Abstract
Introduction
Risk factors (e.g., motor symptom asymmetry) for short- and long-term cognitive and neuropsychiatric symptoms following deep brain stimulation (DBS) of the subthalamic nucleus (STN) in patients with Parkinson’s disease have yet to be fully identified. The objectives of the present study were to determine whether motor symptom asymmetry in Parkinson’s disease is one such risk factor and to identify predictors of subnormal cognitive decline.
Methods
A total of 26 patients receiving STN-DBS (13 with left-sided motor symptoms and 13 with right-sided ones) underwent follow-up neuropsychological, depression and apathy assessments over a 5-year period. Nonparametric intergroup comparisons were performed on raw scores, as well as Cox regression analyses on standardized Mattis Dementia Rating Scale scores.
Results
Compared with patients who had predominantly left-sided symptoms, right-sided patients scored higher on both apathy (at 3 months and 36 months) and depressive symptoms (at 6 months and 12 months) and scored lower on global cognitive efficiency (at 36 months and 60 months). Survival analyses revealed that only right-sided patients had subnormal standardized dementia scores, which were negatively associated with the number of perseverations in the Wisconsin Card Scoring Test.
Conclusion
Right-sided motor symptoms are a risk factor for more severe short- and long-term cognitive and neuropsychiatric symptoms following STN-DBS, confirming literature findings on left hemispheric vulnerability.
Keywords: Motor symptom asymmetry, Cognition, STN-DBS, Cognitive vulnerability, Left hemisphere
Introduction
The long-term cognitive effects of deep brain stimulation (DBS) of the subthalamic nucleus (STN) in Parkinson’s disease (PD) remain a controversial issue, with some authors reporting aggravated cognitive decline and others not. As for long-term psychiatric symptoms, apathy and fatigue seem to increase gradually over the long term (for review see Limousin & Foltynie, 2019), whereas depressive symptoms only seem to increase in the short term (Limousin & Foltynie, 2019). The existence of clinical (e.g., preoperative executive functioning), sociodemographic (e.g., age), and surgical (e.g., hemorrhage) modulating factors is the most plausible explanation for this heterogeneity (for review, see Limousin & Foltynie, 2019). In the present study, we were particularly interested in the impact of motor symptom asymmetry on nonmotor symptoms (i.e., global cognitive efficiency, depressive symptoms, and apathy).
Two recent studies of the effects of STN-DBS 3 months and 12 months after surgery (post-DBS) demonstrated the influence of this variable on cognitive, psychiatric, and affective deficits in PD (Voruz et al., 2020; Voruz et al., 2022). In these studies, normalization of emotion recognition, associated with an improvement in depressive symptoms, was observed in the postoperative condition among patients with left-sided motor symptoms of PD (LPD), compared with the pre-DBS condition, with these patients exhibiting levels of depressive symptoms that were comparable to those of a control sample, but reduced performances for the recognition of neutral emotions. In contrast, this same patient subgroup displayed a decrease in cognitive performance (mainly executive functions) in the post-DBS condition, whereas in the pre-DBS condition, their cognitive performance (global cognitive efficiency and executive functions) did not differ from that of the control sample. Among patients with right-sided motor symptoms of PD (RPD) (Voruz et al., 2020), cognitive performance remained unchanged compared with controls and LPD (except for a decrease in performance on one predominantly verbal task [verbal fluency task]) (Voruz et al., 2022), but emotion recognition performance declined. However, these studies only assessed the short-term effects of STN-DBS as a function of motor symptom asymmetry. To the best of our knowledge, a single study has explored long-term changes in neuropsychiatric symptoms among patients with PD according to motor symptom asymmetry, but not post-DBS (Harris, McNamara, & Durso, 2013). Results showed that RPD is a risk factor for a higher incidence of long-term dementia (Harris et al., 2013). An magnetic resonance imaging (MRI) study showing earlier cortical atrophy in the left versus right hemisphere in patients with PD would appear to reinforce this finding, although it did not draw any links with the lateralization of motor symptoms (Claassen et al., 2016).
In the present retrospective study, we had two main objectives. First, we undertook an extended neuropsychological and psychiatric (depressive symptoms and apathy) follow-up of 26 patients (final assessment at 60-month post-DBS), as a function of motor symptom asymmetry. Second, using the Mattis Dementia Rating Scale (MDRS; (Mattis, 1988), an MDS Task Force-recommended Level I screening instrument (Pirogovsky et al., 2014) that had previously been used to quantify the long-term prevalence of dementia in patients with PD post-DBS (Aviles-Olmos et al., 2014; Krack et al., 2003), we performed a survival analysis to determine which clinical, sociodemographic or neuropsychiatric variables were associated with subnormal scores on the MDRS, both for the whole PD group, and separately for LPD and RPD subgroups.
Based on previous findings on the cognitive and psychiatric impact of motor symptom asymmetry following STN-DBS (Voruz et al., 2020; Voruz et al., 2022), we expected to observe the normalization/maintenance of cognitive performances in the RPD subgroup, and a decrease in cognitive functions in the LPD subgroup in the short term. In contrast to cognition, we expected to observe a decrease (i.e., improvement) in depressive symptoms among LPD patients, but an increase among RPD patients. We expected to observe the opposite pattern for apathy, with a decrease in symptoms in RPD patients (Harris et al., 2013). Finally, over the long term, in line with the hypothesis that RPD patients are vulnerable to long-term cognitive deficits associated with dementia (Harris et al., 2013), we expected to observe a decrease in global cognitive efficiency in RPD (i.e., increased signs of cognitive decline as measured with the MDRS), whereas LPD would more or less maintain their global cognitive efficiency.
