Abstract
Laboratory diagnosis based on genomic amplification methods such as PCR may provide an alternative and more sensitive method than conventional culture for the early detection of deep-seated candidiasis, an increasing cause of morbidity and mortality among immunocompromised patients. A novel method of DNA extraction from clinical samples based on treatment with proteinase K and isolation of DNA on a silica membrane was developed. The targets used for DNA amplification were the Candida albicans-secreted aspartic proteinase (SAP) genes, a multiple-gene family of at least seven members in C. albicans. A single pair of primers was designed in order to detect six of these SAP genes and, subsequently, to increase the sensitivity of the test. Detection of the PCR product by enzyme-linked immunosorbent assay was found to be as sensitive as Southern blotting with an SAP-labeled probe. The sensitivity of the assay was 1 cell/ml from serially diluted Candida cultures and 1 to 4 cells/ml from seeded blood specimens. The sensitivity and specificity of the present assay were tested in a retrospective study performed blindly with 156 clinical samples and were 100 and 98%, respectively, compared with the results of culture. For the subset of blood culture samples (n = 124), the sensitivity and the specificity were 100%. The two false-positive PCR samples came from patients treated with azole antifungal agents, indicating that PCR was probably able to detect damaged organisms that could not be recovered by culture.
Candidemia and deep-seated Candida infections are becoming a serious infectious problem. This has been demonstrated in a recent multicentric study conducted in Holland, whereby the incidence rate doubled between 1987 and 1995 (23), confirming the tendency reported earlier in the United States (1, 2, 21). Invasive Candida infections are among the most common nosocomial infections in immunocompromised patients, particularly in neutropenic patients treated for cancer or lymphoproliferative disorders, and in patients suffering from infectious complications after serious surgery (1, 17). They have been associated with increased morbidity and mortality rates and with increased lengths of hospital stay for the affected patients (25).
The laboratory diagnosis of candidemia, presently based on direct examination and conventional blood culture, is often delayed due to the relatively slow growth of these yeasts from clinical specimens. Because the clinical presentation is usually nonspecific, the clinician must often make an empiric therapeutic decision before culture results are known. A more rapid identification of Candida from clinical specimens would therefore be clinically and epidemiologically helpful.
Several studies seem to indicate that early detection of deep-seated candidiasis based on genomic amplification methods (PCR) may provide an adjunct and may be a more sensitive method than conventional culture. Buchman et al. (4) demonstrated initially that detection of Candida albicans in clinical specimens was possible by PCR by using the lanosterol-demethylase (L1A1) gene as a target for DNA amplification. Other investigators subsequently proposed other DNA targets for Candida or fungal PCR (6, 8, 10, 11, 13, 16, 22). Burgener-Kairuz et al. (5) further developed the L1A1-based PCR assay: a nested amplification of the L1A1 gene allowed the direct detection and species-level identification of four species of Candida in clinical specimens. A retrospective study conducted by this method with clinical specimens demonstrated a sensitivity of 76% and a specificity of 95% compared with the results of culture (24).
It was clear from these results that the observed sensitivity of the test, although encouraging, was insufficient for routine clinical application. The present study was conducted with the objective of increasing the sensitivity and simplifying the methodology of the PCR test so that it could be used as a routine diagnostic test. We felt that these goals could be achieved, first, by changing the DNA target of PCR amplification and, second, by optimizing the DNA preparation method. One of the means of increasing the sensitivity of the PCR is to choose as an amplification target a gene that is present in multiple copies in the organism’s genome and that is also specific for that organism. The secreted aspartic proteinase (SAP) genes fulfill those criteria, since they comprise a multigene family with at least seven members in C. albicans (15). By choosing a unique pair of primers targeted to homologous regions of the SAP genes, an approximate 10-fold increase in the threshold of sensitivity for the detection of C. albicans by PCR could reasonably be expected. In order to augment the detection of the SAP target, an optimized and simple method of preparing Candida genomic DNA was developed, as was a single-step PCR with decontamination procedures and an enzyme-linked immunosorbent assay (ELISA) detection system. The performance of this new protocol was tested with blood artificially seeded with C. albicans and true clinical specimens.
MATERIALS AND METHODS
Yeast strain.
The strain used for the optimization of the amplification procedure and for the preparation of seeded blood specimens was C. albicans SC 5314, isolated from a clinical sample (7) and designated as the reference strain for the sequencing of the C. albicans genome.
Yeast cell dilutions and seeded specimen preparation.
Yeast cells were grown in YEPD broth (1% yeast extract [Difco, Detroit, Mich.], 2% Bacto Peptone [Difco], 2% glucose) and were incubated at 30°C overnight. One milliliter of the culture was centrifuged at 11,000 × g for 10 min, and the pellet was resuspended in 1 ml of H2O. The number of yeasts in the starting suspension, as checked photometrically (A540), was quite reproducible, usually between 1 × 108 and 4 × 108/ml. Tenfold serial dilutions were obtained from this suspension by adding 100 μl of suspension to 900 μl of water to produce suspensions containing 107, 106, 105, 104, 103, 102, 101, and 100 Candida cells per ml. An aliquot of 100 μl of each dilution was plated onto a Sabouraud agar plate, and the numbers of Candida CFU were determined by obtaining colony counts after 48 h of culture. The seeded blood specimens were prepared by adding 100 μl of each of the suspensions in water mentioned above to 900 μl of healthy donor blood to produce blood suspensions containing 106, 105, 104, 103, 102, 101, and 100 Candida cells per ml.
Negative control DNA.
