Abstract
Artificial Intelligence (AI) has emerged as a transformative technology with immense potential in the field of medicine. By leveraging machine learning and deep learning, AI can assist in diagnosis, treatment selection, and patient monitoring, enabling more accurate and efficient healthcare delivery. The widespread implementation of AI in healthcare has the role to revolutionize patients’ outcomes and transform the way healthcare is practiced, leading to improved accessibility, affordability, and quality of care. This article explores the diverse applications and reviews the current state of AI adoption in healthcare. It concludes by emphasizing the need for collaboration between physicians and technology experts to harness the full potential of AI.
Keywords: artificial intelligence, machine learning, deep learning, clinical applications, digital pathology
1. Introduction
Artificial intelligence is increasingly being used as a virtual tool in many countries around the world. With its ability to mimic human cognitive functions, AI has revolutionized industries, improved efficiency, and unlocked new possibilities. During the past few years, governments have adopted a variety of smart applications that can use AI and its subsets provide predictions and recommendations in various fields, such as healthcare, finance, agriculture, education, social media, and data security.
Since the outbreak of COVID-19 in 2019, AI technologies have experienced accelerated adoption and utilization across various domains within the healthcare sector. In response to the pandemic, AI has emerged as a valuable tool and is being used for disease detection and diagnosis, medical imaging and analysis, treatment planning and personalized medicine, drug discovery and development, predictive analytics, and risk assessment. In 2018, Loh E. [1] stated that AI has the potential to significantly transform physicians’ roles and revolutionize the practice of medicine, and it is important for all doctors, in particular those in positions of leadership within the health system, to anticipate the potential changes, forecast their impact and make strategic plans for the medium to long term. In contrast, in 2021, Mistry C. et al. [2] assessed that the necessity for deploying advanced digital devices has become a requirement to offer augmented customer satisfaction, permitting tracking, checking the health status, and achieving better drug adherence.
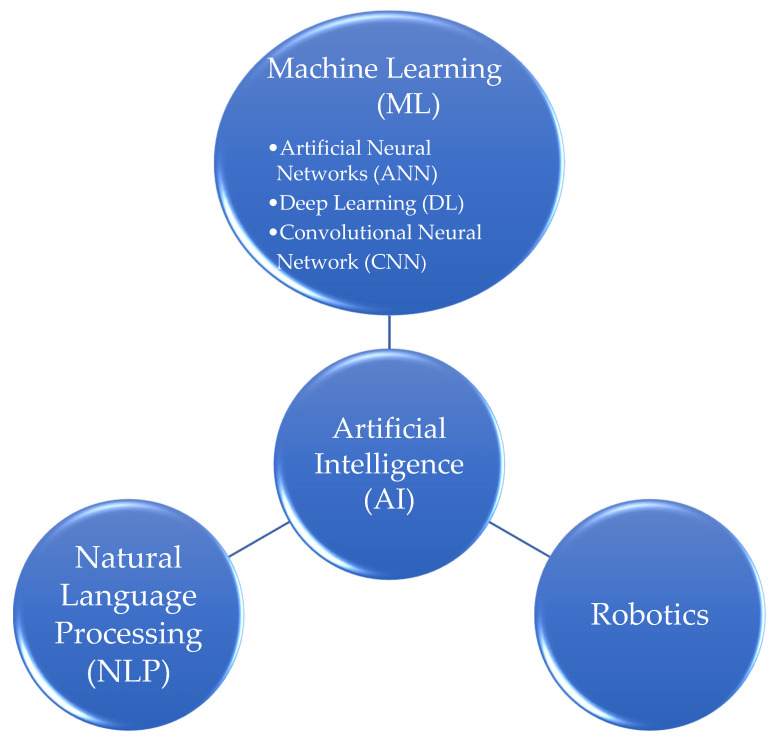
The field of AI is continuously evolving and researchers are exploring various avenues to create intelligent systems with different capabilities. The authors employed a visual representation, in the form of Figure 1, to illustrate the diverse subtypes of AI. Table 1 provides an overview of the definitions of terms related to AI and their integration within the healthcare sector.
Figure 1.
Illustration of the AI subtypes.
Table 1.
Definitions of terms related to AI.
| Term | Definition |
|---|---|
| Artificial Intelligence (AI) | The first definition was been given in 1950 by Alan Turing, the founding father of AI, as the science and engineering of making intelligent machines, especially intelligent computer programs [3]. According to Salto-Tellez M. et al. [4], AI represents a range of advanced machine technologies that can derive meaning and understanding from extensive data inputs, in ways that mimic human capabilities. In the present context of medical practice, a specific definition may be a system’s ability to correctly interpret external data, to learn from such data, and to use those learnings to achieve specific goals and tasks through flexible adaptation [5]. |
| Machine Learning (ML) | ML, a subset of artificial intelligence, exhibits the experiential “learning” associated with human intelligence, while also having the capacity to learn and improve its analyses through the use of computational algorithms [6,7]. Alpaydin E. [8] defined machine learning as the field of programming computers to optimize a performance criterion using example data or past experience. ML-based tools are used in the healthcare system to provide various treatment alternatives and individualized treatments and improve the overall efficiency of hospitals and healthcare systems while lowering the cost of care [9]. |
| Deep Learning (DL) | Deep Learning, a subset of Machine Learning, refers to a deep neural network, which is a specific configuration where neurons are organized in multiple successive layers that can independently learn representations of data and progressively extract complex features, performing tasks such as computer vision and natural language processing (NLP) [10]. In experimental settings across multiple medical specialties, DL performs equivalently to healthcare professionals for detecting diseases from medical imaging [11]. |
| Natural Language Processing (NLP) | Natural Language Processing is a theoretically-motivated range of computational techniques for analyzing and representing naturally-occurring texts at one or more levels of linguistic analysis for the purpose of achieving human-like language processing for a range of tasks or applications [12]. NLP techniques have been used to structure information in healthcare systems by extracting relevant information from narrative texts so as to provide data for decision-making [13]. |
| Robotics | The robot has been defined as “a reprogrammable multifunctional manipulator designed to move material, parts, tools, or specialized devices through variable programmed motions for the performance of a variety of tasks” by the Robot Institute of America [14]. The term “robotics” refers to the study and use of robots. Robotic assistance has been shown to improve the safety and performance of intracorporeal suturing, which is heavily required in urological and gynecological procedures [15]. |
| Artificial Neural Network (ANN) | An Artificial Neural Network, a subset of Machine Learning, is a computational model inspired by the biological neural networks in the human brain. These systems are mainly used for pattern identification and processing and are able to progressively improve their performance based on analytic results from previous tasks [16,17,18]. Many areas have been integrating the use of ANNs to facilitate the diagnosis, prognosis, and treatment of many diseases [19,20,21]. |
| Convolutional Neural Network (CNN) | A Convolutional Neural Network is a Deep Learning algorithm specifically designed for image and video processing, primarily used in medical image analysis and diagnostics. CNNs have demonstrated superior performance as compared with classical machine learning algorithms and in some cases achieved comparable or better performance than clinical experts [22]. |
2. Role of Artificial Intelligence in Healthcare
2.1. Disease Detection and Diagnosis and Medical Imaging
The application of AI within the diagnostic process supporting medical specialists could be of great value for the healthcare sector and the patients’ overall well-being [23]. The fundamental goal of the diagnosis of a disease lies in determining whether a patient is affected by a disease or not [24]. The first step in the diagnostic process involves obtaining a complete medical history and conducting a physical examination. For instance, a technique can use sound analysis to recognize COVID-19 from different respiratory sounds, e.g., cough, breathing, and voice [25]. Additionally, for a precise diagnosis, AI algorithms can be used for the analysis of medical scans and pathology images. Imaging applications include the determination of ejection fraction from echocardiograms [26], the detection and volumetric quantification of lung nodules from radiographs [27], and the detection and quantification of breast densities via mammography [28]. Imaging applications in pathology include an FDA-cleared system for whole-slide imaging (WSI) and their integration into a laboratory offers many benefits over light microscopy [29].