Methodology
Participants
The power analysis for sample was based on the comparison of two independent sample sets (RPD and LPD). Therefore, the analysis was based on the results for apathy in Harris et al. (2013), who measured neuropsychiatric differences according to motor symptom asymmetry in patients with a disease duration (8.98 ± 5.54) that was broadly comparable to ours in the preoperative condition, and who suggested that RPD patients may be vulnerable to dementia in the long term. Moreover, several studies have found significant correlations between symptoms of apathy and cognitive impairment, particularly executive impairment, suggesting that high levels of apathy are significantly associated with more cognitive deficits. In this context, apathy has been shown to contribute to the development of dementia and to have an impact on daily functioning in PD (Dujardin, Sockeel, Delliaux, Destée, & Defebvre, 2009; Pagonabarraga, Kulisevsky, Strafella, & Krack, 2015). In parallel, a previous study has assessed apathy in patients with advanced PD in relation to motor symptom asymmetry, suggesting a potential relationship between increased apathy symptoms in RPD subgroup of patients and the development of dementia (Harris et al., 2013). These data thus seem to suggest that the development of dementia in PD could be mediated by apathy, which would be more predominant in RPD. To achieve the desired statistical power (1 − β) of 80% and risk of Type I error (α) of 0.05, a power calculation indicated that for a one-sided hypothesis, 11.70 (rounded up to 12) participants would be needed in each group. Given the low number of participants, we would have to run nonparametric statistical analyses (Lehmann, 2012). Therefore, in accordance with the literature, we needed to increase the sample by 15%, with 13.46 (rounded down to 13) participants per group.
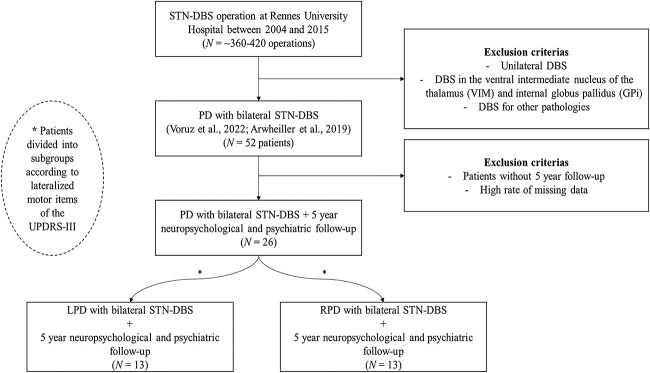
We recruited 26 patients with PD. All of them had undergone surgery for bilateral STN-DBS between 2004 and 2015 (operation performed by CH). All patients were implanted bilaterally in the STN with quadripolar DBS electrodes (monopolar stimulation) (Le Jeune et al., 2009). For the recruitment of these 26 patients, we retrospectively analyzed data from DBS operations performed at Center for Highly Specialized Medicine in Functional Neurosurgery and Movement Disorders at the University Hospital of Rennes (France). The following exclusion criteria were applied: unilateral DBS, DBS in a region other than the STN (Ahrweiller et al., 2019; Voruz et al., 2022). In a second step, we excluded all participants without a 5-year follow-up, as well as those with too many missing data. Analyses revealed no significant differences between the PD subgroup evaluated in this study and the subgroup excluded from the study at 12-month post-DBS (ps > 0.144). A delta score based on the asymmetry of their motor symptoms prior to DBS was retrospectively calculated on the lateralized items (Items 20–26) of the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) (Fahn & Elton, 1987; Stirnimann et al., 2018; Voruz et al., 2020). Patients with a negative score were placed in the LPD subgroup (n = 13; 6 with akinetic-tremor onset, and 7 with akinetic-rigid type onset), and those with a positive score were placed in the RPD subgroup (n = 13; 5 with akinetic-tremor onset and 8 with akinetic-rigid type onset).
Patients were followed up using a battery of neuropsychological tests administered by board-certified clinical neuropsychologists: MDRS (Mattis, 1988) and a series of tests assessing frontal executive functions, including the Modified Wisconsin Card Sorting Test (MCST, Nelson, 1976), Trail Making Test B-A ratio score (TMT, Reitan, 1958), Categorical and Literal Fluency Test (2′) (Cardebat, Doyon, Puel, Goulet, & Joanette, 1990), and the Stroop test (Stroop, 1935), a depression questionnaire, the Montgomery-Åsberg Depression Rating Scale (MADRS, Montgomery & Asberg, 1979), and an apathy questionnaire, the Apathy Evaluation Scale (AES, Marin, Biedrzycki, & Firinciogullari, 1991) over a 5-year period, with assessments at 3-month, 6-month, 12-month, 36-month, and 60-month post-DBS. All patients met the criteria of the Parkinson’s UK Brain Bank for idiopathic PD (Daniel & Lees, 1993). Selection criteria for STN-DBS followed international recommendations published in 1999 (Defer, Widner, Marié, Rémy, & Levivier, 1999) that include disabling levodopa-induced symptoms refractory to medical treatment, disease >5 years, and age < 70 years. At baseline, none of the patients exhibited either cognitive decline, as measured with the MDRS (Fahn & Elton, 1987) (>130/144), severe depressive symptoms, as measured by the MADRS, or DOPA-resistant axial motor signs. All patients underwent neurological screening by board-certified neurologists (MV and SD), and none had a significant neurological event other than PD during the 5-year follow-up. Moreover, no significant differences were observed between PD subgroups on either disease duration [LPD: 12.08 (± 4.94); RPD: 11.00 (± 3.81)] or stimulation parameters (at 12-month and 60-month post-DBS), except for the voltage of the left STN stimulator for the RPD group at 12 months (z = −2.77, p = 0.005), as well as a significant difference on total levadopa equivalent daily dose (LEDD), calculated in accordance with Lozano et al. (1995), which was only observed at 36-month post-DBS (z = −2.05, p = 0.040), with higher levels in RPD patients. No significant differences were observed at the other timepoints.
