Abstract
Eucalyptus spp. are extensively cultivated in southern China because of their adaptability and versatile timber production. Calonectria leaf blight caused by Calonectria species is considered a major threat to Eucalyptus trees planted in China. The GuangXi Zhuang Autonomous Region is the provincial region with the largest distribution of Eucalyptus plantations in China. The present study aimed to expound the species diversity and pathogenicity of Calonectria isolates obtained from the soil of Eucalyptus plantations in GuangXi. A total of 188 Calonectria isolates were recovered from the soil located close to Eucalyptus trees, and the isolates were identified based on the DNA sequence comparisons of the four partial regions of the translation elongation factor 1-alpha (tef1), β-tubulin (tub2), calmodulin (cmdA), and histone H3 (his3) genes. The isolates were identified as Calonectria aconidialis (74.5%), C. hongkongensis (21.3%), C. pseudoreteaudii (2.1%), C. kyotensis (1.6%), and C. chinensis (0.5%). The inoculation results indicated that 40 isolates representing five Calonectria species were pathogenic to the three Eucalyptus genotypes. Two inoculated experiments consistently showed that the longest lesions were produced by the isolates of C. aconidialis. Some isolates of C. aconidialis, C. hongkongensis, and C. kyotensis produced significantly longer lesions than the positive controls, but not the isolates of C. pseudoreteaudii or C. chinensis. These results indicated that Calonectria isolated from the soil may pose a threat to Eucalyptus plantations. Some Calonectria isolates of the same species differed significantly in their virulence in the tested Eucalyptus genotypes. The resistance of different Eucalyptus genotypes to Calonectria isolates within the same species was inconsistent. The inoculation results in this study suggested that many Calonectria isolates in each species had different levels of pathogenicity, and many Eucalyptus genotypes need to be tested to select disease-resistant Eucalyptus genetic materials in the future. The results of the present study enhance our knowledge of species diversity and the potential damage caused by Calonectria in the soil of Eucalyptus plantations. Our results also provide new insights into the breeding of disease-resistant Eucalyptus genotypes for controlling Calonectria leaf blight in China in the future.
Keywords: Calonectria leaf blight, Eucalyptus disease, fungal pathogen, pathogenicity, species diversity
1. Introduction
Eucalyptus L’Hér. (Myrtaceae Juss., Myrtales Juss. ex Bercht. and J.Presl), due to its rapid growth, robust adaptability, and broad applications, is extensively planted in tropical and subtropical regions in China [1]. Eucalyptus was originally introduced to China in 1890 as an ornamental plant [1]. The area covered by Eucalyptus plantations has increased exponentially, from 0.46 million hm2 in 1986 to 5.46 million hm2 in 2018 [2]. In China, Eucalyptus plantations are distributed mainly in GuangXi, GuangDong, YunNan, FuJian, SiChuan, and HaiNan Provinces (or Autonomous Regions). The GuangXi Zhuang Autonomous Region is the provincial region with the largest distribution of Eucalyptus plantations in this country [1]. The area of Eucalyptus plantations in GuangXi is 2.56 million hm2, which is 46.83% of the total area of Eucalyptus plantations in the country [3].
Over the past three decades, Eucalyptus plantations in China have experienced a significant threat of diseases [4,5,6]. Leaf blight caused by Calonectria De Not. species is considered one of the major threats to plantations [5,7,8,9]. Calonectria species primarily infect the leaves of the middle and lower parts of Eucalyptus trees, resulting in water-soaked spots. Under high temperatures and humidity, the spots gradually develop into extended necrotic areas, eventually causing the whole leaves to become blighted and fall off [5,7,9,10,11]. These species also cause cutting rot, damping-off, stem rot, and leaf rot in Eucalyptus nurseries [5,11]. Eucalyptus leaf blight caused by Calonectria species also occurs in other countries, including Australia, Brazil, India, Indonesia, Malaysia, Thailand, and Vietnam [10,12,13,14,15,16,17].
The genus Calonectria includes important pathogens that infect more than 335 plant species, distributed among nearly 100 plant families. These plants include forestry, agricultural, and horticultural crops [11,18,19]. In forestry, Calonectria species mainly attack the families Fabaceae Lindl., Myrtaceae, and Pinaceae Lindl. [11,18].
Calonectria species are soil-borne fungi and their microsclerotia can survive in the soil for extended periods [11]. Currently, 137 Calonectria species have been discovered worldwide [12,15,16,20,21,22,23]. Among these, 84 species have been isolated from the soil near agricultural crops, plantations, natural forests, and unknown forest types in Asia, Africa, North America, and South America [9,12,15,16,20,22,23,24,25,26,27].
Several Calonectria species isolated from blighted Eucalyptus leaves and soil in Eucalyptus plantations in China were pathogenic to the tested Eucalyptus genotypes [7,8,9,28,29]. Some of these species were acquired from diseased Eucalyptus tissues (leaves and branches) and soil close to these trees. In the present study, soil samples were obtained from Eucalyptus plantations in GuangXi. The purposes of this study were to (i) expound the species diversity of Calonectria isolated from these soil samples, and (ii) clarify the pathogenicity of Calonectria species on different Eucalyptus genotypes.
2. Materials and Methods
2.1. Sample Site, Collection, and Fungal Isolation
Soil samples were collected from Eucalyptus plantations between July and August 2019 in GuangXi, southern China. These plantations were located at seven sampling sites across four regions, BeiHai, QinZhou, FangchengGang, and ChongZuo Region (Figure 1, Table 1). The soil in the 3–5-year-old Eucalyptus plantations was relatively moist with thick layers of leaf litter. The upper 0–20 cm of the soil was extracted by removing the thick layers of leaf litter. Fifty-three to sixty-nine soil samples were randomly collected from each sample site (Table 1). The soil samples were first placed in plastic bags to maintain humidity and temperature and then transferred to a laboratory for fungal isolation and further molecular studies.
Figure 1.
Map of GuangXi Zhuang Autonomous Region showing sampling sites in this study. The seven sampling sites are indicated as letters A to G.
Table 1.
Soil samples and recovered Calonectria isolates from Eucalyptus plantations in this study.
| Site No. | Region | Location | GPS Coordinate | Number of Soil Samples | Number of Soil Samples with Calonectria | Percentage of Soil Samples with Calonectria |
|---|---|---|---|---|---|---|
| A | BeiHai | LongJiang Village, BaiSha Town, HePu County, BeiHai Region | 21°46′7.0464″ N, 109°39′27.5256″ E | 62 | 35 | 56.5% |
| B | BeiHai | DongXin Village, ShiWan Town, HePu County, BeiHai Region | 21°47′28.3848″ N, 109°13′14.2608″ E | 60 | 3 | 5.0% |
| C | QinZhou | ChangeDong Village, NaLi Town, QinNan District, QinZhou Region | 21°51′11.83″ N, 108°51′13.18″ E | 60 | 36 | 60.0% |
| D | QinZhou | TunNan Village, HuangwuTun Town, QinNan District, QinZhou Region | 21°58′22.41″ N, 108°29′16.78″ E | 53 | 33 | 62.3% |
| E | FangchengGang | SongBai Village, DongXing Town, FangchengGang Region | 21°34′53.37″ N, 108°04′20.53″ E | 69 | 39 | 56.5% |
| F | FangchengGang | NaYong Village, FangchengGang District, FangchengGang Region | 21°52′49.60″ N, 108°17′46.06″ E | 63 | 38 | 60.3% |
| G | ChongZuo | NaPo Village, FuSui County, ChongZuo Region | 22°35′06.39″ N, 107°57′19.75″ E | 61 | 4 | 6.6% |
| Total | 428 | 188 | 43.9% |
To induce Calonectria isolates, distilled water was utilized to moisten the soil samples in plastic cups. Medicago sativa L. (alfalfa) seeds were surface disinfested in 75% ethanol for 30 s and washed with distilled water. They were then placed on the surface of the moistened soil in plastic cups, as described by Crous [11]. The sampling cups with soil and alfalfa seeds were incubated at 25 °C under 12 h of daylight and 12 h of darkness. After 7 d, the sampling cups with soil and germinating alfalfa seedlings were observed under a dissection microscope. Calonectria isolates were distinguished from other fungi based on the typical morphological characteristics of conidiophores, macroconidia, and vesicles [11,18,30]. A single conidium was transferred from the conidiophores of Calonectria to a 2% (v/v) malt extract agar (MEA) (20 g of malt extract powder and 20 g of agar powder per liter of water) using sterile needles under a stereoscopic microscope. For each soil sample, a culture of one morphologically similar Calonectria isolate was retained for further studies. The obtained cultures were deposited in the culture collection (CSF) located at the Research Institute of Fast-growing Trees (RIFT) of the Chinese Academy of Forestry (CAF) in ZhanJiang, GuangDong Province, China.
2.2. DNA Extraction, PCR Amplification, and Sequencing
The DNA was extracted after the isolates were grown on MEA for 7–10 days. Mycelia were carefully scraped from the surface of the MEA culture medium using a sterilized scalpel and transferred to a 2 mL Eppendorf tube. Total genomic DNA was extracted according to “Extraction method 5: grinding and CTAB” protocols described by van Burik et al. [31]. The extracted DNA was dissolved in 30 µL of TE buffer (1 M Tris-HCl and 0.5 M EDTA, pH 8.0), and then 3 µL of RNase (10 mg/mL) was added at 37 °C for 1 h to degrade the RNA. In the final step, a Nano-Drop 2000 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the DNA concentration.
Consistent with previous studies, the use of four loci, partial gene regions of translation elongation factor 1-alpha (tef1), β-tubulin (tub2), calmodulin (cmdA), and histone H3 (his3), was successful in identifying Calonectria species [5,12,21,22,32,33,34]. These four partial gene regions were amplified using specific primer pairs: EF1-728F/EF2 for the tef1 gene region; fRpb2-5F/fRpb2-7cR or T1/CYLTUB1R for the tub2 gene region; CAL-228F/CAL-2Rd for the cmdA gene region; and CYLH3F/CYLH3R for the his3 gene region [22,24,35]. The PCR reaction mixtures contained 17.5 μL of TopTaq TM master mix, 1 μL of each primer (10 mM), 2 μL of the DNA sample, and RNase-free H2O adjusted to a final volume of 35 μL. The amplification was conducted according to the conditions described by Liu et al. [22].
All the PCR products were sequenced in both the forward and reverse directions of each primer pair at the Beijing Genomics Institute, GuangZhou, China. The sequences were manually edited using MEGA v. 6.0 software [36] and then submitted to GenBank (https://www.ncbi.nlm.nih.gov, accessed on 8 March 2023).
2.3. Phylogenetic Analyses
To preliminarily identify the isolates, a standard nucleotide BLAST search was performed using the tef1, tub2, cmdA, and his3 sequences. The sequences of the available species in the relevant species complexes were downloaded from NCBI for sequence comparisons and phylogenetic analyses. The alignment of sequences for each of the tef1, tub2, cmdA, and his3 gene regions, as well as the combination of these four gene regions, was performed online using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/, accessed on 8 March 2023) with alignment strategy FFT-NS-i (slow; interactive refinement method) [37]. The manual sequence adjustment was performed using MEGA v. 7 software [38].
The maximum likelihood (ML) and Bayesian inference (BI) methods were used for the phylogenetic analysis of the sequence datasets of each of the four gene regions and the combination of these regions. The optimal models of the five sequence datasets for BI analyses were determined using the jModelTest v. 2.1.5 [39]. ML analyses were performed using RaxML v. 8.2.12 [40] on the CIPRES Science Gateway v. 3.3, with the default GTR substitution matrix and 1000 bootstrap runs. The software MrBayes v. 3.2.7 [41] was used for BI analyses with CIPRES Science Gateway v. 3.3. Four Markov chain Monte Carlo (MCMC) chains were executed from a random starting tree for five million generations, and the trees were sampled every 100th generation. The first 25% of the trees were discarded as burn-in, and the rest of the trees were used to confirm the posterior probabilities. Phylogenetic trees were viewed using MEGA v. 7 [38] and FigTree v 1.4.2 for ML and BI trees, respectively. The sequence data for CBS 109167 and CBS 109168 (Curvicladiella cignea Decock and Crous) were treated as outgroups [22].
