Abstract
Objective:
To describe work-related factors, pregnancy, and pregnancy outcomes in female surgeons is the objective of this study.
Background:
Some data suggest surgeon workload may deter pregnancy and adversely affect pregnancy outcomes in female surgeons.
Methods:
A cross-sectional, web-based survey was distributed via e-mail to members of the Society of Obstetrics and Gynaecologists of Canada and to surgical departments of 6 Canadian universities from October 2019 to January 2020.
Results:
A total of 223 surgeons with 451 pregnancies participated. Work hours were reduced in 33.3% of pregnancies, and 28.0% had a policy for pregnancy in their workplace. A total of 57% of surgeons intentionally delayed pregnancy due to heavy workload and 39% to career concerns, and 31% reported work adversely affected their pregnancy. Adverse maternal outcomes included miscarriage (14.9%), preterm labor (6.2%), hypertension (5.5%), pre-eclampsia (2.9%), and placenta praevia (1.3%). Adverse infant outcomes included preterm birth (6.9%), small for gestational age at birth (6.9%), and neonatal intensive care unit admission (4%). Congenital anomalies occurred in 4.2% of pregnancies. Surgeons who reported a policy for working while pregnant were more likely to have reduced their work hours than those without a policy (48.4% vs 28.5% respectively, P < 0.0001). In unadjusted models, those who reduced their work hours while pregnant were less likely to have a miscarriage than those who did not (odds ratio = 0.2, 95% confidence interval, 0.1–0.4).
Conclusions:
Female surgeons reported delays in pregnancy due to work, adverse effects of work on pregnancy, and some elevated rates of adverse outcomes. These data support policies for pregnancy in surgeons and surgical trainees.
INTRODUCTION
The number of female surgeons has increased globally.1 Some studies suggest associations between work-related factors and adverse pregnancy outcomes in physicians.2,3 In a North American study, higher rates of miscarriage (11.8% vs 4.2%), hypertension (10.5% vs 6.3%), placental abruption (1.3% vs 0%), and intrauterine growth restriction (9.2% vs 3.9%) were observed in female surgical residents compared to a reference population of similarly aged obstetrical patients.3 Of 164 gynecological surgeons in Germany, 1 quarter experienced a pregnancy-related complication including intrauterine growth restriction (2.9%), miscarriage (2.9%), preterm birth (7.1%), and premature rupture of membranes (0.6%).4 A large study of 1684 physicians in Japan found that those who worked more than 71 hours per week were more likely to experience threatened abortion (odds ratio [OR] = 3.0, 95% confidence interval [CI], 1.7–6.0) or preterm birth (OR = 2.5, 95% CI, 1.2–5.2) than those who worked 40 hours or less per week.2 In the United States, orthopedic surgeons had a higher risk of preterm delivery (risk ratio = 2.5, 95% CI, 1.3–4.6) compared to the general population, and an increased risk of preterm labor and delivery was observed in those who worked more than 60 hours per week during pregnancy (OR = 4.9, 95% CI, 1.4–36.6).5
A small number of studies have considered workplace policies for pregnancy in female surgeons.6 Policies such as the American Board of Surgery leave policy, which allows for only 4 weeks for maternity or family leave,7 was reported to be deterrent to pregnancy in 82% of general surgeons who had 1 or more pregnancies during residency.8 A Canadian study of general surgery residents found that 84% of 176 participants felt a maternity or parenting policy was necessary, but program directors reported no formal policies in place.6
Higher rates of adverse pregnancy-related outcomes and a lack of workplace policy have been suggested in some studies. Our study of relevant work factors and pregnancy-related outcomes in female surgeons in Canada aimed to add to this growing body of evidence.
METHODS
Survey Development and Distribution
We developed a Qualtrics survey consisting of 24 questions on work-related factors and pregnancy outcomes. Prior instruments6,8 were used to inform our questions, which were then revised following review by 4 female surgeons (2 attending surgeons, 2 fellows). These surgeons reviewed drafts of the survey and provided input regarding content and format. The survey had 3 main areas of focus: work-related factors, pregnancy, and pregnancy outcomes. Data on maternal outcomes including miscarriage, medical abortion, gestational hypertension, gestational diabetes, and preterm labor were collected. Data on infant outcomes including small for gestational age at birth, neonatal intensive care unit (NICU) admission, and congenital anomalies were also collected. Additional survey questions asked about call-time while pregnant, work hour reduction, workplace policies, and obstacles to pursuing pregnancy. A copy of the questionnaire is provided in the Appendix.