These patient subgroups were compared in the pre-DBS condition with 13 healthy controls (HC) matched for mean age [LPD: 58 years (± 8.09); RPD: 57.92 (± 8.38); HC: 57.46 (± 7.80)], sex (LPD: 5 women; RPD: 6 women; HC: 6 women), handedness (LPD: 1 left-handed; RPD: 1 left-handed; HC: 2 left-handed), and mean socio-educational level (categorized according to the total number of years of education) [LPD: 3.38 (± 0.77); RPD: 3.38 (± 1.04); HC: 3.12 (± 0.93)]. HC had no history of neurological disease, head injury or alcohol abuse, and displayed no signs of dementia. They achieved the following scores (mean ± SD) on the neuropsychological (MDRS: 141.54 ± 1.90; Stroop interference: 5.73 ± 7.79; TMT B-A: 41.77 ± 28.75; Categorical fluency: 32.15 ± 8.19; Literal fluency: 19.69 ± 6.74; MCST—number of criteria: 5.92 ± 0.28; MCST—number of errors: 2.85 ± 2.15; MCST—number of perseverations: 0.77 ± 0.93) and psychiatric (MADRS: 2.27 ± 2.45; AES: not done) tests and questionnaires.
Ethics
The study was approved by the Ethics Committee of Rennes University Hospital, and all patients gave their written informed consent to take part in this study, which was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
First, given the distribution of the data, we performed nonparametric analyses. For intergroup comparisons, we ran Kruskal–Wallis tests and, if these were significant, Mann–Whitney U tests (LPD vs. RPD; RPD vs. HC; LPD vs. HC) at each timepoint (3-month pre-DBS, and 6-month, 12-month, 36-month, and 60-month post-DBS) and for each domain (cognition and psychiatry). In the post-DBS conditions, we conducted Mann–Whitney U tests (LPD vs. RPD) at each timepoint (6, 12, 36, and 60 months). There was no follow-up for HC. For the intragroup comparisons, we performed Wilcoxon signed-rank tests. All significant results are annotated in the tables according to the conditions being compared. Benjamini–Hochberg false discovery rate (FDR) corrections were applied for all comparisons as a function of the domain being assessed (cognition, neuropsychiatry) at each timepoint (pre-DBS; 3-month post-DBS; 6-month post-DBS; 12-month post-DBS; 36-month post-DBS, 60-month post-DBS).
Second, to determine whether any variables (sociodemographic, medication, cognitive, psychiatric) could predict the occurrence of subnormal MDRS scores suggesting the probable presence of dementia, we began by measuring the prevalence of these scores by comparing each score against robust norms established for the different age groups within the general population (Pedraza et al., 2010). Any score below the fifth percentile was deemed to be below the norm. We then performed a survival function analysis to ascertain the probability of an event (here, a subnormal MDRS score) occurring at a particular time, first for the whole PD group, and second as a function of motor symptom asymmetry. To this end, and because of our relatively small sample, we calculated a stepwise forward Cox regression model (proportional hazards model) (Væth & Skovlund, 2004) with the following predictors: (i) sociodemographic (age), (ii) medication (LEDD), and (iii) cognitive and psychiatric scores for which inter- and intragroup analyses had revealed significant differences. To determine whether a distinct pattern was present and identify possible distinct associative effects based on motor symptom asymmetry, the same analysis was carried out as a function of motor symptom asymmetry at the onset of the disease.
As the MDRS was only administered in on-medication (pre-DBS) and on-medication + on-DBS stimulation (post-DBS) conditions, we only retained the results obtained under the same conditions for the Cox regression models.
Results
Motor, cognitive, and psychiatric inter- and intragroup comparisons as a function of motor symptom asymmetry
Overall, based on the lateralized items of the UPDRS-III scores, RPD had more severe bilateral motor symptoms than LPD, both before and after DBS (Supplementary Table 3). Moreover, RPD experienced a gradual worsening of their motor symptoms, which were noticeably more severe than those of LPD. Finally, analyses failed to reveal any significant differences between LPD and RPD on tremor, rigidity, and bradykinesia scores, except at 12-month post-DBS for the rigidity score in the on-DOPA and on-STIM conditions, and at 60-month post-DBS for the bradykinesia score in the off-DOPA and on-STIM conditions (in both cases, RPD had higher motor scores) (Supplementary Table 4).
Pre-DBS, no significant difference was observed between groups on any of the cognitive variables. Post-DBS, intragroup comparisons revealed that the MDRS total score of RPD patients was significantly lower at 60 months than at 12-, 6-, or 3-month post-DBS, and pre-DBS, although these differences did not survive FDR correction. No significant intragroup difference was observed for LPD (z < 1.89, p > 0.058). Interestingly, when we analyzed performances on each of the MDRS subtests (Supplementary Table 3), RPD performed significantly worse than LPD on the memory subtest at both 36-month (z = 2.26, p = 0.018) and 60-month (z = 2.97, p = 0.002) post-DBS. No other effects survived FDR correction (Table 1).
