Abstract
Stroke is a global health problem, and survivors of a stroke often suffer from cognitive impairment, which has an essential impact on the rehabilitation of various functions. Repetitive Transcranial Magnetic Stimulation (rTMS) has been widely used in the rehabilitation treatment of stroke patients. There are many investigations into how rTMS impacts motor dysfunction, speech dysfunction and swallowing dysfunction after stroke, but the analysis of rehabilitation effect on stroke patients with cognitive dysfunction is lacking. The purpose of this study was to analyze the effect of different rTMS related therapies on cognitive impairment and to evaluate its clinical effect on cognitive rehabilitation after stroke. Four databases including PubMed, EMBASE, MEDLINE and the Cochrane Library, were searched and a total of 2754 papers were collected. Two reviewers independently completed a review of all papers’ titles and abstracts, screened out the documents that met the criteria, and carried out data extraction, quality assessment, and data analysis. A total of six studies with 197 patients were included. Three studies used the Mini-Mental Status Examination (MMSE) scale to evaluate the cognitive function with a mean effect size of 1.89 (95% CI = −3.08–6.86). Two studies used the Loewenstein Occupational Therapy of Cognitive Assessment (LOTCA) scale with the mean effect size of 1.64 (95% CI = −7.65–10.93). These studies were evaluated separately. Our article provides that rTMS has a positive effect on improving the cognitive ability of stroke patients, but the evidence is still limited, and further large-scale studies are needed to explore the optimal stimulus parameters.
Keywords: Repetitive transcranial magnetic stimulation (rTMS), cognitive impairment, stroke, systematic review, meta-analysis
Introduction
Stroke is a global health problem and in 2015 alone, the global number of deaths related to stroke reached 6.326 million. 1 Cognitive impairment is manifested as inattention, memory loss, executive decline and other aspects. Cognitive impairment often occurs among stroke survivors and has an essential impact on rehabilitation. Between 55% and 75% of patients that had a stroke have functional motor limitations that are present even at 3–6 months after onset, 2 thereby affecting their quality of life or daily activities. 3 Cognitive processes and motor processes are interwoven, as demonstrated by Mireille Bonnard et al. 4 in studies on transcranial magnetic stimulation (TMS) in the regulation of corticospinal cord (CS) excitability. The degree of recovery of motor function after stroke is one of the most intuitive rehabilitation results that patients can feel, and the realization of this result is inseparable from the control and regulation of brain cognitive functions (such as attention, memory, and executive ability). Cognitive function is the driving force of rehabilitation of stroke patients. In the past three decades, growing neurophysiological evidence has shown that cognitive and motor functions are closely related. 5
The uninjured cerebral hemispheres play an important role in the recovery of function after stroke. The interhemispheric competition model considers the presence of mutual inhibition between the hemispheres, and the damage caused by a stroke disrupts this balance, thus producing a reduced inhibition of the unaffected hemisphere by the affected side. To achieve a balance between the two by stimulating the residual function of the injured hemispheres and inhibiting the excessive function of the uninjured hemispheres is a key step in post-stroke rehabilitation. Transcranial magnetic stimulation (TMS) is a painless and non-invasive tool for excitatory regulation of the cerebral cortex. 6 This tool utilizes fluctuating magnetic fields to induce currents that depolarize potential neurons. 7 Repetitive TMS (rTMS) can be used in low or high-frequency applications. The stimulus parameters of rTMS mainly refer to the stimulus frequency, including low frequency (≤1 Hz) and high frequency (≥5 Hz). Low frequency is used to reduce the excitability of the cerebral cortex, while high frequency is used to promote its excitability. 8 According to the interhemispheric competition model, the treatment of rTMS is to up-regulate cortical excitability by delivering HF-rTMS in the affected hemispheres or down-regulate cortical excitatory levels by transporting LF-rTMS in the healthy hemispheres, in order to normalize the imbalance between the affected and unaffected hemispheres.9,10 The size and shape of the coil and the strength of the current were also important parameters. The smaller the coil, the more selective the local stimulus, and the smaller the intensity of the induced magnetic field. The higher the intensity, the wider the cortex was stimulated. The H-shaped probe stimulates more deeply than the eight-shaped or round probe. RTMS regulates excitability in the cerebral cortex and has long-lasting effects on cognitive mechanisms and behavior. These effects are caused by physiological changes caused by different frequencies of stimulation to the stimulated cerebral cortex tissues and their related circuits.11–13
There have been many investigations into the impact of rTMS on motor dysfunction, 14 speech dysfunction, 15 and swallowing dysfunction 16 after stroke, however, the number of studies on the rehabilitation effect of cognitive function in stroke patients by rTMS is very small, and lack strength (the sample size is small, the mechanism of action needs to be further explored, and the degree of technical standardization is not high).
Clinical studies found that cognitive dysfunction caused by cerebral apoplexy was usually characterized by memory, attention, directional force, execution, and other forms existing at the same time. MMSE, MOCA, LOTCA scale is currently widely used in clinical screening scale of cognitive function. The test content covers, memory, attention, executive function, language, abstract thinking, time orientation, place orientation force, computing power, visual spatial ability, and so on a variety of forms of cognitive function, can reflect the comprehensive, accurate and fast the subject degree of cognitive impairments. Therefore, this study based on the cognitive function of a variety of forms (including attention, memory, directional force, execution, etc.), and analyse the clinical efficacy of rTMS treatment in cognitive rehabilitation after stroke.
