Conventional transarterial chemoembolization (c-TACE), a common regimen for initially unresectable hepatocellular carcinoma, provides a chance of resection for 10% patients. This study found that TACE plus hepatic artery chemotherapy infusion (TACE-HAIC) had a higher conversion rate to resection, better survival outcome and comparable advent event, compared with c-TACE.
Keywords: hepatocellular carcinoma, unresectable, conversion therapy, transarterial chemoembolization, hepatic artery chemotherapy infusion
Abstract
Objective:
To evaluate whether this conversion rate to resectability could be increased when patients are treated with transarterial chemoembolization and hepatic arterial infusion chemotherapy (TACE-HAIC) using oxaliplatin plus fluorouracil/leucovorin.
Background:
Conventional TACE (c-TACE) is a common regimen for initially unresectable hepatocellular carcinoma (HCC), which converts to curative-intent resection in about 10% of those patients. It is urgent need to investigated better regimen for those patients.
Methods:
The data of 83 initially unresectable HCC patients were examined, including 41 patients in the TACE-HAIC group and 42 patients in the c-TACE group. Their response rate, conversion rate to resection, survival outcome, and adverse events were compared.
Results:
The conversion rate was significantly better in the TACE-HAIC group than in the c-TACE group (48.8% vs 9.5%; P < 0.001). The TACE-HAIC had marginal superiority in overall response rate as compared to c-TACE (14.6% vs 2.4%; P = 0.107 [RECIST]; 65.9% vs 16.7%; P < 0.001 [mRECIST], respectively). The median progression-free survival was not available and 9.2 months for the TACE-HAIC and cTACE groups, respectively (hazard rate [HR]: 0.38; 95% confidence interval [CI], 0.20–0.70; P = 0.003). The median overall survival was not available and 13.5 months for the TACE-HAIC and c-TACE groups, respectively (HR, 0.63; 95% CI, 0.34–1.17; P = 0.132). The 2 groups had similar rates of grade 3/4 adverse events (all P > 0.05).
Conclusions:
TACE-HAIC demonstrated a higher conversion rate and progression-free survival benefit than c-TACE and could be considered as a more effective regimen for patients with initially unresectable HCC. Future prospective randomized trials are needed to confirm it.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most lethal malignancy on a global scale.1,2 Hepatic resection has been advocated as the standard radical treatment for resectable HCCs, with a 5-year survival rate ranging from 50–70%.3,4 Despite advances in surgical technique, less than 40% of patients with HCC are eligible for resection.4,5
It is generally agreed that successful conversion treatments could provide a chance for longer survival to patients with initially unresectable disease.6,7 However, conversion treatment options are limited. Conventional transarterial chemoembolization (c-TACE) remains the main treatment modality for these patients, aiming to prolong survival, and if possible, resulting in tumor regression and conversion to resection. Unfortunately, this attempt has resulted to low conversion rates (usually <10%).7,8 Therefore, more effective strategies are highly required to improve this conversion resection rate.
Recently, several studies have shown that oxaliplatin plus fluorouracil/leucovorin (FOLFOX)–based hepatic arterial infusion chemotherapy (HAIC) could offer significantly better objective responses and survival benefits than sorafenib or c-TACE for advanced HCC.9–11 As such, FOLFOX-based HAIC seems to be a more attractive regimen for the conversion treatment for unresectable patients. However, the efficacy of HAIC largely relies on the sensitivity of the tumor to the chemoagents used. Different from HAIC, lipiodol-based embolization can occlude the tumor-feeding blood vessels and lead to tumor disease control. The antitumor mechanism of embolization is independent of tumor chemoresistance.12 Thus, it seems that TACE-HAIC could combine the benefits of both TACE and HAIC together to yield an increase in tumor shrinkage and reduction in the risk of chemoresistance-related tumor progression. However, the actual efficacy of conversion to resection and survival for patients who underwent TACE-HAIC is still unknown.
Thus, in this retrospective study, we aimed to compare the conversion rate and survival outcome of TACE-HAIC versus c-TACE in patients with unresectable HCC.
METHODS
Patients and Study Design
The data of patients, who were diagnosed as HCC according to the European Association for the Study of the Liver (EASL) and treated by c-TACE or TACE-HAIC between January 2015 and July 2019, were retrieved. The inclusion criteria were as follows: (a) classified as the Barcelona clinic liver cancer (BCLC) stage A or B; (b) the tumor was not amenable to radical surgical resection, due to insufficient surgical margin, after assessment by 2 experienced hepatobiliary surgeons (BL and YY, with more than 15 years of experience in hepatic resection); or would have an estimated <30% residual liver volume (FLV)13 after resection; (c) classified as Child-Pugh Grade A. Cases were excluded if they met any of the following criteria: (a) a previous history of HCC treatment; (b) signs of vascular invasion or distant metastasis on imaging; (c) severe underlying cardiac, pulmonary, or renal diseases; or (d) a second primary malignancy.
