Abstract
Background
Diabetes is a severe challenge to global public health since it is a leading cause of morbidity, mortality, and rising healthcare costs. 3.0 million Ethiopians, or 4.7% of the population, had diabetes in 2021. Studies on the chronic complications of diabetes in Ethiopia have not been conducted in lower-level healthcare facilities, so the findings from tertiary hospitals do not accurately reflect the issues with chronic diabetes in general hospitals. In addition, there is a lack of information and little research on the complications of chronic diabetes in Ethiopia. The objective of this study was to assess the degree of chronic diabetes complications and associated factors among diabetic patients presenting to general hospitals in the Tigray area in northern Ethiopia.
Methods
As part of a multi-centre cross-sectional study, 1,158 type 2 diabetes (T2D) patients from 10 general hospitals in the Tigray region were randomly chosen. An interviewer-administered questionnaire, a record review, and an SPSS version 20 analysis were used to collect the data. All continuous data were presented as mean standard deviation (SD), while categorical data were identified by frequencies. Using a multivariable logistic regression model, the factors associated with chronic diabetes complications among T2D diabetic patients were found, and linked factors were declared at p 0.05.
Results
Fifty-four of people with diabetes have chronic problems. Hypertension (27%) eye illness, renal disease (19.1%), and hypertension (27%) eye disease were the most common long-term effects of diabetes. Patients with chronic diabetes complications were more likely to be older than 60, taking insulin and an OHGA (Oral Hyperglycemic Agent) (AOR = 3.00; 95% CI 1.73, 5.26), having diabetes for more than five years, taking more than four tablets per day (AOR = 1.63; 95% CI 1.23,2.15), and having high systolic and diastolic blood pressure. Patients with government employment (AOR = 0.48; 95% CI 0.26, 0.90), antiplatelet drug use (AOR = 0.29; 95% CI 0.16, 0.52), and medication for treating dyslipidemia (AOR = 0.54; 95% CI 0.35, 0.84), all had a decreased chance of developing a chronic diabetes problem.
Conclusion
At least one chronic diabetic complication was present in more than half of the patients in this study. Chronic diabetes problems were related to patients’ characteristics like age, occupation, diabetes treatment plan, anti-platelet, anti-dyslipidemia medicine, duration of diabetes, high Systolic BP, high Diastolic BP, and pill burden. To avoid complications from occurring, diabetes care professionals and stakeholders must collaborate to establish appropriate methods, especially for individuals who are more likely to experience diabetic complications.
Background
Hyperglycemia is a metabolic condition associated with diabetes mellitus [1]. There are two basic forms of diabetes: Type 1 diabetes and Type 2 diabetes. Type 1 diabetes mainly affects youngsters and is defined by an insulin shortage that necessitates daily insulin injections [2]. The most prevalent form of diabetes with insulin resistance and a relative insulin deficit is type 2 [3]. There are now more cases of diabetes than ever before due to population growth, age, urbanization, obesity, and inactivity [4]. The prevalence of diabetes was found to be 10.5% (537 million people) worldwide, 4.5% (24 million people) in Africa, and 4.7% (3 million people) in Ethiopia, according to an IDF (International Diabetes Federation) atlas report from 2021 [5].
Diabetes impacts people’s functional skills and quality of life, leading to severe morbidity and premature mortality, and is one of the top public health concerns in the globe [6]. It negatively affects socioeconomic development and public health globally [7]. [8] Diabetes accounts for more than 80% of all early fatalities from non-communicable diseases (NCDs), along with cardiovascular disease, cancer, and respiratory illnesses [8]. In addition, patients with diabetes have a two to three times higher risk of dying from any cause, including cancer, cardiovascular disease, stroke, chronic renal disease, and liver disease [9–11]. Due to insufficient monitoring and skewed or incorrect laboratory data, patients are more likely to have limited compliance with therapies in outpatient settings [12]. Diabetic individuals with poorly controlled hyperglycemia can develop macrovascular diseases such as peripheral artery disease, cardiovascular disease, and cerebrovascular disease as well as microvascular consequences like retinopathy, nephropathy, and neuropathy [13,14].
For instance, studies on the prevalence of chronic diabetic complications revealed that 96% of patients had hypertension, 46% had peripheral neuropathy, 30% had neuropathy, and 7% had neuropathy encountered impotence [15] and the main reason for admission was diabetic foot ulcer (39%) and cardiovascular disease (21%) [16]. Diabetic retinopathy is a prominent cause of blindness, of which 2.6% is attributed to diabetes [17]. In addition, glaucoma, cataract, and other disorder of the eye occur earlier and more frequently in people with diabetes [18]. Similarly, there is a remarkable prevalence of both acute and chronic complications in diabetic cases in Ethiopia [19].
Information on the prevalence of diabetes-related complications is essential to change diabetes management policies and practices to effectively control the disease. However, studies focusing on such topics are rare in Ethiopia [15,20,21] and no studies have been conducted in the study area. In Ethiopia, diabetes-related studies mainly focused on glycemic control, dyslipidemia, self-care management, diabetes prevalence and diabetes complications in hospitalized/high-risk patients [22–26].
Although very few studies on chronic diabetes complications have been conducted in different parts of the country, there has not been a recent comprehensive study of outpatients in general hospitals. On the other hand, because those studies were conducted in tertiary hospitals, they were unable to provide a precise picture of diabetes complications at a lower level, such as in general hospitals. In addition, those studies were carried out among high-risk individuals, the study population of type 1 and 2 diabetes, assessing only acute complications, microvascular or macrovascular complications, and in some studies data were collected through document review only. Furthermore, it is essential to investigate diabetes complications and their contributing factors regularly to pot evolving patterns and formulate diabetes management strategies. Therefore, this study aimed to identify chronic complications related to diabetes and associated factors in general hospitals in the Tigray region of Northern Ethiopia.
Method and materials
Study design, setting and period
A multi-centre cross-sectional study was conducted in the Tigray region from September 2019 to January 2020. Tigray is one of the ten regional states of Ethiopia. The Ethiopian health care system is organized into three-tier: primary, secondary and tertiary levels of care. The primary level of care is provided at a primary hospital, health centre and health post. The Primary Health care Unit (PHCU) is composed of a health centre (HC) and five satellite health posts (HP). These facilities provide service to approximately 25,000 people. A primary hospital provides inpatient and ambulatory service to an average of 100,000 Population and has an inpatient capacity of 25–50 beds. A general hospital provides inpatient and ambulatory services to an average of 1,000,000 people. A tertiary hospital serves an average of five million people. It serves as a referral to the general hospital [27].
In 2019/2020, in the Tigray region, there were 2 referral hospitals, 14 general hospitals, 24 primary hospitals, 230 health centres and 741 health posts. There were more than 310 ambulances and a well-established referral system. There were over 750 private health facilities in the private sector, ranging from drug vendors and clinics to general and specialized hospitals. There were more than 25,000 health workforce ranging from health extension workers to specialists and sub-specialists [28]. This study was done in ten selected public general hospitals namely Alamata, Lemlem Carl, Mekelle, Adigrat, kids Mariam, Adwa, Abiyi Adi, Shule Shire, Sheraro Mayani and Kahsay Abera (Humera). These general hospitals provide basic health services for patients with different diseases including diabetes mellitus. About 4,154 patients with type 2 diabetes received health services at these public general hospitals in 2018/19 [29].
Population
All type 2 diabetic (T2D) patients admitted to the study hospitals and those who attended diabetic clinics during the data collection period participated in the study. To be involved in the study participants had to be adult.
Eligibility
Inclusion criteria
All adult patients aged more than 18 years who were diagnosed to have T2D and had follow-up visits in the study hospitals for ≥1 year. The study did not include patients.
Exclusion criteria
All Patients who were pregnant and critically ill.
