Supplemental Digital Content is available in the text.
Abstract
Objectives:
To investigate how metabolic function of the contralateral liver lobe is affected by unilateral radioembolization (RE), and to compare the changes in volume and metabolic function.
Background:
Unilateral RE induces contralateral liver hypertrophy, but it is unknown if metabolic liver function improves in line with volume increases.
Methods:
This prospective open-label, nonrandomized, therapy-optimizing study included all consecutive patients undergoing right-sided or sequential 90Y-RE for liver malignancies without underlying liver disease or biliary obstruction at a single center in Germany. Magnetic resonance imaging volumetry and hepatobiliary scintigraphy were performed immediately before RE and approximately 6 weeks after RE.
Results:
Twenty-three patients were evaluated (11 metastatic colorectal cancer, 4 cholangiocellular carcinoma, 3 metastatic breast cancer, 1 each of metastatic neuroendocrine tumor, hepatocellular carcinoma, renal cell carcinoma, oesophageal cancer, pancreatic ductal adenocarcinoma). In the untreated contralateral left liver lobe, mean (SD) metabolic function significantly increased from 1.34 (0.76) %/min/m2 at baseline to 1.56 (0.75) %/min/m2 6 weeks after RE (P = 0.024). The mean (SD) functional volume (liver volume minus tumor volume) of the left liver lobe significantly increased from baseline (407.3 [170.3] mL) to follow-up (499.1 [209.8] mL; P < 0.01), with an equivalent magnitude to the metabolic function increase. There were no reports of grade ≥3 adverse events.
Conclusion:
This study indicates that unilobar RE produces a significant increase in the metabolic function, and equivalent volume increase, of the contralateral lobe. RE may be a useful option to induce hypertrophy of the future liver remnant before surgical resection of primary or secondary liver malignancies.
INTRODUCTION
Surgical resection can achieve long-term survival or cure in a substantial proportion of patients with primary or secondary liver malignancies. However, many patients have insufficient future liver remnant (FLR) to allow surgery even when the tumor would otherwise be resectable.1, 2 Such cases may be converted to resectability if the FLR can be improved, and preoperative portal vein embolization (PVE) is currently the standard approach to induce hypertrophy of the FLR.3 However, PVE may also stimulate tumor growth.4, 5
Radioembolization (RE), which delivers high doses of radiation to liver tumors through infusion of 90yttrium (90Y)-labeled glass or resin microspheres into the hepatic arterial circulation of the tumor-bearing liver, may be an interesting alternative to PVE in some settings. Unilobar RE has been shown to lead to substantial contralateral liver hypertrophy, and unlike PVE, RE may simultaneously provide tumor control.6, 7 The FLR volume hypertrophy following RE may be sufficient to enable surgery in many patients but appears to be slower and less pronounced than hypertrophy induced by PVE.8 However, the paucity of controlled trial data means that there are many unknowns surrounding the impact of RE on FLR, which means recommendations for patient selection are not possible.
It is not known if metabolic liver function improves in line with the FLR hypertrophy after RE. Following PVE, 99mTc-labeled mebrofenin hepatobiliary scintigraphy (HBS) has shown that the increase in FLR metabolic function is greater than the FLR volume increase.9–12 Conversely, FLR metabolic function is over-estimated by volumetry after Associating Liver Partitioning with Portal Vein Ligation for Staged Hepatectomy (ALPPS).13 A retrospective pilot study of 13 patients (10 with hepatocellular carcinoma [HCC] and 3 with metastatic colorectal carcinoma [mCRC]) demonstrated the feasibility of using HBS to monitor regional liver metabolic function after unilateral RE, and suggested some correlation between FLR metabolic function and FLR volume but with large individual variation.14 However, unilateral RE did not affect regional liver function in the contralateral lobe in a study of 22 patients with HCC in mostly cirrhotic livers.15
The RadioEmbolization, Volumetry, and liver funcTion measurements (REVoluTion) study aimed to further investigate how metabolic function of the contralateral liver lobe is affected by unilobar RE in patients without underlying liver cirrhosis and how volume changes of the contralateral liver lobe are related to metabolic function.
METHODS
The REVoluTion study was a prospective open-label, nonrandomized, single-center, therapy-optimizing study. All patients underwent treatment and study-related investigations at the University Hospital of Otto von Guericke University, Magdeburg, Germany. The study was registered retrospectively with Deutsches Register Klinischer Studien (ID: DRKS00007162), and this registration included a statistical analysis plan.
The study conformed to the International Conference on Harmonization of Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki. All patients gave written informed consent. The study was approved by the institutional ethics committee at Otto von Guericke University, Magdeburg (Ref. 08/14). Approval for the use of radioisotopes and ionizing radiation (e.g., low-dose-CT for attenuation correction of SPECT data) within the study was obtained from the Federal German radiation protection authority (Bundesamt für Strahlenschutz; Ref. Z 5-22463/2-2014-006).
Patients
All consecutive patients considered for RE between January 2015 and March 2017 were screened and those undergoing right-sided or sequential 90Y-RE for liver malignancies without underlying liver disease or biliary obstruction were eligible to participate if they were aged 18–85 years and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Recruitment continued until a sufficient number of patients had been included (according to sample size calculation).