DNA was extracted from various bacterial and fungal species by the method described below. Except for Candida (Torulopsis) glabrata, one strain of each of the following species was used: Enterococcus sp., Staphylococcus sp., a viridans group streptococcus, Streptococcus pneumoniae, Acinetobacter baumannii, Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella sp., Pasteurella multocida, Proteus vulgaris, and Pseudomonas aeruginosa. They were cultured at the Laboratory of the Hôpital de Zone, Morges, Switzerland. DNA was extracted from other fungal species, including Aspergillus fumigatus, Candida krusei, Candida tropicalis, and Candida parapsilosis. DNAs from Listeria sp., Mycobacterium tuberculosis, and Pneumocystis carinii were obtained from the Laboratory of Clinical Microbiology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland. In addition, 11 strains of C. glabrata (ATCC 90030, ATCC 2001, and 9 clinical strains isolated at CHUV) were also tested. The human and mouse DNA controls were obtained from human leukocytes, human kidney epithelial cells (cell line 293), and mouse tail cells: 10 μl of an extraction product containing 20 ng of human or mouse DNA per μl was used for PCR amplification.
Clinical specimens.
The true clinical specimens (n = 156) investigated in this study were obtained from 27 patients and included blood cultures (n = 124), pleural fluid (n = 4), bile (n = 3), abdominal fluid (n = 22), bronchoalveolar lavage fluid (n = 2), and a skin biopsy specimen (n = 1). Longitudinal samples were obtained from 15 patients, with 2 to 17 samples obtained per patient. All samples were first cultured in the Laboratory of Clinical Microbiology, CHUV, and identification of the yeasts to the species level was carried out by following conventional procedures (12). The positive specimens were collected as follows. When a sample was found to be positive for C. albicans by culture or Gram staining, a 1.8-ml aliquot of the sample was frozen at −80°C until testing by PCR. A 1.8-ml aliquot of all specimens collected from the same patient before (if available) or after this first positive specimen were also kept and frozen. Negative specimens were added randomly and included either sterile specimens or specimens growing bacteria or yeast species other than C. albicans.
Optimization of the DNA preparation.
Five DNA preparation methods published in the literature (3, 4, 9, 19, 20) and two commercially available methods, QiAamp Tissue kit (Qiagen AG, Basel, Switzerland) and Nucleospin Cell & Tissue kit (Macherey-Nagel AG, Oensingen, Switzerland), were compared. The amount of DNA recovered was measured in serially diluted suspensions of C. albicans cells (i) by loading an aliquot of the total extracted DNA on an agarose gel, performing electrophoresis, and comparing the bands for the extract with the bands for known concentrations of a DNA marker, and (ii) after PCR amplification of an aliquot of each DNA extract, as the highest dilution which produced a visible band by agarose gel electrophoresis.
Four lysis solutions for the removal of erythrocytes from blood specimens were compared: (i) distilled water, (ii) a detergent cocktail with DNase (4), (iii) a detergent cocktail without DNase (5), and (iv) 1 N NaOH, 0.2 M citrate, and 0.4 M N-acetylcysteine. The procedure consisted of incubating the specimen on a shaker for 20 min at room temperature in a lysis solution (1 volume of blood and 1 volume of lysis solution). The lysate was centrifuged at 3,000 × g for 10 min, and the pellet was resuspended in 2 ml of the lysis solution, vortexed, and centrifuged again at 3,000 × g for 10 min. For all lysis procedures, the erythrocyte-free pellet was washed in 2 ml of 20 mM Tris-HCl (pH 8.3), centrifuged for 10 min at 3,000 × g, and resuspended in 180 μl of Qiagen ATL lysis buffer for the DNA isolation procedure.
Preparation of DNA from negative control DNA, diluted cultures, seeded blood specimens, and true clinical specimens.
The DNA extraction procedure finally adopted was a modification of the method proposed by the manufacturer of the QiAamp Tissue kit (Qiagen AG). For specimens containing a few or no erythrocytes, an aliquot of 1 ml (diluted yeast culture) or 900 μl (true clinical samples) was centrifuged directly at 11,000 × g for 10 min, and the pellet was resuspended in 180 μl of lysis buffer ATL (Qiagen) and 20 μl of proteinase K (1.7 mg/ml; Qiagen). For blood or specimens containing many erythrocytes, the special lysis procedure described above was a mandatory preliminary step, ending in the resuspension of the sample in 180 μl of lysis buffer ATL (Qiagen) to which 20 μl of proteinase K (1.7 mg/ml; Qiagen) was added. The proteinase K-ATL buffer mixture was incubated at 65°C for 1 h, and then 200 μl of buffer AL (Qiagen) was added and the sample was heated at 70°C for 10 min. After these steps, 200 μl of ethanol was added to each sample, and the suspensions were applied to QiAamp spin columns (Qiagen), centrifuged at 5,000 × g for 1 min, and washed twice with 500 μl of buffer AW (Qiagen). When DNA was extracted from blood cultures, the columns were washed twice with 50 mM EDTA and twice with buffer AW. The two additional washes with EDTA were necessary to chelate the high concentration of divalent cations which were present in the blood culture broth and which inhibited the PCR. DNA was eluted with 200 μl of buffer AE (Qiagen) preheated to 70°C. The DNA eluate obtained was again applied to the same column, incubated at 70°C for 5 min, and recentrifuged. The purified DNA preparation was then kept at −20°C until PCR.
Primers and PCR amplification.