2.2. Treatment Planning and Personalized Medicine
AI tools have the ability to analyze large amounts of data and detect patterns. Therefore, they can make predictions for efficient and personalized treatment strategies. Personalized medicine, as an extension of medical sciences, uses practice and medical decisions to deliver customized healthcare services to patients [30]. For example, CURATE.AI is an AI-derived platform that maps the relationship between an intervention intensity (input-drug) and a phenotypic result (output) for an individual, based exclusively on that individual’s data, creating a profile, which serves as a map to predict the outcome for a specified input and to recommend the intervention intensity that will provide the best result [31].
2.3. Drug Discovery and Development
The use of AI has been increasing in the pharmaceutical industry, and as a result, it has reduced the human workload as well as achieved targets in a short period of time [32]. AI can recognize hit and lead compounds, and provide a quicker validation of the drug target and optimization of the drug structure design [33,34]. In January 2023, Insilico Medicine announced an encouraging topline readout of its phase 1 safety and pharmacokinetics trial of the molecule INS018_055, designed by AI for idiopathic pulmonary fibrosis, a progressive disease that causes scarring of the lungs [35].
2.4. Predictive Analytics and Risk Assessment
Disease risk assessment is the process of evaluating a person’s probability of developing certain diseases, based on risk factors such as genetic predispositions, environmental exposures, and lifestyle choices. AI techniques have been adopted to address the various steps involved in clinical genomic analysis—including variant calling, genome annotation, variant classification, and phenotype-to-genotype correspondence—and perhaps eventually they can also be applied to genotype-to-phenotype predictions [36]. Moreover, Ramazzotti et al. accomplished a successful prognosis prediction for 27 out of 36 cancers by employing AI to analyze various types of biological data such as RNA expression, point mutations, DNA methylation, and omics data of copy number variation. The data used for analysis was sourced from The Cancer Genome Atlas (TCGA) [37].
3. Literature Review
3.1. Methodology
We conducted a comprehensive review of current literature including original articles that studied various clinical applications of AI in healthcare. We performed extensive searches on Google Scholar, PubMed, and ScienceDirect databases to identify relevant manuscripts. As keywords, we used “artificial intelligence”, “deep learning”, and “machine learning”, combined with “clinical applications”, and “healthcare”. We restricted our search to papers published in English between 2013 and 2023 and found more than 200 relevant manuscripts. The inclusion criteria focused on studies that examined the application of artificial intelligence in different medical specialties. We excluded reviews and editorial comments.
3.2. Results
After a thorough review and assessment of the 223 articles, we identified and included a subset of 52 papers that were directly relevant to our research, including four on cardiology, three on dermatology, two on gastroenterology, three on neurology and neuroscience, three on ophthalmology, three on psychiatry, three on forensics and toxicology, four on radiology, 17 on pathology, two on urology, and four on obstetrics and gynecology, listed in Table 2. These selected studies provided valuable insights into the use and impact of AI in various medical specialties, forming the basis of our review.
Table 2.
Scientific articles that analyze the use of artificial intelligence in medical specialties.
| Medical Specialty | Year of Study | Author | Application |
|---|---|---|---|
| Cardiology | 2019 | Attia Z.I. [38] | Screening for cardiac contractile dysfunction |
| 2019 | Attia Z.I. [39] | Detection of left ventricular systolic dysfunction | |
| 2018 | Alsharqi M. [40] | Echocardiography analysis | |
| 2017 | Weng S.F. [41] | Cardiovascular risk prediction | |
| Dermatology | 2020 | Young A.T. [42] | Diagnosis of skin lesions |
| 2019 | Dick V. [43] | Diagnosis of melanoma | |
| 2017 | Esteva A. [44] | Classification of skin cancer | |
| Gastroenterology | 2021 | Kröner P.T. [45] | Detection of various lesions |
| 2020 | Martin D.R. [46] | Detecting current Helicobacter pylori infection |
|
| Neurology and Neuroscience | 2020 | Pedersen M. [47] | Diagnosis of neurological diseases |
| 2017 | Hazlett H.C. [48] | Diagnosis of autism | |
| 2020 | Ienca M. [49] | Diagnosis of Alzheimer’s disease | |
| Ophthalmology | 2017 | Rathi S. [50] | Teleophthalmology for retinopathy and glaucoma |
| 2016 | Gulshan V. [51] | Detection of diabetic retinopathy | |
| 2017 | Long E. [52] | Diagnosis of congenital cataracts | |
| Psychiatry | 2022 | Pham K.T. [53] | Classification of psychiatric disorders |
| 2017 | Vieira S. [54] | Classification of schizophrenia patients | |
| 2018 | Loh E. [1] | Prediction of suicide attempts | |
| Forensics and Toxicology | 2022 | Wankhade T.D. [55] | Detection of various samples |
| 2021 | Thurzo A. [56] | Identification of a cadaver | |
| 2020 | Chary M.A. [57] | Identification of drug use patterns | |
| Radiology | 2018 | Hosny A. [58] | Recognition of complex radiographic patterns |
| 2016 | Chen H. [59] | Detection in ultrasonography | |
| 2017 | Ghafoorian M. [60] | Segmentation in magnetic resonance imaging (MRI) | |
| 2017 | Wang H. [61] | Classification of mediastinal lymph node metastasis | |
| Surgery | 2020 | Zhou X.Y. [62] | Advances in surgery |
| 2018 | Hu Y. [63] | Robotic sewing and knot tying | |
| 2019 | Hu Y. [64] | Suturing robot for transanal endoscopic microsurgery | |
| 2016 | Shademan A. [65] | Robotic soft tissue surgery | |
| Pathology | 2021 | Cui M. [66] | Digitizing histopathology |
| 2019 | Niazi M.K.K. [67] | Whole-slide imaging | |
| 2017 | FDA [68] | IntelliSite Pathology Solution | |
| 2019 | FDA [69] | Summary Aperio AT2 DX system | |
| 2017 | Araújo T. [70] | Classification of breast cancer | |
| 2017 | Tumeh P.C. [71] | Identification of the immune cell populations | |
| 2019 | Bera K. [72] | Quantitative evaluation of histological and morphological patterns | |
| 2018 | Mezheyeuski A. [73] | Classification of lung cancer patients | |
