Abstract
The purpose of this study was to determine whether individual milk samples can replace serum samples for the detection of bovine herpesvirus 1 (BHV1) glycoprotein E (gE)-specific antibodies. Serum and milk samples were collected at the same time from cattle in BHV1-free herds, cattle in unvaccinated herds, and cattle in herds that were vaccinated twice with a BHV1 marker vaccine. The samples were tested in two gE enzyme-linked immunosorbent assay (ELISA) systems. In comparison to serum, the results showed that the gE-blocking ELISA was highly sensitive for testing milk samples (0.96). In contrast, the gE ELISA was less sensitive (0.79). The specificities of the gE-blocking ELISA and the gE ELISA for testing milk samples were very high (1.00 and 0.99, respectively). The presented results indicate that individual milk samples, which can be collected relatively easily and inexpensively, can be used instead of individual serum samples in the gE-blocking ELISA for the screening of cattle for BHV1 gE antibodies.
In 1998, a program to eradicate bovine herpesvirus 1 (BHV1) will start in The Netherlands. Marker vaccines (1, 5–7) will be used in combination with a BHV1 glycoprotein E (gE) enzyme-linked immunosorbent assay (ELISA) to differentiate infected from vaccinated cattle. The companion diagnostic test detects antibodies against wild-type BHV1, whereas antibodies against the marker vaccine from which gE is deleted are not detected. Van Oirschot et al. (21) have described a gE ELISA for the detection of antibodies directed against the gE of BHV1 in serum. However, large-scale epidemiological screening programs involve the collection and testing of millions of serum samples, and these procedures are highly expensive and laborious. Therefore, several studies have investigated the use of milk samples, which can be collected more easily and inexpensively, for the detection of antibodies against several viruses such as bovine respiratory syncytial virus (3), bovine leukemia virus (2, 8, 12), and bovine viral diarrhea virus (13). These results have shown that milk samples can be used as alternatives to serum samples in large-scale screening programs. Also, several ELISAs for the detection of BHV1 in milk have been described (10, 18, 20), but these ELISAs are unable to differentiate infected from vaccinated animals.
The aim of this study was to examine whether individual milk samples can be used instead of serum samples for the detection of BHV1 gE antibodies. We used two different gE ELISA systems and the standard virus neutralization test (VNT) to examine both serum and milk samples from cattle in BHV1-free herds, unvaccinated herds, and vaccinated herds. The addition of a sodium azide mixture as a preservative as well as the influence of storage on the BHV1 gE antibody detection results were examined for milk.
MATERIALS AND METHODS
Test samples.
A serum sample and a milk sample were collected at the same time from cows in four Dutch herds certified to be free of BHV1 (n = 155), cows in four Dutch herds with a history of BHV1 infection (n = 203), and cows in four Dutch herds that had outbreaks of BHV1 prior to being vaccinated twice with the attenuated BHV1 gE-negative marker vaccine (n = 111). The serum and milk samples from the vaccinated herds were collected 6 months after the second vaccination. To all individual milk samples a preservative mixture with final concentrations of 0.02% sodium azide and 0.01% bronopol (preservatives), 0.001% Triton X-100 (detergent), and 4 μg of patent blue (color component) per ml was added. Within 1 day after collection, milk samples were defatted by placing the milk samples in a refrigerator for 12 to 18 h, followed by collecting the fraction below the lipid layer. The elimination of fatty compounds is necessary, because lipids can affect the test results. Serum samples were prepared from blood samples by centrifugation at 1,000 × g for 10 min. The defatted milk samples and serum samples were stored at −20°C.
Prior to the collection and analysis of these milk samples, the influences of the addition of the sodium azide mixture and storage at −20°C were determined. To determine the influence of the preservative on the results of tests with milk samples, 212 milk samples were collected from BHV1-positive herds. The milk samples were divided into two equal parts, and the preservative was added to one part. Milk samples were defatted as described above and were analyzed by the gE-blocking ELISA and the modified gE ELISA. Defatted milk samples were also used to determine the influence of storage at −20°C. Therefore, milk samples were collected from BHV1-positive herds (n = 190) and divided after the addition of the preservative. The divided milk samples were defatted and frozen separately for 1 day or for 32 days at −20°C. After thawing, the milk samples were analyzed in the gE-blocking ELISA. We assumed that the influence of storage on test results for BHV1 gE would be the same for both gE ELISA systems, and therefore, we tested the defatted milk samples in the gE-blocking ELISA only.