This study was approved by the institutional review board of Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China) and was performed following the Declaration of Helsinki of 1975 as revised in 1983. Raw data in our study have been uploaded onto the Research Data Deposit public platform (RDDA2020001594, www.researchdata.org.cn).
c-TACE and TACE-HAIC Treatment
c-TACE was performed as described in our previous studies.14,15 Briefly, chemoembolization was performed using 30 mg/m2 of epirubicin, 200 mg/m2 of carboplatin, and 4 mg/m2 of mitomycin C, mixed with 2–5 mL lipiodol. Then, up to 20 mL of additional pure lipiodol was injected into the tumor-feeding artery until stasis of blood flow in the target artery was observed. Repeated TACE was performed at intervals of 4 weeks.
Compared with c-TACE, the chemoembolization in the TACE-HAIC group was performed as described above but using only 30 mg/m2 of epirubicin with 2–5 mL lipiodol, followed by pure lipiodol. Subsequently, a catheter was placed and fixed in the tumor feeding artery for the FOLFOX-based chemotherapy infusion as the following dosage: 85 mg/m2 oxaliplatin infusion for 2 hours; leucovorin, 400 mg/m2 infusion for 2 hours; and 400 mg/m2 of 5-FU bolus and 2400 mg/m2 of continuous infusion for 46 hours (high 5-FU dose, from August 2017 to November 2018) or 1200 of mg/m2 continuous infusion for 23 hours (low 5-FU dose, from December 2018 to June 2019). TACE-HAIC treatment was repeated every 4 weeks. For easy reference, we designated the above TACE-HAIC procedure as SYSUCC procedure.
Follow-up and Efficacy Assessment
Blood cell counts, liver function tests, and serum alpha-fetoprotein (AFP) levels were determined before each course. Adverse events were graded according to NCI-CTCAE version 5.0 before each course. Contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT) examinations of the upper abdomen and thorax were subsequently performed after every treatment course.
Tumor responses were evaluated by measuring the longest diameter of target lesions according to response evaluation criteria in solid tumors (RECIST) version 1.1.16 Meanwhile, the modified RECIST (mRECIST) was also employed to evaluate the tumor activity.17 Objective response rate (ORR) was defined as the rate of complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as the rate of CR, PR, or stable disease (SD). AFP response, which was defined as the change of AFP level after treatment, was used to further assess the treatment efficacy. Patients with baseline AFP > 20 ng/mL were used to analyze the AFP response.18
Hepatic Resection After Conversion
Hepatic resection was performed after careful assessment when an estimated residual liver volume >30–40% could be remained after resection by 2 experienced surgeons. The procedure of hepatic resection was performed as reported in our previous studies.19,20 Briefly, hepatectomy was performed using cut-ultrasound aspiration (CUSA) and harmonic scalpel. Intraoperative ultrasonography was routinely used to confirm that no tumor remained.
Statistical Analyses
Overall survival (OS) was measured from the date of transarterial therapy to the date of death or the last follow-up. Progression-free survival (PFS) was measured from the date of transarterial therapy to the time of progression or recurrence or last follow-up. A survival curve was generated using the Kaplan-Meier method with a log-rank test.
Categorical variables were analyzed using the chi-square test, and continuous variables were analyzed using the Student’s t-test. P < 0.05 was considered statistically significant. All data were processed with the Statistical Package for Social Sciences version 25.0 (IBM Corp.).
RESULTS
Patient Characteristics
A total number of 83 patients were enrolled in this study, with 41 and 42 patients in the TACE-HAIC and c-TACE groups, respectively (Fig. 1). The baseline data of the patients are shown in Table 1. Clinical characteristics, including AFP, tumor size, tumor number, were comparable between the 2 groups. Furthermore, 22 patients received a high-dose 5-FU and 19 patients received a low-dose 5-Fu in the TACE-HAIC group. The low-dose subgroup had more patients older than 60 years (Table S1, http://links.lww.com/AOSO/A24).
FIGURE 1.