A link to the electronic survey was distributed to all members of the Society of Obstetricians and Gynaecologists of Canada (SOGC), a national organization whose mission is to lead the advancement of women’s health through excellence and collaborative professional practice. Female surgeons of surgical departments at 6 Canadian universities were also invited to participate. The largest 7 Canadian universities with surgical departments were selected. Six of the 7 universities had publicly available contact information for the surgeons, and invitations to participate were sent by e-mail. Females were identified by faculty name and photos when available. Surgeons who had not previously been pregnant could also participate in the study.
Anonymous data collection through software generated e-mails occurred from October 2019 to January 2020. The SOGC sent out 1 e-mail reminder after the original invitation to participate. Female surgeons contacted outside of the SOGC did not receive a reminder e-mail. This study was approved by Queen’s University Health Sciences Research Ethics Board.
Statistical Analysis
Data were exported from Qualtrics as a delimited text file and analyzed in SAS (version 9.4, Cary, NC). Descriptive statistics including frequencies, means, medians, and ranges were generated. Logistic regression was used to identify associations between work-related factors and (1) adverse maternal outcomes, (2) miscarriage, and (3) adverse infant outcomes. In the logistic models, an adverse maternal outcome was defined as any of threatened preterm labor, preterm labor, gestational hypertension, gestational diabetes, antepartum hemorrhage, placental abruption, pre-eclampsia, gestational diabetes, and placenta praevia. An adverse infant outcome was defined as any of NICU admission, preterm birth (<37 weeks of gestation), very preterm birth (<32 weeks of gestation), intrauterine growth restriction, and small for gestational age. OR with 95% CIs were reported. Congenital anomalies were described separately from other adverse infant outcomes.
Open-coded responses for questions were reviewed for common themes and grouped into categories. Survey data were reported using the EQUATOR guidelines.9
RESULTS
Participant Demographics
Two-hundred and twenty-three surgeons with 451 pregnancies completed the survey—192 SOGC members and 31 other surgeons. As we were unable to collect data on who received and read the e-mail, we estimated the lowest response rate to be 32%.
Table 1 summarizes the characteristics of this cohort. Most participants were obstetrician-gynecologist surgeons (85.7%) and reported Canada as their country of residence (94.6%). The mean age at the time of survey completion was 45.1 ± 10.1 years (range, 25–76 years). The mean number of pregnancies per surgeon was 3.3 ±1.9 (range, 0–9). Few pregnancies occurred between 15 and 24 years (1.8%), 16.0% between 25 and 29 years, 47.7% between 30 and 34 years, 29.7% between 35 and 39 years, 3.9% between 40 and 44 years, and 0.9% between 45 and 49 years. The pregnancies occurred before or during medical school (3.1%), during residency (28.6%), during fellowship (7.3%), and while an attending surgeon (57.4%).
TABLE 1.
Demographics of 223 Surgical Trainees and Surgeons With 451 Pregnancies
| Characteristic | N (%) |
|---|---|
| Surgical specialty | |
| Obstetrics and gynecology | 191 (85.7) |
| General surgery | 16 (7.2) |
| Other | 14 (6.2) |
| Missing | 2 (0.9) |
| Mean age at time of survey completion | 45.1 ± 10.1 y |
| Number of pregnancies per woman | |
| 0 | 46 (20.6) |
| 1 | 36 (16.1) |
| 2 | 48 (21.5) |
| 3 | 38 (17.0) |
| ≥4 | 55 (24.8) |
| Age at pregnancy (y) | |
| 15–24 | 8 (1.8) |
| 25–29 | 72 (16.0) |
| 30–34 | 215 (47.7) |
| 35–39 | 134 (29.7) |
| 40–44 | 18 (3.9) |
| 45–49 | 4 (0.9) |
| Level of training during pregnancy | |
| Before/during medical school | 14 (3.1) |
| Residency | 129 (28.6) |
| Fellowship | 33 (7.3) |
| Attending surgeon | 259 (57.4) |
| Other | 14 (3.1) |
| Missing | 2 (0.5) |
| Intentional delay in gravid women | |
| Delay | 220 (48.8) |
| No delay | 230 (51.0) |
| Missing | 1 (0.2) |
| Unintentional delay due to infertility in gravid women | |
| Delay | 59 (13.1) |
| No delay | 389 (86.3) |
| Missing | 3 (0.6) |
| Intentional delay in nulligravid women (n = 46) | |
| Delay | 31 (67.4) |
| No delay | 14 (30.4) |
| Missing | 1 (2.2) |
| Unintentional delay due to infertility in nulligravid women (n = 46) | |
| Delay | 5 (10.9) |
| No delay | 40 (86.9) |
| Missing | 1 (2.2) |
Other surgical specialties included cardiac surgery, orthopedic surgery, pediatric surgery, plastic surgery, thoracic surgery, urological surgery, and neurosurgery.
