Abstract
Introduction:
Comparative data on long-term outcomes of mechanistically different bariatric operations are scarce.
Methods:
In this prospective, observational study, consecutive patients with severe obesity were studied using a predefined reoperation algorithm to determine long-term health outcomes after bariatric surgery (BS): adjustable gastric banding (AGB), Roux-en-Y gastric bypass (RYGB), or biliopancreatic diversion (BPD). All patients were assessed for mortality, postoperative weight loss, rate of reoperation, comorbidities, and quality of life (QoL) 8 years after surgery.
Results:
Between 1996 and 2008, 2364 Swiss patients, with a mean body mass index of 43 ± 7 kg/m2 (mean ± SD) underwent AGB (n = 1404), RYGB (n = 790), or BPD (n = 170). Two thousand two hundred twenty-eight (94%) were followed for 8 years after BS. Eight-year mortality of the whole study group was 34.3 per 104 person-years. Percent excessive weight loss at 8 years was 56.7 ± 1.4% (95% confidence interval) in AGB, 62.5 ± 2.4% in RYGB and 64.8+-3.0% in BPD. The rate of major reoperation was highest in AGB and significantly lower in RYGB and BPD (63.4 vs 54.3 vs 47.2 per 103 person-years, P < 0.001). Remission of comorbidities was observed across all 3 groups, with key improvement (P < 0.01) in esophagitis in the RYGB group, and type 2 diabetes (T2D) (>60%) in procedures involving duodenal exclusion. Total improvement in QoL was similar between the 3 types of operations but was strongly correlated with weight loss preservation (P < 0.001).
Conclusions:
BS, at the expense of a high reoperation rate but low procedural mortality, considerably improves the QoL and results in sustained remission of comorbidities, especially T2D using a predefined reoperation algorithm developed to prevent weight regain and operation-specific complications.
Keywords: bariatric surgery, biliopancreatic diversion, dyslipidemia, gastric banding, gastroesophageal reflux disease, hypertension, obesity management, quality of life, Roux-en-Y gastric bypass, type 2 diabetes, weight loss, weight regain
In this prospective, observational study, the authors describe the results of a predefined surgical reoperation algorithm preventing long-term weight regain and improved health outcomes after bariatric surgery: adjustable gastric banding, Roux-en-Y gastric bypass, or biliopancreatic diversion. At the expense of a high reoperation rate but low procedural mortality, quality of life was improved and sustained remission of comorbidities, especially type 2 diabetes, was observed.
INTRODUCTION
The Look AHEAD study,1 a nonsurgical weight loss program, reported a total body weight loss of 6% maintained over a median follow-up of 9.6 years. Clinical evidence comparing the long-term (>5 years) outcome of different surgical options is still rare.2 Data3–7 show that bariatric surgery (BS) results in greater total body weight loss than nonsurgical treatments (median 20%–30%, 8–12 years after initial operation) and is more effective at reducing comorbidities such as type 2 diabetes mellitus (T2D), hypertension, and dyslipidemia. For nonsurgical and surgical options, there is still uncertainty regarding the durability of long-term weight loss and associated patient management. Most reports of bariatric surgical outcomes have been limited by inadequate and incomplete long-term follow-up, with fewer than 3% of studies reporting results on 80% or more of the original cohort, thus leading to potential overestimation of the effectiveness of the procedure.6 The most comprehensive study with the longest patient follow-up during a period of up to 15 years is the Swedish obesity study;3 however, the procedures investigated are no longer frequently used. Other long-term studies on 6- and 12-year outcomes after Roux-en-Y gastric bypass (RYGB)4,5 and 7-year outcomes of the longitudinal assessment of bariatric surgery (LABS) study,7 comparing gastric banding and RYGB, have shown considerable mean weight regain in patients between years 2 and 7 of more than 9 kg. Furthermore, the LABS study reported a loss to follow-up of 17% of patients resulting in an incomplete picture of how patients fared in these long-term measures. Because weight loss is closely related to improvement in related comorbidities,3,7 prevention of weight regain after bariatric operations is important for the amelioration of long-term health outcomes. We have previously developed a reoperation algorithm to deal with complications after adjustable gastric banding (AGB), RYGB, and biliopancreatic diversion (BPD) (Fig. 1A–C). It was specifically designed to standardize reoperation procedures and prevent the relatively common issue of weight regain.10–14
FIGURE 1.

In-house reoperation algorithm for severely obese patients who did not achieve adequate weight loss after initial bariatric surgery. A, Adjustable gastric band (AGB); *% excessive weight loss, +gastroscopic removal,8 #only port replacement, $due to patients preference, £Roux-en-Y gastric bypass. B, Roux-en-Y gastric bypass (RYGB); *% excessive weight loss, +replaced in favor of lenghtening of biliopancreatic limb,9 §adjustable gastric band (AGB). C, Biliopancreatic diversion (BPD); *% excessive weight loss, +gastroscopic removal,8 $due to patient’s preference, #only port replacement, £Roux-en-Y gastric bypass. Decisions were taking into account patient’s preferences.
Therefore, this study aims to investigate whether long-term improvement of comorbidities and quality of life (QoL) is sustained by durable weight loss, achieved through the use of an algorithm for reoperation described previously.10,11
METHODS
Patients
In this multicenter, observational cohort study, unrelated Swiss Caucasian patients with severe obesity underwent laparoscopic BS between 1996 and 2008 at 4 academically affiliated, urban hospital centers in Switzerland (Table 1) and were followed up for at least 8 years. Inclusion criteria were patients aged between 18 and 70 years and severe obesity (body mass index [BMI] of 35 kg/m2 in the presence of one or more serious comorbidities (eg, T2D, hypertension, dyslipidemia, osteoarthritis, or gastroesophageal reflux disease [GERD]). General exclusion criteria were: open and obsolete operations (eg, vertical banded gastroplasty); large hiatus hernia; geographic factors encumbering regular follow-up (n = 4); inability to comprehend necessary perioperative and follow-up procedures; psychosis; alcohol or drug abuse; serum creatinine level >200 µmol/L; and evidence of liver cirrhosis. Patients were fully informed and gave written consent. The study was approved by the ethics board of Canton Berne, Switzerland, Project ID 2017-02191, and complied with the Declaration of Helsinki.