Materials and methods
Study design and literature search
Our meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Liberati et al., 2009).Four databases (PubMed, EMBASE, MEDLINE, Cochrane Library) were searched systematically and comprehensively, including relevant publications in any language from January 2003 to March 2019. The following keywords were used:[(“Cognition” OR “Cognitions” OR “Cognitive Function” OR “Cognitive Functions” OR “Function, Cognitive” OR “Functions, Cognitive” OR “Cognitive impairment” OR “Cognitive dysfunction” OR “Attention” OR “Memory” OR “Executive Function”) OR (“Stroke” OR “Strokes” OR “Cerebrovascular Accident” OR “Cerebrovascular Accidents” OR “CVA” OR “CVAs” OR “Cerebrovascular Apoplexy” OR “Vascular Accident, Brain” OR “Brain Vascular Accident” OR “Brain Vascular Accidents” OR “Vascular Accidents, Brain” OR “Cerebrovascular Stroke” OR “Cerebrovascular Strokes” OR “Stroke, Cerebrovascular” OR “Strokes, Cerebrovascular” OR “Apoplexy” OR “Cerebral Strokes” OR “Cerebral Strokes” OR “Stroke, Cerebral” OR “Strokes, Cerebral” OR “Stroke, Acute” OR “Acute Stroke” OR “Acute Strokes” OR “Strokes, Acute” OR “Cerebrovascular Accident, Acute” OR “Acute Cerebrovascular Accident” OR “Acute Cerebrovascular Accidents” OR “Cerebrovascular Accidents, Acute” OR “Apoplexy, Cerebrovascula”)] AND (“Transcranial Magnetic Stimulation” OR “TMS” OR “rTMS” OR “Magnetic Stimulation, Transcranial” OR “Magnetic Stimulations, Transcranial OR “Stimulation, Transcranial Magnetic” OR “Stimulations, Transcranial Magnetic” OR “Transcranial Magnetic Stimulations” OR “Transcranial Magnetic Stimulation, Single Pulse” OR “Transcranial Magnetic Stimulation, Paired Pulse” OR “Transcranial Magnetic Stimulation, Repetitive” OR “Repetitive Transcranial Magnetic Stimulation”).
Inclusion and exclusion criteria
The literature inclusion criteria were: (1) human studies, (2) randomized controlled clinical trial including intervention group and control group, (3) cognitive impairment was caused by stroke, (4) the degree of cognitive impairment was measured by relevant scales, such as MMSE, MOCA, LOTCA, (5) rTMS was used as the sole treatment measure or combined with other treatments, and compared with sham-rTMS, drug therapy or cognitive training, (6) sufficient original data was provided in the literature, and (7) the patients were adults(≥18 years). The exclusion criteria were: (1) literature published in the form of abstracts, (2) the degree of cognitive impairment is too severe to cooperate actively with treatment, Coma patients with Glasgow coma scale (GCS) score ≦8, (3) not having carried out any tests to assess the effects of rTMS on cognition in Stroke patients, (4) with several articles from the same study, only the one with the most patients and the latest and most complete data was chosen.
Data extraction
The data of each article were examined independently and extracted by two authors. For each study, the relevant information extracted included: (1) general characteristics: the first author, year of publication, Sample size, sex ratio, mean age, duration of disease, participants’ educational level, medication situation, stroke site, and types of stroke; (2) research design, selection criteria, duration and outcome measurement;(3) intervention therapy methods: stimulation site, intensity, frequency, total pulses of per session, number of session, whether to carry out cognitive function training, and (4) Study results (MMSE/LOTCA Score).
We made our best efforts to contact the authors during the extraction process when the data in the document were incomplete, and we have selected the database with the most detailed data content for the duplicated documents. If there were any disagreement between the two authors in the process of data extraction, they communicated with a professional evaluator to reach consensus.
Statistical analysis and publication bias
All data were assembled using Cochrane Rev-Man 5.3 software (Review Manager of Cochrane Collaboration). The effect size was calculated by mean difference (MD) to estimate the treatment effect, and 95% confidence intervals (CI) were computed. Heterogeneity among the trials was quantified using the I2 statistic, and a value >50% was taken to indicate substantial heterogeneity. If heterogeneity existed, the random effect model was used to obtain a more reliable data analysis. If there was no heterogeneity, the fixed effect model was used. The literature with different outcome indexes was analyzed as necessary. According to the recommendations of the Cochrane Manual for Systematic Evaluation of Interventions, the funnel map was not constructed to assess the publication bias of these studies due to the small number of documents that met the inclusion criteria. The risk of bias for all the included studies was assessed by two statisticians. Although RCT-based evidence is initially considered to be of high quality, our confidence in such evidence may be impaired by the following five factors: (1) Limitations in study design or execution (risk of bias), (2) Inconsistency of results, (3) Indirectness of evidence, (4) Imprecision, (5) Publication bias.
Results
Literature search findings
After searching four databases, 2754 identified references were generated by searching the titles of documents, 20 of which were excluded because of duplicate data. After reading the titles and abstracts, 2720 studies were excluded due to an irrelevance to the current question, description of a basic scientific experiment, not using RCT, or not using rTMS to treat post-stroke cognitive impairment. In the full-text reading review of 14 articles related to the study, three articles were excluded for not using scales to assess the cognitive impact of rTMS on stroke patients, and five articles were excluded for incomplete data. Finally, six studies were selected for meta-analysis and review (Figure 1).
Figure 1.