Sample Size determination
The sample size (n) was estimated using a single population proportion formula proposed by Cochran [30] with an assumption of 95% confidence interval (z = 1.96), a margin of error of 0.03 and the proportion of chronic diabetes complication among T2D patients from a study in Northwestern Ethiopia which was 53.5% (P = 0.535) [21]. The initial sample size was 1,061 which was obtained using the following formula ni = (Z1-α/2)2p (1–p)/d2 = (1.96)20.535(1–0.535)/(0.03)2. 10% of the initial sample size was added for non-response rate and the final sample size was 1,168. Proportional allocation of participants was employed to allocate the sample size among the selected general hospitals based on caseload (Fig 1).
Fig 1. Schematic presentation of the sampling procedure for a study on magnitude of chronic diabetes complication among diabetic patients attending the general hospitals in Tigray region, Northern Ethiopia, 2019/2020.
Sampling procedure
Ten out of fourteen public general hospitals were selected through a simple random sampling technique, and all general hospitals were not included because of budget constraints. Participants were selected using a systematic random sampling method whereby the first patient was selected randomly from the first three by a lottery method, and the next patient was selected every three intervals until the required sample was attained.
Data collection tool and measurement
The data were collected using a pre-tested, interviewer-administered questionnaire that was developed based on relevant literature [12,21,31,32] and record review. It has four parts: part one was used to collect data about socio-demographic characteristics; part two was on clinical characteristics; part three was on behavioural factors; and part four was on chronic diabetes complications. Behavioural variables were assessed based on the WHO TEPwise approach for chronic disease risk factor surveillance [33]. Clinical variables were taken from the patient’s chart/medical record and physical measurements. Body weight was measured to an accuracy of 0.1 kg using a weight scale machine and the patient was barefoot and wearing light clothing. Height was measured in meters, standing upright on a flat surface, by a stadiometer. Body Mass Index (BMI) was calculated as the ratio of weight in kilogram (kg) to the square of height in meters (m2). Systolic blood pressure (BP) and diastolic blood pressure (DBP) was measured from the left arm at the level of the heart using a mercury-based or digital sphygmomanometer after the patient took a rest for more than 10 minute and 1–2 hour for those who took hot drink like coffee [34]. For those patients with BP ≥ 140 mm of mercury (mmHg) and/or DBP ≥ 90 mmHg, blood pressure was measured again, and finally, the average value was taken.
Data collection procedure
The T2D patients attending 10 hospitals during the data collection period were approached by the data collectors, verified for eligibility and then, after informed consent was obtained, data were collected. Data was collected by ten BSc nurses who had either MPH or MSc with multilingual abilities and were supervised by the first and third authors. The type 2 diabetic patients were identified by examining their diagnosis as reported in the medical record. Moreover, clinical and chronic diabetic complications data were extracted from patients’ medical records. The diagnosis of chronic diabetic complication was then confirmed by the physician.
Data management and analysis
To assure data quality, training and orientation of the study were done for the data collectors and supervisors and the questionnaire was pre-tested and checked for its validity and reliability. The pretest of the questionnaire involved 2% of the sample size and it was carried out in Quiha general hospital two weeks before the actual data collection. The questionnaire was revised based on the pre-test results. The questionnaires were checked for completeness and consistency on the daily bases. The data was entered and cleaned, entered in the SPSS version 20 analyses were performed using. A binary logistic regression analysis model was used to identify factors associated with chronic diabetes complications. The Homer-Lemehow goodness-of-fit test was used to check the model fitness and the assumption of a P-value >0.05 was considered a good model fit. Independent variables with p < 0.20 during the bivariate analysis were then included in the multivariable logistic regression for further analysis to control confounding factors. Multicollinearity between independent variables was checked by using the tolerance test and variance inflation factor (VIF). P < 0.05 was considered the cut-off point for reporting an independent variable that shows a statistically significant association with the dependent variable in multivariate analysis. The strength of the association of factors with chronic diabetes complications was demonstrated by computing the adjusted odd ratio (AOR) and its 95% confidence interval (CI).
Variables of the study
Independent variable
Socio-demographic: age, sex, marital status, religion, ethnicity, educational status, occupation, residence, monthly income (UD), family history of diabetes and BMI.
Clinical: diabetes treatment regimen, anti-platelet drug (e.g., AA), anti-dyslipidemia drug, glucometer, duration of diabetes, Fasting Blood Glucose (FBG), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Pill burden, and high health care cost.
Behavioural: adherence to a diabetic diet, saturated fat consumption, vegetable consumption, adherence to diabetic medication, self-blood glucose test, smoking, alcohol consumption, physical activity, and diabetes education.
Dependent variable
Chronic diabetes complications status
Operational definition
The data about chronic diabetes complications were extracted from the patient’s chart/medical record. Only chronic complications that developed after the diagnosis of T2D and could be attributed to diabetes were considered in this study.
Diagnosis of chronic diabetes complication
Hypertension: was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, and/or patient on antihypertensive therapy was taken as the hypertensive patient [35,36].
Coronary artery disease (CAD): The diagnosis criteria for CAD were either a patient with typical anginal pain or equivalent symptoms or an abnormal resting ECG or an asymptomatic patient with the abnormal stress test, either by ECG or echo or a nuclear perfusion imaging test [37].
Peripheral vascular disease: The presence of intermittent claudication and/or an ABI (ankle-brachial/Arm index) value (< 0.9) in any limb was recorded as peripheral vascular disease [38].
Neuropathy: was diagnosed if a change was found in two or more of the three items hypesthesia or anaesthesia in lower and upper limbs when the patient’s lower limp was evaluated [18].
Eye diseases: Eye diseases such as cataracts, glaucoma and diabetic retinopathy were identified based on the report of the ophthalmologist or optometrist from the dilated eye (fundus photography for retinopathy), and comprehensive eye examination, which was recorded on the patient’s chart [18].
Chronic Kidney disease: was diagnosed based on the presence of urinary albumin(micro or macro albuminuria), and/or an abnormally high level of serum creatinine (low glomerular filtration rate) [39].
Foot problem: The diagnosis of foot problem was made through foot examination for any abnormalities (i.e. dry kin, fissure, deformities, callosities, ulceration, prominent vein, and nail lesion) or all patients were asked about a history of foot ulcer, neuro ischemic foot, or amputation [40].
Follow a healthy diet: consuming vegetables, beans and peas, fruit, whole grain, nut, and seeds, seafood, low-fat milk and milk product, and a moderate amount of lean meat, poultry, and egg [41,42].
High fat/oil consumption: eat or consume more than 10% of calories from saturated fat, which means more than 20 grams of saturated fat per day [43,44].
Alcohol consumption: Adult with diabetes who drinks alcohol should do so in moderation (no more than one drink per day for adult women and no more than two drinks per day for adult men). One drink is defined as 12 oz/355ml of beer, 5 oz /148ml glass of wine, or 1.5 oz/44ml of distilled spirit [45].
Physical activity: At least 150 minutes per week of aerobic exercise, plus at least two seasons per week of resistance exercise, are recommended [46].
Ethical considerations
Ethical approval to conduct the study was obtained from the Institutional Review Board (IRB) of Mekelle University (Ref No. ERC 1370/2019). The study received approval from the Tigray regional health bureau and permission from the Medical Directors of the 10 involved hospitals. The study was conducted following the declaration of Helsinki. Study participants were recruited voluntarily after they were informed about the study and that they can withdraw from the study at any stage thereafter they signed the consent form. All data were kept in a safe and secure place anonymously to ensure confidentiality and only the researchers had access to the data.
Results
Socio-demographic characteristics of the participant
Overall, a total of 1,168 diabetic individuals were eligible for the study, but only 1,158 of the participant’s questionnaires were fit for final analysis, which makes the response rate 99.14 per cent. Thirty-four per cent of the participants had an age greater than 60 years, with a mean age of 55.9 (D± 11.9) years. Most of the participants were male (54%), Married (67%), Orthodox Christian (88%), Tigrian ethnicity (96.3%), and urban resident (72.3%). Majority of the participants: 50.5% had no formal education, 80.3% had no family history of diabetes, 29.7% were unemployed, 7.9% had a monthly income of $34.26-$171.16 and 76.5% had a BMI of <25 kg/m2 [Table 1].