According to the recommendations of the Office International des Epizooties (14), standard samples were incorporated into each ELISA plate. For the analysis of serum samples the standards consisted of a strongly positive, a weakly positive, and a negative serum sample. The serum sample strongly positive for BHV1 had a VNT titer of 160. The weakly positive serum was prepared by diluting the “P serum” (15). This BHV1-positive serum, P serum, containing BHV1-specific immunoglobulin G (IgG)-class antibodies, was diluted in BHV1-negative serum (final dilution, 1:512). The value for the prepared weakly positive serum sample (diluted P serum) was similar to the value for the European Union standard reference serum (EU2 serum). This EU2 serum has been prepared to standardize the serological diagnosis of BHV1 (16). The negative serum sample consisted of a pool of four BHV1-negative serum samples (VNT titer, <2).
gE-blocking ELISA.
The gE-blocking ELISA was a commercially available product (Idexx, Westbrook, Maine) primarily developed for the detection of BHV1 gE antibodies in serum. The principle of this gE-blocking ELISA is based on a blocking method in which the reaction of an epitope on the gE of the BHV1 Lam strain with its corresponding monoclonal antibody (MAb; MAb 66) can be blocked by specific antibodies in the test sample. The analysis of test samples was performed according to the instructions of the manufacturer. Briefly, serum samples in a 1:2 dilution in sample diluent (100 μl) or undiluted defatted milk samples (100 μl) were incubated at 2 to 8°C or 18 to 25°C, respectively, for 15 to 18 h. After incubation, the plates were washed six times with washing solution containing 0.05% (vol/vol) Tween 80 in deionized water. A volume of 100 μl of horseradish peroxidase (HRPO)-labelled anti-BHV1 gE MAb (MAb 66) was added to the wells, and the plates were incubated at 18 to 25°C for 30 min. The plates were washed six times with the washing solution, and 100 μl of substrate-chromogen (H2O2-tetramethylbenzidine) solution was added. After an incubation period of 15 min at 18 to 25°C, 100 μl of hydrofluoric acid (0.125%) stop solution was added. The optical density (OD) value was measured at 650 nm with a Bio-Tek Microplate reader (model EL312; Bio-Tek Instruments Inc., Winooski, Vt.). The blocking percentage for each sample was calculated against the OD value for the negative control. The blocking percentages were calculated by the following formula: [(OD650 of the negative control − OD650 of the test sample)/OD650 of the negative control] × 100.
According to the instructions of the manufacturer, serum samples with blocking percentages of ≥40% were classified as positive (antibodies present), those with blocking percentages of between ≥30 and <40% were classified as doubtful, and those with blocking percentages of <30% were classified as negative. For milk samples, blocking percentages of ≥20% were classified as positive and those of <20% were classified as negative.
gE ELISA.
The specificities of the MAbs and the principle and the procedure of the gE ELISA for the detection of antibodies directed against BHV1 in serum have been described by Van Oirschot et al. (21). Briefly, in the gE ELISA two MAbs (MAb 67 and MAb 75) directed against different antigenic epitopes of the gE of BHV1 were used. MAb 67 reacts with the same antigenic domain on gE as MAb 66. To perform the test, ELISA microplates (catalog no. 655092; Greiner) were coated with 100-μl volumes of a 1:4,000 dilution of MAb 75 in phosphate-buffered saline (pH 7.3) for 18 h at 37°C. The plates were stored at −20°C until use.
Serum samples at a dilution of 1:2 (100 μl) in ELISA buffer (containing 0.01 M Na2HPO4, 0.5 M NaCl, 0.005 M KCl, 0.001 M sodium EDTA, 0.05% [vol/vol] Tween 80 [pH 7.3], and 5% fetal bovine serum) were preincubated in round-bottom, uncoated microplates with 50 μl of the Lam strain of BHV1 as antigen at 37°C for 1 h.