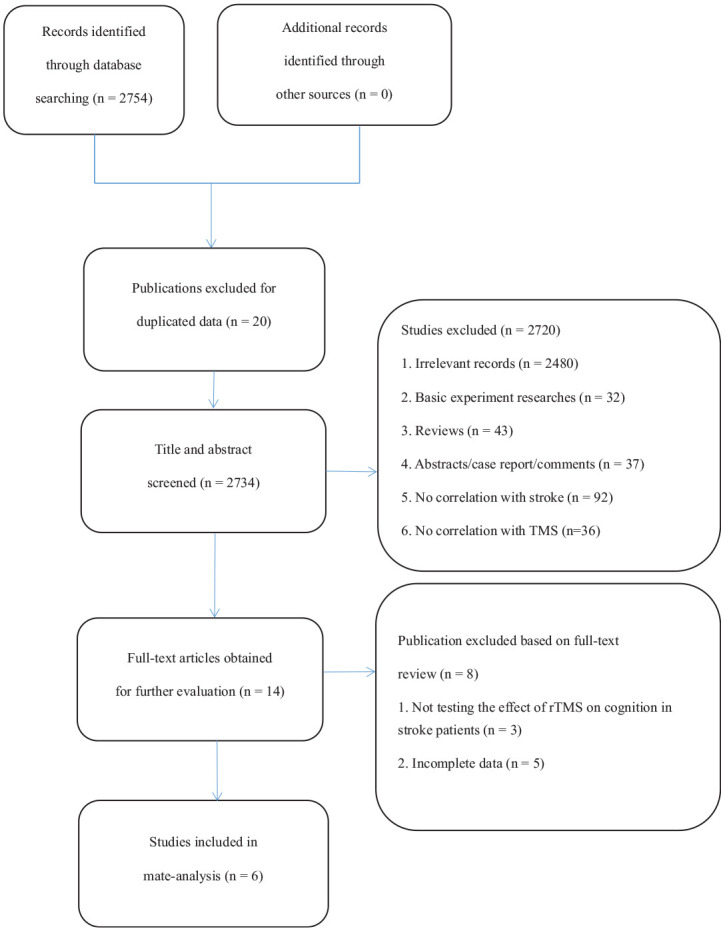
Flowchart of systematic review.
Basic characteristics of studies
The total of six studies included 19717–22 patients with a stroke. There were 10817–22 males and 8917–22 females. Five of the six studies gave the mean age of participants, but one did not mention it.17,18,20,21,22 We tried to contact the author of the original article, but unfortunately, we could not get the relevant data. Two studies indicated that participants took antidepressants such as fluoxetine during the trial.17,18 Four articles clearly described the specific lesion sites of the participants (left, right, or bilateral).17,19,20,22 At the same time, we found that the number of ischemic stroke patients was larger than that of hemorrhagic stroke patients. The basic characteristics included in the literature are described in Table 1.
Table 1.
Demographic characteristics of the included trials.
| Study (year) | Participants (n) | Sex(M/F) | Mean age(years) | Diseaseduration | Education(years) | Medication | stroke site (left/right/bilateral) | Type ofstroke(hemorrhagic/ischemic) |
|---|---|---|---|---|---|---|---|---|
| 1. Du et al. 17 | Total 60 | 34/26 | 57.6 ± 10.8 | Not given | 8.1 ± 3.7 | Fluoxetine | Not given | |
| Treatment group 30 | 15/10/5 | |||||||
| Control group 30 | 16/10/4 | |||||||
| 2. Kim et al. 18 | Total 18 | 10/8 | Not given | Antidepressant | Not given | 4/14 | ||
| Low frequency 6 | 2/4 | 68.3 ± 7.4 | 404.4 ± 71.7(days) | 1/5 | ||||
| High frequency 6 | 4/2 | 53.5 ± 16.9 | 241.2 ± 42.5(days) | 2/4 | ||||
| Sham 6 | 4/2 | 66.8 ± 17.2 | 69.7 ± 39.0(days) | 1/5 | ||||
| 3. Lu et al. 20 | Total 40 | 25/15 | Not given | |||||
| rTMS group 19 | 12/7 | 42.5 ± 12.3 | 67 (30, 365)(days) | 12.8 ± 3.8 | 11/8/0 | 11/8 | ||
| Sham group 21 | 13/8 | 47.3 ± 11.8 | 56 (30, 296)(days) | 11.5 ± 4.5 | 11/10/0 | 11/10 | ||
| 4. In-Seok Park (2015) | Total 20 | 9/11 | Not given | Not given | Not given | Not given | Not given | |
| CACR group 10 | 5/5 | 10/0/0 | ||||||
| rTMS group 10 | 4/6 | 10/0/0 | ||||||
| 5. D’Agata et al. 22 | Total 34 | 15/19 | > 6 months | Not given | ||||
| Intervention 24 | 8/16 | 57 (12) | 10 (4) | 12/12/0 | 7/17 | |||
| Sham 10 | 7/3 | 65 (12) | 10 (4) | 4/6/0 | 3/7 | |||
| 6. Hara et al. 21 | Total 25 | 15/10 | 61.8 ± 14.1 | 45.9 ± 48.5(months) | Not given | Not given | Not given | 14/11 |
| right LF-rTMS 15 | 9/6 | 58.8 ± 15.8 | 49.8 ± 59.4(months) | 7/8 | ||||
| left LF-rTMS 10 | 6/4 | 66.3 ± 10.2 | 40.2 ± 27.2(months) | 7/3 |
n: number; M; male; F: female; LF: low frequency; CACR: computer-assisted cognitive rehabilitation.
