Abstract
Objective:
To suggest the possible indication of proximal gastrectomy for advanced gastric cancer located at the upper third of the stomach.
Background:
Proximal gastrectomy has been an alternative surgical procedure for early proximal gastric cancer due to its benefits for quality of life while maintaining oncological outcomes. However, the oncological safety of proximal gastrectomy for advanced tumors remains unclear.
Methods:
We retrospectively reviewed data from 878 patients who underwent radical total gastrectomy from 2003 to 2018 for pathologic T2–T4 gastric cancer in the upper third of the stomach. We identified risk factors for lymph node metastasis at the distal part of the stomach, which was not dissected in proximal gastrectomy. Subsequently, we evaluated the metastasis rate and therapeutic value index of lymph nodes at the distal part of the stomach in patients with none of these risk factors.
Results:
Multivariable analysis revealed that esophagogastric junction (EGJ)-tumor epicenter distance >30 mm, tumor size >70 mm, macroscopic type IV tumor, and serosal invasion were risk factors for lymph node metastasis at the distal stomach. In patients without risk factors, the therapeutic value index for any lymph nodes at the distal stomach was 0.8, suggesting that lymph node dissection could be omitted in these patients.
Conclusions:
EGJ-tumor epicenter distance ≤ 30 mm, tumor size ≤ 70 mm, not a macroscopic type IV tumor, and no serosal invasion could be an indication of proximal gastrectomy for advanced gastric cancer located at the upper third of the stomach.
Mini-Abstract: Proximal gastrectomy has been performed exclusively for early gastric cancer located in the proximal stomach. This study revealed that patients with advanced proximal gastric cancer could be candidates for proximal gastrectomy if they meet all of these criteria: esophagogastric junction-tumor epicenter distance ≤ 30 mm, tumor size ≤ 70 mm, not a macroscopic type IV tumor, and no serosal invasion. Performing proximal gastrectomy would provide better quality of life while ensuring oncologic safety in patients meeting all 4 criteria.
Supplemental Digital Content is available in the text.
INTRODUCTION
Gastric cancer remains one of the most frequent cancers and the third leading cause of cancer-related mortality worldwide.1 The frequency of proximal gastric cancer, including esophagogastric junction (EGJ) cancer, has been increasing in Western and Asian countries.2,3 Operative options for treatment of proximal gastric cancer are total gastrectomy and proximal gastrectomy. Total gastrectomy is widely accepted as a standard operation for proximal gastric cancer. However, almost all patients who undergo total gastrectomy experience weight reduction and all of them suffer from vitamin B12 deficiency.4,5 Recent studies demonstrated that proximal gastrectomy provides patients with a better quality of life than total gastrectomy, in terms of weight loss and anemia.6,7 Thus, considering the nutritional benefits of proximal gastrectomy, it would be preferable to recommend proximal gastrectomy for patients with gastric cancer located at the upper third of the stomach, if its oncological safety is ensured.
Korean and Japanese gastric cancer treatment guidelines suggest that proximal gastrectomy is an alternative procedure for early proximal gastric cancer due to its similar oncological outcomes compared with total gastrectomy.8,9 However, the oncological safety of proximal gastrectomy for advanced proximal gastric cancer (APGC) remains unclear. The critical factor considered for the oncologic safety of proximal gastrectomy is the probability of lymph node (LN) metastasis at the distal part of the stomach (LN #4d, 5, and 6), which are not dissected during proximal gastrectomy. Although previous studies evaluated the metastasis rate and efficacy of dissection for LNs at the distal part of the stomach in APGC, few studies have identified risk factors for metastasis in these LNs.10–15
We hypothesize that proximal gastrectomy would be sufficient for radical LN dissection to ensure oncologic safety in patients with APGC who have a low risk of LN metastasis at the distal part of the stomach. In this study, we analyzed risk factors of LN metastasis at the distal part of the stomach in patients who underwent total gastrectomy for APGC. Based on the results of these analyses, we subsequently evaluated the therapeutic benefits of dissecting LNs at the distal stomach in patients with none of the risk factors.
