Abstract
Benign paroxysmal positional vertigo is characterized by recurrence, which exposes patients to repeated vertigo attacks. Vitamin D deficiency has been found to be a risk factor in benign paroxysmal positional vertigo, although effect of its elimination on recurrence reduction remains unknown. To determine the effect of vitamin D supplementation in preventing recurrence of benign paroxysmal positional vertigo patients with vitamin D deficiency using a meta-analysis study. We searched and retrieved relevant articles from several databases, then used the Cochrane evaluation system or Methodological Index for Non-Randomized Studies (MINORS) to assess the quality of studies. We adopted risk-ratio (RR) with corresponding 95% confidence interval (CI) to determine effect sizes, and further performed statistical analyses under a randomized- or fixed-effects model. Seven studies, comprising 602 and 731 participants in the case and control group respectively, met our inclusion criteria, and were therefore included in the meta-analysis. Assessment based on Cochrane evaluation system or MINORS revealed that most of the studies had high quality. Moreover, the randomized- model revealed that the vitamin D supplementation group had a lower recurrence rate than the control group which did not accepted vitamin D supplementation (RR = 0.41, 95% CI = 0.26–0.65, p < 0.01). Overall, these findings indicate that vitamin D supplementation can significantly lower recurrence in benign paroxysmal positional vertigo and vitamin D deficiency.
Keywords: BPPV, vitamin D, meta-analysis, recurrence, drug
Introduction
Benign paroxysmal positional vertigo (BPPV) is characterized by transient fierce rotational vertigo and specific nystagmus, which result from otoconias displacement from utricle and flow into semicircular canals. This condition is evoked by head position change in gravity direction. Recently, scanning electron micrographs confirmed the pathology of BPPV to be related to free-floating otoconias that originate from utricle extracted from posterior semicircular canal. 1 Previous studies have shown that particle repositioning maneuvers or canalith repositioning procedure (CRP) is an effective and noninvasive treatment therapy. This approach was found to completely relieve symptoms in 91.3% of patients after one or two treatment courses, 2 and has subsequently been recommended worldwide. BPPV is the most common disease in vertigo patients, with a population lifetime prevalence of 2.4% the disease is more common in the elderly, where it accounts for 8% of patients with moderate or severe dizziness/vertigo. 3
Otoconia is composed by peripheral inorganic calcium carbonate and an organic core which predominantly consists of glycoproteins. 4 BPPV is prone to spontaneous remission and recurrence, because Otoconia goes through degeneration, absorption and regeneration in vivo. A previous epidemiological study reported a 15% BPPV recurrence rate in the first year after CRP treatment, which increased to 50% over 3 years. 2 Calcium homeostasis plays an important role in otoconia formation and absorption. Vitamin D regulates calcium and bone metabolism, which has been implicated in BPPV development. 5 A previous meta-analysis found significantly lower vitamin D levels in recurrence than single-attack patients, affirming that vitamin D plays an important role in BPPV recurrence. 6
A study reported a 78% reduction in BPPV-related falls in a geriatric group of patients, which was attributed to its sudden repeated fierce vertigo attacks and subsequent residual dizziness. 7 The most severe damage caused by BPPV was fall-related injuries and disability, which adversely affected quality of life, including health status, economic resources, work status, social relationships, and leisure activities. 8 Talaat et al. 11 , Jeong et al. 15 showed that improving vitamin D deficiency, via exogenous supplementation, can reduce BPPV recurrence. Conversely, Rhim 14 reported that treating vitamin D deficiency could not reduce the recurrence. Notably, most of the studies on this subject have used small sample sizes, casting doubt on the reliability of the findings. Therefore, there is need to develop approaches to effectively manage BPPV. The present study adopted a meta-analysis to evaluate the effect of eliminating vitamin D deficiency on BPPV recurrence.
Materials and methods
Literature retrieval
We systematically searched for eligible studies from PubMed, Cochrane, Embase, Web of Science, Chinese National Knowledge Infrastructure, Wanfang, and SinoMed Databases. The search string was as follows:[“vitamin D” or “calcifediol” or “25(OH) vitamin D” or “25-hydroxylvitamin D” or “25-hydroxyvitamin D” or “25(OH)D” or “osteoporosis” or “osteopenia” or “drugs” or “Pharmaceutical Preparations”] and [“benign paroxysmal positional vertigo” or “BPPV” or “BPV”]. There was neither language nor date restrictions in our searches.