One hundred and seventy-seven participants (79.4%) had at least 1 pregnancy, for a total of 451 pregnancies. Forty-six participants (20.6%) had never been pregnant, 67.4% of whom had intentionally delayed pregnancy. Among gravid women, 48.8% intentionally delayed pregnancy. Infertility accounted for delay in 13.1% of the pregnancies.
Working While Pregnant
Work hours were reduced in 33.3% of pregnancies (Table 2). This reduction occurred at a mean of 28.6 ± 5.7 weeks of gestation (range, 8–36 weeks of gestation). An institutional policy for work hour reduction existed in the workplace for 28.0% of pregnancies. Surgeons who reported such a policy for working while pregnant were significantly more likely to have reduced their work hours than those without a policy (48.4% vs 28.5%, respectively, P < 0.0001). Call-time was primarily in-hospital during pregnancy (63.4%), with most surgeons having 4–8 nights on call per month (62.1% in first half of pregnancy, 43.2% in second half of pregnancy). Of those who reported a workplace policy, 89.7% reported working ≤8 nights of call per month in the first half of their pregnancy; no association was found between the presence of a workplace policy and the number of nights on call in the first half of the pregnancy. However, surgeons who reported no workplace policy were more likely to work >8 nights of call per month in the second half of their pregnancy than those with such a policy (15.5% vs 4.0%, respectively; P < 0.001). The average maternity leave length was 20.3 ± 14.4 weeks (range, 0 weeks to 3 years). This was perceived to be too short in 44.8% of the pregnancies, but adequate in 38.1% of the pregnancies. Seventy (31.3%) surgeons reported that they perceived their workload to adversely affected their pregnancy.
TABLE 2.
Work Histories During Pregnancy Among 177 Surgical Trainees and Surgeons With 451 Pregnancies
| Work History Characteristic | N (%) |
|---|---|
| Work hour reduction during pregnancy | |
| Yes | 150 (33.3%) |
| No | 296 (65.6%) |
| Missing | 5 (1.1%) |
| Workplace had a policy of work hour reduction during pregnancy | |
| Yes | 126 (28.0%) |
| No | 310 (68.7%) |
| Missing | 15 (3.3%) |
| Mean gestational age when work was reduced (n = 105) | 28.6 wk ± 5.7 (range, 8–36) |
| Location of call throughout pregnancy | |
| At-home | 154 (34.1%) |
| In-house | 286 (63.4%) |
| Missing | 11 (2.5%) |
| Number of nights on call per month for first half of pregnancy | |
| 0 | 21 (4.7%) |
| 1–4 | 81 (17.9%) |
| 4–8 | 280 (62.1%) |
| >8 | 65 (14.4%) |
| Missing | 4 (0.9%) |
| Number of nights on call per month for second half of pregnancy | |
| 0 | 58 (12.9%) |
| 1–4 | 123 (27.2%) |
| 4–8 | 195 (43.2%) |
| >8 | 53 (11.8%) |
| Missing | 22 (4.9%) |
| Mean maternity leave length | 20.3 wk ± 14.4 (range, 0 wk–3 y) |
| Satisfaction with maternity leave length | |
| Too short | 202 (44.8%) |
| Adequate | 172 (38.1%) |
| Too long | 8 (1.8%) |
| Missing | 69 (15.3%) |
| Workload negatively affected their pregnancy | |
| Yes | 141 (31.3%) |
| No | 286 (63.4%) |
| Missing | 24 (5.3%) |
Obstacles to Pursuing Pregnancy
Heavy workload (57.6%) and concern regarding career success (38.8%) were cited as the most common obstacles to pursuing pregnancy. Discouragement from supervisors (8.2%), partners (3.8%), and peers (8.0%) were less frequently reported. Other responses (12.9%) included academic reasons, personal factors, financial reasons, work/colleague concerns, fetal health concerns, or spousal concerns.
