Abstract
Birth weight is a marker that is often referred to determine newborn health, potential growth trajectories and risk of future disease. Accordingly, interventions to promote appropriate and healthy birth weight have been extensively studied and implemented in pregnancy. In particular, physical activity in pregnancy is recommended to promote appropriate fetal development and newborn birth weight. This systematic review and meta-analyses aimed to summarize the effect of physical activity during pregnancy specifically from randomized controlled trials on the following outcomes: birth weight, macrosomia, low birth weight, being large for the gestational age, and being small for the gestational age (Registration No.: CRD42022370729). 63 studies (16,524 pregnant women) were included. There was a significant negative relationship between physical activity during pregnancy and macrosomia (z = 2.16; p = 0.03; RR = 0.79, 95% CI = 0.63, 0.98, I2 = 29%, Pheterogeneity = 0.09). No other significant relationships were found. Promoting physical activity during pregnancy may be an opportune time to reduce the risk of future chronic disease, such as obesity, through the prevention of macrosomia and the promotion of appropriate birth weights.
Keywords: birth weight, macrosomia, low birth weight, gestational age, pregnancy, physical activity
1. Introduction
Birth weight is an important and accessible factor to evaluate newborn health and predict growth trajectories and downstream risk of potential chronic disease such as obesity [1]. The World Health Organization defines low birth weight as the weight of a newborn below 2500 g, typically representing the 10th percentile for its gestational age [2]. Newborns that are small for their gestational age (less than the 10th percentile based on gestational age) are at greater risk of having a low birth weight, and associations have also been found with long-term cognitive deficits in childhood and adolescence [3,4]. On the opposite end of the weight spectrum, macrosomia is defined arbitrarily as a birth weight exceeding 4000 g [5]. Babies born with macrosomia have a higher likelihood of encountering adverse delivery outcomes, including shoulder dystocia, brachial plexus injury, clavicular fracture, birth asphyxia, and neonatal mortality [6,7,8]. Moreover, macrosomia increases the risk of undergoing cesarean section and experiencing vaginal and perineal trauma, as well as postpartum hemorrhage [9,10]. Similar complications are associated with newborns that are large for their gestational age (within the 90th percentile based on their gestational age), including prolonged delivery and the risk of injury at birth. Given the risk of complications at either end of the birth weight spectrum, interventions to promote appropriate birth weight are integral.
Physical activity during pregnancy has been described as a modifiable and accessible health behavior to facilitate maternal and newborn health, including the promotion of an appropriate birth weight [11]. International guidelines for prenatal physical activity suggest that all pregnant individuals without contraindications for being active should aim to accumulate 150 min of moderate-intensity activity per week [12]. Contrary to popular social beliefs that physical activity in pregnancy could result in reduced fetal growth and development, no associations have been found with such factors [13]. In fact, being active throughout pregnancy has been shown to advance placenta blood perfusion [14], which can improve nutrient transport and overall placental function and therefore facilitate fetal development [15]. Furthermore, previous reviews have consistently advised that physical activity in pregnancy does not have adverse effects on fetal development [14,16,17].
However, associations between physical activity in pregnancy and birth weight have been inconclusive. For example, a recent systematic review of 32 studies on prenatal physical activity concluded that there is no association between engagement in physical activity throughout pregnancy and newborn body composition markers [18]. Another study that included objective measures of prenatal physical activity via accelerometry suggested that physical activity was correlated with birth weight, and specifically, that aerobic activity reduced birth weight and increased the number of newborns weighing within the appropriate range [19]. Contrarily, a recent meta-analysis of randomized controlled trials that included studies that had assessed gestational weight gain found no associations between prenatal physical activity and large- or small-for-gestational-age newborns [20]. Despite the inconsistencies in the literature regarding birth weight, prenatal physical activity is still recommended, given the several health benefits for both the pregnant person and future child [16]. In fact, a recent expert review emphasized that physical activity in pregnancy should be integrated into standard care, especially given the high quality and strong evidence base supporting its contribution to reduced perinatal complications and improved labor and delivery outcomes [16].
Rather than negating or minimizing the potential benefits of prenatal physical activity on birth weight due to inconsistent results, they indicate that it may be necessary to further examine this relationship via high-quality randomized controlled trials that specifically target these outcomes. Accordingly, the purpose of this systematic review and meta-analysis was to assess the effect of prenatal physical activity on markers of birth weight (i.e., total birth weight and incidences of being large for the gestational age, being small for the gestational age, low birth weight, and macrosomia) from randomized controlled trials. We hypothesized that the amalgamated findings from randomized controlled trials would be in favor of physical activity in pregnancy for reduced risk of being large for the gestational age, being small for the gestational age, having a low birth weight, and macrosomia, along with showing a significant association with reduced total birth weight.
2. Methods
A systematic review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [21]. The protocol was registered in the International Prospective Registry of Systematic Reviews (PROSPERO), Registration No. CRD42022370729.
2.1. Eligibility Criteria
The eligibility criteria for this systematic review and meta-analysis was guided by the PICOS framework: participants, interventions, comparisons, outcomes, and study design [20]. The participants included pregnant women, the intervention was physical activity, the comparison was no physical activity/control, and the outcomes were birth weight, being small for the gestational age, being large for the gestational age, and macrosomia.
2.2. Population
The population of interest was pregnant individuals without contraindication to exercise or physical activity (following the most recent international clinical guideline about physical activity during pregnancy) [22,23]. Absolute contraindications were characterized by conditions such as ruptured membranes, premature labor, persistent second- or third-trimester bleeding, and other similar factors. On the other hand, relative contraindications were characterized by a history of spontaneous abortion, mild/moderate cardiovascular or respiratory disease, etc. [22,23].
2.3. Intervention (Exposure)
We conducted a search to identify physical activity interventions during pregnancy that involved quantifiable forms of physical activity. The focus was on extracting information regarding the program’s reporting of duration, intensity, type of activities, weekly frequency, session duration, participant adherence, and whether supervision was provided.
2.4. Comparison
The comparator was no exercise or physical activity (i.e., the control group of the selected studies), normally involving pregnant participants who followed a regular obstetrical follow-up in their health centers.
3. Outcome
The main outcomes of the study were birth weight, macrosomia, and low birth weight. Secondary outcomes were being large for the gestational age and being small for the gestational age.
4. Data Sources
A comprehensive search was carried out through the Universidad Politécnica de Madrid software in the following databases: Academic Search Premier, ERIC, MEDLINE, SPORTDiscus, OpenDissertations, Clinicaltrials.gov, Web of Science, Scopus, the Cochrane Database of Systematic Reviews, and the Physiotherapy Evidence Database (PEDro). To ensure equality in the selection process, the same article selection criteria were used for all databases, considering differences in controlled vocabulary and rules of selection syntax. The search terms used were:
English: (physical activity OR exercise OR training OR physical exercise OR fitness OR (strength training) OR physical intervention OR Pilates OR Yoga OR strengthening OR aerobic OR resistance training OR pelvic floor muscle training) AND (pregnancy OR maternal OR antenatal OR pregnant AND (birth weight OR macrosomia OR low birth weight OR large gestational age OR small gestational age) AND (randomized clinical trial OR randomized controlled trial OR RCT).
Spanish: (actividad física O ejercicio O entrenamiento O ejercicio físico O fitness O entrenamiento de fuerza O intervención de actividad física O Pilates O Yoga O fortalecimiento O aeróbico O entrenamiento de resistencia O fortalecimiento del suelo pélvico) Y (embarazo O materno O antenatal O embarazada Y peso de nacimiento O macrosomía O bajo peso al nacer O gran edad gestacional O pequeña edad gestacional) Y (ensayo clínico aleatorizado O ensayo controlado aleatorizado O ECA).
5. Study Selection and Data Extraction
Only randomized controlled trials (RCTs) were selected. Also, systematic reviews previously published in the same field were searched to compare our results. Articles published between 2010 and 2023 written in English and Spanish were considered for the search. Reference lists of selected studies were retrieved to identify other studies that might have been missed by the electronic keyword search.
To ensure compliance with the inclusion criteria, two reviewers (MS and CS) conducted an independent screening of the titles and abstracts retrieved from the electronic searches. The abstracts that met the initial screening were subjected to further analysis. The full texts were screened independently by two reviewers (JG and RB) to identify outcomes of interest for data extraction.
To identify any potential additional studies that were not captured by the electronic searches, the list of references from selected articles was screened. In cases where a study had multiple publications, the most recent or comprehensive publication was chosen as the primary source. However, relevant data from all the publications were extracted to ensure that no valuable information was overlooked.
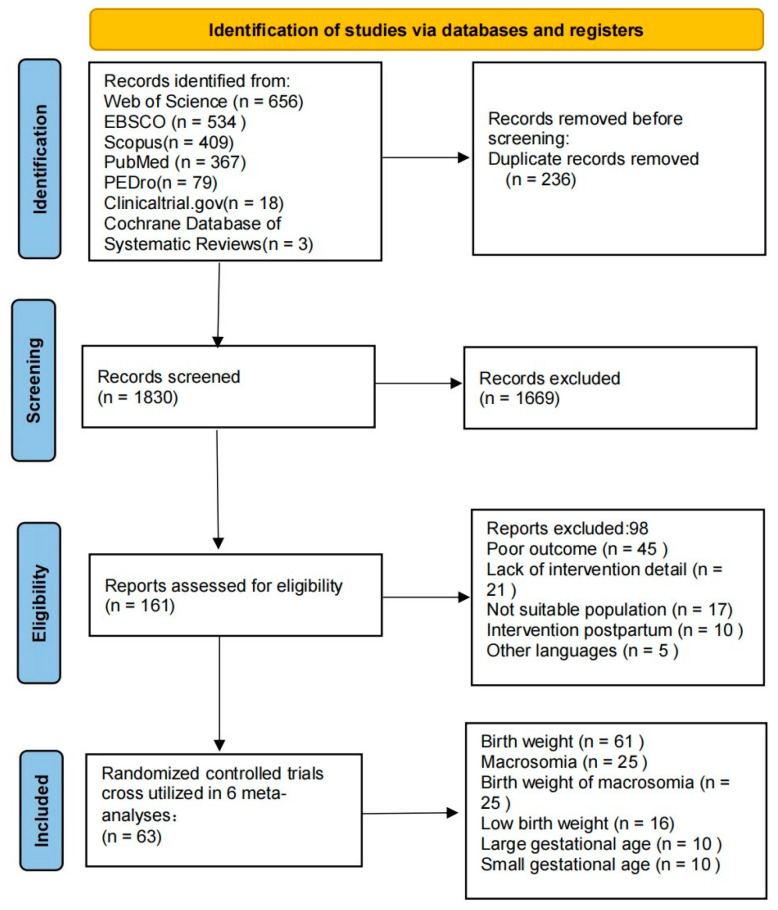
For studies where one reviewer (DZ) recommended exclusion, both reviewers (CS and MS) tried to reach a consensus to make a final decision for exclusion or inclusion. In situations of absolute discrepancy, a third reviewer (RB) provided their expert opinion on whether the study should be included or excluded. The study selection process is detailed in Figure 1.
