Abstract
Cryptococcus neoformans var. gattii is associated with Eucalyptus trees growing in various tropical and subtropical regions of the world. The identification of 13 autochthonous strains of C. neoformans var. gattii in Spain is reported. These strains were isolated from lung (10 samples), liver (1 sample), and brain (2 samples) tissue specimens from six goats suffering from predominantly severe pulmonary disease that were autopsied. The animals were members of five different herds of goats grazing in rural areas of the province of Cáceres (Extremadura, Spain). Between 1990 and 1994, there were five outbreaks, in which between 2.5 and 12% of the goats were affected. Although respiratory symptoms (pneumonia) associated with cachexia were the predominant clinical picture in all outbreaks, brain and liver involvement was also documented in three of the five outbreaks. Biotyping was performed by culturing the isolates on l-canavanine-glycine-bromothymol blue medium and testing them for the assimilation of d-proline and d-tryptophan. Serotyping by agglutination tests confirmed the characterization of all strains as C. neoformans var. gattii serotype B. This is the first confirmation of the presence of this variety in Spain, with a peculiar ability to produce severe pulmonary and systemic disease in normal goats, particularly in the form of outbreaks of pneumonia in association with cachexia.
Cryptococcus neoformans is a capsulated yeast with a worldwide distribution. Since its description at the end of the last century, it has been isolated from different substrata from nature and it has been shown that the basidiomycete Filobasidiella neoformans is its teleomorph (15). Humans and other animals are infected by inhalation, developing cryptococcosis, which is especially severe in immunocompromised individuals, in particular in those infected with the human immunodeficiency virus (4).
Two varieties of C. neoformans have been described, C. neoformans var. neoformans and C. neoformans var. gattii (6). Each variety has its distinctive serotypes based on the antigenic composition of its capsular polysaccharides, which play an important role in pathogenicity. C. neoformans var. neoformans consists of serotypes A, D, and AD, whereas C. neoformans var. gattii has the B and C serotypes (12). Substantial differences in the ecology of the two varieties have been described and noted to influence the epidemiology of cryptococcosis (17).
Cryptococcus neoformans var. neoformans has a worldwide distribution. It is isolated frequently from the droppings of birds, mainly the fecal pigeon (Columba livia), since it can grow in substrates containing high concentrations of creatinine (28). This variety is responsible for the majority of the cases of cryptococcosis in immunocompromised patients (19). By contrast, C. neoformans var. gattii has not been isolated from bird droppings, apparently because it has a lower tolerance for high levels of creatinine. Its optimum growth temperature is 32°C, and plant debris are its natural reservoir, especially those of Eucalyptus camaldulensis (9) and Eucalyptus tereticornis trees (25). More rarely, it has been isolated from bat feces, a wasp’s nest, and other substrata (10, 18). The epidemiology of the infections produced by C. neoformans var. gattii is also different, with infections appearing to occur predominantly in tropical and subtropical areas of Australia, Brazil, Kenya, Zaire, Southeastern Asia, and Southern California, affecting people with no impairment of their immunological status (8).
Cryptococcosis in wild and domestic mammals, with sporadic cases in cats, dogs, goats, horses, and sheep, has also been reported (3). Outbreaks in bovine and caprine livestock have also been identified (1, 23). Information regarding the variety of C. neoformans that causes infection in animals is scarce (11). In Europe, isolation of C. neoformans var. gattii is exceptional (20, 21).
We describe the first identification of C. neoformans var. gattii in Spain. The organism was isolated from lung, liver, and brain tissue specimens of six infected goats from five different herds in which five outbreaks of severe pulmonary and systemic disease occurred from 1990 to 1994.
MATERIALS AND METHODS
Organisms.
Thirteen strains of C. neoformans isolated from tissue samples obtained by autopsy of six goats with subacute or chronic pneumonia associated with cachexia from farms in different districts were studied. These animals, of different Capra hircus races (verata, serrana, and murciana) of local Spanish stock, were members of five different herds which had suffered from epizootic outbreaks of the disease between 1990 and 1994 in Cáceres, Extremadura, Spain. After their isolation and identification, the strains were kept in the collection of the Department of Infectious Diseases of the Faculty of Veterinary Medicine at the University of Extremadura for further studies.