Table 1. Socio-demographic characteristics by chronic diabetes complication among diabetic patients attending the general hospitals in Tigray region, Northern Ethiopian, 2019/2020 (N = 1,158).
| Variable | Category | Chronic DM complication status | Total | |
|---|---|---|---|---|
| No (n = 530) | Yes (n = 628) | |||
| Age | ≤40 year | 88(7.6%) | 49(4.2%) | 137(11.8%) |
| 41–45 year | 59(5.1%) | 41(3.5%) | 100(8.6%) | |
| 46–50 year | 96(8.3%) | 89(7.7%) | 185(16.0%) | |
| 51–55 year | 76(6.6%) | 76(6.6%) | 152(13.1%) | |
| 56–60 year | 93(8.0%) | 103(8.9%) | 196(16.9%) | |
| ≥61 year | 118(10.2%) | 270(23.3%) | 388(33.5%) | |
| Sex | 1. Male | 285(24.6%) | 340(29.4%) | 625(54.0%) |
| 2. Female | 245(21.2%) | 288(24.9%) | 533(46.0%) | |
| Marital status | 1. ingle | 39(3.4%) | 38(3.3%) | 77(6.6%) |
| 2. Married | 380(32.8%) | 394(34.0%) | 774(66.8%) | |
| 3. Divorced | 47(4.1%) | 84(7.3%) | 131(11.3%) | |
| 4. Widowed | 64(5.5%) | 112(9.7%) | 176(15.2%) | |
| Religion | 1. Orthodox | 474(40.9%) | 545(47.1%) | 1019(88.0%) |
| 2. Muslim | 50(4.3%) | 79(6.8%) | 129(11.1%) | |
| 3. Catholic | 6(0.5%) | 4(0.3%) | 10(0.9%) | |
| Ethnicity | 1. Tigrian | 508(43.9%) | 607(52.4%) | 1115(96.3%) |
| 2. Amhara | 19(1.6%) | 19(1.6%) | 38(3.3%) | |
| 3. Afar | 3(0.3%) | 2(0.2%) | 5(0.4%) | |
| Educational status |
1. No formal education | 246(21.2%) | 339(29.3%) | 585(50.5%) |
| 2. Primary school (1–8 grade) | 118(10.2%) | 134(11.6%) | 252(21.8%) | |
| 3. Secondary (9–12 grade) | 89(7.7%) | 80(6.9%) | 169(14.6%) | |
| 4. College/University | 77(6.6%) | 75(6.5%) | 152(13.1%) | |
| Occupation | 1. Farmer | 119(10.3%) | 129(11.1%) | 248(21.4%) |
| 2. Gov’t employee | 103(8.9%) | 62(5.4%) | 165(14.2%) | |
| 3. Private work | 135(11.7%) | 146(12.6%) | 281(24.3%) | |
| 4. Retired | 42(3.6%) | 78(6.7%) | 120(10.4%) | |
| 5. Unemployed | 131(11.3%) | 213(18.4%) | 344(29.7%) | |
| Residence | 1. Urban | 370(32.0%) | 467(40.3%) | 837(72.3%) |
| 2. Rural | 160(13.8%) | 161(13.9%) | 321(27.7%) | |
| Monthly income (UD) |
< $34.23 | 157(13.6%) | 234(20.2%) | 391(33.8%) |
| 2, $34.26–171.16 | 324(28.0%) | 351(30.3%) | 675(58.3%) | |
| 3, >$171.16 | 49(4.2%) | 43(3.7%) | 92(7.9%) | |
| Family history of DM | 1.Ye | 102(8.8%) | 126(10.9%) | 228(19.7%) |
| 2. No | 428(37.0%) | 502(43.4%) | 930(80.3%) | |
| BMI | < 25 kg/m2 | 466(40.2%) | 420(36.3%) | 886(76.5%) |
| ≥ 25 kg/m2 | 162(14.0%) | 110(9.5%) | 272(23.5%) | |
T2D: Type 2 diabetes, DM: Diabetes mellitus, BMI: Body Ma Index, DM: Diabetes Mellitus, UD: United States Dollar.
Clinical and behavioural characteristics of the participants
Of the total patients included in this study, 78.9%, 11.7%, and 16.8% were taking oral hypoglycemic agent (OHGA), anti-coagulant drug, and anti-dyslipidemia drug respectively. The mean duration of diabetes was 6.3 (D ± 4.6) years and 54.6% of participants had diabetes duration of < 5 years. Of all participants, 10.1% had a glucometer at home, 60.7% had FBG of >130.00 mg/dl, 25.8% had SBP of >149.00 mmHg and 9.0% had DBP of > 90.00 mmHg. From the total participants, 42.7% were taking >4 pills per day, 86.4% had a high health care cost, 69.7% consumed a high amount of saturated fat (>20 g per day), and 68.7% were taking less than the recommended amount of vegetable (<4 serving per week). Of all participants, 90.6% and 50.4% adhered to diabetes medication and a healthy diet. Of the total participants, nine out of ten test their blood glucose once per month, 6.2% ever smoke tobacco product, 12.4% consume more than moderate amount of alcohol (≥3 drink per day). Moreover, of the total participants, 61.3% were active physically, and 76.5% were attending diabetes education at the time of follow visit [Table 2].
Table 2. Clinical and behavioural characteristics by chronic diabetes complication among diabetic patients attending the general hospitals in Tigray region, Northern Ethiopian, 2019/2020 (N = 1,158).
| Variable | Category | Chronic DM complication status | Total | |
|---|---|---|---|---|
| No (n = 530) | Yes (n = 628) | |||
| Diabetes treatment regimen | Inulin (injectable) | 78(6.7%) | 61(5.3%) | 139(12.0%) |
| Inulin & OHGA* | 33(2.8%) | 72(6.2%) | 105(9.1%) | |
| OHGA* | 419(36.2%) | 495(42.7%) | 914(78.9%) | |
| Use of anti-platelet drug (e.g. AA) | 1, Ye | 20(1.7%) | 116(10.0%) | 136(11.7%) |
| No | 510(44.0%) | 512(44.2%) | 1022(88.3%) | |
| Use of anti-dyslipidemia drug | Ye | 45(3.9%) | 150(13.0%) | 195(16.8%) |
| No | 485(41.9%) | 478(41.3% | 963(83.2%) | |
| Have glucometer at home | Ye | 47(4.1%) | 70(6.0%) | 117(10.1%) |
| No | 483(41.7%) | 558(48.2%) | 1041(89.9%) | |
| Duration of diabetes since it occurred | < 5 year | 345 | 287(24.8%) | 632(54.6%) |
| ≥ 5 year | 185(16.0%) | 341(29.4%) | 526(45.4%) | |
| FBG test | <130.00 mg/dl | 200(17.3%) | 255(22.0%) | 455(39.3%) |
| ≥ 130.99 mg/dl) | 330(28.5%) | 373(32.2%) | 703(60.7%) | |
| Systolic blood pressure (SBP) | ≤ 139.99 mmHg | 459(39.6%) | 400(34.5%) | 859(74.2%) |
| ≥140.00 mmHg | 71(6.1%) | 228(19.7%) | 299(25.8%) | |
| Diastolic blood pressure (DBP) | ≤ 89.99 mmHg | 503(43.4%) | 551(47.6%) | 1054(91.0%) |
| ≥90.00 mmHg | 27(2.3%) | 77(6.6%) | 104(9.0%) | |
| Pill burden | <4 pills/day | 360(31.1%) | 304(26.3%) | 664(57.3%) |
| ≥ 4 pills /day | 170(14.7%) | 324(28.0%) | 494(42.7%) | |
| High healthcare cot | 1. Ye | 451(38.9%) | 550(47.5%) | 1001(86.4%) |
| 2. No | 79(6.8%) | 78(6.7%) | 157(13.6%) | |
| Adherence to a diabetic diet | Adhere | 264(22.8%) | 320(27.6%) | 264(22.8%) |
| Not adhere | 266(23.0%) | 308(26.6%) | 266(23.0%) | |
| Saturated fat consumption | < 20 gm. fat/day | 143(12.3%) | 208(18.0%) | 351(30.3%) |
| ≥ 20 gm. fat/day | 387(33.4%) | 420(36.3%) | 807(69.7%) | |
| Vegetable consumption per week | <4 serving | 370(32.0%) | 425(36.7%) | 795(68.7%) |
| ≥ 4 serving | 160(13.8%) | 203(17.5%) | 363(31.3%) | |
| Adherence to diabetic Medication | Adhere | 486(42.0%) | 563(48.6%) | 1049(90.6%) |
| Not adhere | 44(3.8%) | 65(5.6%) | 109(9.4%) | |
| Day, in which glucose was measured/wk. | Not measured at all | 488(42.1%) | 566(48.9%) | 1054(91.0%) |
| 1–2 day | 42(3.6%) | 62(5.4%) | 104(9.0%) | |
| Ever smoked tobacco products (smoking) | Ye | 27(2.3%) | 45(3.9%) | 72(6.2%) |
| No | 503(43.4%) | 583(50.3%) | 1086(93.8%) | |
| Alcohol consumption | ≥ 3 drinks per day | 62(5.4%) | 82(7.1%) | 144(12.4%) |
| ≤ 2 drinks per day | 468(40.4%) | 546(47.2%) | 1014(87.6%) | |
| Physical activity | Inactive | 174(15.0%) | 274(23.7%) | 448(38.7%) |
| Active | 356(30.7%) | 354(30.6%) | 710(61.3%) | |
| Attend diabetes education | Ye | 345(36.1%) | 387(40.4%) | 732(76.5%) |
| No | 102(10.7%) | 123(12.9%) | 225(23.5%) | |
*OHGA = Oral Hypoglycemic Agent, T2D: Type 2 diabetes, ASA: Acetylsalicylic Acid, FBG: Fasting Blood Glucose, SBP: systolic Blood Pressure, DBP: Diastolic Blood Pressure.