2.4. Pathogenicity Tests
Representative isolates of each Calonectria species identified in this study were selected for inoculation trials. Three Eucalyptus genotypes were selected for inoculation, E. urophylla S. T. Blake × E. tereticornis Sm. hybrid genotype CEPT1900 and E. urophylla × E. grandis W. Hill hybrid genotypes CEPT1901 and CEPT1902. All inoculated seedlings were similar in size, 3 months old, and approximately 40 cm in height.
Inoculation with mycelial plugs was performed as described by Wu and Chen [9]. For each Eucalyptus genotype, 10 mycelial plugs (5 mm diameter) from 7-day-old MEA cultures of each isolate were inoculated on the abaxial surface of the unwounded leaves of three Eucalyptus seedlings. Ten leaves from three different Eucalyptus seedlings treated with sterile MEA plugs were used as negative controls. The highly pathogenic Calonectria pseudoreteaudii L. Lombard, M.J. Wingf. and Crous, isolate CSF13317 of two Eucalyptus hybrid genotypes, E. urophylla × E. grandis genotype CEPT1878 and E. urophylla × E. tereticornis genotype CEPT1879, as confirmed in a previous study, was used as a positive control [29]. To ensure sufficient humidity for infection development, all Eucalyptus seedlings were placed in moist plastic chambers and maintained under stable climatic conditions (temperature 25–26 °C; humidity 60–70%) for three days. The plastic chambers were removed after three days. To measure the lesion length of each leaf, two diameter measurements of each lesion perpendicular to each other were conducted for each leaf, and the average lesion diameter was computed. The entire experiment was repeated using an identical methodology. The inoculations were conducted in July 2022 at the South China Experimental Nursery (SCEN), located in ZhanJiang, GuangDong Province, China.
To verify Koch’s postulates, re-isolations were conducted. Small pieces of discolored leaf tissue (approximately 0.04 cm2) from the periphery of the generated lesions were cut and placed on a 2% MEA at room temperature. For each inoculated isolate, four leaves of each Eucalyptus genotype were randomly selected, and all the leaves inoculated as positive and negative controls were re-isolated. The re-isolated fungi were identified and confirmed based on the morphological characteristics and disease symptoms exhibited by the leaves with the original fungi. Statistical analyses were performed by one-way analysis of variance (ANOVA) using SPSS Statistics 22 software (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Sample Collection and Fungal Isolation
A total of 428 soil samples were collected from seven sampling sites (A to G) in four regions in GuangXi (Figure 1, Table 1). The fungi with branched conidiophores producing cylindrical conidia and with stipe extensions terminating in a vesicle with a characteristic shape were grouped as Calonectria. A total of 188 soil samples, which accounted for 43.9% of all sampled soil samples, were positive for Calonectria isolates with branched conidiophores, cylindrical macroconidia, and sphaeropedunculate or clavate vesicles. For each sample, a single conidium culture was isolated from white masses of conidiophores with typical morphological characteristics of Calonectria species. In total, 188 Calonectria isolates were obtained from 188 soil samples. The percentage of soil samples that yielded Calonectria ranged from 5.0% to 62.3% at the seven sampling sites (Table 1).
3.2. Sequencing
DNA extraction and sequence comparisons of all 188 Calonectria isolates were performed (Table 2). The tef1, tub2, cmdA, and his3 gene regions of all 188 isolates were amplified. The obtained sequence fragments for the tef1, tub2, cmdA, and his3 gene regions were approximately 520, 600, 690, and 460 bp, respectively. Based on the sequences of the tef1, tub2, cmdA, and his3 loci, the genotypes of all 188 sequenced isolates were determined. A total of 32 genotypes were identified (Table 2).
Table 2.
Isolates obtained in this study used for phylogenetic analyses and pathogenicity tests.
| Species | Isolate No. a,b,c | Genotype d | Sampling Sites | Sample No. | Host | Collector | GenBank Accession No. e | |||
|---|---|---|---|---|---|---|---|---|---|---|
| tef1 | tub2 | cmdA | his3 | |||||||
| C. aconidialis | CSF16467 | AAAA | B | 20190704-2-(26) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261297 | OR261485 | OR261673 | OR261861 |
| C. aconidialis | CSF16470 b,c | AHAA | C | 20190704-3-(1) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261298 | OR261486 | OR261674 | OR261862 |
| C. aconidialis | CSF16473 | AFBA | C | 20190704-3-(2) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261299 | OR261487 | OR261675 | OR261863 |
| C. aconidialis | CSF16477 | AAAA | C | 20190704-3-(4) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261300 | OR261488 | OR261676 | OR261864 |
| C. aconidialis | CSF16479 | AFBA | C | 20190704-3-(5) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261301 | OR261489 | OR261677 | OR261865 |
| C. aconidialis | CSF16481 | AAAA | C | 20190704-3-(6) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261302 | OR261490 | OR261678 | OR261866 |
| C. aconidialis | CSF16484 | AAAA | C | 20190704-3-(11) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261303 | OR261491 | OR261679 | OR261867 |
| C. aconidialis | CSF16488 | AAAA | C | 20190704-3-(13) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261304 | OR261492 | OR261680 | OR261868 |
| C. aconidialis | CSF16490 | AHAA | C | 20190704-3-(14) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261305 | OR261493 | OR261681 | OR261869 |
| C. aconidialis | CSF16493 | AAAA | C | 20190704-3-(15) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261306 | OR261494 | OR261682 | OR261870 |
| C. aconidialis | CSF16499 | AACB | C | 20190704-3-(20) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261307 | OR261495 | OR261683 | OR261871 |
| C. aconidialis | CSF16502 | AAAA | C | 20190704-3-(25) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261308 | OR261496 | OR261684 | OR261872 |
| C. aconidialis | CSF16507 b,c | BAAA | C | 20190704-3-(27) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261309 | OR261497 | OR261685 | OR261873 |
| C. aconidialis | CSF16509 | BAAA | C | 20190704-3-(28) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261310 | OR261498 | OR261686 | OR261874 |
| C. aconidialis | CSF16511 | BAAA | C | 20190704-3-(29) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261311 | OR261499 | OR261687 | OR261875 |
| C. aconidialis | CSF16514 | AAAA | C | 20190704-3-(34) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261312 | OR261500 | OR261688 | OR261876 |
| C. aconidialis | CSF16518 | BAAA | C | 20190704-3-(37) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261313 | OR261501 | OR261689 | OR261877 |
| C. aconidialis | CSF16520 b,c | ABAA | C | 20190704-3-(38) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261314 | OR261502 | OR261690 | OR261878 |
| C. aconidialis | CSF16522 b,c | ADAA | C | 20190704-3-(41) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261315 | OR261503 | OR261691 | OR261879 |
| C. aconidialis | CSF16525 | AHAA | C | 20190704-3-(42) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261316 | OR261504 | OR261692 | OR261880 |
| C. aconidialis | CSF16527 b,c | AGBA | C | 20190704-3-(43) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261317 | OR261505 | OR261693 | OR261881 |
| C. aconidialis | CSF16530 | AAAA | C | 20190704-3-(45) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261318 | OR261506 | OR261694 | OR261882 |
| C. aconidialis | CSF16533 | BAAA | C | 20190704-3-(48) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261319 | OR261507 | OR261695 | OR261883 |
| C. aconidialis | CSF16535 | BAAA | C | 20190704-3-(49) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261320 | OR261508 | OR261696 | OR261884 |
| C. aconidialis | CSF16537 | AAAA | C | 20190704-3-(51) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261321 | OR261509 | OR261697 | OR261885 |
| C. aconidialis | CSF16539 | AGBA | C | 20190704-3-(52) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261322 | OR261510 | OR261698 | OR261886 |
| C. aconidialis | CSF16540 | AAAA | C | 20190704-3-(53) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261323 | OR261511 | OR261699 | OR261887 |
| C. aconidialis | CSF16542 | AAAA | C | 20190704-3-(54) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261324 | OR261512 | OR261700 | OR261888 |
| C. aconidialis | CSF16544 | AAAA | C | 20190704-3-(55) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261325 | OR261513 | OR261701 | OR261889 |
| C. aconidialis | CSF16546 b | ABAA | C | 20190704-3-(56) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261326 | OR261514 | OR261702 | OR261890 |
| C. aconidialis | CSF16549 | AHAA | C | 20190704-3-(57) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261327 | OR261515 | OR261703 | OR261891 |
| C. aconidialis | CSF16551 | AHAA | C | 20190704-3-(58) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261328 | OR261516 | OR261704 | OR261892 |
| C. aconidialis | CSF16552 | BAAA | C | 20190704-3-(59) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261329 | OR261517 | OR261705 | OR261893 |
| C. aconidialis | CSF16555 | AAAA | C | 20190704-3-(60) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261330 | OR261518 | OR261706 | OR261894 |
| C. aconidialis | CSF16557 b,c | CAAA | D | 20190704-4-(1) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261331 | OR261519 | OR261707 | OR261895 |
| C. aconidialis | CSF16561 | AAAA | D | 20190704-4-(2) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261332 | OR261520 | OR261708 | OR261896 |
| C. aconidialis | CSF16562 | BAAA | D | 20190704-4-(4) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261333 | OR261521 | OR261709 | OR261897 |
| C. aconidialis | CSF16564 | BAAA | D | 20190704-4-(5) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261334 | OR261522 | OR261710 | OR261898 |
| C. aconidialis | CSF16566 | AAAA | D | 20190704-4-(7) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261335 | OR261523 | OR261711 | OR261899 |
| C. aconidialis | CSF16568 | AAAA | D | 20190704-4-(8) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261336 | OR261524 | OR261712 | OR261900 |
| C. aconidialis | CSF16571 | AAAA | D | 20190704-4-(9) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261337 | OR261525 | OR261713 | OR261901 |
| C. aconidialis | CSF16573 | AAAA | D | 20190704-4-(10) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261338 | OR261526 | OR261714 | OR261902 |
| C. aconidialis | CSF16575 | AAAA | D | 20190704-4-(11) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261339 | OR261527 | OR261715 | OR261903 |
| C. aconidialis | CSF16578 | AAAA | D | 20190704-4-(12) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261340 | OR261528 | OR261716 | OR261904 |
| C. aconidialis | CSF16580 | AAAA | D | 20190704-4-(15) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261341 | OR261529 | OR261717 | OR261905 |
| C. aconidialis | CSF16582 b,c | BEAA | D | 20190704-4-(18) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261342 | OR261530 | OR261718 | OR261906 |
| C. aconidialis | CSF16584 b,c | BIAA | D | 20190704-4-(22) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261343 | OR261531 | OR261719 | OR261907 |
| C. aconidialis | CSF16586 | AAAA | D | 20190704-4-(23) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261344 | OR261532 | OR261720 | OR261908 |
| C. aconidialis | CSF16588 | BAAC | D | 20190704-4-(24) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261345 | OR261533 | OR261721 | OR261909 |
| C. aconidialis | CSF16591 b | AGBA | D | 20190704-4-(26) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261346 | OR261534 | OR261722 | OR261910 |
