Abstract
Temporal trends of total liver cancer have been well reported in China, especially the trends caused by hepatitis B (HBV); however, the trends of liver cancer attributable to specific etiologies have rarely been reported in China. Thus, this study aims to describe the temporal trends in the incidence, mortality and DALYs of total and etiology-specific liver cancer in China from 1990 to 2019. We extracted the incidence, mortality and disability-adjusted life years (DALYs) of total and etiology-specific liver cancer in China from 1990 to 2019 from global disease burden (GBD) 2019. We plotted the trends in the age-standardized rates for incidence, mortality, and DALYs using locally weighted regression (LOESS)-smoothed data from 1990 to 2019. The age-standardized rate for the incidence of liver cancer was analyzed with an age-period-cohort method. The age-standardized rates for incidence, death, and DALYs decreased by −58.8%, −63.8%, and −65.6%, respectively, between 1990 and 2019. The age-standardized rates of incidence, mortality, and DALYs of total liver cancer showed similar temporal patterns, presenting an overall decline, with the average annual percentage change (AAPC) ranging from −3.3% to −3.8%. People in the period before 2007 had a higher risk, and people after 2007 had a lower risk. The cohort risk ratios (RRs) showed decreasing patterns, with the most rapid decline observed in the 1910 to 1960 cohorts. Our study generally revealed favorable decreasing trends for total and etiology-specific liver cancer in China from 1990 to 2019. Despite the overall decline in liver cancer due to heavy alcohol use and obesity from 1990 to 2019, there have been apparent upward trends since 2006. Planned population-wide interventions targeting heavy alcohol use and obesity may mitigate the increasing trends in liver cancer attributable to alcohol use and NASH.
Keywords: Liver cancer, disease burden, trend, incidence, age-period-cohort
Introduction
Liver cancer is the seventh most common cancer worldwide, with an estimated 841,080 incident cases in 2018, and is the second leading cause of cancer death (781,631 deaths per year). 1 The countries with the highest incidence of liver cancer are mainly those with lower levels of economic development, and most cases of liver cancer are located in several geographically diverse countries, including countries in North and West Africa (Gambia, Egypt, and Guinea) and East and Southeast Asia (Cambodia, Mongolia, and Vietnam). China is the region of the world most affected by liver cancer, accounting for more than 50% of newly diagnosed cases and deaths, despite accounting for only 19% of the world’s population. 1
The main risk factors for liver cancer vary by region. In the most high-risk regions for liver cancer (e.g. China and East Africa), the key risk factors are chronic HBV infection and aflatoxin contamination in food, while in other countries (e.g. Egypt, Japan), HCV infection is probably the main cause. In high-income countries, HCV, alcohol consumption, and obesity/diabetes are common causes of liver cancer. 2 In China, an estimated 83%−92% of liver cancers are hepatocellular carcinoma (HCC). 3 Chronic HBV infection acquired through mother-to-child infection in early life and exposure to dietary aflatoxins are the major causes of liver cancer in China.4,5 Since the introduction of the HBV vaccine in the early 1990s and the nationwide inclusion of HBV vaccination in China’s neonatal immunization program since 2002, the prevalence of HBV and the risk of liver cancer have been declining in the population, especially among young adults.6,7
Temporal trends of total liver cancer have been well reported in China. Zheng et al. 8 reported that the age-standardized incidence rates of liver cancer decreased by approximately 2.2% per year in males and 2.5% per year in females between 2000 and 2014. The young generation, particularly for those under 40 years old, showed a faster downward trend. Li et al. 9 reported that the incidence and mortality of liver cancer were significantly higher in rural areas than in urban areas, and the incidence and mortality of liver cancer declined significantly in both urban and rural areas but was more pronounced in rural areas. Similar trends have also been reported in previous studies.10–12 However, the trends of liver cancer attributed to a specific etiology have rarely been reported and compared. Thus, this study aims to describe and analyze the temporal trends in incidence, mortality and DALYs of total liver cancer and etiology-specific liver cancer in China from 1990 to 2019. This study will provide helpful insights for understanding the changing disease spectrum of liver cancer and allocation of health resources.
Materials and methods
Data source
The Global Burden of Diseases, Injuries, and Risk Factors Study 2019 (GBD 2019) was developed and maintained by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, which aims to provide rigorous and comparable measurements of important health problems across the globe. GBD 2019 estimated 286 causes of death, 369 diseases and injuries, and 87 risk factors across 204 countries and territories from a variety of relevant data sources, including household surveys, censuses, vital statistics, and civil registrations. GBD used DisMod-MR, a Bayesian meta-regression tool that allows the combination of all available data, to estimate prevalence, incidence, and mortality for a disease. More details about the data collection and modeling of GBD 2019 can be found elsewhere.13,14
We extracted the incidence, mortality and disability-adjusted life years (DALYs) of total liver cancer and etiology-specific liver cancer (HBV, HCV, alcohol and nonalcoholic steatohepatitis (NASH)) in China from 1990 to 2019 by gender using the Global Health Data Exchange (GHDx). The population was divided into four age groups: 0–14 years, 15–49 years, 50–69 years, and 70+ years. This study extracted publicly accessible data from the Global Burden of Disease Study 2019, which contains deidentified data not directly linked to individuals. Thus, the institutional review board waived the requirement for ethical approval.