The optimal antigen concentration was determined by checkerboard titration, and this was defined as the concentration that gave an OD value of between 1.500 and 1.800 for BHV1-negative serum samples. After the preincubation period the MAb 75-coated plates were washed six times with washing solution containing 0.05% (vol/vol) Tween 80 in deionized water. A volume of 50 μl of HRPO-labelled MAb 67 was added to the wells of MAb 75-coated ELISA plates, followed by the addition of 90 μl of the preincubated serum-antigen mixture. The solution was incubated at 37°C for 1 h. After this incubation period the plates were washed again by the standard washing procedure described above and 100 μl of substrate-chromogen [H2O2–2,2′-azino-di-(3-ethylbenzthiazoline sulfonate-6)] solution was added to the wells. After an incubation period of 2 h at 18 to 25°C the OD values were measured at 405 nm by using a Bio-Tek Microplate reader but without stopping the reaction.
Defatted milk samples contained a 0.02% sodium azide mixture as a preservative. Sodium azide inactivates conjugated HRPO enzyme (19), and for that reason color development was inhibited in the gE ELISA, which resulted in false-positive reactions (data not shown). Therefore, for the analysis of milk samples, the gE ELISA was modified. In the modified gE ELISA undiluted defatted milk samples (100 μl) were mixed with 50 μl of the Lam strain of BHV1 and preincubated as described above for serum. After the preincubation, 90 μl of the milk-antigen mixture and 50 μl of ELISA buffer were added to the MAb 75-coated ELISA plates, followed by a second incubation period of 1 h at 37°C. After the performance of the standard washing procedure, a volume of 50 μl of HRPO-labelled MAb (MAb 67) and 90 μl of ELISA buffer were added to each well of the ELISA microplate. The microplates were incubated at 37°C for an extra 1 h. The procedure was continued as described above for serum samples. The blocking percentage of each milk or serum sample was calculated against the OD value of the antigen control. The blocking percentages were calculated by the following formula: [(OD405 of the antigen control − OD405 of the test sample)/OD405 of the antigen control] × 100.
Serum samples with blocking percentages of ≥50% were classified as positive (antibodies present), and those with blocking percentages of <50% were classified as negative. The cutoff level for individual milk samples was determined for the modified gE ELISA (see Results).
VNT.
A 24-h VNT was performed as described by Kramps et al. (9). In the VNT, serum samples were analyzed in duplicate in serial twofold dilutions in cell culture medium (starting with a 1:2 dilution). The titer of the test serum was taken as the reciprocal of the highest dilution giving complete inhibition of the cytopathic effect. If both 1:2 serum dilutions did not inhibit the cytopathic effect, the serum was considered negative. In case one of the duplicate samples had a titer of 2 and the other had a titer of 4, the VNT titer was reported as 3. In this study the VNT was used as a standard for the selection of the BHV1-seropositive animals within the unvaccinated herds and to check the response after vaccination, and the VNT results for serum were also used for the evaluation of the value of the BHV1 gE results for milk and serum. The VNT was not suitable for testing milk samples for BHV1 antibodies because of toxic reactions in the 1:2 and 1:4 dilutions.
Detection limit.
For the determination of the detection limits of both gE ELISA systems and the VNT, serum and milk samples were collected from the same animals at the same time (n = 10). Only unvaccinated animals that were BHV1 seropositive in the VNT were used, and these animals were randomly chosen. Serum and milk dilutions were analyzed in serial twofold dilutions by using sample diluent and ELISA buffer (gE-blocking ELISA and gE ELISA, respectively) or cell culture medium (VNT). The titer of the test sample was taken as the reciprocal of the highest dilution giving a positive reaction.
Batch control.
Prior to the analyses of the test samples, the quality of the BHV1 gE ELISA batches was checked. Each new batch was checked for specificity, sensitivity, and detection limit by using a reference panel of 36 defined positive and negative serum samples, including serum obtained from hypervaccinated cattle, sequential serum samples, and a serum sample comparable to the EU2 serum sample. This quality check was performed to ensure the standardization of the test results and to ensure that the new batches were of high quality. Only qualified BHV1 gE ELISA batches were accepted for use in this study.