The magnitude of chronic diabetes complication
Overall, 54% of participants (95% CI 51.35, 57.10) suffered from at least one chronic complication of diabetes. Among all participants who had the complication, 10.5% had a single complication, 16.9% lived with two and 26.8% with more than two types of complications respectively.
Macrovascular complication
Of all types of macrovascular complications, hypertension 27% (95% CI 24.71,29.85) was the most common but cerebrovascular disease 4.31% (95% CI: 3.14, 5.49) was the least common type. Out of all participants who had cerebrovascular complications, 0.3% had TIA and 4% had a history of stroke (Fig 2).
Fig 2. Magnitude of macrovascular complications among diabetic patients attending the general hospitals in Tigray region, Northern Ethiopian, 2019/2020.
Microvascular complication
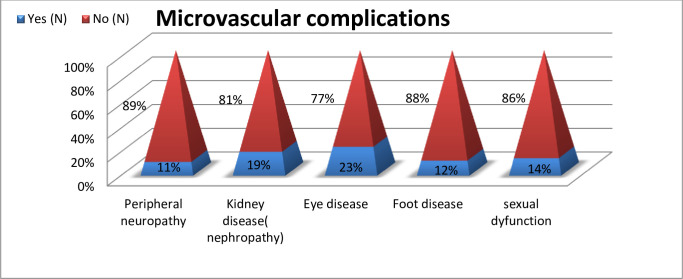
The most common type of microvascular complication was ocular disease 22.62% (95% CI 20.21, 25.03), followed by kidney disease 19.17% (95% CI 16.90, 21.44) and peripheral neuropathy11% (95% CI 8.84, 12.39) respectively. Patients with renal complications consisted of 9% with microalbuminuria,4.0% with macroalbuminuria and 6% with high levels of serum creatinine. Ocular complications included cataracts, retinopathy, diabetes blindness and glaucoma, with magnitudes of 5%, 9%, 1% and 8% respectively. Of the total participant who had a foot disease, 4% had a diabetes-related foot ulcer, 1% had a foot amputation, 3.6% had ischemic pain, 1% had gangrene and 3% had an infection (Fig 3).
Fig 3. Magnitude of microvascular complications among diabetic patients attending the general hospitals in Tigray region, Northern Ethiopian, 2019/2020.
Factor associated with chronic diabetes complication among patients with type 2 diabetes (T2D)
The Homer-Lemehow goodness-of-fit test was done, and its result showed P = 0.0063, which was considered a good model fit. The odds ratio was calculated for factors found to be associated with chronic diabetes complications among type 2 diabetic patients. After considering all assumptions of binary logistic regression and the p-value (≤ 0.05) in the bivariate analysis, fifteen variables were identified as candidates for analysis in the multivariable model. In the multivariable logistic regression analysis, nine variables were found to be factors associated with chronic diabetes complications at a 5% level of significance.
The odds of a chronic diabetes complication was higher among patients age > 60 years (AOR = 3.00; 95% CI 1.73,5.26)) than their counterpart, who took insulin and OHA had a higher chance of developing the complication (AOR = 2.20; 95% CI 1.18, 4.27) than patients taking insulin injection only, with diabetes duration of ≥ 5 years, were at higher risk to develop the complication (AOR = 1.56; 95% CI 1.18, 2.05) than patients with shorter diabetes duration and who took ≥4 pills a day had a greater risk to develop chronic diabetes complication (AOR = 1.63; 95% CI 1.23, 2.15) than their counterpart [Table 3]. Similarly, the odds of a chronic diabetes complication was higher among patient with a higher systolic BP (≥140.00 mm Hg.) or diastolic BP (≥ 90.00 mm Hg.) (AOR = 3.13; 95% CI 2.25, 4.35) and (AOR = 1.39; 95% CI 1.06, 1.81) than participants with lower systolic BP (<139.99 mmHg.) or diastolic BP (<79.99 mm Hg.) respectively. Whereas, patients who were government employees (AOR = 0.48; 95% CI 0.26, 0.90), taking anti-platelet drugs (AOR = 0.29; 95% CI 0.16, 0.52) and anti-dyslipidemia drugs (AOR, = 0.54; 95% CI 0.35, 0.84) were less likely to develop the complication than the patients who were unemployed and did not take anti-platelet and anti-dyslipidemia, respectively [Table 3].
Table 3. Factors associated with chronic diabetes complication among diabetic patients attending the general hospitals in Tigray region, Northern Ethiopian, 2019/2020 (N = 1,158).