Exclusion criteria included: baseline functional volume of the nonembolized lobe >30% of total functional liver volume (TFLV); presence of liver cirrhosis; pre-existing portal vein thrombosis; presence of primary liver malignancies with underlying liver cirrhosis or biliary dilatation; history of locally ablative therapy or surgery on the left liver lobe or if this was planned within 6 weeks of right-lobar 90Y-RE; >10% tumor involvement of the left liver lobe; or contraindications for hepatobiliary magnetic resonance imaging (MRI), 99mTc-mebrofenin HBS, or indocyanine green (ICG) elimination test.
Interventions
All patients received RE using 90Y-resin microspheres (SIR-Spheres, Sirtex Medical, Lane Cove, Australia). Treatment, including preprocedural diagnostic work-up, was performed according to a standard algorithm.16 The activity of 90Y-resin microspheres was calculated by the body surface area (BSA) method. Activity was calculated for whole-liver treatment and then adjusted for right unilobar treatment according to volume of the right lobe.17 90Y-resin microspheres were delivered selectively into the right hepatic artery.
All patients received proton pump inhibitor (pantoprazole, 20 mg/d), low-dose prednisolone (5 mg/d), and ursodeoxycholic acid (500–750 mg/d) (some patients also received enoxaparin [40 mg/d] and pentoxifylline [400 mg tid]) for 8 weeks to attenuate the effect of 90Y-resin microspheres possibly migrating into the gastric vascular bed and the embolization effect to the liver parenchyma.18
Assessments
MRI volumetry, HBS, and ICG elimination test (Pulsion LiMON, PULSION Medical Systems SE, Feldkirchen, Germany) were performed immediately before RE (baseline) and approximately 6 weeks after RE (follow-up).
MRI volumetry (whole liver, left lobe, right lobe, tumor for each lobe) was performed using a 3D-T1-weighted gradient echo sequence (slice thickness 3 mm) obtained 20 minutes after intravenous administration of 0.025 mmol/kg gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA). TFLV was calculated from the total liver volume (TLV) minus the tumor volume or non-Gd-EOB-DTPA-uptaking liver parenchyma. Likewise, right liver lobe functional volume (RFLV) was calculated from right liver lobe volume (RLV) minus right hepatic tumor volume or non-Gd-EOB-DTPA-uptaking liver parenchyma and left liver lobe functional volume (LFLV) was calculated from left liver lobe volume (LLV) minus left hepatic tumor volume or non-Gd-EOB-DTPA-uptaking liver parenchyma. MRI volumetry was completed by an experienced radiologist in collaboration with a hepatobiliary surgeon with consideration of the treated volume as indicated by Bremsstrahlung imaging.
HBS was performed to assess metabolic function in the total liver, right liver lobe, and left liver lobe, using 99mTc-labeled-(2,4,6-trimethyl-3-bromo) iminodiacetic acid (99mTc-mebrofenin, Bridatec; GE Healthcare). The 99mTc-mebrofenin uptake rate was obtained by dynamic planar images within 360 seconds after intravenous administration of 150–370 MBq of 99mTc-mebrofenin, followed by fast 240 seconds single-photon emission computed tomography/computed tomography (SPECT/CT) imaging (combined, these measurements took approximately 12 minutes). SPECT/CT imaging determined the counts within the liver and FLR volume. The percentage of the counts in the FLR in relation to the entire liver was then multiplied by the 99mTc-mebrofenin uptake rate to obtain the actual metabolic function of the FLR. Calculations were performed to normalize the uptake rate to BSA and respective liver volumes (TLV, RLV, LLV, TFLV, RFLV, and LFLV).
To confirm the HBS findings on global metabolic liver function, ICG (0.25 mg/kg) was injected intravenously, and ICG plasma disappearance rate (ICG-PDR) and ICG retention at 15 minutes (ICG-R15) were measured noninvasively using a spectrophotometry clip attached to the patient’s index finger and adjusted for variations in cardiac output between baseline and follow-up measurements.19, 20 Cardiac output was calculated as stroke volume (measured by transthoracic cardiac ultrasound) × heart rate.
Outcomes
The primary study objective was to measure change from baseline in metabolic function of the contralateral liver lobe 6 weeks after right unilateral 90Y RE. If the primary objective yielded a significant result, the coprimary objective was tested, which was to compare the changes in volume and metabolic function of the contralateral liver lobe from baseline to the 6-week follow-up visit.
Secondary objectives included: changes from baseline in total liver and right lobe function and volume and in global metabolic liver function (ICG clearance) 6 weeks after right unilateral 90Y RE; reports of grade ≥3 adverse events; and changes in laboratory variables. Exploratory analyses were conducted on factors potentially influencing left liver lobe volume and function change.