RESULTS
Influence of preservative and storage at −20°C.
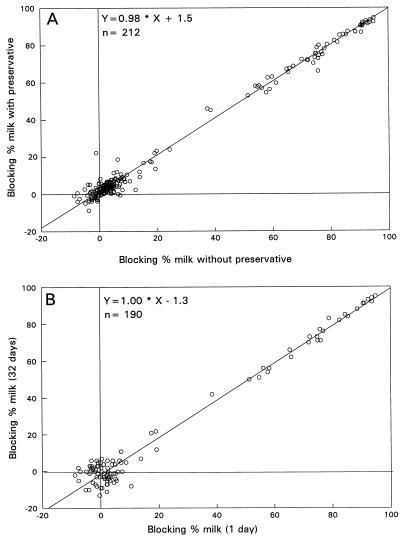
In the gE-blocking ELISA (Fig. 1A) and the modified gE ELISA, the results for the milk samples, with or without preservative, were the same. In the gE-blocking ELISA and the gE ELISA, the correlation coefficients were 0.99 and 0.96, respectively (gE ELISA, y = 0.94 · x + 7.4).
FIG. 1.
Blocking percentages in the gE-blocking ELISA of defatted milk samples with or without sodium azide mix (A) and defatted milk samples after storage for 1 day or 32 days at −20°C (B).
In the gE-blocking ELISA, the correlation coefficient of the test results for milk samples stored for 1 or 32 days at −20°C was 0.98 (Fig. 1B).
Herds certified to be free of BHV1. (i) Serum.
All serum samples from herds certified to be free of BHV1 were negative in the gE-blocking ELISA and the VNT. In the gE ELISA one serum sample reacted positively in two different test runs, with blocking percentages of 83 and 81%, respectively.
(ii) Milk.
All milk samples from herds certified to be free of BHV1 were negative in the gE-blocking ELISA. To assess the cutoff level for milk samples in the modified gE ELISA, the mean blocking percentage for all negative milk samples (26.8%) and the standard deviation (10.2%) were determined. The calculated cutoff value for milk samples was 47.1% (mean value plus 2 times the standard deviation). On the basis of these results, milk samples with a blocking percentage of ≥50% were considered to be positive for the presence of antibodies against BHV1 gE. In the modified gE ELISA one milk sample had a blocking percentage higher than 50%. This milk sample was not from the animal that was seropositive by the gE ELISA.
In comparison to serum samples, the relative specificities of the gE-blocking ELISA and the gE ELISA for the testing of milk samples were 100 and 99%, respectively.
Unvaccinated herds. (i) Serum.
The results of both gE-ELISAs for unvaccinated herds were compared with the VNT results (Table 1). All 45 serum samples that reacted positively in the gE-blocking ELISA were positive in the VNT. One sample with a doubtful reaction in the gE-blocking ELISA had a VNT titer of 6. This serum sample reacted positively in the gE ELISA. Seven serum samples with negative results in the gE-blocking ELISA had VNT titers of 3 and 6. Three of these serum samples were positive by the gE ELISA.
TABLE 1.
Results of the gE-blocking ELISA and the gE ELISA compared to those of VNT for serum from unvaccinated herdsa
| Test method | No. of serum samples with the following result:
|
||
|---|---|---|---|
| Positive | Doubtful | Negative | |
| VNTb | 53 | 0 | 150 |
| gE-blocking ELISAc | 45 | 1 | 157 |
| gE ELISAd | 51 | 0 | 152 |
Sera from 203 cattle were tested.
For VNT, a titer of ≥1:2 indicates a positive sample and a titer of <2 indicates a negative sample.
For the gE-blocking ELISA, blocking percentages of ≥40% are classified as positive, those of ≥30% and <40% are classified as doubtful, and those of <30% are classified as negative.
For the gE ELISA, blocking percentages of ≥50% are classified as positive and those of <50% are classified as negative.
These data indicated that for serum samples the relative sensitivity of the gE-blocking ELISA was 84.9%, while the relative specificity was 100% compared to the results of VNT. For serum samples, the relative sensitivity of the gE ELISA was 92.4%, and the relative specificity was 98.7% compared to the results of VNT.
