Abstract
Invasive candidiasis, including bloodstream infection (candidemia), encompasses the most severe forms of Candida infection. Several species-specific and non-specific serological assays are commercially available to aid in diagnosis. This study compared the performance of five such biomarker assays. Serum samples from 14 patients with proven or probable invasive candidiasis, and from 10 control patients, were included in the analysis. A total of 50 serum samples were tested using C. albicans germ tube antibody (CAGTA) assay (Vircell), C. albicans IgM, C. albicans IgG and Candida mannan assays (Dynamiker Biotechnology). Among these samples, the β-1-3-D-glucan (BDG) assay (Fungitell), a laboratory standard for the diagnosis of invasive candidiasis, was positive in 20 (40%), intermediate in five (10%) and negative in 25 (50%). In cases of proven or probable candidemia, the sensitivity and specificity of the BDG assay was 86% and 80%, respectively; the Candida mannan assay, 14% and 86%; the CAGTA test, 57% and 60%; the C. albicans IgM assay, 71% and 60%; and C. albicans IgG assay 29% and 90%. In 4/8 (50%) cases with multiple serum samples, C. albicans IgM was positive sooner than BDG. Thus, when used as a rule-out test for invasive candidiasis, our data suggest that the C. albicans IgM assay may assist antifungal stewardship (over serum BDG).
Keywords: invasive candidiasis, laboratory diagnostics, assay, serology, immunoglobulin, antigen
1. Introduction
Invasive candidiasis, including candidemia, encompasses the most serious manifestations of Candida infection. It is the most common invasive fungal disease globally [1]. Candidemia is particularly common among critically ill patients receiving antimicrobials and is associated with a significant mortality risk [2]. The diagnosis of candidemia may be challenging, primarily due to a low yield from blood culture specimens and the inconsistent performance of molecular techniques across different patient populations [3]. Indeed, across multiple cohorts of fungaemic patients, an average sensitivity of 38% of has been reported in this setting [4]. Several species-specific and non-specific serological assays are commercially available for the diagnosis of invasive candidiasis: C. (Candida) albicans germ tube antibody (CAGTA) assay [renamed as Invasive Candidiasis (CAGTA) IFA]; C. albicans IgM (immunoglobulin M); C. albicans IgG; and Candida mannan ELISA (enzyme-linked immunosorbent assay). These assays are not in routine clinical use. β-1-3-D-glucan (BDG), a carbohydrate biomarker expressed by many pathogenic fungal species, including Candida, is used widely in clinical practice to exclude a diagnosis of invasive candidiasis [5]. The primary aim of this study was to compare the diagnostic value of serum BDG versus other biomarker and serological tests for the diagnosis of candidemia.
2. Materials and Methods
Serum samples (n = 866), taken between March 2013 and July 2016, for BDG assay were identified retrospectively from the database of the Mycology Reference Centre Manchester (MRCM), Wythenshawe Hospital, Manchester, UK. All samples with a minimum of 500 µL excess serum (stored at −70 °C within four hours of venepuncture—in accordance with the manufacturers’ instructions for all assays) from patients with matching blood culture results and with at least two BDG results were included. Cases of invasive candidiasis were classified as “proven” (based on a positive culture for Candida sp. from blood or another sterile site) or “probable” (based on the presence of risk factors for the development of invasive candidiasis, a compatible clinical syndrome and treated as such but no positive culture)—based on international consensus definitions [6,7]. Additionally, sera from ten patients tested for BDG but for whom the clinical suspicion of a deep-seated Candida infection was very low, and whose blood cultures were negative for Candida spp. were included as controls. For proven and probable cases, day zero was defined as the day of sampling of the first positive culture result, or the onset of clinical suspicion, respectively; for controls, this was defined as the timing of the first BDG sample collection.
