Abstract
This study aimed to evaluate peripherally inserted central catheters (PICCs) and totally implanted venous access devices (TIVADs) as chemotherapy delivery routes. From May 2016 to April 2019, patients with malignancies who had PICCs or TIVADs inserted for chemotherapy were enrolled. We reviewed the patients’ medical records for information concerning demographics, comorbidities, catheter-related complications, and catheter -service days. All patients included in both groups were also assessed for complication-free catheter survival and completion rates of chemotherapy. A total of 467 catheter insertions (185 PICCs and 282 TIVADs) were included in this study. The PICCs were associated with a higher rate of complication-related catheter removal than TIVADs (hazard ratio, 6.5954; 95% confidence interval, 2.394–18.168; p<0.001). The completion of chemotherapy was observed in 77 (41.6%) patients with PICCs and 128 (45.4%) with TIVADs (p = 0.442). The mean duration of catheter service-days was shorter for the patients in the PICC group who completed chemotherapy than those in the TIVAD group (101.3 ± 93.2 vs 245.3 ± 115.9, respectively, p < 0.001). Although PICC was an independent risk factor for complication-related catheter removal, there was no difference in the chemotherapy completion rate between the groups. Therefore, PICCs need to be considered preferentially in patients who require a chemotherapy delivery route for short-term chemotherapy.
Keywords: Peripherally inserted central catheter, totally implanted venous access device, catheter service-days, chemotherapy completion
Introduction
Safe access to chemotherapy delivery and repeated blood sampling in oncology patients is essential in clinical practice. As a reliable route, the use of peripherally inserted central catheters (PICCs) and totally implanted vascular access devices (TIVADs) is common for chemotherapy delivery.1,2
Both TIVADs and PICCs have several advantages. TIVADs can be used for long if appropriately cared for since repeated punctures are made with a non-coring needle when access is necessary. In addition, patients can easily perform daily activities, such as bathing, because these devices are subcutaneously implanted.3–5 However, some oncologists prefer PICCs because they are safer and easier to insert and can be removed in the event of catheter-related complications. 6
Recent guidelines have recommended durable central venous catheters, such as PICCs, TIVADs, and tunneled central catheters for long-term chemotherapy. However, no evidence concerning which type of central venous catheter is optimal or preferable for chemotherapy has been established. 7 Some case series and meta-analyses have demonstrated different incidences of catheter-related complications according to venous access type.8,9 However, in a recent meta-analysis the optimal access type for chemotherapy was still unclear. 10
This study aimed to assess the incidence of catheter-related complications that resulted in catheter removal in patients with malignancies who had PICCs or TIVADs inserted for chemotherapy delivery.
Methods
Study design
A retrospective analysis of all patients who had TIVADs or PICCs inserted for chemotherapy between May 2016 and April 2019 was performed. Medical records were reviewed to identify the demographic characteristics of the patients who underwent chemotherapy. The inclusion criteria were adult patients (over 18 years) who required chemotherapy for solid organ malignancies confirmed by tissue biopsy or radiological studies. All patients were enrolled once during the study period. The procedures were selected based on discussions between patients and oncologists. This study was approved by the local Institutional Review Board (IRB No. 2019-04-026).