(ii) Milk versus serum.
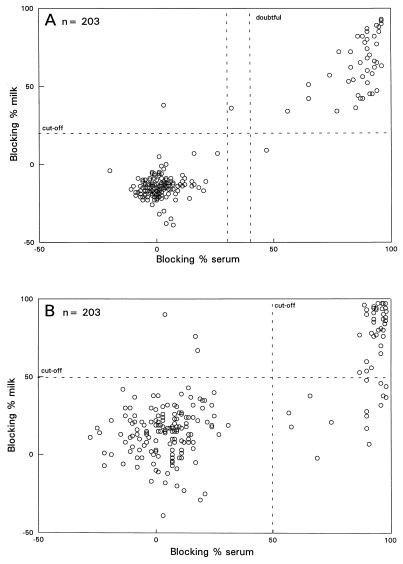
For both gE-ELISA systems the results for the milk samples from the unvaccinated herds were compared with the results for the corresponding serum samples. In the gE-blocking ELISA (Fig. 2A) serum samples from 45 animals were positive, and 1 animal had a doubtful reaction, while milk samples from 46 animals reacted positively. Milk samples and the corresponding serum samples from 44 animals were both positive. By the gE ELISA (Fig. 2B) 51 animals were BHV1 gE seropositive, while 39 milk samples were positive by the modified gE ELISA. For 36 animals the results for both milk and serum were positive. In comparison to serum, the relative sensitivities of the gE-blocking ELISA and the modified gE ELISA for testing milk samples were 98 and 68%, respectively.
FIG. 2.
Blocking percentages of the milk samples versus blocking percentages of the corresponding serum samples analyzed in the gE-blocking ELISA (A) and the gE ELISA (B) (unvaccinated herds).
Vaccinated herds: milk versus serum.
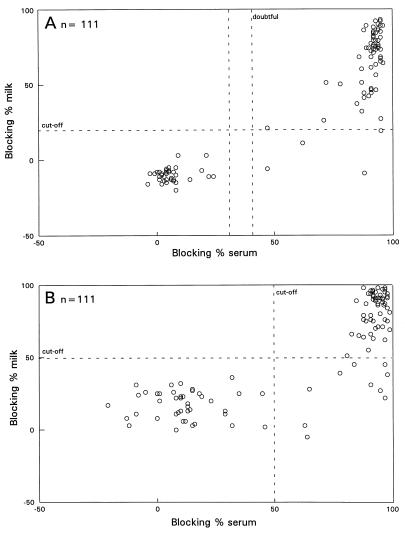
All 111 serum samples from vaccinated herds reacted positively in the BHV1 VNT, indicating that all animals responded to vaccination with the BHV1 (marker) vaccine or have been infected with BHV1. By the gE-blocking ELISA (Fig. 3A), 68 animals were seropositive, while 64 individual milk samples reacted positively. For all 64 animals the corresponding serum samples reacted positively as well. By the gE ELISA, 69 serum samples reacted positively against BHV1 gE antibodies, while 59 milk samples were positive by the modified gE ELISA (Fig. 3B). For all 59 individual milk samples that reacted positively in the gE ELISA, the gE-blocking ELISA test results were positive as well. In comparison to serum, the relative sensitivities of the gE-blocking ELISA and the modified gE ELISA for the testing of milk samples were 94 and 86%, respectively.
FIG. 3.
Blocking percentages of milk samples and blocking percentages of the corresponding serum samples analyzed in the gE-blocking ELISA (A) and the gE ELISA (B) (vaccinated herds).
Total relative sensitivity of both gE ELISA systems for testing of milk versus serum.
To evaluate the sensitivity of testing of milk with both gE ELISA systems, the total relative sensitivity was calculated and was compared to the results for serum. The total relative sensitivity was calculated from the data that were obtained for milk and the corresponding serum samples collected from both unvaccinated herds and vaccinated herds. By the gE-blocking ELISA, 113 (45 + 68) animals were seropositive, and milk from 108 (44 + 64) animals were positive. By the gE ELISA, 120 (51 + 69) animals were seropositive, and milk from 95 (36 + 59) animals was positive. These data indicate that for the testing of milk samples, the total relative sensitivity of the gE-blocking ELISA was 96%, while for the modified gE ELISA the total relative sensitivity was 79% compared to the results for serum.