Statistical analysis
The age-standardized rates for incidence, mortality, and DALYs were all adjusted by the world standardized population to account for differences in population age distribution within countries over time, 15 expressed as the rate per 100,000. The age-standardized incidence, mortality, or DALY rate was calculated using the following formula:
( refers to the incident cases or DALYs in the ith age group, and refers to the number of persons in the same age group)
Absolute values and age-standardized rates for the incidence, mortality, and DALYs were compared between 1990 and 2019. We plotted the trends in the age-standardized rates for the incidence, mortality, and DALYs using locally weighted regression (LOESS)-smoothed data from 1990 to 2019 for the total liver cancer and etiology-specific liver cancer cases. 16 Joinpoint regression analysis was performed to estimate the average annual percentage change (AAPC) over the entire analysis period, the annual percentage change (APC) for each segmented line regression with a maximum number of three joins, and their 95% confidence intervals (95% CIs). 17 The APC is calculated as , where represents the slope of the trend segment. We also estimated the average annual percentage change (AAPC), assuming there was only one segment for the full range of our study periods.
The age-period-cohort model, a parametric statistical model widely used in epidemiological studies, estimates the independent effects of age, period, and cohort on disease incidence or mortality, providing important insights to understand the social, historical, and environmental factors that influence incidence, mortality, and DALYs. The typical APC model was Rap =exp (μ+Aa+Pp+Cc), where Rap is the incidence rate in age group a and in calendar period p. μ denotes the intercept or adjusted mean rate. Aa is the age component for age group a, Pp is the nonlinear period component of period p, and Cc is the nonlinear cohort component of the cohort. The age-period-cohort analysis was conducted using the age-period-cohort model Web Tool (Biostatistics Branch, National Cancer Institute, Bethesda, MD. https://analysistools.nci.nih.gov/apc/). The age-period-cohort model Web Tool allows us to estimate the drift (log-linear trend) representing the average annual change in the age-standardized rates over time based on the birth cohort axis and calendar period axis, which includes the net drift suggesting the overall percentage change per year and the local net representing the change in a specific age group. In addition, we plotted longitudinal age curves that can show the specific age-standardized rates for the reference cohort when controlling for period changes. Risk ratios (RRs) were estimated for cohorts/periods controlling for chronological age and the nonlinear component of the period/cohort, which indicates the relative risk of age standardization for each cohort/period compared to the reference cohort/period.
Results
Table 1 presents the absolute number and rate change in the incidence, mortality and DALYs between 1990 and 2019 for the total number of liver cancer cases by gender and age group and for etiology-specific liver cancer cases. In general, substantial reductions in the numbers and rates of incidence, death, and DALYs of total liver cancer cases were observed between 1990 and 2019. The declines were more pronounced in people at 15–49 years of age and for HBV-attributable and HCV-attributable cancer cases. Despite population growth, the absolute numbers for the incidence, deaths, and DALYs of liver cancer cases decreased in China between 1990 and 2019. However, the age-standardized rates exhibited a more pronounced decline. The age-standardized rates for incidence, death, and DALYs decreased by −58.8%, −63.8%, and −65.6%, respectively. Generally, males had a higher incidence, death, and DALY burden than females, and this disparity was more apparent in 2019 than in 1990. The liver cancer disease burden is the highest in people at 50–69 years of age, followed by people at 15–49 years of age. However, people at 15–49 years of age experienced a larger decline in incidence, deaths, and DALYs between 1990 and 2019 than people at 50–69 years of age. HBV and HCV are the two major etiologies of liver cancer in China, and there were substantial reductions (nearly 60%) in the age-standardized incidence rates for liver cancer due to HBV and HCV, which were larger than the incidence rate reductions for other etiologies of liver cancer.
Table 1.
Change in the incidence, mortality, and DALYs for liver cancer between 1990 and 2019 in China.