| 2020 | Balázs A. [74] | Detection of metastasis and micrometastasis | |
| 2019 | Shaban M. [75] | Prediction of disease-free survival in oral squamous cell carcinoma | |
| 2019 | Hekler A. [76] | Classification of histopathological melanoma images | |
| 2014 | Dong F. [77] | Distinction between benign and malignant intraductal proliferations of the breast | |
| 2015 | Veta M. [78] | Mitosis detection in breast cancer | |
| 2013 | Cireşan D.C. [79] | Mitosis detection in breast cancer | |
| 2018 | Couture H.D. [80] | Prediction of breast cancer grade | |
| 2018 | Sahiner B. [81] | Application to Ki67 staining | |
| 2019 | Hossain M.S. [82] | Automatic quantification of HER2 gene amplification | |
| Urology | 2021 | Kott O. [83] | Diagnosis of prostate cancer and Gleason grading |
| 2020 | Baessler B. [84] | Detection of metastatic testicular germ cell tumors | |
| Obstetrics and Gynecology | 2015 | Idowu I. [85] | Detection of true labor and diagnosis of premature labor |
| 2013 | Manna C. [86] | Identification of most viable oocytes and embryos | |
| 2019 | Zhang L. [87] | Diagnosis of ovarian tumor | |
| 2020 | Hart G. [88] | Early detection of endometrial cancer |
3.2.1. AI in Cardiology
As Attia Z.I. et al. (2019) and Alsharqi M. et al. (2018) declared, using machine learning and deep learning, AI has been deployed to interpret echocardiograms, to automatically identify heart rhythms from an electrocardiogram (ECG), to uniquely identify an individual using the ECG as a biometric signal, and to detect the presence of heart disease such as left ventricular dysfunction from the surface ECG [38,39,40]. In a study conducted in China by Weng S.F. et al. between 2005 and 2015, using routine clinical data of over 350,000 patients, machine learning significantly improved the accuracy of cardiovascular risk prediction, correctly predicting 355 (an additional 7.6%) more patients who developed cardiovascular disease compared with the established algorithm [41].
3.2.2. AI in Dermatology
According to Young AT. et al. (2020), automated AI diagnosis of skin lesions is ready to be tested in clinical environments and has the potential to provide diagnostic support and expanded access to care [42]. A meta-analysis of 70 studies found the accuracy of computer-aided diagnosis of melanoma to be comparable to that of human experts [43]. In 2017, Esteva et al. supported the view that a convolutional neural network (CNN), the leading DL algorithm for image analysis, trained on 129,450 images, achieved performance comparable to dermatologists on two binary classification tasks, carcinomas versus seborrheic keratoses and melanomas versus nevi, for both dermoscopic and non-dermoscopic images [44].
3.2.3. AI in Gastroenterology
Kröner PT. et al. (2021) stated that the clinical applications of AI systems in gastroenterology and hepatology include the identification of premalignant or malignant lesions (e.g., identification of dysplasia or esophageal adenocarcinoma in Barrett’s esophagus, pancreatic malignancies), detection of lesions (e.g., polyp identification and classification, small-bowel bleeding lesion on capsule endoscopy, pancreatic cystic lesions), development of objective scoring systems for risk stratification, predicting disease prognosis or treatment response (e.g., determining survival in patients post-resection of hepatocellular carcinoma), determining which patients with inflammatory bowel disease (IBD) will benefit from biologic therapy, or evaluation of metrics such as bowel preparation score or quality of endoscopic examination [45]. A study conducted by Martin D.R. et al. (2020) using histopathologic images of gastric biopsies as an input had a diagnostic accuracy of 98.9–99.1% for detecting current Helicobacter pylori infection vs. 79.0–79.4% mean accuracy of endoscopists for detecting currently infected H. pylori in two studies [46].
3.2.4. AI in Neurology and Neuroscience
According to Pedersen M. (2020), AI has the potential to create a paradigm shift in the diagnosis, treatment, prediction, and economics of neurological disease [47]. Hazlett HC. et al. (2017) stated that a deep learning algorithm used magnetic resonance imaging (MRI) of the brain of individuals 6 to 12 months old to predict the diagnosis of autism in individual high-risk children at 24 months, with a positive predictive value of 81% [48]. Moreover, Ienca M. and Ignatiadis K. (2020) emphasized that the use of pattern recognition and feature extraction algorithms, for example, can significantly contribute to diagnosing brain diseases, such as brain tumors or Alzheimer’s disease, earlier, more accurately, and at more treatable stages compared to conventional predictive models [49].
3.2.5. AI in Ophthalmology
Rathi S. et al. (2017) declared that teleophthalmology has been well established to aid in the detection of retinopathy of prematurity (ROP), diabetic retinopathy screening, and is being explored for glaucoma screening and other fields of ophthalmology [50]. Furthermore, Gulshan V. et al. (2016) demonstrated the clinical utility of a deep machine-learning algorithm that evaluated retinal fundus photographs from adults that detected referable diabetic retinopathy with high sensitivity and specificity [51]. Long E. et al. (2017) showed that an AI agent, using deep learning and neural networks, accurately diagnosed and provided treatment decisions for congenital cataracts in a multihospital clinical trial, performing just as well as individual ophthalmologists [52].
3.2.6. AI in Psychiatry
The emerging literature has shown that AI is proving to be useful in psychological medicine and psychiatry. According to Pham KT. et al. (2022), within the last two decades, AI began to incorporate neuroimaging studies of psychiatric patients with deep learning models to classify patients with psychiatric disorders [53]. Vieira S. et al. (2017) were able to classify schizophrenia patients and controls with an accuracy of 85.5% by extracting functional connectivity patterns from resting-state functional MRIs of schizophrenia patients and healthy controls [54]. Researchers at the Vanderbilt University Medical Centre created machine-learning algorithms that achieved 80–90% accuracy when predicting whether someone will attempt suicide within the next 2 years, and 92% accuracy in predicting whether someone will attempt suicide within the next week [1].