Procedures
All procedures were performed by two vascular surgeons in the surgical room under fluoroscopic guidance. Trans-jugular TIVADs (Dignity™, Medcomp, Kulpsville, PA, USA or Celsite® Epoxy, B. Braun Medical, Boulogne-Billancourt, France) were implanted via the internal jugular vein. In the supine position, under local anesthesia, the internal jugular vein was accessed with a 21-gauge hyperechoic needle and a 0.018 inch flexible wire (Micropuncture®, Cook Medical, Bloomington, IN, USA) using ultrasound guidance. A peel-away sheath was inserted over the J-tip 0.035 inch guidewire, and the tip of the catheter was placed at the cavoatrial junction (2–2.5 vertebral body units below the carina). A pocket for the portal septum was made in the upper lateral chest, 3 cm below the clavicle. The catheter was placed through a subcutaneous tunnel over the clavicle. A non-coring Huber needle was inserted into the portal septum in the surgical room if requested by the oncologist. The upper arm TIVADs were inserted in the medial upper arm via the basilic vein using the same method as the PICCs insertions. All PICC (POWERPICC®, Bard, Salt Lake City, UT, USA or Turbo-Ject®, Cook Medical, Bloomington, IN, USA) placements were performed in an upper arm vein (basilic or brachial) with a diameter of 3 mm or more on ultrasound. A deep vein was selected if the diameter of the superficial vein was less than 3 mm. On ultrasound guidance, the target vein was punctured 5 cm above the antecubital fossa using a 21-gauge microneedle and a 0.018 inch wire. A 5 Fr peel-away introducer was inserted, and the catheter was advanced through the guidewire into the cavoatrial junction. All TIVADs and PICCs were flushed with heparin-mixed saline after placement.
Endpoints
The primary outcomes measured were complication-free catheter survival and the causes of catheter failure. We reviewed the medical records of the patients to confirm catheter-related adverse events. The complication-free catheter survival interval was defined as the number of days the catheter continued functioning until the completion of therapy, patient death, or the end of the study. Catheter occlusion was defined as a permanent inability to aspirate blood or infuse therapeutics through a PICC lumen or the portal chamber of a TIVAD. 11 Catheter-related infections were categorized as exit site infections, tunnel infections, pocket infections, and bloodstream infections according to clinical practice guidelines. 12 Catheter-related venous thromboembolism (VTE) was defined as clinical signs of venous occlusion and confirmation with imaging studies (computed tomography or ultrasound). 3 Major complications were defined as those that required catheter removal due to occlusion, infection, or VTE. The secondary outcomes studied were the completion rates of chemotherapy and primary and secondary catheter patency throughout the chemotherapy treatment.
Statistical analysis
SPSS software (version 22.0, IBM, Armonk, NY, USA) was used for all analyses. Student’s t-test, Pearson’s χ2 test, and Cox regression analysis were used to analyzed between-group comparisons and determined the risk factors for major complications. The Kaplan-Meier method was used for the complication-free catheter survival analysis. Two-tailed significance testing was used throughout, and statistical significance was set at p < 0.05. The sample size was justified by Freedman’s method using the log-rank test based on a previous study (80% power, significance level 0.05; number needed = 288).3,13
Results
There were 467 catheters, 185 (39,6%) PICCs and 282 (60.4%) TIVADs, for chemotherapy delivery during the study period. The mean age of the patients in the PICC group was higher than that in the TIVAD group (63.7 ± 11.1 vs 57.4 ± 11.2, p < 0.001). Males were significantly more likely to have PICCs placed than TIVADs (67.6% males vs 41.1% males, respectively; p < 0.001). Demographic and baseline characteristics are shown in Table 1.
Table 1.
Demographics and baseline characteristics.
| PICCs |
TIVADs |
p | |
|---|---|---|---|
| n = 185 | n = 282 | ||
| Age, year (mean ± SD) | 63.7 ± 11.1 | 57.4 ± 11.2 | <0.001 |
| Male, n (%) | 125 (67.6) | 116 (41.1) | <0.001 |
| Malignancies, n (%) | |||
| Head and neck | 22 (11.9) | 5 (1.8) | |
| Breast | 7 (3.8) | 93 (33.0) | |
| Lung | 36 (19.5) | 27 (9.6) | |
| Gastrointestinal | 64 (34.6) | 104 (36.9) | |
| Urogenital and gynecologic | 5 (2.7) | 14 (5.0) | |
| Hematologic | 47 (25.4) | 35 (12.4) | |
| Others | 4 (2.2) | 4 (1.4) | |
| Comorbidities, n (%) | |||
| Hypertension | 58 (31.4) | 78 (27.7) | 0.406 |
| Diabetes | 50 (27.0) | 61 (21.6) | 0.184 |
| CAD | 15 (8.1) | 16 (5.7) | 0.344 |
| Stroke | 12 (6.5) | 4 (1.4) | 0.007 |
| COPD | 15 (8.1) | 9 (3.2) | 0.030 |
| VTE | 11 (5.9) | 8 (2.8) | 0.149 |
| Medications, n (%) | |||
| Aspirin | 12 (6.5) | 13 (4.6) | 0.405 |
| Clopidogrel | 5 (2.7) | 16 (5.7) | 0.171 |
| Warfarin | 2 (1.1) | 1 (0.4) | 0.337 |
| NOAC | 17 (9.2) | 13 (4.6) | 0.055 |
CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; NOAC: novel oral anticoagulant; PICC: peripherally inserted central catheter; SD: standard deviation; TIVAD: totally implanted venous access device; VTE: venous thromboembolism.