| Variable | Category | Chronic DM complication | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| No | Yes | COR | P | AOR | ||
| Age | ≤40 year | 88 (7.6%) | 49(4.2%) | 1 | 1 | |
| 41–45 year | 59(5.1%) | 41(3.5%) | 1.2(0.73, 2.12) | 0.423 | 1.26(0.70, 2.26) | |
| 46–50 year | 96(8.3%) | 89(7.7%) | 1.6(1.05, 2.61) | 0.163 | 1.44(0.86, 2.41) | |
| 51–55 year | 76(6.6%) | 76(6.6%) | 1.7(1.12, 2.88) | 0.399 | 1.26(0.73, 2.20) | |
| 56–60 year | 93(8.0%) | 103(8.9%) | 1.9(1.27, 3.11) | 0.208 | 1.39(0.83, 2.35) | |
| ≥61 year | 118(10.2%) | 270(23.3%) | 4.1(2.72, 6.19) | 0.000 | 2.51(1.50, 4.18) *** | |
| Marital status | 1. Single | 39(3.4%) | 38(3.3%) | 1.0(0.66, 1.70) | 0.221 | 1.51(0.77, 2.94) |
| 2. Married | 380(32.8%) | 394(34.0%) | 1.8(1.03, 3.25) | 0.926 | 1.01(0.67, 1.53) | |
| 3. Divorced | 47(4.1%) | 84(7.3%) | 1.7(1.04, 3.08) | 0.059 | 1.68(0.98, 2.89) | |
| 4. Widowed | 64(5.5%) | 112(9.7%) | 1 | 1 | ||
| Educational status |
1. Illiterate | 246(21.2%) | 339(29.3%) | 1.4(0.98, 2.02) | 0.142 | 0.64(0.36, 1.15) |
| 2. Primary school | 118(10.2%) | 134(11.6%) | 1.1(0.77, 1.74) | 0.210 | 0.69(0.39, 1.22) | |
| 3. secondary school | 89(7.7%) | 80(6.9%) | 0.9(0.59, 1.43) | 0.238 | 0.71(0.40, 1.24) | |
| 4. College/University | 77(6.6%) | 75(6.5%) | 1 | 1 | ||
| Occupation | 1. Farmer | 119(10.3%) | 129(11.1%) | 0.6(0.47, 0.92) | 0.946 | 0.98(0.62, 1.54) |
| 2. Gov’t employee | 103(8.9%) | 62(5.4%) | 0.3(0.25, 0.54) | 0.023 | 0.48(0.26, 0.90) * | |
| 3. Private work | 135(11.7%) | 146(12.6%) | 0.6(0.48, 0.91) | 0.552 | 0.87(0.57, 1.35) | |
| 4. Retired | 42(3.6%) | 78(6.7%) | 1.1(0.74, 1.76) | 0.532 | 1.19(0.68, 2.09) | |
| 5. Unemployed | 131(11.3%) | 213(18.4%) | 1 | 1 | ||
| Monthly income (UD) |
< $34.23 | 157(13.6%) | 234(20.2%) | 1.6(1.07, 2.68) | 0.340 | 1.35(0.72, 2.50) |
| 34.26–171.16 | 324(28.0%) | 351(30.3%) | 1.2(0.79, 1.91) | 0.356 | 1.28(0.75, 2.19) | |
| >171.16 | 49(4.2%) | 43(3.7%) | 1 | 1 | ||
| BMI | < 25 kg/m2 | 466(40.2%) | 420(36.3%) | 1 | 1 | |
| ≥ 25 kg/m2 | 162(14.0%) | 110(9.5%) | 1.3(1.00, 1.74) | 0.994 | 0.99(0.72, 1.38) | |
| Diabetes treatment regimen |
Inulin (injectable) | 78(6.7%) | 61(5.3%) | 0.6(0.46, 0.94) | 0.754 | 0.93(0.60, 1.44) |
| Inulin & OHA* | 33(2.8%) | 72(6.2%) | 1.8(1.19, 2.84) | 0.000 | 2.45(1.49, 4.01) *** | |
| OHGA* | 419(36.2%) | 495(42.7%) | 1 | 1 | ||
| Anti-platelet drug | Ye | 20(1.7%) | 116(10.0% | 1 | 1 | |
| No | 510(44.0%) | 512(44.2% | 0.1(0.10, 0.28) | 0.000 | 0.29(0.16, 0.52) *** | |
| Anti-dyslipidemia drug | Ye | 45(3.9%) | 150(13.0%) | 1 | 1 | |
| No | 485(41.9%) | 478(41.3% | 0.2(0.20, 0.42) | 0.006 | 0.54(0.35, 0.84) ** | |
| Duration of diabetes | < 5 year | 345(29.8%) | 287(24.8%) | 1 | 1 | |
| ≥ 5 year | 185(16.0%) | 341(29.4%) | 2.2(1.74, 2.81) | 0.001 | 1.56(1.18, 2.05) ** | |
| Systolic blood pressure (SBP) | < 139.99 mmHg | 459(39.6%) | 400(34.5%) | 1 | 1 | |
| ≥140.00 mmHg | 71(6.1%) | 228(19.7%) | 3.6(2.73, 4.96) | 0.000 | 3.13(2.25, 4.35) *** | |
| Diastolic blood pressure (DBP) | < 89.99 mmHg | 503(43.4%) | 551(47.6%) | 1 | 1 | |
| ≥ 90.00 mmHg | 27(2.3%) | 77(6.6%) | 1.6(1.27, 2.03) | 0.016 | 1.39(1.06, 1.81) * | |
| Pill burden |
<4 pills/day | 360(31.1%) | 304(26.3%) | 1 | 1 | |
| ≥ 4 pills/day | 170(14.7%) | 324(28.0%) | 2.2(1.77, 2.87) | 0.001 | 1.63(1.23, 2.15) ** | |
| High saturated fat consumption | < 20 gm. fat/day | 143(12.3%) | 208(18%) | 1 | 1 | |
| ≥ 20 gm. fat/day | 387(33.4%) | 420(36.3%) | 0.7(0.57, 0.96) | 0.111 | 0.78(0.58, 1.05) | |
| Physical activity | Inactive | 174(15.0%) | 274(23.7%) | 1.5(1.24, 2.01) | 0.070 | 1.31(0.97, 1.77) |
| Active | 356(30.7%) | 354(30.6%) | 1 | 1 | ||
*OHGA: oral hypoglycemic agent
*significant at p<0.05
**significant at p<0.01
***significant at p< 0.0001., Homer-Leme how goodness-of-fit (P = 0.0063), BMI: Body Ma Index.
Discussion
This study aimed to assess the magnitude of chronic diabetes complications and associated factors among diabetic patients attending the general hospitals in the Tigray region, Northern Ethiopia. The overall magnitude of chronic diabetes complications in this study was 54.0% (95% CI: 51.35, 57.10). This is consistent with studies from Bahir Dar, Northwest Ethiopia (54%), and China (52%) [12,21]. The finding of this study was lower than the magnitude reported in Libya(69%), Indonesia (69%), Gurage zone, South Ethiopia(61%), Saudi Arabia(66%) and Nepal (72%) [47–51]. The probable cause for the higher prevalence reported in other studies compared to our study could be due to the presence of age, diabetes duration, and BMI differences. For instance, a study in Indonesia reported that 46% of study participants were aged >60 years old; in Indonesia, Ethiopia, Saudi Arabia, and Nepal, patients with ≥5 years of diabetes duration were 73%, 53%, 46%,46% and 91%, respectively; and participants from a study in Saudi Arabia and Nepal with normal BMI were 17% and 20% respectively.
However, our study showed that 34%, 45% and 77% of the study participants were >60 years of age, with ≥ 5 years of diabetes duration and normal BMI respectively. However, the result of this study is higher than a study in Gurage Zone, Southwest Ethiopia (46%). [21] The probable reason for the higher prevalence reported in our study compared to a study in Gurage Zone, south Ethiopia, could be due to ample size, study setting, and population variation. The study was done using a very small sample size and conducted in both the primary and general hospitals among type 1 and type 2 patients.
In this study, 27.28% (95% CI: 24.71, 29.85) participants had diabetes-related hypertension which is in line with a study done in Jimma, Ethiopia(25%) [15]. This is higher than studies from Palestine (23%), the town of Hosaena, Southern Ethiopia (23.9%) and Saudi Arabia (20%) [52–54]. The reason for the high prevalence in our study as compared to studies conducted in Palestine, Ethiopia and Saudi Arabia might be because of differences in the male-to-female ratio, time at which the study was conducted and study area. The result of this study is lower than studies conducted in Bangladesh (82%), Iraq (38%), Libya (33%), West Ethiopia (42%) and Jimma, Ethiopia (42%) [15,47,55–57]. The variation might have occurred because in this study only participants who became hypertensive after the occurrence of diabetes were considered, whereas the other studies may have included all participants who developed hypertension before and after the diagnosis of diabetes. Moreover, the presence of age, diabetes duration, and BMI differences may contribute to such variation.