3.2.7. AI in Forensics and Toxicology
Forensic medicine and toxicology are important branches of the investigation of crimes. In 2022, Wankhade TD. et al. stated that various procedures of forensic medicine such as analysis of toxins, collection of the various samples of medicolegal importance from body cavities, detection of pathological changes in various organs of the body, detection of various stains on the body, detection of a weapon used in crime, time since death calculations, etc. are the areas where AI will play a key role in framing the various opinions of medicolegal importance [55]. For example, according to Thurzo A. et al. (2021), three-dimensional convolutional neural networks (3D CNN) of artificial intelligence can be used in biological age determination, sex determination, automatized 3D cephalometric landmark annotation, soft-tissue face prediction from the skull and in reverse, and facial growth vectors prediction [56].
In toxicology, deep learning might automatically identify high-level drug use patterns by combining data from social media, poison control logs, published reports, and national surveys [57].
3.2.8. AI in Radiology
According to Hosny A. et al. (2018), AI methods automatically recognize complex patterns in imaging data and provide quantitative, rather than qualitative, assessments of radiographic characteristics [58]. Chen, H et al. (2016) maintained that studies have also shown that deep learning technologies are on par with radiologists’ performance for both detection [59] and segmentation [60] tasks in ultrasonography and MRI, respectively. Additionally, Wang, H. et al. (2017) declared that for the classification tasks of lymph node metastasis in PET–CT (positron emission tomography-computed tomography), deep learning had higher sensitivities but lower specificities than radiologists [61].
3.2.9. AI in Surgery
According to Zhou, XY. et al. (2020), advances in surgery have revolutionized the management of both acute and chronic diseases, prolonging life and extending the boundary of patient survival [62]. Moreover, current robots can already automatically perform some simple surgical tasks, such as suturing and knot tying [63,64]. For example, in 2016, a smart surgical robot stitched up a pig’s small intestines completely on its own and was able to outperform human surgeons who were given the same task [65].
3.2.10. AI in Pathology
In the modern healthcare system, AI and Digital Pathology (DP) have the potential to challenge traditional practice and provide precision for pathology diagnostics. Cui M., and Zhang D.Y. (2021) defined DP as the process of digitizing histopathology, immunohistochemistry, or cytology slides using whole-slide scanners as well as the interpretation, management, and analysis of these images using computational approaches [66]. According to Niazi M. K. K. et al. (2019), whole-slide imaging (WSI) allows entire slides to be imaged and permanently stored at high resolution, a process that provides a vast amount of information, which can be shared for clinical use or telepathology [67]. Two scanners, the Philips IntelliSite Pathology Solution (PIPS) and Leica Aperio AT2 DX, are approved by the Food and Drug Administration (FDA) to review and interpret digital surgical pathology slides prepared from biopsied tissue [68,69].
The use of digital image analysis in pathology can identify and quantify specific cell types quickly and accurately and can quantitatively evaluate histological features, morphological patterns, and biologically relevant regions of interest [72,73,74]. As Balázs et al. (2020) declared, recent groundbreaking results have demonstrated that applications of machine learning methods in pathology significantly improve Ki67 scoring in breast cancer, Gleason grading in prostate cancer, and tumor-infiltrating lymphocyte (TIL) scoring in melanoma [74]. Shaban et al. (2019) trained a novel CNN system to quantify TILs from WSIs of oral squamous cell carcinomas and achieved an accuracy of 96% [75]. Furthermore, Hekler A. et al. conducted a study in 2019 which concluded that a CNN was able to outperform 11 histopathologists in the classification of histopathological melanoma images and thus shows promise to assist human melanoma diagnoses [76]. Table 3 summarize the applications of AI systems in pathology.
Table 3.
Examples of AI systems applications in pathology.
| Examples of AI Systems Applications in Pathology |
|---|
| 1. Differentiate between benign and malignant tumors |
| 2. Grading of dysplasia and in situ lesions [70] |
| 3. Metastasis and micrometastasis detection [74] |
| 4. Relationships between different immune cell populations [70,71] |
| 5. IHC/ISH scoring of multiple biomarkers and topography of the immune response [72] |
| 6. Mitosis detection [78,79] |
In 2014, Dong et al. designed a computational pathology method to identify and quantify nuclear features from diagnostic tumor regions of interest (ROIs) of intraductal proliferative lesions of the breast, with high accuracy for distinguishing between benign breast ductal hyperplasia and malignant ductal carcinoma in situ [77]. Moreover, Coutre et al. (2018) used image analysis with DL to detect breast cancer histologic subtypes [80]. In addition, AI algorithms have been developed to provide quantitative measurements of immunohistochemically stained Ki-67 [81], ER [80], PR, and Her-2/neu images [82].
3.2.11. AI in Urology
AI applications in urology include: utilizing radiomic imaging or ultrasonic echo data to improve or automate cancer detection or outcome prediction, utilizing digitized tissue specimen images to automate detection of cancer on pathology slides, and combining patient clinical data, biomarkers, or gene expression to assist disease diagnosis or outcome prediction [89]. For example, Kott et al. tested an AI-based system for detecting prostate cancer which yielded 91.5% accuracy in classifying slides as either benign or malignant, and 85.4% accuracy in finer classifications of benign vs. Gleason 3 vs. 4 vs. 5 [83]. In another study, Baessler et al. applied ML-based CT radiomics to determine whether the lymph nodes dissected in patients with metastatic or advanced non-seminomatous testicular germ cell tumor were benign or malignant, with 88% sensitivity, and 72% specificity [84].
3.2.12. AI in Obstetrics and Gynecology
AI in Obstetrics
The fields of prenatal diagnosis, labor, and high-risk pregnancy are areas of significant importance in medicine, and they can be associated with medicolegal issues. Studies show that AI tools can be used to reduce these issues and to improve patients’ outcomes (both mothers’ and newborns’). In a study conducted by Idowu et al. [85], electrohysterography signals were employed, and three distinct machine learning algorithms were utilized to assist in the accurate detection of true labor, and the reliable diagnosis of premature labor. In another study, Manna et al. [86] proposed a method that combines AI and ANNs to extract texture descriptors from oocyte or embryo images. This approach enables AI to effectively identify the most viable oocytes and embryos, increasing the likelihood of successful pregnancies.
AI in Gynecology
Numerous research investigations focusing on cervical cancer and cervical intraepithelial neoplasia (CIN) have documented the application of AI. The primary areas where AI has been employed include the assessment of colposcopy, MR imaging (MRI), CT scans, cytology, and data related to human papillomavirus (HPV) [90]. Additionally, Zhang et al. [87] demonstrated in their research that using deep learning on color ultrasound tests as imaging assessments resulted in an impressive accuracy of 0.99 in predicting the definitive diagnosis of ovarian tumors. Moreover, Hart G. et al. emphasized that the application of machine learning shows immense potential in aiding the early detection of endometrial cancer. This approach achieves high-accuracy predictions by primarily relying on personal health information even before the onset of the disease, eliminating the necessity for invasive or costly procedures such as endometrial biopsy [88].