Four proprietary assays, certified for invasive candidiasis in vitro diagnosis, were compared with serum BDG. Serum BDG was measured using a protease zymogen-based, colorimetric assay (Fungitell®, Associates of Cape Cod, East Falmouth, Falmouth, MA, USA). BDG values of <60 pg/mL were defined as negative; 60–79 pg/mL were defined as intermediate; and ≥80 pg/mL as positive. The presence of CAGTA was detected using a specific IgG IFA (immunofluorescence assay) (Virclia®, Vircell S.L., Granada, Spain) with a cut-off titre of ≥1:160 for positives. Candida mannan was measured using a competitive plate ELISA [Dynamiker®, Dynamiker Biotechnology (Tianjin) Co., Ltd., Tianjin, China] using positive and negative cut-off values of 100 and 50 pg/mL, respectively. Readings falling between these values were deemed to be indeterminate and non-diagnostic. C. albicans-specific IgM and IgG was measured using separate indirect ELISAs [Dynamiker®, Dynamiker Biotechnology (Tianjin) Co., Ltd., Tianjin, China] using positive and negative cut-off values of 120 and 80 AU (antibody concentration)/mL, respectively. Readings falling between these values were taken as intermediate. All assays were used in accordance with the manufacturer’s instructions.
Performance characteristics [sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV)] of each assay were calculated. Figures showing BDG and ELISA antigen/antibody levels were plotted using GraphPad Prism® 7 (GraphPad Software, Inc., Avenida De La Playa, CA, USA). Due to the number of patients involved, proven and probable invasive candidiasis groups were merged for data analysis. An indeterminate/intermediate result was regarded as negative for the purpose of sensitivity and specificity analysis.
3. Results
The final analysis included 50 serum samples from 24 patients. Of these, 14 had evidence of invasive candidiasis (proven or probable cases). Of these, 20 (40%) were positive for BDG, 5 (10%) were indeterminate and 25 (50%) were negative. BDG was negative in 2 out of the 14 (14%) proven or probable cases and 8 out of the 10 (80%) cases without evidence of invasive Candida infection (controls).
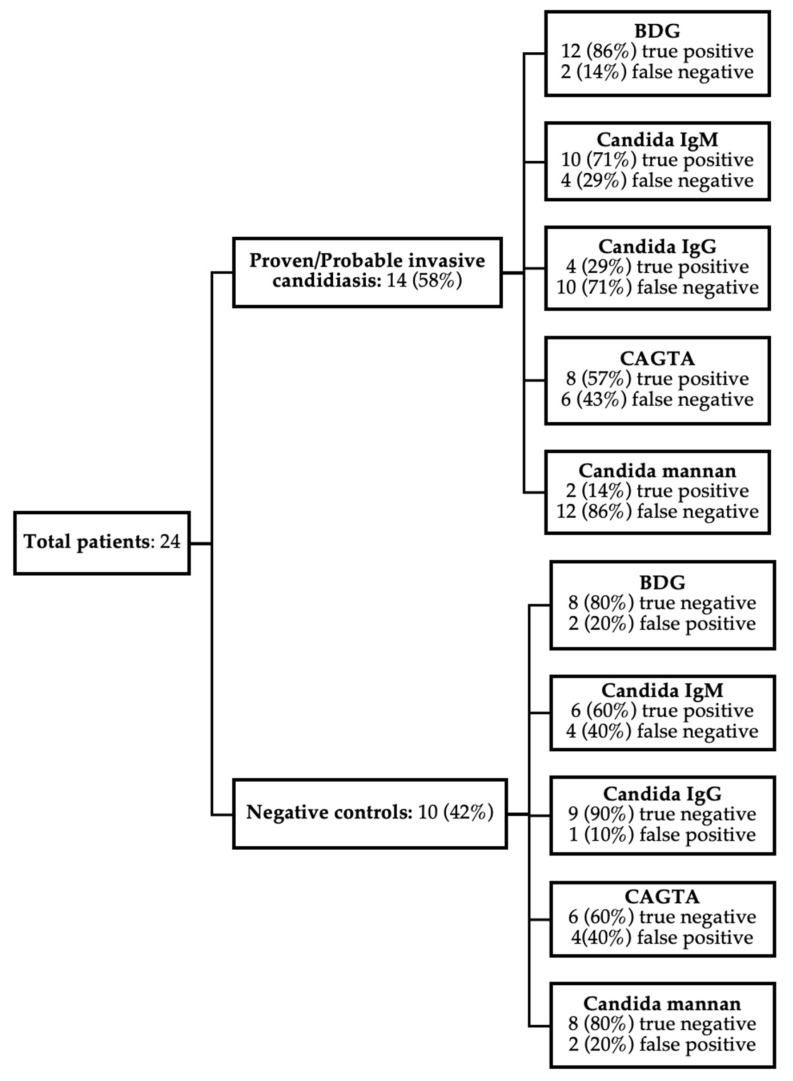
The performance of the five assays is summarised in Figure 1. The sensitivity and specificity of BDG for invasive candidiasis in the study population was 86% and 80%, respectively. For the Candida-specific assays, sensitivity and specificity were as follows: CAGTA, 57% and 60%, respectively; Candida mannan, 14% and 80%, respectively; Candida IgM, 71% and 60%, respectively; and Candida IgG, 29% and 90%, respectively.
