Abstract
Objective
Post-9/11 Veterans endorse greater self-reported functional disability than 80% of the adult population. Previous studies of trauma-exposed populations have shown that increased post-traumatic stress disorder (PTSD) and depressive symptoms are consistently associated with greater disability. Additionally, poorer cognitive performance in the domain of executive functions, particularly inhibitory control, has been associated with disability, though it is unclear if this effect is independent of and/or interacts with PTSD and depression.
Method
Three overlapping samples of n = 582, 297, and 183 combat-deployed post-9/11 Veterans completed comprehensive assessments of executive functions, PTSD and depressive symptoms, and self-reported World Health Organization Disability Assessment Schedule-II (WHODAS II).
Results
Poorer performance on measures of inhibitory control (Delis-Kaplan Executive Functioning System Color-Word Interference-CWI Test and gradual-onset Continuous Performance Test-gradCPT), but not other executive functions, were significantly associated with greater disability on the WHODAS II (ρ’s = −.13 and −.13, p = .002 and .026, respectively). CWI inhibitory control measures accounted for unique variance in disability after controlling for PTSD and depressive symptoms (R2 change = 0.02, p < .001). Further, CWI significantly moderated the effect of depressive symptoms on disability, such that better inhibitory control weakened the relationship between depression and disability.
Conclusions
Inhibitory control deficits are uniquely associated with increased disability in combat-deployed post-9/11 Veterans, and better inhibitory control abilities may serve as a protective factor for depressive symptoms leading to increased disability.
Key Points
Question
In a trauma-exposed Veteran population, does inhibitory control predict functional disability above and beyond PTSD and depressive symptoms?
Findings
After controlling for PTSD and depressive symptoms, inhibitory control explained unique variance in self-reported disability. Inhibitory control also showed a moderation effect on depression where greater inhibitory control on the color-word interference test reduced the association between depression and disability symptoms.
Importance
Inhibitory control represents an important mechanism in understanding and improving daily life functioning in trauma-exposed Veteran populations.
Next Steps
Future research should further characterize the different aspects of inhibitory control deficits in trauma-exposed populations and focus on enhancing inhibitory control paired with more standard psychological distress treatments.
Keywords: Everyday functioning, Executive functions, Disability/handicaps, Posttraumatic stress disorder, Depression
Following combat deployment, Veterans who served in post-9/11 conflicts endorse higher rates of functional disability than approximately 80% of all adults in the general international population (Lippa et al., 2015). Such difficulties may include problems with understanding and communicating, completing work/school activities, and mobility. Post-traumatic stress disorder (PTSD) and depression, two of the most common psychiatric conditions in post-9/11 Veterans, have a particularly deleterious effect on everyday functioning, with PTSD and depression accounting for ~60% of the variance in disability (Bernstein et al., 2022; Fortenbaugh et al., 2020; Lippa et al., 2015). Indeed, after controlling for these two diagnoses, other common psychiatric and neurologic sequelae (i.e., anxiety, impaired sleep, physical pain, alcohol/substance use, and mild traumatic brain injury-mTBI) explain substantially less variance in disability, only collectively explaining an additional ~15% (Bernstein et al., 2022; Fortenbaugh et al., 2020; Lippa et al., 2015). Because post-9/11 Veterans have higher survival rates, longer deployments, and an increased exposure to trauma, they may have more complex disabilities than previous eras of Veterans, requiring additional characterization and interventions (Waszak & Holmes, 2017).