Flow diagram of patients with initially unresectable hepatocellular carcinoma who underwent either TACE-HAIC or c-TACE. c-TACE, conventional transarterial chemoembolization; TACE-HAIC, TACE plus hepatic artery chemotherapy infusion.
TABLE 1.
Baseline Characteristics of Patients With Initially Unresectable Hepatocellular Carcinoma
| Variables | TACE-HAIC (n = 41) | c-TACE (n = 42) | p |
|---|---|---|---|
| Gender | 0.085 | ||
| Male | 30 (73.2%) | 37 (88.1%) | |
| Female | 11 (26.8%) | 5 (11.9%) | |
| Age (years) | 0.189 | ||
| ≥60 | 11 (26.8%) | 17 (40.5%) | |
| <60 | 30 (73.2%) | 25 (59.5%) | |
| PLT (109/L) | 0.249 | ||
| ≥100 | 39 (95.1%) | 37 (88.1%) | |
| <100 | 2 (4.9%) | 5 (11.9%) | |
| ALT (U/L) | 0.097 | ||
| >40 | 15 (36.6%) | 23 (54.8%) | |
| ≤40 | 26 (63.4%) | 19 (45.2%) | |
| ALB (g/L) | 0.309 | ||
| ≥35 | 26 (63.4%) | 22 (52.4%) | |
| <35 | 15 (36.6%) | 20 (47.6%) | |
| TBIL (μmol/L) | 0.353 | ||
| >17.1 | 9 (22.0%) | 13 (31.0%) | |
| ≤17.1 | 32 (78.0%) | 29 (69.0%) | |
| PT (s) | 0.971 | ||
| >13.5 | 4 (9.8%) | 4 (9.5%) | |
| ≤13.5 | 37 (90.2%) | 38 (90.5%) | |
| AFP (ng/mL) | 0.578 | ||
| ≤20 | 6 (14.6%) | 13 (31.0%) | |
| >20 | 35 (85.4%) | 29 (69.0%) | |
| HBsAg | 0.261 | ||
| Positive | 36 (87.8%) | 33 (78.6%) | |
| Negative | 5 (12.2%) | 9 (21.4%) | |
| Main tumor size (cm) | 0.578 | ||
| ≥10 | 23 (56.1%) | 21 (50.0%) | |
| <10 | 18 (43.9%) | 21 (50.0%) | |
| Tumor number | 0.941 | ||
| ≤3 | 28 (68.3%) | 29 (69.0%) | |
| >3 | 13 (31.7%) | 13 (31.0%) | |
| Conversion to resection | <0.001 | ||
| Yes | 20 (48.8%) | 4 (9.5%) | |
| No | 21 (51.2%) | 38 (90.5%) | |
P values were calculated using a 2-sided Chi-square test.
AFP, alpha-fetoprotein; ALT, alanine transaminase; ALB, albumin; c-TACE, conventional transarterial chemoembolization; HBsAg, hepatitis B surface antigen; PT, prothrombin time; TACE-HAIC, TACE plus hepatic artery chemotherapy infusion; TBIL, total bilirubin.
Response and Conversion to Resection
In regards to the resection rate, 48.8% (20/41) of the patients underwent hepatic resection in the TACE-HAIC group, which was significantly higher than that of the c-TACE group (4/42, 9.5%, P < 0.001, Table 1). The images of 2 representative patients before and after conversion in the TACE-HAIC group are shown in Figure 2. There was no CR in both groups according to the RECIST criteria (Table 2). The PR, SD, and DCR in the TACE-HAIC group were higher than the c-TACE group (14.6% vs 2.4%, P = 0.107; 78.0% vs 59.5%, P = 0.069; 92.7% vs 54.8%, P < 0.001, respectively, Figs. 3A, B). Moreover, the TACE-HAIC group had lower PD than the c-TACE group (7.3% vs 38.1%, P < 0.001).
FIGURE 2.