Literature selection
Studies that met the following criteria were included in our analysis: (1) analyzed supplementation of vitamin D in BPPV in combination with vitamin D deficiency (deficiency <20 ng/mL); (2) BPPV was idiopathic without any secondary cause; (3) BPPV was not accompanied by renal disease, Ménière disease, or any ear disease, including chronic otitis media, vestibular neuritis, and otitis media on the same side; (4) One outcome compared recurrence and presented the data; and (5) clearly defined cases and controls. Studies that were reviews, editorials, case reports, commentaries, or critiques, and those not written in the language, were excluded from our analysis.
Data extraction and quality assessment
Two researchers (Zhiling Yang and Min Xie) independently searched the databases and collected data, based on the aforementioned criteria. The reviewers consulted each other in case of discordance, and a third researcher (Juanli Li) was consulted to resolve any inconsistencies. The reviewers extracted several pieces of information, including name of the first author, publication date, study type, country, duration of follow-up, p-value of recurrence difference, patient information such as age, sex, subtype of BPPV, number of participants, clinical course, repositioning maneuver, recurrence number, vitamin D concentrations (ng/mL) before and after treatment, as well as medical administration parameters, namely drug components, approach, dosage, and duration. Quality of the selected studies was assessed using the Cochrane evaluation system or MINORS items according to the different design of trials.
Statistical analysis
Effect size of the outcomes was presented as risk ratio (RR), with corresponding 95% confidence intervals (CI), whereas heterogeneity across studies was evaluated using Cochrane-based Q and I 2 tests. Data followed by p < 0.05 or I 2 > 50% were considered to denote statistically significant heterogeneity, and were subjected to a randomized-effects model. Otherwise, a fixed-effects model was used. All statistical analyses were conducted using Revman 5.3 software.
Results
Characterization of the selected studies
Our search strategy resulted in 2732 articles. The first screening allowed exclusion of 670 duplicate articles, while a further 2052 were excluded after reading the titles and abstracts. This is because 1639 were irrelevant, 58 were reviews, 351 did not report vitamin D supplementation, and 4 did not have full texts. Full texts for the remaining 10 articles were intensively read, and 2 of these that had not adopted recurrence as observational indicator subsequently excluded. One article sought to determine pharmacological history of patients with idiopathic BPPV in relation to the risk of developing recurrence, with one of the pharmacological histories describing vitamin D. This article did not satisfy our inclusion criteria and was therefore eliminated from the analysis. Finally, seven articles were included in the our meta-analysis.9–15 These articles comprised 602 and 731 participants in the case and control groups, respectively. A flow diagram describing study search is shown in Figure 1, while characteristics of the seven included articles are summarized in Table 1.
Figure 1.
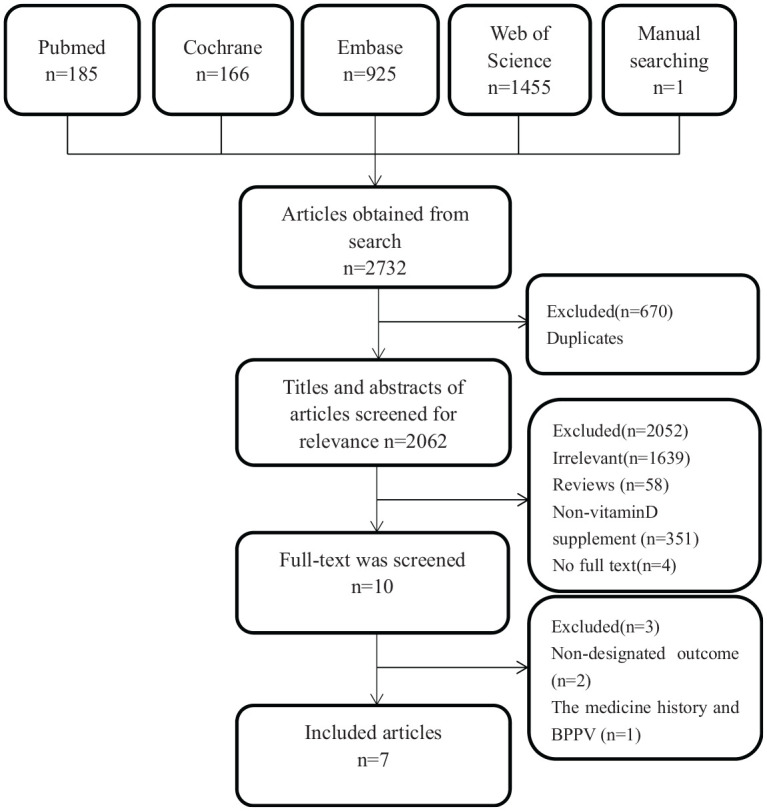
The screening flow chart.