Sample Size Calculation
The first hypothesis (increase in contralateral liver function) is powered at 95% to ensure that the hierarchical test procedure does not stop early. The second test (to show that the increase in contralateral liver function is larger than the increase in contralateral liver volume) is powered at 85%, resulting in an overall power to reject both null hypotheses of ≥80%. The basis of the sample size calculations was the publication by de Graaf et al.12 and a conservative assumption of a 30% increase in liver function. A minimal sample size of 21 patients for a paired t test was calculated. To compensate for up to 25% of patients being lost to follow-up, or otherwise not evaluable, a sample size of 30 patients was planned.
Statistical Methods
Statistical analysis was performed using SPSS (SPSS 23, IBM, Armonk, NY). Descriptive analysis of patient and therapy characteristics was performed. All metric and ordinal variables are reported as median (interquartile range). Categorical variables are presented as frequency and percentage.
The hierarchical (a priori ordered) procedure allowed for testing of both corresponding null hypotheses at an unadjusted level of 0.025 (one-sided). Tests were carried out as one-sided Wilcoxon matched-pairs signed-rank tests. Total liver and right lobe function and volume, global liver function, and laboratory analyses were carried out as two-sided Wilcoxon matched-pairs signed-rank tests. Univariate analysis for factors with influence on volume and function changes was done using analysis of variance. P values below 0.05 were considered to indicate statistical significance. Safety analyses were performed for all patients receiving RE. Severe adverse events were listed and expressed as percentage of all treated patients.
RESULTS
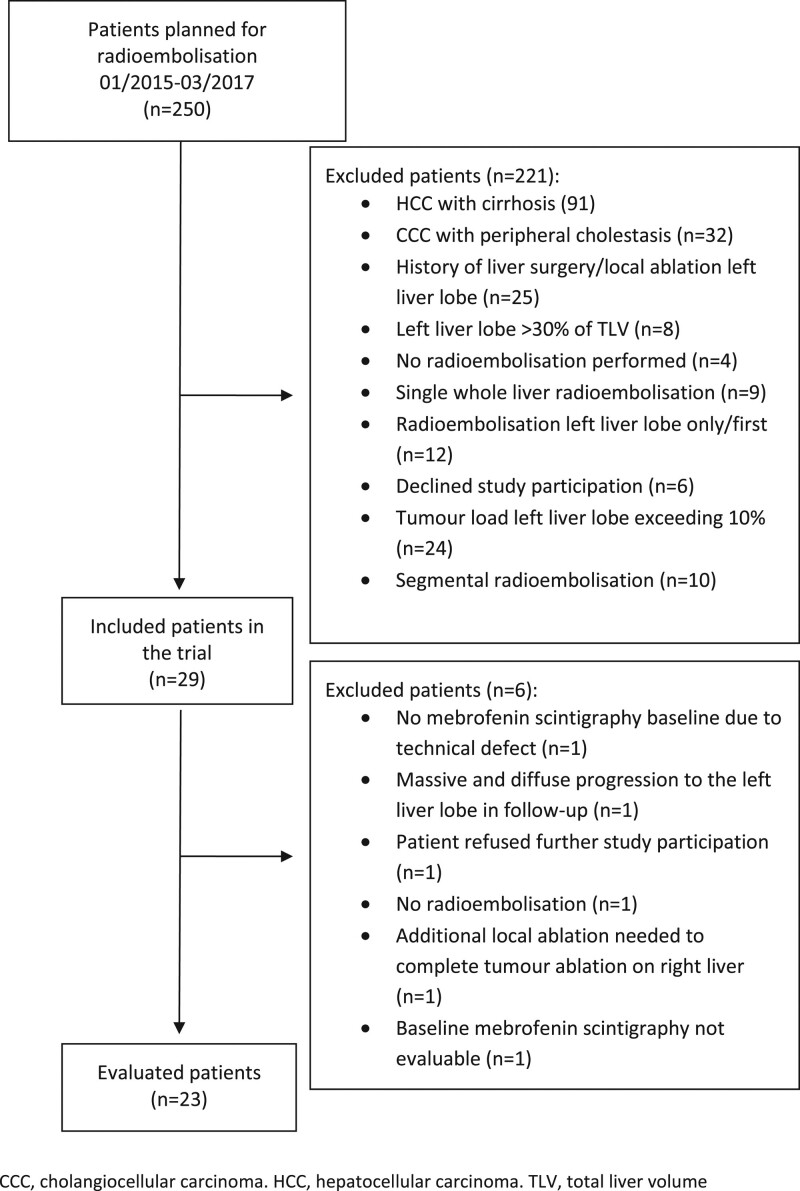
Between January 2015 and March 2017, 250 patients had RE planned at the University Hospital of Otto von Guericke University, Magdeburg, Germany. Twenty-nine patients were eligible for inclusion in this study, and 23 were evaluated (Figure 1).
FIGURE 1.
Study patient participation and exclusion.
Baseline and treatment characteristics of these 23 patients are summarized in Table 1. Median time from baseline MRI to RE was 20 days (IQR 20–40 days), and median time from RE to follow-up was 39 days (IQR 30–42 days).
TABLE 1.