Figure 1.
Summary of BDG, Candida IgG + IgM, CAGTA and mannan assay results. True/false positive negative rates for 24 patients were shown. Proven and probable cases were grouped together for data analysis.
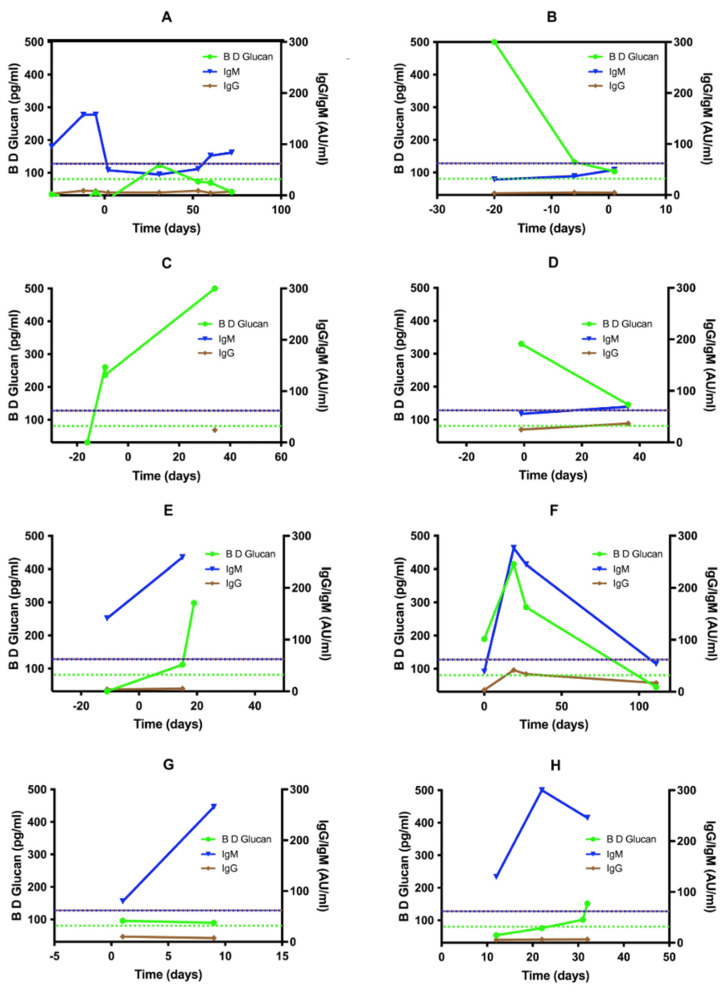
The temporal relationship between BDG, Candida IgM/IgG levels and the timing of the first positive deep culture result are outlined in Figure 2. Six proven (A–E) and two probable (F–G) cases (with at least two test results available for analysis) are presented. Time zero represented the date when the first positive sterile site sample was reported. Of the 14 proven or probable cases, 12 had one or more positive biomarker or serological test results before the first sterile site culture sample was collected, which later became positive. Among these, BDG was positive in six (50%), Candida IgM in four (33%), CAGTA in three (25%) and Candida IgG in one (8%). Candida mannan was negative in all such cases. We modelled assay performance against an assumed invasive candidiasis incidence rate of five cases/100,000 bed days (with a mean length-of-stay (LOS) of seven days)—a metric quoted previously in data from our unit [8]. The positive PPV and NPV for BDG were 0.15% and 99.9%, respectively; and 0.062% and 99.8%, respectively, for Candida IgM.
