcinoma: a case report. Gynecol Oncol Rep 2019; 29: 58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]