TABLE 1.
Patient Characteristics at Baseline (Before Gastric Banding [AGB], RYGB and BPD)
| AGB | RYGB | BPD | Statistics | |
|---|---|---|---|---|
| N* | 1404 | 790 | 170 | P < 0.001 |
| Between groups† | ||||
| Female (%) | 81 | 68 | 69 | P < 0.01 |
| AGB vs RYGB or BPD† | ||||
| BMI (kg/m2) | 42.9 ± 5.4 | 45.8 ± 6.0 | 53.2 ± 8.7 | P < 0.001 |
| Between groups† | ||||
| Weight (kg) | 119.5 ± 18.0 | 128.1 ± 20.0 | 151.3 ± 27.2 | P < 0.001 |
| Between groups† | ||||
| Height (cm) | 166.7 ± 8.3 | 167.1 ± 9.3 | 168.1 ± 9.8 | NS |
| Age (years) | 42.5 ± 12.1 | 43.9 ± 11.6 | 43.5 ± 10.1 | NS |
| Hypertension (%) | 76 | 90 | 90 | P < 0.001 |
| AGB vs RYGB or BDP‡ | ||||
| Type-2 diabetes (%) | 23 | 40 | 25 | P < 0.001 |
| RYGB vs AGB or BPD‡ | ||||
| Dyslipidemia (%) | 65 | 66 | 80 | P < 0.001 |
| AGB or RYGB vs BPD‡ | ||||
| Osteoarthritis (%) | 78 | 83 | 80 | NS |
| Esophagitis (%) | 29 | 25 | 26 | NS |
Values are given as mean ± SD.
*N, number of patients including lost to follow up (n = 136) and death (n = 71).
†ANOVA, analysis of variance.
‡Kruskall-Wallis Test
AGB indicates adjustable gastric banding; BPD, biliopancreatic diversion; BMI, body mass index; RYGB, Roux-en-Y gastric bypass.
Procedures
A multidisciplinary team, consisting of an internist specializing in obesity and his associate physician, a bariatric surgeon, a dietitian, and a psychologist assessed each eligible patient before surgery. An array of diverse phenotypic data and blood for subsequent analysis were routinely obtained as previously described.11
Patients received 1 of 3 bariatric procedures listed below to manage their obesity. AGB consists of a small proximal gastric reservoir (~25 mL) and stoma that limit the volume and speed with which solid food empties, and has been described in detail elsewhere.10,11 RYGB creates a stapled small proximal gastric reservoir attached to the jejunum, bypassing stomach, pylorus, duodenum, and the first part of the jejunum as previously described.12–15 The BPD group comprised of banded and nonbanded patients. The banded operation combines AGB, as above, with a pylorus-sparing duodenal-jejunal bypass, “duodenal switch,” dividing and closing the proximal duodenum attaching the post-pyloric stomach to the ileum.16 The nonbanded BPD patients were those with BPD by Scopinaro’s procedure.17
Postoperative complications and insufficient weight loss (<50% excessive weight loss [EWL] more than 3 years after primary operation) were defined and determined the choice of reoperation (Fig. 1A–C). Options and risk/benefit balance were discussed in the multidisciplinary team with every eligible patient and the type of reoperation was selected considering the patients’ preference and physicians’ judgment.
Definitions of Complications and Indications for Reoperation
After initial BS, patients were followed up every 6 months for at least 8 years at the private practice of the obesity specialist (F.F.H.). Complications, reoperations, vital signs and physical examinations, band adjustments, and medications were recorded at each visit.
Major complications were defined as pulmonary (pneumonia, edema, respiratory insufficiency or adult respiratory distress syndrome), cardiovascular (myocardial infarct, congestive heart failure, stroke), renal, psychiatric (depression, psychosis), abdominal (anastomotic/marginal ulcers, peritonitis, intestinal obstruction, gastric dilatation, deep wound infections, and internal hernia).10,11,18 Postoperative complications and “insufficient” weight loss were defined according to published criteria and governed the choice of reoperation19–21 (Fig. 1A–C). Insufficient weight was loss was defined as having lost less than 50% EWL or regained above 50% EWL from lowest weight ever achieved (NADIR) more than 3 years after primary operation.
Major reoperations were defined as those requiring laparoscopy or laparotomy under general anesthesia, whereas minor reoperations included interventional gastroscopy and port-tube–related abdominal wall procedures not requiring laparotomy or general anesthesia.19–21 Reoperations were performed using the algorithm previously described and illustrated in Figure 1A–C.10–14
The rate of reoperation was calculated by dividing the absolute amount of reoperations by total person-years at end of study (8 years) or death.
Variables
Comorbidities and Their Long-Term Reporting
Comorbidities were reported using the following criteria: hypertension was defined as blood pressure ≥130/85 mm Hg or taking antihypertensive drugs. T2D was reported when HbA1c ≥6.5% or taking antidiabetic drugs; dyslipidemia was reported with triglyceride levels >200 mg/dL, HDL-cholesterol <35 mg/dL, LDL-cholesterol levels >100mg/dL or taking lipid-lowering drugs. At 8 years follow-up, improvement was defined as a 10% change in measured values compared with baseline (as appropriate) or reducing the dosage of respective drug treatment. A change in measured values of 10% at 8 years compared with values at baseline or increasing dosage of respective drug treatment was considered a treatment failure. Patients were considered in remission if measured values were within normal limits and medication was ceased.
Osteoarthritis was evaluated based on symptoms, mobility and use of painkillers by oral interview before and 8 years after BS. Esophageal pathology was assessed preoperatively and again before end of study by esophagogastroscopy described in detail previously.22 Other comorbidities, for example, sleep apnea and urinary incontinence were not routinely studied before surgery; therefore, no data are available.