Baseline Characteristics (n = 23)
| Characteristic | Baseline Value |
|---|---|
| Sex, n (%) | |
| Female | 6 (26.1) |
| Male | 17 (73.9) |
| Median (IQR) age, years | 66 (59–74) |
| Age, n (%) | |
| ≤65 years | 10 (43.5) |
| >65 years | 13 (56.5) |
| Mean (SD) BSA, m2 | 1.97 (0.21) |
| Diabetes, n (%) | 2 (8.7%) |
| Primary tumor, n (%): | |
| Colorectal cancer | 11 (47.8) |
| Breast | 3 (13.0) |
| CCC‡ | 4 (17.3) |
| NET | 1 (4.3) |
| HCC* | 1 (4.3) |
| Other† | 3 (13.0) |
| Previous chemotherapy, n (%): | 17 (73.9) |
| 1 line | 9 (52.9) |
| 2 lines | 6 (35.3) |
| 3 lines | 2 (11.8) |
| Median (IQR) duration, months | 8 (5.5–16) |
| Platinum containing chemotherapy | 13 (56.9) |
| Median (IQR) duration of platinum-containing chemotherapy, months | 6 (5–8.5) |
| Previous liver resection, n (%) | 4 (17.4) |
| Evidence of liver steatosis, n (%) | 5 (21.7) |
| RE characteristics: | |
| Treated segment, n (%) | |
| S4–8 | 9 (39.1) |
| S1, 4–8 | 7 (30.4) |
| S5–8 | 5 (21.7) |
| S1, 5–8 | 2 (8.7) |
| Mean (SD) administered 90Y activity, MBq | 1444.7 (315.6) |
| Mean (SD) 90Y dose to right liver lobe, Gy/mL | 45.7 (7.5) |
| ≤45 Gy/mL, n (%) | 11 (47.8) |
| >45 Gy/mL, n (%) | 12 (52.2) |
| Stasis during application, n (%) | 0 (0) |
*No liver cirrhosis.
†one each of renal cell carcinoma, oesophageal carcinoma, pancreatic ductal adenocarcinoma (tail).
‡No concomitant biliary dilatation.
BAS indicates body surface area; CCC, cholangiocellular carcinoma; GBq, giga-becquerel; Gy, gray; HCC, hepatocellular carcinoma; IQR, inter-quartile range; NET, neuroendocrine tumor; RE, radioembolization.
Liver Function—HBS Assessment
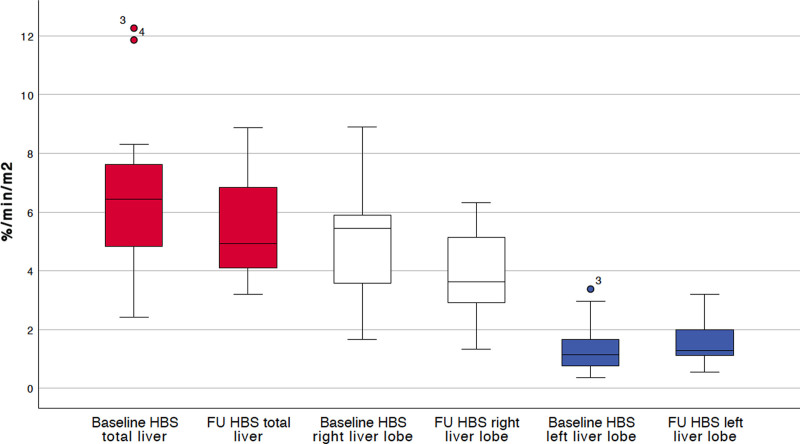
In the untreated contralateral left liver lobe, mean (SD) metabolic function (normalized to BSA) significantly increased from 1.34 (0.76) %/min/m2 at baseline to 1.56 (0.75) %/min/m2 6 weeks after RE (P = 0.024; Figure 2). Total liver metabolic function did not significantly change between baseline and follow-up (6.32 [2.48] % min/m2 and 5.5 [1.77] % min/m2, respectively, P = 0.068; Figure 2). There was, however, a significant metabolic function decrease in the treated right liver lobe, from a mean of 4.99 (1.94) %/min/m2 to 3.95 (1.36) %/min/m2 (P = 0.023; Figure 2).
FIGURE 2.
Liver metabolic function normalized for body-surface area at baseline (before radioembolization) and 6 weeks after radioembolization (n = 23) measured by hepatobiliary scintigraphy.
When the change in metabolic function of the left liver lobe was adjusted for the left liver lobe volume, the change from baseline was diminished (P > 0.05; Table 2), indicating that the function increase was no greater than would be expected from the volume increase, and indeed the increase in volume and metabolic function were of a similar magnitude (Table 2). The decrease in metabolic function from baseline of total liver and right liver lobe was significant when adjusted for total liver and right liver lobe volume, respectively (Table 2), indicating that the functional decrease was greater than the volume decrease.
TABLE 2.