Detection limit.
The detection limit, defined as the highest dilution that scored a positive reaction, of both gE ELISA systems was determined for serum and milk samples, and the detection limits were compared with the detection limit of VNT for serum. For serum samples the geometric mean titers (reciprocal of the mean logarithmic titers) of the VNT, the gE-blocking ELISA, and the gE ELISA were 269, 69, and 87, respectively. The geometric mean titers in milk samples were 9.8 for the gE-blocking ELISA and 4.9 for the gE ELISA. This means that the mean BHV1 gE antibody titer in milk was 7 times (gE-blocking ELISA) or 18 times (gE ELISA) lower than that in serum (Table 2).
TABLE 2.
BHV1 antibody titers detected in serum by the standard VNT, the gE-blocking ELISA, and the gE ELISA in comparison to the titers in milka
| Herd and cow no. | Titer
|
||||
|---|---|---|---|---|---|
| VNT with serum | gE-blocking ELISA
|
gE ELISA
|
|||
| Serum | Milk | Serum | Milk | ||
| Herd A | |||||
| Cow 1 | 256 | 128 | 32 | NDb | 8 |
| Cow 2 | 192 | 128 | 32 | 128 | 8 |
| Herd B | |||||
| Cow 1 | 2,048 | 256 | 32 | ≥256 | 8 |
| Cow 2 | 256 | 128 | 16 | 128 | 2 |
| Cow 3 | 512 | 64 | 8 | 128 | 4 |
| Herd C | |||||
| Cow 1 | 12 | 8 | 2 | 8 | <1 |
| Cow 2 | 96 | 64 | 16 | 128 | 2 |
| Cow 3 | 64 | 16 | 8 | 16 | <1 |
| Herd D | |||||
| Cow 1 | 128 | 64 | 8 | 128 | <1 |
| Cow 2 | ≥2,048 | 128 | 1 | ≥256 | 8 |
| Geometric mean | 269 | 69 | 9.8 | 87 | 4.9 |
Serum and milk from 10 animals were tested.
ND, not done (not enough serum).
DISCUSSION
Our study demonstrated that individual milk samples are suitable for use in the detection of antibodies directed against BHV1 gE. In the gE-blocking ELISA, milk samples can be used instead of serum samples for the detection of BHV1-positive animals in infected herds whether they have been vaccinated with the BHV1 marker vaccine or not.
The main problem with the use of milk in the antibody detection tests is the lower concentration of immunoglobulins in comparison with that in serum. According to Mach and Pahud (11), the total amount of immunoglobulin G1 in milk could be 30 times less than the amount in serum (in milligrams per milliliter). Also, the ELISA antibody titers against bovine leukemia virus (8) and bovine respiratory syncytial virus (3) in milk are lower than those in serum. Although the titers of immunoglobulins directed against the BHV1 gE epitopes in milk are lower than those in serum (Table 2), the results indicate that, in comparison to serum, the gE-blocking ELISA is highly sensitive for the testing of milk samples (sensitivity, 0.96), irrespective of whether the samples are collected from unvaccinated or vaccinated cows. In contrast, the modified gE ELISA is less sensitive for the testing of milk samples (sensitivity, 0.76). The results of the determination of the detection limit for milk samples (Table 2) also showed a higher geometric mean titer for the gE-blocking ELISA than for the gE ELISA. These data underline the fact that the gE-blocking ELISA is more sensitive than the gE ELISA for the testing of individual milk samples.
The specificities of both gE ELISA systems for the testing of milk samples is very high. This indicates that the number of false-positive reactions during the eradication program will be very low in both gE ELISA systems.
In case milk samples will be used in large-scale screening programs, the addition of a preservative to the milk and storage at −20°C are necessary, because milk samples cannot always be analyzed on the day of collection. In the gE-blocking ELISA and in the modified gE ELISA, the addition of the sodium azide mixture has no influence on the BHV1 gE results. The results of the gE-blocking ELISA also indicate that defatted milk samples can be stored at −20°C for at least 32 days without having any influence on the BHV1 gE antibodies.