Figure 2.
BDG, Candida IgM and IgG levels relative to the time of first positive deep culture result. Six proven (A–F) and two probable (G,H) cases with multiple sample results are presented. Time zero was defined as the date when positive blood culture was first reported.
4. Discussion
Blood cultures remain the gold-standard method for diagnosing Candida bloodstream infections, whilst the direct culture of specimens from other sterile sites (such as joint or cerebrospinal fluid) is preferred for non-fungaemic patients [4]. However, the poor real-world performance of direct culture methods—namely, a low specificity and protracted turn-around times—limits their utility to diagnose clinical infection and, therefore, to direct antifungal stewardship (AFS) interventions [9]. BDG is a sensitive fungal biomarker, validated across several patient populations, and currently used as a component of the diagnostic criteria for invasive fungal diseases, including candidemia [6,7,10]. However, BDG is a non-specific biomarker, and false positives due to antigenemia in the absence of an invasive mycosis are relatively common [11]. Moreover, many fungal species express BDG, reducing the utility of the assay for the specific diagnosis of invasive candidiasis [12,13]. Indeed, our data confirm the non-specific nature of BDG for the diagnosis of invasive candidiasis. Candida-specific tests (including PCR (polymerase chain reaction) and T2 MR (magnetic resonance) assays) are available. However, due to the low concentration of fungal cells per mL of blood during candidemia, their sensitivity is moderate at best. They also lack sufficient validation and standardisation data to support their use in routine clinical practice [14,15]. Whilst such novel assays may offer advantages by reducing inappropriate anti-fungal use, significant up-front costs and the need for specialist technical and interpretative expertise are likely to preclude their use in non-specialist settings and developing countries for the foreseeable future. Thus, there is a pressing need for specific and cost-effective assays for the diagnosis of invasive candidiasis.
Whilst the NPV of BDG (99.9%) and Candida IgM (99.8%) is comparable and within a range acceptable for use in a clinical setting, our data demonstrate that Candida IgM is detectable earlier in the course of infection compared to other biomarkers (Figure 2). However, this may be confounded by other factors, including IgM cross-reactivity, which may account for the low specificity of this test when used in this setting. Whilst the CAGTA assay has been suggested as an effective diagnostic marker for invasive candidiasis [16,17], its performance across different populations is questionable [18]. Also, it is specific to C. albicans, whilst candidemia due to other species is relatively frequent. However, other data suggest that the CAGTA assay may be more reliable when used as an adjunct to either BDG or mannan testing and may add diagnostic value to either assay [17]. Nevertheless, a review of observational studies highlights the importance of combining mannan antigen and anti-mannan antibody to optimise diagnostic performance, possibly a strategy that prevents assay underestimation of serum antibody-bound mannan versus free mannan [19]. Similar data have been reported regarding combining BDG with Candida PCR in patients with invasive candidiasis [15,20]. Thus, a single-assay approach may not represent the optimal strategy.
Regarding biomarker and antibody kinetics during invasive infection, our data suggest that mannan antigen and Candida IgG are not readily detectable during the earliest stages of invasive candidiasis. Whilst this is in keeping with the normal immunological response in respect of IgG, which is typically delayed following initial antigenic exposure, it is noteworthy that mannan antigen detection is even less sensitive than Candida IgG, despite mannan being one of the main components of the Candida cell wall [21,22]. Thus, it would seem intuitive that mannan should be abundant during the earliest stages of bloodstream infections. However, given the clear presence of anti-mannan IgM, it is possible that mannan agglutination by specific IgM isoforms, with or without the formation of immune complexes, may confound early antigenic detection by obscuring epitope–antibody binding within the assay—as has been demonstrated in earlier data from studies involving cases of human disease [23] and in a murine model [24]. Moreover, mannan was found to bind to plasma albumin in the latter study, leading to sequestration. Thus, both phenomena would explain the low sensitivity of mannan detection in early fungaemia, putatively as low mannan levels are sequestered initially, becoming detectable by such techniques only when saturation of physiological sites occurs as the infection progresses and the fungaemic load increases. It would be useful to assess whether such phenomena are indeed important in the laboratory detection of mannan, through assays with extraction or dissociation steps to detect free antigen. This may increase the sensitivity of mannan detection early in invasive infection, thus increasing the value of mannan as a rule-out test. However, assessing the impact of early antifungal therapy, which may reduce the burden of circulating free (unbound) antigen, would be imperative for the diagnostic value of such assays.