In the included studies, the sites of rTMS stimulation varied, including the right side of the dorsolateral prefrontal cortex (R-DLPFC), the left prefrontal cortex (L-DLPFC), the dorsolateral prefrontal cortex (DLPFC) and the bilateral frontal lobe (BFL). The minimum stimulus intensity was 60% MT, and the maximum stimulus intensity was 120% MT. Four studies used a low frequency of 0.5 or 1 Hz.17,20,21,22 One study used high frequency with a frequency of 10 Hz. 19 Another study also compared the high frequency with the low frequency with a false stimulus. 18 Only one study conducted cognitive training for patients at the same time during the experiment. 20 Among the six studies included, three17–19 were assessed with the Mini-Mental Status Examination (MMSE) scale, and two19,20 were assessed with the Loewenstein Occupational Therapy of Cognitive Assessment (LOTCA) scale. One 19 was assessed with both the MMSE and LOTCA scale. The other two articles21,22 selected sub-items from other cognitive function assessment scales to record the changes in participants’ cognitive function. A description of the rTMS intervention in the included studies is provided in Table 2.
Table 2.
Description of RTMS intervention in the included studies.
| Study (year) | Stimulationposition | Intensity(%MT) | Frequency(Hz) | Total pulsesper session | Number ofsessions | Cognitivetraining | MMSE/LOTCA (mean ± SD) |
|---|---|---|---|---|---|---|---|
| 1. Du et al. 17 | BFL | 60 | 0.5 | 20 trains (100 µs,30times/ 20 days) | 20 | No | Active: MMSE Before 14 ± 4 |
| After 24 ± 7 | |||||||
| Control: MMSE Before 15 ± 4 | |||||||
| After 18 ± 6 | |||||||
| 2.Kim et al. 18 | DLPFC | LF:80 | LF:1 | LF: 900pulses | 10 | No | Low Frequency: MMSE 20.0 ± 6.1 |
| HF:90 | HF:10 | HF: 450pulses | High Frequency: MMSE 20.5 ± 5.5 | ||||
| Sham group: MMSE 19.7 ± 5.2 | |||||||
| 3. Lu et al. 20 | R-DLPFC | 100 | 1 | 600 pulses | 20 | Yes | rTMS group: LOTCA 67.16 ± 9.26 |
| Sham group: LOTCA 67.86 ± 8.69 | |||||||
| 4. Park andYoon 19 | L-DLPFC | 100 | 10 | Not Given | 12 | No | rTMS group: |
| MMSE Before 17.90 ± 2.470 After 19.50 ± 2.369 | |||||||
| LOTAC: Before 68.70 ± 6.464 After 70.50 ± 6.223CACR group: | |||||||
| MMSE Before 18.00 ± 1.886 After20.30 ± 2.058 | |||||||
| LOTAC: Before 71.70 ± 4.945 After76.80 ± 4.442 | |||||||
| 5.D’Agata et al. 22 | Not Given | 120 | 1 | 900 pulses | 20 | No | Not Given |
| 6.Hara et al. 21 | Not Given | 90 | 1 | 2400 pulses | 11 | No | Not Given |
RTMS: repetitive transcranial magnetic stimulation; LF: low frequency; hf: high frequency; R-DLPFC: the right side of the dorsolateral prefrontal cortex; L-DLPFC: the left prefrontal cortex; DLPFC: the dorsolateral prefrontal cortex; BFL: bilateral frontal lobe; MMSE: the mini-mental status examination; LOTCA: Loewenstein occupational therapy of cognitive assessment; CACR: computer-assisted cognitive rehabilitation.
Study quality
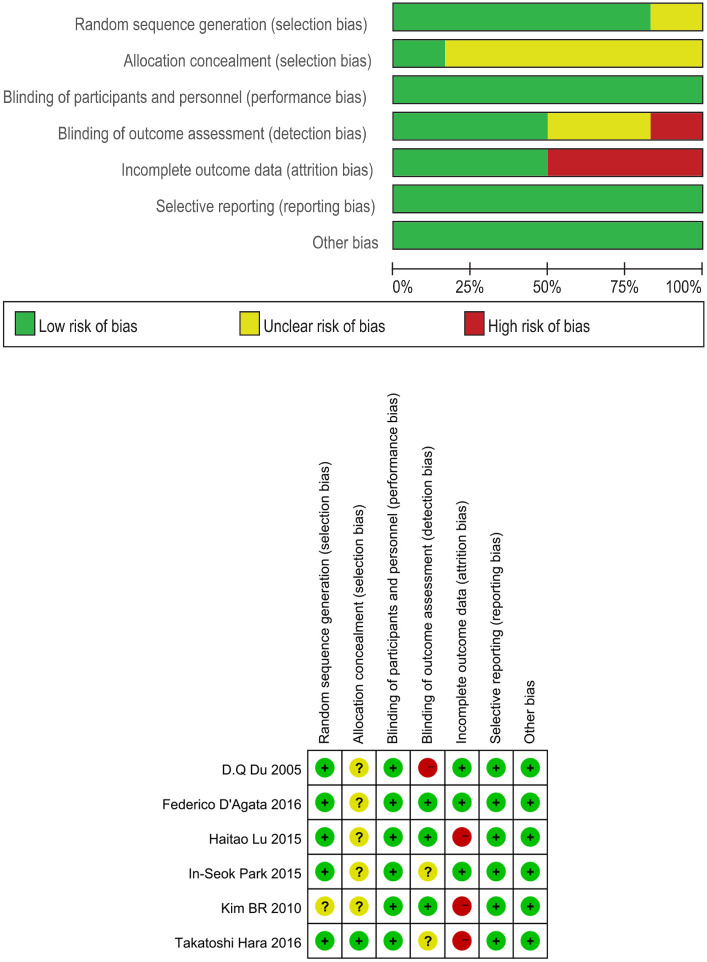
The Cochrane Handbook was used to evaluate the risk of bias for the included studies, and the results are summarized in Table 3. Randomization was used in all included studies.17–22 Five trials17,19–22 described adequate sequence randomization, based on random sequence generation using random number tables or computer programs. One study 21 reported allocation procedures with adequate concealment. Most studies were double-blind for both participants and evaluators. Most of the studies reported the data in detail. Thus, all the included studies were judged to have a mild risk of bias.