A technical success rate of 100% was achieved in both groups. All PICCs were placed in the medial upper arm. The brachial vein was approached if the diameter of the basilic vein was <2.0 mm. Additionally, six TIVADs were implanted in the basilic vein of the upper arm because of the tracheostomy status of the patients. There were significant differences in laterality preferences and the number of catheter lumens between the two groups. The details of the two procedures are presented in Table 2.
Table 2.
Procedure details.
| PICCs |
TIVADs |
p | |
|---|---|---|---|
| n = 185 | n = 282 | ||
| Technical success rate, % | 100.0 | 100.0 | |
| Access vein, n (%) | <0.001 | ||
| Internal jugular | 0 (0) | 276 (97.9) | |
| Brachial | 85 (45.7) | 0 (0) | |
| Basilic | 100 (54.3) | 6 (2.1) | |
| Laterality, n (%) | <0.001 | ||
| Right | 25 (12.6) | 225 (79.8) | |
| Left | 160 (87.4) | 57 (20.2) | |
| Lumen, n (%) | <0.001 | ||
| Single | 18 (9.7) | 282 (100) | |
| Dual | 167 (90.3) | 0 (0) | |
| Length, cm (mean ± SD) | 42.8 ± 3.7 | 25.7 ± 3.3 | <0.001 |
PICC: peripherally inserted central catheter; SD: standard deviation; TIVAD: totally implanted venous access device.
The mean duration of catheter service-day differed significantly between the groups (PICCs, 94.3 ± 89.2 vs TIVADs, 304.6 ± 197.7; p < 0.001). However, the number of functioning catheters at the completion of planned chemotherapy was not significantly different between the two groups (77 PICCs, 41.6% vs 128 TIVADs, 45.4%; p = 0.422). In contrast, there were differences between the two groups in terms of the causes of complication-related catheter removal. Catheter-related infections (PICC, 10.3% vs TIVAD, 3.9%; p = 0.008), catheter-related venous thromboses (PICC, 5.4% vs TIVAD, 0.4%; p < 0.001), and catheter occlusions (PICC, 23.8% vs TIVAD, 0.7%; p < 0.001) were more frequently found in the PICC group. The catheter-related complications and reasons for removal are summarized in Tables 3 and 4.
Table 3.
Reason for catheter removal.
| PICCs |
TIVADs |
p | |
|---|---|---|---|
| n = 185 | n = 282 | ||
| Total catheter service-days | 17,346 | 85,598 | |
| Mean ± SD | 94.3 ± 89.2 | 304.6 ± 197.7 | <0.001 |
| Completion, planned chemotherapy | 77 (41.6) | 128 (45.4) | 0.422 |
| Catheter service-days (mean ± SD) | 101.3 ± 93.2 | 245.3 ± 115.9 | <0.001 |
| n/1000 catheter-days | 4.4 | 1.5 | |
| Removal, complication | 53 (28.6) | 13 (4.6) | |
| Infection, catheter-related | 19 (10.3) | 11 (3.9) | |
| VTE, catheter-related | 9 (4.9) | 1 (0.4) | |
| Catheter occlusion | 10 (5.4) | 2 (0.7) | |
| Insertion site pain | 3 (1.6) | 1 (0.4) | |
| Edema, arm | 2 (1.1) | 0 (0) | |
| Accidental withdrawal | 10 (5.4) | 0 (0) | |
| Current use | 1 (0.5) | 46 (16.3) | |
| Death, with catheter functioning | 22 (11.9) | 50 (17.7) | |
| Unknown | 6 (3.2) | 6 (2.1) | |
| Lost to follow-up | 26 (14.1) | 39 (13.8) |
PICC: peripherally inserted central catheter; SD: standard deviation; TIVAD: totally implanted venous access device; VTE: venous thromboembolism.