BMI was calculated as body weight divided by height in m2. Percent Excessive BMI lost (% EBMIL) was calculated as follows:
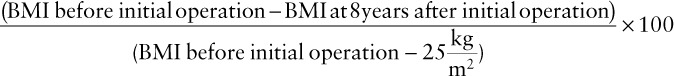 |
Percent excessive body weight loss (% EWL) was calculated as follows:
 |
MLIC, Metropolitan Life Insurance Company.
Interquartile range depicts all values between the 25th and 75th percentile of the respective study group.
Quality of Life Assessment and Bariatric Analysis and Reporting Outcome System Score
The Moorehead-Ardelt Quality of Life Questionnaire II23 was used to assess the subjective QoL. It uses simple drawings for each of the 6 QoL questions: self-esteem, physical activity, social life, work conditions, eating behavior, and sexual activity, each to be filled out on a 10-point Likert scale from −0.5 to +0.5. The Bariatric Analysis and Reporting Outcome System Score,24,25 is a scoring system that allows comparisons between patients and outcomes of different types of BS as previously described.11
Statistics
Means ± SD are presented throughout the manuscript unless specified otherwise. Intergroup differences are calculated using analysis of variance (ANOVA), ANOVA for repeated measures, or Kruskal-Wallis test when appropriate. Patients who underwent a revision or reversal of their bariatric procedure were included in the analysis of their original bariatric procedure, consistent with intention to treat. Logistic regression models were used for the analysis of remission of metabolic syndrome, esophagitis, or osteoarthritis and the following predictors: sex, baseline BMI (BMI0), age, and operation type (AGB, RYGB, or BPD). Patients who died were removed from all analyses except mortality analyses, and patients lost to follow-up were excluded from weight loss, comorbidity, and QoL analyses. All analyses were performed using IBM SPSS (version 22).
RESULTS
Baseline Characteristics
Of 2364 patients (75% women, 25% men), 1404 underwent AGB (59%), 790 underwent RYGB (33%), and 170 underwent BPD (7%) as their first bariatric procedure. Two thousand two hundred twenty-eight patients (94%) had 8-year follow-up data (Table 1).
Age, height, prevalence of osteoarthritis, and esophagitis were similar in all groups. BMI and weight before the operation were highest in BPD patients. Patients with a higher BMI at baseline were more likely to have RYGB or BPD operations, whereas those with a lower BMI more frequently had AGB. RYGB and BPD patients presented with a higher prevalence of hypertension compared with AGB patients. Furthermore, patients with BPD were more dyslipidemic than those with RYGB or AGB. Prevalence of T2D was higher in RYGB than in BPD or AGB.
Mortality
There were 71 deaths during the 8 years of observation: corresponding to a mortality of 26.1, 36.7, and 104.2 per 104 patient-years, in AGB, RYGB, and BPD, respectively (Table 2). Age at time of death was similar between patient cohorts (Table 2).
TABLE 2.
Mortality During ≥8 Years of Follow-Up
| AGB | RYGB | BPD | Statistics | |
|---|---|---|---|---|
| Person-years (N) | 12648 (1404) | 6320 (790) | 1440 (170) |
P < 0.01 Between groups |
| Age at time of death (years) | 59.9 ± 11.0 | 57.0 ± 10.9 | 57.5 ± 10.8 | NS |
| BMI at primary operation | 43.7 ± 7.7 | 45.6 ± 7.8 | 51.0 ± 11.8 |
P < 0.04 BPD vs others |
| BMI at time of death | 32.6 ± 7.5 | 37.9 ± 8.0 | 41.1 ± 13.0 |
P < 0.02 Between groups |
| Total death/104 person-years (N) | 26.1 (33) | 36.7 (23) | 104.2 (15) | P < 0.01*BPD vs others |
| Procedural mortality/104 person-years (N) | 3.2 (4) | 14.3 (9) | 48.6 (7) | NA |
| Pimary operation | 0 (0) | 4.7 (3) | 27.7 (4) | NA |
| Revisonal operation | 3.2 (4) | 9.6 (6) | 20.8 (3) | NA |
| Nonprocedural mortality/104 person-years (N) | 22.9 (29) | 22.4 (14) | 55.6 (8) | NA |
| Cardiovascular | 11 (14) | 11.2 (7) | 27.8 (4) | NA |
| Malignant neoplasia | 7.9 (10) | 8 (5) | 13.9 (2) | NA |
| Suicide | 1.6 (2) | 1.6 (1) | 13.9 (2) | NA |
| Others | 2.4 (3) | 1.6 (1) | 0 (0) | NA |
N, number of patients including lost to follow up (n = 136) and death (n = 71).
Values are given as mean ± SD.
*ANOVA, analysis of variance or Kruskall-Wallis was used, where appropriate.
AGB indicates adjustable gastric banding; BPD, biliopancreatic diversion; BMI, body mass index; RYGB, Roux-en-Y gastric bypass.
Weight Trajectories
Percent EBMIL at 8 years was lower in patients treated with AGB (65.0%, 95% confidence interval [CI] 62.9–67.1) compared with RYGB (69.9% [95% CI 67.4–72.4]) or BPD (70.6% [95% CI 66.2–74.8]; both P < 0.0001, ANOVA for repeated measures). When adjusting for age, sex, and BMI at baseline, these differences were still significant (P < 0.001 for RYGB vs AGB and P < 0.01 for BPD vs AGB) (Fig. 2).
FIGURE 2.

Effect of different bariatric operations on % excessive BMI lost (% EBMIL). AGB indicates adjustable gastric band; BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass.
In the present study, weight NADIR for group AGB and BPD was at year 8, whereas patients in the RYGB group reached their lowest weight after 2 years. Mean weight regain in the RYGB group between years 2 and 6 was 5.1% EBMIL (95% CI 2.9–7.3), corresponding to 3.3kg (95% CI 1.4–5.2).
AGB patients of 40.1% lost less than 50% of excess weight, while in patients with RYGB or BPD the percentage of subjects losing less than 50% EWL was 23% and 18% respectively (P < 0.001 vs AGB). Mean weight remained stable between 6 and 8 years after primary operation in all 3 groups.