In this study, peripheral vascular disease was seen among 9.13% (95% CI: 7.49, 10.81) participants, which is higher than the finding from a survey conducted in Sri Lanka (4.7%) [32,58]. The reason for the higher value of our study might be because of genetic variability, patients’ poor adherence to medication, and practice related to lifestyle recommendations. However, the result of this study is lower than India (12%), Bangladesh (14%) and Libya (15%) [59–61]. This variation occurred due to our study population remained without a diagnosis for years because of a lack of awareness and access to advanced diagnostic tests.
Coronary artery disease (CAD)occurred among 3.28% (95% CI 2.25, 4.30) participants in this study, which is lower than studies done in Sri Lanka (11%), Saudi Arabia (23%), Bangladesh (26%), India (8%), Nepal (23%), and Iraq (15%) [32,60,62–64]. The reasons for the lower result of this study compared to the other studies are our study participants might have had a late diagnosis of diabetes, late initiation of treatment, or lifestyle modification, and a lack of access to advanced diagnostic studies like stress tests, echocardiography and nuclear perfusion imaging test. Moreover, genetic and racial variability may play a role in such discrepancy.
Of the total participants in this study, 4.31% (95% CI 3.14, 5.49) had a stroke, which is higher than studies done in Libya (1.9%), Saudi Arabia (0.19%), Nepal (1%) and Iraq (0.7%) [47,51,54,56]. The reason for the higher value of our result might be due to over-reporting, a larger sample size, and the late initiation of diabetes treatment or lifestyle recommendations compared to other studies, but this result is lower than India (7%), Bangladesh (11%), and Indonesia (18%) [59,60,65]. The reason for our study’s lower figure could be participants in other studies were older, had a higher rate of obesity, and had diabetes for a longer time. In addition, socio-cultural variations and genetic predisposition play a role in the variation.
Peripheral neuropathy was seen among 11% (95% CI 8.84, 12.39) participants; this finding is almost similar to that of studies conducted in India (11%) and West Ethiopia (10%) [66,67]. The reason for the similarity could be due to the population characteristics, mainly the socio-demographic characteristics, being relatively similar.
This result is higher than the result of Saudi Arabia (1.4%) and Iraq (6.5%) [54,56]. The reason for the higher figure in this study could be due to socio-demographic characteristics variation; for instance, in this study, only T2D patients were included, and 28% of participants were from the rural area, whereas the study from Iraq included both types 1 and type 2 diabetes, and more than 53% of participants in the Saudi Arabia study were rural resident.
However, it is lower than the result of Sri Lanka (63%), Southwest Ethiopia (15%), Nepal (15%), Bangladesh (28%), India (19%), Tanzania (29%), and Egypt (22%) [32,49,51,60,62,68,69]. The probable reason for the lower figure in our study could be the lower rate of progression and the low onset of the disease, which discourage patients from seeking treatment early. In addition, a lack of routine foot examinations by senior experts could result in underreporting or miss diagnosis of such cases.
In this study the magnitude of diabetes nephropathy was 19.17%(95%CI: 16.90,21.44) this figure is in line with the study conducted in Ethiopia [15]. Unlike our study, Sri Lanka (51%), Nepal (25%), Bangladesh (43%), India (41.1%), Egypt (67%) and Sudan (39%) Studies have found higher percentages of participants with nephropathy [32,51,60,62,69,70]. The reason for the lower finding of our study might be ethnicity, racial, and socio-demographic characteristics difference, which play a major role in the development of diabetic nephropathy. However, our result is higher than the findings of studies in Tanzania (12%), China (11%), India (10.5%), Ethiopia (11.4%), Saudi Arabia (4.2%), and Iraq (14.2%) [12,21,63,64,66,68]. The higher result in our study could be because in this study all forms of kidney disease were included, whereas in the other studies, microalbuminuria, macro albuminuria and high level of serum creatinine were reported separately. Moreover, genetic and socio-demographic variability may play a role.
In this study, retinopathy was seen in 9% (95% CI: 7.25–10.53) of participants, which is in line with studies conducted in Indonesia (7%), Tunisia (8.1%), Iraq (7.5%), and Ethiopia (10%) [16,64,65,69] and higher than a survey done in India (4.8%) and Saudi Arabia (3%) [63,66]. The probable reason for the higher value in this study could be attributed to the lack of awareness among our patients about the importance of regular eye examinations. Moreover, the patient’s poor medication adherence, knowledge, and attitude might have contributed to such a difference.
However this result is lower than research findings done in Ethiopia (26%), Sri Lanka (26%), SSA (15%), Nepal (29%), Korea (38%), India (15.4%), Bangladesh (38%), Libya (31%), Tanzania (50%), Sudan (14%), and Egypt (21%) [21,32,35,51,58–61,68,70,71]. The rationale for the lower figure of this study compared to the other studies might be the lack of access to the regular dilated fundus examination technique due to it is not available at general hospitals. Hence, diabetic patients rarely check on their visual status in the absence of a symptom.
In this study, 4.14% (95% CI 2.99, 5.29) of the participants had a diabetes-related foot ulcer, which is similar to the study finding in Sri Lanka (2.6%), Korea (4.4%), Saudi Arabia (2.17%), Nepal (5%), and Ethiopia (5%) [15,32,51,58,63] and higher than China, Iraq (0.8%), Libya (1.1%), Sri-Lanka (1.3%), and Ethiopia. [12,32,61,64,67] (1.2%). This difference may be due to the small sample size and study population variation. However, this finding is lower than studies done in the North, South, and BLH, Ethiopia (21.2%, 20.4%, and 17%, respectively) [16,21,49]. The justification for the lower finding of our study could be due to underreporting and patients were not received regular foot examinations by a senior expert.
In this study, age, occupation, diabetes treatment regimen, anti-platelet drug, anti-dyslipidemia drug, duration of diabetes, systolic BP, diastolic BP and Pill burden were variables showing statistical association with the occurrence of diabetes complications. Similarly, the age of the participant, diabetes treatment regimen and/or duration of diabetes were also identified as factors associated with chronic diabetes complications in studies in, China, Ethiopia, and Libya [12,21,61].
However, gender, marital status, BMI, poor glycemic control, dyslipidemia, and family history of diabetes do not show association in this study, but gender in Libya and Iraq [61,64], marital status and BMI in Ethiopia [49], poor glycemic control in Ethiopia and Libya [49,61], dyslipidemia [61] and family history of diabetes in Libya [61] were identified as factors associated with chronic diabetes complication.
Some of the major limitations of this study that should be mentioned are: First of all, as it was hospital-based cross-sectional in its design, this only allows for the identification of variables that have an association with the dependent variable rather than causation. Second, it could not be generalizable to the entire population and to diabetic patients who don’t receive care in the study area’s public general hospital. Third, some independent variables, such as lipid profile, HgbA1C, and eating pattern for each day, were not taken into consideration. Finally, the target population of this study included diabetic patients who were treated at the general hospital by physicians with limited competence to diagnose and the lack of some diagnostic tests even though the patient had a significantly more complex disease burden.
Conclusion
In this study, more than half participants had at least one chronic diabetes complication. Diabetes-related hypertension was the most prevalent type of chronic diabetes complication, followed by eye and renal disease. Furthermore, chronic diabetes complication was associated with age, occupation, diabetes treatment regimen, an anti-platelet drug, anti-dyslipidemia drug, diabetes duration, systolic BP, diastolic BP, and pill burden. Therefore, healthcare providers managing patients with diabetes should work collaboratively with other stakeholders to ensure that all patients with diabetes are screened for early detection and treatment of complications of diabetes using different approaches and strategies. A future follow-up study should be carried out to investigate the cause of chronic diabetes complications among diabetic patients.
Supporting information
(SAV)
Acknowledgments
We would like to acknowledge all staff of referral clinic of the elected public general hospital for their support, the participant for kindly giving the required information, supervisor and data collector.