We acknowledge limitations to this study. Firstly, this work is limited by the relatively small sample size, especially for proven and probable cases. This is a function of the low sensitivity of blood culture in diagnosing candidemia and our institution’s relatively low incidence-rate [4,8]. Furthermore, sampling was not conducted at fixed time points in the course of infection; thus, a cogent assessment of biomarker kinetics during invasive candidiasis cannot be drawn from our data. However, this study represents a pilot study to observe the quantitative changes in Candida biomarkers and antibodies during antifungal treatment. Thus, further studies should explore the serum samples from patients with proven IC and be employed in future studies to develop a complete picture of the changes in Candida-related biomarker levels. We also acknowledge the lack of a patient-specific dataset, a necessity given the retrospective and multi-site nature of the samples received in our unit.
Acknowledgments
The authors are very grateful to the National Institute of Health and Care Research (NIHR) Biomedical Research Centre (NIHR203308) Manchester for their support. The view(s) expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author Contributions
Conceptualization, A.R.B.A.B., M.D.R. and R.R.-R.; methodology, A.R.B.A.B., C.P.E., J.C.Y.L. and R.R.-R.; software, A.R.B.A.B. and C.P.E.; validation, C.P.E., M.D.R., R.R.-R. and L.N.-F.; formal analysis, A.R.B.A.B., C.P.E. and J.C.Y.L.; investigation, A.R.B.A.B., J.C.Y.L. and C.P.E.; resources, A.R.B.A.B.; data curation, A.R.B.A.B. and C.P.E.; writing—original draft preparation, A.R.B.A.B.; writing—review and editing, C.P.E., L.N.-F., C.M, R.R.-R. and M.D.R.; supervision, M.D.R., R.R.-R., C.B.M. and L.N.-F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study as data were based on residual patient samples (obtained in the course of standard clinical care) for the purposes of laboratory assay evaluation and validation.
Informed Consent Statement
Not applicable.
Data Availability Statement
A published dataset is not available due to patient confidentiality concerns.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and Multi-national prevalence of fungal diseases—Estimate precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koehler P., Stecher M., Cornely O., Vehreschild M., Bohlius J., Wisplinghoff H., Vehreschild J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019;25:1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 3.White P.L. Recent advances and novel approaches in laboratory-based diagnostic mycology. Med. Mycol. 2019;57((Suppl. S3)):S259–S266. doi: 10.1093/mmy/myy159. [DOI] [PubMed] [Google Scholar]
- 4.Clancy C.J., Nguyen M.H. Finding the “missing 50%” of invasive candidiasis: How non-culture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013;56:1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 5.Theel E.S., Doern C.D. Point-counterpoint: β-d-glucan testing is important for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2013;51:3478–3483. doi: 10.1128/JCM.01737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassetti M., Azoulay E., Kullberg B.-J., Ruhnke M., Shoham S., Vazquez J., Giacobbe D.R., Calandra T. EORTC/MSGERC definitions of invasive fungal diseases: Summary of activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. 2021;72:S121–S127. doi: 10.1093/cid/ciaa1751. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rautemaa-Richardson R., Rautemaa V., Al-Wathiqi F., Moore C.B., Craig L., Felton T.W., Muldoon E.G. Impact of a diagnostics-driven antifungal stewardship programme in a UK tertiary referral teaching hospital. J. Antimicrob. Chemother. 2018;73:3488–3495. doi: 10.1093/jac/dky360. [DOI] [PubMed] [Google Scholar]
- 9.Rouzé A., Estella Á., Timsit J.F. Is (1, 3)-β-D-glucan useless to guide antifungal therapy in ICU? Intensive Care Med. 2022;48:930–932. doi: 10.1007/s00134-022-06766-2. [DOI] [PubMed] [Google Scholar]