| Incidence |
Mortality |
DALYs |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 |
2019 |
% in rates | 1990 |
2019 |
% in rates | 1990 |
2019 |
% in rates | |||||||
| Number | ASR | Number | ASR | Number | ASR | Number | ASR | Number | ASR | Number | ASR | ||||
| Overall | 236,824.6 | 25.7 | 210,462.4 | 10.6 | −58.8 | 232,449.2 | 26.0 | 187,699.6 | 9.4 | −63.8 | 7,577,768.1 | 769.1 | 5,325,460.7 | 264.3 | −65.6 |
| Gender | |||||||||||||||
| Male | 170,115.4 | 36.4 | 159,787.3 | 16.4 | −55.1 | 164,905.4 | 36.7 | 139,025.6 | 14.6 | −60.3 | 5,590,399.0 | 1102.8 | 4,145,795.1 | 414.9 | −62.4 |
| Female | 66,709.3 | 15.0 | 50,675.1 | 4.9 | −67.0 | 67,543.8 | 15.6 | 48,674.0 | 4.8 | −69.4 | 1,987,369.1 | 420.1 | 1,179,665.6 | 115.9 | −72.4 |
| Age groups | |||||||||||||||
| 0–14 | 1731.6 | 10.3 | 504.2 | 6.2 | −40.2 | 62,017.8 | 9.3 | 33,735.1 | 4.7 | −49.5 | 155,190.7 | 452.2 | 30,889.7 | 221.8 | −51.0 |
| 15–49 | 68,811.4 | 79.7 | 44,399.3 | 29.1 | −63.5 | 118,222.7 | 76.7 | 92,453.3 | 25.1 | −67.3 | 3,022,653.2 | 2328.2 | 1,598,256.6 | 751.0 | −67.7 |
| 50–69 | 122,718.3 | 113.9 | 107,222.0 | 54.0 | −52.5 | 50,374.2 | 131.7 | 61,143.4 | 56.6 | −57.0 | 3,586,711.5 | 2125.6 | 2,770,211.2 | 857.8 | −59.6 |
| 70+ | 43,563.3 | 0.5 | 58,336.9 | 0.2 | −58.2 | 1834.5 | 0.6 | 367.8 | 0.2 | −71.2 | 813,212.7 | 48.1 | 926,103.2 | 13.7 | −71.4 |
| Etiology | |||||||||||||||
| HBV | 158,410.0 | 16.5 | 135,027.6 | 6.6 | −59.8 | 152,613.7 | 16.2 | 116,997.8 | 5.8 | −64.5 | 5,264,530.9 | 520.6 | 3,597,724.6 | 177.7 | −65.9 |
| HCV | 35,446.6 | 4.5 | 340,36.4 | 1.8 | −61.3 | 37,163.0 | 5.0 | 33,078.9 | 1.8 | −64.8 | 909,454.8 | 105.9 | 714,427.1 | 35.1 | −66.9 |
| Alcohol | 17,553.6 | 2.0 | 19,187.8 | 0.9 | −52.8 | 17,525.6 | 2.0 | 17,435.7 | 0.8 | −57.4 | 508,949.3 | 54.0 | 451,771.3 | 21.4 | −60.5 |
| NASH | 8809.0 | 1.0 | 10,034.5 | 0.5 | −50.0 | 8866.1 | 1.1 | 9401.1 | 0.5 | −54.4 | 256,934.3 | 27.3 | 229,176.0 | 11.3 | −58.5 |
ASR: age-standardized rates; DALYs: disability-adjusted life years; HBV: hepatitis B; HCV: hepatitis C; NASH: nonalcoholic steatohepatitis.
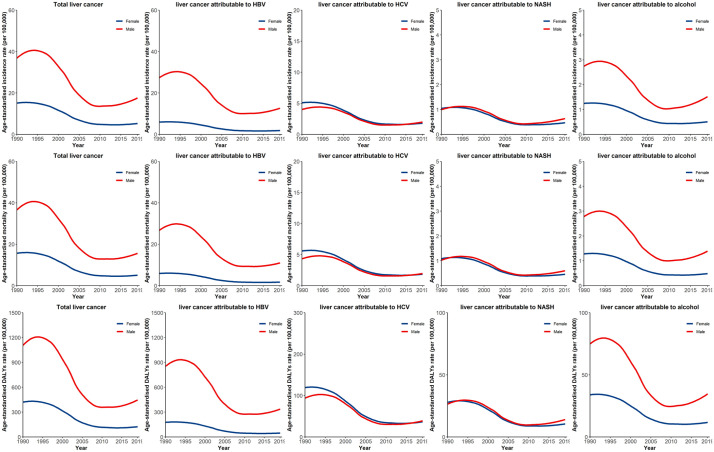
Figure 1 presents the temporal trends of the age-standardized incidence rates for the total number of liver cancer cases and etiology-specific liver cancer cases in males and females between 1990 and 2019. In general, males and females present different temporal patterns of total liver cancer and etiology-specific liver cancer. For example, males generally had higher incidence rates for total liver cancer, liver cancer attributable to HBV, and liver cancer attributable to alcohol use than females. The incidence rates of liver cancer due to HCV and liver cancer due to NASH were comparable between the two sexes; females had higher incidence rates of liver cancer attributable to HCV in the early 1990s than males. Our analysis reveals a rapid decline between 1995 and 2010 and a small upward trend in 2019 for total liver cancer and etiology-specific liver cancer in both males and females. However, for total liver cancer, liver cancer attributable to HBV, and liver cancer attributable to alcohol use, there was also an upward trend in the early 1990s, whereas the other liver cancers had a flat trend in the early 1990s.
Figure 1.
Temporal trends of age-standardized incidence rates for liver cancer and etiology-specific liver cancer by gender between 1990 and 2019 in China.