Two C. albicans-specific oligonucleotides in the N-terminal region of the SAP product (15) were selected as primers and were prepared with a DNA synthesizer by Microsynth (Balgach, Switzerland). The sequences of these oligonucleotides are 5′-CTGCTGATATTACTGTTGGTTC-3′ (upper primer A1-6; bp 495 to 516 on SAP6 from C. albicans) and 5′-CCACCAATACCAACGGTATC-3′ (lower primer B1-6; bp 759 to 740 on SAP6 from C. albicans). These primers amplify a 263-bp fragment in the SAP genes of C. albicans. The same lot of primers was used throughout the study.
PCR was performed in a 50-μl reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.2 mM (each) deoxynucleotide triphosphates (dATP, dCTP, and dGTP; Pharmacia Biotech, Dübendorf, Switzerland), 0.4 mM dUTP (Pharmacia Biotech), 0.2 μM (each) primer, 1.5 U of Taq DNA polymerase (Perkin-Elmer International, Rotkreuz, Switzerland), and 0.5 U of uracil-DNA-glycosylase (UNG; Boehringer Mannheim, Rotkreuz, Switzerland). A 10-μl aliquot of the extracted DNA was added to the mixture. PCR was performed in a thermocycler (GeneAmp PCR system 9600; Perkin-Elmer), as follows. The activity of UNG was initiated by incubation at 50°C for 5 min. Then, the first cycle included 5 min of denaturation at 94°C, 1 min of annealing at 58°C, and 1 min of primer extension at 72°C. This first step was followed by 35 cycles of 30 s of denaturation at 94°C, 30 s of annealing at 58°C, and 1 min of primer extension at 72°C. The PCR product was then maintained at 72°C, and 5 μl of 0.5 mM EDTA was added to inactivate the UNG. A 10-μl aliquot of the amplified product was immediately analyzed on a 2% agarose gel stained with ethidium bromide (EtBr). The rest of the material was frozen at −20°C. In order to detect the presence of inhibitors of the PCR, several dilutions of the DNA samples were amplified. The undiluted DNA samples were amplified in triplicate, and 5 μl of a positive DNA control was added to one of the three samples. Several negative controls were included in each series in order to detect contamination.
Detection of amplified products by Southern blot analysis.
An oligonucleotide probe was designed and prepared with a DNA synthesizer by Microsynth. This probe was specific for SAP6 from C. albicans, and the sequence was 5′-GTTATTGTTGACACTGGGTCTTCTGATTT-3′ (555SP6; bp 536 to 564 on SAP6 from C. albicans). It was labeled with T4 polynucleotide kinase and [γ-32P]dATP. A 10-μl aliquot of the PCR product was blotted onto a nylon membrane (GeneScreen Plus; Dupont, Boston, Mass.), hybridized overnight at 42°C with the [γ-32P]dATP-labeled probe, and washed three times in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1% sodium dodecyl sulfate at a low-stringency temperature, i.e., 50°C. After the washes the membrane was directly exposed in an Instant Imager (Packard Instrument Company, Meriden, Conn.) for 30 min and then to X-ray film (Fuji Film) at −70°C for 4 to 16 h.
Microtitration plate hybridization assay.
The single-stranded PCR product was hybridized simultaneously with a 5′-biotin-(AAATCAGAAGACCCAGTGTCAACAAAAC-3′) and a digoxigenin (DIG)-labeled (5′-DIG-GGGTATTCAAATTTTTGGAAG-3′) oligonucleotide probe and was detected on a microtitration plate coated with streptavidin (PCR ELISA; DIG Detection kit [Boehringer Mannheim]). A 10-μl aliquot of the PCR product was denaturated with 40 μl of the denaturation solution in a 1.5-ml tube and was incubated for 10 min at room temperature. After denaturation, 200 μl of hybridization solution containing 20 and 30 pmol of biotin- and DIG-labeled probes per ml, respectively, was added to the wells of the streptavidin-coated microtitration plate. The hybridization reaction was performed directly in the plate for 1 h at 37°C on a shaker (Shaker-Incubator; Microtec Produkte AG, Embrach-Embrachport, Switzerland). The hybridization solution was then discarded, and each well was washed six times with 200 μl of washing solution. The anti-DIG–peroxidase conjugate was prepared by diluting the antibody in the conjugate buffer to a concentration of 10 μl per ml (10 mU/ml) and adding 200 μl to each well. The plates were incubated for 30 min at 37°C on a shaker (Microtec Produkte AG), the wells were washed six times with 200 μl of washing solution, and 200 μl of the colorimetric substrate was added to each well. After 30 min of incubation at 37°C, the A405 of each well was read on a microtitration plate reader (MR 5000; Microtec Produkte AG). A negative control and two positive controls (one PCR control and one detection control) were included in each series.
Control of the amplification with plasmids containing SAP genes.
Five plasmids containing the six SAP genes (pCA1-4 for SAP1 and SAP4, pCA2 for SAP2, pCA3 for SAP3, pCA5 for SAP5, and pCA6 for SAP6) were constructed by Monod et al. (15). They were introduced into competent E. coli DH5α cells by electroporation (Electro Cell Manipulator 600; BTX Inc., San Diego, Calif.). The transformed cells were plated onto Luria-Bertani agar with ampicillin (LBamp agar) and were grown overnight at 37°C. One colony was then inoculated in 2 ml of Luria-Bertani medium with ampicillin (LBamp medium) and was grown overnight at 37°C. A 1-ml aliquot of the culture was used for the miniprep extraction of plasmid DNA. Plasmid DNAs from SAP1 and SAP4 were cut overnight at 37°C with BamHI and NcoI. Two fragments were obtained: the large fragment contained SAP1 and the small fragment contained SAP4. The DNA of each fragment was then purified from a gel. Aliquots of 1 μl of the purified DNAs from SAP1 to SAP6 were tested in the PCR system.