Figure 1.
Flow chart of the retrieved and analyzed articles.
Data extraction tables were created in an Excel sheet. One researcher extracted the data, and then data extraction was independently verified by a content expert to facilitate further analysis. Extracted data were study characteristics (i.e., author last name, year and country), type of article (RCT), total sample size and group sample size, intervention/exposure (exercise prescribed and/or measured), including: frequency, intensity, time and type, supervision of intervention, duration of intervention and adherence of intervention, primary and secondary outcomes (Table 1).
Table 1.
Characteristics of the studies analyzed.
| Author | Year | Country | Type | N | EG | CG | Intervention Physical Exercise Program | Main Variables Analyzed | Secondary Variables Analyzed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq | Intensity | Duration of Program | Type of Exercise | Superv. Class | Duration of Class | Adh. | |||||||||
| Aktan [24] | 2021 | Turkey | RCT | 43 | 21 | 22 | 2 | Mod | 8 w | Clinical Pilates exercise | Yes | 60 min | - | General anxiety, gestational weight gain | Type of delivery, birth weight |
| Atkinson [25] | 2022 | Canada | RTC | 241 | 119 | 122 | 3–4 | Mod | 22 w | Walking | No | 25–40 min | 80% | Gestational weight gain | Depression, type of delivery, and birth weight |
| Babbar [26] | 2016 | USA | RCT | 46 | 23 | 23 | 3 | Mod | 8 w | Yoga | Yes | 60 min | 80% | Umbilical artery, type of delivery, birth weight | Gestational weight gain |
| Bacchi [27] | 2018 | Argentina | RTC | 111 | 49 | 62 | 3 | Low–Mod | 28 w | Aquatic activities | Yes | 55–60 min | 80% | Gestational weight gain and birth weight | - |
| Backhausen [28] | 2017 | Denmark | RCT | 516 | 258 | 258 | 2 | Low | 12 w | Water exercise | No | 70 min | 76% | Low back pain, birth weight | Type of delivery |
| Barakat [29] | 2012 | Spain | RCT | 290 | 138 | 152 | 3 | Mod | 28 w | Aerobic exercise | Yes | 40–45 min | - | Type of delivery | Gestational weight gain and birth weight |
| Barakat [30] | 2013 | Spain | RCT | 510 | 255 | 255 | 3 | Mod | 28 w | Aerobic, strength, and flexibility exercise | Yes | 50–55 min | 95% | Gestational diabetes | Gestational weight gain and birth weight |
| Barakat [31] | 2016 | Spain | RCT | 765 | 382 | 383 | 3 | Mod | 28 w | Aerobic, strength, and flexibility exercise | Yes | 50–55 min | 80% | Hypertension | Type of delivery, gestational weight gain, birth weight |
| Barakat [32] | 2018a | Spain | RCT | 429 | 227 | 202 | 3 | Mod | 28 w | Aerobic exercise | Yes | 55–60 min | 80% | Duration of labor | Type of delivery, use of epidural, birth weight |
| Barakat [33] | 2018b | Spain | RCT | 65 | 33 | 32 | 3 | Mod | 28 w | Aerobic, pelvic floor strength, and flexibility exercise | Yes | 55–60 min | 85% | Placenta weight | Gestational age, type of delivery, birth weight |
| Barakat [34] | 2018c | Spain | RCT | 456 | 234 | 222 | 3 | Mod | 28 w | Aerobic exercise | Yes | 50–55 min | 80% | Gestational weight gain | Gestational age, type of delivery, birth weight |
| Bhartia [35] | 2019 | India | RCT | 78 | 38 | 40 | 1 | Mod | 12 w | Yoga | Yes | 50 min | - | Maternal stress, type of delivery, birth weight | - |
| 2 | No | ||||||||||||||
| Bjøntegaard [36] | 2021 | Norway | RCT | 281 | 164 | 117 | 1 | Mod | 12 w | Aerobic, strength, and balance exercise | Yes | 60 min | - | Type of delivery, birth weight | Physical activity of children at age of seven |
| 2 | No | 45 min | |||||||||||||
| Brik [37] | 2019 | Spain | RTC | 85 | 42 | 43 | 3 | 55–60% Max HR | 29 w | Aerobic, strength, coordination and balance, and pelvic floor exercise | Yes | 60 min | 70% | Gestational weight gain, fetal cardiac function | Type of delivery, birth weight, gestational age |
| Bruno [38] | 2016 | Italy | RTC | 131 | 69 | 62 | 3 | Mod | 16 w | Walking, dietary counselling | No | 30 min | - | Gestational diabetes | Gestational weight gain, type of delivery, birth weight |
| Clark [39] | 2018 | USA | RTC | 36 | 14 | 22 | 3 | Mod | 20 w | Aerobic | Yes | 60 min | - | Gestational weight gain | Type of delivery, birth weight |
| Cordero [40] | 2015 | Spain | RCT | 257 | 101 | 156 | 2 | 50–55% Max HR | 26 w | Aerobics in gym hall | Yes | 50–60 min | 80% | Gestational diabetes | Gestational weight gain, type of delivery, birth weight |
| 1 | Aquatic activity | ||||||||||||||
| Da Silva [41] | 2017 | Brazil | RTC | 639 | 213 | 426 | 3 | Mod | 16 w | Aerobic, strength training | Yes | 60 min | 70% | Preterm birth and pre-eclampsia | Gestational weight gain, birth weight |
| Daly [42] | 2017 | Ireland | RCT | 88 | 44 | 44 | 3 | Mod | 26 w | Aerobic, pelvic floor exercise | Yes | 50–60 min | 80% | Maternal fasting plasma glucose | Type of delivery and birth weight |
| De Oliveria [43] | 2012 | Brazil | RTC | 111 | 54 | 57 | 3 | 60–80% Max HR | 25 w | Walking | Yes | 15–40 min | 85% | VO2max, birth weight and gestational age | - |
| Ellingsen [44] | 2020 | Norway | RTC | 279 | 164 | 115 | 1 | Mod | 12 w | Aerobic activity and strength exercise | Yes | 60 min | - | Neurodevelopment in 7-year-old children | Gestational age, birth weight, type of delivery |
| 2 | No | 45 min | |||||||||||||
| Fritel [45] | 2015 | France | RCT | 282 | 140 | 142 | 1 | Low | 8 w | Pelvic floor training | Yes | 20–30 min | - | Urinary incontinence | Type of delivery and birth weight |
| Garnaes [46] | 2017 | Norway | RCT | 74 | 38 | 36 | 3 | Mod | 20 w | Aerobic, strength training | Yes | 60 min | - | Birth weight | Type of delivery, perineal tears, gestational age |
| 2 | - | No | 50 min | ||||||||||||
| 7 | Pelvic floor training | No | 1 min | ||||||||||||
| Guelfi [47] | 2016 | Australia |
RCT | 172 | 85 | 87 | 3 | Mod | 14 w | Home-based stationary cycling program | Yes | 20–60 min | - | Gestational diabetes | Type of delivery, birth weight |
| Haakstad [48] | 2011 | Norway | RCT | 105 | 52 | 53 | 2 | Mod | 12 w | Aerobic dance and strength training | Yes | 60 min | 80% | Birth weight | Gestational age, type of delivery |
| 1 | No | 30 min | |||||||||||||
| Hellenes [49] | 2015 | Norway | RCT | 336 | 188 | 148 | 1 | Mod | 16 w | Aerobic activity | Yes | 30 + min | - | Cognitive, language and motor domains of children | Gestational age, birth weight, and type of delivery |
| 2 | No | ||||||||||||||
| Hopkins [50] | 2010 | New Zealand | RCT | 84 | 47 | 37 | 5 | 65% VO2max |
16 w | Stationary cycling program | No | 40 min | - | Birth weight, gestational age | - |
| Johannessen [51] | 2021 | Norway | RCT | 722 | 383 | 339 | 1 | Mod | 12 w | Aerobic, strength and pelvic floor exercise | Yes | 55–70 min | - | Urinary incontinence at 3 months postpartum | Type of delivery, episiotomy, epidural, duration of labor, birth weight |
| 2 | No | 45 min | |||||||||||||
| Karthiga [52] | 2022 | India | RCT | 234 | 121 | 113 | 7 | Mod | 20 w | Yoga 5 sessions of Yoga techniques |
No | 60 min | - | Gestational hypertension | Type of delivery, duration of labor, birth weight |
| Labonte-Leymoyne [53] | 2017 | Canada | RCT | 18 | 10 | 8 | 3 | 55% VO2max |
24 w | Aerobic exercise | Yes | 20 + min | - | Neuroelectric response of the neonatal brain | Maternal weight gain, birth weight |
| Leon-Larios [54] | 2017 | Spain | RCT | 466 | 254 | 212 | 5 | Low | 6 w | Perineal massage and pelvic floor exercise | No | 18–23 min | - | Perineal tear and episiotomy | Type of delivery, duration of labor, birth weight, and epidural analgesia |
| McDonald [55] | 2018 | USA | RCT | 90 | 49 | 41 | 5 | 55–69% Max HR | 20 w | Walking program | No | 40 min | - | Preeclampsia and pathophysiological progress of preeclampsia | Gestational weight gain and birth weight |
| McDonald [56] | 2022 | USA | RCT | 192 | 131 | 61 | 3 | Mod | 24 w | Aerobic and resistance training | Yes | 50 min | 80% | Gestational weight gain, type of delivery and birth weight | - |
| Murtezani [57] | 2014 | Republic of Kosovo | RCT | 63 | 30 | 33 | 3 | Mod | 20 w | Aerobic and strength exercise | Yes | 40–45 min | 85% | Birth weight and gestational age | - |