Although our data indicate that milk can be used instead of serum, it should be noted that for the detection of BHV1 antibodies VNT is more sensitive than both gE ELISA systems. This study indicates that serum samples with low VNT titers (≤8) could be negative in both gE ELISA systems. Perrin et al. (17) also showed that serum samples with low VNT titers can give negative results in the gE-blocking ELISA. The results of the detection limit for serum (Table 2) underline these published data and indicate that in both gE ELISA systems the titers in serum are lower than the VNT titers in serum. It must be assumed that some of the negative BHV1 gE reactions are not due to an intrinsic lack of sensitivity of both gE ELISA systems but are mainly due to a lower antibody response to the antigenic epitope of BHV1 gE than to neutralizing epitopes. However, VNT can obviously not be used to detect infected cattle in vaccinated herds. For that purpose only gE ELISA systems, which detect gE-specific antibodies against wild-type BHV1, are suitable. During BHV1 eradication programs, wherein cattle must be monitored for the presence of BHV1, the lower sensitivity of the gE ELISAs compared with that of VNT can be compensated for by a more frequent testing of the cattle (4). For that purpose milk is the specimen of choice because it is much cheaper and easier to collect milk instead of blood.
In summary, this study shows that in spite of the lower BHV1 gE antibody levels in milk samples than in serum samples, individual milk samples can replace serum samples for the detection of BHV1-positive animals in infected herds, irrespective of whether the herd is vaccinated with a BHV1 gE-negative marker vaccine or not.
ACKNOWLEDGMENT
We thank J. A. Kramps for critical comments.
REFERENCES
- 1.Bosch J C. Bovine herpesvirus 1 marker vaccines: tools for eradication? Ph.D. thesis. Utrecht, The Netherlands: University of Utrecht; 1997. [Google Scholar]
- 2.Brenner J, Moss S, Moalem U. A comparative study of the ELISA and AGID technique for the detection of bovine leucosis virus antibodies in bovine serum and milk. Isr J Vet Med. 1994;49:165–167. [Google Scholar]
- 3.Elvander M, Edwards S, Naslund K, Linde N. Evaluation and application of an indirect ELISA for the detection of antibodies to bovine respiratory syncytial virus in milk, bulk milk and serum. J Vet Diagn Invest. 1995;7:1677–1682. doi: 10.1177/104063879500700202. [DOI] [PubMed] [Google Scholar]
- 4.Graat L, de Jong M C M, Frankena K. Proceedings of the IBR workshop, 26 and 27 June, Maastricht, The Netherlands. 1997. The IBR eradication campaign: surveillance of certified herds; pp. 1–33. [Google Scholar]
- 5.Kaashoek M. Marker vaccines against bovine herpesvirus 1 infections. Ph.D. thesis. Utrecht, The Netherlands: University of Utrecht; 1995. [Google Scholar]
- 6.Kaashoek M J, Moerman A, Madic J, Rijsewijk F A M, Quak J, Gielkens A L J, Van Oirschot J T. A conventionally attenuated glycoprotein E-negative strain of bovine herpesvirus type 1 is an efficacious and safe vaccine. Vaccine. 1994;12:439–444. doi: 10.1016/0264-410x(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 7.Kaashoek M J, Weerdmeester K, Maris-Veldhuis M A, Rijsewijk F A M, Van Oirschot J T. An inactivated vaccine based on a glycoprotein E-negative strain of bovine herpesvirus 1 induces protective immunity and allows serological differentiation. Vaccine. 1995;13:342–346. doi: 10.1016/0264-410x(95)98254-8. [DOI] [PubMed] [Google Scholar]