Table1.
The characteristics of the seven included articles.
| Study | Age, years | n(M/F) | Course | Subtype of BPPV | Vitamin D concentration leverl (ng·mL−1) | Medication | n (Recurrence) | p-value (Recurrence difference) | Study type | Follow up | Quality assessment levels | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | |||||||||||
| Büki et al. 9 | Case:58.5a control:58.5a | Case:4(0/4) control:4(0/4) | Over several years | Recurrent PC-BPPV | Case: 14 control: 14 | NA | Case: daily 8000 IU cholecalciferol for 2 weeks, and daily 4000 IU cholecalciferol for the next 2 weeks, then a weekly dose of 8000 IU was given as recommended control: before medication | 0; 4 | NA | Comparative before-after study | 8 months | 15△ |
| S heikhzadeh et al. 10 | Case: 47.8 ± 5.7a control: 48.2 ± 4.8a | Case: 27(12/15)cotrol:27(13/14) | over 6 months | Recurrent PC-BPPV | Case: 11.41 ± 1.9 control: 10.7 ± 2.3 | Case: first month 19.37 ± 3.3; 10.6 ± 2.2 second month 34.2 ± 3.3; 10.6 ± 2.2 second month 35.5 ± 2.9; 11.1 ± 2.3 control: NA | Case: 50,000 IU cholecalciferol weekly for 2 months. Control: no medication. | 4/26 | p = 0.001 | Case-control | 6 months | 22△ |
| Talaat et al. 11 | Case:50.8 ± 12.8a control:50.5 ± 11.4a | Case:28(13/15) control: 65(29/36) | NA | Non-recurrent PC-BPPV HC-BPPV | Case:6.7 ± 2 control:6.8 ± 2.1 | Case:28.3 ± 5; control:8.7 ± 3.5 | Case:50,000 IU oral vitamin D3, three times weekly, maintenance dose: 50,000 IU oral vitamin D3, once every 2 weeks, Calcium citrate 600 mg tablets twice daily control: no medication. | 4; 28 | p < 0.001 | non-RCT | 18 months | 22△ |
| Carneiro de Sousa et al. 13 | Case: 71c control:57c | Case: 5(NA) control:5(NA) | Over 2 years | Recurrent PC-BPPV HC-BPPV AC-BPPV | Case: 14.0 control: 13.2 | Case: third month 33.8 sixth month 31.8 ninth month 30.6 12th month 35.2 control: NA | Case: eight oral drops per day( 5000 IU of vitamin D per day) control: no medication | 0; 5 | NA | non-RCT | 1 year | 16△ |
| Califano et al. 12 | Case: 60.2 ± 12.41a control:60.2 ± 12.41a | case: 68(40/28) control:68(40/28) | Over 1 year | Recurrent PC-BPPV HC-BPPV | Case: 18.2 ± 10.43 control: 18.2 ± 10.43 | NA | Case: cholecalciferol (vitamin D3) per os, from 10,000 to 50,000 IU, weekly, with the overall limit of 600,000 IU in a year control: before medication | 13; 28 | p < 0.001 | Comparative before-after study | 1 year | 22△ |
| Rhim 14 | Case: 40b control: 45b | Case:25(4/21) control: 50(8/42) | NA | Non-recurrent PC-BPPV HC-BPPV AC-BPPV MC-BPPV | Case: 6.06 control: 7.14 | Case: 31.1 control: NA | Case: intramuscular injection 200,000 IU three to four injections in the first year, The injection solution contains 200,000 IU (5 mg) of cholecalciferol. control: no medication. | 7/13; | p < 0.883 | non-RCT | 2 years | 22△ |
| Jeong et al. 15 | Case: 62.2 ± 11.7a control:61.6 ± 12.2a | Case:445(162/283) control:512(179/333) | NA | Non-recurrent PC-BPPV HC-BPPV AC-BPPV MC-BPPV | Case: 13.3 ± 3.9 control: 13.3 ± 3.9 | Case:24.2 ± 8.4 control: NA | Case: vitamin D 400 IU and 500 mg of calcium carbonate twice a day for 1 year. Control: no medication. | 168; 239 | p = 0.005 | RCT | 1 year | Low risk of bias☆ |
a: Mean ± SD; AC-BPPV: anterior semicircular canal BPPV; b: median age; c: mean (range); F: female; HC-BBPV: lateral semicircular canal BPPV; M: male; MC-BPPV: multiple semicircular canal BPPV; NA: (not available); PC-BBPV: posterior semicircular canal BPPV; RCT: randomized controlled trail; △: MINORS items used for quality assessment; ☆: Cochrane bias evaluation system used for quality assessment.