Metabolic Function of Total Liver and Right and Left Liver Lobes, Adjusted for Volume or BSA, at Baseline (Before Radioembolization) and 6 weeks After Radioembolization (n = 23) Measured by Hepatobiliary Scintigraphy
| Baseline | Follow-up | ||
|---|---|---|---|
| Metabolic Function Endpoint | Mean (SD) | Mean (SD) | p |
| Applied 99mTc-Mebrofenin activity, MBq | 152.8 (15.6) | 157.6 (16.5) | 0.171 |
| Left liver lobe adjusted for BSA, %/min/m2 | 1.34 (0.76) | 1.56 (0.75) | 0.024* |
| Change from baseline, % | NA | 28.8 (51.9) | 0.429†0.855‡ |
| As a proportion of total liver adjusted for BSA, % | 21.4 (8.4) | 28.6 (10.2) | <0.01* |
| Total liver adjusted for BSA, %/min/m2 | 6.32 (2.48) | 5.50 (1.77) | 0.068 |
| Right liver lobe adjusted for BSA, %/min/m2 | 4.99 (1.94) | 3.95 (1.36) | 0.023 |
| Left liver lobe adjusted for LLV, %/min/l | 6.90 (3.93) | 6.01 (2.82) | 0.096* |
| Change from baseline, % | NA | 0.06 (43.3) | 0.005† |
| In relation to total liver adjusted for TLV, % | 91.4 (27.9) | 95.2 (20.1) | 0.392* |
| Total liver adjusted for TLV, %/min/l | 7.69 (3.74) | 6.32 (2.54) | 0.024 |
| Right liver lobe adjusted for RLV, %/min/l | 8.03 (3.99) | 6.44 (2.61) | 0.024 |
| Left liver lobe adjusted for LFLV, %/min/l | 7.06 (4.04) | 6.75 (3.99) | 0.347* |
| Change from baseline, % | NA | 7.0 (47.1) | 0.073‡ |
| In relation to total liver adjusted for TFLV, % | 84.1 (26.0) | 85.2 (27.1) | 0.215* |
| Total liver adjusted for TFLV, %/min/l | 8.36 (3.82) | 7.91 (3.22) | 0.429 |
| Right liver lobe adjusted for RFLV, %/min/l | 8.88 (4.11) | 10.62 (10.39) | 0.808 |
*By one-sided test (as defined in statistical analysis plan).
†Compared with volume change of LLV.
‡Compared with volume change LFLV.
BSA indicates body surface area; LFLV, left functional liver volume (LLV minus left hepatic tumor volume or non-Gd-EOB-DTPA uptaking liver parenchyma); LLV, left liver volume; NA; not applicable; RFLV, right functional liver volume (RLV minus right hepatic tumor volume or non-Gd-EOB-DTPA uptaking liver parenchyma); RLV, right liver volume; TFLV, total functional liver volume (TLV minus tumor volume or non-Gd-EOB-DTPA uptaking liver parenchyma); TLV, total liver volume.
The univariate analysis showed that with lower baseline left liver lobe function, a greater increase in left liver lobe metabolic function at follow-up was observed (see Table, Supplemental Digital Content 1, http://links.lww.com/AOSO/A60). There was also a trend indicating that a higher applied 90Y activity (in GBq) to the right liver lobe may correlate with a greater increase in left liver lobe function (normalized to BSA), but this trend was not evident with the delivered dose per mL of right liver lobe (see Table, Supplemental Digital Content 1 http://links.lww.com/AOSO/A60).
Global Liver Function—ICG Assessment
The global, but noninvasive, measure of liver metabolic function, the ICG test, also showed no change between baseline and the 6-week follow-up in global metabolic liver function (Table 3).
TABLE 3.
Global Liver Metabolic Function at Baseline (Before Radioembolization) and 6 weeks After Radioembolization Measured by the Indocyanine Green Test
| Baseline | Follow-up | ||
|---|---|---|---|
| Outcome | Mean (SD) | Mean (SD) | P |
| ICG plasma disappearance rate, %/min | 21.9 (8.1)* | 19.5 (5.9)† | 0.177 |
| ICG plasma disappearance rate:cardiac output ratio | 4.1 (2.0)* | 4.4 (2.3)‡ | 0.777 |
| Residual ICG after 15 min, % | 6.8 (7.6)* | 7.6 (6.4)† | 0.295 |
| Cardiac output l/min¶ | 5.6 (1.2)* | 4.9 (1.4)† | 0.376 |
ICG, indocyanine green. ICG R15: residual indocyanine green after 15 minutes.
*n = 21.
†n = 19.
‡n = 18.
¶Measured by echocardiography.
ICG indicates indocyanine green.
Liver Volume
The mean LLV and LFLV significantly increased from baseline to follow-up from 419.1 mL to 536.9 mL and from 407.3 mL to 499.1 mL, respectively (Table 4). Mean TLV and mean TFLV did not significantly change from baseline to follow-up (Table 4). The change in mean RLV was also not significant but RFLV did show a significant decrease (Table 4). The net result of these changes was that the LLV as a percentage of the TLV significantly increased from 23.86% to 29.87% (P < 0.01; Table 4), and the LFLV as a percentage of the TFLV significantly increased from 25.81% to 36.00% (P < 0.01; Table 4).
TABLE 4.