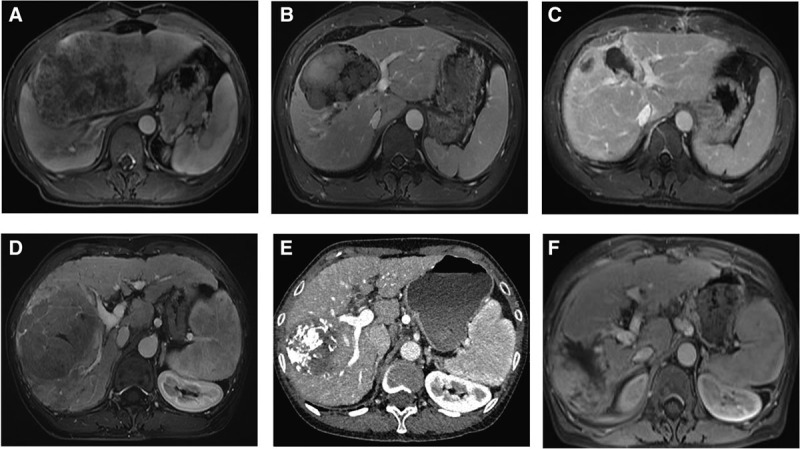
Images of 2 representative patients who received TACE-HAIC treatment. Case 1, before (A), after 2 cycles of TACE-HAIC (B) and after resection (C). Case 2, before (D), after 2 cycles of TACE-HAIC (E) and after resection (F). TACE-HAIC, TACE plus hepatic artery chemotherapy infusion.
TABLE 2.
Efficacy Evaluated by RECIST and mRECIST Criteria After TACE-HAIC and c-TACE Treatments
| RECIST Criteria | mRECIST Criteria | |||||
|---|---|---|---|---|---|---|
| TACE-HAIC (n = 41) | c-TACE (n = 42) | P | TACE-HAIC (n = 41) | c-TACE (n = 42) | P | |
| Complete response | 0 (0.0%) | 0 (0.0%) | 1.000 | 2 (4.9%) | 2 (4.8%) | 1.000 |
| Partial response | 6 (14.6%) | 1 (2.4%) | 0.107 | 25 (61.0%) | 3 (7.1%) | <0.001 |
| Stable disease | 32 (78.0%) | 25 (59.5%) | 0.069 | 11 (26.8%) | 25 (59.5%) | 0.003 |
| Progressive disease | 3 (7.3%) | 16 (38.1%) | <0.001 | 3 (7.3%) | 12 (28.6%) | 0.012 |
| Overall response | 6 (14.6%) | 1 (2.4%) | 0.107 | 27 (65.9%) | 7 (16.7%) | <0.001 |
| Disease control | 38 (92.7%) | 23 (54.8%) | <0.001 | 38 (92.7%) | 30 (71.4%) | 0.012 |
P values were calculated using a 2-sided Chi-square test.
c-TACE, conventional transarterial chemoembolization; RECIST, response evaluation criteria in solid tumors; TACE-HAIC, transarterial chemoembolization-hepatic artery infusion chemotherapy.
FIGURE 3.
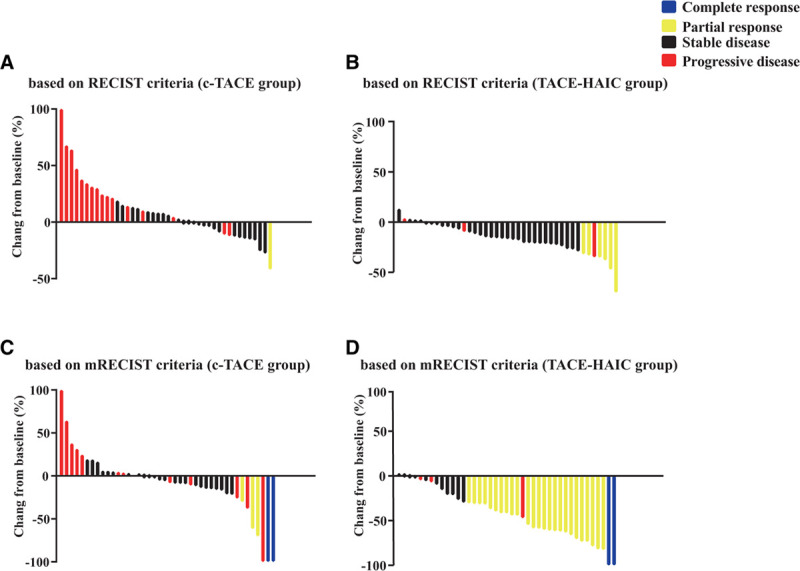
Waterfall plots depicting maximum response of target lesions using the criteria of RECIST in the TACE (A) and TACE-HAIC groups (B), modified RECIST (mRECIST) criteria in the TACE (C) and TACE-HAIC groups (D). RECIST, response evaluation criteria in solid tumors; TACE, transarterial chemoembolization; TACE-HAIC, TACE plus hepatic artery chemotherapy infusion.