Quality assessment
Methodological quality of the randomized controlled trial (RCT) was assessed using the Cochrane evaluation tool for assessing risk of bias. This assessment comprised seven items, namely random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Moreover, we included three levels of risk assessment, namely low, unclear, and high risk of bias. One, out of the seven included studies, represented a single-blind RCT, although the Cochrane evaluation system revealed that it was a high quality RCT with a low risk of bias.
We adopted MINORS guidelines to assess methodological quality in non-randomized controlled trials. These guidelines consisted of 12 indices, namely (i) a clearly stated aim; (ii) inclusion of consecutive patients; (iii) prospective collection of data; (iv) endpoints appropriate to the aim of the study; (v) unbiased assessment of the study endpoint; (vi) follow-up period appropriate to the aim of the study; (vii) loss to follow-up less than 5%; (viii) prospective calculation of the study size; (ix) adequate control group; (x) contemporary groups (control and studied group should be managed during the same time period, no historical comparison); (xi) baseline equivalence of groups; and (xii) adequate statistical analyses. The total score is 24. The high quality was defined as the score is ≥16 points, otherwise the quality is low (<16 points). Since most of the studies herein were non-randomized controlled trials, we adopted MINORS to assess their quality. Four non-RCT trials had scores of 22 points, and were subsequently classified as high quality. The rest two studies were classified as median quality. Quality results are summarized in Table 1, while a detailed description of the scores is presented in Table 2.
Table 2.
The detailed MINORS scores of the six non-randomized trials.
| Items Study | Bükiet al. 9 | Sheikhzadehet al. 10 | Talaatet al. 11 | Carneirode Sousaet al. 13 | Califanoet al. 12 | Rhim 14 |
|---|---|---|---|---|---|---|
| A stated aim of the study | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutivepatients | 2 | 2 | 2 | 2 | 2 | 2 |
| Prospective collectionof data | 2 | 2 | 2 | 2 | 2 | 2 |
| Endpoint appropriateto the study aim | 1 | 2 | 2 | 2 | 2 | 2 |
| Unbiased evaluationof endpoints | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow-up periodappropriateto the major endpoint | 2 | 2 | 2 | 2 | 2 | 2 |
| Loss to follow up notexceeding 5% | 2 | 2 | 2 | 2 | 2 | 2 |
| A control group having thegold standard intervention | 2 | 2 | 2 | 2 | 2 | 2 |
| Contemporary groups | 0 | 2 | 2 | 2 | 2 | 2 |
| Baseline equivalenceof groups | 2 | 2 | 2 | 0 | 2 | 2 |
| Prospective calculation f the sample size | 0 | 2 | 2 | 0 | 2 | 2 |
| Statistical analyses adaptedto the study design | 0 | 2 | 2 | 0 | 2 | 2 |
| total points | 15 | 22 | 22 | 16 | 22 | 22 |
Outcomes
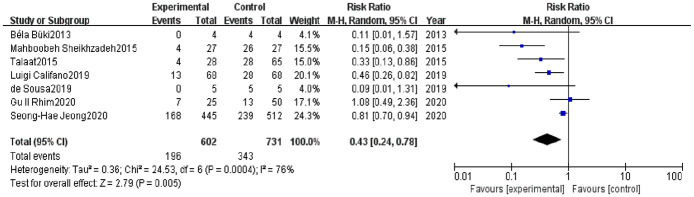
We applied the random effects model to analyze recurrence events between groups due to presence of remarkable heterogeneity (I 2 = 76%, p < 0.0004) (Figure 2). Forest plots revealed significant differences between vitamin D supplementation case groups and controls (RR = 0.43, 95% CI = 0.24–0.78, p <0.01; Figure 2).