Volume of Total Liver and Right and Left Liver Lobes at Baseline (Before Radioembolization) and 6 Weeks After Radioembolization (n = 23) Measured by MRI
| Parameter | Baseline | Follow-up | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| TLV, mL | 1757.8 (448.4) | 1805.7 (465.9) | 0.25 |
| TFLV, mL | 1567.1 (335) | 1456.3 (382.2) | 0.056 |
| Right liver lobe volume, mL | 1338.7 (368.8) | 1268.8 (366) | 0.064 |
| Right liver lobe functional volume, mL | 1166.9 (276) | 956.8 (346.3) | <0.01 |
| LLV, mL | 419.1 (178.7) | 536.9 (206.6) | <0.01 |
| LFLV, mL | 407.3 (170.3) | 499.1 (209.8) | <0.01 |
| LLV percentage of TLV | 23.86 (7.83) | 29.87 (8.22) | <0.01 |
| LFLV percentage of TFLV | 25.81 (8.31) | 36 (16.6) | <0.01 |
TFLV, TLV minus tumor volume or non-Gd-EOB-DTPA uptaking liver parenchyma. RFLV, RLV minus right hepatic tumor volume or non-Gd-EOB-DTPA uptaking liver parenchyma. LFLV, LLV minus left hepatic tumor volume or non-Gd-EOB-DTPA uptaking liver parenchyma. Lack of Gd-EOB-DTPA uptake may be due to treatment-related liver parenchymal damage.
LFLV indicates left liver lobe functional volume; LLV, left liver lobe volume; RFLV, right liver lobe functional volume; RLV, right liver lobe volume; TFLV, total functional liver volume; TLV, total liver volume.
Univariate analyses showed that the increase from baseline in LLV and LFLV was reduced if the patient had undergone a higher number of chemotherapy lines or had a higher baseline LLV (see Table, Supplemental Digital Content 1, http://links.lww.com/AOSO/A60), whereas patients whose baseline left liver function (adjusted by LFLV and LLV) was high had a greater increase of LLV (than when baseline liver function was low, see Table, Supplemental Digital Content 1, http://links.lww.com/AOSO/A60). There was a trend for greater LFLV increase with a longer time from RE to follow-up MRI (see Table, Supplemental Digital Content 1, http://links.lww.com/AOSO/A60).
Safety and Laboratory Measures
There were no reports of grade ≥3 adverse events during the study. There was a small but significant decrease in mean albumin levels (P = 0.016); however, the mean albumin value remained within the normal range (see Table, Supplemental Digital Content 2, http://links.lww.com/AOSO/A61).
DISCUSSION
The results from the REVoluTion study primarily show that unilobar RE produces a significant increase in the metabolic function of the contralateral lobe in patients with liver malignancies without underlying liver disease or biliary obstruction. This is the first prospective and appropriately powered study to assess liver metabolic function changes after RE. RE also induced an increase in left lobar volume, and as demonstrated by the non-significant change in metabolic function when adjusted for volume, the FLR function, and volume increases were grossly equivalent. These results suggest that the liver lobe function increases induced by RE are not greater or smaller than would be expected by the volume increase (in contrast to the findings with PVE12 and ALPPS,13 respectively). However, care is required in comparing results from different studies—for example, the proportion of patients receiving previous chemotherapy was higher in our study than in the previous study of PVE.12 Another important difference between our study and others is that patients were given prophylaxis to reduce radiation-induced toxicity in liver parenchyma,21 and this may have had an impact on the volumetric or functional changes observed.
The exploratory univariate analyses suggested that if higher 90Y activity was applied to the right liver lobe, greater left liver lobe functional increases may be observed. As reported in a pilot study,14 there was no such relationship between applied activity and volume. However, this finding could be incidental as higher delivered doses to the treated liver volume were not associated with higher left liver lobe functional increases. Furthermore, patients included in the REVoluTion study were given RE with palliative intent and the applied 90Y activity was calculated with this in mind, rather than with the aim of achieving ablation. Therefore, one cannot conclude that delivering higher 90Y activity would achieve greater functional increases of the FLR or that this could be achieved without the risk of liver decomposition. There is a lack of evidence to define optimum mean absorbed dose cutoffs to induce contralateral hypertrophy, but this is an important aspect in the personalization of RE with the intent of improving FLR and thus requires more study.
A higher baseline left liver lobe function was associated with greater volume increases in the untreated lobe. This may suggest that volume increases require a reasonable level of liver function, but in such cases, the univariate analysis suggests that function increases are lower than may be expected from the relative volume increases. Therefore, despite the similar function and volume increases in this population as a whole, there would appear to be factors that result in large inter-individual differences in the relationship between volume and metabolic function changes. This confirms earlier findings,14 and means that further study is needed to identify factors that may influence the improvement in FLR metabolic function after RE. While these factors are unknown, there may be a role for preoperative HBS when RE is used for hypertrophy generation in preparation for liver surgery, as volumetry alone does not accurately predict FLR metabolic function.