Table 1.
Performances of the LPD (n = 13) and RPD (n = 13) subgroups of patients with PD before (preoperative condition; M-3) and after (postoperative condition; M + 3, M + 6, M + 12, Y + 3, Y + 5) STN-DBS
| Pre-DBS | Post-DBS | ||||||
|---|---|---|---|---|---|---|---|
| 3 months Mean ± SD |
3 months Mean ± SD |
6 months Mean ± SD |
12 months Mean ± SD |
36 months Mean ± SD |
60 months Mean ± SD |
||
| RPD (n = 13)a | MDRS | 139.46 ± 2.88 | 139.83 ± 3.24 | 138.69 ± 4.63 | 140.46 ± 3.20 | 136.46 ± 8.49 | 132.92 ± 8.26 |
| Stroop—interference | 0.97 ± 6.65 | −0.45 ± 8.14 | −0.70 ± 5.42 | 1.81 ± 4.66 | 2.58 ± 4.87 | −1.10 ± 5.80 | |
| TMT B-A | 80.85 ± 45.86 | 103.25 ± 83.80 | 90.00 ± 69.98 | 77.38 ± 60.01 | 98.83 ± 94.53 | 138.00 ± 124.84 | |
| Categorical fluency | 26.08 ± 9.61 | 23.83 ± 10.33 | 23.23 ± 9.98 | 22.62 ± 8.05 | 23.92 ± 8.80 | 23.67 ± 10.17 | |
| Literal fluency | 20.85 ± 8.81 | 18.17 ± 7.35 | 20.31 ± 7.33 | 18.31 ± 7.80 | 17.92 ± 6.16 | 16.25 ± 9.37 | |
| MCST—Number of criteria | 5.81 ± 0.38 | 5.63 ± 0.48 | 5.54 ± 0.66 | 5.15 ± 1.68 | 4.66 ± 1.73 | 3.83 ± 2.01 | |
| MCST—Number of errors | 4.85 ± 4.04 | 4.75 ± 4.20 | 5.08 ± 4.86 | 5.92 ± 4.82 | 10.17 ± 10.63 | 11.25 ± 6.14 | |
| MCST—Number of perseverations | 1.23 ± 1.30 | 1.08 ± 1.31 | 1.62 ± 2.40 | 1.31 ± 1.75 | 3.42 ± 4.83 | 4.17 ± 3.16 | |
| LPD (n = 13) | MDRS | 140.15 ± 2.88 | 138.31 ± 4.73 | 139.31 ± 6.16 | 140.08 ± 3.30 | 138.38 ± 5.90 | 137.15 ± 6.16 |
| Stroop—interference | 2.54 ± 5.84 | 1.69 ± 8.86 | 3.00 ± 6.97 | 3.72 ± 8.41 | 3.86 ± 6.86 | 4.51 ± 7.18 | |
| TMT B-A | 69.54 ± 79.24 | 72.92 ± 76.96 | 85.62 ± 121.67 | 81.31 ± 92.22 | 74.58 ± 63.77 | 91.08 ± 111.47 | |
| Categorical fluency | 27.77 ± 13.04 | 26.77 ± 11.12 | 27.54 ± 9.36 | 27.54 ± 10.98 | 25.46 ± 10.66 | 26.17 ± 10.40 | |
| Literal fluency | 19.46 ± 7.81 | 16.77 ± 7.77 | 19.08 ± 6.81 | 18.00 ± 4.90 | 19.38 ± 7.70 | 17.83 ± 6.01 | |
| MCST—Number of criteria | 5.63 ± 0.77 | 5.17 ± 1.75 | 5.50 ± 1.19 | 4.83 ± 1.97 | 4.98 ± 1.39 | 4.85 ± 1.76 | |
| MCST—Number of errors | 4.33 ± 5.07 | 4.42 ± 6.78 | 6.00 ± 8.74 | 8.33 ± 8.35 | 9.33 ± 10.65 | 9.50 ± 11.04 | |
| MCST—Number of perseverations | 1.42 ± 2.27 | 1.67 ± 3.31 | 2.00 ± 3.05 | 2.58 ± 3.32 | 2.83 ± 5.10 | 4.20 ± 7.24 | |
aData were missing at 6-month post-DBS for one of the RPD patients.
Note. HC: healthy controls; MCST: Modified Card Scoring Test; MDRS: Mattis Dementia Rating Scale; LPD: patients with PD exhibiting predominantly left-sided motor symptoms; pre-DBS: preoperative condition; post-DBS: postoperative condition; RPD: patients with PD exhibiting predominantly right-sided motor symptoms; SD: standard deviation; TMT: Trail Making Test.
FDR corrections were applied pre-DBS (NA) 3-month post-DBS (NA), 6-month post-DBS (NA), 12-month post-DBS (p < 0.025), 36-month post-DBS (p < 0.006), and 60-month post-DBS (p < 0.002).
Pre-DBS, no significant differences were observed between groups on any of the psychiatric variables (Table 2 and Fig. 1). Post-DBS, for depressive symptoms (MADRS), intergroup analysis revealed a significant difference between RPD and LPD at both 6 months (z = −2.30, p = 0.022) and 12 months (z = −2.01, p = 0.045). Intragroup analysis revealed significant differences for LPD patients between 60-month post-DBS and both 6-month post-DBS and pre-DBS, suggesting an increase in depressive symptoms over time. In contrast, no significant intragroup effects were observed among RPD patients for depressive symptoms. For apathy (AES), intergroup analysis revealed significant differences between LPD and RPD at both 3-month (z = −1.97, p = 0.049) and 36-month (z = −2.31, p = 0.021) post-DBS, suggesting more severe symptoms of apathy in RPD versus LPD patients. No intragroup effect survived FDR correction (see Fig. 2).