The mRECIST criteria were also used to evaluate the viable tumor for the 2 groups. As were shown in Table 2, CR was comparable between the groups (4.9% vs 4.8%, P = 1.000). The PR and ORR for the TACE-HAIC group were higher than the c-TACE group when using mRECIST criteria (61.0% vs 7.1%, P < 0.001; 65.9% vs 16.7%, P < 0.001, respectively; Figs. 3C, D). Moreover, the TACE-HAIC group had lower PD and SD than the c-TACE group (7.3% vs 28.6%, P = 0.012; 26.8% vs 59.5%, P = 0.006; respectively). The data of FLV/LV after conversion therapy was shown in Figure S1, http://links.lww.com/AOSO/A24.
Thirty-five patients had a high baseline AFP level in the TACE-HAIC and 19 in c-TACE groups, respectively (Table S2, http://links.lww.com/AOSO/A24). The 2 groups had similar rate of mild AFP responses (>20%, <50% decrease), while the TACE-HAIC group had a higher rate of significant AFP decrease (≥50% decrease, P = 0.002) and a lower rate of AFP increase (>20% increase, P = 0.007) than the c-TACE group.
Characteristics of Resection
The median time from the start of treatment to resection was 2.2 ± 1.0 months in the TACE-HAIC group and 1.3 ± 0.2 months in the c-TACE group. The mean cycles from start of treatment to resection were 1.6 ± 0.6 cycles in the TACE-HAIC group and 1 cycle in the c-TACE group. Seven patients (30.4%) underwent a major hepatectomy (more than 3 Couinaud’s segments) in the TACE-HAIC group, while only 2 (50.4%) in the c-TACE group. The mean operative time was 193.2 ± 38.0 and 202.0 ± 57.4 minutes for the TACE-HAIC and c-TACE groups, respectively. The mean estimated blood loss was 440.0 ± 265.9 and 732.0 ± 320.2 mL for the TACE-HAIC and c-TACE groups, respectively. There was no 90-day mortality in this series. One patient in the TACE-HAIC group had a biliary leak and recovered within 1 month after resection.
Potential factors predicting conversion hepatectomy in the initially unresectable patients are shown in Table S3, http://links.lww.com/AOSO/A24. The conversion rate was significantly higher for patients with lesions no more than 3, as compared to those with >3 lesions (85.0% vs 15.0%; P = 0.025). In contrast, other factors were not significantly associated with conversion resection (Table S3, http://links.lww.com/AOSO/A24).
Survival Outcome
The median follow-up period was 27.6 months (c-TACE: 47.8 months; TACE-HAIC: 19.6 months). The median PFS was not available for the TACE-HAIC group and 9.2 months for the c-TACE group, respectively. The OS events were 29.3% and 66.7% in the TACE-HAIC and c-TACE groups, respectively. The median OS was not available for the TACE-HAIC group and 13.5 months for the c-TACE group, respectively. The superiority of TACE-HAIC over c-TACE was shown in PFS (HR: 0.38, 95% CI: 0.20–0.86, P = 0.003, Fig. 4). OS did not reach the statistical difference, although it has trend to favor in the TACE-HAIC group (HR: 0.63, 95% CI: 0.34–1.17, P = 0.142, Fig. 4). Furthermore, Kaplan-Meier survival curve showed no significant difference in OS (HR: 0.46, 95% CI: 0.14–1.54, P = 0.132, Fig. 5) and PFS (HR: 1.39, 95% CI: 0.39–5.02, P = 0.616, Fig. 5) between the high dose and low dose of 5-Fu subgroups.
FIGURE 4.
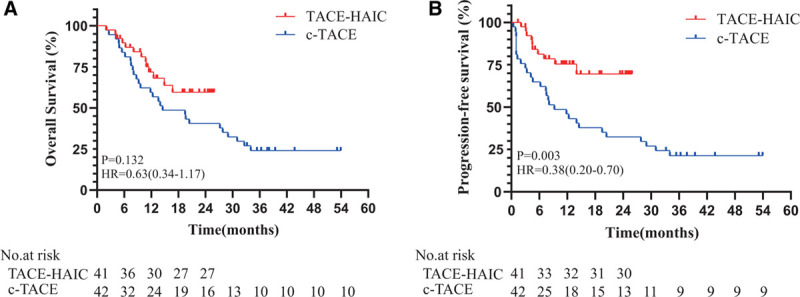
Kaplan-Meier curves for OS (A) and PFS (B) in the TACE-HAIC and c-TACE groups. Vertical bars indicate censoring of patients alive at their last follow-up. c-TACE, conventional transarterial chemoembolization; OS, overall survival; PFS, progression-free survival; TACE-HAIC, TACE plus hepatic artery chemotherapy infusion.