Figure 2.
Forest plot comparison of vitamin D supplementation in preventing recurrences of benign paroxysmal positional vertigo and vitamin D deficiency.
Sensitivity analysis and publication bias
Sensitivity analyses, conducted using a leave-one-out analysis approach, revealed that none of the exclusions altered results from present analyses. This indicated excellent reliability and stability of our results. Furthermore, we found significant heterogeneity among the studies (I 2 = 76%, p < 0.0004), with no evidence of a sharp decline after exclusion any of studies. Moreover, funnel plots showed that a majority of spots were concentrated and located top of the plot, indicative of low publication bias (Figure 3).
Figure 3.
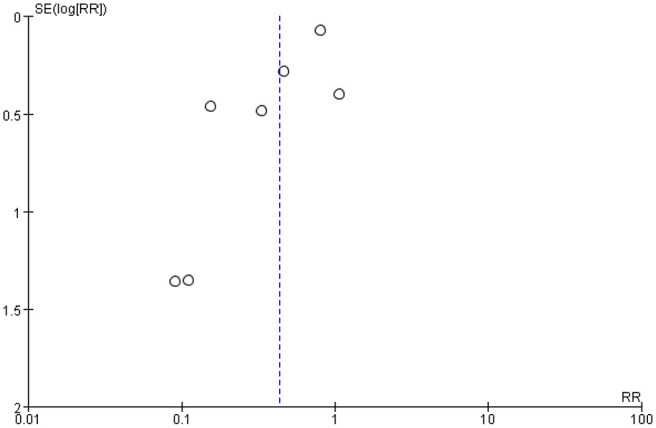
Funnel plot comparison of vitamin D supplementation in preventing recurrences of benign paroxysmal positional vertigo and vitamin D deficiency.
Discussion
BPPV is the most common peripheral vestibule vertigo disease. Its symptoms include vestibular disorders and vegetative nerve response reactions, namely rotational vertigo, oscillopsia, imbalance, fear of falling, nausea, and vomit. In the present study, we focused on idiopathic BPPV, since this represents the largest proportion of the disease. Quality of life in BPPV patients is severely influenced, both physically and psychologically, due to vertigo recurrent attacks. Results of the present study indicated that supplementation of vitamin D can significantly reduce recurrence in BPPV combined with vitamin D deficiency. Moreover, supply treatment is beneficial for BPPV patients, hence could be a precautionary medication for managing recurrence in these patients. Notably, we observed obvious heterogeneity among the included studies, possibly due to the following reasons:(1) There were differences in the experiment design among studies; (2) There was no uniform therapeutic medication across the studies; and (3) The BPPV subtypes were not consistent among studies. Previous studies by Sheikhzadeh et al. 10 and Carneiro de Sousa et al. 13 used non-randomized controlled trials to emphatically study recurrent BPPV and found significantly rising levels of vitamin D in serum along with vitamin D supplementation in case group. These two studies revealed significant reduction in vertigo attacks in the supplementation group. Although Carneiro de Sousa et al. 13 extracted just 10 recurrent BPPV patients in the study, and found elevated serum levels (greater than 30 ng/mL) after the supplementation, there was no relapse in participants in the case group, all those participants in the control group exhibited relapses in the tracking 1 year. A research indicated that multi-canal BPPV is prone to recurrence and needs several rounds of CRP treatment. 16 Two other studies by Büki et al. 9 and Califano et al. 12 also focused on recurrent BPPV in patients. Specifically, they adopted a self-comparative as well as before and after study to compare recurrence over the course of 1 year following vitamin D supplementation, and found that BPPV patients did not encounter relapses in the follow up period of 8 months to 1 year. Talaat et al. 11 conducted medication supplementation in all patients, then divided them into sufficiency case and deficiency control groups, according to the serum level of vitamin D. A comparison in recurrence between the groups revealed significantly lower rates in the case group, owing to an increase in the level of vitamin D in serum to sufficiency following treatment. The trial concluded that the recurrence was closely associated with the level of vitamin D in the serum. A multicenter, large sample, single-blinded, randomized trial was conducted in Korea, 15 the result indicated that the supplementation of vitamin D in BPPV can reduce significantly recurrence, especially in those patients with vitamin D deficiency. Rhim’s 14 study reported an opposite conclusion, where vitamin D supplementation did not help in reducing the recurrence within several tracking time nodes including 6 months, 1 year, and 2 years. The negative result was possibly due to the fact that most of the included patients were lateral canalolithiasis, cupulolithiasis, and multi-canal BPPV rather than the posterior canal BPPV. A study on epidemiology of vitamin D deficiency indicated that this is a global public health problem across all age groups. 17