Table 3.
The risks of bias of included studies based on the Cochrane’s handbook.
|
There were two major outcomes in this study, the effect of rTMS on MMSE and LOTCA scale scores in patients with poststroke cognitive dysfunction, and the GRADE system recommended for each outcome was medium GRADE (Table 4).
Table 4.
Summary of finding table from GRADE profiler.
| rTMS for cognitve impairment in stroke patients | ||||||
|---|---|---|---|---|---|---|
| Patient or population: patients with cognitive impairment in stroke patients | ||||||
| Settings: | ||||||
| Intervention: rTMS | ||||||
| Outcomes | Illustrative comparative risks*(95% CI) |
Relative effect(95% CI) | No ofParticipants(studies) | Quality of theevidence(GRADE) | Comments | |
| Assumed risk |
Corresponding risk |
|||||
| Control | RTMS | |||||
| MMSE | The mean mmse in the intervention groups was1.89 higher (3.08 lower to 6.86 higher) | 98 (3 studies) | ⊕⊕⊕Θ moderate 1 | |||
| LOTCA | The mean lotca in the intervention groups was 1.64 higher (7.65 lower to 10.93 higher) | 60 (2studies) | ⊕⊕⊕Θ moderate 1 | |||
CI: Confidence interval;
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk(and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
The inaccuracy of the results was reflected in the inclusion of less than 100 samples for each outcome.
Meta-analyses
We summarized and analyzed the results of all the studies and found that not every study used the same scale because of the variety of cognitive function evaluation scales. Among them, MMSE and LOTCA scales were used more frequently. Therefore, we conducted a meta-analysis of the studies using MMSE and LOTCA scales.
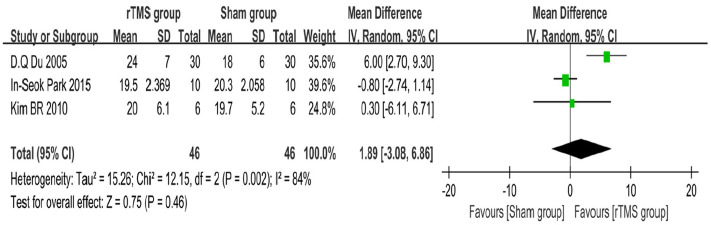
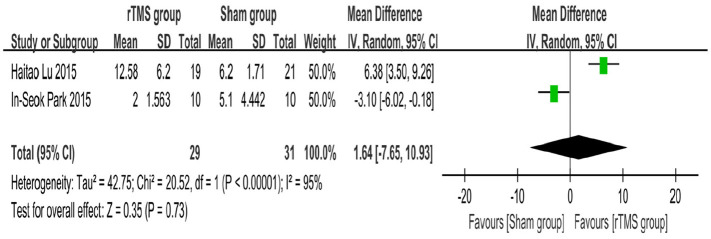
We choose which meta-analysis model should be used according to the value of I2: I2 < 50% choose fixed effect model, I2 > 50%, use random effect model. The results of the analysis showed that three studies (including 87 participants)17–19 used the MMSE scale to evaluate the cognitive function of participants, the average effect size was 1.89 (95% CI = −3.08–6.86), I2 = 84%, and the random effect model was used for meta analysis (Figure 2). Two studies (including 60 participants)19,20 used the LOTCA scale to assess the cognitive function of participants, with an average effect size of 1.64 (95% CI = −7.65–10.93), I2 = 95%, and a random effect model for meta analysis (Figure 3). One of the studies was included in both meta-analyses because it used both the MMSE and LOTCA scales. Two studies used scales other than MMSE and LOTCA to assess participants’ cognitive function and were not included in the meta-analysis. The results observed are not statistically significant, as showed in the figures.
Figure 2.
Forest plot: mean differences in the effect of rTMS on patients with stroke with 95% CI (MMSE).
Figure 3.
Forest plot: mean differences in the effect of rTMS on patients with stroke with 95% CI (LOTCA).
Discussion
Discussion of the main results
During literature retrieval, we found that the research on rTMS in the treatment of cognitive impairment induced by other causes are well-reported, but the research on rTMS in the treatment of cognitive impairment caused by stroke is limited, which is likely why there is no published meta-analysis except for a sub-analysis there. The purpose of this systematic review and meta-analysis was to evaluate the effects of rTMS on Stroke patients with mild to moderate cognitive impairment. In this meta-analysis, six randomized controlled trials including 197 patients compared the efficacy of rTMS with that of sham rTMS. Our results showed that rTMS has a positive effect on cognitive function in stroke patients (MD from MMSE = 1.89, MD from LOTCA = 1.64). According to the quality evaluation of GRADE methodology, the above two results all belong to the medium level of evidence, suggesting that rTMS has a positive effect on cognitive function in stroke patients. The insignificance of the results may be due to the fact that the number of subjects included in each outcome was less than 100. Due to the limited number of studies included for each result, there may be publication biases.
Because there are many kinds of scales used to assess cognitive function, six studies utilized different scales to evaluate cognitive function in their investigation. Therefore, we summarized the studies using the same scale (MMSE or LOTCA) to conduct a meta-analysis. Du et al. 17 treated patients with cognitive impairment with low-frequency rTMS based on neurology and limb function training. The results showed that the cognitive function of patients was improved. This effect may be because repetitive transcranial magnetic stimulation increases cerebral blood flow and metabolism in the frontal region after it acts on brain tissue, thereby improving cognition. 23 rTMS may also have a significant effect on gene expression of neurotransmitters and their receptors in the brain. However, this observation requires further observation and confirmation.