FIGURE 5.
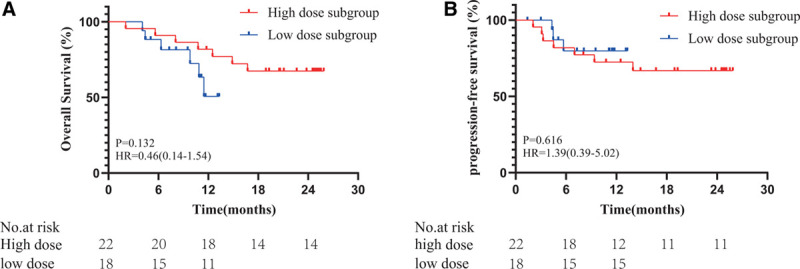
Kaplan-Meier curves for OS (A) and PFS (B) in the high-dose and low-dose subgroups. OS, overall survival; PFS, progression-free survival.
The median postprogression survival was 7.2 months and 10.1 months for the TACE-HAIC and c-TACE groups (P = 0.169), respectively. The median postprogression survival was 15.8 months and 5.6 months for the high-dose and low-dose subgroups (P = 0.167), respectively. In the high-dose subgroup, 6 patients had disease recurrence after resection, and 1 patients received TACE-HAIC, 1 patients received radiotherapy, and 2 patients received sorafenib, respectively. In the low-dose subgroup, 2 patients had disease progression and 2 patients relapsed; all of them received TACE-HAIC treatment thereafter. In the c-TACE group, 2 patients had disease progression. Six patients received repeat c-TACE, 1 patient received c-TACE plus radiotherapy, and 1 patient received c-TACE plus ablation, respectively.
To analyze the effects of resection on the 2 groups, we compared survival of patients who failed to conversion between the TACE-HAIC and the c-TACE group. The results showed that the overall survival rates between 2 groups were similar (HR 1.06, 0.50–2.12, P = 0.876, Figure S2, http://links.lww.com/AOSO/A24).
Adverse Effects
The adverse effects (AEs) observed in this study are shown in Table S4, http://links.lww.com/AOSO/A24. Although the TACE-HAIC group had a higher rate of hypoalbuminemia (P = 0.011), TACE-HAIC group was comparable with the c-TACE group in any grade AEs and AEs grade ≥ 3 (all P > 0.05). Furthermore, there were no significantly different AEs between the high-dose and low-dose 5-FU subgroups, although a relatively high rate of elevated alanine aminotransferase (ALT) level and vomiting were observed in the high-dose subgroup (Table S4, http://links.lww.com/AOSO/A24).
DISCUSSION
This study aimed to compare the efficacy of TACE-HAIC to that of c-TACE for patients with initially unresectable HCC. We observed that 48.8% of the patients received a conversion resection in the TACE-HAIC group, while the patients from the c-TACE group had a conversion rate of 9.5%. The treatment response and patient survival were also more favorable in the TACE-HAIC group. Furthermore, the administration of TACE-HAIC had a manageable toxicity profile similar with that of c-TACE alone.
Over the past 2 decades, little improvement has been made in the conversion treatments for patients with unresectable diseases. c-TACE was a major treatment for these patients, with the conversion rate at only ~10%,7, 10 which was consistent with this finding. Our study showed that the TACE-HAIC treatment provided a conversion rate of 48.8%, significant improvement over that from the past decades.7, 21 Distinct with downstaging of tumor burden, increasing remnant liver volume is another strategy to increase resectability. ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) is a primary method to increase liver volume.22 However, the reported 90-day mortality rate was up to 11.1%, much higher than that in most hospitals with a high volume of surgical hepatectomy.23 Furthermore, studies showed that ALPPS focused on liver hypertrophy while neglecting the factor of oncological selection and increasing tumor proliferative activity.24, 25 Unlike ALPPS, the TACE-HAIC-based conversion treatment could provide a therapeutic window to assess tumor biology and predict outcomes. In our study, the postoperative 90-day mortality rate was 0%, and the PD rate was 7.3% in the TACE-HAIC group. These data indicated that TACE-HAIC is beneficial for patients with initially unresectable HCC.