Cloning and sequencing of the PCR products.
PCRs were performed by the PCR protocol described above, except that UTP was replaced by TTP and UNG was omitted. The PCR products were cloned into a pCR 2.1 vector and transformed into E. coli (INVαF′ One Shot Competent cells) with the TA cloning kit (Invitrogen BV, Leek, The Netherlands) according to the instructions of the manufacturer. Twenty clones from two PCR products were selected on LBamp agar for purification. One isolated colony for each clone was resuspended in 100 μl of H2O, and 5 μl of the suspension was amplified by PCR. The positive clones containing the insert of interest were inoculated into 100 ml of LBamp medium and were grown overnight at 37°C. Plasmid DNA was extracted with the Nucleobond AX100 kit (Macherey-Nagel AG) and was sequenced by a standard protocol with an AutoRead kit (Pharmacia). All the reactions were analyzed on an ALF automated station (Pharmacia).
RESULTS
Choice and specificity of a target DNA based on the SAP genes.
The sequences of the first six SAP genes from C. albicans were compared. A pair of primers which amplified a 263-bp fragment in a conserved region close to the deduced N-terminal segments of the proteins encoded by the SAP1 to SAP6 genes and common to the SAP1 to SAP6 genes was chosen in order to amplify the six genes and thus obtain an increase in the sensitivity with the starting material: the upper primer is homologous to SAP5 and SAP6 and presents some mismatches (MMs) with the other genes, i.e., SAP1, 3 MMs; SAP2, 2 MMs; SAP3, 1 MM; and SAP4, 2 MMs. The lower primer is homologous to SAP4, SAP5, and SAP6 and displays the following MMs with the other genes: SAP1, 2 MMs; SAP2, 2 MMs; and SAP3, 4 MMs.
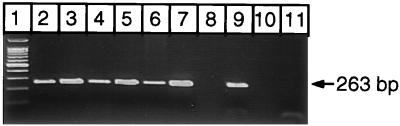
The different parameters of the amplification protocol were then optimized with pure C. albicans genomic DNA, replacing TTP by UTP in the presence of UNG in order to prevent cross-contamination from previous amplifications. The chosen pair of primers amplified the DNAs of the SAP1 to SAP6 genes, as shown by separate amplifications of the six plasmids containing these genes (Fig. 1). In order to check the specificities of the primers, two PCR products from a C. albicans-positive control and a positive blood sample were cloned as described above. The nucleotide sequences for nine positive clones (positive control, four clones; clinical sample, five clones) were highly homologous to the published SAP sequences. One clone had a nucleotide sequence homologous to that of the SAP2, two clones had nucleotide sequences homologous to that of the SAP4 gene, and two pairs of three clones had nucleotide sequences homologous to the SAP5 and SAP6 gene (data not shown).
FIG. 1.
EtBr-stained agarose gel of PCR products obtained from five plasmids containing the SAP1 to SAP6 genes. DNAs from SAP1 and SAP4 were obtained from pCA1-4 as explained in Materials and Methods. Lanes: 1, molecular size marker (100-bp ladder; Promega Corporation, Madison, Wis.); 2, SAP1 DNA insert isolated and purified from pCA1-4; 3, SAP2 DNA insert from pCA2; 4, SAP3 DNA insert from pCA3; 5, SAP4 DNA insert isolated and purified from pCA1-4; 6, SAP5 DNA insert from pCA5; 7, SAP6 DNA insert from pCA6; 8, void; 9, positive control; 10, void; 11, negative control. The measured length (∼263 bp) of the PCR products corresponded to the length expected from available N-terminal sequences.
The specificities of the SAP primers was also checked by using DNAs of various origins: human leukocytes, human kidney epithelial cells, mouse tail cells, and various bacterial and fungal species as detailed in Materials and Methods. The chosen primers were truly specific for C. albicans: no cross amplification with any other tested DNA and particularly no cross amplification with DNA from C. krusei (n = 1), C. glabrata (n = 11), C. tropicalis (n = 1), and C. parapsilosis (n = 1) was observed. The specificity was also confirmed by the results with the blood culture samples from the clinical study, in which no cross-reactions were observed with non-C. albicans DNA, i.e., human, bacterial, or yeast DNA.
Detection of the PCR product: ELISA versus Southern blotting.
Amplification with a biotinylated primer followed by hybridization with a DIG-labeled oligonucleotide probe was found to be less sensitive than hybridization of the nonlabeled PCR product simultaneously with a biotin- and a DIG-labeled probe (data not shown). Heat denaturation of the PCR product prior to hybridization was less efficient and was more difficult to perform than alkaline denaturation. There also was no significant difference between a 1-h and a 3-h incubation time or between a 37°C and a 55°C hybridization temperature. Although the hybridization probes were specific for SAP6, they hybridized with each of the cloned DNA inserts from SAP1 to SAP6 isolated in the six plasmids described above.
The sensitivities of using an EtBr-stained gel, Southern blotting with a [γ-32P]dATP-labeled probe, and ELISA for the detection of the PCR product obtained from C. albicans genomic DNA are shown in Table 1. Detection by ELISA was found to be as sensitive as detection by Southern blotting. The mean ± 1 standard deviation (SD) optical density (OD) corresponding to a 10−8 dilution of DNA (200 fg/ml of DNA) was 0.646 ± 0.420 (n = 2).
TABLE 1.