| Nagpal [58] | 2020 | Canada | RCT | 40 | 23 | 17 | 3 | Mild | 11 w | Walking program + nutrition | Yes | 25–40 min | 80.2% | Scoring women on meeting the intervention goals | Gestational weight gain, birth weight, macrosomia, and low birth weight |
| Nascimento [59] | 2011 | Brazil | RCT | 80 | 39 | 41 | 5 | Low–Mod | 17 w | Aerobic exercise Walking |
No | 40 min | 62.5% | Gestational weight gain | Birth weight |
| Navas [60] | 2021 | Spain | RCT | 294 | 148 | 146 | 3 | 55–65% Max HR | 20 w | Aquatic exercise | Yes | 45 min | - | Postpartum depression, quality of life, and quality of sleep | Gestational age, birth weight |
| Pais [61] | 2021 | India | RCT | 124 | 61 | 63 | 7 | Low | 20 w | Yoga One-to-one Yoga session for 7 days |
No | 45 min | - | Preeclampsia and gestational diabetes | Gestational age, duration of labor, type of delivery, birth weight |
| Perales [62] | 2014 | Spain | RCT | 167 | 90 | 77 | 3 | 55–60% Max HR | 29 w | Aerobic activity | Yes | 55–60 min | - | Prenatal depression | Gestational weight gain, birth weight, and type of delivery |
| Perales [63] | 2015 | Spain | RCT | 63 | 38 | 25 | 3 | 55–60% Max HR | 28 w | Aerobic dance, pelvic floor muscle training | Yes | 55–60 min | 80% | Fetal and maternal heart rate | Gestational weight gain, birth weight, type of delivery |
| Perales [64] | 2020 | Spain | RCT | 1348 | 688 | 660 | 3 | Light–Mod | 30 w | Aerobic, pelvic floor exercise | Yes | 50–55 min | 95% | Gestational weight gain, hypertension, and gestational diabetes | Type of delivery, birth weight, gestational age |
| Pereira [65] | 2022 | Portugal | RCT | 126 | 63 | 63 | 3 | 55–69% Max HR | 3 w | Walking | Yes | 30 min | - | Rate of labor induction | Type of delivery, birth weight |
| Phelan [66] | 2011 | USA | RCT | 363 | 179 | 184 | 7 | Low | 26 w | Walking | No | 30 min | - | Gestational weight gain | Gestational hypertension, birth weight, and type of delivery |
| Price [67] | 2012 | USA | RCT | 62 | 31 | 31 | 4 | Mod | 23 w | Aerobic exercise | Yes | 45–60 min | - | Birth weight | Duration of labor, type of delivery |
| Prabhu [68] | 2015 | India | RCT | 105 | 52 | 53 | 2 | Mod | 12 w | Aerobic dance | Yes | 45 min | 80% | Birth weight | - |
| 1 | No | 30 min | |||||||||||||
| Raper [69] | 2021 | USA | RCT | 125 | 58 | 67 | 3 | Mod | 24 w | Aerobic | Yes | 50 min | 80% | Gestational diabetes, type of delivery, and birth weight | - |
| Rodriguez-Blanque [70] | 2019 | Spain | RTC | 129 | 65 | 64 | 3 | Mod | 17 w | Aquatic physical exercise | Yes | 60 min | - | Laceration and episiotomy rates | Type of delivery, birth weight, and anesthesia |
| Rodriguez-Diaz [71] | 2017 | Spain | RCT | 100 | 50 | 50 | 2 | Mod | 8 w | Pilates | Yes | 40–45 min | 90% | Gestational weight gain, blood pressure, strength, flexibility, and spinal curvature | Type of delivery, episiotomy, analgesia, and birth weight |
| Ruchat [72] | 2012 | Canada | RCT | 71 | 26 | 45 | 1 | Mod | 22 w | Walking | Yes | 25–40 min | - | Gestational weight gain, birth weight | - |
| 2–3 | No | ||||||||||||||
| Ruiz [73] | 2013 | Spain | RCT | 962 | 481 | 481 | 3 | Light–Mod | 28 w | Aerobic and resistance exercise | Yes | 50–55 min | 97% | Gestational weight gain | Birth weight, type of delivery |
| Sagedal [74] | 2017 | Norway | RCT | 591 | 296 | 295 | 2 | Mod | 24 w | Aerobic, strength training. Dietary counselling | Yes | 60 min | - | Gestational weight gain, birth weight | Gestational age, perineal tear |
| Seneviratne [75] | 2015 | New Zealand | RCT | 75 | 38 | 37 | 3–5 | Mod | 16 w | Stationary cycling program | No | 15–30 min | - | Birth weight, type of delivery | Gestational weight gain, gestational age |
| Silva-Jose [76] | 2022 | Spain | RCT | 139 | 69 | 70 | 3 | 55–65% Max HR | 30 w | Aerobic exercise | Yes | 55–60 min | 80% | Gestational weight gain |
Birth weight, type of delivery |
| Sobhgol [77] | 2022 | Australia | RCT | 200 | 100 | 100 | 7 | Low | 16 w | Pelvic floor muscle exercise | No | 30 min | 50% | Female sexual function | Type of delivery, perineal tear, episiotomy, duration of labor, and birth weight |
| Stafne [78] | 2012 | Norway | RCT | 761 | 396 | 365 | 2 | Mod–High | 12 w | Aerobic, strength, pelvic floor training | Yes | 60 min | 55% | Urinary and anal incontinence |
Type of delivery, birth weight |
| 1 | No | 45 min | |||||||||||||
| Szumilewicz [79] | 2020 | Poland | RCT | 260 | 133 | 127 | 3 | Low–Mod | 24 w | Aerobic, resistance, pelvic floor muscle training | Yes | 60 min | - | Urinary incontinence 2 months and 1 year postpartum | Type of delivery, birth weight, duration of labor, analgesia |
| Taniguchi [80] | 2016 | Japan | RCT | 118 | 60 | 58 | 3 | Mod | 6 + w | Walk briskly | Yes | 30 min | 80% | Type of delivery, birth weight | - |
| Tomic [81] | 2013 | Croatia | RCT | 334 | 166 | 168 | 3 | 60–75% Max HR | 28 w | Aerobic exercise | Yes | 50 min | 80% | Macrosomia, birth weight, type of delivery, gestational weight gain | - |
| Uria-Minguito [82] | 2022 | Spain | RCT | 203 | 102 | 101 | 3 | 65–70% Max HR | 28 w | Aerobic exercise | Yes | 50–60 min | - | Gestational diabetes | Gestational weight gain, type of delivery, birth weight |
| Ussher [83] | 2015 | UK | RCT | 789 | 394 | 395 | 3–4 | Low | 6 w | Exercise on a treadmill |
Yes | 20 min | 88.8% | Continuous smoking abstinence | Type of delivery, birth weight |
| Vinter [84] | 2011 | Denmark | RCT | 304 | 150 | 154 | 7 | Mod | 24 w | Aerobic exercise, dietary counselling | No | 30–60 min | - | Gestational weight gain | Birth weight |
| Wang [85] | 2017 | China | RCT | 226 | 112 | 114 | 3 | 55–65% Max HR | 24 w | Stationary cycling program | Yes | 45–60 min | 75% | Gestational diabetes | Birth weight, macrosomia |
| Yekefallah [86] | 2021 | Iran | RCT | 70 | 35 | 35 | 2 | Low–Mod | 11 w | Yoga | Yes | 75 min | - | Episiotomy, perineal tear, type of delivery | Birth weight, gestational age, duration of labor |
Author: first author last name; Year: year of study; Country: country where the article was developed (usually in the method part); Type: type of article, if it is a randomized clinical (or controlled) trial, RCT is indicated. N: total number of women analyzed. Those of the GI and those of the CG have to coincide. EG: Number of women analyzed in the intervention group. CG: number of women analyzed in the control group. Freq: weekly frequency of exercise sessions (3 days a week, 2, etc.). Intensity: moderate, high...; Duration of program: program time. If the program lasted 10 weeks, or if it started in week 12 and ended in week 28, it is described as being 16 weeks long. Type of exercise: aerobic, muscle strengthening, etc. Superv. Classes: whether or not there was supervision. Duration of class: minutes of each session. Adh.: adherence of the participants to the intervention (%). This indicates how many women attended. Main variables analyzed: lists all the main variables of the study. This is usually in the method section in “outcomes”, and they appear as “main outcomes”. If main does not appear, they are the first. You will find it in several places. Secondary variables: the same as before, but secondary. In the same study, this may involve different types of exercises, varying durations for each exercise, and both supervised and unsupervised exercises.
6. Quality of Evidence and Risk of Bias Assessments
To evaluate the quality of evidence for each study design and outcome, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used. This framework provides a standardized and comprehensive approach to assess the strength of the evidence across multiple studies [87].
To evaluate the risk of bias, the Cochrane Handbook was utilized. The potential sources of bias evaluated are selection bias (inadequate randomization procedures for RCTs), performances bias (compliance with the intervention for RCTs), detection bias (flawed outcome measurement), attrition bias (incomplete follow-up and high loss to follow-up), and reporting bias (selective or incomplete outcome reporting) [88].