Abbreviations
- AOR
Adjusted Odd ratio
- COR
Crud Odd Ratio
- CI
Confidence Interval
- BMI
Body Ma Index
- EFY
Ethiopian Fiscal Year
- FB
Fasting Blood Sugar
- HDL
High-Density Lipoprotein
- LDL
Low-Density Lipoprotein
- AMP
Amputation
- OHA
Oral Hypoglycaemic Agent
- RB
Random Blood Glucose
- SD
Standard Deviation
- SPSS
Statistical Package for Social Science
- T2D
Type 2 Diabetes
- TIA
Transit Ischemic Attach
- WHO
World Health Organization
- BP
Blood Pressure
- BP
Systolic Blood Pressure
- DBP
Dystonic Blood Pressure
- HgbA1C
Glycolated Hgb
- IRB
Institutional Review Board
- ERC
Ethical Review Committee
- IDF
International Diabetes Federation and mmHg: mm of mercury
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, et al. Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2019;127(S 01):S1–S7. doi: 10.1055/a-1018-9078 [DOI] [PubMed] [Google Scholar]
- 2.WHO. Classification of diabetes mellitus. 2019. [Google Scholar]
- 3.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. The lancet. 2017;389(10085):2239–51. [DOI] [PubMed] [Google Scholar]
- 4.Gassasse Z, Smith D, Finer S, Gallo V. Association between urbanisation and type 2 diabetes: an ecological study. BMJ global health. 2017;2(4):e000473. doi: 10.1136/bmjgh-2017-000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Scientific reports. 2020;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramtahal R, Khan C, Maharaj-Khan K, Nallamothu S, Hinds A, Dhanoo A, et al. Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. Journal of Epidemiology and global health. 2015;5(4):S35–S43. doi: 10.1016/j.jegh.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1659–724. doi: 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu X-O, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA network open. 2019;2(4):e192696-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragg F, Holmes MV, Iona A, Guo Y, Du H, Chen Y, et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. Jama. 2017;317(3):280–9. doi: 10.1001/jama.2016.19720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Policardo L, Seghieri G, Anichini R, De Bellis A, Franconi F, Francesconi P, et al. Effect of diabetes on hospitalization for ischemic stroke and related in‐hospital mortality: a study in Tuscany, Italy, over years 2004–2011. Diabetes/metabolism research and reviews. 2015;31(3):280–6. doi: 10.1002/dmrr.2607 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients-a cross-sectional hospital-based survey in urban China. Health and Quality of Life Outcomes. 2010;8(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brutsaert E. Complications of diabetes mellitus. Endocrine and Metabolic Disorders. MSD Manual Professional Edition.[homepage on the internet] c2019. [Google Scholar]
- 14.Stolar M. Glycemic control and complications in type 2 diabetes mellitus. The American Journal of Medicine. 2010;123(3):S3–S11. doi: 10.1016/j.amjmed.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Worku D, Hamza L, Woldemichael K. Patterns of diabetic complications at jimma university specialized hospital, southwest Ethiopia. Ethiopian Journal of health sciences. 2010;20(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gizaw M, Harries A, Ade S, Tayler-Smith K, Ali E, Firdu N, et al. Diabetes mellitus in Addis Ababa, Ethiopia: admissions, complications and outcomes in a large referral hospital. Public Health Action. 2015;5(1):74–8. doi: 10.5588/pha.14.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. The lancet global health. 2013;1(6):e339–e49. doi: 10.1016/S2214-109X(13)70113-X [DOI] [PubMed] [Google Scholar]
- 18.Retinopathy ADAPP C., neuropathy, and foot care: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S185–S94. [DOI] [PubMed] [Google Scholar]
- 19.Epidemiology Nigatu T., complications and management of diabetes in Ethiopia: a systematic review. Journal of diabetes. 2012;4(2):174–80. [DOI] [PubMed] [Google Scholar]
- 20.Abejew AA BA, Kerie MW. Diabetic complications among adult diabetic patients of a Tertiary Hospital in Northeast Ethiopia. Adv Public Health 2015;2015. [Google Scholar]
- 21.Kidist Reba Lebeta Zeleke Argaw, Birhane. BW. Prevalence of Diabetic Complications and Its Associated Factors Among Diabetes Mellitus Patients Attending Diabetes Mellitus Clinics; Institution Based Cross-Sectional Study American Journal of Health Research. 2017;5 (2):38–43. [Google Scholar]
- 22.Mariye T, Tasew H, Teklay G, Gerensea H, Daba W. Magnitude of diabetes self-care practice and associated factors among type two adult diabetic patients following at public Hospitals in the central zone, Tigray Region, Ethiopia, 2017. BMC research notes. 2018;11(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishak NH, Yusoff SSM, Rahman RA, Kadir AA. Diabetes self-care and its associated factors among elderly diabetes in primary care. Journal of Taibah University Medical Sciences. 2017;12(6):504–11. doi: 10.1016/j.jtumed.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonger Z, Shiferaw S, Tariku EZ. Adherence to diabetic self-care practices and its associated factors among patients with type 2 diabetes in Addis Ababa, Ethiopia. Patient preference and adherence. 2018;12:963. doi: 10.2147/PPA.S156043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padma K, Bele SD, Bodhare TN, Valsangkar S. Evaluation of knowledge and self-care practices in diabetic patients and their role in disease management. National Journal of Community Medicine. 2012;3(1):3–6. [Google Scholar]
- 26.Mukeshimana M, Hakizimana G, Mwali C, Umuhoza C, Uwambajimana J, Asingizwe D. The knowledge and practice of self-care management among patients attending a diabetes clinic in Kigali, Rwanda. Rwanda Journal. 2015;2(1):24–30. [Google Scholar]
- 27.HSTP F. Health Sector Transformation Plan. Addis Ababa: Federal Ministry of Health (FMOH). 2015;100. [Google Scholar]
- 28.TRHB. Tigray health sector 2021 annual bulletin Tigray, Ethiopia: 2022. [Google Scholar]
- 29.TRHB. Tigray Region Health Bureau annual health services report. Tigray, Ethiopia: 2020. [Google Scholar]
- 30.Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Archives of orofacial Sciences. 2006;1:9–14. [Google Scholar]
- 31.Abejew AA, Belay AZ, Kerie MW. Diabetic complications among adult diabetic patients of a tertiary hospital in northeast Ethiopia. Advances in Public Health. 2015;2015. [Google Scholar]
- 32.Arambewela MHS, Jayasekara Noel P, Kumbukage Hettiarachchige Buddhi Pradeep Ranjan, Jayasena Mahesh P et al. Prevalence of Chronic Complications, Their Risk Factors, and the Cardiovascular Risk Factors among Patients with Type 2 Diabetes Attending the Diabetic Clinic at a Tertiary Care Hospital in Sri Lanka. Journal of Diabetes Research. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. American Journal of public health. 2016;106(1):74–8. doi: 10.2105/AJPH.2015.302962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mort KH JR. Timing of Blood Pressure Measurement Related to Caffeine Consumption. Annals of Pharmacotherapy. 2008;42(1):105–10. doi: 10.1345/aph.1K337 [DOI] [PubMed] [Google Scholar]
- 35.Ekoru K, Doumatey A, Bentley AR, Chen G, Zhou J, Shriner D, et al. Type 2 diabetes complications and comorbidity in Sub-Saharan Africans. EClinicalMedicine. 2019;16:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the Management of arterial hypertension: The Task Force for the Management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European heart journal. 2018;39(33):3021–104. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman RS. Diabetes mellitus: management of microvascular and macrovascular complications. J Cleveland Clinic: Centers for Continuing Education. 2016. [Google Scholar]
- 38.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909. doi: 10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