Outbreaks.
Between 1990 and 1994, five outbreaks of severe pulmonary disease associated with cachexia were registered in which the members of five different herds of goats grazing in different geographical areas of the countryside in the province of Cáceres were affected. Although respiratory symptoms associated with cachexia were the predominant clinical picture in all outbreaks, liver and brain involvement was also documented in three of the five outbreaks. The infected animals showed congestive nasal mucosae with mucopurulent exudate, cough, dyspnea, anorexia, and severe weight loss causing cachexia and leading to death after a variable period of 2 to 6 weeks. Neurological symptoms included ataxia, mydriasis, blindness, and progressive paralysis. No mastitis was observed in the infected animals. The number of affected animals varied from 2.5 to 12% of the herd, depending on the outbreak. No specific antifungal treatment was given, although empiric antimicrobials (penicillin) were administered. All animals with clinical manifestations died, with a lethality rate of 100%. It is unknown whether there were animals with subclinical infections.
Cryptococcal isolates.
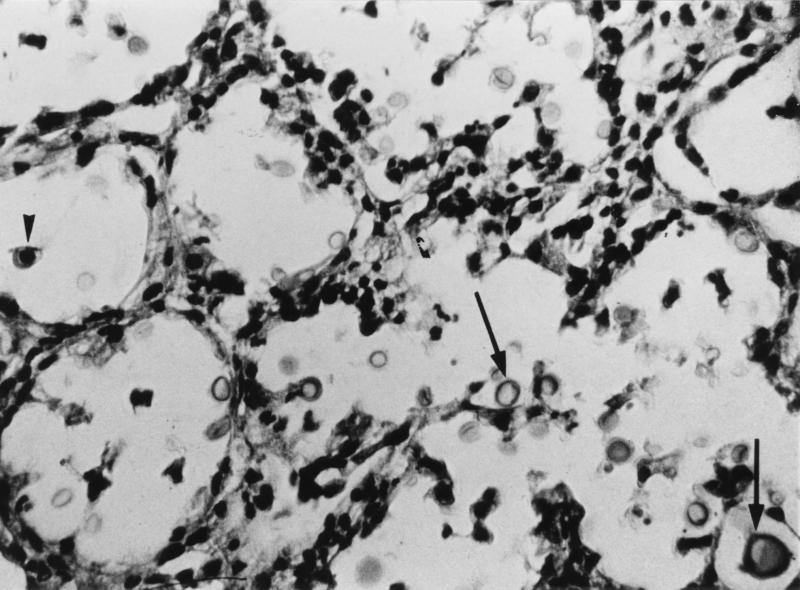
One or two animals from each herd that had died spontaneously were selected for autopsy. Multiple bilateral lung infiltrates were found. In three animals, enlarged mediastinal lymph nodes were also observed, as well as edema in the brain and multiple whitish focal infiltrates in the hepatic parenchyma. Various samples were taken under sterile conditions for histopathological and microbiological studies. Direct microscopy of samples treated with 20% potassium hydroxide and Gram staining of the lung tissue revealed the presence of spherical yeast cells, some of which had buds. Staining with India ink showed a high number of capsulated yeast cells. Upon microscopic examination, lung (Fig. 1) and brain samples showed masses of yeast cells with no inflammatory or fibrotic margins. Pulmonary, hepatic, and cerebral tissue specimens were cultured in Sabouraud dextrose agar medium with chloramphenicol (Difco, Detroit, Mich.). After 48 h at 37°C, several mucoid whitish colonies were observed. India ink mounts revealed capsulated yeast cells.
FIG. 1.
Histopathology of lung tissue from an autopsied goat showing a high number of encapsulated yeast cells inside the alveoli. Hematoxylin-eosin stain. Original magnification, ×400.
Identification procedures.