Table 2.
Depressive Symptoms and Apathy Scores of LPD (n = 13) and RPD (n = 13) subgroups before (preoperative condition; M-3) and after (postoperative condition; M + 3, M + 6, M + 12, Y + 3, Y + 5) STN-DBS
| PRE-DBS | Post-DBS | ||||||
|---|---|---|---|---|---|---|---|
| 3 months Mean ± SD |
3 months Mean ± SD |
6 months Mean ± SD |
12 months Mean ± SD |
36 months Mean ± SD |
60 months Mean ± SD |
||
| RPD (n = 13)a | Depression (MADRS) | 4.62 ± 4.87 | 9.27 ± 13.68 | 7.00 ± 4.40 | 8.30 ± 5.42 | 4.83 ± 3.56 | 6.92 ± 6.12 |
| Apathy (AES—total score) | 33.85 ± 6.41 | 37.67 ± 10.87 | 35.00 ± 6.47 | 37.11 ± 7.41 | 37.08 ± 6.58 | 33.92 ± 8.53 | |
| LPD (n = 13)b | Depression (MADRS) | 4.00 ± 4.29 | 2.23 ± 2.09 | 2.91 ± 2.59* | 3.55 ± 3.91* | 3.92 ± 2.43 | 5.36 ± 3.50″,# |
| Apathy (AES—total score) | 28.85 ± 6.26 | 28.69 ± 6.02* | 30.36 ± 7.22 | 29.82 ± 8.08 | 30.54 ± 5.41* | 33.00 ± 8.92 | |
aData were missing at 3- and at 12-month post-DBS for two of the RPD patients.
Data were missing at 12- and at 60-month post-DBS for two of the RPD patients.
Significant after FDR correction when compared with RPD.
Significant after FDR correction when compared with 3-month pre-DBS condition.
Significant after FDR correction when compared with 6-month post-DBS condition.
Note. HC: healthy controls; MDRS: Mattis Dementia Rating Scale; LPD: patients with PD exhibiting predominantly left-sided motor symptoms; pre-DBS: preoperative condition; post-DBS: postoperative condition; RPD: patients with PD exhibiting predominantly right-sided motor symptoms; SD: standard deviation.
FDR corrections were applied pre-DBS (NA), 3-month post-DBS (p < 0.050), 6-month post-DBS (p < 0.050), 12-month post-DBS (p < 0.050), 36-month post-DBS (p < 0.050), and 60-month post-DBS (p < 0.025).
Fig. 1.
Study flowchart. Note. GPi: internal globus pallidus; LPD: patients with PD exhibiting predominantly left-sided motor symptoms; PD: patients with Parkinson’s disease; DBS: deep brain stimulation; RPD: patients with PD exhibiting predominantly right-sided motor symptoms; SD: standard deviation; STN-DBS: subthalamic nucleus deep brain stimulation; VIM: ventral intermediate nucleus of the thalamus.
Fig. 2.

Psychiatric changes over 5-year period following STN-DBS among patients living with Parkinson’s disease, differentiated according to motor symptom asymmetry (predominantly left-sided or right-sided PD). (A) Changes in apathy symptoms (AES) from pre-DBS to 60-month post-DBS. (B) Changes in depressive symptoms (MADRS) from pre-DBS to 60-month post-DBS. Note. AES: Apathy Evaluation Scale; MADRS: Montgomery-Åsberg Depression Rating Scale; pre-DBS: preoperative condition; post-DBS: postoperative condition; SD: standard deviation. ** Significant results after FDR correction for within- and between-group differences are marked with colored asterisks.
Predictive variables of global cognitive efficiency
Analysis of MDRS scores showed that no patients had subnormal scores pre-DBS and 3-month post-DBS. At 6-month and 12-month post-DBS, 8.33% of each group (LPD and RPD) had subnormal scores. In contrast, at 36-month post-DBS, 16.33% of LPD patients and 32.66% of RPD patients had subnormal scores. Finally, at 60-month post-DBS, 24.99% of LPD and 41.66% of RPD had subnormal scores. If patients had a low MDRS score at time X, this low score persisted over time (Supplementary Table 4).
Stepwise forward Cox regression analyses (omnibus tests of model coefficients) on the whole PD group revealed a significant negative association between a subnormal score for MDRS and WCST—number of perseverations (B = 0.164, SE = 0.60, Wald = 7.430, p = 0.006). When the analyses were performed as a function of motor symptom asymmetry at the onset of the disease, analyses revealed a significant association with WCST—number of perseverations for RPD patients (B = 0.340, SE = 0.14, Wald = 5.647, p = 0.017), whereas no significant results (WCST—number of errors: B = 0.633, SE = 0.43, Wald = 2.212, p = 0.137; LEDD: B = −0.010, SE = 0.01, Wald = 2.035, p = 0.154) were revealed for LPD patients, suggesting that the whole group effects may have been driven by the relationship between the MDRS score and WCST—number of perseverations in RPD patients (see Fig. 3).
Fig. 3.