- 39.Committee APP. Chronic kidney disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S175–S84. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Pai DR, Yuhhui C. Diabetic foot ulcer–diagnosis and management. Clin Res Foot Ankle. 2013;1(3):120. [Google Scholar]
- 41.NIDDK. Diabetes Diet, Eating, & Physical Activity. U.S. Department of Health and Human Services; 2016. [cited 2023 March 20]; Available from: https://www.niddk.nih.gov/health-information/diabetes/overview/diet-eating-physical-activity. [Google Scholar]
- 42.McGuire S. US Department of Agriculture and US Department of Health and human services, dietary guidelines for Americans, 2010. Washington, DC: US government printing office, January 2011. Advances in nutrition. 2011;2(3):293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kathy W. Warwick CC. How Much Fat Can People with Diabetes Have Each Day?: Daily guidelines for dietary fat. Healthline Media 2022. [updated September 8, 2022; cited 2023 March 24]; Available from: https://www.healthline.com/health/diabetes/how-much-fat-can-a-diabetic-have-a-day#blood-sugar-effect. [Google Scholar]
- 44.Twenefour D, Shields E. Saturated fats and the management of diabetes: the debates, controversies and consensus. Practical Diabetes. 2020;37(4):115–20. [Google Scholar]
- 45.ADA. 5. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S46–S60. doi: 10.2337/dc19-S005 [DOI] [PubMed] [Google Scholar]
- 46.Sigal RJ, Armstrong MJ, Colby P, Kenny GP, Plotnikoff RC, Reichert SM, et al. Physical activity and diabetes. Canadian Journal of Diabetes. 2013;37:S40–S4. doi: 10.1016/j.jcjd.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 47.Roaeid R KO. Prevalence of long-term complications among Type 2 diabetic patients in Benghazi, Libya. J Diabetol. 2011;3(5):1–8. [Google Scholar]
- 48.Tarigan TJ YE, Subekti I, Pramono LA, Martina D. Profile and analysis of diabetes chronic complications in Outpatient Diabetes Clinic of Cipto Mangunkusumo Hospital, Jakarta. Medical Journal of Indonesia. 2015;;24(3):156–62. [Google Scholar]
- 49.Gebre BB, Assefa ZM. The magnitude and associated factors of diabetic complication among diabetic patients attending Gurage zone hospitals, South West Ethiopia. BMC Research Notes. 2019;12(1):780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hossam Al-Esawi SAA. Prevalence of Complications among Saudi Males Type 2 Diabetic Patients in Riyadh Primary Health Care Centers,2019. Diabetes Updates. 2021. 7:1–11. [Google Scholar]
- 51.Adhikari S, Shrestha S, Shakya R, N. K. Prevalence of Chronic Complications and Drug Utilization Pattern of Type II Diabetes Mellitus. MJ Diab. 2017;2(1):006. [Google Scholar]
- 52.Al-Halaweh AA DN, Almdal TP, Cowan A, Khatib S, Nasser-Eddin L, et al. Prevalence of type 2 diabetes mellitus complications among Palestinians with T2DM. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2017;11:S783–S7. [DOI] [PubMed] [Google Scholar]
- 53.Dawit Jember Tesfaye FT, Mohammed Taha. Coexistence of Chronic Complications among Diabetic Patients at Nigist Eleni Mohammed Memorial Hospital, Hossana, South Ethiopia. Open Access Library Journal. Open Access Library Journal. 2015;2 e1218. [Google Scholar]
- 54.Ataur Rahman Khan ZNA-AL, Fatima Shaheen, Yousuf Sana Abdullah Ahmad Al, Afghan Syed Zakaullah Khan, Marghani Salah Al. Prevalence of Chronic Complication among Type 2 Diabetics Attending Primary Health Care Centers of Al Ahsa District of Saudi Arabia: A Cross-Sectional Survey. Global Journal of Health Science 2014;6(4). doi: 10.5539/gjhs.v6n4p245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haque HF AF, Afroze SR, Mitra P, Rahim MA, Ahmed AS, et al. Frequency and Risk Factors of Diabetic Complications among Selected Group of Diabetic Patients: Real-life Scenario from a Developing Country, Bangladesh. BIRDEM Medical Journal. 2017;7(2):143–7. [Google Scholar]
- 56.Ali NSM AO, Salih HM, Ahmed IH. Prevalence of Type 2 Diabetes Associated Complications ion Kurdistan Region Iraq J Basic Clin Pharma 2019;10:1–6. [Google Scholar]
- 57.Ayana Tadesse Korsa ESG, Bayisa Habte Gebeyehu, Dedefo Mohammed Gebre. Diabetes Mellitus Complications and Associated Factors Among Adult Diabetic Patients in Selected Hospitals of West Ethiopia. The Open Cardiovascular Medicine Journal. 2019;13:41–8. [Google Scholar]
- 58.Kim JH, Kim DJ, Jang HC, Choi SH. Epidemiology of micro-and macrovascular complications of type 2 diabetes in Korea. Diabetes & Metabolism Journal. 2011;35(6):571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaz NC, Ferreira A, Kulkarni M, Vaz FS, Pinto N. Prevalence of diabetic complications in rural Goa, India. Indian Journal of community medicine: official publication of Indian Association of Preventive & Social Medicine. 2011;36(4):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haque HF, Afroz F, Afroze SR, Mitra P, Rahim MA, Ahmed AS, et al. Frequency and risk factors of diabetic complications among the selected group of diabetic patients: real-life scenario from a developing country, Bangladesh. BIRDEM Medical Journal. 2017;7(2):143–7. [Google Scholar]
- 61.Roaeid R, Kadiki O. Prevalence of long-term complications among Type 2 diabetic patients in Benghazi, Libya. J Diabetol. 2011;3(5):1–8. [Google Scholar]
- 62.Maniarasu K, Muthunarayanan L. Prevalence of certain chronic complications of diabetes among type 2 diabetic patients in rural population of Kancheepuram district, Tamil Nadu-A cross-sectional study. International Journal of Medicine and Public Health. 2017;7(1). [Google Scholar]
- 63.Khan AR, Lateef ZNA-A, Fatima S, Al Yousuf SAA, Afghan SZK, Al Marghani S. Prevalence of chronic complication among type 2 diabetics attending primary health care centres of Al Ahsa district of Saudi Arabia: a cross-sectional survey. Global journal of health science. 2014;6(4):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ali NSM, Allela OQ, Salih HM, Ahmed IH. Prevalence of Type 2 Diabetes Associated Complications in Kurdistan Region Iraq. Journal of Basic and Clinical Pharmacy. 2019;10(1). [Google Scholar]
- 65.Tarigan TJ, Yunir E, Subekti I, Pramono LA, Martina D. Profile and analysis of diabetes chronic complications in Outpatient Diabetes Clinic of Cipto Mangunkusumo Hospital, Jakarta. Medical Journal of Indonesia. 2015;24(3):156–62. [Google Scholar]
- 66.Raman R, Gupta A, Krishna S, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 27). Journal of Diabetes and its Complications. 2012;26(2):123–8. doi: 10.1016/j.jdiacomp.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 67.Korsa AT, Genemo ES, Bayisa HG, Dedefo MG. Diabetes Mellitus Complications and Associated Factors Among Adult Diabetic Patients in Selected Hospitals of West Ethiopia. The Open Cardiovascular Medicine Journal. 2019;13(1). [Google Scholar]
- 68.Stanifer JW, Cleland CR, Makuka GJ, Egger JR, Maro V, Maro H, et al. Prevalence, risk factors, and complications of diabetes in the Kilimanjaro region: a population-based study from Tanzania. PloS one. 2016;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bos M, Agyemang C. Prevalence and complications of diabetes mellitus in Northern Africa, a systematic review. BMC public health. 2013;13(1):1–7. doi: 10.1186/1471-2458-13-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hussein M, Menasri S. Prevalence of microvascular complications in type 2 diabetics attending a primary healthcare centre in Sudan. Dubai Diabetes and Endocrinology Journal. 2019;25(3–4):127–33. [Google Scholar]
- 71.Macky TA, Khater N, Al-Zamil MA, El Fishawy H, Soliman MM. Epidemiology of diabetic retinopathy in Egypt: a hospital-based study. Ophthalmic research. 2011;45(2):73–8. doi: 10.1159/000314876 [DOI] [PubMed] [Google Scholar]