METHODS
Patients
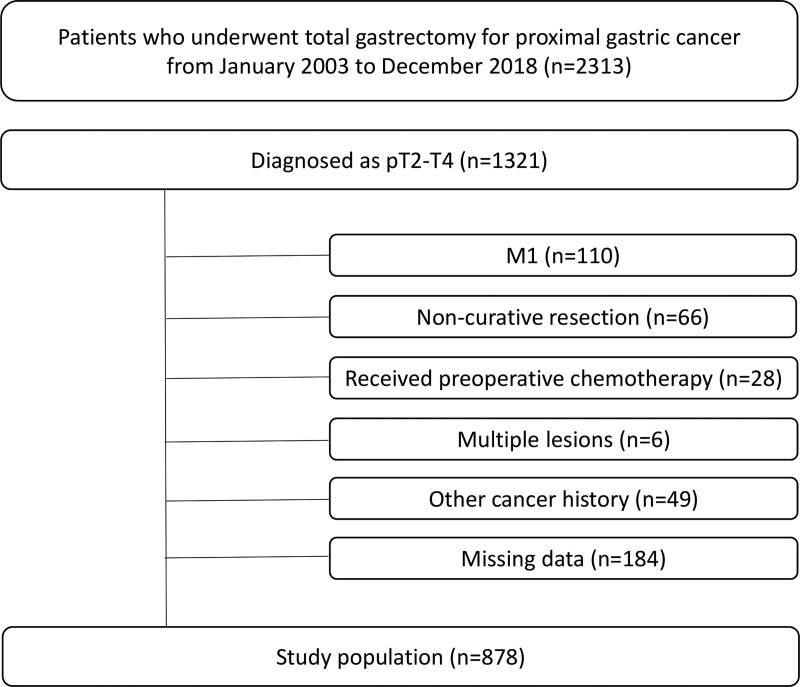
We retrospectively reviewed a prospective database of 2313 patients who underwent total gastrectomy with LN dissection for gastric cancer in the upper third of the stomach. All procedures were performed from January 2003 to December 2018 at the Department of Surgery, Yonsei University College of Medicine. We included 1321 patients whose tumors were diagnosed pathologically as T2-T4. Individuals meeting any the following criteria were excluded from this study: distant metastases (n = 110), preoperative chemotherapy (n = 28), noncurative resection (n = 66), synchronous gastric cancer (n = 6), history of another cancer (n = 49), and incomplete pathologic data regarding the LN station (n = 184). A total of 878 patients were included in the final analysis (Fig. 1).
FIGURE 1.
Flow diagram of the study.
We collected preoperative patient information, including age, sex, body mass index (BMI), and American Society of Anesthesiologists classification, from our database. The Institutional Review Board of Severance Hospital, Yonsei University Health System approved the protocol (No. 4-2021-0336) and waived the requirement for informed consent for this retrospective study.
Surgical Procedures
Eight surgeons performed total gastrectomy via open, laparoscopic, or robotic approaches. According to the preoperative staging, the extent of LN dissection was D1+ for early gastric cancer and D2 for advanced gastric cancer.9 D1+ dissection involved removal of the following LN bearing tissues: right pericardial LNs (LN #1); left pericardial LNs (LN #2); LNs along the lesser curvature (LN #3); left greater curvature LNs along the short gastric arteries (LN #4sa); left greater curvature LNs along the left gastroepiploic artery (LN #4sb); right greater curvature LNs along the right gastroepiploic artery (LN #4d); suprapyloric LNs (LN #5); infrapyloric LNs (LN #6); LNs along the left gastric artery (LN #7); LNs along the common hepatic artery (LN #8a); LNs around the celiac artery (LN #9); and LNs along the proximal splenic artery (LN #11p). D2 dissection involved removal of all LNs included in the D1+ dissection plus removal of LN-bearing tissues along the distal splenic artery (LN #11d), hepatic artery (LN #12a), and splenic hilum (LN #10). Based on the results of the JCOG0110 trial,16 if the tumor invaded the greater curvature of the stomach or if LN metastasis was suspected around the splenic hilum, we performed splenic hilar LN dissection (LN #10) from 2017.