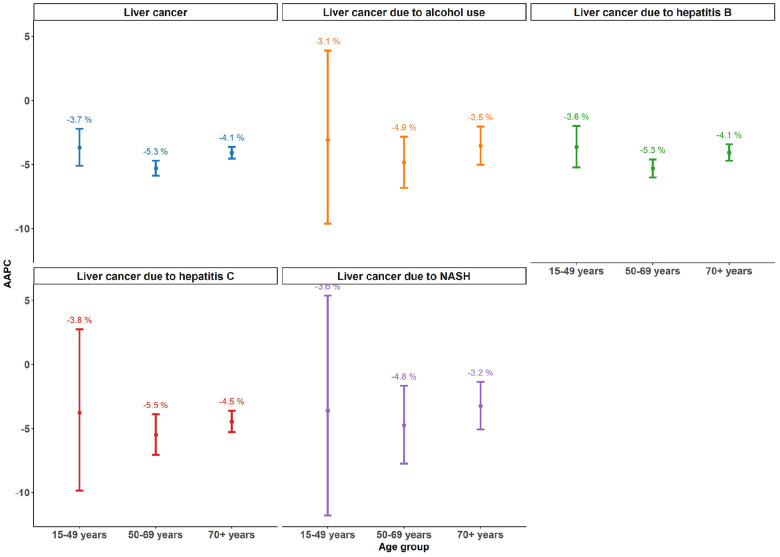
Figure 2 presents the AAPC for etiology-specific liver cancer and age groups between 1990 and 2019. Total liver cancer and etiology-specific liver cancer cases showed a negative AAPC, which indicates declining trends between 1990 and 2019. Among the total liver cancer and etiology-specific liver cancer cases, people at 50–69 years of age had the largest AAPC, indicating the largest decline in age-standardized incidence rates. People at 15–49 years of age and people at 70+ years of age had similar AAPC values, yet the 95% CI of the AAPC for people at 15–49 years of age was substantially wide, indicating the low reliability of the AAPC estimate. The AAPC was larger in liver cancer due to HBV and HCV than in liver cancer due to other etiologies, and this finding was consistent for all age groups.
Figure 2.
Average annual percent of change in age-standardized incidence rates for liver cancer and etiologyspecific liver cancer by age group between 1990 and 2019 in China.
Table 2 presents the results of joinpoint regression analysis for liver cancer between 1990 and 2019. In general, the temporal liver cancer trends are characterized by three distinct periods (approximately 1990–1999; 1999–2006; and 2006–2019). The age-standardized rates of incidence, mortality, and DALYs of the total liver cancer cases showed similar temporal patterns, presenting an overall decline in the AAPC ranging from −3.3% to −3.8%, which was mainly driven by rapid declines between 1999 and the mid-2000s, with AAPC values of approximately −15%. The temporal pattern of etiology-specific liver cancer generally resembled that of total liver cancer. Of note, liver cancer attributable to HBV appeared to have a more pronounced decline (a larger AAPC) between 1999 and the mid-2000s than liver cancer of other etiologies. In addition, we observed upward trends from 2006 to 2019 for liver cancer due to alcohol and NASH.
Table 2.
Joinpoint regression analysis of liver cancer between 1990 and 2019 in China.
| Disease | Metric | Statistics | Period | Values |
|---|---|---|---|---|
| Total liver cancer | Incidence rates | APC | 1990–1999 | −0.2 (−0.6, 0.9) |
| APC | 1999–2006 | −13.7 (−14.9, −12.4) | ||
| APC | 2006–2019 | −0.4 (−0.1, 0.8) | ||
| AAPC | 1990–2019 | −3.3 (−3.7, −2.9) | ||
| Mortality rates | APC | 1990–1999 | 0.4 (−0.5, 1.3) | |
| APC | 1999–2005 | −15.7 (−17.6, −13.7) | ||
| APC | 2005–2019 | −0.8 (−1.3, −0.3) | ||
| AAPC | 1990–2019 | −3.7 (−4.3, −3.2) | ||
| DALYs rates | APC | 1990–1999 | −14.7 (−16.2, −13.1) | |
| APC | 1999–2006 | −0.1 (−0.6, 0.5) | ||
| APC | 2006–2019 | −3.9 (−4.4, −3.3) | ||
| AAPC | 1990–2019 | −3.8 (−5.5, −2.1) | ||
| Liver cancer attributable to HBV | Incidence rates | APC | 1990–1999 | 0.4 (−0.4, 1.2) |
| APC | 1999–2006 | −14.0 (−12.7, −21.1) | ||
| APC | 2006–2019 | 0.3 (−0.2, 0.7) | ||
| AAPC | 1990–2019 | −3.3 (−3.8, −2.9) | ||
| Mortality rates | APC | 1990–1999 | 0.7 (−0.2, 1.6) | |
| APC | 1999–2005 | −17.8 (−14.1, −16.5) | ||
| APC | 2005–2019 | −0.9 (−1.4, −0.5) | ||
| AAPC | 1990–2019 | −3.8 (−4.3, −3.3) | ||
| DALYs rates | APC | 1990–1999 | 0.4 (−0.6, 1.4) | |
| APC | 1999–2005 | −16.6 (−18.5, −14.5) | ||
| APC | 2005–2019 | −0.8 (−1.3, −0.3) | ||
| AAPC | 1990–2019 | −3.9 (−4.5, −3.4) | ||
| Liver cancer attributable to HCV | Incidence rates | APC | 1990–1999 | −0.5 (−1.2, 0.3) |
| APC | 1999–2006 | −13.0 (−14.2, −11.8) | ||
| APC | 2006–2019 | −0.1 (−0.5, 0.3) | ||