Many trauma-exposed Veterans also experience cognitive dysfunction (35.1% demonstrate objective mild or major cognitive dysfunction, Riley et al., 2019), especially those with PTSD and depression diagnoses (Jurick et al., 2022; Riley et al., 2019). Though cognitive deficits have been shown to span across domains in this population (e.g., memory, attention, Vasterling & Arditte Hall, 2018), the domain of executive functions (EFs), processes that allow an individual to plan, monitor, and successfully execute their goals, may be particularly affected (DeGutis et al., 2015; Dolan et al., 2012; Dutra, Marx, McGlinchey, DeGutis, & Esterman, 2018; Esterman et al., 2013a, 2019; Stetz et al., 2007; Swick, Honzel, Larsen, Ashley, & Justus, 2012). According to the influential Miyake model (Miyake et al., 2000), EFs consist of three major subdomains: inhibitory control, the ability to inhibit or override task-irrelevant automatic responses; working memory, manipulating information held in mind; and cognitive flexibility, switching between tasks. Inhibitory control has further shown to have multiple components, including response inhibition, the ability to withhold a prepotent response; and distractor suppression, the ability to ignore or filter out distracting information while performing a goal-directed task (Friedman & Miyake, 2004). Of the three EF subdomains, studies have found that inhibitory control is most consistently linked with PTSD and depression in trauma-exposed populations (Cotrena, Branco, Shansis, & Fonseca, 2016; DeGutis et al., 2015; Qureshi et al., 2011). Better inhibitory control is also related to several important daily life activities in non-trauma-exposed populations, including being associated with better emotional regulation (Tang & Schmeichel, 2014), impulse control (Enticott, Ogloff, & Bradshaw, 2006), and automobile driving safety (Jongen, Brijs, Komlos, Brijs, & Wets, 2011; Sani, Tabibi, Fadardi, & Stavrinos, 2017). Better inhibitory control may generally maintain task-oriented behaviors conducive to functioning across one’s lifespan (Bessette et al., 2020). Because Veterans may have both more prevalent inhibitory control deficits and greater disability than the general population, expanded ranges in these measures may reveal particularly robust inhibitory control/disability associations.
Besides its direct relationship with daily life functioning, poorer inhibitory control may also indirectly influence daily life functioning by playing a critical role in the symptomatology of PTSD (e.g., risk-taking behavior, re-experiencing trauma memories) and/or depression (e.g., poor concentration/distractibility), and may serve as a risk factor and/or consequence of one or both conditions (DeGutis et al., 2015; Dutra & Sadeh, 2018; Joormann & Gotlib, 2010). With regard to PTSD, poorer predeployment inhibitory control ability on the go/no-go gradual-onset continuous performance task (gradCPT) has been associated with a greater likelihood of Veterans developing PTSD post-deployment (Samuelson et al., 2020). Additionally, other disorders associated with inhibitory control impairments, such as ADHD and anxiety, have shown to be risk factors for the development of PTSD (Howlett et al., 2018). Inhibitory control abilities may allow individuals to disengage from or inhibit their responses to trauma-related triggers in their environment or memory, which may reduce the likelihood of developing more persistent PTSD symptoms (Aupperle, Melrose, Stein, & Paulus, 2012; Catarino, Küpper, Werner-Seidler, Dalgleish, & Anderson, 2015; DeGutis et al., 2022). Further, in those with PTSD, trauma-related or emotional stimuli may become considerably more salient and may overwhelm inhibitory control abilities (Ashley, Honzel, Larsen, Justus, & Swick, 2013; Bardeen & Read, 2010), which could further exacerbate pre-existing inhibitory control deficits. For depression, poorer inhibitory control may impair reappraisal strategies (Joormann, 2010; Joormann & Gotlib, 2010) or make it difficult to disengage from ruminative thoughts (Yang, Cao, Shields, Teng, & Liu, 2017; Zetsche, D’Avanzato, & Joormann, 2012). In short, inhibitory control deficits play an important role in developing and maintaining symptoms of PTSD and depression, which can lead to impairments in everyday functioning (Fortenbaugh et al., 2020). Despite this, there remain aspects of both PTSD and depression that are less likely to be associated with inhibitory control, such as the presence of negative emotions, including fear, anger, guilt, or shame.