The vitamin D endocrine system is essential for calcium and bone homeostasis, in the metabolism process of otoconia, the direct effect of vitamin D would be controlling calcium concentration by regulating calcium absorption and uptake, and by influencing ion channel/pump expression which similarly affect calcium and subsequently otoconia formation/maintenance. In vitamin D receptor null mice, otoconia showed degenerative features such as fissure, fusion, and smaller particles. These findings suggested that there was a strong bonding between BPPV and vitamin D deficiency. 18 Vitamin D is also involved in several other physiological functions, such as regulation of arterial blood pressure, modulation of immunological responses, regulation of insulin production, protection against certain cancers, reno-protection, among other beneficial actions. 19 Because of these functions, deficiency, or severe deficiency of vitamin D can produce adverse effects and negatively affect health.
In conclusion, on the basis of the patients had not the diseases which influenced the intake, conversion, or absorption process of vitamin D, we recommend a propose that clinicians should provide a choice for BPPV patients to examine the vitamin D was sufficient or not, and suggest dietary supplementation if the vitamin D was insufficient, or drug supplementation if the vitamin D was deficient. For the insufficient or less patients, the serum of vitamin D should be monitored every 3 months, in order to allow the clinicians to adjust the therapeutic schedule till the vitamin D was sufficient. There were some reasons as follow: Firstly, vitamin D deficiency and insufficiency were very common in the worldwide, it was a global health issue; 18 Secondly, Based on the result of the present work, the supplementation of vitamin D can significantly reduce the recurrence in BPPV; Thirdly, some international societies like the Endocrine Society, the International Osteoporosis Foundation, the National Osteoporosis Foundation and the American Geriatrics Society stated that at least 30 ng/mL was needed for disease prevention. 18 Eliminating the risk factor for BPPV can reduce recurrence and the associated complications, and help to prevent injury caused by vitamin D deficiency to other body organs.
The present study also had some limitations: Firstly, relevant studies were limited. Secondly, the relevant high quality RCT trials were too few. Thirdly, there was no consistent vitamin D supplementation project in BPPV combined with vitamin D deficiency. It was difficult to ascertain the reason for the observed heterogeneity between studies. These limitations and their potential influence on the results, warrant future investigations to validate our findings.
Author biographies
Zhiling Yang worked in the department of Otolaryngology Head and Neck Surgery for eight years in the Gansu Provincial People’s Hospital, who acquired graduate degree in the ENT major and is currently an attending physician.
Juanli Li worked in the department of Otolaryngology Head and Neck Surgery for six years in the Gansu Provincial People’s Hospital, who acquired graduate degree in the ENT major and is currently an attending physician.
Zhenzhen Zhu worked in the department of Otolaryngology Head and Neck Surgery for seven years in the Gansu Provincial People’s Hospital, who acquired graduate degree in the ENT major and is currently an attending physician.
Jian He worked in the department of Otolaryngology Head and Neck Surgery over thirty years in the Gansu Provincial People’s Hospital, who is the head chief and an archiater of ENT department. He graduated as undergraduate degree, who published over ten papers as the first author or the corresponding author in the Chinese Science Citation Database.