Pathologic Examination of Resected Stomach and LNs
After surgery, a surgeon separated the soft tissues containing LNs from the main specimen for each LN station. All nodal specimens and resected stomach were delivered to the pathology department after the operation. Pathologists obtained photographs of the resected stomach and recorded the tumor size and proximal and distal margins. Based on the pathologic and photographic findings, we measured the distance from the EGJ to the epicenter of the tumor. The tumor stage was defined according to the eighth edition of American Joint Committee on Cancer Staging System. Macroscopic type and histologic classification were also described, according to the Japanese Classification of Gastric Carcinoma (third English edition).17
Therapeutic Value Index of Estimated Benefit From LN dissection
We used the therapeutic value index (TVI) as previously defined18 to evaluate the benefit of dissecting LNs at the distal part of the stomach. The TVI was calculated by multiplying the incidence of LN metastasis at the station by the 5-year overall survival (OS) rate of patients with metastasis at that station. The incidence of LN metastasis and 5-year OS rate of patients with metastasis were calculated independently for each LN station.
Statistical Analysis
Categorical variables were presented as frequency (percentage), and continuous variables were presented as mean (standard deviation [SD]). Univariable and multivariable logistic regression analyses were performed to identify risk factors for LN metastasis at the distal part of the stomach. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Optimal cutoff values were determined using Youden’s index. The 5-year OS rate was calculated using the Kaplan–Meier method. All tests were two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics software for Windows, version 25.0 (IBM Corp., Armonk, NY) and SAS for Windows, version 9.4 (SAS Inc., Cary, NC).
RESULTS
Clinicopathologic Characteristics
The mean patient age was 58.4 years (SD, 12.4), and the majority of patients were male (69.0%). The mean BMI was 23.3 kg/m2 (SD, 3.0). The mean distance from EGJ to tumor epicenter was 35.4 mm (SD, 20.0), and the mean tumor size was 52.9 mm (SD, 33.0). A total of 114 patients (13.0%) had a macroscopic type IV tumor, and 626 patients (71.3%) had an undifferentiated tumor. The proportion of tumors invading the serosa was 44.5%, whereas 23.1% and 32.3% of tumors were pathologically T2 and T3, respectively. LN metastases were detected in 557 patients (63.4%), whereas 321 patients (36.6%) had no LN metastasis. A total of 101 patients (11.5%) had LN metastases at the distal part of the stomach, meaning any LN metastases at LN #4d, 5, and 6. The LN metastasis rates at LN #4d, 5, and 6 were 7.1, 3.8, and 4.4%, respectively (Table 1).
TABLE 1.
Clinicopathologic Characteristics
| Variables | Value (N = 878) |
|---|---|
| Age (y) | 58.4 ± 12.4 |
| Sex | |
| Male | 606 (69.0) |
| Female | 272 (31.0) |
| BMI (kg/m2) | 23.3 ± 3.0 |
| ASA classification | |
| 1 | 356 (40.5) |
| 2 | 413 (47.0) |
| 3 | 100 (11.4) |
| 4 | 9 (1.0) |
| Distance from EGJ to tumor epicenter (mm) | 35.4 ± 20.0 |
| Tumor size (mm) | 52.9 ± 33.0 |
| Tumor location, circular | |
| Lesser curvature | 378 (43.1) |
| Greater curvature | 93 (10.6) |
| Anterior wall | 133 (15.1) |
| Posterior wall | 245 (27.9) |
| Circular | 29 (3.3) |
| Macroscopic type | |
| I | 70 (8.0) |
| II | 214 (24.4) |
| III | 480 (54.7) |
| IV | 114 (13.0) |
| Histologic type | |
| Differentiated | 252 (28.7) |
| Undifferentiated | 626 (71.3) |
| Pathologic T stage | |
| 2 | 203 (23.1) |
| 3 | 284 (32.3) |
| 4 | 391 (44.5) |
| Pathologic N stage | |
| 0 | 321 (36.6) |
| 1 | 138 (15.7) |
| 2 | 163 (18.6) |
| 3a | 167 (19.0) |
| 3b | 89 (10.1) |
| LN metastasis at the distal part of the stomach * | 101 (11.5) |
| #4d | 62 (7.1) |
| #5 | 33 (3.8) |
| #6 | 39 (4.4) |
Data are presented as mean ± standard deviation or number (percentage).
*Distal part of the stomach, which encompasses LN #4d, #5, and #6.