4. Discussion and Challenges
The literature review underscores the remarkable potential of AI in different medical specialties, to revolutionize screening and diagnostic procedures, and therefore, improving patient care. AI can improve diagnostic accuracy while limiting errors and impact patient safety such as assisting with prescription delivery [91,92,93]. Nevertheless, there are some challenges that need to be considered as AI usage increases in healthcare, such as ethical, social and technical challenges. For example, AI processes may lack transparency, making accountability problematic, or may be biased, leading to unfair, discriminatory behavior or mistaken decisions [94]. Moreover, AI algorithms are unable to perform a holistic approach to clinical scenarios and are not fully able to take into consideration the psychological and social aspects of human nature, which are often considered by a skilled healthcare professional [95]. Addressing those challenges requires collaboration between healthcare professionals, researchers, policymakers and technology developers to ensure that AI tools are implemented responsibly, ethically and safely in the healthcare sector.
5. Conclusions
Artificial intelligence systems powered by machine learning and deep learning are rapidly implemented in medicine. Moreover, combining AI with actual knowledge in various medical specialties could result in dramatic changes, such as advanced diagnostics, correct risk and prognosis evaluation, and even treatment suggestions. Thus far, AI is proving to be effective and the research will continue to improve, as more applications are discovered and explored. The integration of digital pathology based on AI systems in our current practice will help enhance patient care. In conclusion, AI’s role in medicine will continue to expand. In collaboration with experts in technology and ethics, we can revolutionize health care, making it more precise and we can pave the way for a healthier future with the right implementations of AI.
Author Contributions
Methodology, data curation, writing—original draft preparation, D.G.P.; writing—review and editing, C.L.M., A.I.N., M.N., A.F. and A.I.P.; supervision, conceptualization and funding, I.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the “Dunărea de Jos” University of Galati, VAT 27232142, and The APC was paid by the “Dunărea de Jos” University of Galati, VAT 27232142.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Loh E. Medicine and the rise of the robots: A qualitative review of recent advances of artificial intelligence in health. BMJ Lead. 2018;2:59–63. doi: 10.1136/leader-2018-000071. [DOI] [Google Scholar]
- 2.Mistry C., Thakker U., Gupta R., Obaidat M.S., Tanwar S., Kumar N., Rodrigues J.J.P.C. MedBlock: An AI-Enabled and Blockchain-Driven Medical Healthcare System for COVID-19; Proceedings of the IEEE International Conference Communication; Montreal, QC, Canada. 14–23 June 2021; pp. 1–6. [Google Scholar]
- 3.Turing A.M. I. Computing machinery and intelligence. Mind. 1950;236:433–460. doi: 10.1093/mind/LIX.236.433. [DOI] [Google Scholar]
- 4.Salto-Tellez M., Maxwell P., Hamilton P. Artificial intelligence-the third revolution in pathology. Histopathology. 2019;74:372–376. doi: 10.1111/his.13760. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan A., Haenlein M. Siri, Siri, in my hand: Who’s the fairest in the land? On the interpretations, illustrations, and implications of artificial intelligence. Bus. Horiz. 2019;62:15–25. doi: 10.1016/j.bushor.2018.08.004. [DOI] [Google Scholar]
- 6.Bini S.A. Artificial intelligence, machine learning, deep learning, and cognitive computing: What do these terms mean and how will they impact health care? J. Arthroplast. 2018;33:2358–2361. doi: 10.1016/j.arth.2018.02.067. [DOI] [PubMed] [Google Scholar]
- 7.Naylor C.D. On the prospects for a (deep) learning health care system. JAMA. 2018;320:1099–1100. doi: 10.1001/jama.2018.11103. [DOI] [PubMed] [Google Scholar]
- 8.Alpaydin E. Introduction to Machine Learning. 3rd ed. The MIT Press; Cambridge, MA, USA: 2014. p. 3. [Google Scholar]
- 9.Javaid M., Haleem A., Singh R.P., Suman R., Rab S. Significance of machine learning in healthcare: Features, pillars and applications. Int. J. Intell. Netw. 2022;3:58–73. doi: 10.1016/j.ijin.2022.05.002. [DOI] [Google Scholar]
- 10.LeCun Y., Bengio Y., Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 11.Liu X., Faes L., Kale A.U., Wagner S.K., Fu D.J., Bruynseels A., Mahendiran T., Moraes G., Shamdas M., Kern C., et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit. Health. 2019;1:e271–e297. doi: 10.1016/S2589-7500(19)30123-2. [DOI] [PubMed] [Google Scholar]
- 12.Liddy E.D. Encyclopedia of Library and Information Science. 2nd ed. Marcel Decker, Inc.; New York, NY, USA: 2001. Natural Language Processing. [Google Scholar]
- 13.Iroju O.G., Olaleke J.O. A Systematic Review of Natural Language Processing in Healthcare. Int. J. Inf. Technol. Comput. Sci. 2015;7:44–50. doi: 10.5815/ijitcs.2015.08.07. [DOI] [Google Scholar]
- 14.Bann S., Khan M., Hernandez J., Munz Y., Moorthy K., Datta V., Rockall T., Darzi A. Robotics in Surgery. J. Am. Coll. Surg. 2003;196:784–795. doi: 10.1016/S1072-7515(02)01750-7. [DOI] [PubMed] [Google Scholar]
- 15.Hussain A., Malik A., Halim M.U., Ali A.M. The use of robotics in surgery: A review. Int. J. Clin. Pract. 2014;68:1376–1382. doi: 10.1111/ijcp.12492. [DOI] [PubMed] [Google Scholar]
- 16.Jain A.K., Mao J., Mohiuddin K.M. Artificial neural networks: A tutorial. Computer. 1996;29:31–44. doi: 10.1109/2.485891. [DOI] [Google Scholar]
- 17.Papik K., Molnár B., Schaefer R., Dombóvári Z., Tulassay Z., Féher J. Application of neural networks in medicine—A review. Med. Sci. Monit. 1998;4:538–546. [Google Scholar]