| AAPC | 1990–2019 | −3.5 (−3.9, −3.1) | ||
| Mortality rates | APC | 1990–1999 | −0.6 (−1.6, 0.4) | |
| APC | 1999–2006 | −13.3 (−14.9, −11.7) | ||
| APC | 2006–2019 | −0.6 (−1.6, 0.4) | ||
| AAPC | 1990–2019 | −3.8 (−4.4, −3.3) | ||
| DALYs rates | APC | 1990–1999 | −0.9 (−1.9, −0.2) | |
| APC | 1999–2006 | −14.0 (−15.7, −12.3) | ||
| APC | 2006–2019 | −0.5 (−1.1, 0.1) | ||
| AAPC | 1990–2019 | −4.0 (−4.6, −3.5) | ||
| Liver cancer attributable to alcohol use | Incidence rates | APC | 1990–1999 | −0.2 (−1.0, 0.6) |
| APC | 1999–2006 | −13.2 (−14.5, −11.9) | ||
| APC | 2006–2019 | 1.5 (1.1, 2.0) | ||
| AAPC | 1990–2019 | −2.8 (−3.2, −2.3) | ||
| Mortality rates | APC | 1990–1999 | −0.3 (−1.3, 0.7) | |
| APC | 1999–2006 | −13.5 (−15.1, −11.9) | ||
| APC | 2006–2019 | 1.0 (0.4, 1.6) | ||
| AAPC | 1990–2019 | −3.1 (−3.7, −2.6) | ||
| DALYs rates | APC | 1990–1999 | −0.7 (−1.7, 0.4) | |
| APC | 1999–2006 | −14.2 (−15.8, −12.5) | ||
| APC | 2006–2019 | 1.0 (0.4, 1.6) | ||
| AAPC | 1990–2019 | −3.4 (−4.0, −2.8) | ||
| Liver cancer attributable to NASH | Incidence rates | APC | 1990–2000 | −0.5 (−1.2, 0.1) |
| APC | 2000–2006 | −14.3 (−15.9, −12.7) | ||
| APC | 2006–2019 | 1.7 (1.3, 2.1) | ||
| AAPC | 1990–2019 | −2.6 (−3.0, −2.2) | ||
| Mortality rates | APC | 1990–1999 | 0.3 (−0.5, 1.2) | |
| APC | 1999–2006 | −13.3 (−14.7, −11.9) | ||
| APC | 2006–2019 | 1.1 (0.6, 1.6) | ||
| AAPC | 1990–2019 | −2.8 (−3.3, −2.4) | ||
| DALYs rates | APC | 1990–1999 | 0.1 (−0.8, 1.1) | |
| APC | 1999–2006 | −14.3 (−15.8, −12.8) | ||
| APC | 2006–2019 | 1.0 (0.5, 1.6) | ||
| AAPC | 1990–2019 | −3.2 (−3.7, −2.7) |
AAPC: the average annual percentage change; APC: the annual percentage change; DALYs: disability-adjusted life years; HBV: hepatitis B; HCV: hepatitis C.
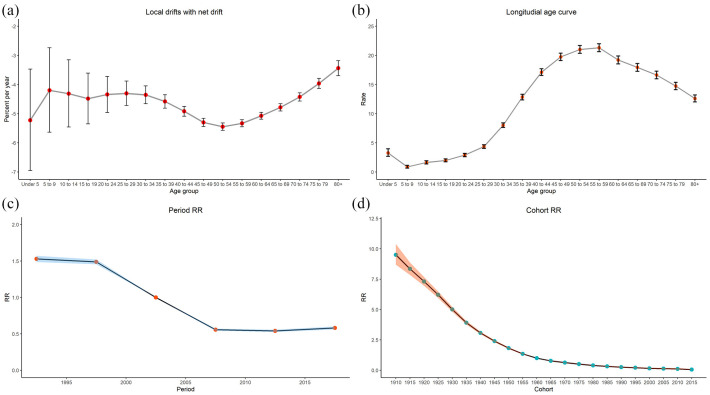
Figure 3 presents the results of the age-period-cohort analysis of the incidence rate of total liver cancer between 1990 and 2019. Age-period-cohort analysis generally revealed the overall decline in the age-standardized incidence rates of the total liver cancer cases, the total liver cancer risk peak in people at 55–59 years of age, the period RRs of the liver cancer incidence rates in China changed in the 2000s, with higher RRs before 2007 and reduced RRs after 2007. Figure 3(a) shows the local net age-specific range of change between 1990 and 2019. The local drifts were negative in all age groups, representing a decline in the age-standardized incidence rates of the total liver cancer cases. The local net drifts were relatively lower among people at 40–59 years of age, which may suggest that the decline in liver cancer incidence rates was more apparent in those age groups. Figure 3(b) illustrates the longitudinal age curves of the incidence rates for the total liver cancer cases between 1990 and 2019. In the same birth cohort, total liver cancer risk increased rapidly in people at age 30 and above and then peaked in people at 55–59 years of age. Although people at age 60 and above had a relatively lower risk than people at 55–59 years of age, they still had a substantially higher risk than people at a young age. The period RRs showed decreasing trends for liver cancer incidence rates in China (Figure 3(c)). People in the period before 2007 had higher risk, people after 2007 had lower risk, and after 2007, there was no further decline in risk. The cohort RRs showed decreasing patterns, with the most rapid decline observed in the 1910 to 1960 cohorts (Figure 3(d)).