ASA indicates American Society of Anesthesiologists.
Univariable Analysis to Identify Risk Factors for LN Metastasis at the Distal Part of the Stomach
In univariable analysis, LN metastasis at the distal part of the stomach was significantly associated with lower BMI (P = 0.004), longer distance from EGJ to tumor epicenter (P < 0.001), larger tumor size (P < 0.001), macroscopic type IV tumor (P < 0.001), undifferentiated-type tumor (P = 0.001), and serosal invasion (P < 0.001) (Table 2).
TABLE 2.
Univariable Analysis of Risk Factors for Lymph Node Metastasis at the Distal Part of the Stomach
| Variables | Univariable Analyses | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Age | 0.984 | 0.968–1.000 | 0.052 |
| Sex | |||
| Male | Reference | ||
| Female | 1.400 | 0.910–2.154 | 0.126 |
| BMI | 0.899 | 0.836–0.966 | 0.004 |
| ASA classification | |||
| 1 | Reference | ||
| 2 | 1.010 | 0.649–1.574 | 0.964 |
| 3 | 0.950 | 0.469–1.923 | 0.886 |
| 4 | 0.960 | 0.117–7.875 | 0.970 |
| Distance from EGJ to tumor epicenter | 1.047 | 1.036–1.057 | <0.001 |
| Size | 1.031 | 1.025–1.038 | <0.001 |
| Macroscopic type | |||
| Not type IV | Reference | ||
| Type IV | 4.964 | 3.104–7.940 | <0.001 |
| Histologic type | |||
| Differentiated | Reference | ||
| Undifferentiated | 2.744 | 1.529–4.924 | 0.001 |
| Pathologic T stage | |||
| 2/3 | Reference | ||
| 4 | 6.537 | 3.889–10.987 | <0.001 |
ASA indicates American Society of Anesthesiologists.
Cutoff Value Determination for Continuous Variables
We used Youden’s index to determine optimal cutoff values for continuous variables associated with LN metastasis at the distal part of the stomach (BMI, tumor size, and EGJ–tumor epicenter distance). The optimal cutoff for BMI was 22 kg/m2, based on a maximum Youden’s index of 21.89 kg/m2, and the optimal cutoff value for tumor size was 70 mm, based on a maximum Youden’s index of 70.11 mm. The cutoff value for EGJ–tumor epicenter distance was 37.5 mm, based on a maximum Youden’s index; however, this value was not practical for clinical applications. We therefore evaluated the LN metastasis rate and TVI according to EGJ–tumor epicenter distance (Table 3). The results showed that TVI at the distal part of the stomach was lowest (0.9) when the tumor epicenter was located within 30 mm from EGJ and highest (≥2.5) when the epicenter was located within 40 mm or within 50 mm from EGJ. Based on these results, we set the cutoff value for EGJ-tumor epicenter distance as 30 mm.
TABLE 3.
Comparison of Therapeutic Value Indices at the Distal Part of the Stomach According to Esophagogastric Junction–Tumor Epicenter Distance
| EGJ–Tumor Epicenter Distance (mm) | Patients (n) | Metastatic rate at LN at Distal Stomach* (%) | 5-year OS With LN Metastasis at Distal Stomach* (%) | Therapeutic Value Index |
|---|---|---|---|---|
| ≤20 | 191 | 1.0 | 100 | 1.0 |
| ≤30 | 403 | 3.0 | 30.9 | 0.9 |
| ≤40 | 621 | 5.6 | 44.5 | 2.5 |
| ≤50 | 722 | 6.5 | 40.2 | 2.6 |
*Distal part of the stomach, which encompasses LN stations #4d, #5, and #6.
Multivariable Analysis to Identify Risk Factors for LN Metastasis at the Distal Part of the Stomach
Using the cutoff values for BMI, tumor size, and distance from EGJ to tumor epicenter, we performed multivariable analysis to identify risk factors for LN metastasis at the distal part of the stomach. Multivariable analysis revealed that EGJ–tumor epicenter distance > 30 mm (P < 0.001), tumor size > 70 mm (P < 0.001), macroscopic type IV tumor (P = 0.022), and serosal invasion (P < 0.001) were independent risk factors for LN metastasis at the distal part of the stomach (Table 4).