Adverse Maternal and Infant Outcomes
Adverse maternal outcomes as described in Figure 1 were reported in 289 of the pregnancies (64.1%). The most common adverse outcome was miscarriage (≤23.5 weeks of gestation), which occurred in 67 pregnancies (14.9%). No stillbirths were reported. While 282 pregnancies (62.5%) were full-term, 32 pregnancies (7.1%) experienced threatened preterm labor, and 28 pregnancies (6.2%) experienced preterm labor. Other adverse maternal outcomes included gestational hypertension (5.5%) and diabetes (2.7%), antepartum hemorrhage (4.7%), and placental abruption (3.5%). Pre-eclampsia was reported in 13 pregnancies (2.9%), and eclampsia in none. One molar pregnancy was reported. Medical abortion occurred in 37 pregnancies (8.2%).
FIGURE 1.
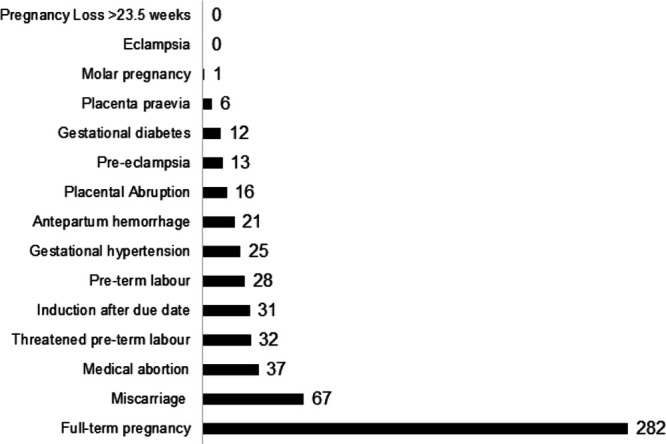
Maternal outcomes among 177 surgical trainees and surgeons with 451 pregnancies.
Adverse infant outcomes as described in Figure 2 were reported in 18 (26.2%) pregnancies. These included preterm birth (6.9%), small for gestational age at birth (6.9%), intrauterine growth restriction (4%), and NICU admission (4%). Congenital anomalies were observed in 19 infants (4.2%); 6 infants had more than 1 anomaly for a total of 26 anomalies.
FIGURE 2.

Infant outcomes among 177 surgical trainees and surgeons with 451 pregnancies. Nineteen infants had congenital anomalies, with 6 infants having more than 1 reported for a total of 26 congenital anomalies. These included anomalies of the skin (n = 1), anomalies of the eye (n = 1), anomalies of the ear, face, or neck (n = 1), musculoskeletal anomalies (n = 1), respiratory anomalies (n = 1), anomalies of the nervous system (n = 2), gastrointestinal anomalies (n = 3), cardiovascular anomalies (n = 3), and genitourinary anomalies (n = 4). Nine anomalies were reported as “other,” including chromosomal abnormalities, endocrine abnormalities, a developmental coordination disorder, a sacrococcygeal teratoma, a sacral dimple and an abdominal wall abnormality.
Associations Between Work-Related Factors and Adverse Outcomes
In unadjusted models (Table 3), work reduction was associated with a twofold higher risk of adverse maternal and infant outcomes. In contrast, those who reduced their work hours while pregnant were less likely to have a miscarriage than those who did not (OR = 0.2, 95% CI, 0.1–0.4). There also was a suggested protective effect on miscarriage from working ≤8 hours call per month versus more (OR = 0.6, 95% CI, 0.3–1.2).
TABLE 3.