Figure 3.
Age-period-cohort analysis of age-standardized incidence rates for liver cancer between 1990 and 2019 in China (a) Local drifts with net drift. (b) Longitudial age curve. (c) Period RR. (d) Cohort RR.
Discussion
To our limited knowledge, this study is the first to describe the long-term secular trends in the incidence, mortality, and DALYs of etiology-specific liver cancer in China. Several key messages could be derived from our investigation. Our study revealed that total liver cancer and etiology-specific liver cancer cases all showed a decreasing trend in incidence, mortality, and DALYs from 1990 to 2019 in China. Remarkably, the most rapid decline in liver cancer and etiology-specific liver cancer cases occurred between 1999 and the mid-2000s. For etiology-specific liver cancer cases, the magnitude and rate of decline were more pronounced for liver cancer attributable to HBV and HCV than for liver cancer attributable to other etiologies. There were recent upward trends in liver cancer due to alcohol use and NASH. The age-period-cohort analysis results revealed that the period RRs of the liver cancer incidence rates in China changed in the 2000s, with higher RRs in the years before 2007 and reduced RRs after 2007. People born after 2000 had a low risk, and there was no further decline in risk thereafter.
Liver cancer used to be one of the most frequently diagnosed cancers in China and has claimed many lives. Chronic HBV infection has been widely acknowledged as the leading cause of liver cancer in China. 18 In 1992, the prevalence of HBV among the population at 1–59 years of age was reported to be as high as 9.75%. 19 To control the disease, China included HBV vaccination for newborns in its national immunization program beginning in 1992; however, vaccine coverage was still suboptimal. Subsequently, China’s Ministry of Public Health included neonatal HBV vaccination in the national immunization program beginning in 2002, with the vaccine provided entirely by the government, and since then, the overall coverage of hepatitis B vaccine in infants has increased steadily and reached more than 95.0% in urban and 97.0% in rural areas. 20 Neonatal hepatitis B vaccination has proven efficient in many countries, including China, to prevent HBV-related liver cancer, and this effect does not necessarily take a long time; the decline in related liver cancer cases usually occurs after the vaccination program begins. 21 Our study revealed that a rapid decline in HBV-related liver cancer was observed between 1990 and the mid-2000s, which approximately coincided with the timeline of China’s HBV immunization plan. In addition, abundant epidemiological evidence suggests that aflatoxin exposure synergizes with chronic hepatitis B virus (HBV) infection to increase liver cancer. 22 Food policy reforms in China resulted in a dramatic decrease in aflatoxin exposure, which was independent of the HBV vaccination or combined effects involving HBV vaccination, reducing liver cancer incidence in endemic areas.12,23–25
Chronic HCV infection is an increasingly common cause of liver cancer in China. The prevalence rate of HCV was only 0.4% among the population less than 60 years old in China, which is substantially lower than the prevalence of the surface antigen of the hepatitis B virus (HBsAg). 26 However, because of the large population size, there are still many liver cancer cases due to HCV. Unlike HBV, there is no efficient vaccine for HCV prevention. Since the early 1990s, China implemented routine screening for HCV, and many HCV-infected people have been diagnosed. However, early treatment of HCV is not efficient, and only 6%–16% of patients can be effectively treated. 27 PEG IFN/RBV treatment, as the mainstream treatment for hepatitis C, significantly improved the patient sustained virologic response (SVR) from 2004 to 2014. China’s rapid decline in HCV-attributable liver cancer may be the result of the combination of routine screening for HCV and rapid improvements in HCV treatment. However, after 2010, there was no further decline in HCV-attributable liver cancer. Novel direct-acting antivirals (DAAs) represent a significant breakthrough in HCV treatment, with reported SVR rates as high as 97%.28,29 Previous modeling studies have suggested that with the wide use of DAAs, there will be a tremendous decline in HCV-related complications, including liver cancer. 30
The changing pattern of liver cancer attributable to other etiologies has rarely been reported in the literature. Generally, liver cancer attributable to NASH and alcohol use constitute only a small fraction of the total liver cancer cases and is declining overall. However, it is notable that liver cancer attributable to NASH and alcohol use showed increasing trends after 2006. Alcohol use and obesity are common risk factors for liver cancer in Western countries. 31 Many studies have revealed that there are increasing trends in alcohol-related or obesity-related liver cancer in high-income countries.32,33 The recent increase in liver cancer attributable to NASH and alcohol use in China may reflect the changing lifestyles of Chinese people. Many studies have suggested that an increasing number of people have engaged in harmful drinking behaviors over the past decade, especially younger adults.34,35 Meanwhile, the prevalence of obesity has increased in most areas of the world, including China. 36 Note that a NASH diagnosis may require liver biopsy, which is difficult to implement to determine liver cancer etiologies in the real world. Liver biopsy for NASH diagnosis is usually recommended for people who have certain indications, including elevated liver enzyme levels or clinical signs of liver disease. 37 Therefore, many people with NASH that does not necessitate liver biopsy may miss the opportunity to get diagnosed. Due to a lack of awareness about NASH and due to missed diagnoses, the actual incidence of NASH-related liver cancer might be underestimated. There is no valid pharmaceutical intervention or treatment for the control of alcohol or obesity. Lifestyle interventions for heavy alcohol use and obesity are urgently needed in China to address the increasing burden of liver cancer related to alcohol and NASH.