| C. aconidialis | CSF16593 | AAAA | D | 20190704-4-(27) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261347 | OR261535 | OR261723 | OR261911 |
| C. aconidialis | CSF16594 | BAAA | D | 20190704-4-(28) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261348 | OR261536 | OR261724 | OR261912 |
| C. aconidialis | CSF16597 | AAAA | D | 20190704-4-(32) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261349 | OR261537 | OR261725 | OR261913 |
| C. aconidialis | CSF16599 b,c | AACB | D | 20190704-4-(34) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261350 | OR261538 | OR261726 | OR261914 |
| C. aconidialis | CSF16602 | AACB | D | 20190704-4-(36) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261351 | OR261539 | OR261727 | OR261915 |
| C. aconidialis | CSF16604 | BAAA | D | 20190704-4-(37) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261352 | OR261540 | OR261728 | OR261916 |
| C. aconidialis | CSF16607 b | AACB | D | 20190704-4-(38) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261353 | OR261541 | OR261729 | OR261917 |
| C. aconidialis | CSF16609 b,c | AEAA | D | 20190704-4-(40) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261354 | OR261542 | OR261730 | OR261918 |
| C. aconidialis | CSF16612 | BAAA | D | 20190704-4-(44) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261355 | OR261543 | OR261731 | OR261919 |
| C. aconidialis | CSF16614 | AAAA | D | 20190704-4-(45) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261356 | OR261544 | OR261732 | OR261920 |
| C. aconidialis | CSF16618 | AACB | D | 20190704-4-(46) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261357 | OR261545 | OR261733 | OR261921 |
| C. aconidialis | CSF16621 | BAAA | D | 20190704-4-(47) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261358 | OR261546 | OR261734 | OR261922 |
| C. aconidialis | CSF16625 | BAAA | D | 20190704-4-(48) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261359 | OR261547 | OR261735 | OR261923 |
| C. aconidialis | CSF16627 b | AFBA | D | 20190704-4-(50) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261360 | OR261548 | OR261736 | OR261924 |
| C. aconidialis | CSF16631 | BAAA | D | 20190704-4-(51) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261361 | OR261549 | OR261737 | OR261925 |
| C. aconidialis | CSF16633 | AAAA | D | 20190704-4-(52) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261362 | OR261550 | OR261738 | OR261926 |
| C. aconidialis | CSF16640 | BAAC | E | 20190705-1-(4) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261363 | OR261551 | OR261739 | OR261927 |
| C. aconidialis | CSF16643 b,c | AGBD | E | 20190705-1-(10) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261364 | OR261552 | OR261740 | OR261928 |
| C. aconidialis | CSF16645 | AAAA | E | 20190705-1-(11) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261365 | OR261553 | OR261741 | OR261929 |
| C. aconidialis | CSF16648 b,c | BACA | E | 20190705-1-(12) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261366 | OR261554 | OR261742 | OR261930 |
| C. aconidialis | CSF16651 | BAAC | E | 20190705-1-(13) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261367 | OR261555 | OR261743 | OR261931 |
| C. aconidialis | CSF16653 b,c | BACB | E | 20190705-1-(14) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261368 | OR261556 | OR261744 | OR261932 |
| C. aconidialis | CSF16655 | AAAA | E | 20190705-1-(15) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261369 | OR261557 | OR261745 | OR261933 |
| C. aconidialis | CSF16657 | BAAB | E | 20190705-1-(17) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261370 | OR261558 | OR261746 | OR261934 |
| C. aconidialis | CSF16659 | BAAA | E | 20190705-1-(22) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261371 | OR261559 | OR261747 | OR261935 |
| C. aconidialis | CSF16661 | AAAA | E | 20190705-1-(23) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261372 | OR261560 | OR261748 | OR261936 |
| C. aconidialis | CSF16663 | AAAA | E | 20190705-1-(27) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261373 | OR261561 | OR261749 | OR261937 |
| C. aconidialis | CSF16666 b | AGBD | E | 20190705-1-(28) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261374 | OR261562 | OR261750 | OR261938 |
| C. aconidialis | CSF16668 | AAAA | E | 20190705-1-(31) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261375 | OR261563 | OR261751 | OR261939 |
| C. aconidialis | CSF16672 | AFBA | E | 20190705-1-(38) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261376 | OR261564 | OR261752 | OR261940 |
| C. aconidialis | CSF16675 b,c | AACA | E | 20190705-1-(39) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261377 | OR261565 | OR261753 | OR261941 |
| C. aconidialis | CSF16677 | BAAB | E | 20190705-1-(40) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261378 | OR261566 | OR261754 | OR261942 |
| C. aconidialis | CSF16682 | AAAA | E | 20190705-1-(43) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261379 | OR261567 | OR261755 | OR261943 |
| C. aconidialis | CSF16686 | AAAA | E | 20190705-1-(45) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261380 | OR261568 | OR261756 | OR261944 |
| C. aconidialis | CSF16689 | BAAB | E | 20190705-1-(46) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261381 | OR261569 | OR261757 | OR261945 |
| C. aconidialis | CSF16691 | AAAA | E | 20190705-1-(48) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261382 | OR261570 | OR261758 | OR261946 |
| C. aconidialis | CSF16693 b,c | BAAB | E | 20190705-1-(49) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261383 | OR261571 | OR261759 | OR261947 |
| C. aconidialis | CSF16695 | BAAB | E | 20190705-1-(50) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261384 | OR261572 | OR261760 | OR261948 |
| C. aconidialis | CSF16697 | BAAA | E | 20190705-1-(52) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261385 | OR261573 | OR261761 | OR261949 |
| C. aconidialis | CSF16702 | BAAA | E | 20190705-1-(54) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261386 | OR261574 | OR261762 | OR261950 |
| C. aconidialis | CSF16704 | BAAA | E | 20190705-1-(55) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261387 | OR261575 | OR261763 | OR261951 |
| C. aconidialis | CSF16706 b,c | BAAC | E | 20190705-1-(56) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261388 | OR261576 | OR261764 | OR261952 |
| C. aconidialis | CSF16707 | AAAA | E | 20190705-1-(58) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261389 | OR261577 | OR261765 | OR261953 |
| C. aconidialis | CSF16710 | AAAA | E | 20190705-1-(59) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261390 | OR261578 | OR261766 | OR261954 |
| C. aconidialis | CSF16712 | AAAA | E | 20190705-1-(60) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261391 | OR261579 | OR261767 | OR261955 |
| C. aconidialis | CSF16714 | AAAA | E | 20190705-1-(61) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261392 | OR261580 | OR261768 | OR261956 |
| C. aconidialis | CSF16716 | AAAA | E | 20190705-1-(62) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261393 | OR261581 | OR261769 | OR261957 |
| C. aconidialis | CSF16718 b,c | ACAA | E | 20190705-1-(64) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261394 | OR261582 | OR261770 | OR261958 |
| C. aconidialis | CSF16720 | BAAC | E | 20190705-1-(67) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261395 | OR261583 | OR261771 | OR261959 |
| C. aconidialis | CSF16722 | BAAA | E | 20190705-1-(69) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261396 | OR261584 | OR261772 | OR261960 |
| C. aconidialis | CSF16728 | AAAA | F | 20190705-2-(5) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261397 | OR261585 | OR261773 | OR261961 |
| C. aconidialis | CSF16729 | BAAA | F | 20190705-2-(8) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261398 | OR261586 | OR261774 | OR261962 |
| C. aconidialis | CSF16735 b | BAAA | F | 20190705-2-(14) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261399 | OR261587 | OR261775 | OR261963 |
| C. aconidialis | CSF16739 | AAAA | F | 20190705-2-(17) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261400 | OR261588 | OR261776 | OR261964 |
| C. aconidialis | CSF16742 b,c | AFBA | F | 20190705-2-(18) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261401 | OR261589 | OR261777 | OR261965 |
| C. aconidialis | CSF16751 | AGBA | F | 20190705-2-(21) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261402 | OR261590 | OR261778 | OR261966 |
| C. aconidialis | CSF16760 | BAAA | F | 20190705-2-(26) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261403 | OR261591 | OR261779 | OR261967 |
| C. aconidialis | CSF16762 | AFBA | F | 20190705-2-(27) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261404 | OR261592 | OR261780 | OR261968 |
| C. aconidialis | CSF16767 | BAAA | F | 20190705-2-(29) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261405 | OR261593 | OR261781 | OR261969 |
| C. aconidialis | CSF16770 | AFBA | F | 20190705-2-(30) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261406 | OR261594 | OR261782 | OR261970 |
| C. aconidialis | CSF16774 | AAAA | F | 20190705-2-(31) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261407 | OR261595 | OR261783 | OR261971 |
| C. aconidialis | CSF16779 | AAAA | F | 20190705-2-(33) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261408 | OR261596 | OR261784 | OR261972 |
| C. aconidialis | CSF16788 | AAAA | F | 20190705-2-(38) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261409 | OR261597 | OR261785 | OR261973 |
| C. aconidialis | CSF16792 b | BAAC | F | 20190705-2-(41) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261410 | OR261598 | OR261786 | OR261974 |
| C. aconidialis | CSF16809 b | BAAB | F | 20190705-2-(55) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261411 | OR261599 | OR261787 | OR261975 |
| C. aconidialis | CSF17104 | AHAA | A | 20190806-2-(1) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261412 | OR261600 | OR261788 | OR261976 |
| C. aconidialis | CSF17110 b,c | AAAA | A | 20190806-2-(5) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261413 | OR261601 | OR261789 | OR261977 |
| C. aconidialis | CSF17112 | BAAA | A | 20190806-2-(7) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261414 | OR261602 | OR261790 | OR261978 |
| C. aconidialis | CSF17114 | AAAA | A | 20190806-2-(8) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261415 | OR261603 | OR261791 | OR261979 |
| C. aconidialis | CSF17116 | AHAA | A | 20190806-2-(9) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261416 | OR261604 | OR261792 | OR261980 |
| C. aconidialis | CSF17130 c | AAAA | A | 20190806-2-(24) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261417 | OR261605 | OR261793 | OR261981 |
| C. aconidialis | CSF17133 | AAAA | A | 20190806-2-(25) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261418 | OR261606 | OR261794 | OR261982 |
| C. aconidialis | CSF17135 | AHAA | A | 20190806-2-(27) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261419 | OR261607 | OR261795 | OR261983 |
| C. aconidialis | CSF17137 | AAAA | A | 20190806-2-(28) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261420 | OR261608 | OR261796 | OR261984 |
| C. aconidialis | CSF17140 | AAAA | A | 20190806-2-(31) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261421 | OR261609 | OR261797 | OR261985 |
| C. aconidialis | CSF17142 b,c | AAAA | A | 20190806-2-(38) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261422 | OR261610 | OR261798 | OR261986 |
| C. aconidialis | CSF17144 | AAAA | A | 20190806-2-(41) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261423 | OR261611 | OR261799 | OR261987 |
| C. aconidialis | CSF17146 | AAAA | A | 20190806-2-(42) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261424 | OR261612 | OR261800 | OR261988 |