Inhibitory control has been shown to be an important predictor of functional disability in other populations (e.g., adults with cardiovascular disease, Jefferson, Paul, Ozonoff, & Cohen, 2006; post-acute stroke, Shea-Shumsky, Schoeneberger, & Grigsby, 2019; adult drivers, Sani et al., 2017) and is associated with symptoms of PTSD and depression (DeGutis et al., 2015; Esterman et al., 2019). However, the relationships between inhibitory control, PTSD/depression, and functional disability in trauma-exposed populations remain unclear. On the one hand, stronger inhibitory control may serve as a resiliency factor in those with PTSD and depressive symptoms (i.e., moderation effect), such that greater inhibitory control abilities reduce the impact of depressive and PTSD symptoms on daily life functioning. For example, better inhibitory control abilities such as enhanced distractor suppression may reduce the impact of depression and PTSD symptoms on disrupting driving and/or work performance (Cunningham & Regan, 2016; McIntyre et al., 2013). In contrast, some studies have suggested that inhibitory control may at least partially mediate the relationship between psychiatric symptoms (e.g., depressive symptoms) and daily functioning, as has been observed in studies of other clinical populations (e.g., older adults, Bell, Pope, & Stavrinos, 2018; Bell-McGinty, Podell, Franzen, Baird, & Williams, 2002). Better elucidating the relationships between inhibitory control, PTSD and depression, and daily functioning could provide important mechanistic insights as well as inform clinical practice. For example, this could provide evidence for the usefulness of targeting inhibitory control to improve functioning (e.g., cognitive training: Crocker et al., 2018, Haaland, Sadek, Keller, & Castillo, 2016; Maraver, Bajo, & Gomez-Ariza, 2016; pharmacological interventions: McAllister et al., 2016). Indeed, Crocker et al. (2022) recently found that, in post-9/11 Veterans undergoing cognitive processing therapy for PTSD, poorer inhibitory control was associated with decreased quality of life improvements after treatment.
The present study sought to extend previous studies by using a large group of trauma-exposed Veterans to determine whether inhibitory control is uniquely associated with functional disability independent of other executive functions and PTSD/depressive symptoms. We also sought to assess if inhibitory control significantly moderates or mediates the relationship between PTSD/depression and disability, or alternatively, PTSD/depressive symptoms moderate or mediate the relationship between inhibitory control and functional disability. To address these aims, Veterans were assessed using validated clinical (Clinician Administered PTSD Scale for the DSM-IV, CAPS-IV, Blake et al., 1995; Depression Anxiety Stress Scale, DASS, Lovibond & Lovibond, 1995) and functional outcome measures (World Health Organization Disability Assessment Schedule-II, WHODAS II, Federici, Meloni, & Presti, 2009). Participants were also assessed on a cognitive battery which included multiple measures of inhibitory control (gradual onset Continuous Performance Task-gradCPT, Esterman, Noonan, Rosenberg, & DeGutis, 2013b; Color-Word Interference test-CWI, Delis, Kaplan & Kramer, 2001) working memory (e.g., Digit Span, Weschler, 1997), cognitive flexibility (Trail Making Test Number-Letter Switching, Delis et al., 2001; Intra-Extra Dimensional Set Shift-IED, De Luca et al., 2003), mixed cognitive flexibility and inhibition measures (Letter Fluency and Verbal Fluency, Delis et al., 2001), and adult reading ability (Wechsler Test of Adult Reading-WTAR, Wechsler, 1997). We separately examined three partially overlapping samples of post-9/11 Veterans who were administered slightly different cognitive batteries: 582 performing the CWI along with a battery of additional EF tasks, 297 performing the gradCPT, and 183 performing the CWI, gradCPT, and an additional EF battery. In these samples, to avoid issues of floor effects in functional disability, we also separately examined subgroups of Veterans (72%–80% of the full samples) reporting significant interference in daily life on the WHODAS (n = 420/235/145, respectively; see below for details). These different samples provided important robustness checks of the relationships observed between inhibitory control, functional disability, and PTSD/depressive symptoms.