7. Statistical Analysis
Statistical analyses were performed with the software RevMan in its 5.3 version. For continuous variables, birth weight (grams), mean, and standard deviations were recorded. The overall confidence interval (CI) was calculated using the mean difference (MD) [89]. All dichotomous outcomes, macrosomia, low birth weight, being large for the gestational age, and being small for the gestational age were expressed as categorical variables (Yes/No) to calculate the relative risk (RR) [90]. Random effect models were applied. To establish the compensated average in both dichotomous and continuous analyses, a weight system was used that considered the sample size per group, and, generally, these were contributed by each study. To assess the variation in study results between studies (i.e., the degree of heterogeneity), the I2 statistic was interpreted using established thresholds: 25% for low heterogeneity, 50% for moderate heterogeneity, and >75% for high heterogeneity [91].
8. Results
8.1. Study Characteristics
In total, 63 studies that met the inclusion criteria were identified, involving 16,524 pregnant women across 23 countries on five continents. All of the studies were randomized control trials, including 59 exercise interventions only and 4 of exercise and dietary counselling. Studies varied in frequency from 2 to 7 days per week, with low to moderate intensities lasting 15 to 75 min per session. These interventions were carried out during the first, second, and third trimesters, and lasted from 3 to 30 weeks. The types of exercise included walking, stationary cycling, water aerobics, swimming, resistance training, stretching, Pilates, Yoga, pelvic floor muscle training, and combinations of various exercise types. Additional details about the studies can be found in the Table 1. The results of mean birth weight, macrosomia, low birth weights, being large for the gestational age, and being small for the gestational age are presented below.
8.2. Risk of Bias Assessment
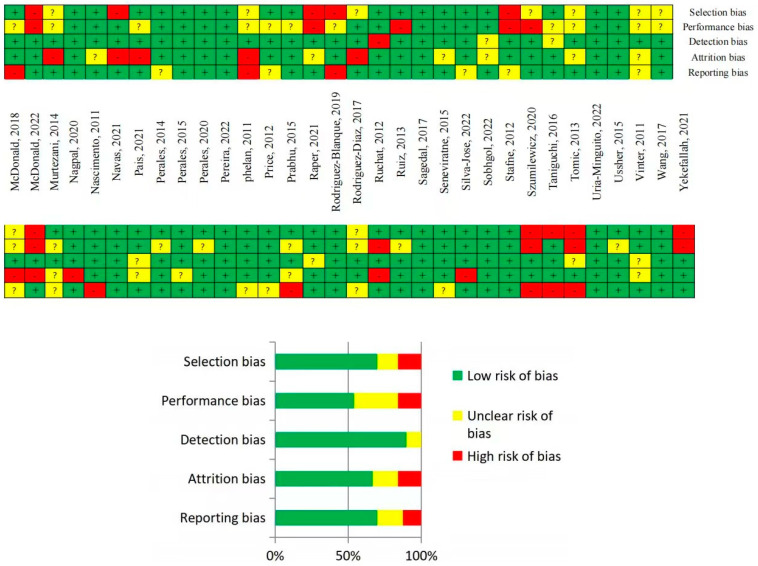
Collectively, the quality of evidence varied from low to high. In some situations, the blinding of participants to the group (intervention or control group) was not feasible, and it is typically impossible to achieve due to the intervention characteristics (physical activity intervention), resulting in unclear or high risk of bias (performance bias), depending on how it was recorded. Other sources of bias in some cases were the impossibility of finding the published article protocol (to compare the planned and measured outcomes), but also a lack of reporting (or having an uncertain definition) of the randomization process. Overall, the majority of the studies presented a low risk of bias within the five types of bias assessed. The risk of bias analysis is reported in Figure 2.
Figure 2.
Risk of bias of the included studies [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
8.3. Effect of Physical Activity during Pregnancy on Birth weight
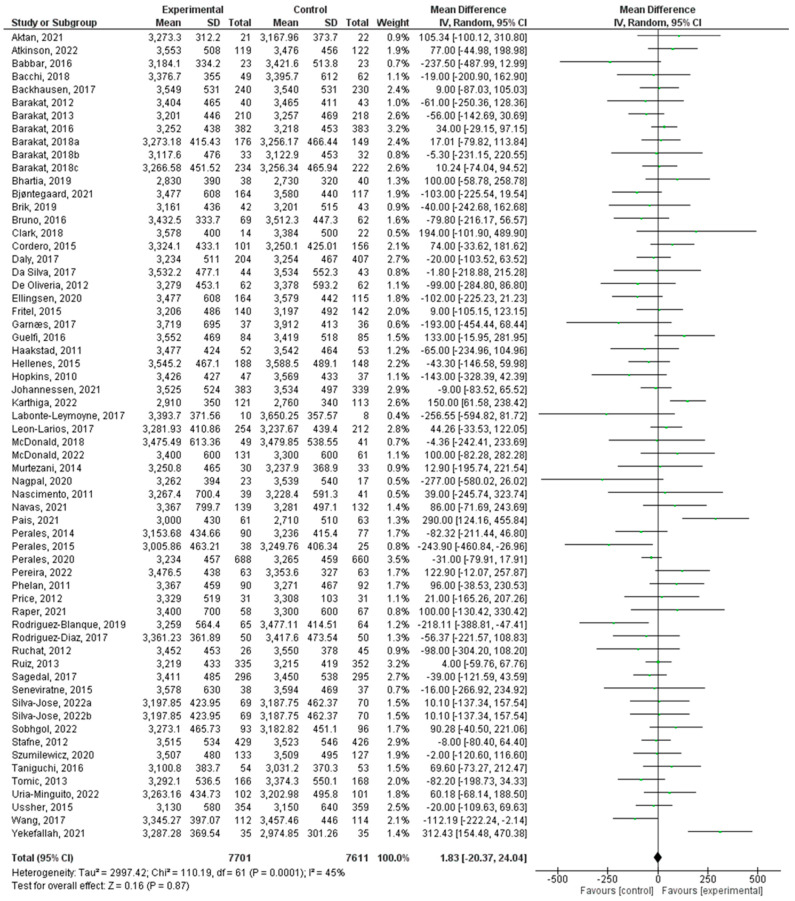
There were a total of 61 studies that were incorporated in this analysis [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,85,86]. Regular exercise or physical activity during pregnancy did not have a significant relationship with birth weight (z = 0.11; p = 0.91) (Std. Mean Dif., Random, 95% CI = 0.00 (−0.04, 0.05) I2 = 43%, Pheterogeneity = 0.0003). The forest plot corresponding to the current meta-analysis is illustrated in Figure 3.
Figure 3.
Effect of exercise during pregnancy on birth weight [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86].
8.4. Effect of Physical Activity during Pregnancy on Macrosomia
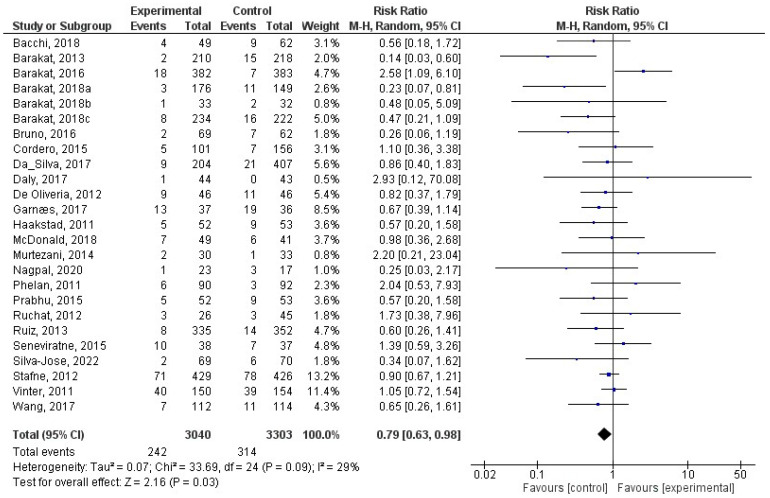
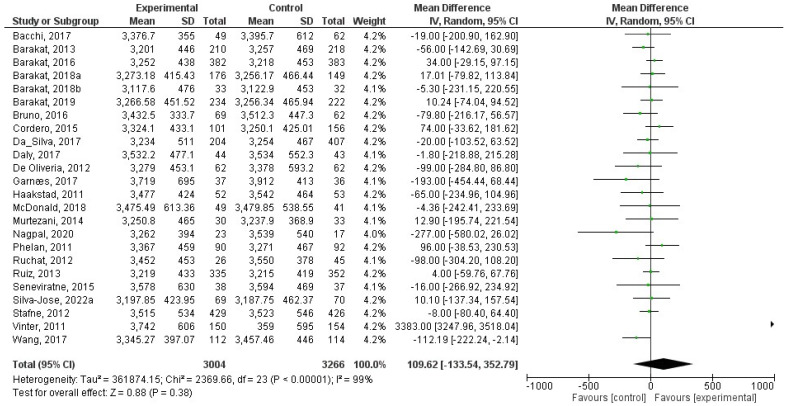
This analysis included a total of 25 studies [27,30,31,32,33,34,38,40,41,42,43,46,48,55,57,58,66,67,72,73,75,76,78,84,85]. There was a significant relationship (z = 2.16; p = 0.03) between physical activity during pregnancy and macrosomia (RR = 0.79, 95% CI = 0.63, 0.98, I2 = 29%, Pheterogeneity = 0.09) such that macrosomia occurred less frequently in the exercise group. In Figure 4, the forest plot pertaining to the current meta-analysis is depicted. In addition, we tested only the studies that reported on macrosomia for differences in birth weight, and none were observed (z = 0.86; p = 0.39) (Figure 5).
Figure 4.
Effect of exercise during pregnancy on macrosomia [27,30,31,32,33,34,38,40,41,42,43,46,48,55,57,58,66,67,72,73,75,76,78,84,85].
Figure 5.
Effect of exercise during pregnancy on birth weight in studies reporting on macrosomia [27,30,31,32,33,38,40,41,42,43,46,48,55,57,58,66,72,73,75,78,84,85].