Stepwise forward Cox regression survival analyses for whole PD group and motor symptom asymmetry subgroups (left-sided PD and right-sided PD). Analysis revealed similar patterns of results for the whole group and right-sided subgroup. Note. pre-DBS: preoperative condition; post-DBS: postoperative condition.
Discussion
The first aim of the present longitudinal study was to investigate whether the cognitive and psychiatric symptoms of patients with PD after STN-DBS were impacted by motor symptom asymmetry over a 5-year period. Our second aim was to determine which factors were longitudinally associated with a subnormal MDRS score (suggestive of dementia), again according to the asymmetry of the motor symptoms. Previous studies (Voruz et al., 2020; Voruz et al., 2022) had suggested that motor symptom asymmetry can predict the short-term emotional, cognitive and psychiatric outcome in patients undergoing STN-DBS, but its role in long-term nonmotor dysfunction has yet to be demonstrated. In the present study, we confirmed (Harris et al., 2013) the presence of higher levels of apathy and depressive symptoms among RPD in the short-term post-STN-DBS, with no DBS improvement effect. Over the longer term, we observed an increase in depressive symptoms for LPD, whereas RPD remained statistically stable. Regarding cognition and the emergence of dementia, results failed to reveal any significant differences in the pre-DBS condition, but we observed a long-term decrease in global cognitive efficiency that was more pronounced in RPD patients and predicted by the WCST—number of perseverations score.
The long-term cognitive and psychiatric outcomes we observed post-DBS appear to confirm the findings and hypotheses of Harris et al. (2013), with lower neuropsychological scores and higher levels of apathy in RPD patients. They also support previous neuroimaging data indicating earlier cortical atrophy in the left hemisphere in PD (Claassen et al., 2016). That said, we failed to observe either a significant difference on cognition in the pre-DBS condition or a modification (increase or decrease) in cognitive performance on specific neuropsychological tasks, in contrast to what was observed in a larger cohort study by Voruz et al. (2022) from which our patients were extracted. This can be explained precisely by the fact that our cohort was smaller than the one in Voruz et al. (2022). Nevertheless, trends were observed, suggesting that we would have observed results had the size of our cohort been larger. One hypothesis is that DBS transiently restored/maintained some cognitive performances, but these then resumed their pathological trajectory, without any compensatory effects of DBS. We can therefore surmise that there is a greater deterioration in nigrostriatal structures in the left hemisphere (i.e., in RPD), leading to poorer performances and even cognitive impairment. This was suggested by the power of the WCST—number of perseverations to predict a global cognitive decline in the whole PD group (seemingly driven by RPD patients). These effects seem to corroborate previous results suggesting that poor executive function is a risk factor for dementia following STN-DBS (Lezcano et al., 2016), as well as a previous study indicating that that the WCST—number of perseverations score of patients with PD (without STN-DBS) is predictive of the incidence of dementia at 1-year follow-up. In contrast, LPD patients were less prone to a worsening of cognitive performance, and even if a decrease was observed, it seemed to be less marked than in RPD patients. Motor symptom asymmetry with predominant left-hemisphere involvement (RPD patients) can be regarded as a risk factor for the development of cognitive impairment in PD over the long term, and a risk factor for psychiatric symptoms in the short term. This may explain the greater decline in verbal fluency over the long-term post-DBS (Zangaglia et al., 2012), as well as discrepant longitudinal results in the literature for depressive symptoms (Limousin & Foltynie, 2019). Interestingly, this left-hemisphere susceptibility to neurodegeneration explaining subsequent cognitive and motor worsening is not unique to STN-DBS patients and patients with PD in general, as it has been reported in several neurodegenerative disorders, including Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis (Lubben, Ensink, Coetzee, & Labrie, 2021). That being said, LPD patients displayed a significant increase in depressive symptoms over the long term, even though they had significantly lower MADRS scores at 6- and 12-month post-DBS, compared with RPD patients. This suggests a potentially beneficial effect of STN-DBS on depressive symptoms in LPD in the short term, whereas in RPD a negative effect was observed (mean score decreases for LPD, but increases for RPD, which may explain the intergroup results). That said, more longitudinal studies are needed to explore this fascinating left-hemisphere vulnerability for cognitive decline and right-hemisphere vulnerability for depressive symptoms.
Limitations
First, our patient sample could be considered small, possibly compromising the generalization of the results. However, our power analysis, based on a study testing the hypothesis that patients undergoing DBS are vulnerable to dementia (Harris et al., 2013), indicated that we had a sufficient number of participants. Moreover, as patients were matched on age, sex, and sociocultural level and did not differ from HC in the pre-DBS condition, we were able to undertake valid interpretations. Second, we did not have a longitudinally assessed HC group, so we only ran comparisons between the results of patients with PD and those of HC pre-DBS. We also did not have a PD control group without STN-DBS, thus preventing us from interpreting an effect of surgery dissociated from the effects of long-term pathology. Third, STN-DBS stimulation parameters could only be extracted for 12- and 60-month post-DBS, meaning that they could not be included in the regressions, despite their potential effects. Nevertheless, despite a difference in voltage in the left STN at 12-month post-DBS, the parameters were comparable on all points 60-month post-DBS. Fourth, the MDRS is an effective screening instrument, but does not provide a comprehensive assessment of cognitive functioning. Moreover, some of its subscales can be cognitively multidetermined, as is the case for every neuropsychological test. Regarding the neuropsychological test battery, it did not exhaustively probe all cognitive functions, and mainly focused on executive functions. Future studies will need to feature more exhaustive batteries. Consequently, our interpretation must be generalized with caution owing to the limited sample size. Nevertheless, the results of our survival analysis on the subnormal MDRS score yielded results consistent with previous observations in the literature.