Thirteen isolates were identified as C. neoformans by the API 20 C Aux system (BioMérieux, Marcy L’Etoile, France). The strains were maintained by periodic subculturing in Sabouraud agar glucose tubes until they were sent to the Mycology Laboratory of the Institut Municipal d’Investigació Mèdica (IMIM) in Barcelona, Spain, for typing. The strains were grown on niger seed (Guizotia abyssinica) agar medium and in Pal’s medium with sunflower seeds (Helianthus annus) (26). The production of a brown pigment due to the action of phenoloxidase was observed in both media. The positive urease tests, the auxanograms for sugars, and the sensitivity to cycloheximide were all characteristic of C. neoformans (Auxocolor; Sanofi, Pasteur, Paris, France). The biovariety study was performed by culture in CGB medium (l-canavanine-glycine-bromothymol blue) to determine the use of glycine as the unique source of carbon (16, 27) by the d-proline and the d-tryptophan assimilation tests as unique sources of nitrogen (7, 22). Serotyping was performed with the Crypto Check agglutination test (Iatron Labs Inc., Tokyo, Japan) (13). The serotyping results were confirmed by the Mycological Laboratory of the Institute of Tropical Medicine in Antwerp, Belgium. C. neoformans reference strains ATCC 90112, RV 56164 serotype A, RV 20185 serotype B, RV 45978 serotype C, and RV 68038 serotype D were used as quality controls.
RESULTS
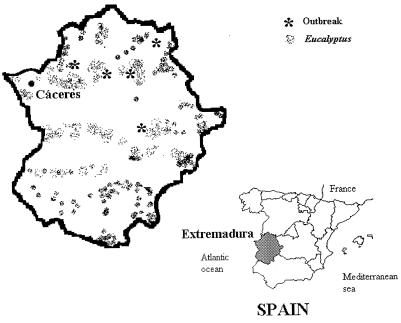
Data on the five outbreaks of predominantly severe pulmonary disease which occurred in different geographical areas of the province of Cáceres, Extremadura, are summarized in Table 1 and Figure 2. All infected animals presented severe pulmonary symptoms associated with cachexia as the most relevant clinical manifestations, although in three of the outbreaks, symptoms of central nervous system involvement were also present. Involvement of brain and liver tissues was confirmed by histopathologic examination and culture. With the use of Indian ink, it was possible to observe encapsulated yeast cells characteristic of C. neoformans in pulmonary and brain tissue specimens. Thirteen very mucinoid strains were isolated from the 10 lung tissue samples as well as from the brain and liver tissues of the six autopsied goats. The identification showed that the 13 strains were C. neoformans, since the patterns of assimilation of sugars, cycloheximide tolerance, production of urease, and growth at 37°C were characteristic of this species. Strains were stained brown in the H. annus and G. abyssinica media. All of them were positive by the CGB test, and they assimilated d-proline and d-tryptophan, indicating that they belonged to C. neoformans var. gattii. Agglutination tests with the Crypto Check system demonstrated that all of the strains were serotype B. These results were confirmed by the Tropical Medicine Institute of Antwerp, Belgium.
TABLE 1.
Characteristics of the different outbreaks of predominantly severe pulmonary disease associated with cachexiaa
| Date of outbreak | No. of animals per herd | Clinical prevalence (%)b | Necropsy (no. of animals) | Localization in central nervous system | Isolation of C. neoformansc |
|---|---|---|---|---|---|
| 1990 | 140 | 12 | 2 | No | 5 |
| 1991 | 250 | 2 | 1 | Yes | 2 |
| 1994 | 300 | 10 | 1 | No | 2 |
| 1994 | 200 | ? | 1 | Yes | 2 |
| 1994 | 120 | 2.5 | 1 | Yes | 2 |
The outbreaks in goats in different geographical areas of the province of Cáceres, Extremadura, Spain.
Lethality rate, 100%.
Number of strains from different tissues.
FIG. 2.
Map of Extremadura, Spain, showing the locations of the five outbreaks of cryptococcal infections in goats and forested areas with Eucalyptus spp.
DISCUSSION
Since Ellis and Pfeiffer (8, 9) isolated C. neoformans var. gattii from the environment in Australia, its natural habitat has been associated with E. camaldulensis (river red gum tree). This native species of eucalyptus is widespread in the south of Australia, and it has been exported and planted in areas of California, Mexico, and other parts of the world (24). The isolation of C. neoformans var. gattii from nature has been reported from the tropical zones of central Africa and Brazil (18). However, it has also been found in temperate zones of countries, such as Uruguay (10), and has been shown to cause some cases of human cryptococcosis in Argentina (2). It has also been isolated from material obtained from another Eucalyptus tree, E. tereticornis, and from different substrata, including a wasp’s nest (10).