8.5. Effect of Physical Activity during Pregnancy on Low Birth Weight
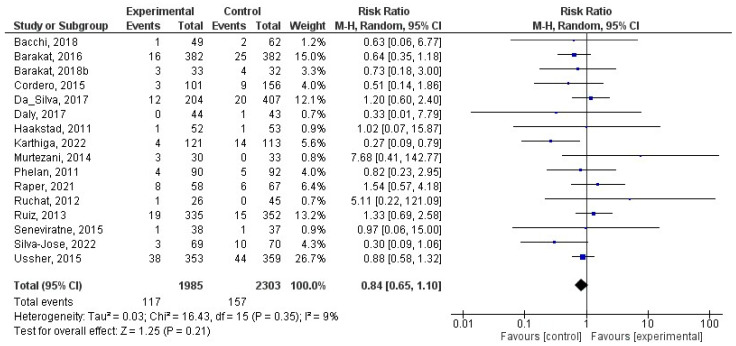
This analysis comprised a total of 16 studies [27,31,33,40,41,42,48,52,57,66,69,72,73,75,76,83]. There was no statistically significant association (z = 1.25; p = 0.21) between physical activity during pregnancy and the likelihood of low birth weight (RR = 0.84, 95% CI = 0.65, 1.10, I2 = 9%, Pheterogeneity = 0.35). Figure 6 displays the forest plot for the present meta-analysis.
Figure 6.
Effect of exercise during pregnancy on low birth weight [27,31,33,40,41,42,48,52,57,66,69,72,73,75,76,83].
8.6. Effect of Physical Activity during Pregnancy on Large for Gestational Age
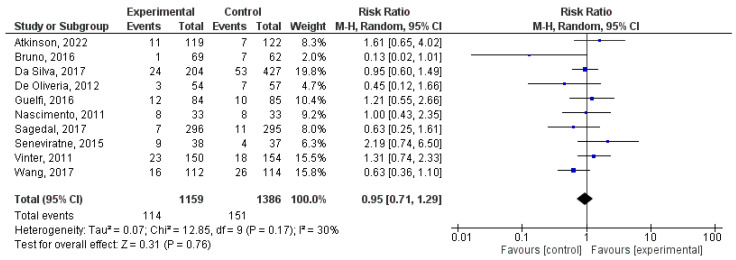
There was a total of 10 studies that were incorporated into this analysis [25,38,41,43,47,59,74,75,84,85]. There was no difference (z = 0.31; p = 0.76) between intervention and control for being large for the gestational age (RR = 0.95, 95% CI = 0.71, 1.29, I2 = 30%, Pheterogeneity = 0.17). Figure 7 visually presents the results of the meta-analysis through a forest plot.
Figure 7.
Effect of exercise during pregnancy on being large for gestational age [25,38,41,43,47,59,74,75,84,85].
8.7. Effect of Physical Activity during Pregnancy on Small for Gestational Age
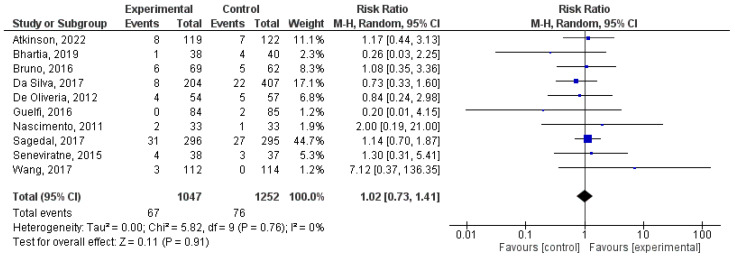
There was a total of 10 studies that were incorporated into this analysis [25,35,38,41,43,47,59,74,75,85]. Incorporating regular exercise during pregnancy did not cause a significant difference (z = 0.11; p = 0.91) for being small for the gestational age (RR = 1.02, 95% CI = 0.73, 1.41, I2 = 0%, Pheterogeneity = 0.76). The meta-analysis results are visually presented in Figure 8 through a forest plot.
Figure 8.
Effect of exercise during pregnancy on being small for gestational age [25,35,38,41,43,47,59,74,75,85].
9. Discussion
This systematic review and meta-analysis demonstrated the positive effect of physical activity in pregnancy in reducing the risk of macrosomia by referring specifically to evidence from randomized controlled trials. No other significant associations were found with indices of birth weight, including birth weight as a continuous variable, risk of being small for the gestational age, and having a low birth weight. Birth weight is often used as an accessible marker of newborn health and as an assessment of potential growth trajectories and downstream risk of chronic disease [1,92]. Physical activity during pregnancy may be a key factor in promoting appropriate birth weight, especially contributing to the prevention of macrosomia, and therefore is an important behavior that will facilitate both maternal and child health.
The only significant finding in relation to prenatal physical activity and birth weight was the reduced odds of developing macrosomia. This finding is consistent with previous reviews that have shown reduced odds of macrosomia with physical activity in pregnancy [13,93]. In fact, one systematic review that conducted a sub-analysis of randomized controlled trials only found a 39% reduced risk of macrosomia [13], and our findings further underscored this, thus supporting the effectiveness of prenatal physical activity in the prevention of macrosomia. Macrosomia has shown strong associations with the risk of downstream childhood obesity, and there have been both physiological and environmental mechanisms that have been proposed [94,95]. For example, it is theorized that macrosomia can be a proxy measure for potential adipose tissue function, including overactivity and therefore excess energy storage that can increase the risk for later-life obesity [96]. Moreover, early food restriction practices have been associated with infants who are larger, potentially as an effort to bring their weight into expected trajectories; however, this may be a detriment to appetite regulation [97,98] later on in life. In line with the Developmental Origins of Health and Disease, uterine and early life environments can program downstream childhood obesity, and perhaps prenatal physical activity is a viable factor that can prevent this by reducing the risk of macrosomia [99]. Notably, we also tested if birth weight was different only amongst the studies that reported on macrosomia, and this was not significant. We postulate that this may be due to the fact that macrosomia is defined in absolute values as >4000 g, whereas birth weight is continuous. It may also be due to the heterogeneity amongst included studies in the measurement of weight, as well as the types of exercises performed. Similar findings have been noted about nutrition and exercise interventions in pregnancy, where favorable prevalence outcomes have been found, such as the prevention of excessive gestational weight gain and macrosomia, but no differences were found when weight was measured continuously between intervention and control groups [24,57,70].
This review did not find any significant relationship with prenatal physical activity and birth weight, large- or small-for-gestational-age newborns, and low birth weight. These null findings are consistent with previous systematic reviews, meta-analyses, and observational studies [13,17,93,100]. Previous research has suggested that the relationship between physical activity during pregnancy and birth weight may have an inverted U-shape, such that higher frequencies and intensities of activity may be associated with being small for the gestational age or low birth weight, whereas lower frequencies and intensities of activity could increase risk of being large for the gestational age or macrosomia [101]. Notably, though, engagement in regular moderate levels of physical activity does not increase the risk for small-for-gestational-age and low-birth-weight newborns [13]. Therefore, although our results found no relationship with the lower end of the weight spectrum, it should be highlighted that engagement in physical activity throughout pregnancy does not increase the risk for smaller newborns. A common misconception is that physical activity in pregnancy could be unsafe for fetal development, as energy reserves would be diverted from the placenta, or there is a risk of physical harm [102]. In order to improve knowledge on the safety of maternal physical activity, it is essential that public health messaging should debunk stereotypes or myths that suggest physical activity in pregnancy can deplete or divert energy reserves for fetal growth and development.
Physical activity throughout pregnancy elicits several benefits for maternal and newborn health, including the prevention of perinatal complications such as excessive gestational weight gain and gestational diabetes [103,104]. In the present review, we assessed the direct relationship between engagement in a physical activity intervention and markers of birth weight; however, it should be acknowledged that benefits pertaining to birth weight could be moderated by improvements in other markers of perinatal health. For example, gestational weight gain and birth weight are positively correlated, and therefore, preventing excessive gestational weight gain may also affect the reduction in birth weight [105]. Similarly, gestational diabetes increases the risk for large-for-gestational-age newborns [106]. Physical activity in pregnancy reduces the risk for excessive gestational weight gain by 32% and gestational diabetes by 38% [102,103], and accordingly, may be attributed to also improving newborn birth weight. Physical activity also improves labor and delivery outcomes, including the reduced risk of cesarean section, thus preventing potential complications associated with higher-birth-weight newborns [107]. Taken together, the benefits of physical activity extend beyond indices of weight and affect both the pregnant person and newborn.
Strengths of this review include the inclusion of both English and Spanish articles, expanding the scope of our search in comparison to previous reviews that were restricted to one language, and the inclusion specifically of randomized controlled trials, allowing for the assessment of the features of physical activity interventions that may not be captured through observational studies (e.g., frequency and type of activity). Moreover, randomized controlled trials are deemed to provide more high-quality evidence. However, these results should be interpreted with caution due to the inclusion of studies deemed to be of low quality, as well as the heterogeneity in the contents of the included interventions. Future research should aim to further extrapolate findings based on the intensity of the intervention and types of physical activity. Future comprehensive research should also expand on the inclusion of additional languages.
10. Conclusions
By referring to evidence from randomized controlled trials, this review identified that prenatal physical activity could reduce the risk for macrosomia. Prenatal physical activity did not have a significant effect on mean birth weight, small- or large-for-gestational-age newborns, or low birth weight. Importantly, though, prenatal physical activity does not also increase the risk for smaller newborns, and this is an important message that should be widely disseminated to debunk myths associated with reduced or diverted energy reserves for fetal growth and development with active pregnancies. Further research is needed to examine the effect of prenatal physical activity on birth weight, including specific recommended intensity and types of activity, and whether birth weight is moderated through the prevention of other perinatal complications by physical activity, such as excessive gestational weight gain and gestational diabetes.
Acknowledgments
The authors would like to thank Ane Uría-Minguito and Alejandro Barrera for their collaboration in the preparation of this study.