Kim et al. 18 studied the effects of rTMS with different stimulus frequencies applied to left DLPFC on working memory, attention and executive function in the cognitive function of patients after stroke. The study found that the improvement of cognitive function was not obvious. The MMSE score of low-frequency stimulation group was slightly higher than that of the sham stimulation group. High-frequency rTMS has a positive effect on the emotional state. Rektorova et al. 24 conducted a high-frequency rTMS on patients’ left DLPFC in a Stroop test of executive function in patients with cerebrovascular diseases and mild cognitive impairment, which produced a significant stimulating effect. However, they did not observe any effect on executive function and working memory, which is similar to what the present investigation revealed. 18 Many areas of the brain participate in and influence cognitive function, including the left dorsolateral prefrontal cortex (DLPFC), right dorsolateral prefrontal cortex (DLPFC), bilateral inferior parietal lobe, Broca’s area, and anterior cingulate area.25,26 In this study, rTMS stimulation was limited to left DLPFC, which may not be enough to induce positive cognitive effects. Hao Z et al. 27 assessed randomized controlled trials of rTMS on functional recovery in patients with stroke. Their meta-analysis covered 19 trials in which 588 patients and two RCTs are included. Hao and colleagues found no significant effect on global cognitive functioning indexed by the Mini Mental State Examination score, and the limited data revealed rTMS may have effects on aphasia and neglect in patients with stroke.
Lu et al. 20 used low-frequency rTMS on the right side of DLPFC of stroke patients and found that their cognitive and memory functions could be improved and maintained for some time. Two months after treatment, the scores of MOCA and LOTCA in rTMS group were higher than those in sham operation group (MOCA: 6.17 ± 2.55 vs 4.14 ± 0.95, p = 0.002; LOTCA: 12.58 ± 6.20 vs 6.20 ± 1.71, p < 0.001). Memory is supported by multiple cognitive nervous systems. The prefrontal lobe is the key area of memory, especially the DLPFC. 28 It is essential for memory encoding and retrieval. 29 Previous studies have shown that stimulation of the prefrontal cortex can improve cognitive and memory functions, and that low-frequency rTMS reverses AB1-42-mediated memory impairment in rats. 30 Other studies have shown that healthy individuals and patients with mild cognitive impairment show significant improvement in language and non-language recognition after 1 Hz rTMS stimulation on the right side of the DLPFC. 31 This improvement in cognitive function may depend on the regulation of excitability and accessory structures on the right side of the DLPFC, 32 because stimulation can activate the hippocampus by affecting subcortical and posterior cortical structures, thereby regulating the memory retrieval process. In the present investigation, cognitive and memory functions were improved after 2 months of rTMS treatment. Because each rTMS stimulus may be stored as a “memory” in the stimulus area, the effect of the next stimulus will be generated based on the previous stimulus and stored again. When all stimuli are completed, the cumulative biological reactions produced by these stimuli may last for some time. 33 Memory is an integral part of the overall cognitive level of the brain. Improvement of memory performance can enable patients to complete training tasks more efficiently during the rehabilitation process, thereby improving the cognitive function of the whole brain.
Park and Yoon 19 studied the effects of ACR and rTMS on the improvement of cognitive function in stroke patients. According to the K-MMSE and LOTCA-G scores, the cognitive function of rTMS group and computer-assisted cognitive rehabilitation (CACR) group improved significantly after the intervention. In the CACR group and rTMS group, the improvement of LOTCA-G score was greater than that of K-MMSE score. This effect is similar to the results of Kim et al., 34 Appelros et al. 35 and Cho. 36 Park et al. used CACR in stroke patients and showed that all subjects scored significantly higher in the cognitive and visual motor organization, and that CACR had a greater improvement in cognitive function than rTMS. This effect suggests that if studies combining the two interventions (CACR and rTMS) can be applied to both subjects and appropriate assessment tools are used to verify the effect, then the cognitive improvement of stroke patients may be improved.
Trail-Making Test (TMT) is a group of easy and inexpensive neuropsychological tests to evaluate several cognitive functions that consist of both TMT-A and TMT-B. Hara et al. 21 observed that rTMS significantly improved TMT-B performance in stroke patients. From the perspective of cognitive function, TMT-B is considered to reflect executive function or collective transferability, which is related to the connection between various cognitive functions. Current studies have shown that TMT-B performance has improved in right hemiplegic patients after low-frequency rTMS intervention. Zakzanis et al. 37 argued that TMT-B was more active in the left hemisphere than in the right hemisphere when measuring the movement of cognitive set, which provided a basis for this study.
Event-Related Potentials (ERP) are a reproducible electrophysiological response to an external stimulus (visual or auditory), representing the brain activity associated with various cognitive processes such as selective attention, memory, or decision making. Interestingly, ERP can be valuable in the diagnosis of cognitive impairment and can track the cognitive changes during the follow-up in stroke patients. 38 Recently, Non-Invasive Brain Stimulation (NIBS) techniques have been proposed as support of standard cognitive and motor rehabilitation.39,40 rTMS and transcranial Direct Current Stimulation (tDCS) are the most used NIBS techniques in rehabilitation.41,42 D’Agata et al. 22 showed that exercise and cognitive ability of patients with chronic stroke improved after NIBS treatment. NIBS stimulation (rTMS or dual TDCS) has a long-term effect on cortical plasticity and can also promote motor and cognitive improvement in chronic disease patients. Endogenous components of ERP (N200 and P300) reflect perceptual and cognitive processes and play an important role in testing stroke patients. Prieto et al. 43 demonstrated that P300 is highly sensitive in detecting continuous attention changes in stroke patients with right parietal lobe lesions. The effect of NIBS treatment on P300 is related to the function of the attention network, which is achieved by temporarily changing the plasticity of neurons. Although not permanent, it can be used for cognitive rehabilitation of patients with chronic stroke, improving their compliance and promoting simultaneous cognitive training (such as visual-spatial skills training and motor ability training).