Methods
Participants And Procedure
Participants in the present study were post-9/11 Veterans enrolled in the longitudinal study at the Translational Research Center for TBI and Stress Disorders (TRACTS), a Research Center of Excellence at the Veterans Affairs (VA) Boston Healthcare System. Participants enrolled in TRACTS complete the entire assessment battery in person and are asked to complete it again approximately two years later. This ongoing project recruits a community-dwelling cohort with a range of psychiatric diagnoses and levels of functional impairment as well as those with no diagnoses. The present study included three samples of consecutively enrolled Veterans between the years 2011 and 2016 who met inclusion and exclusion criteria (see below). Veterans enrolled in the TRACTS longitudinal study completed comprehensive psychiatric and neuropsychological assessments, including diagnostic interviews conducted by doctoral-level psychologists (McGlinchey, Milberg, Fonda, & Fortier, 2017). This study was approved by the VA Boston Healthcare System institutional review board, IRB protocol #2354, and was carried out in accordance with the Declaration of Helsinki.
Inclusion criteria for the TRACTS cohort included service in the US military, deployment to a post-9/11 conflict area, and between ages 18 and 65 years old. Exclusionary criteria included prior seizure disorder, cognitive disorder due to a general medical condition or neurological illness unrelated to TBI, active suicidal or homicidal ideation requiring intervention, and current diagnosis of bipolar disorder or psychotic disorder unrelated to PTSD. To be included in the present study, participants were required to have completed at least one inhibitory control measure. Notably, individuals who completed just one or both inhibitory control measures did not differ from each other in any demographic variables, other EF performance measures, psychiatric variables, or functional disability (see Tables 2 and 4, all p’s > .05). Consistent with our previous work, participants were excluded from the present study if they had not been deployed to a combat zone (n = 18) in order to maintain a focus on trauma-exposed individuals (Lippa et al., 2015). Participants were excluded for a history of moderate/severe TBI (n = 38) as measured by the Boston Assessment of TBI-Lifetime (Fortier et al., 2014), since this represents a qualitatively different injury than the more commonly reported mild TBIs. A total of 51 participants who did not complete the WHODAS II were also excluded. Additionally, consistent with previous studies from our group (McGlinchey et al., 2017), participants were further excluded due to failure on the Medical Symptom Validity Test (Green, 2004), a stand-alone measure of performance validity (n = 67). Individuals that scored less than the recommended cutoff on the immediate recall, delayed recall, or consistency, according to manual guidelines were removed from analyses. Similarly, participants were excluded based on a measure of symptom validity (n = 29; Neurobehavioral Symptom Inventory-NSI Validity-10 scale, see Somatic and Psychiatric Symptoms). Some participants were excluded for more than one reason.