Author Contributions
Conceptualization R.B., J.G.-A. and M.S.-P.; methodology, T.S.N., C.S.-J., R.B. and M.S.-P.; software, J.G.-A.; validation, D.Z. and C.S.-J.; formal analysis, D.Z., T.S.N., C.S.-J., R.B. and M.S.-P.; investigation, D.Z., T.S.N. and M.S.-P.; resources, R.B.; data curation, J.G.-A.; writing—original draft preparation, D.Z., T.S.N. and M.S.-P.; writing—review and editing, D.Z., R.B. and M.S.-P.; visualization, J.G.-A.; supervision, M.S.-P.; project administration, R.B. and M.S.-P.; funding acquisition, R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding. The APC was funded by Project UPM C2311580017. Instituto de las Mujeres. Ministerio de Igualdad de España.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chiavaroli V., Derraik J.G., Hofman P.L., Cutfield W.S. Born large for gestational age: Bigger is not always better. J. Pediatr. 2016;170:307–311. doi: 10.1016/j.jpeds.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Sebayang S.K., Dibley M.J., Kelly P.J., Shankar A.V., Shankar A.H., Group S.S. Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: Analyses of the birthweight cohort of the SUMMIT trial. Trop. Med. Int. Health. 2012;17:938–950. doi: 10.1111/j.1365-3156.2012.03039.x. [DOI] [PubMed] [Google Scholar]
- 3.Upadhyay R.P., Naik G., Choudhary T.S., Chowdhury R., Taneja S., Bhandari N., Martines J.C., Bahl R., Bhan M.K. Cognitive and motor outcomes in children born low birth weight: A systematic review and meta-analysis of studies from South Asia. BMC Pediatr. 2019;19:35. doi: 10.1186/s12887-019-1408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stålnacke S.R., Tessma M., Böhm B., Herlenius E. Cognitive development trajectories in preterm children with very low birth weight longitudinally followed until 11 years of age. Front. Physiol. 2019;10:307. doi: 10.3389/fphys.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamorski M.A., Biggs W.S. Management of suspected fetal macrosomia. Am. Fam. Physician. 2001;63:302. [PubMed] [Google Scholar]
- 6.Boulet S.L., Alexander G.R., Salihu H.M., Pass M. Macrosomic births in the United States: Determinants, outcomes, and proposed grades of risk. Am. J. Obstet. Gynecol. 2003;188:1372–1378. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- 7.Boulet S.L., Salihu H.M., Alexander G.R. Mode of delivery and the survival of macrosomic infants in the United States, 1995–1999. Birth. 2006;33:278–283. doi: 10.1111/j.1523-536X.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Decker A., Platt R.W., Kramer M.S. How big is too big? The perinatal consequences of fetal macrosomia. Am. J. Obstet. Gynecol. 2008;198:517.e1–517.e6. doi: 10.1016/j.ajog.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs F., Bouyer J., Rozenberg P., Senat M.-V. Adverse maternal outcomes associated with fetal macrosomia: What are the risk factors beyond birthweight? BMC Pregnancy Childbirth. 2013;13:90. doi: 10.1186/1471-2393-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomić V., Bošnjak K., Petrov B., Đikić M., Knežević D. Macrosomic births at Mostar Clinical Hospital: A 2-year review. Bosn. J. Basic Med. Sci. 2007;7:271. doi: 10.17305/bjbms.2007.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagpal T.S., Mottola M.F. Physical activity throughout pregnancy is key to preventing chronic disease. Reproduction. 2020;160:R111. doi: 10.1530/REP-20-0337. [DOI] [PubMed] [Google Scholar]
- 12.Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., Carty C., Chaput J.P., Chastin S., Chou R., et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport M.H., Meah V.L., Ruchat S.-M., Davies G.A., Skow R.J., Barrowman N., Adamo K.B., Poitras V.J., Gray C.E., Garcia A.J. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018;52:1386–1396. doi: 10.1136/bjsports-2018-099836. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Fernandez J., Ochoa J.J., Lopez-Frias M., Diaz-Castro J. Impact of early nutrition, physical activity and sleep on the fetal programming of disease in the pregnancy: A narrative review. Nutrients. 2020;12:3900. doi: 10.3390/nu12123900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everest C., da Silva D.F., Puranda J., Souza S.C., Goudreau A.D., Nagpal T.S., Edwards C.M., Gupta R., Adamo K.B. Physical Activity and Weight Gain Throughout Pregnancy Are Associated With Umbilical Cord Markers. J. Obstet. Gynaecol. Can. 2022;44:1262–1270. doi: 10.1016/j.jogc.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Gascoigne E.L., Webster C.M., Honart A.W., Wang P., Smith-Ryan A., Manuck T.A. Physical activity and pregnancy outcomes: An expert review. Am. J. Obstet. Gynecol. MFM. 2023;5:100758. doi: 10.1016/j.ajogmf.2022.100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forczek W., Curylo M., Forczek B. Physical activity assessment during gestation and its outcomes: A review. Obstet. Gynecol. Surv. 2017;72:425–444. doi: 10.1097/OGX.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 18.Menke B.R., Duchette C., Tinius R.A., Wilson A.Q., Altizer E.A., Maples J.M. Physical Activity during Pregnancy and Newborn Body Composition: A Systematic Review. Int. J. Environ. Res. Public Health. 2022;19:7127. doi: 10.3390/ijerph19127127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins C.C., Pivarnik J.M., Paneth N., Stein A.D. Physical activity and fetal growth during pregnancy. Obstet. Gynecol. 2007;109:81–87. doi: 10.1097/01.AOG.0000249605.11458.ac. [DOI] [PubMed] [Google Scholar]
- 20.Teede H.J., Bailey C., Moran L.J., Khomami M.B., Enticott J., Ranasinha S., Rogozińska E., Skouteris H., Boyle J.A., Thangaratinam S. Association of antenatal diet and physical activity–based interventions with gestational weight gain and pregnancy outcomes: A systematic review and meta-analysis. JAMA Intern. Med. 2022;182:106–114. doi: 10.1001/jamainternmed.2021.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015;126:e135–e142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 23.Davies G.A., Wolfe L.A., Mottola M.F., MacKinnon C. Joint SOGC/CSEP clinical practice guideline: Exercise in pregnancy and the postpartum period. Can. J. Appl. Physiol. 2003;28:329–341. doi: 10.1139/h03-024. [DOI] [PubMed] [Google Scholar]
- 24.Aktan B., Kayıkçıoğlu F., Akbayrak T. The comparison of the effects of clinical Pilates exercises with and without childbirth training on pregnancy and birth results. Int. J. Clin. Pract. 2021;75:e14516. doi: 10.1111/ijcp.14516. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson S.A., Maran A., Dempsey K., Perreault M., Vanniyasingam T., Phillips S.M., Hutton E.K., Mottola M.F., Wahoush O., Xie F. Be Healthy in Pregnancy (BHIP): A randomized controlled trial of nutrition and exercise intervention from early pregnancy to achieve recommended gestational weight gain. Nutrients. 2022;14:810. doi: 10.3390/nu14040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babbar S., Hill J.B., Williams K.B., Pinon M., Chauhan S.P., Maulik D. Acute feTal behavioral Response to prenatal Yoga: A single, blinded, randomized controlled trial (TRY yoga) Am. J. Obstet. Gynecol. 2016;214:399.e1–399.e8. doi: 10.1016/j.ajog.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Bacchi M., Mottola M.F., Perales M., Refoyo I., Barakat R. Aquatic activities during pregnancy prevent excessive maternal weight gain and preserve birth weight: A randomized clinical trial. Am. J. Health Promot. 2018;32:729–735. doi: 10.1177/0890117117697520. [DOI] [PubMed] [Google Scholar]
- 28.Backhausen M.G., Tabor A., Albert H., Rosthøj S., Damm P., Hegaard H.K. The effects of an unsupervised water exercise program on low back pain and sick leave among healthy pregnant women–A randomised controlled trial. PLoS ONE. 2017;12:e0182114. doi: 10.1371/journal.pone.0182114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barakat R., Pelaez M., Lopez C., Montejo R., Coteron J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: Results of a randomized controlled trial. J. Matern. Fetal. Neonatal. Med. 2012;25:2372–2376. doi: 10.3109/14767058.2012.696165. [DOI] [PubMed] [Google Scholar]
- 30.Barakat R., Pelaez M., Lopez C., Lucia A., Ruiz J.R. Exercise during pregnancy and gestational diabetes-related adverse effects: A randomized controlled trial. Br. J. Sports Med. 2013;47:630–636. doi: 10.1136/bjsports-2012-091788. [DOI] [PubMed] [Google Scholar]
- 31.Barakat R., Pelaez M., Cordero Y., Perales M., Lopez C., Coteron J., Mottola M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016;214:649.e1–649.e8. doi: 10.1016/j.ajog.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Barakat R., Franco E., Perales M., Lopez C., Mottola M.F. Exercise during pregnancy is associated with a shorter duration of labor. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018;224:33–40. doi: 10.1016/j.ejogrb.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Barakat R., Vargas M., Brik M., Fernandez I., Gil J., Coteron J., Santacruz B. Does Exercise During Pregnancy Affect Placental Weight?: A Randomized Clinical Trial. Eval. Health Prof. 2018;41:400–414. doi: 10.1177/0163278717706235. [DOI] [PubMed] [Google Scholar]
- 34.Barakat R., Refoyo I., Coteron J., Franco E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz. J. Phys. Ther. 2018;23:148–155. doi: 10.1016/j.bjpt.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhartia N., Jain S., Shankar N., Rajaram S., Gupta M. Effects of antenatal yoga on maternal stress and clinical outcomes in north indian women: A randomised controlled trial. J. Indian Acad. Clin. Med. 2019;20:10–14. [Google Scholar]