TABLE 4.
Multivariable Analysis of Risk Factors for Lymph Node Metastasis at the Distal Part of the Stomach
| Variables | Multivariable Analyses | ||
|---|---|---|---|
| OR | 95% CI | P | |
| BMI (kg/m2) | |||
| ≤22 | Reference | ||
| >22 | 0.715 | 0.447–1.144 | 0.162 |
| Distance from EGJ to tumor epicenter (mm) | |||
| ≤30 | Reference | ||
| >30 | 3.456 | 1.772–6.742 | <0.001 |
| Size (mm) | |||
| ≤70 | Reference | ||
| >70 | 3.613 | 2.173–6.009 | <0.001 |
| Macroscopic type | |||
| Not type IV | Reference | ||
| Type IV | 1.884 | 1.09–3.237 | 0.022 |
| Histologic type | |||
| Differentiated | Reference | ||
| Undifferentiated | 1.533 | 0.803–2.925 | 0.195 |
| T stage | |||
| 2/3 | Reference | ||
| 4 | 3.734 | 2.150–6.486 | <0.001 |
TVI With Regional LNs Among All Patients and Patients With None of Risk Factors
A total of 247 patients (28.1%) had none of the 4 identified risk factors for LN metastasis at the distal part of the stomach. Specifically, they met these criteria: EGJ–tumor epicenter distance ≤ 30 mm; tumor size ≤ 70 mm; macroscopic type I, II, or III tumor (ie, not a type IV tumor); and no serosal invasion. In the patients with none of these risk factors, there was one patient with LN metastasis at LN #5 and one patient with LN metastasis at LN #6. None of the patients had LN metastasis at LN #4d. Calculated TVIs of LN #4d, #5, and #6 were 0, 0.4, and 0.4, respectively. TVI of being metastatic to any LN station among #4d, #5, and #6 was 0.8 (Table 5).
TABLE 5.
Metastasis Rate, Survival of Patients With Nodal Metastasis, and Therapeutic Value Index of Lymph Node Stations for All and Patients With None of the Risk Factors
| Station No. | All Patients | Patients With None of the Risk Factors* | ||||
|---|---|---|---|---|---|---|
| Metastasis Rate (%) | 5-y OS of Patients With LN Metastasis (%) | Therapeutic Value Index | Metastasis Rate (%) | 5-y OS of Patients With LN Metastasis (%) | Therapeutic Value Index | |
| 1 | 23.5 | 44.9 | 10.6 | 15.0 | 56.0 | 8.4 |
| 2 | 15 | 40.9 | 6.1 | 7.3 | 80.8 | 5.9 |
| 3 | 40.8 | 58.9 | 24.0 | 23.9 | 81.2 | 19.4 |
| 4sa | 6 | 35.7 | 2.1 | 0 | - | 0 |
| 4sb | 7.5 | 36.7 | 2.8 | 0.4 | 100 | 0.4 |
| 4d | 7.1 | 34.5 | 2.4 | 0 | - | 0 |
| 5 | 3.8 | 29 | 1.1 | 0.4 | 100 | 0.4 |
| 6 | 4.4 | 32.7 | 1.4 | 0.4 | 100 | 0.4 |
| 7 | 15.4 | 51.1 | 7.9 | 6.1 | 73.3 | 4.5 |
| 8 | 8.2 | 37.3 | 3.1 | 2.8 | 100 | 2.8 |
| 9 | 133 | 48.1 | 6.4 | 7.7 | 57.4 | 4.4 |
| 10 | 29.1 | 28.8 | 8.4 | 20.8 | 100 | 20.8 |
| 11p | 9.3 | 42.2 | 3.9 | 4.5 | 77.8 | 3.5 |
| 11d | 4.2 | 59.3 | 2.5 | 2.8 | 100 | 2.8 |
| 12a | 4.2 | 29.1 | 1.2 | 2.1 | 66.7 | 1.4 |
*Patients with none of the risk factors: tumor size ≤ 70 mm, esophagogastric junction-tumor epicenter distance ≤ 30 mm, not a macroscopic type IV tumor, and no serosal invasion.