Comparison of the sensitivities of PCR assays with dilutions of DNA or DNA prepared from diluted cultures or seeded blood
| Dilution and origin of template for PCR | Detection by the following method:
|
||
|---|---|---|---|
| EtBr-stained agarose gel | Southern blot (cpm) | ELISA (OD) | |
| 10−4 | |||
| DNAa | +b | 22,249c | 2.901c |
| Cultured | + | 25,226 | 2.762 |
| Seeded bloodd | + | NDe | ND |
| 10−5 | |||
| DNA | + | 16,891 | 2.798 |
| Culture | + | 16,748 | 2.802 |
| Seeded blood | + | 2,282 | 2.625 |
| 10−6 | |||
| DNA | + | 7,734 | 2.917 |
| Culture | + | 8,802 | 2.621 |
| Seeded blood | + | 1,936 | 2.739 |
| 10−7 | |||
| DNA | + | 1,039 | 2.596 |
| Culture | + | 1,835 | 2.411 |
| Seeded blood | + | 342 | 1.294 |
| 10−8 | |||
| DNA | − | 152 | 0.944 |
| Culture | − | 44 | 0.304 |
| Seeded blood | − | 125 | 0.655 |
| 10−9 | |||
| DNA | − | 11 | 0.112 |
| Culture | − | 5 | 0.105 |
| Seeded blood | − | 11 | 0.106 |
| Negative control | |||
| DNA | − | 0.3 | 0.115 |
| Culture | − | 2 | 0.118 |
| Seeded blood | − | 3 | 0.120 |
One milliliter of an overnight C. albicans culture was diluted to 10−1. The DNA was extracted as described in Materials and Methods and was then serially diluted to 10−9. The 10−1 DNA solution contained 2 μg of DNA per ml, as estimated by comparison with a marker on an EtBr-stained gel.
A visible band (+) or no visible band (−) on agarose gel electrophoresis with EtBr staining.
The background (Southern blot) and blank values (ELISA) were not deduced from the displayed values. The sensitivity of the assay in this experiment, as indicated by the calculated DNA or cell content of the 10−8 dilutions, was 200 fg of DNA per ml, 1 cell/ml (culture dilution), and 4 cells/ml (seeded blood).
Tenfold serial dilutions of the C. albicans culture diluted 10−1 in water or blood were obtained as explained in Materials and Methods. Quantification of cell numbers in the 10−1 was obtained by measuring the A540 of the cultures, and the quantities were 1.2 × 10−7 and 3.75 × 10−7 cells/ml, respectively. A 10-μl aliquot of each dilution was used for PCR amplification. A 10-μl aliquot of the PCR product was used for detection by agarose gel detection electrophoresis with EtBr staining, Southern blotting, or ELISA.
ND, not determined.
Optimization of C. albicans DNA preparation.
Optimization and simplification of the DNA preparation protocol are crucial for the application of PCR as a routine test. The methodology used for this step was reexamined systematically by using recently developed and currently available technical improvements. The sensitivity of each DNA extraction method was tested with serial dilutions of Candida cells.
Five published methods (3, 4, 9, 19, 20) and three commercially available methods of DNA preparation, as mentioned in Materials and Methods, were tested and compared (Table 2). Some of them were combined with physical factors such as glass beads or thermal shock, microwave, or sonication treatment. The effect of the addition or removal of treatments with enzymes such as zymolyase and proteinase K on the recovery of DNA were also compared.
TABLE 2.
Comparison of methods of extracting DNA from diluted cultures of C. albicans
| Method | Reference or source | Sensitivity (cells/ml)a | Time (h) | Ease of use and recovery |
|---|---|---|---|---|
| Zymolyase, SDS, proteinase K, phenol | Buchman et al. (4) | 10 (1) or inhibition (1) | 2 | Handy, phenol, two enzymatic steps, good recovery or total inhibition |
| Zymolyase, proteinase K, phenol, RNase | Holm et al. (9) | 10 (1) and 102 (1) | 4 | Tedious, phenol, three enzymatic steps, good recovery |
| Glass beads, phenol | Sanglard et al. (19) | 102 (2) | 0.75 | Handy, phenol, medium recovery |
| Zymolyase, proteinase K, lysing solution, RNase | Sanglard et al. (20) | 106 (2) | 2 | Handy, no phenol, three enzymatic steps, low recovery |
| Zymolyase, silica beads | Boom et al. (3) | 102 (2) | 2.5 | Handy, no phenol, one enzymatic step, medium recovery, some inhibition |
| Zymolyase, proteinase K, silica membrane | Qiagen | 102 (1) | 2 | Very handy, no phenol, two enzymatic steps, medium recovery |
| Zymolyase, proteinase K, silica membrane | Macherey & Nagel | 10 (3) | 2 | Very handy, no phenol, two enzymatic steps, good recovery |
| Proteinase K, silica membraneb | Qiagen (modified protocol) | 10 (23) | 1.5 | Simple, rapid, no phenol, one enzymatic step, good recovery |
Tenfold serial dilutions of an overnight C. albicans culture in water were obtained as described in Materials and Methods, and cell quantification was performed as explained in Materials and Methods. A 10-μl aliquot of the DNA extracted from each dilution was used for PCR amplification. A 10-μl aliquot of the PCR product was used for detection by agarose gel electrophoresis with EtBr staining. The sensitivity was defined as the highest dilution which produced a visible band by EtBr staining after agarose gel electrophoresis with the SAP primers and the PCR protocol described in the text. The number of separate experiments is indicated in parentheses.
This method was adopted for the present study.
The following observations were made. (i) None of the physical treatments mentioned above increased the amount of DNA recovered (data not shown). (ii) Proteinase K treatment alone led to the recovery of DNA in amounts equal to those recovered after treatment with zymolyase plus proteinase K. The DNA preparation protocol was therefore simplified: a single enzymatic digestion step with proteinase K was used and the zymolyase digestion step was omitted.