The survival benefit observed in this study may also be partly due to the FOLFOX-based infusion chemotherapy. Exerting the hepatic first-pass effect, HAIC can deliver a higher concentration of chemotherapeutic agents to the liver tumors, exhibiting lower toxicity than intravenous chemotherapy.26,27 Therefore, the FOLFOX-based artery infusion chemotherapy has shown promising efficacy for the downsizing HCC tumor. However, it is noteworthy that 22.4% of patients undergoing the HAIC-only treatment had disease progression.9 In this study, only 7.3% of the patients in the TACE-HAIC group exhibited disease progression, consistent with a recently published paper analyzing the TACE-HAIC treatments.28 It was noticeable that embolization could reduce the risk of FOLFOX-related resistance. Several studies have found that embolization was the basis and core of a combination therapy.29, 30 Despite a lack of direct comparative analysis, we believe that TACE-HAIC treatment can exert better disease than the HAIC-only treatment.
Previous studies have shown that a steady-state plasma concentration of 5-Fu can be achieved after 15 hours of continuous infusion.31–33 Accordingly, we modified the treatment using a low-dose, 23-hour continuous infusion of 5-Fu in 19 patients. Notably, the patients’ compliance in the 23-hour subgroup was much better than those in the 46-h subgroup. All the patients in the 23-hour subgroup had completed treatment according to the schedule. While 2 patients in the 46-hour high-dose subgroup refused to receive the second cycle treatment because of the mandatory immobilization of the right leg for at least 50 hours. Although the high-dose subgroup provided marginally better OS, the low-dose subgroup shared a similar conversion resection rate (P = 0.427) and PFS (P = 0.616), likely due to the relative extended postprogression survival in the high-dose subgroup. Hence, we believe that the modified 23-hour continuous infusion of 5-Fu is reasonable and applicable in clinical practice.
We also investigated whether the addition of HAIC would increase the observed AEs. The frequency of AEs was increased in the TACE-HAIC group. The AEs were mainly observed to be grade 1 or 2 hypoalbuminemia, which were manageable. However, no statistically significant differences were found in terms of grades 3–4 adverse reactions between the TACE-HAIC and c-TACE groups. Therefore, our study results have demonstrated that the TACE-HAIC treatment had good safety and tolerance.
Interestingly, although the partial response in the TACE-HAIC group was only 14.6%, the conversion rate was as high as 48.8%. In other words, more than 30% of patients fulfilled the resection criteria but not the PR’s criteria. Of note, these studies showed that PR were 40.8–59.2% for patients after the HAIC treatment,9, 11, 32 according to the mRECIST-based assessment. Although these could not be directly compared, our SYSUCC procedure exhibited higher PFS and mRECIST-based DCR than the 3 previous studies.
Our study had some limitations. First, the number of enrolled patients was relatively small. The patients also had a relatively short follow-up period, potentially contributing to the lack of significant difference in the OS. The tendency is clear to favor the TACE-HAIC group, which may reach significance after long-term follow-up. Second, this study only compared the efficacy between the TACE-HAIC and c-TACE treatment directly. There is still lack of a direct comparison of the efficacy between TACE-HAIC and HAIC treatments. Therefore, we have started a prospective randomized study to compare the TACE-HAIC to the HAIC (ClinicalTrials.gov identifier: NCT03591705) to identify the optimal conversion treatment for these unresectable patients.
CONCLUSIONS
In conclusion, we have found that TACE-HAIC demonstrates a significantly higher conversion rate than the c-TACE regimen. Therefore, TACE-HAIC may be a safe and promising treatment for patients with initially unresectable HCC. Randomized studies are needed to confirm the value of TACE-HAIC in increasing patient survival.
Footnotes
B. Li, J. Qiu, and Y. Zheng are cofirst authors.
This work was funded by grants from the National Natural Science Foundation of China (No. 81772598 and 81802421); the Guangzhou Science and Technology Program of China (No. 201804020093); and the Central Universities in China (No. 18ykpy36).
Disclosure: The authors declare that they have nothing to disclose.
All the authors approved this version manuscript to be submitted. Y.F. Yuan, B.K. Li, J.L. Qiu, Y. Zheng, and W. He did concept and design. J.L. Qiu, Y.X. Shi, Y.C. Yuan, and Y.P. Zhang participated in acquisition, analysis, or interpretation of data. B.K. Li, Y.X. Shi and J.L. Qiu did drafting of the manuscript. Y.X. Shi and Z.Y. Qiu did statistical analysis. W. He, C.W. Wang, C.R. Zhong, and K. Li participated in administrative, technical, or material support. Y.F. Yuan, B.K. Li, and J.L. Qiu did supervision.