DISCUSSION
In this study, 4 risk factors, specifically longer distance from EGJ to tumor epicenter, larger tumor size, macroscopic type IV tumor, and serosal invasion, were risk factors for LN metastasis at the distal part of the stomach in patients with APGC. Approximately one-quarter of patients had no risk factors in the study population, and their TVIs at the distal part of the stomach were very low. Therefore, these results suggest that dissecting LNs at the distal part of the stomach is not essential for patients without risk factors. Hypothetically, the oncologic safety of proximal gastrectomy was not inferior to that of total gastrectomy for patients with APGC without risk factors.
The distance from EGJ to tumor epicenter had been the most relevant clinical parameter for surgical planning. For Siewert type II EGJ cancers, proximal gastrectomy is recommended as an acceptable procedure since the benefit of dissecting LNs at the distal part of the stomach is low for these tumors.13,15,19–22 However, for advanced tumors located lower than Siewert type II, including Siewert type III EGJ cancers, the appropriate extent of gastric resection is controversial.15,19,21,22 Our results demonstrate that proximal gastrectomy for advanced tumors would be an acceptable procedure for not only Siewert type II EGJ cancers but also for some patients with Siewert type III EGJ cancers, if the tumor epicenter is within 30 mm from EGJ.
The tumor size affects not only LN metastasis to the distal part of stomach but also the size of remnant stomach. Several studies have reported that the LN metastasis rate and TVI at the distal part of the stomach were low in advanced Siewert type II EGJ cancer, even though they included large tumors over 100 mm.13,15,19 On the other hand, a prospective multicenter study in Japan reported that the LN metastasis rate at the distal part of the stomach was more than 10% in clinically advanced Siewert type II EGJ cancer, if the tumor size was more than 60 mm.23 Based on the results of our study, we recommend total gastrectomy for large tumors due to the risk of LN metastasis at the distal stomach. Moreover, a larger tumor size results in a smaller remaining stomach volume after proximal gastrectomy, leading to poorer nutritional outcomes.24 Insufficient remnant stomach volume reduces the nutritional advantages of proximal gastrectomy, even if oncologic safety is achieved.
Serosal invasion, which is also a risk factor identified in this study, is corroborated by a previous study that reported LN metastasis at the distal part of the stomach in only T4 proximal gastric cancer.25 Another study, which excluded T4 tumors and macroscopic type IV tumors, also demonstrated that LN metastasis rate at the distal part of the stomach was low and TVI was zero in APGC.10 These 4 risk factors, EGJ–tumor epicenter distance, tumor size, macroscopic type, and serosal invasion can be assessed during preoperative investigations and intraoperative surgical findings.
Proximal gastrectomy is advantageous over total gastrectomy because of preservation of the distal portion of the stomach. The remaining distal stomach functions as a food reservoir to improve oral digestion and maintain weight after proximal gastrectomy.26 Since the parietal cells essential for vitamin B12 absorption are located primarily in the body of the stomach, vitamin B12 supplements are required for life after total gastrectomy but are often not required after proximal gastrectomy.24 Proximal gastrectomy also allows food passage through the duodenum, providing a route for iron absorption.27 Given the advantages of function preservation and maintained oncologic safety, proximal gastrectomy would be preferable to total gastrectomy for patients with APGC and no risk factors.
No published prospective clinical trials have compared survival outcomes after proximal gastrectomy and total gastrectomy for APGC, although several retrospective reports have been published, as summarized in Supplement 1, http://links.lww.com/AOSO/A86.28–35 Except for one report,30 survival rates after proximal gastrectomy were comparable32–35 or even better (although not significantly)28,29,31 than those after total gastrectomy. However, it should be noted that esophageal reflux and anastomotic strictures were common after proximal gastrectomy.28,31 Thus, a strategy to keep the motility of residual stomach, preserving more than half of the original stomach, is necessary.36 Otherwise, protection of the esophageal mucosa using a flap formation37 or double tract reconstruction should be considered.38
When applying our results as an indication for proximal gastrectomy, it will be necessary to evaluate metastasis at LN #3b (LNs along the right gastric artery located at the distal lesser curvature) because this station is not adequately dissected during proximal gastrectomy. The possibility of LN #3b metastasis was reported with APGC when tumor size was >40 mm25 or gastric invasion distance was > 40 mm.11 In this study, we did not evaluate metastasis at LN #3b because the LN #3 is not routinely separated into LN #3a and LN #3b in our practice. Thus, further investigations are necessary to evaluate metastasis at LN #3b when using our results as an indication for proximal gastrectomy.