- 36.Bjontegaard K.A., Stafne S.N., Morkved S., Salvesen K.A., Evensen K.A.I. Body mass index and physical activity in seven-year-old children whose mothers exercised during pregnancy: Follow-up of a multicentre randomised controlled trial. BMC Pediatr. 2021;21:496. doi: 10.1186/s12887-021-02952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brik M., Fernández-Buhigas I., Martin-Arias A., Vargas-Terrones M., Barakat R., Santacruz B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet. Gynecol. 2019;53:583–589. doi: 10.1002/uog.20147. [DOI] [PubMed] [Google Scholar]
- 38.Bruno R., Petrella E., Bertarini V., Pedrielli G., Neri I., Facchinetti F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern. Child Nutr. 2017;13:e12333. doi: 10.1111/mcn.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark E., Isler C., Strickland D., McMillan A.G., Fang X., Kuehn D., Ravisankar S., Strom C., May L.E. Influence of aerobic exercise on maternal lipid levels and offspring morphometrics. Int. J. Obes. 2019;43:594–602. doi: 10.1038/s41366-018-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordero Y., Mottola M.F., Vargas J., Blanco M., Barakat R. Exercise Is Associated with a Reduction in Gestational Diabetes Mellitus. Med. Sci. Sports Exerc. 2015;47:1328–1333. doi: 10.1249/MSS.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 41.da Silva S.G., Hallal P.C., Domingues M.R., Bertoldi A.D., Silveira M.F.d., Bassani D., da Silva I.C.M., da Silva B.G.C., Coll C.d.V.N., Evenson K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int. J. Behav. Nutr. Phys. Act. 2017;14:175. doi: 10.1186/s12966-017-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daly N., Farren M., McKeating A., O’Kelly R., Stapleton M., Turner M.J. A Medically Supervised Pregnancy Exercise Intervention in Obese Women: A Randomized Controlled Trial. Obstet. Gynecol. 2017;130:1001–1010. doi: 10.1097/AOG.0000000000002267. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveria Melo A.S., Silva J.L.P., Tavares J.S., Barros V.O., Leite D.F., Amorim M.M. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012;120:302–310. doi: 10.1097/AOG.0b013e31825de592. [DOI] [PubMed] [Google Scholar]
- 44.Ellingsen M.S., Pettersen A., Stafne S.N., Morkved S., Salvesen K.A., Evensen K. Neurodevelopmental outcome in 7-year-old children is not affected by exercise during pregnancy: Follow up of a multicentre randomised controlled trial. BJOG. 2020;127:508–517. doi: 10.1111/1471-0528.16024. [DOI] [PubMed] [Google Scholar]
- 45.Fritel X., De Tayrac R., Bader G., Savary D., Gueye A., Deffieux X., Fernandez H., Richet C., Guilhot J., Fauconnier A. Preventing urinary incontinence with supervised prenatal pelvic floor exercises: A randomized controlled trial. Obstet. Gynecol. 2015;126:370–377. doi: 10.1097/AOG.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 46.Garnaes K.K., Nyrnes S.A., Salvesen K.Å., Salvesen Ø., Mørkved S., Moholdt T. Effect of supervised exercise training during pregnancy on neonatal and maternal outcomes among overweight and obese women. Secondary analyses of the ETIP trial: A randomised controlled trial. PLoS ONE. 2017;12:e0173937. doi: 10.1371/journal.pone.0173937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guelfi K.J., Ong M.J., Crisp N.A., Fournier P.A., Wallman K.E., Grove J.R., Doherty D.A., Newnham J.P. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016;128:819–827. doi: 10.1097/AOG.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 48.Haakstad L.A., Bø K. Exercise in pregnant women and birth weight: A randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:66. doi: 10.1186/1471-2393-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellenes O.M., Vik T., Løhaugen G.C., Salvesen K.Å., Stafne S.N., Mørkved S., Evensen K.A.I. Regular moderate exercise during pregnancy does not have an adverse effect on the neurodevelopment of the child. Acta Paediatr. 2015;104:285–291. doi: 10.1111/apa.12890. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins S.A., Baldi J.C., Cutfield W.S., McCowan L., Hofman P.L. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J. Clin. Endocrinol. Metab. 2010;95:2080–2088. doi: 10.1210/jc.2009-2255. [DOI] [PubMed] [Google Scholar]
- 51.Johannessen H.H., Froshaug B.E., Lysaker P.J.G., Salvesen K.A., Lukasse M., Morkved S., Stafne S.N. Regular antenatal exercise including pelvic floor muscle training reduces urinary incontinence 3 months postpartum-Follow up of a randomized controlled trial. Acta Obstet. Gynecol. Scand. 2021;100:294–301. doi: 10.1111/aogs.14010. [DOI] [PubMed] [Google Scholar]
- 52.Karthiga K., Pal G.K., Dasari P., Nanda N., Velkumary S., Chinnakali P., Renugasundari M., Harichandrakumar K.T. Effects of yoga on cardiometabolic risks and fetomaternal outcomes are associated with serum nitric oxide in gestational hypertension: A randomized control trial. Sci. Rep. 2022;12:11732. doi: 10.1038/s41598-022-15216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labonte-Lemoyne E., Curnier D., Ellemberg D. Exercise during pregnancy enhances cerebral maturation in the newborn: A randomized controlled trial. J. Clin. Exp. Neuropsychol. 2017;39:347–354. doi: 10.1080/13803395.2016.1227427. [DOI] [PubMed] [Google Scholar]
- 54.Leon-Larios F., Corrales-Gutierrez I., Casado-Mejia R., Suarez-Serrano C. Influence of a pelvic floor training programme to prevent perineal trauma: A quasi-randomised controlled trial. Midwifery. 2017;50:72–77. doi: 10.1016/j.midw.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 55.McDonald S.M., Yeo S., Liu J., Wilcox S., Sui X., Pate R.R. Associations between maternal physical activity and fitness during pregnancy and infant birthweight. Prev. Med. Rep. 2018;11:1–6. doi: 10.1016/j.pmedr.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDonald S.M., Mouro S., Wisseman B., Isler C., DeVente J., Newton E., Hildebrand J., Kuehn D., Kelley G., Chasan-Taber L. Influence of prenatal exercise on the relationship between maternal overweight and obesity and select delivery outcomes. Sci. Rep. 2022;12:17343. doi: 10.1038/s41598-022-22283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murtezani A., Paçarada M., Ibraimi Z., Nevzati A., Abazi N. The impact of exercise during pregnancy on neonatal outcomes: A randomized controlled trial. J. Sports Med. Phys. Fitness. 2014;54:802–808. [PubMed] [Google Scholar]
- 58.Nagpal T.S., Prapavessis H., Campbell C.G., de Vrijer B., Bgeginski R., Hosein K., Paplinskie S., Manley M., Mottola M.F. Sequential introduction of exercise first followed by nutrition improves program adherence during pregnancy: A randomized controlled trial. Int. J. Behav. Med. 2020;27:108–118. doi: 10.1007/s12529-019-09840-0. [DOI] [PubMed] [Google Scholar]
- 59.Nascimento S.L., Surita F.G., Parpinelli M.A., Siani S., Pinto e Silva J.L. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: A randomised clinical trial. BJOG. 2011;118:1455–1463. doi: 10.1111/j.1471-0528.2011.03084.x. [DOI] [PubMed] [Google Scholar]
- 60.Navas A., Carrascosa M.d.C., Artigues C., Ortas S., Portells E., Soler A., Yañez A.M., Bennasar-Veny M., Leiva A. Effectiveness of moderate-intensity aerobic water exercise during pregnancy on quality of life and postpartum depression: A multi-center, randomized controlled trial. J. Clin. Med. 2021;10:2432. doi: 10.3390/jcm10112432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pais M., Pai M.V., Kamath A., Bhat R., Bhat P., Joisa G.H. A Randomized Controlled Trial on the Efficacy of Integrated Yoga on Pregnancy Outcome. Holist. Nurs. Pract. 2021;35:273–280. doi: 10.1097/HNP.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 62.Perales M., Refoyo I., Coteron J., Bacchi M., Barakat R. Exercise during pregnancy attenuates prenatal depression: A randomized controlled trial. Eval. Health Prof. 2015;38:59–72. doi: 10.1177/0163278714533566. [DOI] [PubMed] [Google Scholar]
- 63.Perales Santaella M., Mateos S., Vargas M., Sanz I., Lucía Mulas A., Barakat Carballo R.O. Fetal and maternal heart rate responses to exercise in pregnant women. A randomized Controlled Trial. Arch. Med. Deporte. 2015;170:361–367. [Google Scholar]
- 64.Perales M., Valenzuela P.L., Barakat R., Cordero Y., Pelaez M., Lopez C., Ruilope L.M., Santos-Lozano A., Lucia A. Gestational Exercise and Maternal and Child Health: Effects until Delivery and at Post-Natal Follow-up. J. Clin. Med. 2020;9:379. doi: 10.3390/jcm9020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pereira I.B., Silva R., Ayres-de-Campos D., Clode N. Physical exercise at term for enhancing the spontaneous onset of labor: A randomized clinical trial. J. Matern. Fetal. Neonatal. Med. 2022;35:775–779. doi: 10.1080/14767058.2020.1732341. [DOI] [PubMed] [Google Scholar]
- 66.Phelan S., Phipps M.G., Abrams B., Darroch F., Schaffner A., Wing R.R. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for Delivery Study. Am. J. Clin. Nutr. 2011;93:772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price B.B., Amini S.B., Kappeler K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012;44:2263–2269. doi: 10.1249/MSS.0b013e318267ad67. [DOI] [PubMed] [Google Scholar]
- 68.Prabhu N. Effect of a exercise by pregnant women and birth weight: A randomized controlled trial. J. Evol. Med. Dent. Sci. 2015;4:1509–1517. doi: 10.14260/jemds/2015/212. [DOI] [Google Scholar]