Table 4.
Major complications.
| PICCs |
TIVADs |
p | |
|---|---|---|---|
| n = 185 | n = 282 | ||
| Infection, n (%) | 19 (10.3) | 11 (3.9) | 0.011 |
| n/1000 catheter-days | 1.10 | 0.13 | |
| CRBSI | 8 (4.3) | 1 (0.4) | |
| CC | 9 (4.9) | 1 (0.4) | |
| Pocket/exit site with CC | 0 (0) | 6 (2.1) | |
| Pocket/exit site without CC | 2 (1.1) | 3 (1.1) | |
| Occlusion, n (%) | 44 (23.8) | 2 (0.7) | <0.001 |
| n/1000 catheter-days | 2.54 | 0.02 | |
| Failed, thrombolysis with urokinase | 8 (4.3) | 0 (0) | |
| VTE, n (%) | 9 (4.9) | 1 (0.4) | <0.001 |
| n/1000 catheter-days | 0.58 | 0.01 |
CC, catheter colonization; CRBSI, catheter-related blood stream infection; PICC, peripherally inserted central catheter; TIVAD, totally implanted venous access device; VTE, venous thromboembolism.
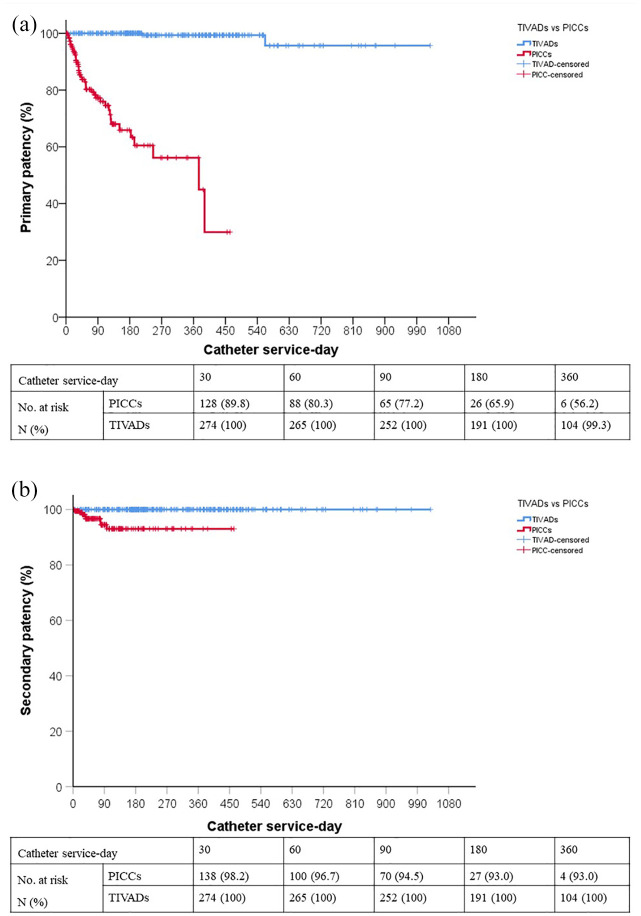
Figure 1 presents the overall complication-free catheter survival using the Kaplan-Meier method. There was a significant difference in catheter survival between the two groups. In addition, the primary and secondary patency rates at 360 days were lower in the PICC group than in the TIVAD group. However, 81.8% were successfully recanalized using urokinase thrombolysis, and the improvement in patency at 180 days was 27.1% of PICC occlusions (Figure 2).