Our study has the limitations of a retrospective study, including the potential for selection bias, which could limit the general applicability of our results. Randomized, prospective studies are required to assess the clinical applicability of these criteria. Moreover, we cannot evaluate survival outcomes of our indication for proximal gastrectomy, since all the patients underwent radical total gastrectomy. To identify long-term outcomes of our indication, future study is required comparing the patients who underwent total gastrectomy and proximal gastrectomy within the indication. Last, we analyzed risk factors for LN metastasis at the distal part of the stomach based on postoperative pathologic data, including EGJ-tumor epicenter distance, tumor size, macroscopic tumor type, and T stage. Although these risk factors can be assessed before or during surgery, accuracy of preoperative assessments was not considered in this study.
In conclusion, our results suggest that proximal gastrectomy may be indicated for APGC meeting all of these criteria: EGJ-tumor epicenter distance ≤30 mm, tumor size ≤70 mm, not a macroscopic type IV tumor (ie, types I, II, or III), and no serosal invasion. By performing proximal gastrectomy, it would be possible to provide a better quality of life while ensuring oncologic safety in patients meeting all four criteria.
Supplementary Material
Footnotes
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (MSIP; No. 2016R1A2B4014984). The funding source had no role in the design or conduct of the study; data collection, analysis, or interpretation; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure: The authors declare that they have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Dassen AE, Lemmens VE, van de Poll-Franse LV, et al. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer. 2010;46:1101–1110. [DOI] [PubMed] [Google Scholar]
- 3.Deans C, Yeo MS, Soe MY, et al. Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J Surg. 2011;35:617–624. [DOI] [PubMed] [Google Scholar]
- 4.Fujiya K, Kawamura T, Omae K, et al. Impact of malnutrition after gastrectomy for gastric cancer on long-term survival. Ann Surg Oncol. 2018;25:974–983. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Kim HI, Hyung WJ, et al. Vitamin B(12) deficiency after gastrectomy for gastric cancer: an analysis of clinical patterns and risk factors. Ann Surg. 2013;258:970–975. [DOI] [PubMed] [Google Scholar]
- 6.Takiguchi N, Takahashi M, Ikeda M, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407–416. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki M, Takiguchi S, Omori T, et al. Multicenter prospective trial of total gastrectomy versus proximal gastrectomy for upper third cT1 gastric cancer. Gastric Cancer. 2021;24:535–543. [DOI] [PubMed] [Google Scholar]
- 8.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yura M, Yoshikawa T, Otsuki S, et al. Oncological safety of proximal gastrectomy for T2/T3 proximal gastric cancer. Gastric Cancer. 2019;22:1029–1035. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y, Katai H, Ito M, et al. Can proximal gastrectomy be justified for advanced adenocarcinoma of the esophagogastric junction? J Gastric Cancer. 2018;18:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song W, Liu Y, Ye J, et al. Proximal gastric cancer: lymph node metastatic patterns according to different T stages dictate surgical approach. Chin Med J (Engl). 2014;127:4049–4054. [PubMed] [Google Scholar]
- 13.Fujitani K, Miyashiro I, Mikata S, et al. Pattern of abdominal nodal spread and optimal abdominal lymphadenectomy for advanced Siewert type II adenocarcinoma of the cardia: results of a multicenter study. Gastric Cancer. 2013;16:301–308. [DOI] [PubMed] [Google Scholar]
- 14.Mine S, Kurokawa Y, Takeuchi H, et al. Distribution of involved abdominal lymph nodes is correlated with the distance from the esophagogastric junction to the distal end of the tumor in Siewert type II tumors. Eur J Surg Oncol. 2015;41:1348–1353. [DOI] [PubMed] [Google Scholar]