- 18.Abraham T.H. Integrating mind and brain: Warren S. McCulloch, cerebral localization, and experimental epistemology. Endeav. 2003;27:32–36. doi: 10.1016/S0160-9327(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 19.Itchhaporia D., Snow P.B., Almassy R.J., Oetgen W.J. Artificial neural networks: Current status in cardiovascular medicine. J. Am. Coll. Cardiol. 1996;28:515–521. doi: 10.1016/S0735-1097(96)00174-X. [DOI] [PubMed] [Google Scholar]
- 20.Baxt W.G. Application of artificial neural networks to clinical medicine. Lancet. 1995;346:1135–1138. doi: 10.1016/S0140-6736(95)91804-3. [DOI] [PubMed] [Google Scholar]
- 21.Lisboa P.J., Taktak A.F. The use of artificial neural networks in decision support in cancer: A systematic review. Neural Netw. 2006;19:408–415. doi: 10.1016/j.neunet.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Chassagnon G., Vakalopolou M., Paragios N., Revel M.-P. Deep learning: Definition and perspectives for thoracic imaging. Eur. Radiol. 2020;30:2021–2030. doi: 10.1007/s00330-019-06564-3. [DOI] [PubMed] [Google Scholar]
- 23.Mirbabaie M., Stieglitz S., Frick N.R.J. Artificial intelligence in disease diagnostics: A critical review and classification on the current state of research guiding future direction. Health Technol. 2021;11:693–731. doi: 10.1007/s12553-021-00555-5. [DOI] [Google Scholar]
- 24.Ransohoff D.F., Feinstein A.R. Problems of Spectrum and Bias in Evaluating the Efficacy of Diagnostic Tests. N. Engl. J. Med. 1978;299:926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 25.Lella K.K., Pja A. A literature review on COVID-19 disease diagnosis from respiratory sound data. AIMS Bioeng. 2021;8:140–153. doi: 10.3934/bioeng.2021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asch F.M., Abraham T., Jankowski M., Cleve J., Adams M., Romano N., Polivert N., Hong H., Lang R. Accuracy and reproducibility of a novel artificial intelligence deep learning-based algorithm for automated calculation of ejection fraction in echocardiography. J. Am. Coll. Cardiol. 2019;73((Suppl. S1)):1447. doi: 10.1016/S0735-1097(19)32053-4. [DOI] [Google Scholar]
- 27.Retson T.A., Besser A.H., Sall S., Golden D., Hsiao A. Machine Learning and Deep Neural Networks in Thoracic and Cardiovascular Imaging. J. Thorac. Imaging. 2019;34:192–201. doi: 10.1097/RTI.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le E.P.V., Wang Y., Huang Y., Hickman S., Gilbert F.J. Artificial intelligence in breast imaging. Clin. Radiol. 2019;74:357–366. doi: 10.1016/j.crad.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Evans A.J., Bauer T.W., Bui M.M., Cornish T.C., Duncan H., Glassy E.F., Hipp J., McGee R.S., Murphy D., Myers C., et al. US Food and Drug Administration approval of whole slide imaging for primary diagnosis: A key milestone is reached and new questions are raised. Arch. Pathol. Lab. Med. 2018;142:1383–1387. doi: 10.5858/arpa.2017-0496-CP. [DOI] [PubMed] [Google Scholar]
- 30.Awwalu J., Garba A.G., Ghazvini A., Atuah R. Artificial intelligence in personalized medicine application of AI algorithms in solving personalized medicine problems. Int. J. Comput. Theory Eng. 2015;7:439–443. doi: 10.7763/IJCTE.2015.V7.999. [DOI] [Google Scholar]
- 31.Blasiak A., Khong J., Kee T. CURATE.AI: Optimizing Personalized Medicine with Artificial Intelligence. Slas Technol. 2020;25:95–105. doi: 10.1177/2472630319890316. [DOI] [PubMed] [Google Scholar]
- 32.Paul D., Sanap G., Shenoy S., Kalyane D., Kalia K., Tekade R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today. 2021;26:80–93. doi: 10.1016/j.drudis.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak K.K., Pichika M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today. 2019;24:773–780. doi: 10.1016/j.drudis.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Sellwood M.A. Artificial intelligence in drug discovery. Fut. Sci. 2018;10:2025–2028. doi: 10.4155/fmc-2018-0212. [DOI] [PubMed] [Google Scholar]
- 35.Arnold C. Inside the nascent industry of AI-designed drugs. Nat. Med. 2023;29:1292–1295. doi: 10.1038/s41591-023-02361-0. [DOI] [PubMed] [Google Scholar]
- 36.Dias R., Torkamani A. Artificial intelligence in clinical and genomic diagnostics. Genome Med. 2019;11:70. doi: 10.1186/s13073-019-0689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramazzotti D., Lal A., Wang B., Batzoglou S., Sidow A. Multi-omic tumor data reveal diversity of molecular mechanisms that correlate with survival. Nat. Commun. 2018;9:4453. doi: 10.1038/s41467-018-06921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attia Z.I., Kapa S., Lopez-Jimenez F., McKie P.M., Ladewig D.J., Satam G., Pellikka P.A., Enriquez-Sarano M., Noseworthy P.A., Munger T.M., et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019;25:70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 39.Attia Z.I., Kapa S., Yao X., Lopez-Jimenez F., Mohan T.L., Pellikka P.A., Carter R.E., Shah N.D., Friedman P.A., Noseworthy P.A. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019;30:668–674. doi: 10.1111/jce.13889. [DOI] [PubMed] [Google Scholar]
- 40.Alsharqi M., Woodward W.J., Mumith J.A., Markham D.C., Upton R., Leeson P. Artificial intelligence and echocardiography. Echo Res. Pract. 2018;5:R115–R125. doi: 10.1530/ERP-18-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng S.F., Reps J., Kai J., Garibaldi J.M., Qureshi N. Can machine learning improve cardiovascular risk prediction using routine clinical data? PLoS ONE. 2017;12:e0174944. doi: 10.1371/journal.pone.0174944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young A.T., Xiong M., Pfau J., Keiser M.J., Wei M.L. Artificial Intelligence in Dermatology: A Primer. J. Investig. Dermatol. 2020;140:1504–1512. doi: 10.1016/j.jid.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Dick V., Sinz C., Mittlböck M., Kittler H., Tschandl P. Accuracy of Computer-Aided Diagnosis of Melanoma: A Meta-analysis. JAMA Dermatol. 2019;155:1291–1299. doi: 10.1001/jamadermatol.2019.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esteva A., Kuprel B., Novoa R.A., Ko J., Swetter S.M., Blau H.M., Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kröner P.T., Engels M.M., Glicksberg B.S., Johnson K.W., Mzaik O., van Hooft J.E., Wallace M.B., El-Serag H.B., Krittanawong C. Artificial intelligence in gastroenterology: A state-of-the-art review. World J. Gastroenterol. 