The results also showed that the RRs of liver cancer incidence rates in China changed in the 2000s, with higher RRs in the years before 2007 and lower RRs after 2007. The reduced RR period after the 2000s was largely attributable to the nationwide HBV immunization program beginning in 2002, the improvement of HCV screening and treatment for HCV in the early 2000s. Our analysis also revealed a consistent decline in cohort effects, which can be explained by improvements in socioeconomic status, access to treatment, sanitation, hygiene, and clean water in China. There are no hepatitis data in China from the early 20th century, yet life expectancy soared by approximately 30 years after the establishment of New China in 1949. 38 We also found that people born after 2000 have low risk, and there is therefore no further decline in this risk. This finding is consistent with the timeline of HBV vaccine implementation in newborns, which may substantially reduce the risk of HBV-related liver cancer in China.
There are several limitations that must be acknowledged before interpreting our results. First, detailed data within China, including provinces and urban or rural areas, were not included in the GBD datasets. Therefore, further studies involving comprehensive and detailed liver cancer data are warranted. Second, the GBD study used sophisticated statistical modeling to derive estimates from limited raw data, which depends on heavy assumptions on the modeling process. Therefore, the temporal trends of liver cancer in China should be interpreted with caution. Finally, we could only study the temporal trend in liver cancer by each etiology, and the interactions of several etiologies, including the interactions of HBV and/or HCV and alcohol and/or obesity, were not considered in this study.
Our study generally revealed favorable decreasing trends for total liver cancer and etiology-specific liver cancer in China from 1990 to 2019. The decline in liver cancer attributable to HBV and HCV may be the result of the HBV immunization program and massive screening and treatment for HCV. Despite the overall decline in liver cancer due to heavy alcohol use and obesity from 1990 to 2019, there have been apparent upward trends since 2006. Planned population-wide interventions targeting heavy alcohol use and obesity may mitigate the increasing trends in liver cancer attributable to alcohol use and NASH.
Author biographies
Songxia Yu, MD, is a clinical research scientist in Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, Research Center for Clinical Pharmacy, The First Affiliated Hospital, College of Medicine, Zhejiang University
Haowen Wang, MD, is a clinical research scientist in Department of Vascular Surgery and Vascular Interventional Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College
Tingyang Hu, MD, is a clinical research scientist in Department of Vascular Surgery and Vascular Interventional Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College
Chengbo Yu, MD, is a clinical research scientist in State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Department of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University
Hanbo Liu, MD, is a clinical research scientist in Department of Vascular Surgery and Vascular Interventional Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College
Xudong Chen, MD, is a clinical research scientist in Department of Vascular Surgery and Vascular Interventional Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College
Jingsong Jiang, MD, is a clinical research scientist in Department of Vascular Surgery and Vascular Interventional Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College
Min Deng, MD, is a clinical research scientist in Department of Vascular Surgery and Vascular Interventional Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College
Footnotes
Author Contributions: Songxia Yu and Min Deng conceived and designed the study, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Haowen Wang conceived and designed the study, authored or reviewed drafts of the paper, and approved the final draft.
Tingyang Hu and Chengbo Yu analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Hanbo Liu, Xudong Chen and Jingsong Jiang prepared figures and/or tables, and approved the final draft.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Medical & Health Technology Program of Zhejiang Province (grant No. 2016KYA072 and 2018KY652) and Hangzhou Xiaoshan District Social Development Major Science and Technology Project (grant No.2017201).
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
This study used publicly available deidentified data accessed from the Global Burden of Disease Study 2019 repository. Therefore, for the secondary analysis, ethical approval, and statement from an ethics committee or institutional review board were not required.
Informed consent: Informed consent for patient information to be published in this article was not obtained because *REASON*.
This study used publicly available deidentified data accessed from the Global Burden of Disease Study 2019 repository. Informed consent was not required for this study.