| C. aconidialis | CSF17150 | AAAA | A | 20190806-2-(44) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261425 | OR261613 | OR261801 | OR261989 |
| C. aconidialis | CSF17153 | AAAA | A | 20190806-2-(45) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261426 | OR261614 | OR261802 | OR261990 |
| C. aconidialis | CSF17155 | AAAA | A | 20190806-2-(46) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261427 | OR261615 | OR261803 | OR261991 |
| C. aconidialis | CSF17158 | AAAA | A | 20190806-2-(47) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261428 | OR261616 | OR261804 | OR261992 |
| C. aconidialis | CSF17160 | AAAA | A | 20190806-2-(49) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261429 | OR261617 | OR261805 | OR261993 |
| C. aconidialis | CSF17163 b | AHAA | A | 20190806-2-(51) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261430 | OR261618 | OR261806 | OR261994 |
| C. aconidialis | CSF17166 | BAAA | A | 20190806-2-(52) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261431 | OR261619 | OR261807 | OR261995 |
| C. aconidialis | CSF17169 | AAAA | A | 20190806-2-(53) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261432 | OR261620 | OR261808 | OR261996 |
| C. aconidialis | CSF17172 | AAAA | A | 20190806-2-(54) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261433 | OR261621 | OR261809 | OR261997 |
| C. aconidialis | CSF17181 | AAAA | A | 20190806-2-(59) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261434 | OR261622 | OR261810 | OR261998 |
| C. aconidialis | CSF17184 | AAAA | A | 20190806-2-(60) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261435 | OR261623 | OR261811 | OR261999 |
| C. aconidialis | CSF17187 | AAAA | A | 20190806-2-(61) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261436 | OR261624 | OR261812 | OR262000 |
| C. hongkongensis | CSF16463 b,c | AGAA | B | 20190704-2-(6) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261437 | OR261625 | OR261813 | OR262001 |
| C. hongkongensis | CSF16464 | AAAA | B | 20190704-2-(14) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261438 | OR261626 | OR261814 | OR262002 |
| C. hongkongensis | CSF16486 | AAAA | C | 20190704-3-(12) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261439 | OR261627 | OR261815 | OR262003 |
| C. hongkongensis | CSF16611 | AAAA | D | 20190704-4-(41) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261440 | OR261628 | OR261816 | OR262004 |
| C. hongkongensis | CSF16637 | AAAA | E | 20190705-1-(3) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261441 | OR261629 | OR261817 | OR262005 |
| C. hongkongensis | CSF16670 | AAAA | E | 20190705-1-(36) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261442 | OR261630 | OR261818 | OR262006 |
| C. hongkongensis | CSF16680 | AAAA | E | 20190705-1-(42) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261443 | OR261631 | OR261819 | OR262007 |
| C. hongkongensis | CSF16699 | AAAA | E | 20190705-1-(53) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261444 | OR261632 | OR261820 | OR262008 |
| C. hongkongensis | CSF16726 b,c | AAAA | F | 20190705-2-(3) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261445 | OR261633 | OR261821 | OR262009 |
| C. hongkongensis | CSF16731 b,c | AABA | F | 20190705-2-(11) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261446 | OR261634 | OR261822 | OR262010 |
| C. hongkongensis | CSF16733 | AAAA | F | 20190705-2-(13) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261447 | OR261635 | OR261823 | OR262011 |
| C. hongkongensis | CSF16737 b,c | ABAA | F | 20190705-2-(16) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261448 | OR261636 | OR261824 | OR262012 |
| C. hongkongensis | CSF16745 | AAAA | F | 20190705-2-(19) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261449 | OR261637 | OR261825 | OR262013 |
| C. hongkongensis | CSF16748 | AAAA | F | 20190705-2-(20) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261450 | OR261638 | OR261826 | OR262014 |
| C. hongkongensis | CSF16754 b,c | BAAA | F | 20190705-2-(22) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261451 | OR261639 | OR261827 | OR262015 |
| C. hongkongensis | CSF16756 c | AAAA | F | 20190705-2-(25) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261452 | OR261640 | OR261828 | OR262016 |
| C. hongkongensis | CSF16765 b,c | AFAA | F | 20190705-2-(28) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261453 | OR261641 | OR261829 | OR262017 |
| C. hongkongensis | CSF16781 b,c | ADAA | F | 20190705-2-(35) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261454 | OR261642 | OR261830 | OR262018 |
| C. hongkongensis | CSF16786 b,c | AEAA | F | 20190705-2-(36) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261455 | OR261643 | OR261831 | OR262019 |
| C. hongkongensis | CSF16790 | AAAA | F | 20190705-2-(39) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261456 | OR261644 | OR261832 | OR262020 |
| C. hongkongensis | CSF16795 | AAAA | F | 20190705-2-(44) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261457 | OR261645 | OR261833 | OR262021 |
| C. hongkongensis | CSF16797 | AAAA | F | 20190705-2-(46) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261458 | OR261646 | OR261834 | OR262022 |
| C. hongkongensis | CSF16803 | AAAA | F | 20190705-2-(49) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261459 | OR261647 | OR261835 | OR262023 |
| C. hongkongensis | CSF16805 | AAAA | F | 20190705-2-(52) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261460 | OR261648 | OR261836 | OR262024 |
| C. hongkongensis | CSF16811 | AAAA | F | 20190705-2-(56) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261461 | OR261649 | OR261837 | OR262025 |
| C. hongkongensis | CSF16813 b | ABAA | F | 20190705-2-(57) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261462 | OR261650 | OR261838 | OR262026 |
| C. hongkongensis | CSF16816 | AAAA | F | 20190705-2-(58) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261463 | OR261651 | OR261839 | OR262027 |
| C. hongkongensis | CSF16819 | AAAA | F | 20190705-2-(62) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261464 | OR261652 | OR261840 | OR262028 |
| C. hongkongensis | CSF16821 | AAAA | G | 20190705-4-(5) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261465 | OR261653 | OR261841 | OR262029 |
| C. hongkongensis | CSF16823 b,c | AAAB | G | 20190705-4-(14) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261466 | OR261654 | OR261842 | OR262030 |
| C. hongkongensis | CSF17107 | AAAA | A | 20190806-2-(4) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261467 | OR261655 | OR261843 | OR262031 |
| C. hongkongensis | CSF17118 b,c | ACAA | A | 20190806-2-(11) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261468 | OR261656 | OR261844 | OR262032 |
| C. hongkongensis | CSF17120 | AAAA | A | 20190806-2-(13) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261469 | OR261657 | OR261845 | OR262033 |
| C. hongkongensis | CSF17122 | AAAA | A | 20190806-2-(18) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261470 | OR261658 | OR261846 | OR262034 |
| C. hongkongensis | CSF17125 b,c | AAAA | A | 20190806-2-(19) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261471 | OR261659 | OR261847 | OR262035 |
| C. hongkongensis | CSF17127 | AAAA | A | 20190806-2-(23) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261472 | OR261660 | OR261848 | OR262036 |
| C. hongkongensis | CSF17148 | AAAA | A | 20190806-2-(43) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261473 | OR261661 | OR261849 | OR262037 |
| C. hongkongensis | CSF17174 | AAAA | A | 20190806-2-(55) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261474 | OR261662 | OR261850 | OR262038 |
| C. hongkongensis | CSF17176 b | ACAA | A | 20190806-2-(56) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261475 | OR261663 | OR261851 | OR262039 |
| C. hongkongensis | CSF17178 | AAAA | A | 20190806-2-(57) | soil under 4-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, L.L. Liu, Y. Liu, Y.C. Qu, Y.L. Li & X.Y. Liang | OR261476 | OR261664 | OR261852 | OR262040 |
| C. pseudoreteaudii | CSF16497 | AAAA | C | 20190704-3-(16) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261477 | OR261665 | OR261853 | OR262041 |
| C. pseudoreteaudii | CSF16505 b,c | AAAA | C | 20190704-3-(26) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261478 | OR261666 | OR261854 | OR262042 |
| C. pseudoreteaudii | CSF16635 c | AAAA | E | 20190705-1-(2) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261479 | OR261667 | OR261855 | OR262043 |
| C. pseudoreteaudii | CSF16826 b,c | AAAA | G | 20190705-4-(20) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261480 | OR261668 | OR261856 | OR262044 |
| C. kyotensis | CSF16724 b,c | AAAA | F | 20190705-2-(1) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261481 | OR261669 | OR261857 | OR262045 |
| C. kyotensis | CSF16776 c | AAAA | F | 20190705-2-(32) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261482 | OR261670 | OR261858 | OR262046 |
| C. kyotensis | CSF16801 b,c | AAAA | F | 20190705-2-(47) | soil under 5-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261483 | OR261671 | OR261859 | OR262047 |
| C. chinensis | CSF16829 b,c | AAAA | G | 20190705-4-(21) | soil under 3-year-old E. urophylla × E. grandis | S.F. Chen, Q.C. Wang, W.X. Wu, Y.X. Zheng & L.F. Liu | OR261484 | OR261672 | OR261860 | OR262048 |
a CSF: Culture collection located at the Research Institute of Fast-growing Trees (RIFT), Chinese Academy of Forestry, ZhanJiang, GuangDong Province, China. b Isolates used for phylogenetic analyses. c Isolates used for pathogenicity tests. d Genotype within each Calonectria species, determined by sequences of the tef1, tub2, cmdA, and his3 regions. e tef1 = translation elongation factor 1-alpha; tub2 = β-tubulin; cmdA = calmodulin; his3 = histone H3.
3.3. Phylogenetic Analyses
For the 188 isolates sequenced in this study, one to two isolates of each genotype determined by tef1, tub2, cmdA, and his3 sequences were selected for phylogenetic analyses. A total of 47 representative isolates representing 32 genotypes were selected (Table 2). The sequences of 69 isolates presenting 40 published Calonectria species closely related to the Calonectria isolates obtained in the present study were downloaded from GenBank and used for phylogenetic analyses based on four individual gene regions and the combination of those regions (Table 3).
Table 3.
Isolates from other studies used for phylogenetic analyses in this study.
| Species Code a | Species | Isolate No. b,c | Other Collection Number c | Host | Area of Occurrence | Collector | GenBank Accession Numbers d | References or Source of Data | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cmdA | his3 | tef1 | tub2 | ||||||||
| B1 |
Calonectria
acaciicola |
CMW 47173T | CBS 143557 | Soil (Acacia auriculiformis
plantation) |
Do Luong, Nghe An, Vietnam | N.Q. Pham & T.Q. Pham | MT335160 | MT335399 | MT412690 | MT412930 | [15,22] |
| CMW 47174 | CBS 143558 | Soil (A. auriculiformis plantation) | Do Luong, Nghe An, Vietnam | N.Q. Pham & T.Q. Pham | MT335161 | MT335400 | MT412691 | MT412931 | [15,22] | ||
| B2 | C. acicola | CMW 30996T | – | Phoenix canariensis | Northland, New Zealand | H. Pearson | MT335162 | MT335401 | MT412692 | MT412932 | [22,34,42] |
| CBS 114812 | CMW 51216 | P. canariensis | Northland, New Zealand | H. Pearson | MT335163 | MT335402 | MT412693 | MT412933 | [22,34,42] | ||
| B4 | C. aconidialis | CMW 35174T | CBS 136086; CERC 1850 |
Soil (Eucalyptus plantation) | HaiNan, China | X. Mou & S.F. Chen | MT335165 | MT335404 | MT412695 | OK357463 | [22,24,43] |
| CMW 35384 | CBS 136091; CERC 1886 |
Soil (Eucalyptus plantation) | HaiNan, China | X. Mou & S.F. Chen | MT335166 | MT335405 | MT412696 | OK357464 | [22,24,43] | ||