REFERENCES
- 1.Cao M, Li H, Sun D, et al. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (Lond). 2020; 40:205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019; 156:477–491.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu J, Peng B, Tang Y, et al. CpG methylation signature predicts recurrence in early-stage hepatocellular carcinoma: results from a multicenter study. J Clin Oncol. 2017; 35:734–742 [DOI] [PubMed] [Google Scholar]
- 4.Cucchetti A, Zhong J, Berhane S, et al. The chances of hepatic resection curing hepatocellular carcinoma. J Hepatol. 2020; 72:711–717 [DOI] [PubMed] [Google Scholar]
- 5.Shaya FT, Breunig IM, Seal B, et al. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-medicare. Pharmacoeconomics. 2014; 32:63–74 [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZF, Luo YJ, Lu Q, et al. Conversion therapy and suitable timing for subsequent salvage surgery for initially unresectable hepatocellular carcinoma: what is new? World J Clin Cases. 2018; 6:259–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma–a strategy to increase resectability. Ann Surg Oncol. 2007; 14:3301–3309 [DOI] [PubMed] [Google Scholar]
- 8.Goto Y, Hisaka T, Sakai H, et al. Salvage surgery for initially unresectable locally advanced hepatocellular carcinoma downstaged by hepatic arterial infusion chemotherapy. Anticancer Res. 2020; 40:4773–4777 [DOI] [PubMed] [Google Scholar]
- 9.Lyu N, Lin Y, Kong Y, et al. FOXAI: a phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018; 67:395–396 [DOI] [PubMed] [Google Scholar]
- 10.He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017; 36:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019; 5:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943 [DOI] [PubMed] [Google Scholar]
- 13.Kianmanesh R, Piardi T, Tamby E, et al. Liver angulometry: a simple method to estimate liver volume and ratios. HPB (Oxford). 2013; 15:976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Zou R, Zheng Y, et al. Lipiodol deposition in portal vein tumour thrombus predicts treatment outcome in HCC patients after transarterial chemoembolisation. Eur Radiol. 2019; 29:5752–5762 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Liao Y, Qiu J, et al. Transcatheter arterial chemoembolization alone or combined with ablation for recurrent intermediate-stage hepatocellular carcinoma: a propensity score matching study. J Cancer Res Clin Oncol. 2020; 146:2669–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247 [DOI] [PubMed] [Google Scholar]
- 17.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009; 27:446–452 [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Qiu J, Li J, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016; 263:778–786 [DOI] [PubMed] [Google Scholar]
- 20.Qiu J, Zheng Y, Shen J, et al. Resection versus ablation in hepatitis B virus-related hepatocellular carcinoma patients with portal hypertension: a propensity score matching study. Surgery. 2015; 158:1235–1243 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Peng Y, Hu J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg. 2020; 271:534–541 [DOI] [PubMed] [Google Scholar]
- 22.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012; 255:405–414 [DOI] [PubMed] [Google Scholar]
- 23.Chapman BC, Paniccia A, Hosokawa PW, et al. Impact of facility type and surgical volume on 10-year survival in patients undergoing hepatic resection for hepatocellular carcinoma. J Am Coll Surg. 2017; 224:362–372 [DOI] [PubMed] [Google Scholar]
- 24.Borger P, Schneider M, Frick L, et al. Exploration of the transcriptional landscape of ALPPS reveals the pathways of accelerated liver regeneration. Front Oncol. 2019; 9:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka K, Matsuo K, Murakami T, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol. 2015; 41:506–512 [DOI] [PubMed] [Google Scholar]
- 26.Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol. 2014; 25:742–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983; 10:176–182 [PubMed] [Google Scholar]
- 28.Guo W, Gao J, Zhuang W, et al. Efficacy and safety of hepatic arterial infusion chemotherapy combined with transarterial embolization for unresectable hepatocellular carcinoma: a propensity score-matching cohort study. JGH Open. 2020; 4:477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao S, Zhang PJ, Guo JH, et al. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol. 2015; 21:10443–10452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moradian S, Aledavood SA, Tabatabaee A. Iranian cancer patients and their perspectives: a qualitative study. Eur J Cancer Care (Engl). 2012; 21:377–383 [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Zhen R, Liao H, et al. Pharmacokinetics of continuous transarterial infusion of 5-fluorouracil in patients with advanced hepatocellular carcinoma. Oncol Lett. 2018; 15:7175–7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaldate RR, Haregewoin A, Grier CE, et al. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist. 2012; 17:296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu BJ, Gao S, Zhu X, et al. Sorafenib combined with embolization plus hepatic arterial infusion chemotherapy for inoperable hepatocellular carcinoma. World J Gastrointest Oncol. 2020; 12:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]