Rate of Reoperation During 8 Years of Study
Patients of 57.8% (n = 736) in the AGB group did not need a reoperation. Of the 42% of patients (n = 538) who did need one, 72.3% had a single reoperation and 27% had more than one reoperation (Table 3). Therefore, the reoperation rate per 103 person-years was 63.4 for AGB (Table 3). The most frequent reoperations were conversion to RYGB (43%), band replacement (34.4%), and conversion to banded BPD (12.8%) (Table 3). Six bands eroded and were removed endoscopically.8 Consecutive treatment was done according to our algorithm (Fig. 1A).
TABLE 3.
Reoperation Characteristics During 8 Years Observational Period
| Type of Reoperation | AGB | RYGB | BPD | Statistics* |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Total patients† | 1274 (100%) | 785 (100%) | 169 (100%) | |
| Patients without reoperation | 736 (57.8%) | 500 (63.7%) | 111 (65.7%) | %: P < 0.001 AGB vs other groups |
| Patients with reoperation | 538 (42.2%) | 285 (36.3%) | 58 (34.3%) | %: P < 0.001 AGB vs other groups |
| Reoperations | ||||
| Total number | 646 | 341 | 68 |
P < 0.001 Between groups |
| Per 103 person-years | 63.4 | 54.3 | 47.2 | |
| 1 reoperation | 467 | 276 | 57 | |
| Per 103 person-years | 45.8 | 43.9 | 39.6 | |
| >1 reoperation | 179 | 65 | 9 | |
| Per 103 person-years | 17.6 | 10.3 | 7.5 | |
| Type of reoperations | ||||
| Band replacement due to slippage and leakage | 221 (34.3%) | — | 22 (32.4%) | |
| Conversion to RYGB due to band intolerance | 278 (43.0%) | — | 25 (36.8%) | NA |
| Conversion to banded BPD due to insufficent weight loss | 83 (12.8%) | — | — | |
| Conversion to nonbanded BPD due to insufficient weight loss | — | 36 (10.5%) | 10 (14.7%) | NA |
| Fobi-ring ± pouch-resizing due to loss of restriction | 4 (0.6%) | 212 (62.3%) | 1 (1.5%) | %: P < 0.001 RYGB vs other groups |
| Band/Fobi removal only due toband intolerance | 11 (1.7%) | 20 (5.9%) | — | |
| Internal hernia | 45 (7.0%) | 60 (17.5%) | 4 (5.9%) | %: P < 0.001 RYGB vs other groups |
| Various | 4 (0.6%) | 13 (3.8%) | 6 (8.7%) | %: P < 0.001 AGB vs other groups |
*Kruskall-Wallis Test.
†Including all patients, who died during ≥8 years of study (n = 71), but not those, lost to follow-up (n = 136).
AGB indicates adjustable gastric banding; BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass.
In the RYGB group, of the 36.3% of patients that were reoperated (n = 341), 80.8% had a single reoperation and 19.2% had more than one reoperation (Table 3). Therefore, the reoperation rate per 103 person-years was 54.3, being significantly lower than that observed in AGB (P < 0.01) (Table 3). The most frequent reoperations were a Fobi-ring with pouch resizing (62.3%) due to weight regain after NADIR and reoperations due to internal hernia (17.5%) (Table 3). Thirty patients (3.8%) experienced 43 marginal ulcers during 8 years of follow-up, which were all treated nonsurgically with high dose proton pump inhibitors and sucralfate.
In the BPD group, of the 34.3% of patients that had reoperations, 86% had a single reoperation and 14% had more than one reoperation (Table 3) Therefore, the reoperation rate per 103 person-years was 47.2, significantly lower compared with AGB and RYGB (P < 0.001) (Table 3). The most frequent reoperations were conversion to RYGB due to band intolerance or band slippage (36.8%), band replacement due to band leakage or slippage (32.4%), and conversion to nonbanded BPD due to band intolerance (12.8%) (Table 3).
Age and sex were not related to the need for reoperation, whereas patients with a higher BMI at baseline and patients treated with AGB had higher reoperation rates. This effect was still significant after adjusting for age, sex, BMI at baseline, and operation type (P < 0.02).
Effect of Reoperation on Weight Loss 8 Years After Initial Operation
Patients in the AGB group with no reoperation (n = 736, 58%) tended to have greater (P < 0.09) % EWL than those who had a reoperation (Table 4). Patients with band removal lost about 25% less weight than patients still carrying a band after 8 years (Table 4). Patients with and without a reoperation after RYGB lost similar amounts of weight. Interestingly, conversion from AGB to RYGB resulted in less %EWL when compared to patients with primary RYGB (Table 4).
TABLE 4.
Percent of Excessive Weight Loss (EWL) During ≥8 Years in 2157* Patients After Bariatric Surgery
| Primary Surgery | Type of Intervention/Reoperation | Number | % EWL at 8 years (95% CI) | IQR† 25%–75% | ANOVA Reop vs No Reop |
|---|---|---|---|---|---|
| AGB | Total patients | 56.7 ± 1.4 | 29.7–83.7 | NS | |
| No reoperation | 736 | 57.8 ± 1.5 | 30.0–85.6 | — | |
| Reoperation | 538 | 55.1 ± 1.3 | 29.6–80.6 | P = 0.09 | |
| Band replacement | 166 | 56.7 ± 1.3 | 32.3–81.1 | NS | |
| Conversion to RYGB | 278 | 53.5 ± 1.4 | 26.5–80.5 | P = 0.04 | |
| Conversion to BPD | 83 | 56.7 ± 1.2 | 34.5–78.9 | NS | |
| Band removal | 11 | 42.7 ± 1.5 | 14.0–70.4 | P = 0.03 | |
| RYGP | Total patients | 62.5 ± 2.5 | 40.1–84.9 | NS | |
| No reoperation | 500 | 62.7 ± 2.4 | 40.6–84.8 | NS | |
| Reoperation | 285‡ | 62.2 ± 2.6 | 39.0–85.4 | — | |
| Fobi-ring ± Pouch- resizing | 212 | 61.4 ± 2.6 | 38.2–84.6 | NS | |
| Enterotranspostion/distalization | 36 | 56.5 ± 2.9 | 30.4–82.6 | NS | |
| Fistula revision | 12 | 72.1 ± 2.3 | 50.9–93.3 | P < 0.01 | |
| BPD | Total patients | 64.8 ± 3.0 | 45.9–83.7 | NS | |
| No reoperation | 111 | 63.2 ± 2.9 | 45.0–81.4 | — | |
| Reoperation | 58‡ | 68.6 ± 3.2 | 48.7–88.5 | P = 0.09 | |
| Band replacement | 22 | 73.5 ± 3.4 | 51.8–95.2 | P = 0.03 | |
| Conversion to RYGB | 25 | 70.1 ± 2.8 | 52.5–87.7 | NS | |
| Conversion to BPD nonbanded | 10 | 56.1 ± 2.6 | 39.8–72.4 | NS |
Values are given as mean ± SD.