- 10.De Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T., Pappas P.G., Maertens J., Lortholary O., Kauffman C.A., et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of al-lergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racil Z., Kocmanova I., Lengerova M., Weinbergerova B., Buresova L., Toskova M., Winterova J., Timilsina S., Rodriguez I., Mayer J. Difficulties in using 1, 3-β-D-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies–high frequency of false-positive results and their analysis. J. Med. Microbiol. 2010;59:1016–1022. doi: 10.1099/jmm.0.019299-0. [DOI] [PubMed] [Google Scholar]
- 12.Karageorgopoulos D., Qu J.-M., Korbila I., Zhu Y.-G., Vasileiou V., Falagas M. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: A meta-analysis. Clin. Microbiol. Infect. 2013;19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 13.Nucci M., Barreiros G., Reis H., Paixão M., Akiti T., Nouér S. Performance of 1,3-beta-D-glucan in the diagnosis and monitoring of invasive fusariosis. Mycoses. 2019;62:570–575. doi: 10.1111/myc.12918. [DOI] [PubMed] [Google Scholar]
- 14.Zacharioudakis I.M., Zervou F.N., Mylonakis E. T2 Magnetic Resonance Assay: Overview of Available Data and Clinical Implications. J. Fungi. 2018;4:45. doi: 10.3390/jof4020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White P.L., Archer A.E., Barnes R.A. Comparison of non-culture-based methods for detection of systemic fungal infections, with an emphasis on invasive Candida infections. J. Clin. Microbiol. 2005;43:2181–2187. doi: 10.1128/JCM.43.5.2181-2187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Jiménez M.C., Muñoz P., Guinea J., Valerio M., Alonso R., Escribano P., Bouza E. Potential role of Candida albicans germ tube antibody in the diagnosis of deep-seated candidemia. Med. Mycol. 2014;52:270–275. doi: 10.1093/mmy/myt025. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Jiménez M.C., Muñoz P., Valerio M., Alonso R., Martos C., Guinea J., Bouza E. Candida biomarkers in patients with candidaemia and bacteraemia. J. Antimicrob. Chemother. 2015;70:2354–2361. doi: 10.1093/jac/dkv090. [DOI] [PubMed] [Google Scholar]
- 18.Wei S., Wu T., Wu Y., Ming D., Zhu X. Diagnostic accuracy of Candida albicans germ tube antibody for invasive candidiasis: Systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2018;93:339–345. doi: 10.1016/j.diagmicrobio.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Mikulska M., Calandra T., Sanguinetti M., Poulain D., Viscoli C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care. 2015;14:R222. doi: 10.1186/cc9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeating C., White P.L., Posso R., Palmer M., Johnson E., McMullan R. Diagnostic accuracy of fungal PCR and β-d-glucan for detection of candidaemia: A preliminary evaluation. J. Clin. Pathol. 2017;71:420–424. doi: 10.1136/jclinpath-2017-204692. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rubio R., De Oliveira H.C., Rivera J., Trevijano-Contador N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020;10:2993. doi: 10.3389/fmicb.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y., Kang M., Li D., Wang T., Kuang Z., Ma Y. Performance of a new Candida anti-mannan IgM and IgG assays in the diagnosis of candidemia. Rev. Inst. Med. Trop. São Paulo. 2020;62:e25. doi: 10.1590/s1678-9946202062025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappe R. Coexistence of Free Antigens, Free Antibodies and Immune Complexes in Sera from Patients with Suspected Deep-seated Candidosis. Mycoses. 1989;32:24–32. doi: 10.1111/j.1439-0507.1989.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 24.Han Y., Ulrich M.A., Cutler J.E. Candida albicans mannan extract—Protein conjugates induce a protective immune response against experimental candidiasis. J. Infect. Dis. 1999;179:1477–1484. doi: 10.1086/314779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A published dataset is not available due to patient confidentiality concerns.