In Europe, C. neoformans var. gattii has been isolated in Germany from a patient working with wood of imported tropical trees (14); from environmental samples in Apulia, a region of Italy (21); and from Eucalyptus sp. samples in Portugal (20). Up to the present time, there have been no reports of the isolation of C. neoformans var. gattii from the environment or authochthonous infections in humans or other animals in Spain, a country with a temperate climate. Eucalyptus trees coming from France had been introduced in Extremadura since the last century, but an extensive reforestation with Eucalyptus sp. took place between 1955 and 1977. E. camaldulensis is by far the predominant species, followed by Eucalyptus globulus (5). Since 1980, no more reforestation campaigns with Eucalyptus have been promoted. Whether C. neoformans var. gattii was brought into Spain by infected imported trees is unknown.
It should be noted that autochthonous strains of C. neoformans var. gattii were isolated from goats of local Spanish stock suffering from severe subacute or chronic respiratory symptoms associated in some cases with systemic disease. The various animals from which this variety has been isolated were all found to be grazing free in different zones of the countryside in a region located in the central western part of the Iberian peninsula. The five outbreaks occurred in different geographical zones from 1990 to 1994. This suggests that there is wide distribution of this pathogenic yeast in the geographical area to which reference has been made. Inhalation was the most likely mode of infection. The animals in one of the herds had been grazing in a eucalyptus grove, in which the predominant species was E. camaldulensis.
In all cases, serotype B was identified. The prevalence of serotype B over serotype C in C. neoformans var. gattii everywhere except in the south of California is well known (9, 15, 17). Exhaustive studies to isolate this variety from the environment have still not been conducted in Spain, but undoubtedly this will become a primary objective in the near future.
ACKNOWLEDGMENTS
This work was supported by grant 96/1991-01 from Fondo de Investigaciones Sanitarias (FIS) of the Spanish Ministry of Health.
We are grateful to Danielle Swinne of the Tropical Medicine Institute of Antwerp for confirmation of serotypes, Libero Ajello for constructive criticisms and critical review of the manuscript, and Marta Pulido for editing the manuscript and editorial assistance.
REFERENCES
- 1.Aller B, Santiago E, Escudero A, Rey M. Criptococosis pulmonar en cabras. Rev Patron Biol Anim. 1971;15:387–397. [Google Scholar]
- 2.Bava A J, Negroni R. Características epidemiológicas de 105 casos de criptococosis diagnosticados en la República Argentina entre 1981–1990. Rev Inst Med Trop Sao Paulo. 1992;34:335–340. [PubMed] [Google Scholar]
- 3.Chapman H M, Robinson W F, Bolton J R, Robertson J P. Cryptococcus neoformans infection in goats. Aust Vet J. 1990;67:263–265. doi: 10.1111/j.1751-0813.1990.tb07783.x. [DOI] [PubMed] [Google Scholar]
- 4.Chuck S L, Sande M A. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 5.Devesa J A. Vegetación y flora de Extremadura. Badajoz, Spain: Ed. Universitas; 1995. pp. 402–403. [Google Scholar]
- 6.De Vroey C, Gatti F. Cryptococcus neoformans var. gattii. Vanbreuseghem and Takashio, 1970. Mycoses. 1989;32:675. doi: 10.1111/j.1439-0507.1989.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 7.Dufait R, Velho R, De Vroey C. Rapid identification of the two varieties of Cryptococcus neoformans by d-proline. Mykosen. 1987;30:483. [PubMed] [Google Scholar]
- 8.Ellis D H. Cryptococcus neoformans var. gattii in Australia. J Clin Microbiol. 1987;25:430–431. doi: 10.1128/jcm.25.2.430-431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis D H, Pfeiffer T J. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol. 1990;28:1642–1644. doi: 10.1128/jcm.28.7.1642-1644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gezuele E, Calegari L, Sanabria D, Davel G, Civila E. Isolation in Uruguay of Cryptococcus neoformans var. gattii from a nest of the wasp Polybia occidentalis. Rev Iberoam Micol. 1993;10:5–6. [Google Scholar]