Several periods of incubation with proteinase K were tested, and a 1-h incubation time was finally chosen because longer incubations did not increase the amount of DNA recovered. Two commercially available silica-based column treatments for DNA purification (Macherey-Nagel versus Qiagen) were tested and led to the recovery of the same amount of DNA. The Qiagen column was preferred because the individual packing of each column seemed to guarantee better protection against potential contamination.
The method of DNA preparation eventually adopted simply consisted of a single enzymatic incubation step, followed by a Qiagen column treatment, for an overall DNA preparation time of 1.5 h. The sensitivity of this method of DNA preparation obtained with serial dilutions of C. albicans cells in water was 10 cells/ml (EtBr staining and agarose gel electrophoresis; n = 23) (Table 2) and 1 cell/ml (detection by Southern blotting or ELISA) as shown in Table 1. The mean ± 1 SD OD corresponding to 10−8 dilution of Candida cells in water (1 cell/ml) was 0.508 ± 0.287 (n = 2).
Sensitivity of the PCR for C. albicans in seeded blood specimens.
Because hemoglobin is an inhibitor of the Taq DNA polymerase, blood specimens required additional preparation and washing steps in order to lyse erythrocytes and remove hemoglobin. These additional lysis steps lasted approximately 1 h, hence leading to an overall DNA preparation time of 2.5 h for samples containing blood. Among the four lysis protocols tested with C. albicans cells serially diluted in human donor blood, the best sensitivity and practicability were obtained with the alkaline citrate-cysteine solution. Specifically, for the following erythrocyte lysis methods for the recovery of C. albicans DNA from seeded blood specimens, the indicated sensitivities were achieved: distilled water, 104 cells/ml; detergent cocktail with DNase (4), 104 cells/ml; detergent cocktail without DNase (5), 102 to 103 cells/ml; and NaOH, citrate, and N-acetylcysteine (the method adopted for this study), 10 cells/ml. Tenfold serial dilutions of an overnight C. albicans culture in donor blood and cell quantification were obtained as explained in Materials and Methods. Each erythrocyte lysis method was tested and was combined with the proteinase K-silica membrane DNA extraction method (Table 2). A 10-μl aliquot of the DNA extracted from each dilution was used for PCR amplification. A 10-μl aliquot of PCR product was used for detection by agarose gel electrophoresis with EtBr staining. The sensitivity was defined as the highest dilution which produced a visible band by EtBr staining after agarose gel electrophoresis with the SAP primers and the PCR protocol described above. As expected, detection by ELISA increased the sensitivity of the alkaline citrate-cysteine lysis protocol by 1 order of magnitude. The sensitivity observed with serial dilutions of C. albicans cells in blood was 1 to 4 cells/ml, corresponding to a 10−8 dilution of seeded blood, with a mean ± 1 SD OD of 0.799 ± 0.360 (n = 3). Table 1 presents the values for one experiment, comparing detection by EtBr staining and agarose gel electrophoresis, Southern blotting, and ELISA.
Performance of the optimized test with clinical samples.
The method of preparing DNA from clinical samples described in Materials and Methods was applied by using a 900-μl volume for each clinical sample, with a preliminary lysis protocol for blood culture samples and other clinical samples containing blood (i.e., abdominal fluid). In order to detect inhibition due to excess DNA, each DNA extract was diluted 1:10, 1:100, and, in some cases, 1:1,000. In order to detect the presence of other inhibitory substances, the undiluted DNA samples were amplified in triplicate, with 5 μl of a positive DNA control added to one of the three samples. The DNA extraction procedure was performed once for clinical samples and was repeated only for samples showing inhibition. Each DNA dilution was amplified, with up to five to six PCR tests performed per clinical sample. Each PCR product was observed by agarose gel electrophoresis. ELISA was then performed once with each PCR product from all samples negative by agarose gel electrophoresis and once with the PCR product showing the faintest signal on agarose gel electrophoresis with the positive samples. Thus, for each negative sample, there were at least four replicates for detection by PCR and agarose gel electrophoresis and at least three replicates for detection by ELISA. The first 61 samples were analyzed in parallel by Southern blotting and ELISA. The reproducibility was 100% for samples with an OD of >0.500. When the OD was <0.500, the reproducibility was lower, a fact which can be expected from the Poisson distribution in the low DNA concentration expected from these OD values (i.e., 10 pg/10 μl). When duplicate samples gave discordant results, they were retested in duplicate or triplicate in order to confirm the positive or negative result.
The ELISA cutoff value was estimated empirically from values obtained with the various negative controls (blank) and by comparing the values obtained by Southern blot analysis and ELISA. A positive ELISA value was defined as being confirmed by the presence in the same PCR products of a band on the Southern blot. Statistical analysis of the observed blank values obtained with the various negative controls demonstrates that the values were quite reproducible: there were no differences between the mean blank values (mean ± 1 SD) obtained with water (ELISA blank OD, 0.109 ± 0.0046; n = 17), PCR-negative control DNA (PCR blank OD, 0.112 ± 0.0073; n = 16), extraction- and PCR-negative control DNA (extraction control blank OD, 0.108 ± 0.0053; n = 50), or negative clinical samples (sample blank OD, 0.109 ± 0.0065; n = 102). The cutoff value was calculated to be the highest mean blank value, i.e., mean ± 1 SD OD of 0.112 plus 3 SDs of the highest SD value (OD = 0.022). The rounded cutoff value was hence 0.140. All values higher than 0.140 were considered positive, a definition validated with the first 61 clinical samples by comparing detection of the PCR amplification product by ELISA with detection by Southern blotting, in which a 100% correlation between the two methods was observed.