| B5 | C. aeknauliensis | CMW 48253T | CBS 143559 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT335180 | MT335419 | MT412710 | OK357465 | [15,22,24] |
| CMW 48254 | CBS 143560 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT335181 | MT335420 | MT412711 | OK357466 | [15,22,24] | ||
| B8 | C. asiatica | CBS 114073T | CMW 23782; CPC 3900 |
Debris (leaf litter) | Prathet Thai, Thailand | N.L. Hywel-Jones | AY725741 | AY725658 | AY725705 | AY725616 | [34,44] |
| B10 | C. australiensis | CMW 23669T | CBS 112954; CPC 4714 |
Ficus pleurocarpa | Queensland, Australia | C. Pearce & B. Paulus | MT335192 | MT335432 | MT412723 | MT412946 | [22,34,45] |
| B17 | C. brassicicola | CBS 112841T | CMW 51206; CPC 4552 |
Soil at Brassica sp. | Indonesia | M.J. Wingfield | KX784561 | N/A e | KX784689 | KX784619 | [26] |
| B19 | C. bumicola | CMW 48257T | CBS 143575 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT335205 | MT335445 | MT412736 | OK357467 | [15,22,24] |
| B20 | C. canadiana | CMW 23673T | CBS 110817; STE-U 499 |
Picea sp. | Canada | S. Greifenhagen | MT335206 | MT335446 | MT412737 | MT412958 | [11,22,46,47] |
| CERC 8952 | – | Soil | HeNan, China | S.F. Chen | MT335290 | MT335530 | MT412821 | MT413035 | [22,32] | ||
| B23 | C. chinensis | CMW 23674T | CBS 114827; CPC 4101 |
Soil | Hong Kong, China | E.C.Y. Liew | MT335220 | MT335460 | MT412751 | MT412972 | [22,34,44] |
| CMW 30986 | CBS 112744; CPC 4104 |
Soil | Hong Kong, China | E.C.Y. Liew | MT335221 | MT335461 | MT412752 | MT412973 | [22,34,44] | ||
| B26 | C. cochinchinensis | CMW 49915T | CBS 143567 | Soil (Hevea brasiliensis plantation) | Duong Minh Chau, Tay Ninh, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT335225 | MT335465 | MT412756 | MT412977 | [15,22] |
| CMW 47186 | CBS 143568 | Soil (A. auriculiformis plantation) | Song May, Dong Nai, Vietnam | N.Q. Pham & T.Q. Pham | MT335226 | MT335466 | MT412757 | MT412978 | [15,22] | ||
| B29 | C. colombiensis | CMW 23676T | CBS 112220; CPC 723 |
Soil (E. grandis trees) | La Selva, Colombia | M.J. Wingfield | MT335228 | MT335468 | MT412759 | MT412980 | [22,44] |
| CMW 30985 | CBS 112221; CPC 724 |
Soil (E. grandis trees) | La Selva, Colombia | M.J. Wingfield | MT335229 | MT335469 | MT412760 | MT412981 | [22,44] | ||
| B30 | C. crousiana | CMW 27249T | CBS 127198 | E. grandis | FuJian, China | M.J. Wingfield | MT335230 | MT335470 | MT412761 | MT412982 | [22,48] |
| CMW 27253 | CBS 127199 | E. grandis | FuJian, China | M.J. Wingfield | MT335231 | MT335471 | MT412762 | MT412983 | [22,48] | ||
| B31 | C. curvispora | CMW 23693T | CBS 116159; CPC 765 |
Soil | Tamatave, Madagascar | P.W. Crous | MT335232 | MT335472 | MT412763 | OK357468 | [11,22,24,34,43,49] |
| CMW 48245 | CBS 143565 | Soil (Eucalyptus plantation) | Aek Nauli, North Sumatra, Indonesia | M.J. Wingfield | MT335233 | MT335473 | MT412764 | N/A | [15,22] | ||
| B46 | C. heveicola | CMW 49913T | CBS 143570 | Soil (Hevea brasiliensis plantation) | Bau Bang, Binh Duong, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT335255 | MT335495 | MT412786 | MT413004 | [15,22] |
| CMW 49928 | CBS 143571 | Soil | Bu Gia Map National Park, Binh Phuoc, Vietnam | N.Q. Pham, Q.N. Dang & T.Q. Pham | MT335280 | MT335520 | MT412811 | MT413025 | [15,22] | ||
| B48 | C. hongkongensis | CBS 114828T | CMW 51217; CPC 4670 |
Soil | Hong Kong, China | M.J. Wingfield | MT335258 | MT335498 | MT412789 | MT413007 | [22,44] |
| CERC 3570 | CMW 47271 | Soil (Eucalyptus plantation) | BeiHai, GuangXi, China | S.F. Chen, J.Q. Li & G.Q. Li | MT335260 | MT335500 | MT412791 | MT413009 | [21,22] | ||
| B51 | C. ilicicola | CMW 30998T | CBS 190.50; IMI 299389; STE-U 2482 |
Solanum tuberosum | Bogor, Java, Indonesia | K.B. Boedijn & J. Reitsma | MT335266 | MT335506 | MT412797 | OK357469 | [11,22,24,34,50] |
| B52 | C. indonesiae | CMW 23683T | CBS 112823; CPC 4508 |
Syzygium aromaticum | Warambunga, Indonesia | M.J. Wingfield | MT335267 | MT335507 | MT412798 | MT413015 | [22,44] |
| CBS 112840 | CMW 51205; CPC 4554 |
S. aromaticum | Warambunga, Indonesia | M.J. Wingfield | MT335268 | MT335508 | MT412799 | MT413016 | [22,44] | ||
| B55 | C. kyotensis | CBS 114525T | ATCC 18834; CMW 51824; CPC 2367 |
Robinia pseudoacacia | Japan | T. Terashita | MT335271 | MT335511 | MT412802 | MT413019 | [11,22,26,51] |
| CBS 114550 | CMW 51825; CPC 2351 |
Soil | China | M.J. Wingfield | MT335246 | MT335486 | MT412777 | MT412995 | [22,26] | ||
| B57 | C. lantauensis | CERC 3302T | CBS 142888; CMW 47252 |
Soil | LiDao, Hong Kong, China | M.J. Wingfield & S.F. Chen | MT335272 | MT335512 | MT412803 | OK357470 | [21,22,24] |
| CERC 3301 | CBS 142887; CMW 47251 |
Soil | LiDao, Hong Kong, China | M.J. Wingfield & S.F. Chen | MT335273 | MT335513 | MT412804 | OK357471 | [21,22,24] | ||
| B58 | C. lateralis | CMW 31412T | CBS 136629 | Soil (Eucalyptus plantation) | GuangXi, China | X. Zhou, G. Zhao & F. Han | MT335274 | MT335514 | MT412805 | MT413020 | [22,43] |
| B63 | C. lombardiana | CMW 30602T | CBS 112634; CPC 4233; Lynfield 417 |
Xanthorrhoea australis | Victoria, Australia | T. Baigent | MT335395 | MT335635 | MT412926 | MT413133 | [11,22,35,45] |
| B66 | C. malesiana | CMW 23687T | CBS 112752; CPC 4223 |
Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335286 | MT335526 | MT412817 | MT413031 | [22,44] |
| CBS 112710 | CMW 51199; CPC 3899 |
Leaf litter | Prathet, Thailand | N.L. Hywel-Jones | MT335287 | MT335527 | MT412818 | MT413032 | [22,44] | ||
| B74 | C. multiseptata | CMW 23692T | CBS 112682; CPC 1589 |
E. grandis | North Sumatra, Indonesia | M.J. Wingfield | MT335299 | MT335539 | MT412830 | MT413044 | [22,34,44] |
| B80 | C. pacifica | CMW 16726T | A1568; CBS 109063; IMI 354528; STE-U 2534 |
Araucaria heterophylla | Hawaii, USA | M. Aragaki | MT335311 | MT335551 | MT412842 | OK357472 | [11,22,24,44,46] |
| CMW 30988 | CBS 114038 | Ipomoea aquatica | Auckland, New Zealand | C.F. Hill | MT335312 | MT335552 | MT412843 | OK357473 | [11,22,34,44] | ||
| B86 | C. penicilloides | CMW 23696T | CBS 174.55; STE-U 2388 |
Prunus sp. | Hatizyo Island, Japan | M. Ookubu | MT335338 | MT335578 | MT412869 | MT413081 | [11,22,52] |
| B97 | C. pseudoreteaudii | CMW 25310T | CBS 123694 | E. urophylla × E. grandis | GuangDong, China | M.J. Wingfield & X.D. Zhou | MT335354 | MT335594 | MT412885 | MT413096 | [22,35] |
| CMW 25292 | CBS 123696 | E. urophylla × E. grandis | GuangDong, China | M.J. Wingfield & X.D. Zhou | MT335355 | MT335595 | MT412886 | MT413097 | [22,35] | ||
| B104 | C. queenslandica | CMW 30604T | CBS 112146; CPC 3213 |
E. urophylla | Lannercost, Queensland, Australia | B. Brown | MT335367 | MT335607 | MT412898 | MT413108 | [22,35,53] |
| CMW 30603 | CBS 112155; CPC 3210 |
E. pellita | Lannercost, Queensland, Australia | P.Q Thu & K.M. Old | MT335368 | MT335608 | MT412899 | MT413109 | [22,35,53] | ||
| B106 | C. reteaudii | CMW 30984T | CBS 112144; CPC 3201 |
E. camaldulensis | Chon Thanh, Binh Phuoc, Vietnam | M.J. Dudzinski & P.Q. Thu | MT335370 | MT335610 | MT412901 | MT413111 | [11,22,45,53] |
| CMW 16738 | CBS 112143; CPC 3200 |
Eucalyptus leaves | Binh Phuoc, Vietnam | M.J. Dudzinski & P.Q. Thu | MT335371 | MT335611 | MT412902 | MT413112 | [11,22,45,53] | ||
| B112 | C. sumatrensis | CMW 23698T | CBS 112829; CPC 4518 |
Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335382 | MT335622 | MT412913 | OK357474 | [22,24,44] |
| CMW 30987 | CBS 112934; CPC 4516 |
Soil | Northern Sumatra, Indonesia | M.J. Wingfield | MT335383 | MT335623 | MT412914 | OK357475 | [22,24,44] | ||
| B113 | C. syzygiicola | CBS 112831T | CMW 51204; CPC 4511 |
Syzygium aromaticum | Sumatra, Indonesia | M.J. Wingfield | N/A | N/A | KX784736 | KX784663 | [26] |
| B116 | C. uniseptata | CBS 413.67T | CMW 23678; CPC 2391; IMI 299577 |
Paphiopedilum callosum | Celle, Germany | W. Gerlach | GQ267379 | GQ267248 | GQ267307 | GQ267208 | [26] |
| B120 | C. yunnanensis | CERC 5339T | CBS 142897; CMW 47644 |
Soil (Eucalyptus plantation) | YunNan, China | S.F. Chen & J.Q. Li | MT335396 | MT335636 | MT412927 | MT413134 | [21,22] |
| CERC 5337 | CBS 142895; CMW 47642 |
Soil (Eucalyptus plantation) | YunNan, China | S.F. Chen & J.Q. Li | MT335397 | MT335637 | MT412928 | MT413135 | [21,22] | ||
| B124 | C. singaporensis | CBS 146715T | MUCL 048320 | leaf litter (submerged in a small stream) | South East Asian rainforest, Mac Ritchie Reservoir, Singapore | C. Decock | MW890042 | MW890055 | MW890086 | MW890124 | [54] |
| CBS 146713 | MUCL 048171 | leaf litter (submerged in a small stream) | South East Asian rainforest, Mac Ritchie Reservoir, Singapore | C. Decock | MW890040 | MW890053 | MW890084 | MW890123 | [54] | ||
| B127 | C. borneana | CMW 50782T | CBS 144553 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635067 | OL635043 | OL635019 | N/A | [16] |
| CMW 50832 | CBS 144551 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635065 | OL635041 | OL635017 | N/A | [16] | ||
| B128 | C. ladang | CMW 50776T | CBS 144550 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635075 | OL635051 | OL635027 | N/A | [16] |
| CMW 50775 | CBS 144549 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635074 | OL635050 | OL635026 | N/A | [16] | ||
| B129 | C. pseudomalesiana | CMW 50821T | CBS 144563 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635076 | OL635052 | OL635028 | OL635137 | [16] |
| CMW 50779 | CBS 144668 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635077 | OL635053 | OL635029 | OL635138 | [16] | ||
| B130 | C. tanah | CMW 50777T | CBS 144562 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635088 | OL635064 | OL635040 | OL635146 | [16] |
| CMW 50771 | CBS 144560 | Soil (Eucalyptus plantation) | Sabah, Tawau, Brumas, Malaysia | N.Q. Pham, Marincowitz & M.J. Wingfield | OL635086 | OL635062 | OL635038 | OL635144 | [16] | ||
| C. cassiae | ZHKUCC 210011 T | – | Cassia surattensis | Guangzhou CityGuangDong, China | Y. X. Zhang, C. T. Chen, Manawas., & M. M. Xiang | ON260790 | N/A | MZ516860 | MZ516863 | [55] | |
| ZHKUCC 210012 | – | Cassia surattensis | Guangzhou CityGuangDong, China | Y. X. Zhang, C. T. Chen, Manawas., & M. M. Xiang | ON260791 | N/A | MZ516861 | MZ516864 | [55] | ||
| C. guangdongensis | ZHKUCC 21-0062T | – | Heliconia metallica | GuangDong, China | Y. X. Zhang, C. T. Chen, Manawas., & M. M. Xiang | MZ491127 | N/A | MZ491149 | MZ491171 | [55] | |
| ZHKUCC 21-0063 | – | Heliconia metallica | GuangDong, China | Y. X. Zhang, C. T. Chen, Manawas., & M. M. Xiang | MZ491128 | N/A | MZ491150 | MZ491172 | [55] | ||
| Curvicladiella cignea | CBS 109167T | CPC 1595; MUCL 40269 |
Decaying leaf | French Guiana | C. Decock | KM231287 | KM231461 | KM231867 | KM232002 | [30,45,56] | |
| CBS 109168 | CPC 1594; MUCL 40268 |
Decaying seed | French Guiana | C. Decock | KM231286 | KM231460 | KM231868 | KM232003 | [30,45,56] | ||
a Codes (B1 to B120) of the 120 accepted Calonectria species from [22]. b T: ex-type isolates of the species. c ATCC: American Type Culture Collection, Virginia, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CERC: China Eucalypt Research Centre, ZhanJiang, GuangDong Province, China; CMW: Culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; CPC: Pedro Crous working collection housed at Westerdijk Fungal Biodiversity Institute; IMI: International Mycological Institute, CABI Bioscience, Egham, Bakeham Lane, UK; MUCL: Mycotheque, Laboratoire de Mycologie Systematique st Appliqee, I’Universite, Louvian-la-Neuve, Belgium; STE-U: Department of Plant Pathology, University of Stellenbosch, South Africa; ZHKUCC: Zhongkai University of Agriculture and Engineering Culture Collection; –: no other collection number. d tef1: translation elongation factor 1-alpha; tub2: β-tubulin; cmdA: calmodulin; his3: histone H3. e N/A: information is not available.