Table 2.
Demographics between no interference and interference subsamples
| Variable | No interference CWI |
Interference CWI |
p-value | No interference gradCPT |
Interference gradCPT |
p-value | No interference combined |
Interference combined |
p-value |
|---|---|---|---|---|---|---|---|---|---|
| n | 145 | 420 | 58 | 235 | 36 | 145 | |||
| Gender (M:F) | 132:13 | 376:44 | .603 | 53:5 | 213:22 | .862 | 33:3 | 130:15 | .720 |
| Age | 33.93 (8.89) | 34.47 ( 9.10) | .529 | 37.52 (9.23) | 37.06 (8.48) | .732 | 36.56 (8.33) | 37.87(8.7) | .414 |
| Race | |||||||||
| Black | 10% | 15% | .447 | 5% | 19% | .011 | 8% | 19% | .119 |
| White | 74% | 71% | .440 | 74% | 68% | .332 | 72% | 69% | .706 |
| Other | 16% | 14% | .051 | 15% | 13% | .022 | 20% | 12% | .010 |
| Education (years) | 14.44 (2.05) | 14.23 (2.12) | .304 | 15.09 (2.17) | 14.52 (2.35) | .099 | 15.17 (2.00) | 14.72 (2.38) | .297 |
| Estimated premorbid IQ (WTAR) | 105.71 ( 11.89) | 104.36 (11.50) | .229 | 108.39 ( 13.79) | 105.91 (11.05) | .250 | 108.39 ( 13.79) | 106.38 (10.74) | .345 |
| PTSD symptoms severity (CAPS-IV) | 22.57 ( 19.83) | 53.12 (26.08) | <.001 | 20.71 (20.27) | 51.17 (27.42) | <.001 | 17.94 (18.26) | 48.08 (26.16) | <.001 |
| Depression symptoms severity (DASS) | 1.86 ( 3.43) | 11.47 (9.58) | <.001 | 1.93 (3.94) | 12.07 ( 9.69) | <.001 | 1.50 (2.84) | 11.37 (9.54) | <.001 |
| Current PTSD diagnosis (%) | 20% | 62% | <.001 | 14% | 57% | <.001 | 11% | 53% | <.001 |
| Current mood disorder (%) | 3% | 35% | <.001 | 2% | 36% | <.001 | 0% | 35% | <.001 |
| Current anxiety disorder (%) | 12% | 19% | .044 | 9% | 16% | .109 | 8% | 17% | .218 |
| Current substance use disorder (%) | 10% | 19% | .012 | 7% | 21% | .013 | 6% | 21% | .074 |
| Military mTBI (%) | 29% | 57% | <.001 | 41% | 61% | .007 | 36% | 59% | .012 |
| Lifetime mTBI (%) | 57% | 74% | <.001 | 62% | 78% | .013 | 61% | 76% | .075 |
| Overall daily life functioning (WHODAS II) | 4.26 (5.80) | 25.02 (15.45) | <.001 | 5.27 (5.80) | 26.89 (16.18) | <.001 | 4.45 (5.41) | 27.24 ± 16.00 | <.001 |
Table 4.
Executive functioning measures between no interference and interference subgroups
| No interference CWI |
interference CWI |
p-value | No interference gradCPT |
interference gradCPT |
p-value | No interference combined |
interference combined | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | n | Mean (SD) | n | Mean (SD) |
n | Mean (SD) |
n | Mean (SD) |
n | Mean (SD) |
n | Mean (SD) |
|||
| CWI Composite of Inhibition and Inhibition/Switching | 144 | 10.23 (2.42) |
420 | 10.10 (2.52) |
.589 | — | — | — | — | — | 36 | 10.49 (2.31) |
145 | 9.92 (2.70) |
.252 |
| CWI Inhibition | 145 | 10.44 (2.62) |
420 | 10.32 (2.85) |
.650 | — | — | — | — | — | 36 | 10.53 (2.72) |
145 | 10.09 (3.33) |
.466 |
| CWI Inhibition/Switching | 144 | 10.04 (2.83) |
420 | 9.89 (2.79) |
.564 | — | — | — | — | — | 36 | 10.44 (2.55) |
145 | 9.76 (2.73) |