Possible neurobiological effects of rTMS
In vitro studies of the role of rTMS, low intensity pulsed magnetic field stimulation leads to an increase in intracellular calcium, changes in cytoskeletal structure, metabolism, and neuronal homeostasis. Animal studies have shown that rTMS has a physiological effect on synaptic plasticity, which leads to the activation of mechanisms regulating the concentrations of 5-ht, epinephrine and dopaminergic neurotransmitters, the expression of membrane receptors, and the expression of genes related to neuronal plasticity, thereby activating growth factors44,45 Regulating the activity of cerebral cortex in a painless and noninvasive environment has aroused great clinical interest.
Limitations
Our current meta-analysis has several limitations. Firstly, the number of studies and samples in the meta-analysis are minimal. Secondly, although we have assessed the efficacy of rTMS, there is no follow-up study on whether there is a long term impact on cognitive improvement due to insufficient data. Thirdly, heterogeneity inevitably exists due to different evaluation scales, different stimulation parameters and various types of stroke (hemorrhagic or ischemic). This inconsistency may have impacted our overall results. Also, the effect of drugs taken by patients and other rehabilitation training on the outcome of the study cannot be ruled out. Finally, because there are relatively few clinical studies on rTMS in the treatment of cognitive impairment after stroke, the number of investigations we have included in the meta-analysis is minimal, so that the analysis of this issue is not comprehensive enough. To avoid these problems, we have formulated strict criteria for selection and exclusion and selected two scales with high frequency for analysis, but there is still a lack of comprehensive evaluation and analysis.
Future perspectives
In the future, we hope to use larger sample sizes, longer follow-up times, and some sensitive assessment tools to detect the cognitive function of rTMS in stroke patients based on more literature studies, and to provide a more precise and more accurate reference for clinical rehabilitation.
Conclusion
In conclusion, rTMS has a positive effect on improving the cognitive ability of stroke patients, but the evidence is still limited, and further large-scale studies are needed to explore the optimal stimulus parameters.
Author biographies
Mengting Liu is a Rehabilitation Physician, has long been committed to the prevention and treatment of stroke, paraplegia, neck, shoulder, waist and leg pain and other diseases. She integrates acupuncture, massage, traditional Chinese medicine, fitness Qigong and other traditional Chinese medicine treatment methods with modern rehabilitation treatment techniques, and combines traditional Chinese and western medicine to prevent and cure diseases.
Guanai Bao is a Master of Traditional Chinese Medicine, has been engaged in rehabilitation work for many years.She is good at treating cancer rehabilitation diseases with traditional Chinese medicine combined with modern rehabilitation therapy.
Lu Bai, Master of Science, is a Psychologist and Secretary Of Zhejiang Psychological Oncology Committee.
Enyan Yu is a Psychiatrist and Professor, Chair of Psychology of aging group of Chinese Association for Mental Hygiene. Member of the standing committee in Psychiatry group of Chinese Medical Association.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval was not sought for the present study because this was a review article and did not involve any patients.
Informed consent: Informed consent was not sought for the present study because this was a review article and did not involve any subjects.
ORCID iDs: Mengting Liu  https://orcid.org/0000-0003-1736-1681
https://orcid.org/0000-0003-1736-1681
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and causespecific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CP, Tsai PY, Yang TF, et al. Differential effect of conditioning sequences in coupling inhibitory/facilitatory repetitive transcranial magnetic stimulation for poststroke motor recovery. CNS Neurosci Ther 2014; 20: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvão SCB, Dos Santos RBC, Dos Santos PB, et al. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil 2014; 95: 222–229. [DOI] [PubMed] [Google Scholar]
- 4.Mireille Bonnard, Camus Mickael, de Graaf Jozina, et al. Direct evidence for a binding between cognitive and motor functions in humans: a tms study. J Cogn Neurosci 2003; 15(8): 1207–1216. [DOI] [PubMed] [Google Scholar]
- 5.Bonnard M, Camus M, de Graaf J, et al. Direct evidence for a binding between cognitive and motor functions in humans: a TMS study. J Cogn Neurosci 2003; 15: 1207–1216. [DOI] [PubMed] [Google Scholar]
- 6.Rastgoo M, Naghdi S, Nakhostin Ansari N, et al. Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. Disabil Rehabil 2016; 38: 1918–1926. [DOI] [PubMed] [Google Scholar]
- 7.Wassermann E, Epstein C, Ziemann U. Oxford handbook of transcranial stimulation. Oxford, UK: Oxford University Press, 2008. [Google Scholar]
- 8.Dionisio A, Duarte IC, Patricio M, et al. The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: a systematic review. J Stroke Cerebrovasc Dis 2018; 27: 1–31. [DOI] [PubMed] [Google Scholar]
- 9.Fisicaro F, Lanza G, Grasso AA, et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord 2019; 12: 1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10(10): 597–608. [DOI] [PubMed] [Google Scholar]
- 11.Rodger J, Mo C, Wilks T, et al. Transcranial pulsed magnetic field stimulation facilitates reorganization of abnormal neural circuits and corrects behavioral deficits without disrupting normal connectivity. FASEB J 2012; 26: 1593–1606. [DOI] [PubMed] [Google Scholar]