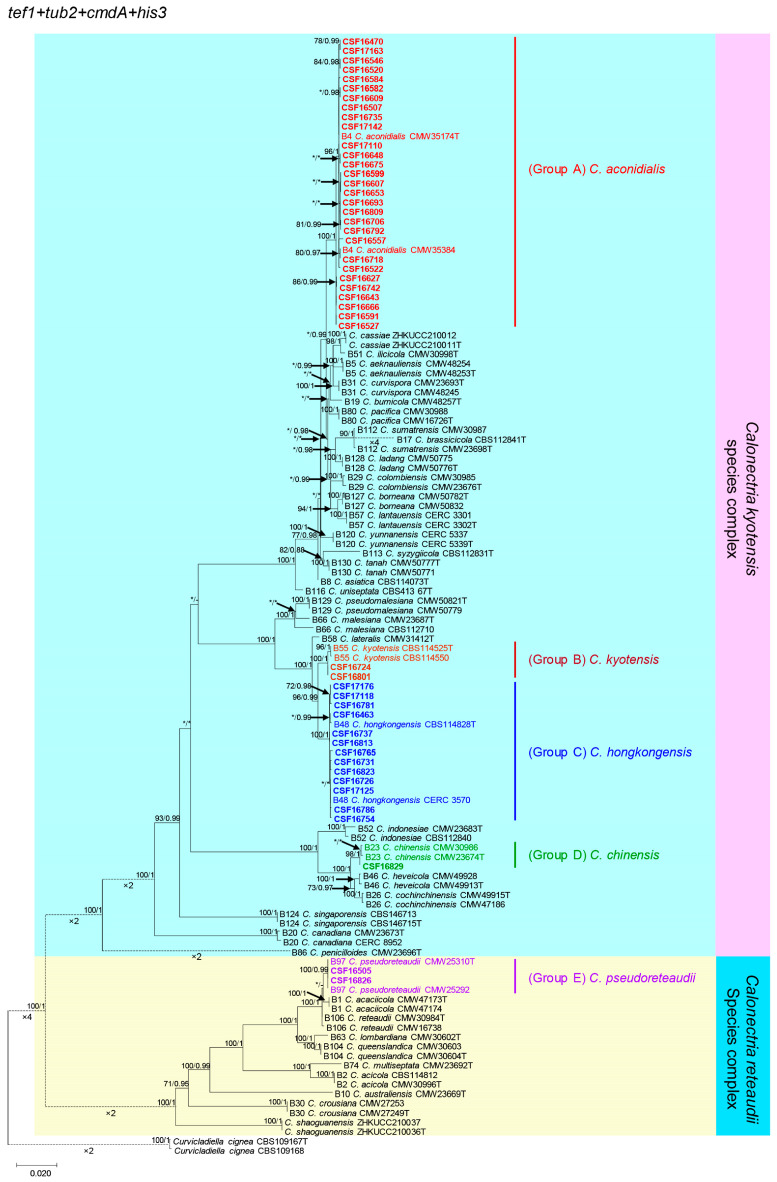
For BI phylogenetic analyses of each dataset, GTR+I, TPM2uf+I+G, TIM1+G, TPM2uf+I+G, and GTR+I+G models were selected for tef1, tub2, cmdA, his3, and the combination of those regions, respectively. The overall topologies generated from the ML analyses and the BI analyses for each dataset were similar. The ML tree with bootstrap support values and the posterior probabilities obtained from BI are presented in Figure 2 and Supplementary Figures S1–S4.
Figure 2.
Phylogenetic tree of Calonectria species based on maximum likelihood (ML) analyses of a combined DNA dataset of tef1, tub2, cmdA, and his3 gene sequences. Bootstrap support value ≥ 70% for ML and posterior probabilities values ≥ 0.95 for Bayesian inference (BI) analyses are presented above the branches as follows: ML/BI. Bootstrap values < 70% or probabilities values < 0.95 are marked with “*”, and absent analysis values are marked with “-” Ex-type isolates are marked with “T”. Isolates sequenced in this study are highlighted in bold and shown in color. Two isolates of Curvicladiella cignea (CBS 109167 and CBS 109168) were used as outgroups.
The 47 Calonectria isolates were divided into five groups (Groups A to E) based on tef1, tub2, cmdA, his3, and combined tef1/tub2/cmdA/his3 analyses (Figure 2 and Supplementary Figures S1–S4). The phylogenetic analyses showed that the isolates in Groups A, B, C, and D belong to the C. kyotensis species complex, while the isolates in Group E belong to the C. reteaudii species complex.
The isolates in Group A represented 19 genotypes based on the sequences of four gene regions (Table 2). The phylogenetic analyses showed that these isolates were grouped with Calonectria aconidialis L. Lombard, Crous and S.F. Chen based on the tef1, cmdA, and his3 trees (Supplementary Figures S1, S3 and S4). In the tub2 tree, the isolates were clustered directly with or most closely to C. aconidialis, Calonectria asiatica Crous and Hywel-Jones, and Calonectria uniseptate Gerlach (Supplementary Figure S2), and were grouped with C. aconidialis according to the combined tef1/tub2/cmdA/his3 tree (Figure 2). Therefore, the isolates in Group A were identified as C. aconidialis. The isolates in Group B represented one genotype (Table 2). These isolates were clustered with Calonectria kyotensis Terash. in the tef1, tub2, and his3 trees (Supplementary Figures S1, S2, and S4), and were clustered directly with or most closely to C. kyotensis and C. uniseptate in the cmdA tree (Supplementary Figure S3). According to the combined tef1/tub2/cmdA/his3 tree, these isolates were grouped with C. kyotensis (Figure 2), and therefore isolates in Group B were identified as C. kyotensis. The isolates in Group C represented 10 genotypes (Table 2) and were clustered with Calonectria hongkongensis Crous in the tef1, tub2, cmdA, and his3 trees and the four-gene combined phylogenetic tree (Figure 2 and Supplementary Figures S1–S4). The isolates in Group C were identified as C. hongkongensis. The isolate in Group D represented one genotype (Table 2). This isolate was clustered with Calonectria chinensis (Crous) L. Lombard, M.J. Wingf. and Crous in the cmdA and his3 trees (Supplementary Figures S3 and S4). The isolate was clustered directly with or most closely to C. chinensis in the tef1 and tub2 trees (Supplementary Figures S1 and S2). The isolate was clustered with C. chinensis based on the combined tef1/tub2/cmdA/his3 tree (Figure 2). Consequently, the isolate was identified as C. chinensis.
The isolates in Group E represented one genotype (Table 2). These isolates were clustered with C. pseudoreteaudii in the tef1, tub2, and his3 trees (Supplementary Figures S1, S2 and S4). These isolates were grouped with C. pseudoreteaudii and Calonectria reteaudii (Bugnic.) C. Booth in the cmdA tree (Supplementary Figure S3). According to the combined tef1/tub2/cmdA/his3 tree, these isolates were grouped with C. pseudoreteaudii (Figure 2). Therefore, the isolates in Group E were identified as C. pseudoreteaudii.
3.4. Diversity and Distribution of Calonectria Species
Based on the sequence comparisons of the four gene region sequences, the 188 Calonectria isolates were identified as five species, C. aconidialis (74.5%), C. hongkongensis (21.3%), C. pseudoreteaudii (2.1%), C. kyotensis (1.6%), and C. chinensis (0.5%) (Figure 3). Calonectria hongkongensis was isolated from all seven sampling sites (Sites A to G) (Table 2). Calonectria aconidialis was isolated from six sampling sites (Sites A to F) (Table 2). Calonectria pseudoreteaudii was detected at sites C, E, and G (Table 2). Calonectria kyotensis and C. chinensis were found only at sites F and G, respectively (Table 2).
Figure 3.
The isolate number and percentage of each Calonectria species in the GuangXi Zhuang Autonomous Region. Different species are indicated by numbers with different colors.
3.5. Pathogenicity Tests
Forty isolates representing the five Calonectria species, C. aconidialis (21 isolates), C. hongkongensis (12 isolates), C. kyotensis (three isolates), C. pseudoreteaudii (three isolates), and C. chinensis (one isolate), were used for pathogenicity tests on the leaves of three Eucalyptus genotypes (Table 2, Figure 4 and Figure 5). All 40 isolates and the positive control produced disease spots and lesions on the leaves of the inoculated seedlings. No disease symptoms were observed in the leaves of the negative control seedlings (Figure 4 and Figure 5). Calonectria species with the same morphological characteristics as the originally inoculated fungi were successfully re-isolated from the diseased tissues of the inoculated leaves. No Calonectria isolates were re-isolated from the leaves of the negative control seedlings. Thus, Koch’s postulates were fulfilled. Two pathogenicity tests were performed, and ANOVA showed that the two pathogenicity tests were significantly different (p < 0.05). Consequently, the data from each experiment were analyzed separately.
Figure 4.
The pathogenicity results of experiment one. Column chart indicating the average lesion length (mm) on leaves resulting from inoculation trials of three Eucalyptus hybrid genotypes inoculated with 40 isolates of five Calonectria species and positive and negative controls. Horizontal bars represent the standard error of the means. Different numbers on the right of the bars indicate treatment means that were significantly different (p = 0.05). The “***” represents no lesions produced by the negative controls.
Figure 5.
The pathogenicity results of experiment two. Column chart indicating the average lesion length (mm) on leaves resulting from inoculation trials of three Eucalyptus hybrid genotypes inoculated with 40 isolates of five Calonectria species and positive and negative controls. Horizontal bars represent the standard error of the means. Different numbers on the right of the bars indicate treatment means that were significantly different (p = 0.05). The “***” represents no lesions produced by the negative controls.
The results of the pathogenicity tests showed that some isolates of C. aconidialis, C. hongkongensis, and C. kyotensis generated significantly longer lesions than the positive control on each of the three Eucalyptus genotypes in both experiments (p < 0.05). For example, C. aconidialis isolates (CSF16507, CSF16520, CSF16557, CSF16582, CSF16648, CSF16693, CSF16706, CSF17110, CSF17130, and CSF17142), C. hongkongensis isolate CSF17125, and C. kyotensis isolate CSF16776 produced significantly longer lesions than positive control isolate CSF13317 (C. pseudoreteaudii) on the three Eucalyptus genotypes in both experiments (Figure 4 and Figure 5).
Significant differences in pathogenicity were also observed among isolates of the same Calonectria species in both experiments (p < 0.05). For example, C. aconidialis isolate CSF16706 produced significantly longer lesions (p < 0.05) than the other C. aconidialis isolates (CSF16470, CSF16507, CSF16522, CSF16527, CSF16582, CSF16584, CSF16599, CSF16609, CSF16643, CSF16653, CSF16675, CSF16718, and CSF16742) on each of the three Eucalyptus genotypes in both experiments. Calonectria hongkongensis isolate CSF16754 produced significantly longer lesions (p < 0.05) than the other C. hongkongensis isolates (CSF16726, CSF16737, CSF16781, CSF16786, and CSF16823). Calonectria kyotensis isolate (CSF16776 produced significantly longer lesions (p < 0.05) than the other C. kyotensis isolates CSF16724 and CSF16801) (Figure 4 and Figure 5).
The pathogenicities of the same genotype determined by the sequences of tef1, tub2, cmdA, and his3 loci of the same Calonectria species were significantly different in the three Eucalyptus genotypes (p < 0.05). For example, C. aconidialis isolates (genotype: AAAA) CSF17130 and CSF17142 produced significantly longer lesions (p < 0.05) than those caused by CSF17110 in experiment one (Table 2, Figure 4), and CSF17110 produced significantly longer lesions (p < 0.05) than CSF17142 in experiment two (Table 2, Figure 5). Calonectria hongkongensis (genotype: AAAA) CSF17125 produced significantly longer lesions (p < 0.05) than CSF16726 and CSF16756 in experiment one (Table 2, Figure 4), and C. kyotensis (genotype: AAAA) isolate CSF16776 produced significantly longer lesions (p < 0.05) than CSF16724 and CSF16801 in both experiments (Table 2, Figure 4 and Figure 5).