The volume increase in the left liver lobe following RE was lower with a higher number of previous chemotherapy lines, and when the baseline left liver volume was higher. These effects of previous chemotherapy (in particular platinum-containing chemotherapy) and baseline left liver volume are known to occur with PVE,22–25 and the possible explanation of this has been discussed previously.8 Univariate analysis also suggested that a longer time between RE and the follow-up MRI was associated with greater volume increases in the left liver lobe. Many studies have shown that the volume increases induced in the contralateral lobe by unilobar RE continue to increase for up to 12 months.26–28 The 6-week follow-up in the current study may not have captured the maximal volume increases (and possibly function increases), and this could be viewed as a limitation to our findings. However, although there are no data on this issue, there is no clear rationale to believe that volumetric and functional changes in the liver develop at different rates beyond 6 weeks and that their relation to each other changes over time. Hence, it is unlikely that a longer follow-up interval would substantially have changed our results. For the present study, it was pragmatic to use a 6-week interval because patients who received RE as a palliative treatment were studied. For these patients, it is standard practice at our center to perform follow-up imaging to evaluate the treatment response after 6 weeks. In the case of bilobar liver lesions, these are treated sequentially with a 6-week interval between RE of both liver lobes, and imaging is performed before treatment of the second lobe. Moreover, as this study was the first prospective study of its kind and because 6 weeks is the usual waiting period after PVE in preparation for major hepatectomy, the 6-week follow-up was deemed appropriate. From a surgical perspective, it must also be taken into account that, if the delay between RE and surgery is extended, many patients will require chemotherapy while they are waiting for surgery, which may potentially compromise the parenchymal quality of the liver and increase surgical morbidity.
Other potential limitations of this study include the heterogeneous population that received a variety of previous therapies, which may have confounded some of the outcome measurements. Furthermore, some patients were scheduled to receive a second RE to the left liver (i.e., sequential treatment of the whole liver), but as the treatment of the left liver was conducted after follow-up assessment of volumes and function, this did not influence the analysis. Surgical outcomes were not part of this study aimed at determining liver function changes. Suggestions of further study include an assessment of unilobar 90Y RE to induce hypertrophy of the contralateral lobe in a surgical trial investigating secondary liver resection rates in patients with primarily unresectable, but potentially resectable, liver malignancies. Certainly, surgical safety will have to be a major focus of such a trial, as Y90 RE can potentially complicate liver resection by causing more perihepatic adhesions and inflammation than PVE, most likely through radiation effects which may induce sinusoidal congestion and subsequent liver fibrosis within the field of radiation. However, while this phenomenon is frequently observed, it has never been a major obstacle to safe resection in our own practice, nor has it been shown to compromise surgical safety in previous studies, including a retrospective study on 100 patients undergoing partial hepatectomy following RE, which did not show any unexpected surgical morbidity.29–31 These previous studies further warrant the evaluation of RE as a preconditioning treatment before liver surgery.
In conclusion, unilobar RE may be a useful alternative to PVE to induce hypertrophy of the FLR before surgical resection of primary or secondary liver malignancies. Unlike PVE, RE is unlikely to induce tumor growth in the treated liver lobe, and the results presented from this prospective study show that both volume and function of the contralateral lobe increase by the same magnitude after unilobar RE.
Supplementary Material
Footnotes
B.G. did study design, literature research, data collection, data analysis, data interpretation, article writing, and editing. H.A. did data collection, data interpretation, and article review. D.K. data collection, data analysis, data interpretation, and article editing. O.G. did data collection, data analysis, data interpretation, and article editing. J.J., J.O., R.D., M.P., M.F., O.O., P.S., C.B., R.S., J.R., and M.P. did data collection, data interpretation, article review. M.S. did study design, literature research, data collection, data analysis, data interpretation, article writing, and editing.
B.G. has received research grants from Sirtex Medical as well as lecture honoraria and travel grants from Sirtex Medical, Amgen, Merck, Roche, Novartis and B. Braun Travacare, and receives honoraria for participation on advisory boards from Terumo, Sirtex Medical and Amgen. H.A. receives lecture fees from Norgine, Pfizer, Novartis, GE, Sirtex Medical as well as research grants from Pfizer. R.D. receives lecture honoraria from Sirtex Medical. M.P. receives research grants from Bayer and Sirtex Medical as well as lecture honoraria from Sirtex Medical. M.S. receives research grants from Bayer and Sirtex Medical as well as lecture honoraria from Siemens, Cook, Boston Scientific, Sirtex Medical, Falk Foundation, Bayer and receives honoraria for participation on advisory boards from Sirtex Medical, Bayer and Siemens. The remaining authors declare that they have nothing to disclose.
Editorial support was provided by Martin Gilmour of Empowering Strategic Performance (ESP) Ltd, Crowthorne, UK.