*Excluding lost to follow-up (n = 136) and dead patients (n = 71).
†Interquartile range (IQR) 25th to 75th percentile of observed values.
‡Including internal hernia, band or Fobi-ring removal.
%EWL indicates percent excessive weight loss; AGB, adjustable gastric banding; ANOVA, analysis of variance for repeated measures; BPD, biliopancreatic diversion; , RYGB: Roux-en-Y gastric bypass.
Patients with no reoperation after BPD (n = 111, 65.7%) tended to have greater (P < 0.09) % EWL than those with reoperation, mostly due to replacement of leaking band (32.4% of operated patients) (Table 4).
Remission Rates of Comorbidities
Remission was observed in all 5 comorbid conditions at 8 years after initial procedure (T2D 53.5%, hypertension 30.1%, dyslipidemia 62.8%, esophagitis 42.7%, and osteoarthritis 35%) (Table 5). Only some patients experienced a worsening in their comorbid conditions (T2D 5.4%, hypertension 9.9%, dyslipidemia 7.9%, and osteoarthritis 33.5%) (Table 5).
TABLE 5.
Effect of Operation Type on Obesity-Related Comorbidities in 2157* Bariatric Surgery Patients Followed ≥8 Years26
| Comorbidities | Type of Operation | Preoperatively, N (%) | Remission (%) | Improved (%) | No Change or Worse (%) | OR (95% CI)‡ | Statistics† |
|---|---|---|---|---|---|---|---|
| T2D | All patients | 629 (29) | 53.8 | 40.8 | 5.4 | ||
| AGB | 285 (23) | 45.6 | 46.5 | 8.9 | |||
| RGB (vs AGB) | 305 (40) | 60.9 | 36.4 | 2.7 | 0.5 (0.3–0.8) | P < 0.001 | |
| BPD (vs AGB) | 39 (25) | 62.1 | 35.1 | 2.8 | 0.6 (0.4–0.9) | P < 0.01 | |
| Hypertension | All patients | 1768 (82) | 30.1 | 60 | 9.9 | ||
| AGB | 943 (76) | 30.2 | 57.1 | 12.7 | |||
| RYGB (vs AGB) | 686 (90) | 30.7 | 62.6 | 6.6 | NS | NS | |
| BPD (vs AGB) | 139 (90) | 25.6 | 66.9 | 7.2 | 0.6 (0.4–0.9). | P < 0.01 | |
| Dyslipidemia | All patients | 1590 (74) | 62.8 | 29.2 | 7.9 | ||
| AGB | 918 (72) | 63.1 | 28.4 | 8.5 | |||
| RGB | 555 (73) | 65 | 29.4 | 5.7 | NS | NS | |
| BPD (vs AGB, RYGB) | 117 (84) | 51.2 | 35.1 | 13.7 | 0.6 (0.4–0.9) | P < 0.01 | |
| Esophagitis | All patients | 590 (27) | 42.7 | NA | NA | ||
| AGB | 359 (29) | 35.1 | NA | NA | |||
| RGB (vs AGB) | 191 (25) | 56.1 | NA | NA | 2.4 (1.3–4.2) | P = 0.003 | |
| BPD (vs AGB) | 40 (26) | 48.1 | NA | NA | NS | NS | |
| Osteoarthritis | All patients | 1729 (80) | 35 | 31.5 | 33.5 | ||
| AGB | 973 (78) | 38.1 | 29 | 32.9 | |||
| RGB (vs AGB) | 632 (83) | 30.8 | 35.2 | 33.9 | NS | NS | |
| BPD (vs AGB) | 124 (80) | 32.4 | 31.5 | 36.3 | NS | NS |
*Excluding lost to follow-up (n = 136) and dead patients (n = 71).
†According to unadjusted logistic regression model.
‡OR (95% CI): odd’s ratio with 95% confidence interval for % remission.
AGB indicates adjustable gastric banding; BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass; T2D, type-2 diabetes.
The remission rate of patients with RYGB and BPD was much higher in T2D than that observed in patients treated with AGB (45.6%, P < 0.01 vs BPD [62.1%] and P < 0.001 vs RYGB [60.9%]) (Table 5). No patient had a worsening glycemic control as a result of their bariatric operation after 8 years. Interestingly, remission rates of hypertension were lower in BPD (25.6%) when compared with RYGB (30.2%) or AGB (30.7%, P < 0.01) (Table 5). Prevalence of dyslipidemia improved similarly in the 3 patient groups (AGB 63.1%, RYGB 65.0%, and BPD 67.0%) (Table 5). The remission rate of esophagitis was significantly higher after RYGB than after AGB (56.1% vs 35.1%, P = 0.003) (Table 5). The remission rate of osteoarthritis was similar in all 3 groups investigated (AGB 38.1%, RYGB 30.8%, and BPD 32.4%) (Table 5). Reported worsening of osteoarthritis was 2-fold higher in BPD compared with RYGB patients (13.3 vs 6%, P < 0.01) (Table 5).
The remission rate of T2D, hypertension, and esophagitis remained significant after adjusting for sex, age, and BMI before operation (P < 0.005).