Figure 1.
Overall complication-free catheter survival.
Figure 2.
(a) Primary and (b) secondary patency.
In the univariate analysis, male sex, history of previous central catheter insertion, catheter insertion on the left side, and having a PICC were all associated with an increased risk of major complications, with a trend toward a higher risk of major complications in patients aged >75 years. Multivariate stepwise logistic regression analysis showed that of these five covariates, only having a PICC (hazard ratio, 6.595; 95% confidence interval, 2.394–18.168; p < 0.001) independently affected catheter removal due to complications. The analysis of risk factors for major complications is shown in Table 5.
Table 5.
Univariate and multivariate analyses of risk factors for major complications.
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 75 yearsvs < 75 years | 1.931 (0.907–4.112) | 0.088 | – | – |
| Male vs female | 2.310 (1.222–4.368) | 0.010 | – | – |
| Previous catheter vs noprevious catheter | 2.442 (1.194–4.998) | 0.015 | – | – |
| Hematologic vsnon-hematologic | 1.312 (0.567–3.034) | 0.526 | ||
| Anticoagulant vs noanticoagulants | 2.021 (0.790–5.168) | 0.142 | ||
| Antiplatelet vs noantiplatelets | 1.037 (0.351–3.062) | 0.948 | ||
| Left vs right | 2.350 (1.265–4.366) | 0.007 | – | – |
| PICC vs TIVAD | 5.625 (2.846–11.117) | <0.001 | 6.595 (2.394–18.168) | <0.001 |
CI: confidence interval; HR: hazard ratio; PICC: peripherally inserted central catheter; TIVAD: totally implanted venous access device.
Discussion
In this single-center retrospective observational study, we compared the differences in catheter survival, catheter-related complications, and chemotherapy completion rates between PICCs and TIVADs in patients with malignancies. Complication-free catheter survival was significantly higher in the TIVAD group than in the PICC group. In addition, a significantly higher primary patency rate was observed in the TIVAD group. Although secondary patency was still significantly lower in the PICC group, urokinase thrombolysis effectively improved catheter patency. However, there was no statistically significant difference in the rates of chemotherapy completion.
In this study, the catheter-related infection rates were 10.3% (1.10/1000 catheter service-days) in the PICC group and 3.9% (0.13/1000 catheter service-days) in the TIVAD group. This result is consistent with the findings of similar publications.14,15 However, a recent randomized analysis reported a very low incidence of catheter-related infections in oncology patients with PICCs (0.16/1000 catheter service-days). 3 In addition, the incidence of catheter-related infections in the PICC group was lower than that in the TIVAD group. The authors proposed that extensive programs to prevent catheter-related infections are important for a consistently low rate of catheter-related infections.
The patterns of catheter-related infections differed between the groups in our study. Pocket and exit site infections were more common in the TIVAD group, whereas catheter colonization and catheter-related bloodstream infections were observed more frequently in the PICC group. This is presumed to be related to repetitive non-coring needle punctures in the chamber of the TIVADs. Local infection of the TIVAD port pocket or exit site is a major reason for catheter removal. Cherifi et al. 16 have suggested that local signs of infection were independent predictive factors for failure to retain TIVAD in patients with device-related bacteremia. We have a policy to remove TIVADs or PICCs in patients with signs of pocket or exit site infections.