- 12.Gersner R, Kravetz E, Feil J, et al. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci 2011; 31: 7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funke K, Benali A. Modulation of cortical inhibition by rTMS – findings obtained from animal models. J Physiol 2011; 18: 4423–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang H, Sun J, Tang X, et al. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33(5): 847–867. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Qu Y, Yuan M, et al. Low-frequency repetitive transcranial magnetic stimulation for patients with aphasia after stoke: A meta-analysis. J Rehabil Med 2015; 47: 675–681. [DOI] [PubMed] [Google Scholar]
- 16.Liao X, Xing G, Guo Z, et al. Repetitive transcranial magnetic stimulation as an alternative therapy for dysphagia after stroke: a systematic review and meta-analysis. Clin Rehabil 2017; 31: 289–298. [DOI] [PubMed] [Google Scholar]
- 17.Du DQ, Wu YB. Living ability and cognitive function ameliorated by low frequency repetitive transcranial magnetic stimulation in patients with post-stroke depression: Comparison with drug plus psychological treatment. Chin J Clin Rehabil 2005; 16: 22–23. [Google Scholar]
- 18.Kim BR, Kim DY, Chun MH, et al. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil 2010; 89: 362–368. [DOI] [PubMed] [Google Scholar]
- 19.Park IS, Yoon J. The effect of computer-assisted cognitive rehabilitation and repetitive transcranial magnetic stimulation on cognitive function for stroke patients. J Phys Ther Sci 2015; 27: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Zhang T, Wen M, et al. Impact of repetitive transcranial magnetic stimulation on post-stroke dysmnesia and the role of BDNF Val66Met SNP. Med Sci Monit 2015; 21: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara T, Abo M, Kakita K, et al. Does a combined intervention program of repetitive transcranial magnetic stimulation and intensive occupational therapy affect cognitive function in patients with post-stroke upper limb hemiparesis? Neural Regen Res 2016; 11: 1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Agata F, Peila E, Cicerale A, et al. Cognitive and neurophysiological effects of non-invasive brain stimulation in stroke patients after motor rehabilitation. Front Behav Neurosci 2016; 10: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010; 3: 95–118. [DOI] [PubMed] [Google Scholar]
- 24.Rektorova I, Megova S, Bares M, et al. : Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci 2005; 229: 157–161. [DOI] [PubMed] [Google Scholar]
- 25.Bahlmann J, Beckmann I, Kuhlemann I, et al. Transcranial magnetic stimulation reveals complex cognitive control representations in the rostral frontal cortex. Neuroscience 2015; 300: 425–431. [DOI] [PubMed] [Google Scholar]
- 26.Gerton BK, Brown TT, Meyer-Lindenberg A, et al. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia 2004; 42: 1781–1787. [DOI] [PubMed] [Google Scholar]
- 27.Hao Z, Wang D, Zeng Y, et al. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013; 2013: CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balconi M. Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull 2013; 29: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumenfeld RS, Lee TG, D’Esposito M. The effects of lateral prefrontal transcranial magnetic stimulation on item memory encoding. Neuropsychologia 2014; 53: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan T, Xie J, Liu T, et al. : Low-frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) reverses Abeta(1-42)-mediated memory deficits in rats. Exp Gerontol 2013; 48: 786–794. [DOI] [PubMed] [Google Scholar]
- 31.Turriziani P, Smirni D, Zappala G, et al. : Enhancing memory performance with rTMS in healthy subjects and individuals with mild cognitive impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci 2012; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia 2008; 46: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valero-Cabre A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur J Neurosci 2008; 27: 765–774. [DOI] [PubMed] [Google Scholar]
- 34.Kim YG. The effects of Korean computer-based cognitive rehabilitation program (CoTras) for the cognition and ADL in stroke. J Korean Soc Occup Ther 2011, 19: 75–88. [Google Scholar]
- 35.Appelros P: Characteristics of mini-mental state examination 1 year after stroke. Acta Neurol Scand 2005; 112: 88–92. [DOI] [PubMed] [Google Scholar]
- 36.Cho YN, Kim HK, Kwon HC. The effects of computerized cognitive rehabilitation on cognitive function post-stroke patients. J Kor Special Edu Rehabil Sci 2012; 51: 261–278. [Google Scholar]
- 37.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the trail making test. Neuropsychologia 2005; 43: 1878–1886. [DOI] [PubMed] [Google Scholar]
- 38.Stahlhut L, Grotemeyer K-H, Husstedt I-W, et al. The impact of stroke on cognitive processing - a prospective event-related potential study. J Neurol Sci 2014; 339: 157–163. [DOI] [PubMed] [Google Scholar]
- 39.Miniussi C, Cappa SF, Cohen LG, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul 2008; 1: 326–336. [DOI] [PubMed] [Google Scholar]
- 40.Sandrini M, Cohen LG. Noninvasive brain stimulation in neurorehabilitation. Handb Clin Neurol 2013; 116: 499–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005; 128: 490–499. [DOI] [PubMed] [Google Scholar]
- 42.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil 2009; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonso-Prieto E, Alvarez-González MA, Fernández-Concepción O, et al. Usefulness of P300 as a tool for diagnosing alterations in sustained attention in ischemic cerebrovascular disease. Rev Neurol 2002; 34: 1105–1109. [PubMed] [Google Scholar]
- 44.Yue L, Xiao-lin H, Tao S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res 2009; 1260: 94–99. [DOI] [PubMed] [Google Scholar]
- 45.Aydin-Abidin S, Trippe J, Funke K, et al. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res 2008; 188: 249–261. [DOI] [PubMed] [Google Scholar]