- 15.Goto H, Tokunaga M, Miki Y, et al. The optimal extent of lymph node dissection for adenocarcinoma of the esophagogastric junction differs between Siewert type II and Siewert type III patients. Gastric Cancer. 2015;18:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano T, Sasako M, Mizusawa J, et al. ; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265:277–283. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd english edition. Gastric Cancer. 2011;14:101–112. [DOI] [PubMed] [Google Scholar]
- 18.Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346–351. [DOI] [PubMed] [Google Scholar]
- 19.Cao H, Ooi M, Yu Z, et al. Should pyloric lymph nodes be dissected for siewert type II and III adenocarcinoma of the esophagogastric junctions: experience from a high-volume center in China. J Gastrointest Surg. 2019;23:256–263. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita H, Katai H, Morita S, et al. Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg. 2011;254:274–280. [DOI] [PubMed] [Google Scholar]
- 21.Ooki A, Yamashita K, Kikuchi S, et al. Clinical significance of total gastrectomy for proximal gastric cancer. Anticancer Res. 2008;28(5B):2875–2883. [PubMed] [Google Scholar]
- 22.Kodera Y, Yamamura Y, Shimizu Y, et al. Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert’s classification applied to 177 cases resected at a single institution. J Am Coll Surg. 1999;189:594–601. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa Y, Takeuchi H, Doki Y, et al. Mapping of lymph node metastasis from esophagogastric junction tumors: a prospective nationwide multicenter study. Ann Surg. 2021;274:120–127. [DOI] [PubMed] [Google Scholar]
- 24.Cho M, Son T, Kim HI, et al. Similar hematologic and nutritional outcomes after proximal gastrectomy with double-tract reconstruction in comparison to total gastrectomy for early upper gastric cancer. Surg Endosc. 2019;33:1757–1768. [DOI] [PubMed] [Google Scholar]
- 25.Haruta S, Shinohara H, Hosogi H, et al. Proximal gastrectomy with exclusion of no. 3b lesser curvature lymph node dissection could be indicated for patients with advanced upper-third gastric cancer. Gastric Cancer. 2017;20:528–535. [DOI] [PubMed] [Google Scholar]
- 26.Asaoka R, Irino T, Makuuchi R, et al. Changes in body weight, skeletal muscle and adipose tissue after gastrectomy: a comparison between proximal gastrectomy and total gastrectomy. ANZ J Surg. 2019;89:79–83. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Hyung WJ, Kim HI, et al. Method of reconstruction governs iron metabolism after gastrectomy for patients with gastric cancer. Ann Surg. 2013;258:964–969. [DOI] [PubMed] [Google Scholar]
- 28.Rosa F, Quero G, Fiorillo C, et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG). Gastric Cancer. 2018;21:845–852. [DOI] [PubMed] [Google Scholar]
- 29.Sugoor P, Shah S, Dusane R, et al. Proximal gastrectomy versus total gastrectomy for proximal third gastric cancer: total gastrectomy is not always necessary. Langenbecks Arch Surg. 2016;401:687–697. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Park SS, Kim J, et al. Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg. 2006;30:1870–1876. [DOI] [PubMed] [Google Scholar]
- 31.Yoo CH, Sohn BH, Han WK, et al. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004;36:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998;123:127–130. [PubMed] [Google Scholar]
- 33.Kitamura K, Yamaguchi T, Nishida S, et al. The operative indications for proximal gastrectomy in patients with gastric cancer in the upper third of the stomach. Surg Today. 1997;27:993–998. [DOI] [PubMed] [Google Scholar]
- 34.Jakl RJ, Miholic J, Koller R, et al. Prognostic factors in adenocarcinoma of the cardia. Am J Surg. 1995;169:316–319. [DOI] [PubMed] [Google Scholar]
- 35.Moreaux J, Msika S. Carcinoma of the gastric cardia: surgical management and long-term survival. World J Surg. 1988;12:229–235. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Kinami S, Fushida S, et al. Evaluation of residual stomach motility after proximal gastrectomy for gastric cancer by electrogastrography. Dig Dis Sci. 2006;51:268–273. [DOI] [PubMed] [Google Scholar]
- 37.Hayami M, Hiki N, Nunobe S, et al. Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double-flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol. 2017;24:1635–1642. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SH, Jung DH, Son SY, et al. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer. 2014;17:562–570. [DOI] [PubMed] [Google Scholar]