Conclusion
The results of the present study support the notion that motor symptom asymmetry, together with right-sided motor symptoms at PD onset, can be a risk factor for the development of cognitive impairment and apathy following STN-DBS. They therefore highlight a left-hemisphere vulnerability to neurodegeneration and open up a new avenue for research in the field of personalized medicine.
Supplementary Material
Acknowledgements
We would like to thank the patients and healthy controls for contributing their time to this study.
Contributor Information
Philippe Voruz, Clinical and Experimental Neuropsychology Laboratory, Faculty of Psychology, Geneva, Switzerland; Neurology Department, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Claire Haegelen, Neurosurgery Department, Pontchaillou University Hospital, Rennes, France; MediCIS, INSERM-University of Rennes 1, Rennes, France.
Frédéric Assal, Neurology Department, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Sophie Drapier, ‘Behavior and Basal Ganglia’ Research Unit, University of Rennes 1, Rennes, France; Neurology Department, Pontchaillou University Hospital, Rennes, France.
Dominique Drapier, ‘Behavior and Basal Ganglia’ Research Unit, University of Rennes 1, Rennes, France; Adult Psychiatry Department, Guillaume Régnier Hospital, Rennes, France.
Paul Sauleau, ‘Behavior and Basal Ganglia’ Research Unit, University of Rennes 1, Rennes, France; Physiology Department, Pontchaillou University Hospital, Rennes, France.
Marc Vérin, ‘Behavior and Basal Ganglia’ Research Unit, University of Rennes 1, Rennes, France; Neurology Department, Pontchaillou University Hospital, Rennes, France.
Julie A Péron, Clinical and Experimental Neuropsychology Laboratory, Faculty of Psychology, Geneva, Switzerland; Neurology Department, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Funding
The present study was performed at the Neurology Department of Rennes University Hospital, France (Prof. Marc Vérin). The first author was funded by Swiss National Foundation grant no. 105314_182221 (PI: Prof. Julie Péron). The funders had no role in data collection, discussion of content, preparation of the manuscript, or decision to publish.
Authors’ Contributions
P.V.: Conception; Statistical execution; Design; Writing of the first draft; Manuscript review and critique C.H.: Execution; Manuscript review and critique F.A.: Manuscript review and critique S.D.: Execution; Manuscript review and critique D.D.: Execution P.S.: Execution; Manuscript review and critique M.V.: Conception; Organization; Execution; Statistical review and critique; Manuscript review and critique J.P.: Conception; Organization; Execution; Statistical review and critique; Writing of the first draft; Manuscript review and critique.
Data availability
The data were extracted from the "B@bel" database from the neurology department of the CHU PONTCHAILLOU (Rennes, France). Access to the data is allowed subject to a request to Dr. Sophie Drapier.
References
- Ahrweiller,K., Houvenaghel, J., Riou, A., Drapier, S., Sauleau, P., Haegelen, C., et al. (2019). Postural instability and gait disorders after subthalamic nucleus deep brain stimulation in Parkinson’s disease: A PET study. Journal of Neurology, 266(11), 2764–2771. 10.1007/s00415-019-09482-y. [DOI] [PubMed] [Google Scholar]
- Aviles-Olmos, I., Kefalopoulou, Z., Tripoliti, E., Candelario, J., Akram, H., Martinez-Torres, I., et al. (2014). Long-term outcome of subthalamic nucleus deep brain stimulation for Parkinson's disease using an MRI-guided and MRI-verified approach. Journal of Neurology, Neurosurgery & Psychiatry, 85(12), 1419–1425. 10.1136/jnnp-2013-306907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardebat, D., Doyon, B., Puel, M., Goulet, P., & Joanette, Y. (1990). Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurologica Belgica, 90(4), 207–217. [PubMed] [Google Scholar]
- Claassen, D. O., McDonell, K. E., Donahue, M., Rawal, S., Wylie, S. A., Neimat, J. S., et al. (2016). Cortical asymmetry in Parkinson's disease: Early susceptibility of the left hemisphere. Brain and behavior, 6(12), e00573. 10.1002/brb3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, S., & Lees, A. (1993). Parkinson's Disease Society Brain Bank, London: Overview and research. Journal of Neural Transmission. Supplementum, 39, 165–172. [PubMed] [Google Scholar]
- Defer, G. L., Widner, H., Marié, R. M., Rémy, P., & Levivier, M. (1999). Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD). Movement disorders: official journal of the Movement Disorder Society, 14(4), 572–584. . [DOI] [PubMed] [Google Scholar]
- Dujardin, K., Sockeel, P., Delliaux, M., Destée, A., & Defebvre, L. (2009). Apathy may herald cognitive decline and dementia in Parkinson's disease. Movement Disorders, 24(16), 2391–2397. 10.1002/mds.22843. [DOI] [PubMed] [Google Scholar]
- Fahn, S., & Elton, R. (1987). UPDRS program members. Unified Parkinsons disease rating scale. Recent Developments in Parkinson’s Disease, 2, 153–163. [Google Scholar]
- Harris, E., McNamara, P., & Durso, R. (2013). Apathy in patients with Parkinson disease as a function of side of onset. Journal of Geriatric Psychiatry and Neurology, 26(2), 95–104. 10.1177/0891988713481267. [DOI] [PubMed] [Google Scholar]