Xudong Wei worked in the department of Otolaryngology Head and Neck Surgery over twenty years in the Gansu Provincial People’s Hospital, who is an archiater of ENT department. He graduated as doctor degree, who published over five papers as the first author in the Science Citation Index in the recent five years. Otherwise, he presided two National Natural Science Foundation of China projects.
Min Xie worked in the department of department of Neurosurgery for eight years in the First Hospital of Lanzhou University, who acquired graduate degree in the Neurosurgery major and is currently an attending physician.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We received a grant from the research that the relevance and clinical significance between NO concentration of nose expiratory and allergic rhinitis.
ORCID iD: Zhiling Yang  https://orcid.org/0000-0002-1970-6113
https://orcid.org/0000-0002-1970-6113
References
- 1.Instrum RS, Parnes LS. Benign paroxysmal positional vertigo. Adv Otorhinolaryngol 2019; 82: 67–76. [DOI] [PubMed] [Google Scholar]
- 2.Nunez RA, Cass SP, Furman JM. Short- and long-term outcomes of canalith repositioning for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 2000; 122(5): 647–652. [DOI] [PubMed] [Google Scholar]
- 3.Neuhauser HK, von Brevern M, Radtke A, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology 2005; 65(6): 898–904. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg YW, Zhao X, Yamoah EN.Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain Res 2006; 1091(1): 47–57. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka T, Shirota S, Sawai Y, et al. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope 2013; 123(11): 2813–2816. [DOI] [PubMed] [Google Scholar]
- 6.AlGarni MA, Mirza AA, Althobaiti AA, et al. Association of benign paroxysmal positional vertigo with vitamin D deficiency: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2018; 275(11): 2705–2711. [DOI] [PubMed] [Google Scholar]
- 7.Oghalai JS, Manolidis S, Barth JL, et al. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg 2000; 122(5): 630–634. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Escamez JA, Gamiz MJ, Fernandez-Perez A, et al. Long-term outcome and health-related quality of life in benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol 2005; 262(6): 507–511. [DOI] [PubMed] [Google Scholar]
- 9.Büki B, Ecker M, Jünger H, et al. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med Hypotheses 2013; 80(2): 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikhzadeh M, Lotfi Y, Mousavi A, et al. The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: a case-control study. Caspian J Intern Med 2016l; 7(3): 173–177. [PMC free article] [PubMed] [Google Scholar]
- 11.Talaat HS, Kabel AMH, Khaliel LH, et al. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx, 2016; 43(3): 237–241. [DOI] [PubMed] [Google Scholar]
- 12.Califano L, Salafia F, Melillo MG, et al. Is hypovitaminosis D a risk factor for either the onset or the recurrence of Benign Paroxysmal Positional Vertigo. Frontiera ORL 2014; 144 Pt A:138–145. [Google Scholar]
- 13.Carneiro de Sousa PJM, Abreu Pereira DM, Pereira CM, et al. Vitamin D deficiency and benign paroxysmal positioning vertigo. Hearing Balance Commun 2019; 17(2): 179–181. [Google Scholar]
- 14.Rhim GI. Effect of vitamin D injection in recurrent benign paroxysmal positional vertigo with vitamin D deficiency. Int Arch Otorhinolaryngol 2020; 24(4): e423–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong S, Kim J-S, Kim H-J, et al. Prevention of benign paroxysmal positional vertigo with vitamin D supplementation: a randomized trial. Neurology 2020; 95(9): e1117–e1125. [DOI] [PubMed] [Google Scholar]
- 16.Pal’Chun VT, Guseva AL, Olimpieva SP. Clinical features and treatment of multi-canal benign paroxysmal positional vertigo. Vestn Otorinolaringol 2019; 84(6): 28–32. [DOI] [PubMed] [Google Scholar]
- 17.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 2014; 144(Pt A): 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Büki B, Jünger H, Zhang Y, et al. The price of immune responses and the role of Vitamin D in the inner ear. Otol Neurotol 2019; 40(6): 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang S, Lee H. Vitamin D and health - The missing vitamin in humans. Pediatr Neonatol 2019; 60(3): 237–244. [DOI] [PubMed] [Google Scholar]