- 11.Hill F I. Cryptococcosis in a North Island brown Kiwi (Apteryx australis mantelli) in New Zealand. J Med Vet Mycol. 1995;33:305–309. doi: 10.1080/02681219580000621. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda R, Shinoda T, Fukazawa Y, Kaufman L. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping clinical isolates. J Clin Microbiol. 1982;16:22–29. doi: 10.1128/jcm.16.1.22-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagasawa K, Itagaki H, Ikeda R, Shinoda T, Kagaya K, Fukazawa Y. Evaluation of a new method for identification of Cryptococcus neoformans which uses serologic tests aided by selected biologic tests. J Clin Microbiol. 1991;29:2873–2876. doi: 10.1128/jcm.29.12.2873-2876.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khol K H, Hof H, Schrettenbrunner A, Seeliger H P R, Kwon-Chung K J. Cryptococcus neoformans var. gattii in Europe. Lancet. 1985;29:1515. doi: 10.1016/s0140-6736(85)92300-1. [DOI] [PubMed] [Google Scholar]
- 15.Kwon-Chung K J. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia. 1975;67:1197–1200. [PubMed] [Google Scholar]
- 16.Kwon-Chung K J, Polacheck I, Bennett J E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C) J Clin Microbiol. 1982;15:535–537. doi: 10.1128/jcm.15.3.535-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon-Chung K J, Bennett J E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120:126–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- 18.Lazera M S, Wanke B, Nishikwa M N. Isolation of both varieties of Cryptococcus neoformans from saprophyic sources in the city of Rio de Janeiro, Brazil. J Med Vet Mycol. 1993;31:449–454. [Google Scholar]
- 19.Levitz S M. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis. 1991;13:1163–1169. doi: 10.1093/clinids/13.6.1163. [DOI] [PubMed] [Google Scholar]
- 20.Martins H M, Bernardo F M, Martins M L. Abstracts of the Third Meeting of the European Confederation of Medical Mycology, Lisboa, Portugal. 1996. Cryptococcus spp. associated with Eucalyptus trees in Lisboa, Portugal, abstr. 7.1. [Google Scholar]
- 21.Montagna M T, Tortorano A M, Fiore L, Ingletti A M, Barbuti S. Cryptococcus neoformans var. gattii en Italie. Not I. Premier cas autochtone de méningite à sérotype B chez un sujet VIH positif. J Mycol Med. 1997;7:90–92. [Google Scholar]
- 22.Mukamurangwa P, Raes-Wuytack C, De Vroey C H. Cryptococcus neoformans var. gattii can be separated from the var. neoformans by its ability to assimilate D-tryptophan. J Med Vet Mycol. 1995;33:419–420. [PubMed] [Google Scholar]
- 23.Pal M. Studies on the prevalence of cryptococcosis in respiratory disorders of domestic animals. Rev Iberoam Micol. 1989;6:29–33. [Google Scholar]
- 24.Pfeiffer T, Ellis D. Environmental isolation of Cryptococcus neoformans var. gattii from California. J Infect Dis. 1991;163:929–930. doi: 10.1093/infdis/163.4.929. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer T, Ellis D. Environmental isolation of Cryptococcus neoformans var. gattii from Eucalyptus tereticornis. J Med Vet Mycol. 1992;30:407–408. [PubMed] [Google Scholar]
- 26.Staib F, Seibold M, Artweiler E, Frohlich B, Weber S, Blisse A. The brown colour effect (BCE) of Cryptococcus neoformans in the diagnosis, control and epidemiology of C. neoformans infections in AIDS patients. Zentralbl Bakteriol Hyg A. 1987;266:167–177. doi: 10.1016/s0176-6724(87)80030-5. [DOI] [PubMed] [Google Scholar]
- 27.Swinne D. Study of Cryptococcus neoformans varieties. Mykosen. 1984;27:137–141. doi: 10.1111/j.1439-0507.1984.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 28.Swinne D, De Vroey C. Epidemiologie de la cryptococcose. Rev Iberoam Micol. 1987;4:77–83. [Google Scholar]