Unadjusted Odds Ratio of Work-Related Factors and Maternal, Infant Adverse Outcomes, and Miscarriage in Surgical Trainees and Surgeons
| Maternal Adverse Outcome | Infant Adverse Outcome | Miscarriage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Work-Related Factor | Adverse Outcome (N) | No Adverse Outcome (N) | Unadjusted Odds Ratio (95% CI) | Adverse Outcome (N) | No Adverse Outcome (N) | Unadjusted Odds Ratio (95% CI) | Adverse Outcome (N) | No Adverse Outcome (N) | Unadjusted Odds Ratio (95% CI) |
| Workplace policy for work hour reduction while pregnant* | |||||||||
| Policy | 32 | 94 | 1.0 (0.6–1.6) | 17 | 109 | 0.8 (0.4–1.4) | 14 | 112 | 0.7 (0.4–1.3) |
| No policy | 80 | 230 | 1.0 | 53 | 257 | 1.0 | 49 | 261 | 1.0 |
| Reduced work hours† | |||||||||
| Reduced | 51 | 99 | 2.0 (1.3–3.1) | 33 | 117 | 2.0 (1.2–3.4) | 6 | 144 | 0.2 (0.1–0.4) |
| Not reduced | 60 | 236 | 1.0 | 36 | 260 | 1.0 | 60 | 236 | 1.0 |
| Stage of training while pregnant‡ | |||||||||
| Before + during medical school | 3 | 11 | 0.9 (0.2–3.4) | 2 | 12 | 0.9 (0.2–4.1) | 2 | 12 | 0.9 (0.2–4.2) |
| Fellowship + residency | 46 | 116 | 1.3 (0.8–2.1) | 24 | 138 | 0.9 (0.5–1.6) | 22 | 140 | 0.9 (0.5–1.5) |
| Attending | 60 | 199 | 1.0 | 41 | 218 | 1.0 | 40 | 219 | 1.0 |
| Intentional delay§ | |||||||||
| Delay | 59 | 161 | 1.2 (0.8–1.9) | 39 | 181 | 1.4 (0.8–2.3) | 32 | 188 | 0.9 (0.6–1.6) |
| No delay | 53 | 177 | 1.0 | 31 | 199 | 1.0 | 35 | 195 | 1.0 |
| Call-time‖ | |||||||||
| In-hospital | 75 | 211 | 1.2 (0.8–1.9) | 50 | 236 | 1.6 (0.9–2.9) | 38 | 248 | 0.7 (0.4–1.2) |
| At-home | 35 | 119 | 1.0 | 18 | 136 | 1.0 | 27 | 127 | 1.0 |
| No. nights on call in first half of pregnancy¶ | |||||||||
| <8 per month | 95 | 287 | 0.9 (0.5–1.7) | 58 | 324 | 0.9 (0.4–1.8) | 54 | 327 | 0.8 (0.4–1.7) |
| ≥8 per month | 17 | 48 | 1.0 | 11 | 54 | 1.0 | 11 | 55 | 1.0 |
| No. nights on call in second half of pregnancy# | |||||||||
| <8 per month | 95 | 281 | 0.9 (0.5–1.6) | 59 | 317 | 0.9 (0.4–2.0) | 45 | 331 | 0.6 (0.3–1.2) |
| ≥8 per month | 15 | 38 | 1.0 | 9 | 44 | 1.0 | 10 | 43 | 1.0 |
An adverse maternal outcome was defined as any of threatened preterm labor, preterm labor, gestational hypertension, gestational diabetes, antepartum hemorrhage, placental abruption, pre-eclampsia, gestational diabetes, placenta praevia. An adverse infant outcome was defined as any of NICU admission, preterm birth (<37 weeks of gestation), very preterm birth (<32 weeks of gestation), intrauterine growth restriction, and small for gestational age.
*Missing 15 observations.
†Missing 5 observations.
‡Missing 16 observations.
§Missing 1 observation.
‖Missing 11 observations.
¶Missing 4 observations.
#Missing 22 observations.
DISCUSSION
Our study of 451 pregnancies from 1981 to 2019 documented that many female surgeons encounter workplace barriers to pregnancy and perceived adverse effects of work on pregnancy. In addition, few (28%) of our study participants reported a workplace policy for pregnant surgeons and this has been reported in other studies.
Among general surgery residents, a lack of maternity and parenting policies, obstacles to breastfeeding and increased workload have been identified as pregnancy concerns.10 A lack of parental leave is also noted in academic surgeons, with only ~50% of the highest ranked academic medical centers in the United States offering paid leave.11 Similarly, in a qualitative study of family medicine residents, the changing demands of work and guilt regarding increasing colleagues’ workload were identified stressors throughout pregnancy. Solutions suggested included flexibility in scheduling and providing adequate breaks, privacy, and refrigerators for breastfeeding.12 In a survey distributed to surgery residents in the United States investigating the perceptions of parental leave, 30.4% of participants felt a lack of support from other residents, and 32.7% felt a lack of support from faculty when taking such leave. Those who took leave reported a lack of a universal leave policy and program flexibility, with perceived lack of support a large obstacle.13 This was also reported in our study, with discouragement from peers reported in 8% of pregnancies and concerns about colleagues frequently reported.