A single blinded evaluation of the 156 samples demonstrated the following results (Table 3): 51 were both culture and PCR positive for C. albicans, hence, an observed sensitivity of 100%. The minimum number of C. albicans yeast cells measured by PCR in a true clinical specimen (blood culture) was 20 CFU/ml, as quantitated by plating 100 μl of the blood onto a Sabouraud dextrose agar plate.
TABLE 3.
Retrospective study of PCR versus culture for the detection of C. albicans in clinical samples
| Origin of clinical samples | No. of samples
|
|||||
|---|---|---|---|---|---|---|
| Total | Positive by culture | Positive by agarose gel electrophoresis with EtBr staining | Positive by ELISA | False negative by PCR | False positive by PCR | |
| Blood culture | 124 | 31 | 29 | 31 | 0 | 0 |
| Othersa | 32 | 20 | 18 | 22 | 0 | 2b |
| Total | 156 | 51 | 47 | 53 | 0 | 2b |
Abdominal fluid (n = 22), pleural fluid (n = 4), bile (n = 3), bronchoalveolar lavage fluid (n = 2), and a skin biopsy specimen (n = 1).
The two samples false positive by PCR were two abdominal fluid samples from patients treated with azole antifungal agents.
While there were no false-positive results for the subset of blood culture samples, among the other specimens, there were false-positive results by PCR with two samples from two different patients. A closer examination of these two specimens indicated that both specimens were abdominal drainage fluid from surgical patients. The first patient suffered from a C. glabrata infection. The second patient suffered from a mixed C. albicans and C. glabrata infection, and with a previous specimen from the second patient, both species were recovered by culture. Both patients were treated with azole antifungal agents at the time of sampling. A cross-reaction of the SAP-specific primers with C. glabrata DNA could be excluded since 14 blood culture specimens from the retrospective study were culture positive for C. glabrata and PCR negative for C. albicans and since the DNAs extracted from 11 strains of C. glabrata did not cross-react with the C. albicans-specific SAP primers.
DISCUSSION
There is no doubt that molecular biology-based techniques, and particularly PCR DNA amplification methods, will become increasingly popular in clinical microbiology laboratories in the near future. A prerequisite for the use of these techniques in the routine clinical laboratory, however, is that they be at least as sensitive and specific as conventional culture and that they be rapid, simple, and reliable. The method described in this study offers all the needed characteristics: it is simple, robust, sensitive, and reproducible. No special enzymes except proteinase K are used for the DNA preparation procedure, and phenol extraction is avoided. The total time required for the procedure is about 8 h: 2.5 h for DNA preparation, 2.5 h for PCR, and 3 h for ELISA. A good sensitivity is obtained with a single-step PCR, a significant improvement compared with our previous assay, which used nested PCR. Although some investigators (26) report that nested PCR guarantees a higher specificity for the amplification procedure, nested PCR is subject to many contamination artifacts, lengthens the PCR procedure, and delays the time until the final results can be obtained, while the single-step PCR procedure can use decontaminating procedures such as decontamination with UTP and UNG. Furthermore, use of an ELISA format for the detection of the PCR product provides the same increase in sensitivity, relative to that of agarose gel electrophoresis, as the radioactive Southern blotting technique. In addition, ELISA offers the potential for automation, a highly desirable feature for a routine laboratory test.
The sensitivity of the present assay is increased by 2 orders of magnitude in comparison with the sensitivity of our previous assay, for which we reported a detection level of 100 to 200 cells/ml (5) and which is 1 order of magnitude more sensitive than most other published methods. Miyakawa et al. (14), Holmes et al. (10), and Fujita et al. (8) reported sensitivities of 30, 15, and 10 cells/ml, respectively. The increased sensitivity observed in this study probably results not only from the choice of the novel PCR amplification target but also from the optimization of the DNA preparation method and the PCR product detection method.
Sugita et al. (22) previously described a PCR assay with SAP primers based on the amplification of a single gene copy, since the SAP1 gene was the only SAP gene described at that time. They could detect C. albicans in three clinical cerebrospinal fluid samples, but they presented no quantitative data about the sensitivity of their assay. Amplification of common regions of a multigene family such as the C. albicans SAP genes is interesting not only because it will increase the sensitivity of the assay but also because one or several of these genes are candidate genes encoding virulence factors for C. albicans (18). Thus, it may be possible in the future to measure directly by reverse transcription-PCR the relative levels of expression of these putative virulence factors in clinical specimens.
The aim of this retrospective study was mainly to compare the sensitivity and specificity of PCR with those of conventional culture methods and not to establish the true sensitivity and specificity of the PCR assay compared with clinical data and clinical outcome. The patient samples were included arbitrarily by the routine clinical laboratory. A panel of positive samples was mixed with negative samples from the same or different patients in order to obtain a relatively high proportion of C. albicans-positive samples. The overall sensitivity and specificity of the assay observed with the clinical samples were 100 and 98%, respectively, with a specificity of 100% obtained with the subset of blood culture samples. A closer examination of the samples that had discordant PCR results and that were false positive by PCR indicates, however, that they were probably due to the presence of mixtures of organisms, in which C. albicans growth was overlooked or inhibited by C. glabrata or by azole antifungal agent treatment. Thus, the specificity of the PCR assay is likely to be higher than 98%.
Given the high sensitivity demonstrated by the present method with retrospective clinical specimens, a prospective clinical study is under way in order to compare the performance of PCR and culture methods for the detection of C. albicans in patients at risk, particularly from blood and other normally sterile sites. Finally, the sequences of the SAP genes from other Candida species, particularly C. glabrata, will be investigated in order to develop a PCR amplification system allowing the detection of the main clinically significant species of Candida.