- 69.Raper M.J., McDonald S., Johnston C., Isler C., Newton E., Kuehn D., Collier D., Broskey N.T., Muldrow A., May L.E. The influence of exercise during pregnancy on racial/ethnic health disparities and birth outcomes. BMC Pregnancy Childbirth. 2021;21:258. doi: 10.1186/s12884-021-03717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez-Blanque R., Sanchez-Garcia J.C., Sanchez-Lopez A.M., Exposito-Ruiz M., Aguilar-Cordero M.J. Randomized Clinical Trial of an Aquatic Physical Exercise Program During Pregnancy. J. Obstet. Gynecol. Neonatal. Nurs. 2019;48:321–331. doi: 10.1016/j.jogn.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Rodríguez-Díaz L., Ruiz-Frutos C., Vázquez-Lara J.M., Ramírez-Rodrigo J., Villaverde-Gutiérrez C., Torres-Luque G. Effectiveness of a physical activity programme based on the Pilates method in pregnancy and labour. Enferm. Clín. 2017;27:271–277. doi: 10.1016/j.enfcli.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Ruchat S.-M., Davenport M.H., Giroux I., Hillier M., Batada A., Sopper M.M., Hammond J., Mottola M.F. Nutrition and exercise reduce excessive weight gain in normal-weight pregnant women. Med. Sci. Sports Exerc. 2012;44:1419–1426. doi: 10.1249/MSS.0b013e31825365f1. [DOI] [PubMed] [Google Scholar]
- 73.Ruiz J.R., Perales M., Pelaez M., Lopez C., Lucia A., Barakat R. Supervised exercise–based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin. Proc. 2013;88:1388–1397. doi: 10.1016/j.mayocp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 74.Sagedal L., Øverby N., Bere E., Torstveit M., Lohne-Seiler H., Småstuen M., Hillesund E., Henriksen T., Vistad I. Lifestyle intervention to limit gestational weight gain: The Norwegian Fit for Delivery randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2017;124:97–109. doi: 10.1111/1471-0528.13862. [DOI] [PubMed] [Google Scholar]
- 75.Seneviratne S.N., Jiang Y., Derraik J., McCowan L., Parry G.K., Biggs J.B., Craigie S., Gusso S., Peres G., Rodrigues R.O., et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG. 2016;123:588–597. doi: 10.1111/1471-0528.13738. [DOI] [PubMed] [Google Scholar]
- 76.Silva-Jose C., Sánchez-Polán M., Barakat R., Díaz-Blanco Á., Mottola M.F., Refoyo I. A Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic Modifies Maternal Weight Gain, Smoking Habits and Birth Weight—Randomized Clinical Trial. J. Clin. Med. 2022;11:4045. doi: 10.3390/jcm11144045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sobhgol S.S., Smith C.A., Thomson R., Dahlen H.G. The effect of antenatal pelvic floor muscle exercise on sexual function and labour and birth outcomes: A randomised controlled trial. Women Birth. 2022;35:e607–e614. doi: 10.1016/j.wombi.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Stafne S.N., Salvesen K.A., Romundstad P.R., Eggebo T.M., Carlsen S.M., Morkved S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet. Gynecol. 2012;119:29–36. doi: 10.1097/AOG.0b013e3182393f86. [DOI] [PubMed] [Google Scholar]
- 79.Szumilewicz A., Kuchta A., Kranich M., Dornowski M., Jastrzebski Z. Prenatal high-low impact exercise program supported by pelvic floor muscle education and training decreases the life impact of postnatal urinary incontinence: A quasiexperimental trial. Medicine. 2020;99:e18874. doi: 10.1097/MD.0000000000018874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taniguchi C., Sato C. Home-based walking during pregnancy affects mood and birth outcomes among sedentary women: A randomized controlled trial. Int. J. Nurs. Pract. 2016;22:420–426. doi: 10.1111/ijn.12453. [DOI] [PubMed] [Google Scholar]
- 81.Tomic V., Sporis G., Tomic J., Milanovic Z., Zigmundovac-Klaic D., Pantelic S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat. Med. J. 2013;54:362–368. doi: 10.3325/cmj.2013.54.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uria-Minguito A., Silva-Jose C., Sanchez-Polan M., Diaz-Blanco A., Garcia-Benasach F., Carrero Martinez V., Alzola I., Barakat R. The Effect of Online Supervised Exercise throughout Pregnancy on the Prevention of Gestational Diabetes in Healthy Pregnant Women during COVID-19 Pandemic: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health. 2022;19:14104. doi: 10.3390/ijerph192114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ussher M., Lewis S., Aveyard P., Manyonda I., West R., Lewis B., Marcus B., Riaz M., Taylor A.H., Barton P., et al. The London Exercise And Pregnant smokers (LEAP) trial: A randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technol. Assess. 2015;19:1–135. doi: 10.3310/hta19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vinter C.A., Jensen D.M., Ovesen P., Beck-Nielsen H., Jørgensen J.S. The LiP (Lifestyle in Pregnancy) study: A randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34:2502–2507. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C., Wei Y., Zhang X., Zhang Y., Xu Q., Sun Y., Su S., Zhang L., Liu C., Feng Y., et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017;216:340–351. doi: 10.1016/j.ajog.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 86.Yekefallah L., Namdar P., Dehghankar L., Golestaneh F., Taheri S., Mohammadkhaniha F. The effect of yoga on the delivery and neonatal outcomes in nulliparous pregnant women in Iran: A clinical trial study. BMC Pregnancy Childbirth. 2021;21:351. doi: 10.1186/s12884-021-03794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Granholm A., Alhazzani W., Møller M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019;123:554–559. doi: 10.1016/j.bja.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 88.Higgins J.P., Savović J., Page M.J., Elbers R.G., Sterne J.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Chichester, UK: 2019. Assessing risk of bias in a randomized trial; pp. 205–228. [Google Scholar]
- 89.Hedges L.V., Tipton E., Johnson M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods. 2010;1:39–65. doi: 10.1002/jrsm.5. [DOI] [PubMed] [Google Scholar]
- 90.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 91.Higgins J. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.6. John Wiley & Sons; Chichester, UK: 2011. Analysing data and undertaking meta-analyses. [Google Scholar]
- 92.Saigal S., Stoskopf B., Streiner D., Paneth N., Pinelli J., Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: A longitudinal, population-based study. Pediatr. Res. 2006;60:751–758. doi: 10.1203/01.pdr.0000246201.93662.8e. [DOI] [PubMed] [Google Scholar]
- 93.Bisson M., Lavoie-Guénette J., Tremblay A., Marc I. Physical activity volumes during pregnancy: A systematic review and meta-analysis of observational studies assessing the association with infant’s birth weight. Am. J. Perinatol. Rep. 2016;6:e170–e197. doi: 10.1055/s-0036-1583169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Most J., Marlatt K.L., Altazan A.D., Redman L.M. Advances in assessing body composition during pregnancy. Eur. J. Clin. Nutr. 2018;72:645–656. doi: 10.1038/s41430-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y., Gao E., Wu J., Zhou J., Yang Q., Walker M., Mbikay M., Sigal R., Nair R., Wen S. Fetal macrosomia and adolescence obesity: Results from a longitudinal cohort study. Int. J. Obes. 2009;33:923–928. doi: 10.1038/ijo.2009.131. [DOI] [PubMed] [Google Scholar]
- 96.Sauder K.A., Ritchie N.D. Reducing intergenerational obesity and diabetes risk. Diabetologia. 2021;64:481–490. doi: 10.1007/s00125-020-05341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beall M.H., El Haddad M., Gayle D., Desai M., Ross M.G. Adult obesity as a consequence of in utero programming. Clin. Obstet. Gynecol. 2004;47:957–966. doi: 10.1097/01.grf.0000135668.61661.9c. [DOI] [PubMed] [Google Scholar]
- 98.Sarr O., Yang K., Regnault T.R. In utero programming of later adiposity: The role of fetal growth restriction. J. Pregnancy. 2012;2012:134758. doi: 10.1155/2012/134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silveira P.P., Portella A.K., Goldani M.Z., Barbieri M.A. Developmental origins of health and disease (DOHaD) J. Pediatr. 2007;83:494–504. doi: 10.2223/JPED.1728. [DOI] [PubMed] [Google Scholar]
- 100.Santos P.C., Leirós-Rodríguez R., Abreu S., Ferreira M., Alves O., Mota J. Physical activity during pregnancy and its effects on neonatal outcomes. Placenta. 2022;128:9–17. doi: 10.1016/j.placenta.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Jantunen H., Wasenius N., Salonen M.K., Perälä M.-M., Kautiainen H., Simonen M., Pohjolainen P., Kajantie E., von Bonsdorff M., Eriksson J. Relationship between physical activity and physical performance in later life in different birth weight groups. J. Dev. Orig. Health Dis. 2018;9:95–101. doi: 10.1017/S2040174417000575. [DOI] [PubMed] [Google Scholar]
- 102.Walasik I., Kwiatkowska K., Kosińska Kaczyńska K., Szymusik I. Physical activity patterns among 9000 pregnant women in poland: A cross-sectional study. Int. J. Environ. Res. Public Health. 2020;17:1771. doi: 10.3390/ijerph17051771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davenport M.H., Ruchat S.-M., Poitras V.J., Garcia A.J., Gray C.E., Barrowman N., Skow R.J., Meah V.L., Riske L., Sobierajski F. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018;52:1367–1375. doi: 10.1136/bjsports-2018-099355. [DOI] [PubMed] [Google Scholar]
- 104.Ruchat S.-M., Mottola M.F., Skow R.J., Nagpal T.S., Meah V.L., James M., Riske L., Sobierajski F., Kathol A.J., Marchand A.-A. Effectiveness of exercise interventions in the prevention of excessive gestational weight gain and postpartum weight retention: A systematic review and meta-analysis. Br. J. Sports Med. 2018;52:1347–1356. doi: 10.1136/bjsports-2018-099399. [DOI] [PubMed] [Google Scholar]
- 105.Currie L.M., Woolcott C.G., Fell D.B., Armson B.A., Dodds L. The association between physical activity and maternal and neonatal outcomes: A prospective cohort. Matern. Child Health J. 2014;18:1823–1830. doi: 10.1007/s10995-013-1426-3. [DOI] [PubMed] [Google Scholar]
- 106.Mou S.S., Gillies C., Hu J., Danielli M., Al Wattar B.H., Khunti K., Tan B.K. Association between HbA1c Levels and Fetal Macrosomia and Large for Gestational Age Babies in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of 17,711 Women. J. Clin. Med. 2023;12:3852. doi: 10.3390/jcm12113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119. doi: 10.1136/bmj.j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.