Concerns regarding career success were reported in 38.8% of our participants’ pregnancies. US studies have described the impact that pregnancy has on the decision to pursue a career in surgery and subsequent satisfaction in that career, with data illustrating higher rates of career dissatisfaction after maternity leave.14,15 These studies support the need for concrete workplace policies addressing childcare, leave, and scheduling.14–16 The American College of Surgeons suggest creating a schedule that is “flexible and equitable,” accommodating “call schedule, duty hours and operative schedules late in the third trimester” and breastfeeding support.17 In Germany, once a pregnancy is disclosed, surgical duties are restricted by regulations that prevent the use of puncturing tools, limit surgical performances to 4 hours, and do not allow night shifts or lead roles during emergent cases. However, 3 quarters of the German female gynecological surgeons surveyed described the need for more flexibility in this policy,4 suggesting that even existing policy may benefit from review.
In our unadjusted models, work reduction was associated with adverse maternal and infant outcomes. This is most likely an issue of temporality, with the work reduction occurring after an obstetrical risk was identified. A higher risk of miscarriage in female surgeons who did not reduce work hours while pregnant compared to those who did reduce their work hours was observed and a lower risk suggested among those who worked 8 hours of call or less per month. Our findings support associations between longer work hours (>70 hours per week) and increased threatened abortion and preterm birth in female physicians2 and a higher risk of adverse obstetrical outcomes in surgical residents who worked more than 6 nights on call per month3 observed in other studies.
It is important to note that our study questionnaire asked participants if their workplace had a policy for reduced workload, or reduced call-time, while pregnant and did not discuss the details such as duration of work hours, limitations of call-time or cross-coverage schemes. These are potential aspects of policy that could benefit pregnant surgeons. Additional data on the impact and perceptions of stigma for burdening colleagues with additional duties, and intentional delay in pregnancy as reported by 48.8% of the participants in our study, could be useful in policy development.
The average maternity leave length in our study was 20.3 weeks compared to a pregnancy leave of 1 year in the general Canadian population. This was perceived to be too short in 44.8% of pregnancies. Surgeons’ comments also suggested that lactation support and childcare support were underdeveloped in study participants’ workplaces. This was observed in a study of 347 general surgeons in the United States, of whom 78% had a maternity leave of 6 weeks or less and 72% perceiving this as too short.8 Sixty-four percent of women surgeons in this American study were concerned that their work schedule adversely affected their own health and the health of their infant,8 while surgeons in our study perceived workload to negatively affect 31.3% of pregnancies. The development of maternity leave and call-time policies has been suggested to greatly improve pregnancy outcomes for working surgeons.18
Rates of maternal and infant adverse outcomes in our cohort tended to be like those of the general population. The congenital anomaly prevalence was 4.2% among pregnancies of surgeons in our study, comparable to the expected prevalence of 3%–5% in the general Canadian population.19 The prevalence of unintended pregnancy loss in our study population (14.9%) was as observed in the general Canadian population.20 Additionally, our study population had an infertility rate of 13%, similar to the estimated rate of 11.5%–15.7% in Canada.21
Although our numbers were small, adverse outcomes that may be higher in surgeons included pre-eclampsia in 2.9% compared to 1.2% in the general population in 2011,22 placenta praevia in 1.3% of surgeon pregnancies compared to 0.3% of the general Canadian population,23 and medical abortion in 8.7% of surgeons compared to 1.17% in the general Canadian population.24 These differences may reflect the higher age of surgeons at pregnancy than the general pregnancy population, and possibly differences in denominators used for estimating risk (ie, pregnancies in our study vs live births other reports).
Some limitations of our study include a lack of data collection on familial support, childcare, gender roles, and other cultural factors that may have affected the pregnancy outcomes studied. Study participants were primarily SOGC members, and therefore obstetrician-gynecologist surgeons. While it is possible these data may not be generalizable to other female surgeons, similarities among operating times, workload, and training requirements across surgical specialties in Canada and the US support generalizability. Given our e-mail recruitment approach, it is difficult to accurately estimate the number of obstetricians and gynecologists that received and read the e-mail. The true response rate may be higher than our conservative estimate. Surgeons with concerns regarding workplace barriers to pregnancy and/or adverse outcomes may have been more likely to respond. The wide age ranges, career stages, gravid and outcomes, however, do suggest a diverse group of participants. Given that the source population consisted of female surgeons, many of whom had a specialty devoted to reproductive health, reported clinical outcomes are expected to be accurate in this population. To describe both the gravida and parity of the surgeons, we included all pregnancies. This considers each pregnancy an independent event; however, statistical independence may not be met.
CONCLUSIONS
In conclusion, our findings add to growing evidence of a lack of institutional policy for pregnancy and postpartum leaves as a persistent concern of female surgeons. We also support prior findings of an increased risk of miscarriage with greater workload. A study of workplace policies for pregnant surgeons could inform the strategic development of such policy based on the protective role suggested by our data.