The overall data showed that Eucalyptus genotypes CEPT1900 and CEPT1901 were relatively more tolerant than CEPT1902 to the Calonectria isolates tested in this study. The majority of the tested Calonectria isolates generated longer lesions on genotype CEPT1902 than on CEPT1900 and CEPT1901 in both experiments, except for C. aconidialis isolates (CSF16599, CSF16648, and CSF16675), C. hongkongensis isolates (CSF16463, CSF16731, and CSF16754), and C. kyotensis isolate CSF16776 in experiment one (Figure 4), and C. aconidialis isolates (CSF16520, CSF16718, and CSF17110), C. hongkongensis isolates (CSF16726, CSF16731, CSF16754, CSF16756, and CSF16823), C. pseudoreteaudii isolate CSF16826, C. kyotensis isolate CSF16801, and C. chinensis isolate CSF16829 in experiment two (Figure 5).
The resistance of different Eucalyptus genotypes to Calonectria isolates within the same species was inconsistent. For example, Eucalyptus genotypes CEPT1900 and CEPT1901 were significantly more tolerant than genotype CEPT1902 in both experiments (p < 0.05) to C. aconidialis isolates (CSF16470, CSF16522, CSF16527, CSF16742, and CSF17130), and C. hongkongensis isolate CSF17118 was significantly more tolerant than genotype CEPT1902 in both experiments (p < 0.05). Eucalyptus genotype CEPT1901 was significantly more tolerant to C. aconidialis isolate CSF16599 than to the other two Eucalyptus genotypes in both experiments (p < 0.05) (Figure 4 and Figure 5).
4. Discussion
In this study, 428 soil samples were collected from seven Eucalyptus plantations in multiple regions of GuangXi in southern China. Based on their morphological characteristics, 188 Calonectria isolates were obtained. Of these, 188 isolates were identified based on multi-gene phylogenetic inferences. These isolates were identified as C. aconidialis, C. hongkongensis, C. pseudoreteaudii, C. kyotensis, and C. chinensis. Pathogenicity tests indicated that all five Calonectria species were pathogenic among the three tested Eucalyptus genotypes.
This study showed that Calonectria fungi in the C. kyotensis species complex were widely distributed in the soil of Eucalyptus plantations in southern China. Calonectria fungi were isolated from 43.9% of the soil samples. Except for C. pseudoreteaudii, which reside in the C. reteaudii species complex, the other four species resided in the C. kyotensis species complex. The four species in the C. kyotensis species complex accounted for 97.9% of all the isolates obtained in this study. This is consistent with the results of previous studies showing that Calonectria species in the C. kyotensis species complex, especially C. aconidialis, C. hongkongensis, and C. kyotensis, are the dominant species distributed in the soil of Eucalyptus plantations in southern China [9,23,24,25]. In addition to soil isolation, C. aconidialis, C. kyotensis, C. hongkongensis, and C. chinensis were also occasionally isolated from diseased Eucalyptus tissues [8,28,55]. Calonectria hongkongensis also caused fruit rot in rambutan (Nephelium lappaceum L.) in Puerto Rico [57]. The Calonectria species in the C. kyotensis species complex isolated from the soil in this study can cause disease in Eucalyptus trees.
In this study, one species in the C. reteaudii species complex, C. pseudoreteaudii, was isolated from the soil of three Eucalyptus plantation sites. This fungus has been extensively isolated from diseased Eucalyptus tissues (leaves and branches) in plantations in FuJian, GuangXi, GuangDong, and HaiNan Provinces in southern China [5,7,8,9,21,43,58]. Calonectria pseudoreteaudii is considered one of the key causal agents of Eucalyptus leaf blight in southern China. Except for Eucalyptus trees, C. pseudoreteaudii caused leaf spots in Macadamia F. Muell. sp. in China and Laos [59,60] and caused leaf spot and stem blight in Vaccinium corymbosum L. in China [61]. The results of this and previous studies suggested that C. pseudoreteaudii is an important pathogen in many plant species with a wide geographic distribution.
The inoculation results indicated that isolates of C. aconidialis, C. hongkongensis, and C. kyotensis produced significantly longer lesions than those of the positive control on the three Eucalyptus genotypes. These results highlighted that Calonectria species dominantly distributed in the soil were potential threats to Eucalyptus plantations in southern China.
One of the most effective measures to control Eucalyptus leaf blight caused by Calonectria species is selecting disease-resistant Eucalyptus genotypes. Eucalyptus genotypes resistant to Eucalyptus leaf blight have been selected in Australia, Brazil, China, India, and South Africa [62,63,64,65,66,67].
The pathogenicity tests in this study indicated that some Calonectria isolates within the same species were significantly different in their virulence from the tested Eucalyptus genotypes. The resistance of different Eucalyptus genotypes to Calonectria isolates within the same species was inconsistent. This is consistent with the results of previous studies [9,29]. Variations in plant pathogen intra-species pathogenicity and differences in plant pathogen resistance are common in some pathogens and plants [68,69,70]. This is the result of the evolution of both pathogenicity and virulence of plant pathogens, pathogen and plant genetic regulation, plant pathogen co-evolution, and other factors [71,72,73]. The results of this and previous studies suggested that in the process of selecting disease-resistant Eucalyptus genotypes, many isolates of each Calonectria species with different pathogenicities and many Eucalyptus genotypes should be tested.
Acknowledgments
We thank Quanchao Wang, Yuxiong Zheng, Linfang Liu, Lingling Liu, Ying Liu, Xueying Liang, Yancheng Qu, and Yanglong Li for their assistance in collecting samples. We thank Ying Liu, Xueying Liang, Bingyin Chen and Linqin Lu for their assistance in conducting inoculations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9080802/s1, Figure S1: Phylogenetic tree of Calonectria species based on maximum likelihood (ML) analyses of the tef1 gene sequences in this study; Figure S2: Phylogenetic tree of Calonectria species based on maximum likelihood (ML) analyses of the tub2 gene sequences in this study; Figure S3: Phylogenetic tree of Calonectria species based on maximum likelihood (ML) analyses of the cmdA gene sequences in this study; Figure S4: Phylogenetic tree of Calonectria species based on maximum likelihood (ML) analyses of the his3 gene sequences in this study.
Author Contributions
S.C. conceived and designed the experiments. S.C. collected the samples. W.W. collected the samples and performed laboratory work, pathogenicity tests, and data analysis. All authors analyzed and checked the data. All authors wrote and revised the paper. All authors agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was initiated by the National Ten-thousand Talents Program (Project No. W03070115), the bilateral agreement between the Governments of South Africa and China, and supported by the National Key R&D Program of China (China–South Africa Forestry Joint Research Centre Project; Project No. 2018YFE0120900), and the GuangDong Top Young Talents Program in China (Project No. 20171172).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xie Y.J., Arnold R.J., Wu Z.H., Chen S.F., Du A.P., Luo J.Z. Advances in eucalypt research in China. Front. Agric. Sci. Eng. 2017;4:380–390. doi: 10.15302/J-FASE-2017171. [DOI] [Google Scholar]
- 2.Xie Y.J., Du A.P. Eucalyptus in South China. Party School of the CPC Central Committee Press; Beijing, China: 2019. (In Chinese) [Google Scholar]
- 3.Liu T., Xie Y.J. Studies on the Causes of Rapid Development of Eucalyptus Plantations in China. Eucalypt Sci. Technol. 2020;37:38–47. (In Chinese) [Google Scholar]
- 4.Li G.Q., Liu F.F., Li J.Q., Liu Q.L., Chen S.F. Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia. 2018;40:63–95. doi: 10.3767/persoonia.2018.40.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q.C., Chen S.F. Calonectria pentaseptata causes severe leaf disease on cultivated Eucalyptus in Leizhou Peninsula of southern China. Plant Dis. 2020;104:493–509. doi: 10.1094/PDIS-05-19-1009-RE. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X.D., Wingfield M.J. Eucalypt diseases and their management in China. Australas. Plant Pathol. 2011;40:339–345. doi: 10.1007/s13313-011-0053-y. [DOI] [Google Scholar]
- 7.Li W.W., Chen S.F., Wingfield M.J., Duong T.A. Calonectria queenslandica: Causal agent of Eucalyptus leaf blight in Southern China. Plant Dis. 2023;107:730–742. doi: 10.1094/PDIS-01-22-0196-RE. [DOI] [PubMed] [Google Scholar]
- 8.Liang X.Y., Wang Q.C., Chen S.F. Phylogeny, Morphology, Distribution, and Pathogenicity of Seven Calonectria Species from Leaf Blighted Eucalyptus in HaiNan Island, China. Plant Dis. 2023 doi: 10.1094/PDIS-12-22-2802-RE. [DOI] [PubMed] [Google Scholar]
- 9.Wu W.X., Chen S.F. Species diversity, mating strategy and pathogenicity of Calonectria species from diseased leaves and soils in the Eucalyptus plantation in Southern China. J. Fungi. 2021;7:73. doi: 10.3390/jof7020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose R., Banerjee S., Negi N., Pandey A., Bhandari M.S., Pandey S. Identification and pathogenicity of Calonectria pseudoreteaudii causing leaf blight of Eucalyptus—A new record for India. Physiol. Mol. Plant Pathol. 2022;122:101917. doi: 10.1016/j.pmpp.2022.101917. [DOI] [Google Scholar]
- 11.Crous P.W. Taxonomy and Pathology of Cylindrocladium (Calonectria) and Allied Genera. APS Press; St. Paul, MN, USA: 2002. [Google Scholar]
- 12.Alfenas R.F., Lombard L., Pereira O.L., Alfenas A.C., Crous P.W. Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil. Stud. Mycol. 2015;80:89–130. doi: 10.1016/j.simyco.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth T.H., Jovanovic T., Old K.M., Dudzinski M.J. Climatic mapping to identify high-risk areas for Cylindrocladium quinqueseptatum leaf blight on eucalypts in mainland South East Asia and around the world. Environ. Pollut. 2000;108:365–372. doi: 10.1016/S0269-7491(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 14.Old K.M., Wingfield M.J., Yuan Z.Q. A Manual of Diseases of Eucalyptus in South East Asia. Center for International Forestry Research; Jakarta, Indonesia: 2003. [Google Scholar]
- 15.Pham N.Q., Barnes I., Chen S.F., Liu F.F., Dang Q.N., Pham T.Q., Lombard L., Crous P.W., Wingfield M.J. Ten new species of Calonectria from Indonesia and Vietnam. Mycologia. 2019;111:78–102. doi: 10.1080/00275514.2018.1522179. [DOI] [PubMed] [Google Scholar]
- 16.Pham N.Q., Marincowitz S., Chen S.F., Yaparudin Y., Wingfield M.J. Calonectria species, including four novel taxa, associated with Eucalyptus in Malaysia. Mycol. Prog. 2022;21:181–197. doi: 10.1007/s11557-021-01768-8. [DOI] [Google Scholar]
- 17.Sanchez-Gonzalez E.I., Soares T.D.P.F., Zarpelon T.G., Zauza E.A.V., Mafia R.G., Ferreira M.A. Two new species of Calonectria (Hypocreales, Nectriaceae) causing Eucalyptus leaf blight in Brazil. MycoKeys. 2022;91:169–197. doi: 10.3897/mycokeys.91.84896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lombard L., Zhou X.D., Crous P.W., Wingfield B.D., Wingfield M.J. Calonectria species associated with cutting rot of Eucalyptus. Persoonia. 2010;24:1–11. doi: 10.3767/003158510X486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale A., Crous P.W., Lombard L., Polizzi G. Calonectria diseases on ornamental plants in Europe and the Mediterranean basi: An overview. J. Plant Pathol. 2003;95:463–476. [Google Scholar]