The rate of catheter-related VTE was 5.4% (0.58/1000 catheter service-days) in the PICC group and 0.4% (0.01/1000 catheter service-days) in the TIVAD group. In oncology patients, VTE is a common complication that increases the mortality rate due to the risk of fatal pulmonary embolism. In particular, VTEs in patients with cancer are related to venous access devices such as PICCs. 17 Our findings regarding the high VTE rate in patients with PICCs compared to those in patients with TIVADs is consistent with previous studies.18–20 In this study, most of the TIVADs were inserted into the jugular vein, which is relatively large with a short intravenous length; therefore, fewer VTEs are thought to occur with TIVADs than with PICCs. Recent clinical practice guidelines recommend selecting veins less than 45% of the catheter-to-vein ratio (CVR) calculated using vessel diameter to prevent VTEs. 21 In our study, a 5 Fr PICC was inserted in veins >3 mm in diameter. According to the guidelines, some patients had CVR >45%. This is a limitation of the present study. If the CVR was lowered according to the guidelines, the incidence of thrombotic complications of PICCs would have been lower. More recently, Spencer and Mahoney 22 proposed the 45% rule of CVR by occupying a three-dimensional percentage catheter area. Based on the three-dimensional theoretical assessment, 5 Fr PICC is a safe catheter size in patients with veins >3 mm in diameter. This aspect requires further evaluation in future studies.
Our data suggest that the catheter occlusion rate in PICCs was higher than that in TIVADs. These results are consistent with those reported in the literature. 23 Luminal occlusion of PICCs is the most common complication and reason for catheter removal. 24 However, in our study, recanalization was achieved in 81% (26/44) of patients through thrombolysis using urokinase, and the improvement in patency at 180 days was 27.1% of PICC occlusions. These findings indicate that active recanalization can prevent the premature removal of chemotherapy delivery devices in oncology patients.
In the multivariate analysis of risk factors for major complications, PICC was the only independent risk factor for catheter removal due to major complications. However, when the chemotherapy completion rate and the number of catheter-implanted days were analyzed, the rate of chemotherapy completion was 44.6% in the PICC group and 45.4% in the TIVAD group in our study. These results are higher than those of other studies published previously.25,26 Of the patients who completed their chemotherapy regimens, the mean duration of catheter service-days was shorter in the PICC group than the TIVAD group. Therefore, it can be inferred that PICCs are reliable for short-term chemotherapy regimens.
This study had several limitations. First, this was a retrospective, nonrandomized, single-institute, observational study. Therefore, selection bias may limit the generalizability of our results. Second, data regarding chemotherapy regimens were not analyzed. Third, more than 10% of the patients in each group failed to follow-up.
Conclusion
This study suggests that TIVAD is associated with a low rate of catheter removal as a result of complications in oncology patients. However, in patients who needed short-term chemotherapy, the completion rate was similar to that of PICCs. The findings we report can be used to select the most appropriate catheter for chemotherapy delivery in patients who have planned short-term chemotherapy regimens.
Author biographies
Woo-Sung Yun has high expertise in vascular surgery and is now an associated professor at Kyungpook National University.
Shin-Seok Yang is Clinical Associated Professor at Samsung Medical Center and his research interest include vascular surgery and endovascular therapy for peripheral arterial and venous disease.
Footnotes
Author’s Note: Woo-Sung Yun is currently affiliated with Division of Transplantation and Vascular Surgery, Department of Surgery, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from INSTITUTIONAL REVIEW BOARD (APPROVAL NUMBER IRB No. 2019-04-026)* of Yeungnam University Hospital.
Informed consent: Informed consent was not sought for the present study because retrospecive study using medical records.