APPENDIX
This survey collects anonymous data on whether work-related factors during pregnancy and delivery increase the risk of adverse outcomes in female surgeons. Thank you for your time and participation.
*Questions 6 to 24 will be repeated for each of the pregnancies reported by the participant in Question 5. They will read “pregnancy in the year x” depending on the years reported.
1. What is your country of residence?
Afghanistan _____
Albania _____
Algeria _____
etc….
Zimbabwe _____
2. What is your surgical speciality?
General Surgery ____
Obstetrics and Gynecology Surgery ____
Cardiac Surgery ____
Plastic Surgery ____
Neuro Surgery ____
Orthopedic Surgery ____
Pediatric Surgery ____
Thoracic Surgery ____
Vascular Surgery ____
Urological Surgery ____
Ophthalmological Surgery ____
3. How old are you currently? _______
4. How many pregnancies have you had that resulted in a delivery, unintended pregnancy loss, or intended pregnancy loss? _______
5. What year(s) did this delivery, unintended pregnancy loss or intended pregnancy loss occur?
1970 _____
1971 _____
1972 _____
etc…
2019 _____
6. How old were you during your pregnancy in year x? _____
7. At what stage in your medical training/practice were you during your pregnancy in year x?
Before medical school____
During medical school____
Residency____
Fellowship____
Attending surgeon____
Other, please specify: ____________
8. Did your pregnancy in year x end with intended loss/therapeutic abortion? Yes/No
9. Did you intentionally delay becoming pregnant before your pregnancy in year x? Yes/No
10. Which of the following barriers, if any, were applicable to your pregnancy in year x. Check all that apply:
Heavy workload ____
Discouraged by workplace supervisor ____
Discouraged by peers ____
Discouraged by partner ____
Concern regarding career success ____
Other (please specify) _____________
11. Was your pregnancy in year x unintentionally delayed due to infertility? Yes/No
12. Was your call-time during your pregnancy in year x at-home, or in-house? At home/In-house
13. How many nights on call, on average, did you work per month for the first four months of your pregnancy for delivery or unintended loss in year x?
0 ____
1–4 ____
4–8 ____
>8 ____
14. How many nights on call, on average, did you work per month for the remaining months (approximately 5) of your pregnancy for delivery or unintended loss in year x?
0 ____
1–4 ____
4–8 ____
>8 ____
15. Did you reduce your work hours during your pregnancy in year x? Yes/No
16. Did your workplace have a policy for reduced workload, or reduced call-time, during your pregnancy in year x? Yes/No
17. If you answered yes to Question 16, at what gestational age was the workload reduced during your pregnancy in year x? _____ weeks
18. How long was your maternity leave for your pregnancy in year x? ______ weeks
19. Was the length of your maternity leave for your pregnancy in year x:
Too short ____
Too long ____
Adequate ____
20. In your opinion, did your workload negatively impact your pregnancy and delivery in year x? Yes/No
21. Please check off which, if any, of the following complications applied to your pregnancy in year x.
Placental abruption ____
Placenta praevia ____
Gestational diabetes ____
Gestational hypertension ____
NICU admission ____
Preterm labor ____
Pre-eclampsia ____
Eclampsia ____
Threatened preterm labor ____
Antepartum hemorrhage ____
22. Please check off which, if any, of the following applied to your pregnancy in year x.
Full-term pregnancy ____
Induction after due date ____
Preterm birth (<37 weeks of gestation) ____
Very preterm birth (<32 weeks of gestation) ____
Unintended pregnancy loss (≥23.5 weeks of gestation) ____
Unintended pregnancy loss (<23.5 weeks of gestation) ____
Intrauterine growth restriction ____
Small for gestational age ____
23. Did your infant from your pregnancy in year x have any congenital abnormalities? Yes/No
24. If you answered yes to Question 23, please specify the type(s) of anomaly.
Nervous system ____
Cardiovascular system ____
Respiratory system ____
Gastrointestinal system ____
Genitourinary system ____
Musculoskeletal system ____
Eye ____
Ear, face, or neck ____
Skin ____
Other, please specify: _____________
Thank you for completing this survey!
Footnotes
Disclosure: The authors declare that they have nothing to disclose.