- 8.Klinteval K, Naslund K, Svedlund G, Najdu L, Linde N, Klingeborn B. Evaluation of an indirect ELISA for the detection of antibodies to bovine leukemia virus in milk and serum. J Virol Methods. 1991;33:319–333. doi: 10.1016/0166-0934(91)90032-u. [DOI] [PubMed] [Google Scholar]
- 9.Kramps J A, Magdalena J, Quak J, Weerdmeester K, Kaashoek M J, Maris-Veldhuis M A, Rijsewijk F A M, Keil G, Van Oirschot J T. A simple, specific, and highly sensitive blocking enzyme-linked immunosorbent assay for the detection of antibodies to bovine herpesvirus 1. J Clin Microbiol. 1994;32:2175–2181. doi: 10.1128/jcm.32.9.2175-2181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause H P, Achilles H, Lehmann M, Stammler M. Comparison of three ELISA systems for the detection of BHV1 antibodies in serum and milk samples. Tieraerzt Umsch. 1989;44:487–488. [Google Scholar]
- 11.Mach J P, Pahud J J. Bovine secretory immune system. J Dairy Sci. 1971;54:1327. [PubMed] [Google Scholar]
- 12.Nguyen V K, Maes R F. Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to bovine leukemia virus in serum and milk. J Clin Microbiol. 1993;31:979–981. doi: 10.1128/jcm.31.4.979-981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niskanen R, Alenius S, Larsson B, Juntti N. Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to bovine virus diarrhoea virus in milk. J Vet Med (B) 1989;36:113–118. doi: 10.1111/j.1439-0450.1989.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 14.Office International des Epizooties. Meeting of the OIE standards commission, 64th General Session, report no. 64 SG/12/CS2B. Paris, France: Office International des Epizooties; 1996. [Google Scholar]
- 15.Perrin B, Bitsch V, Cordioli P, Edwards S, Eliot M, Guérin B, Lenihan P, Perrin M, Rønsholt L, Van Oirschot J T, Vanopdenbosch E, Wellemans G, Wizigmann G, Thibier M. A European comparative study of serological methods for the diagnosis of infectious bovine rhinotracheitis. Rev Sci Tech Off Int Epizoot. 1993;12:969–984. doi: 10.20506/rst.12.3.724. [DOI] [PubMed] [Google Scholar]
- 16.Perrin B, Calvo T, Cordioli P, Coudert M, Edwards S, Eliot M, Guérin B, Kramps J A, Lenihan P, Perrin M, Lenihan P, Paschaleri E, Perrin M, Schon J, Van Oirschot J T, Vanopdenbosch E, Wellemans G, Wizigmann G, Thibier M. Selection of European Union standard reference sera for use in the serological diagnosis of infectious bovine rhinotracheitis. Rev Sci Tech Off Int Epizoot. 1994;13:947–960. doi: 10.20506/rst.13.3.810. [DOI] [PubMed] [Google Scholar]
- 17.Perrin B, Perrin M, Moussa A, Coudert M. Evaluation of a commercial gE blocking ELISA test for detection of antibodies to infectious bovine rhinotracheitis virus. Vet Rec. 1996;138:520. doi: 10.1136/vr.138.21.520. [DOI] [PubMed] [Google Scholar]
- 18.Rosskopf M, Straub E, Ackermann M. Comparison of two ELISA systems for detection of antibodies against IBR/IPV and against bovine leukosis virus. Schweiz Arch Tierheilkd. 1994;136:58–67. [PubMed] [Google Scholar]
- 19.Saine P K, Webert D W, Judkins J C. Role of sodium azide in reducing nonspecific color development in enzyme immunoassays. J Vet Diagn Invest. 1995;7:509–514. doi: 10.1177/104063879500700415. [DOI] [PubMed] [Google Scholar]
- 20.Stuker G, Giger T. Detection of IBR/IPV antibodies in milk. Schweiz Arch Tierheilkd. 1980;122:707–710. [PubMed] [Google Scholar]
- 21.Van Oirschot J T, Kaashoek M J, Maris-Veldhuis M A, Weerdmeester K, Rijsewijk F A M. An enzyme linked immunosorbent assay to detect antibodies against glycoprotein gE of bovine herpesvirus 1 allows differentiation between infected and vaccinated cattle. J Virol Methods. 1997;67:23–34. doi: 10.1016/s0166-0934(97)00073-6. [DOI] [PubMed] [Google Scholar]