2021;27:6794–6824. doi: 10.3748/wjg.v27.i40.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin D.R., Hanson J.A., Gullapalli R.R., Schultz F.A., Sethi A., Clark D.P. A Deep Learning Convolutional Neural Network Can Recognize Common Patterns of Injury in Gastric Pathology. Arch. Pathol. Lab. Med. 2020;144:370–378. doi: 10.5858/arpa.2019-0004-OA. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen M., Verspoor K., Jenkinson M., Law M., Abbott D.F., Jackson G.D. Artificial intelligence for clinical decision support in neurology. Brain Commun. 2020;2:fcaa096. doi: 10.1093/braincomms/fcaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazlett H.C., Gu H., Munsell B.C., Kim S.H., Styner M., Wolff J.J., Elison J.T., Swanson M.R., Zhu H., Botteron K.N., et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ienca M., Ignatiadis K. Artificial Intelligence in Clinical Neuroscience: Methodological and Ethical Challenges. AJOB Neurosci. 2020;11:77–87. doi: 10.1080/21507740.2020.1740352. [DOI] [PubMed] [Google Scholar]
- 50.Rathi S., Tsui E., Mehta N., Zahid S., Schuman J.S. The Current State of Teleophthalmology in the United States. Ophthalmology. 2017;124:1729–1734. doi: 10.1016/j.ophtha.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulshan V., Peng L., Coram M., Stumpe M.C., Wu D., Narayanaswamy A., Venugopalan S., Widner K., Madams T., Cuadros J., et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316:2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 52.Long E., Lin H., Liu Z., Wu X., Wang L., Jiang J., An Y., Lin Z., Li X., Chen J., et al. An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nat. Biomed. Eng. 2017;1:0024. doi: 10.1038/s41551-016-0024. [DOI] [Google Scholar]
- 53.Pham K.T., Nabizadeh A., Selek S. Artificial Intelligence and Chatbots in Psychiatry. Psychiatr. Q. 2022;93:249–253. doi: 10.1007/s11126-022-09973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira S., Pinaya W.H.L., Mechelli A. Using Deep Learning to Investigate the Neuroimaging Correlates of Psychiatric and Neurological Disorders: Methods and Applications. Neurosci. Biobehav. Rev. 2017;74:58–75. doi: 10.1016/j.neubiorev.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Wankhade T.D., Ingale S.W., Mohite P.M., Bankar N.J. Artificial Intelligence in Forensic Medicine and Toxicology: The Future of Forensic Medicine. Cureus. 2022;14:e28376. doi: 10.7759/cureus.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thurzo A., Kosnáčová H.S., Kurilová V., Kosmeľ S., Beňuš R., Moravanský N., Kováč P., Kuracinová K.M., Palkovič M., Varga I. Use of Advanced Artificial Intelligence in Forensic Medicine, Forensic Anthropology and Clinical Anatomy. Healthcare. 2021;9:1545. doi: 10.3390/healthcare9111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chary M.A., Manini A.F., Boyer E.W., Burns M. The Role and Promise of Artificial Intelligence in Medical Toxicology. J. Med. Toxicol. 2020;16:458–464. doi: 10.1007/s13181-020-00769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosny A., Parmar C., Quackenbush J., Schwartz L.H., Aerts H.J.W.L. Artificial intelligence in radiology. Nat. Rev. Cancer. 2018;18:500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen H., Zheng Y., Park J.H., Heng P.A., Zhou S.K. Iterative Multi-Domain Regularized Deep Learning for Anatomical Structure Detection and Segmentation from Ultrasound Images; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Athens, Greece. 17–21 October 2016; pp. 487–495. [DOI] [Google Scholar]
- 60.Ghafoorian M., Karssemeijer N., Heskes T., van Uden I.W.M., Sanchez C.I., Litjens G., de Leeuw F.E., van Ginneken B., Marchiori E., Platel B. Location Sensitive Deep Convolutional Neural Networks for Segmentation of White Matter Hyperintensities. Sci. Rep. 2017;7:5110. doi: 10.1038/s41598-017-05300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H., Zhou Z., Li Y., Chen Z., Lu P., Wang W., Liu W., Yu L. Comparison of machine learning methods for classifying mediastinal lymph node metastasis of non-small cell lung cancer from 18F-FDG PET/CT images. EJNMMI Res. 2017;7:11. doi: 10.1186/s13550-017-0260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X.Y., Guo Y., Shen M., Yang G.Z. Application of artificial intelligence in surgery. Front. Med. 2020;14:417–430. doi: 10.1007/s11684-020-0770-0. [DOI] [PubMed] [Google Scholar]
- 63.Hu Y., Zhang L., Li W., Yang G.Z. Robotic Sewing and Knot Tying for Personalized Stent Graft Manufacturing; Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS); Madrid, Spain. 1–5 October 2018; pp. 754–760. [DOI] [Google Scholar]
- 64.Hu Y., Li W., Zhang L., Yang G.Z. Designing, prototyping, and testing a flexible suturing robot for transanal endoscopic microsurgery. IEEE Robot. Autom. Lett. 2019;4:1669–1675. doi: 10.1109/LRA.2019.2896883. [DOI] [Google Scholar]
- 65.Shademan A., Decker R.S., Opfermann J.D., Leonard S., Krieger A., Kim P.C.W. Supervised autonomous robotic soft tissue surgery. Sci. Transl. Med. 2016;8:337ra64. doi: 10.1126/scitranslmed.aad9398. [DOI] [PubMed] [Google Scholar]
- 66.Cui M., Zhang D.Y. Artificial intelligence and computational pathology. Lab. Investig. 2021;101:412–422. doi: 10.1038/s41374-020-00514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niazi M.K.K., Parwani A.V., Gurcan M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019;20:e253–e261. doi: 10.1016/S1470-2045(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Food and Drug Administration IntelliSite Pathology Solution (PIPS, Philips Medical Systems) [(accessed on 8 June 2023)];2017 Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/intellisite-pathology-solution-pips-philips-medical-systems.
- 69.Food and Drug Administration 510(k) Summary Aperio AT2 DX System, U.S. Department of Health and Human Services (ed) 2019. [(accessed on 8 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K190332.pdf.