- Krack, P., Batir, A., Van Blercom, N., Chabardes, S., Fraix, V., Ardouin, C., et al. (2003). Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. New England Journal of Medicine, 349(20), 1925–1934. 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Le Jeune, F., Drapier, D., Bourguignon, A., Péron, J., Mesbah, H., Drapier, S., et al. (2009). Subthalamic nucleus stimulation in Parkinson disease induces apathy. A PET Study, 73(21), 1746–1751. 10.1212/WNL.0b013e3181c34b34. [DOI] [PubMed] [Google Scholar]
- Lehmann, E. L. (2012). Parametric versus nonparametrics: two alternative methodologies. In Selected works of EL Lehmann (pp. 437-445). Springer, 10.1007/978-1-4614-1412-4_38. [DOI] [Google Scholar]
- Lezcano, E., Gómez-Esteban, J. C., Tijero, B., Bilbao, G., Lambarri, I., Rodriguez, O., et al. (2016). Long-term impact on quality of life of subthalamic nucleus stimulation in Parkinson’s disease. Journal of Neurology, 263(5), 895–905. 10.1007/s00415-016-8077-4. [DOI] [PubMed] [Google Scholar]
- Limousin, P., & Foltynie, T. (2019). Long-term outcomes of deep brain stimulation in Parkinson disease. Nature Reviews Neurology, 15(4), 234–242. 10.1038/s41582-019-0145-9. [DOI] [PubMed] [Google Scholar]
- Lozano, A. M., Lang, A. E., Galvez-Jimenez, N., Miyasaki, J., Duff, J., Hutchison, W., et al. (1995). Effect of GPi pallidotomy on motor function in Parkinson's disease. The Lancet, 346(8987), 1383–1387. 10.1016/S0140-6736(95)92404-3. [DOI] [PubMed] [Google Scholar]
- Lubben, N., Ensink, E., Coetzee, G. A., & Labrie, V. (2021). The enigma and implications of brain hemispheric asymmetry in neurodegenerative diseases. Brain. Communications, 3(3), fcab211. 10.1093/braincomms/fcab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, R. S., Biedrzycki, R. C., & Firinciogullari, S. (1991). Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research, 38(2), 143–162. 10.1016/0165-1781(91)90040-V. [DOI] [PubMed] [Google Scholar]
- Mattis, S. (1988). Dementia rating scale: Professional manual. Odessa: Psychological Assessment Resources, Incorporated. [Google Scholar]
- Montgomery, S., & Asberg, M. (1979). MADRS scale. Brit J Psychiatry, 134(4), 382–389. 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nelson, H. E. (1976). A modified card sorting test sensitive to frontal lobe defects. Cortex, 12(4), 313–324. 10.1016/S0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga, J., Kulisevsky, J., Strafella, A. P., & Krack, P. (2015). Apathy in Parkinson's disease: Clinical features, neural substrates, diagnosis, and treatment. The Lancet Neurology, 14(5), 518–531. 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- Pedraza, O., Lucas, J. A., Smith, G. E., Petersen, R. C., Graff-Radford, N. R., & Ivnik, R. J. (2010). Robust and expanded norms for the Dementia Rating Scale. Archives of Clinical Neuropsychology, 25(5), 347–358. 10.1093/arclin/acq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky, E., Schiehser, D. M., Litvan, I., Obtera, K. M., Burke, M. M., Lessig, S. L., et al. (2014). The utility of the Mattis Dementia Rating Scale in Parkinson's disease mild cognitive impairment. Parkinsonism & Related Disorders, 20(6), 627–631. 10.1016/j.parkreldis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Reitan, R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8(3), 271–276. 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- Stirnimann, N., N'Diaye, K., Le Jeune, F., Houvenaghel, J.-F., Robert, G., Drapier, S., et al. (2018). Hemispheric specialization of the basal ganglia during vocal emotion decoding: Evidence from asymmetric Parkinson's disease and 18FDG. PET., 119, 1–11. 10.1016/j.neuropsychologia.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. 10.1037/h0054651. [DOI] [Google Scholar]
- Væth, M., & Skovlund, E. (2004). A simple approach to power and sample size calculations in logistic regression and Cox regression models. Statistics in Medicine, 23(11), 1781–1792. 10.1002/sim.1753. [DOI] [PubMed] [Google Scholar]
- Voruz, P., Le Jeune, F., Haegelen, C., N'Diaye, K., Houvenaghel, J.-F., Sauleau, P., et al. (2020). Motor symptom asymmetry in Parkinson's disease predicts emotional outcome following subthalamic nucleus deep brain stimulation. Neuropsychologia, 144, 107494. 10.1016/j.neuropsychologia.2020.107494. [DOI] [PubMed] [Google Scholar]
- Voruz, P., Pierce, J., Ahrweiller, K., Haegelen, C., Sauleau, P., Drapier, S., et al. (2022). Motor symptom asymmetry predicts non-motor outcome and quality of life following STN DBS in Parkinson's disease. Scientific Reports, 12(1), 1–9. 10.1038/s41598-022-07026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangaglia, R., Pasotti, C., Mancini, F., Servello, D., Sinforiani, E., & Pacchetti, C. (2012). Deep brain stimulation and cognition in Parkinson's disease: An eight-year follow-up study. Movement Disorders, 27(9), 1192–1194. 10.1002/mds.25047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data were extracted from the "B@bel" database from the neurology department of the CHU PONTCHAILLOU (Rennes, France). Access to the data is allowed subject to a request to Dr. Sophie Drapier.