ORCID iD: Min Deng  https://orcid.org/0000-0003-1240-8612
https://orcid.org/0000-0003-1240-8612
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011; 15: 223–243, vii–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Wang Y, Feng X, et al. Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China north areas: experience of the Chinese National Cancer Center. Int J Infect Dis 2017; 65: 15–21. [DOI] [PubMed] [Google Scholar]
- 4.Luo R.-H. Risk factors for primary liver carcinoma in Chinese population. World J Gastroenterol 2005; 11: 4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groopman JD, Johnson D, Kensler TW. Aflatoxin and hepatitis B virus biomarkers: a paradigm for complex environmental exposures and cancer risk. Cancer Biomark 2005; 1: 5–14. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CJ, Yang YW, You SL, et al. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013; 310: 974–976. [DOI] [PubMed] [Google Scholar]
- 7.Qu C, Chen T, Fan C, et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: a cluster randomized controlled trial. PLoS Med 2014; 11: e1001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res 2018; 30: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Deng Y, Tang W, et al. Urban-rural disparity in cancer incidence, mortality, and survivals in Shanghai, China, during 2002 and 2015. Front Oncol 2018; 8: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Yang S, Xu K, et al. Patterns and trends of liver cancer incidence rates in eastern and southeastern Asian countries (1983-2007) and predictions to 2030. Gastroenterology 2018; 154: 1719–1728.e5. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Xie L, Chen WQ, et al. Rural-urban, sex variations, and time trend of primary liver cancer incidence in China, 1988-2005. Eur J Cancer Prev 2013; 22: 448–454. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Chen T, Thorgeirsson SS, et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 2013; 34: 1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new who standard. Geneva: World Health Organization, 2001. Available at: http://www.who.int/healthinfo/paper31.pdf (accessed 7 May 2021). [Google Scholar]
- 16.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979; 74: 829–836. [Google Scholar]
- 17.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–351. [DOI] [PubMed] [Google Scholar]
- 18.de Martel C, Maucort-Boulch D, Plummer M, et al. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 2015; 62: 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Men P, Xiao Y, et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis 2019; 19: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis 2012; 16: e82–e88. [DOI] [PubMed] [Google Scholar]
- 21.Chang MH, Chen CJ, Lai MS, et al.; Taiwan Childhood Hepatoma Study Group. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med 1997; 336: 1855–1859. [DOI] [PubMed] [Google Scholar]
- 22.Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev 1994; 3: 3–10. [PubMed] [Google Scholar]
- 23.Chen JG, Egner PA, Ng D, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013; 6: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Zhu J, Wang G, et al. Qidong: a crucible for studies on liver cancer etiology and prevention. Cancer Biol Med 2019; 16: 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JG, Zhu J, Zhang YH, et al. Liver cancer mortality over six decades in an epidemic area: what we have learned. PeerJ 2021; 9: e10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YS, Li L, Cui FQ, et al. [A sero-epidemiological study on hepatitis C in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2011; 32: 888–891. [PubMed] [Google Scholar]
- 27.Muir AJ. The rapid evolution of treatment strategies for hepatitis C. Am J Gastroenterol 2014; 109: 628–635. [DOI] [PubMed] [Google Scholar]
- 28.Das D, Pandya M. Recent advancement of direct-acting antiviral agents (DAAs) in hepatitis C therapy. Mini Rev Med Chem 2018; 18: 584–596. [DOI] [PubMed] [Google Scholar]
- 29.Pecoraro V, Banzi R, Cariani E, et al. New direct-acting antivirals for the treatment of patients with hepatitis C virus infection: a systematic review of randomized controlled trials. J Clin Exp Hepatol 2019; 9: 522–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Zhou Y, Fu X, et al. The burden of chronic hepatitis C in China from 2004 to 2050: an individual-based modeling study. Hepatology 2019; 69: 1442–1452. [DOI] [PubMed] [Google Scholar]
- 31.Saitta C, Pollicino T, Raimondo G. Obesity and liver cancer. Ann Hepatol 2019; 18: 810–815. [DOI] [PubMed] [Google Scholar]
- 32.Julien J, Ayer T, Bethea ED, et al. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019–40: a modelling study. Lancet Public Health 2020; 5: e316–e323. [DOI] [PubMed] [Google Scholar]
- 33.Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017; 67: 302–309. [DOI] [PubMed] [Google Scholar]
- 34.Im PK, Millwood IY, Guo Y, et al. Patterns and trends of alcohol consumption in rural and urban areas of China: findings from the China Kadoorie Biobank. BMC Public Health 2019; 19: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manthey J, Shield KD, Rylett M, et al. Obesity: global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 2019; 393: 2493–2502. [DOI] [PubMed] [Google Scholar]
- 36.Morgen CS, Sørensen TI. Global trends in the prevalence of overweight and obesity. Nat Rev Endocrinol 2014; 10: 513–514. [DOI] [PubMed] [Google Scholar]
- 37.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 38.Hipgrave D. Communicable disease control in China: from Mao to now. J Glob Health 2011; 1: 224–238. [PMC free article] [PubMed] [Google Scholar]