Quality of Life, Measuring Patient’s Subjective Satisfaction and Bariatric Analysis and Reporting Outcome System Score
Overall, perceived QoL improvement by patients was independent of the type of bariatric operation used. Patients with the highest amount of absolute weight loss—that is, patients treated with BPD (delta BMI 20.0 ± 8.3 kg/m2)—reported the greatest improvement in physical activity 8 years after their operation (P < 0.001), compared with AGB (delta BMI 11.5 ± 5.3 kg/m2) and RYGB (delta BMI 14.4 ± 5.8 kg/m2) (Table 6). Perceived eating behavior was better in RYGB than AGB or BPD (P < 0.001) (Table 6). Pleasure related to sexuality was highest in ABG and was substantially higher than that observed in RYGB (P < 0.01) (Table 6). Interestingly, weight regain of more than 10% after NADIR in RYGB patients resulted in lower improvement of social contacts (0.06 ± 0.20 vs 0.24 ± 0.20, P < 0.001) and self esteem (0.18 ± 0.20 vs 0.30 ± 0.20, P < 0.001) (Table 6).
TABLE 6.
Quality of Life Assessment ≥8 Years After Initial Bariatric Surgery
| AGB (N = 1241*) | RYGB (N = 762*) | BPD (N = 154*) | Statistics ANOVA | |
|---|---|---|---|---|
| Quality of life (QoL) total† (−3 to +3) | 1.10 ± 0.69 | 1.08 ± 0.73 | 1.12 ± 0.74 | NS |
| Improvement in physical activity | 0.24 ± 0.20 | 0.22 ± 0.20 | 0.29 ± 0.20 | P < 0.001 |
| BPD vs others | ||||
| Improving social contacts | 0.20 ± 0.20 | 0.18 ± 0.20 | 0.19 ± 0.20 | NS |
| Satisfaction concerning work | 0.19 ± 0.21 | 0.19 ± 0.24 | 0.20 ± 0.24 | NS |
| Pleasure related to sexuality | 0.06 ± 0.19 | 0.02 ± 0.20 | 0.04 ± 0.21 | P < 0.01 |
| AGB vs RYGB | ||||
| General self esteem improvement | 0.25 ± 0.20 | 0.26 ± 0.20 | 0.21 ± 0.18 | P < 0.001 |
| PBD vs others | ||||
| Perceived eating behavior | 0.19 ± 0.15 | 0.25 ± 0.13 | 0.21 ± 0.17 | P < 0.001 |
| RYGB vs others | ||||
| Total BAROS Score | 3.5 ± 1.5 | 3.8 ± 1.7 | 3.7 ± 1.9 | P < 0.001 |
| AGB vs others | ||||
| % good or better | 67.9 | 72.3 | 71.3 | P < 0.001 |
| AGB vs others |
Values are given as mean ± SD.
*Excluding lost to follow-up (n = 136) and dead patients (n = 71).
†Range after addition of all subscores −3 to +3. Subscores range between −0.5 and +0.5 on a visual analogue scale.
AGB indicates adjustable gastric banding; ANOVA, analysis of variance; BAROS, Bariatric Analysis and Reporting Outcome System; BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass.
Bariatric Analysis and Reporting Outcome System score was lower in AGB compared with RYGB and BPD (P < 0.001) (Table 6).
DISCUSSION
Reliable long-term data comparing the effectiveness of different bariatric operations (and reoperations) on weight management and their significance in the management of patients with severe obesity are rare.5,27 Landmark bariatric studies have had important limitations that interfere with the generalization of their findings.6,7 Our study adds to the current literature, providing valuable long-term results with an exceptional follow-up of more than 94% for multiple bariatric operations.
Our reoperation algorithm (Fig. 1A–C) allowed us to identify patients who were not on the appropriate weight-loss trajectory. We could suggest and perform beneficial secondary procedures, which enhance weight loss efficacy and durability. The consistently better long-term weight management results in the present study can be attributed to the implementation of operation algorithms applied in all 3 BS groups (Table 4).3–7,28 A recent meta-analysis of O’Brien et al28 analyzing BS outcomes after 10 years found a mean % EWL of 45.9% in AGB compared with 56.7% in the present study. In the LABS study with a follow-up of 7 years,7 percent total body weight lost (% TBL) in gastric banding patients was 14.9% corresponding to a mean weight loss of 18.8 kg. In the present study, AGB patients continuously lost weight during the observational period, resulting in a mean weight loss of 32.0 kg (26.8% TBL) compared with 18.8 kg (14.9% TBL) in the LABS study;7 however, this 70% increase in long-term weight loss was achieved at the expense of a 50% higher reoperation rate (63.4 vs 43.3 per 103 person-years, present study vs LABS7), but lower overall mortality (2.6 vs 3.9 per 103 person-years).
Maximum weight loss (% TBL) after RYGB at about 2 years was similar in the present study with 35.2% TBL compared with LABS7 (35.0% TBL) and Adams et al5 (34.9% TBL). In contrast, weight regain in RYGB patients starting 2 years after initial operation was considerably less in the present study (3.3 kg or 2.6% TBL), compared with weight regain at 6 and 12 years in Adams et al4,5 (6 years 8.7 kg or 7.0% TBL, 12 years 10 kg or 8.1% TBL) and LABS7 (7 years 8.8 kg or 6.6.% TBL). The consistently better long-term weight management results (Fig. 2) in the present study are due to the implemented reoperation algorithm (Fig. 1).10,11,13,14 Patients with weight regain of ≥10% after NADIR or %EWL <50% were offered revisional surgery (Table 4 and Fig. 1b) resulting in stable weight 6–8 years after surgery with beneficial long-term outcomes (Tables 5 and 6).
Despite the high reoperation rates (Table 3), overall mortality was similar in RYGB in the present study when compared with the results reported by Adams et al29 (36.7 vs 37.6 per 104 person-years). However, mortality was lower than reported in LABS7 (36.7 vs 53.3 104 per person-years) despite a considerably higher reoperation rate in the present study (54.3 vs 1.2 per 103 person-years, present study vs LABS.7
For BPD, O’Brien et al28 found 74% EWL at 10 years, which was higher than the 64.8% observed in the present study. Because two-thirds of the patients of the BPD group were initially operated with gastric banding with a duodenal switch, restriction might not have been optimal during the 8 years of study. However, weight was maintained after 6 years of study with 64.8% EWL, which is similar to findings by Sethi et al.30 This result was achieved with the lowest reoperation rate of the 3 BS procedure types (Table 3) but the highest mortality rate (Table 2). The higher mortality rate was possibly due to the relatively small number of BPD patients in that study group and their much higher BMI before the first operation (Table 2).