All anonymized data are available upon request to the corresponding author.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denys A, Prior J, Bize P, et al. Portal vein embolization: what do we know? Cardiovasc Intervent Radiol. 2012;35:999–1008. [DOI] [PubMed] [Google Scholar]
- 4.Elias D, De Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–788. [DOI] [PubMed] [Google Scholar]
- 5.Hoekstra LT, van Lienden KP, Doets A, et al. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812–817. [DOI] [PubMed] [Google Scholar]
- 6.Birgin E, Rasbach E, Seyfried S, et al. Contralateral liver hypertrophy and oncological outcome following radioembolization with (90)Y-microspheres: a systematic review. Cancers (Basel). 2020;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teo JY, Allen JC, Ng DCE, et al. Prospective study to determine early hypertrophy of the contra-lateral liver lobe after unilobar, Yttrium-90, selective internal radiation therapy in patients with hepatocellular carcinoma. Surgery. 2018;163:1008–1013. [DOI] [PubMed] [Google Scholar]
- 8.Garlipp B, de Baere T, Damm R, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59:1864–1873. [DOI] [PubMed] [Google Scholar]
- 9.Hirai I, Kimura W, Fuse A, et al. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495–506. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama Y, Yamamoto Y, Hino I, et al. 99mTc galactosyl human serum albumin liver dynamic SPET for pre-operative assessment of hepatectomy in relation to percutaneous transhepatic portal embolization. Nucl Med Commun. 2003;24:809–817. [DOI] [PubMed] [Google Scholar]
- 11.Yumoto Y, Yagi T, Sato S, et al. Preoperative estimation of remnant hepatic function using fusion images obtained by (99m)Tc-labelled galactosyl-human serum albumin liver scintigraphy and computed tomography. Br J Surg. 2010;97:934–944. [DOI] [PubMed] [Google Scholar]
- 12.de Graaf W, van Lienden KP, van den Esschert JW, et al. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825–834. [DOI] [PubMed] [Google Scholar]
- 13.Olthof PB, Tomassini F, Huespe PE, et al. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: liver volume overestimates liver function. Surgery. 2017;162:775–783. [DOI] [PubMed] [Google Scholar]
- 14.van der Velden S, Braat MNGJA, Labeur TA, et al. A pilot study on hepatobiliary scintigraphy to monitor regional liver function in 90Y radioembolization. J Nucl Med. 2019;60:1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labeur TA, Cieslak KP, Van Gulik TM, et al. The utility of 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT/CT for selective internal radiation therapy in hepatocellular carcinoma. Nucl Med Commun. 2020;41:740–749. [DOI] [PubMed] [Google Scholar]
- 16.Seidensticker R, Seidensticker M, Damm R, et al. Hepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35:1109–1118. [DOI] [PubMed] [Google Scholar]
- 17.Dezarn WA, Cessna JT, DeWerd LA, et al. ; American Association of Physicists in Medicine. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys. 2011;38:4824–4845. [DOI] [PubMed] [Google Scholar]
- 18.Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57:1078–1087. [DOI] [PubMed] [Google Scholar]
- 19.de Liguori Carino N, O’Reilly DA, Dajani K, et al. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol. 2009;35:957–962. [DOI] [PubMed] [Google Scholar]
- 20.Inal MT, Memiş D, Kargi M, et al. Prognostic value of indocyanine green elimination assessed with LiMON in septic patients. J Crit Care. 2009;24:329–334. [DOI] [PubMed] [Google Scholar]
- 21.Seidensticker M, Seidensticker R, Damm R, et al. Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity. PLoS One. 2014;9:e112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Baere T, Teriitehau C, Deschamps F, et al. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17:2081–2089. [DOI] [PubMed] [Google Scholar]
- 23.Denys A, Lacombe C, Schneider F, et al. Portal vein embolization with N-butyl cyanoacrylate before partial hepatectomy in patients with hepatocellular carcinoma and underlying cirrhosis or advanced fibrosis. J Vasc Interv Radiol. 2005;16:1667–1674. [DOI] [PubMed] [Google Scholar]
- 24.Beal IK, Anthony S, Papadopoulou A, et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol. 2006;79:473–478. [DOI] [PubMed] [Google Scholar]
- 25.Sturesson C, Keussen I, Tranberg KG. Prolonged chemotherapy impairs liver regeneration after portal vein occlusion - an audit of 26 patients. Eur J Surg Oncol. 2010;36:358–364. [DOI] [PubMed] [Google Scholar]
- 26.Ong F, Tibballs J. Hepatic volume changes post-selective internal radiation therapy with (90) Y microspheres. J Med Imaging Radiat Oncol. 2020;64:347–352. [DOI] [PubMed] [Google Scholar]
- 27.Orcutt ST, Abuodeh Y, Naghavi A, et al. Kinetic analysis of contralateral liver hypertrophy after radioembolization of primary and metastatic liver tumors. Surgery. 2018;163:1020–1027. [DOI] [PubMed] [Google Scholar]
- 28.Goebel J, Sulke M, Lazik-Palm A, et al. Factors associated with contralateral liver hypertrophy after unilateral radioembolization for hepatocellular carcinoma. PLoS One. 2017;12:e0181488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shehta A, Lee JM, Suh KS, et al. Bridging and downstaging role of trans-arterial radio-embolization for expected small remnant volume before liver resection for hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg. 2020;24:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardo F, Sangro B, Lee RC, et al. The post-SIR-spheres surgery study (P4S): retrospective analysis of safety following hepatic resection or transplantation in patients previously treated with selective internal radiation therapy with Yttrium-90 resin microspheres. Ann Surg Oncol. 2017;24:2465–2473. [DOI] [PubMed] [Google Scholar]