ACKNOWLEDGMENTS
This work was mainly supported by a grant from the Swiss National Foundation for Scientific Research (3200-043402.95) and was partly supported by a grant from the Roche Foundation.
We thank K. Jaton Ogay, P. Rudaz, G. Togni, D. Firsov, and H.-P. Gäggeler for helpful collaboration and C. Durussel for helping to collect the clinical samples.
REFERENCES
- 1.Banerjee, S. N., T. G. Emori, D. H. Culver, R. P. Gaynes, W. R. Jarvis, T. Horan, J. R. Edwards, J. Tolson, T. Henderson, W. J. Martone, and the National Nosocomial Infections Surveillance System. 1991. Secular trends of nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am. J. Med. 91(Suppl. 3B):86S–89S. [DOI] [PubMed]
- 2.Beck Sague C M, Jarvis W R. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 3.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-Van Dillen P M E, Van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman T G, Rossier M, Merz W G, Charache P. Detection of surgical pathogens by in vitro DNA amplification. Part 1. Rapid identification of Candida albicans by in vitro amplification of a fungus-specific gene. Surgery. 1990;108:338–347. [PubMed] [Google Scholar]
- 5.Burgener-Kairuz P, Zuber J P, Jaunin P, Buchman T G, Bille J, Rossier M. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P-450 lanosterol-alpha-demethylase (L1A1) gene fragment. J Clin Microbiol. 1994;32:1902–1907. doi: 10.1128/jcm.32.8.1902-1907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crampin A C, Matthews R C. Application of the polymerase chain reaction to the diagnosis of candidosis by amplification of an HSP-90 gene fragment. J Med Microbiol. 1993;39:233–238. doi: 10.1099/00222615-39-3-233. [DOI] [PubMed] [Google Scholar]
- 7.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in C. albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita S-I, Lasker B A, Lott T J, Reiss E, Morrison C J. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33:962–967. doi: 10.1128/jcm.33.4.962-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holm C, Meeks-Wagner D W, Fangman W L, Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42:169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- 10.Holmes A R, Cannon R D, Shepherd M G, Jenkinson H F. Detection of Candida albicans and other yeasts in blood by PCR. J Clin Microbiol. 1994;32:228–231. doi: 10.1128/jcm.32.1.228-231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopfer R L, Walden P, Setterquist S, Highsmith W E. Detection and differentiation of fungi in clinical specimens using polymerase chain reaction (PCR) amplification and restriction enzyme analysis. J Med Vet Mycol. 1993;31:65–75. doi: 10.1080/02681219380000071. [DOI] [PubMed] [Google Scholar]
- 12.McGinnis M R. Mycology. In: McGinnis M R, editor. Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. pp. 6.0.1–6.12.4. [Google Scholar]
- 13.Miyakawa Y, Mabuchi T, Kagaya K, Fukazawa Y. Isolation and characterization of a species-specific DNA fragment for detection of Candida albicans by polymerase chain reaction. J Clin Microbiol. 1992;30:894–900. doi: 10.1128/jcm.30.4.894-900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyakawa Y, Mabushi T, Fukasawa Y. New method for detection of Candida albicans in human blood by polymerase chain reaction. J Clin Microbiol. 1993;31:3344–3347. doi: 10.1128/jcm.31.12.3344-3347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monod M, Togni G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol Microbiol. 1994;13:357–368. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 16.Niesters H G M, Goessens W H F, Meis J F M G, Quint W G V. Rapid, polymerase chain reaction-based identification assays for Candida species. J Clin Microbiol. 1993;31:904–910. doi: 10.1128/jcm.31.4.904-910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittet D, Wenzel R P. Nosocomial bloodstream infections. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 18.Sanglard D, Hube B, Monod M, Odds F C, Gow N A R. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;9:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 20.Sanglard D, Togni G, de Viragh P, Monod M. Disruption of the gene encoding the secreted acid protease (ACP) in the yeast Candida tropicalis. FEMS Microbiol Lett. 1992;95:149–156. doi: 10.1016/0378-1097(92)90421-j. [DOI] [PubMed] [Google Scholar]
- 21.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 22.Sugita Y, Kanaizuka I, Nakajima H, Ibe M, Yokota S, Matsuyama S. Detection of Candida albicans DNA in cerebrospinal fluid. J Med Vet Mycol. 1993;31:353–358. [Google Scholar]
- 23.Voss A, Kluytmans J A J W, Koeleman J G M, Spanjaard L, Vandenbroucke-Grauls C M J E, Verbrugh H A, Vos M C, Weersink A Y L, Hoogkamp-Korstanje J A A, Meis J F G M. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur J Clin Microbiol Infect Dis. 1997;15:909–912. doi: 10.1007/BF01690507. [DOI] [PubMed] [Google Scholar]
- 24.Walsh T J, Pizzo P A. Nosocomial fungal infections: a classification for hospital acquired fungal infections and mycoses arising from endogenous flora or reactivation. Annu Rev Microbiol. 1988;42:517–545. doi: 10.1146/annurev.mi.42.100188.002505. [DOI] [PubMed] [Google Scholar]
- 25.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 26.Wildfeuer A, Schlenk R, Friedrich W. Detection of Candida albicans DNA with yeast-specific primer system by polymerase chain reaction. Mycoses. 1996;39:341–346. doi: 10.1111/j.1439-0507.1996.tb00150.x. [DOI] [PubMed] [Google Scholar]