Comorbidities, especially hypertension and dyslipidemia, were present in around 90% of patients before BS (Table 5). Independent of the BS procedure chosen, a considerable number of patients were in remission for various comorbidities 8 years after surgery. Change in BMI is an important predictor of comorbidity resolution. Patients with persistently elevated BMI have higher rates of persistent diabetes, hypertension, and dyslipidemia. T2D remission was >60% after 8 years in patients that had operations including surgical duodenal exclusion. These rates are similar to those observed in a recent meta-analysis for RYGB,18 Adams et al,5 the LABS study,7 and a 10-year study31 in BPD patients. In contrast to recent data reported by Aminian et al32 where 32% of patients experienced a relapse of T2D after more than 5 years of observation, less than 3% of patients showed worsening of T2D in the present study. This could be possibly explained by effectively preventing weight regain in BPD and RYGB patients (Fig. 1 and Table 4) as recently demonstrated by Yoshino et al.33 Their finding is supported by the fact, that in the present study remission rates of T2D were highly correlated with mean % EWL of the 3 types of operations investigated (r = 0.98; Tables 4 and 5).
By contrast, only 45% of AGB patients were in remission for T2D 8 years after surgery, a rate which is higher than that observed in the comparable LABS study,7 but lower than that observed in RYGB and BPD in the present study (>60%). One explanation could be the lower mean percentage of total weight lost in AGB patients (14.9% TBL in LABS7 and 26.8% in the present study) when compared to RYGB and BPD patients (Fig. 1 and Table 4). Therefore, our data would suggest, that patients with T2D should undergo duodenal exclusion5,7,11,31,32,34 rather than AGB, as they achieve greater weight loss long term. Previous studies on sleeve gastrectomy have had a maximum duration of 5 years.35–37 The reported remission rates of T2D were up to over 80% and %EWL of over 80%.37 Since the remission of T2D depends largely on EWL, preventing weight regain for sustained remission in subsequent years is extremely important. These long-term data are lacking so far for sleeve gastrectomy.
Banding procedures showed a higher persistence of esophagitis. This is a consequence of operation-induced anatomical changes that bear an increased predisposition for GERD. Therefore, subjects with preexisting GERD (esophagitis) should receive RYGB as the bariatric procedure of choice (Table 5).
Especially noteworthy, overall improvement in health-related QoL was similar in all 3 BS procedures, a finding not yet reported to our knowledge. Kolotkin et al38 showed that after 12 years, RYGB patients had improved weight-related and physical QoL with a peak at 2 years and declining thereafter as weight regain occurred. O’Brian et al39 assessed 10-year QoL in a randomized controlled trial of laparoscopic AGB versus intensive medical therapy with no difference between the groups; however, baseline values of QoL were only slightly lower than normal values before the beginning of the study. Furthermore, in the Swedish obesity study intervention study40 investigating various bariatric operations, QoL improvement corresponded closely to phases of weight loss and remained stable during years 6–10; a timeframe in which stable weight was observed. As such, the high QoL in the present study points to the fact that preservation of QoL might depend on the prevention of long-term weight regain. Reoperations might, therefore, be essential for maintaining weight loss and thus sustaining improved long-term health outcomes. The often-described decrease in health-related QoL over time after BS might be driven by weight regain. This is supported by the fact that patients with reoperations aimed to prevent weight regain of ≥10% after NADIR had perceived lower self-esteem and less social contacts than those who had preserved their weight at 8 years (P < 0.001).
There are several limitations to this complex study, foremost being selection and ascertainment bias. This Swiss population lacks ethnic diversity, limiting the generalization of the findings. Moreover, sleeve gastrectomy, currently the most commonly performed bariatric procedure,41 was not included in the study since it was only introduced in 2009 as primary bariatric procedure in our center. Furthermore, the universal health care system in Switzerland offers unique support that might not apply to other systems imposing socioeconomic limitations not encountered here. Patient selection, although standardized, reflected a degree of surgeon bias, yet represents medical practice. It was mitigated by the relatively small group of surgeons and the consolidating role of one obesity expert and associate screening and following up with all patients, neither of whom had conflicts of interest such as industry ties or surgical fees related to the study.
To conclude, weight regain can be prevented by using a reoperation algorithm in a population with severe obesity treated with BS. This goes along with low procedural mortality independent of the primary bariatric procedure used, resulting in a sustained improvement in the QoL and comorbidities. RYGB should be the procedure of choice for patients with preoperative esophagitis. Procedures with duodenal exclusion should be preferred over gastric banding for patients with T2D as they result in greater long-term weight loss. Whether comparative long-term data using sleeve gastrectomy will result in similar long-term T2D remission awaits further study.
ACKNOWLEDGMENTS
We are sincerely indebted to all participants in the present study. We thank all surgeons for their surgical expertise and consultation and the late John Kral for his inspiring expertise and scientific support over the years.
Footnotes
Disclosure: The authors declare that they have nothing to disclose.