- 70.Araújo T., Aresta G., Castro E., Rouco J., Aguiar P., Eloy C., Polónia A., Campilho A. Classification of breast cancer histology images using convolutional neural networks. PLoS ONE. 2017;12:e0177544. doi: 10.1371/journal.pone.0177544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tumeh P.C., Hellmann M.D., Hamid O., Tsai K.K., Loo K.L., Gubens M.A., Rosenblum M., Harview C.L., Taube J.M., Handley N., et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bera K., Schalper K.A., Rimm D.L., Velcheti V., Madabhushi A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019;16:703–715. doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mezheyeuski A., Bergsland C.H., Backman M., Djureinovic D., Sjöblom T., Bruun J., Micke P. Multispectral imaging for quantitative and compartment-specific immune infiltrates reveals distinct immune profiles that classify lung cancer patients. J. Pathol. 2018;244:421–431. doi: 10.1002/path.5026. [DOI] [PubMed] [Google Scholar]
- 74.Balázs A., Rantalainen M., Hartman J. Artificial intelligence as the next step towards precision pathology. J. Intern. Med. 2020;288:62–81. doi: 10.1111/joim.13030. [DOI] [PubMed] [Google Scholar]
- 75.Shaban M., Khurram S.A., Fraz M.M., Alsubaie N., Masood I., Mushtaq S., Hassan M., Loya A., Rajpoot N.M. A Novel Digital Score for Abundance of Tumour Infiltrating Lymphocytes Predicts Disease Free Survival in Oral Squamous Cell Carcinoma. Sci. Rep. 2019;9:13341. doi: 10.1038/s41598-019-49710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hekler A., Utikal J.S., Enk A.H., Solass W., Schmitt M., Klode J., Schadendorf D., Sondermann W., Franklin C., Bestvater F., et al. Deep learning outperformed 11 pathologists in the classification of histopathological melanoma images. Eur. J. Cancer. 2019;118:91–96. doi: 10.1016/j.ejca.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Dong F., Irshad H., Oh E.Y., Lerwill M.F., Brachtel E.F., Jones N.C., Knoblauch N.W., Montaser-Kouhsari L., Johnson N.B., Rao L.K.F., et al. Computational pathology to discriminate benign from malignant intraductal proliferations of the breast. PLoS ONE. 2014;9:e114885. doi: 10.1371/journal.pone.0114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veta M., van Diest P.J., Willems S.M., Wang H., Madabhushi A., Cruz-Roa A., Gonzalez F., Larsen A.B., Vestergaard J.S., Dahl A.B., et al. Assessment of algorithms for mitosis detection in breast cancer histopathology images. Med. Image Anal. 2015;20:237–248. doi: 10.1016/j.media.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 79.Cireşan D.C., Giusti A., Gambardella L.M., Schmidhuber J. Mitosis detection in breast cancer histology images with deep neural networks. Pt 2Med. Image Comput. Comput.-Assist. Interv. 2013;16:411–418. doi: 10.1007/978-3-642-40763-5_51. [DOI] [PubMed] [Google Scholar]
- 80.Couture H.D., Williams L.A., Geradts J., Nyante S.J., Butler E.N., Marron J.S., Perou C.M., Troester M.A., Niethammer M. Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. NPJ Breast Cancer. 2018;4:30. doi: 10.1038/s41523-018-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahiner B., Tozbikian G., Lozanski G., Gurcan M., Senaras C. Creating synthetic digital slides using conditional generative adversarial networks: Application to Ki67 staining. Med. Imaging 2018 Digit. Pathol. 2018;10581:1058103. doi: 10.1117/12.2294999. [DOI] [Google Scholar]
- 82.Hossain M.S., Hanna M.G., Uraoka N., Nakamura T., Edelweiss M., Brogi E., Hameed M.R., Yamaguchi M., Ross D.S., Yagi Y. Automatic quantification of HER2 gene amplification in invasive breast cancer from chromogenic in situ hybridization whole slide images. J. Med. Imaging. 2019;6:047501. doi: 10.1117/1.JMI.6.4.047501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kott O., Linsley D., Amin A., Karagounis A., Jeffers C., Golijanin D., Gershman B. Development of a deep learning algorithm for the histopathologic diagnosis and Gleason grading of prostate cancer biopsies: A pilot study. Eur. Urol. Focus. 2021;7:347–351. doi: 10.1016/j.euf.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baessler B., Nestler T., Pinto dos Santos D., Paffenholz P., Zeuch V., Pfister D., Heidenreich A. Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur. Radiol. 2020;30:2334–2345. doi: 10.1007/s00330-019-06495-z. [DOI] [PubMed] [Google Scholar]
- 85.Idowu I.O., Fergus P., Hussain A., Dobbins C., Khalaf M., Eslava R.V.C., Keight R. Artificial Intelligence for Detecting Preterm Uterine Activity in Gynecology and Obstetric Care; Proceedings of the 2015 IEEE International Conference on Computer and Information Technology; Ubiquitous Computing and Communications; Dependable, Autonomic and Secure Computing; Pervasive Intelligence and Computing; Liverpool, UK. 26–28 October 2015; pp. 215–220. [DOI] [Google Scholar]
- 86.Manna C., Nanni L., Lumini A., Pappalardo S. Artificial intelligence techniques for embryo and oocyte classification. Reprod. Biomed. Online. 2013;26:42–49. doi: 10.1016/j.rbmo.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L., Huang J., Liu L. Improved deep learning network based in combination with cost-sensitive learning for early detection of ovarian cancer in color ultrasound detecting system. J. Med. Syst. 2019;43:251. doi: 10.1007/s10916-019-1356-8. [DOI] [PubMed] [Google Scholar]
- 88.Hart G.R., Yan V., Huang G.S., Liang Y., Nartowt B.J., Muhammad W., Deng J. Population-based screening for endometrial cancer: Human vs. machine intelligence. Front. Artif. Intell. 2020;3:539879. doi: 10.3389/frai.2020.539879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J., Remulla D., Nguyen J.H., Dua A., Liu Y., Dasgupta P., Hung A.J. Current status of artificial intelligence applications in urology and their potential to influence clinical practice. BJU Int. 2019;124:567–577. doi: 10.1111/bju.14852. [DOI] [PubMed] [Google Scholar]
- 90.Sone K., Toyohara Y., Taguchi A., Miyamoto Y., Tanikawa M., Uchino-Mori M., Iriyama T., Tsuruga T., Osuga Y. Application of artificial intelligence in gynecologic malignancies: A review. J. Obstet. Gynaecol. Res. 2021;47:2577–2585. doi: 10.1111/jog.14818. [DOI] [PubMed] [Google Scholar]
- 91.He J., Baxter S.L., Xu J., Xu J., Zhou X., Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang F., Jiang Y., Zhi H., Dong Y., Li H., Ma S., Wang Y. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017;2:230–243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alabdulkareem A. Artificial intelligence and dermatologists: Friends or foes? [(accessed on 15 July 2023)];J. Dermatol. Dermatol. Surg. 2019 23:57–60. doi: 10.4103/jdds.jdds_19_19. Available online: link.gale.com/apps/doc/A596299970/HRCA?u=anon~9a29d018&sid=googleScholar&xid=e1fd2a5d. [DOI] [Google Scholar]
- 94.Mittelstadt B.D., Allo P., Taddeo M., Wachter S., Floridi L. The ethics of algorithms: Mapping the debate. Big Data Soc. 2016;3:2053951716679679. doi: 10.1177/2053951716679679. [DOI] [Google Scholar]
- 95.Cabitza F., Rasoini R., Gensini G.F. Unintended Consequences of Machine Learning in Medicine. JAMA. 2017;318:517–518. doi: 10.1001/jama.2017.7797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.