ORCID iDs: Woo-Sung Yun  https://orcid.org/0000-0001-8956-8310
https://orcid.org/0000-0001-8956-8310
Shin-Seok Yang  https://orcid.org/0000-0003-4957-3080
https://orcid.org/0000-0003-4957-3080
References
- 1.Bertoglio S, Faccini B, Lalli L, et al. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: aprospective study on the incidence of complications and overall failures. J Surg Oncol 2016; 113: 708–714. [DOI] [PubMed] [Google Scholar]
- 2.Tsuruta S, Goto Y, Miyake H, et al. Late complications associated with totally implantable venous access port implantation via the internal jugular vein. Support Care Cancer 2020; 28: 2761–2768. [DOI] [PubMed] [Google Scholar]
- 3.Taxbro K, Hammarskjold F, Thelin B, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth 2019; 122: 734–741. [DOI] [PubMed] [Google Scholar]
- 4.Clemons M, Stober C, Kehoe A, et al. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support Care Cancer 2020; 28: 4891–4899. [DOI] [PubMed] [Google Scholar]
- 5.Robinson A, Stober C, Fergusson D, et al. A multicentre, randomized pilot trial comparing vascular access strategies for early stage breast cancer patients receiving non-trastuzumab containing chemotherapy. Breast Cancer Res Treat 2019; 178: 337–345. [DOI] [PubMed] [Google Scholar]
- 6.Zaghal A, Khalife M, Mukherji D, et al. Update on totally implantable venous access devices. Surg Oncol 2012; 21: 207–215. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013; 31: 1357–1370. [DOI] [PubMed] [Google Scholar]
- 8.Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009; 374: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pu YL, Li ZS, Zhi XX, et al. Complications and costs of peripherally inserted central venous catheters compared with implantable port catheters for cancer patients: a meta-analysis. Cancer Nurs 2020; 43: 455–467. [DOI] [PubMed] [Google Scholar]
- 10.Robinson A, Souied O, Bota AB, et al. Optimal vascular access strategies for patients receiving chemotherapy for early-stage breast cancer: a systematic review. Breast Cancer Res Treat 2018; 171: 607–620. [DOI] [PubMed] [Google Scholar]
- 11.Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: the 3P-O study. J Vasc Interv Radiol 2017; 28: 749–756 e2. [DOI] [PubMed] [Google Scholar]
- 12.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1982; 1: 121–129. [DOI] [PubMed] [Google Scholar]
- 14.Kagan E, Salgado CD, Banks AL, et al. Peripherally inserted central catheter-associated bloodstream infection: risk factors and the role of antibiotic-impregnated catheters for prevention. Am J Infect Control 2019; 47: 191–195. [DOI] [PubMed] [Google Scholar]
- 15.Tang TT, Liu L, Li CX, et al. Which is better for patients with breast cancer: totally implanted vascular access devices (TIVAD) or peripherally inserted central catheter (PICC)? World J Surg 2019; 43: 2245–2249. [DOI] [PubMed] [Google Scholar]
- 16.Cherifi S, Jacobs F, Strale H, et al. Outcome of totally implantable venous access device-related bacteraemia without device removal. Clin Microbiol Infect 2007; 13: 592–598. [DOI] [PubMed] [Google Scholar]
- 17.Chopra V, Ratz D, Kuhn L, et al. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost 2014; 12: 847–854. [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre L, Noyon E, Georgescu D, et al. Port catheter versus peripherally inserted central catheter for postoperative chemotherapy in early breast cancer: a retrospective analysis of 448 patients. Support Care Cancer 2016; 24: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 19.Cotogni P, Barbero C, Garrino C, et al. Peripherally inserted central catheters in non-hospitalized cancer patients: 5-year results of a prospective study. Support Care Cancer 2015; 23: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J, Chen W, Sun W, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access 2017; 18: 153–157. [DOI] [PubMed] [Google Scholar]
- 21.Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice, 8th edition. J Infus Nurs 2021; 44(1S): S1–S169. [DOI] [PubMed] [Google Scholar]
- 22.Spencer TR, Mahoney KJ. Reducing catheter-related thrombosis using a risk reduction tool centered on catheter to vessel ratio. J Thromb Thrombolysis 2017; 44: 427–434. [DOI] [PubMed] [Google Scholar]
- 23.Patel GS, Jain K, Kumar R, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer 2014; 22: 121–128. [DOI] [PubMed] [Google Scholar]
- 24.Chopra V, Montoya A, Joshi D, et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc 2015; 63: 1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voog E, Campion L, du Rusquec P, et al. Totally implantable venous access ports: a prospective long-term study of early and late complications in adult patients with cancer. Support Care Cancer 2018; 26: 81–89. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki A, Suminoe A, Koga Y, et al. Long-term use of peripherally inserted central venous catheters for cancer chemotherapy in children. Support Care Cancer 2006; 14: 153–160. [DOI] [PubMed] [Google Scholar]