REFERENCES
- 1.The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. NEJM. 2013; 369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe BM, Belle SH. Long-term risks and benefits of bariatric surgery: a research challenge. JAMA. 2014; 312:1792–1793. [DOI] [PubMed] [Google Scholar]
- 3.Sjöström L, Narbro K, Sjöström CD, et al. ; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007; 357:741–752. [DOI] [PubMed] [Google Scholar]
- 4.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012; 308:1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams TD, Davidson LE, Litwin SE, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017; 377:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puzziferri N, Roshek TB, III, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014; 312:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg. 2018; 153:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldinger R, Mluench R, Steffen R, et al. Conservative management of intragastric migration of Swedish adjustable gastric band by endoscopic retrieval. Gastrointest Endosc. 2001; 53:98–101. [DOI] [PubMed] [Google Scholar]
- 9.Boerboom A, Homan J, Aarts E, et al. A long biliopancreatic and short alimentary limb results in more weight loss in revisional RYGB surgery. Outcomes of the randomized controlled ELEGANCE REDO trial. Surg Obes Relat Dis. 2019; 15:60–69. [DOI] [PubMed] [Google Scholar]
- 10.Biertho L, Steffen R, Branson R, et al. Management of failed adjustable gastric banding. Surgery. 2005; 137:33–41. [DOI] [PubMed] [Google Scholar]
- 11.Steffen R, Potoczna N, Bieri N, et al. Successful multi-intervention treatment of severe obesity: a 7-year prospective study with 96% follow-up. Obes Surg. 2009; 19:3–12. [DOI] [PubMed] [Google Scholar]
- 12.Kral JG. Overview of surgical techniques for treating obesity. Am J Clin Nutr. 1992; 55(2 suppl):552S–555S. [DOI] [PubMed] [Google Scholar]
- 13.Bonnefond A, Keller R, Meyre D, et al. Eating behavior, low-frequency functional mutations in the melanocortin-4 receptor (MC4R) gene, and outcomes of bariatric operations: a 6-year prospective study. Diabetes Care. 2016; 39:1384–1392. [DOI] [PubMed] [Google Scholar]
- 14.Horber FF, Steffen R. Reversal of long-term weight regain after roux-en-y gastric bypass using liraglutide or surgical revision. A prospective study. Obes Surg. 2021; 31:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamdi A, Julien C, Brown P, et al. Midterm outcomes of revisional surgery for gastric pouch and gastrojejunal anastomotic enlargement in patients with weight regain after gastric bypass for morbid obesity. Obes Surg. 2014; 24:1386–1390. [DOI] [PubMed] [Google Scholar]
- 16.Gagner M, Steffen R, Biertho L, Horber F. Laparoscopic adjustable gastric banding with duodenal switch for morbid obesity: technique and preliminary results. Obes Surg. 2003; 13:444–449. [DOI] [PubMed] [Google Scholar]
- 17.Scopinaro N, Marinari GM, Camerini, et al. Laparoscopic standard biliopancreatic diversion: technique and preliminary results Obes Surg. 2002. 12:241–4. [DOI] [PubMed] [Google Scholar]
- 18.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium; Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009; 361:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang S-H, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014; 149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarr MG. Reoperative bariatric surgery. Surg Endosc. 2007; 21:1909–1913. [DOI] [PubMed] [Google Scholar]
- 21.Coakley BA, Deveney CW, Spight DH, et al. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis. 2008; 4:581–586. [DOI] [PubMed] [Google Scholar]
- 22.Potoczna N, Branson R, Kral JG, et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J Gastrointest Surg. 2004; 8:971–981. [DOI] [PubMed] [Google Scholar]
- 23.Moorehead MK, Ardelt-Gattinger E, Lechner H, et al. The validation of the moorehead-ardelt quality of life questionnaire II. Obes Surg. 2003; 3:684–692. [DOI] [PubMed] [Google Scholar]
- 24.Oria HE, Moorehead MK. Bariatric analysis and reporting outcome system (BAROS). Obes Surg. 1998; 8:487–499. [DOI] [PubMed] [Google Scholar]
- 25.Oria HE, Moorehead MK., Oria HE, et al. Updated Bariatric Analysis and Reporting Outcome System (BAROS). Surg Obes Relat Dis. 2009; 5:60–66. [DOI] [PubMed] [Google Scholar]
- 26.Zar JH.Biostatistical Analysis. 5th ed. Prentice-Hall/Pearson; 2010. [Google Scholar]
- 27.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006; 244:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019; 29:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007; 357:753–761. [DOI] [PubMed] [Google Scholar]
- 30.Sethi M, Chau E, Youn A, et al. Long-term outcomes after biliopancreatic diversion with and without duodenal switch: 2-, 5-, and 10-year data. Surg Obes Relat Dis. 2016; 12:1697–1705. [DOI] [PubMed] [Google Scholar]
- 31.Kapeluto JE, Tchernof A, Masckauchan D, et al. Ten-year remission rates in insulin-treated type 2 diabetes after biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2020; 16:1701–1712. [DOI] [PubMed] [Google Scholar]
- 32.Aminian A, Vidal J, Salminen P, et al. Late relapse of diabetes after bariatric surgery: not Rare, but not a Failure. Diabetes Care. 2020; 43: 534–540. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino M, Kayser BD, Yoshino J, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med. 2020; 383:721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batterhamand Rl, Cummings DE. Mechanisms of Diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016; 39:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schauer Ph R, Bhatt DL, Kinwan JP, et al. Batriatric surgery versus intensive medical therapy for Diabetes-5-year outcomes. N Engl J Med. 2017; 376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallenius V, Alaraj A, Björnfot N, et al. Sleeve gastrectomy and Roux-en-Y gastric bypass in the treatment of type 2 diabetes. Two-year results from a Swedish multicenter randomized controlled trial. Surg Obes Relat Dis. 2020; 16:1035–1044. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz-Tovar J, Carbajo MA, Jimenez JM, et al. Long-term follow-up after sleeve gastrectomy versus Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc. 2019; 33:401–410. [DOI] [PubMed] [Google Scholar]
- 38.Kolotkin RL, Kim J, Davidson LE, et al. 12-year trajectory of health-related quality of life in gastric bypass patients versus comparison groups. Surg Obes Relat Dis. 2018; 14:1359–1365. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien PE, Brennan L, Laurie C, et al. Intensive medical weight loss or laparoscopic adjustable gastric banding in the treatment of mild to moderate obesity: long-term follow-up of a prospective randomised trial. Obes Surg. 2013; 23:1345–1353. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson J, Taft C, Rydén A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond). 2007; 31:1248–1261. [DOI] [PubMed] [Google Scholar]
- 41.Rogers AM. Current state of bariatric surgery: procedures, data and patient management. Tech Vasc Interv Radiol. 2020; 23:100654. [DOI] [PubMed] [Google Scholar]