REFERENCES
- 1.Wirtzfeld DA. The history of women in surgery. Can J Surg. 2009; 52:317–320. [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi M, Rahman M, Ishiguro A, et al. Long working hours and pregnancy complications: women physicians survey in Japan. BMC Pregnancy Childbirth. 2014; 14:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behbehani S, Tulandi T. Obstetrical complications in pregnant medical and surgical residents. J Obstet Gynaecol Can. 2015; 37:25–31. [DOI] [PubMed] [Google Scholar]
- 4.Knieper C, Ramsauer B, Hancke K, et al. “Pregnant and operating”: evaluation of a Germany-wide survey among female gynaecologists and surgeons. Geburtshilfe Frauenheilkd. 2014; 74:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton AR, Tyson MD, Braga JA, et al. Childbearing and pregnancy characteristics of female orthopaedic surgeons. J Bone Joint Surg Am. 2012; 94:e77. [DOI] [PubMed] [Google Scholar]
- 6.Merchant SJ, Hameed SM, Melck AL. Pregnancy among residents enrolled in general surgery: a nationwide survey of attitudes and experiences. Am J Surg. 2013; 206:605–610. [DOI] [PubMed] [Google Scholar]
- 7.The American Board of Surgery. Training & Certification. 2019. Available at: http://www.absurgery.org/default.jsp?policygsleave. Accessed March 10, 2020.
- 8.Rangel EL, Smink DS, Castillo-Angeles M, et al. Pregnancy and motherhood during surgical training. JAMA Surg. 2018; 153:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley K, Clark B, Brown, et al. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003; 15:261–266. [DOI] [PubMed] [Google Scholar]
- 10.Merchant S, Hameed M, Melck A. Pregnancy among residents enrolled in general surgery (PREGS): a survey of residents in a single Canadian training program. Can J Surg. 2011; 54:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itum DS, Oltmann SC, Choti MA, et al. Access to paid parental leave for academic surgeons. J Surg Res. 2019; 233:144–148. [DOI] [PubMed] [Google Scholar]
- 12.Walsh A, Gold M, Jensen P, et al. Motherhood during residency training: challenges and strategies. Can Fam Physician. 2005; 51:990–991. [PMC free article] [PubMed] [Google Scholar]
- 13.Altieri MS, Salles A, Bevilacqua LA, et al. Perceptions of surgery residents about parental leave during training. JAMA Surg. 2019; 154:952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scully RE, Davids JS, Melnitchouk N. Impact of procedural specialty on maternity leave and career satisfaction among female physicians. Ann Surg. 2017; 266:210–217. [DOI] [PubMed] [Google Scholar]
- 15.Davids JS, Scully RE, Melnitchouk N. Impact of procedural training on pregnancy outcomes and career satisfaction in female postgraduate medical trainees in the United States. J Am Coll Surg. 2017; 225:411–418.e2. [DOI] [PubMed] [Google Scholar]
- 16.Mayer KL, Ho HS, Goodnight JE, Jr. Childbearing and child care in surgery. Arch Surg. 2001; 136:649–655. [DOI] [PubMed] [Google Scholar]
- 17.Cole S, Arnold M, Sanderson A, et al. Pregnancy during otolaryngology residency: experience and recommendations. Am Surg. 2009; 75:411–415. [PubMed] [Google Scholar]
- 18.American College of Surgeons. Statement on the Importance of Pregnancy, Parental Leave, and Workplace Accommondations for Surgical Residents and Fellows. 2020. Available at: http://www.facs.org/ABOUT-ACS/Statements/121-parental-leave. Accessed February 18, 2021.
- 19.Public Health Infobase: 2017. Congenital Anomalies in Canada: 2017. 2019. Available at: http://health-infobase.canada.ca/congenital-anomalies/data/tool. Accessed February 20, 2020.
- 20.National Health Service. Miscarriage. 2013. Available at: http://www.nhs.uk/Conditions/Miscarriage/Pages/Introduction.aspx. Accessed February 17, 2021.
- 21.Bushnik T, Cook JL, Yuzpe AA, et al. Estimating the prevalence of infertility in Canada. Hum Reprod. 2012; 27:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health Agency of Canada. Maternal hypertension in Canada. 2016. Available at: http://www.canada.ca/en/public-health/services/publications/healthy-living/maternal-hypertension-canada.html. Accessed February 20, 2020.
- 23.Rowe T. Placenta previa. J Obstet Gynaecol Can. 2014; 36:667–668. [DOI] [PubMed] [Google Scholar]
- 24.Abortion Rights Coalition of Canada. Statistics – Abortion in Canada. Available at: https://www.arcc-cdac.ca/backrounders/statistics-abortion-in-canada.pdf. Accessed March 1, 2020.