- 20.Pham N.Q., Marincowitz S., Chen S.F., Rodas C., Wingfield M.J. Soil-borne Calonectria (Hypocreales, Nectriaceae) associated with Eucalyptus plantations in Colombia. MycoKeys. 2022;94:17–35. doi: 10.3897/mycokeys.94.96301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J.Q., Wingfield M.J., Liu Q.L., Barnes I., Roux J., Lombard L., Crous P.W., Chen S.F. Calonectria species isolated from Eucalyptus plantations and nurseries in South China. IMA Fungus. 2017;8:259–286. doi: 10.5598/imafungus.2017.08.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q.L., Li J.Q., Wingfield M.J., Duong T.A., Wingfield B.D., Crous P.W., Chen S.F. Reconsideration of species boundaries and proposed DNA barcodes for Calonectria. Stud. Mycol. 2020;97:100106. doi: 10.1016/j.simyco.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q.L., Wingfield M.J., Duong T.A., Wingfield B.D., Chen S.F. Diversity and Distribution of Calonectria Species from Plantation and Forest Soils in FuJian Province, China. J. Fungi. 2022;8:811. doi: 10.3390/jof8080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L.L., Wu W.X., Chen S.F. Species diversity and distribution characteristics of Calonectria in five soil layers in a Eucalyptus plantation. J. Fungi. 2021;7:857. doi: 10.3390/jof7100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Chen S.F. Diversity and Distribution of Calonectria Species in Soils from Eucalyptus urophylla × E. grandis, Pinus massoniana, and Cunninghamia lanceolata Plantations in Four Provinces in Southern China. J. Fungi. 2023;9:198. doi: 10.3390/jof9020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombard L., Wingfield M.J., Alfenas A.C., Crous P.W. The forgotten Calonectria collection: Pouring old wine into new bags. Stud. Mycol. 2016;85:159–198. doi: 10.1016/j.simyco.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei F., Zhou L., Pan W., Lin H., Zhou X. First reports of Calonectria pacifica and Ca. kyotensis in the soil from Camellia oleifera plantations in China. For. Pathol. 2022;52:e12760. doi: 10.1111/efp.12760. [DOI] [Google Scholar]
- 28.Li W.W., Chen S.F., Wingfield M.J., Duong T.A. Calonectria species associated with diseased leaves and soils in southern China Eucalyptus plantations. Phytopathol. Res. 2023;5:29. doi: 10.1186/s42483-023-00183-z. [DOI] [Google Scholar]
- 29.Liu L.L., Chen S.F. Pathogenicity of six Calonectria species isolated from five soil layers in a Eucalyptus plantation. J. Phytopathol. 2022;170:445–452. doi: 10.1111/jph.13096. [DOI] [Google Scholar]
- 30.Lombard L., van der Merwe N.A., Groenewald J.Z., Crous P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015;80:189–245. doi: 10.1016/j.simyco.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Burik J.A.H., Schreckhise R.W., White T.C., Bowden R.A., Myerson D. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Med. Mycol. 1998;36:299–303. doi: 10.1046/j.1365-280X.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q.L., Chen S.F. Two novel species of Calonectria isolated from soil in a natural forest in China. MycoKeys. 2017;26:25–60. doi: 10.3897/mycokeys.26.14688. [DOI] [Google Scholar]
- 33.Lombard L., Crous P.W., Wingfield B.D., Wingfield M.J. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Stud. Mycol. 2010;66:15–30. doi: 10.3114/sim.2010.66.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lombard L., Crous P.W., Wingfield B.D., Wingfield M.J. Phylogeny and systematics of the genus Calonectria. Stud. Mycol. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombard L., Crous P.W., Wingfield B.D., Wingfield M.J. Species concepts in Calonectria (Cylindrocladium) Stud. Mycol. 2010;66:1–14. doi: 10.3114/sim.2010.66.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis v. 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posada D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 40.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronquist F., Teslenko M., Van Der Mark P., Ayres D., Darling A., Höhna S., Larget B., Liu L., Suchard M., Huelsenbeck J. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadgil P., Dick M. Fungi silvicolae novazelandiae: 10. N. Zeal. J. For. Sci. 2004;44:30. doi: 10.1186/s40490-014-0030-7. [DOI] [Google Scholar]
- 43.Lombard L., Chen S.F., Mou X., Zhou X.D., Crous P.W., Wingfield M.J. New species, hyper–diversity and potential importance of Calonectria spp. from Eucalyptus in South China. Stud. Mycol. 2015;80:151–188. doi: 10.1016/j.simyco.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crous P.W., Groenewald J.Z., Risède J.M., Hyweljones N. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crous P.W., Groenewald J.Z., Risede J.M., Simoneau P., Hyde K.D. Calonectria species and their Cylindrocladium anamorphs: Species with clavate vesicles. Stud. Mycol. 2006;55:213–226. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang J.C., Crous P.W., Schoch C.L. Species concepts in the Cylindrocladium floridanum and Cy. spathiphylli complexes (Hypocreaceae) based on multi-allelic sequence data, sexual compatibility and morphology. Syst. Appl. Microbiol. 2001;24:206–217. doi: 10.1078/0723-2020-00026. [DOI] [PubMed] [Google Scholar]
- 47.Lechat C., Crous P.W., Groenewald J.Z. The enigma of Calonectria species occurring on leaves of Ilex aquifolium in Europe. IMA Fungus. 2010;1:101–108. doi: 10.5598/imafungus.2010.01.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S.F., Lombard L., Roux J., Xie Y.J., Wingfield M.J., Zhou X.D. Novel species of Calonectria associated with Eucalyptus leaf blight in Southeast China. Persoonia. 2011;26:1–12. doi: 10.3767/003158511X555236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Victor D., Crous P.W., Janse B.J.H., Wingfield M.J. Genetic variation in Cylindrocladium floridanum and other morphologically similar Cylindrocladium species. Syst. Appl. Microbiol. 1997;20:268–285. doi: 10.1016/S0723-2020(97)80074-4. [DOI] [Google Scholar]
- 50.Boedijn K.B., Reitsma J. Notes on the genus Cylindrocladium (Fungi: Mucedineae) Reinwardtia. 1950;1:51–60. [Google Scholar]
- 51.Terashita T. A new species of Calonectria and its conidial state. Trans. Mycol. Soc. Jpn. 1968;8:124–129. [Google Scholar]
- 52.Tubaki K. Studies on the Japanese Hyphomycetes. V. Leaf & stem group with a discussion of the classification of Hyphomycetes and their perfect stages. J. Hattori Bot. Lab. 1958;20:142–244. [Google Scholar]
- 53.Kang J.C., Crous P.W., Old K.M., Dudzinski M.J. Non-conspecificity of Cylindrocladium quinqueseptatum and Calonectria quinqueseptata based on a β-tubulin gene phylogeny and morphology. Can. J. Bot. 2001;79:1241–1247. doi: 10.1139/cjb-79-10-1241. [DOI] [Google Scholar]
- 54.Crous P.W., Hernández-Restrepo M., Schumacher R.K., Cowan D.A., Maggs-Kölling G., Marais E., Wingfield M.J., Yilmaz N., Adan O.C.G., Akulov A., et al. New and Interesting Fungi. 4. Fungal Syst. Evol. 2021;7:255–343. doi: 10.3114/fuse.2021.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y., Chen C., Chen C., Chen J., Xiang M., Wanasinghe D.N., Hsiang T., Hyde K.D., Manawasinghe I.S. Identification and Characterization of Calonectria Species Associated with Plant Diseases in Southern China. J. Fungi. 2022;8:719. doi: 10.3390/jof8070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Decock C., Crous P.W. Curvicladium gen. nov., a new hyphomycete genus from French Guiana. Mycologia. 1998;90:276–281. doi: 10.1080/00275514.1998.12026907. [DOI] [Google Scholar]
- 57.Serrato-Diaz L.M., Latoni-Brailowsky E.I., Rivera-Vargas L.I., Goenaga R., Crous P.W., French-Monar R.D. First report of Calonectria hongkongensis causing fruit rot of rambutan (Nephelium lappaceum) Plant Dis. 2013;97:1117. doi: 10.1094/PDIS-01-13-0008-PDN. [DOI] [PubMed] [Google Scholar]
- 58.Chen Q.Z., Guo W.S., Ye X.Z., Huang X.P., Wu Y.Z. Identification of Calonectria associated with Eucalyptus leaf blight in Fujian Province. J. Fujian Coll. For. 2013;33:176–182. (In Chinese) [Google Scholar]
- 59.Jiang G.Z., Gao F., Yue H., He X.Y. First Report of Fruit Spot of Macadamia sp. Caused by Calonectria pentaseptata in China. Plant Dis. 2020;104:575. doi: 10.1094/PDIS-05-19-0963-PDN. [DOI] [Google Scholar]
- 60.Phanthavong S., Daly A., Weir B., Lee D., Park D., Balmas V., Burgess L. First report of Calonectria pseudoreteaudii in Lao PDR associated with a leaf spot disease of Macadamia integrifolia. Australas. Plant Pathol. 2023;52:23–26. doi: 10.1007/s13313-022-00900-w. [DOI] [Google Scholar]
- 61.Chen C., Liang X., Lin Y., Hsiang T., Xiang M., Zhang Y. First Report of Leaf Spot and Stem Blight on Blueberry (Vaccinium corymbosum ‘Bluerain’) Caused by Calonectria pseudoreteaudii in China. Plant Dis. 2023;107:1951. doi: 10.1094/PDIS-09-22-2125-PDN. [DOI] [Google Scholar]
- 62.Alfenas R.F., Freitas R.G., Pereira O.L., Coutinho M.M., Zarpelon T.G., Cândido T.S., Alfenas A.C. Screening of Corymbia and Eucalyptus species for resistance to Calonectria pteridis leaf blight. For. Pathol. 2016;46:76–81. doi: 10.1111/efp.12222. [DOI] [Google Scholar]
- 63.Bolland L., Tierney J., Tierney B. Studies on leaf spot and shoot blight of Eucalyptus caused by Cylindrocladium quinqueseptatum. Eur. J. For. Pathol. 1985;15:385–397. doi: 10.1111/j.1439-0329.1985.tb00894.x. [DOI] [Google Scholar]
- 64.Blum L.E.B., Dianese J.C., Costa C.L. Comparative pathology of Cylindrocladium clavatum and C. scoparium on Eucalyptus spp. and screening of Eucalyptus provenances for resistance to Cylindrocladium damping-off. Trop. Pest Manag. 1992;38:155–159. doi: 10.1080/09670879209371674. [DOI] [Google Scholar]
- 65.Li G.Q., Li J.Q., Liu F.F., Li T.H., Chen S.F. Preliminary analysis on the pathogenicity of 12 pathogens causing Eucalyptus blight to 10 Eucalyptus clones. Eucalypt Sci. Technol. 2014;31:1–7. (In Chinese) [Google Scholar]
- 66.Sharma J.K., Mohanan C. Relative susceptibility of Eucalyptus provenances to Cylindrocladium leaf blight in Kerala, India. Eur. J. For. Pathol. 1992;22:257–265. doi: 10.1111/j.1439-0329.1992.tb00792.x. [DOI] [Google Scholar]
- 67.Wang Q.C., Chen S.F. Resistance of eight Eucalyptus genotypes to Calonectria pentaseptata in South China. Eucalypt Sci. Technol. 2020;37:1–9. (In Chinese) [Google Scholar]
- 68.Pasco C., Jouan B., Andrivon D. Resistance of potato genotypes to common and netted scab-causing species of Streptomyces. Plant Pathol. 2005;54:383–392. doi: 10.1111/j.1365-3059.2005.01178.x. [DOI] [Google Scholar]
- 69.Pouzoulet J., Pivovaroff A.L., Santiago L.S., Rolshausen P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014;5:253. doi: 10.3389/fpls.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bellee A., Comont G., Nivault A., Abou-Mansour E., Coppin C., Dufour M.C., Corio-Costet M.F. Life traits of four Botryosphaeriaceae species and molecular responses of different grapevine cultivars or hybrids. Plant Pathol. 2017;66:763–776. doi: 10.1111/ppa.12623. [DOI] [Google Scholar]
- 71.Sacristán S., García-Arenal F. The evolution of virulence and pathogenicity in plant pathogen populations. Mol. Plant Pathol. 2008;9:369–384. doi: 10.1111/j.1364-3703.2007.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang R.H., Tyler B.M. Mechanisms and evolution of virulence in oomycetes. Annu. Rev. Phytopathol. 2012;50:295–318. doi: 10.1146/annurev-phyto-081211-172912. [DOI] [PubMed] [Google Scholar]
- 73.Pagán I., García-Arenal F. Tolerance of plants to pathogens: A unifying view. Annu. Rev. Phytopathol. 2020;58:77–96. doi: 10.1146/annurev-phyto-010820-012749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.