.173 |
| gradCPT | — | — | — | — | — | 58 | 3.32 (0.65) |
235 | 3.02 (0.80) |
.010 | 36 | 3.33 (0.68) |
145 | 3.01 (0.80) |
.029 |
| FAS: Letter Fluency total score | 131 | 11.19 (3.26) |
306 | 10.99 (3.69) |
.584 | 47 | 12.26 (3.44) |
137 | 10.96 (4.00) |
.048 | 27 | 12.15 (3.33) |
71 | 10.82 (4.30) |
.150 |
| Trails: Number/Letter Switching total time | 143 | 10.41 (2.45) |
413 | 10.21 (2.57) |
.269 | 57 | 10.30 (2.54) |
229 | 10.35 (2.35) |
.721 | 35 | 10.69 (1.94) |
142 | 10.30 (2.26) |
.240 |
| Digit Span total | 132 | 10.45 (2.96) |
309 | 9.86 (2.84) |
.051 | 47 | 10.87 (3.03) |
141 | 10.06 (2.72) |
.085 | 27 | 11.15 (3.49) |
71 | 9.49 (2.70) |
.014 |
| Intra/Extra Dimensional Set Shift: stages completed | 123 | −0.10 (1.02) |
288 | −0.16 (0.99) |
.579 | 41 | 0.08 (0.64) |
115 | −0.04 (0.78) |
.344 | 22 | 0.11 (0.64) |
57 | −0.16 (0.86) |
.198 |
Participants completed all assessments in person (e.g., CWI, EFs, psychiatric, WHODAS II, validity test, etc.) at time 1 and a subset of participants returned to complete all assessments at time 2. Since we used the entire time 1 sample to examine relationships between the CWI Test and disability, we call this the “CWI sample” (n = 582). Part way through data collection, the gradCPT was added to the assessment battery. In some participants, the gradCPT was administered at the time 1 assessment (n = 183) and in others, it was administered at time 2 (n = 114). We combined all the occasions where participants were given the gradCPT for the first time to create the “gradCPT sample” (n = 297). Finally, because of potential practice effects in the cognitive battery, we only compared and combined the gradCPT with CWI when both measures were given at time 1 (n = 183). We call this the “overlapping sample.” Finally, as the aim of this study was to examine functional disability, we considered these full samples as well as subgroups of participants who reported that their functional difficulties interfered with their daily life (WHODAS H2 > 0; interference subgroup). Unlike the full samples, the interference subgroups did not show severe floor effects on the WHODAS II (see Fig. 1A and B).
Fig. 1.
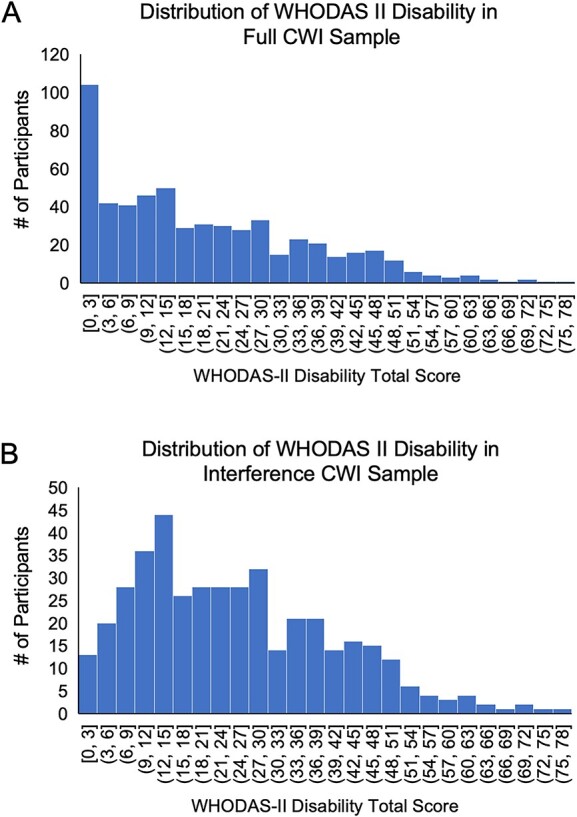
(A) Distribution of WHODAS II disability in full CWI sample. (B) Distribution of WHODAS II disability in interference CWI subgroup. Note. Higher disability scores indicate greater disability. Below each histogram bar, the ranges are within the values specified wile excluding the highest value, e.g., [0,3] represents all numbers ≥0 and <3.
The current study therefore includes the CWI samples (full sample n = 582; interference subgroup n = 420), the gradCPT samples (full sample n = 297; interference subgroup n = 235), and the overlapping samples in which Veterans completed both the CWI and gradCPT (full sample n = 183; interference subgroup n = 145; see Supplementary material online, Fig. S1) where both inhibition tasks were completed during their first data collection visit. Examining the consistency of results across these multiple samples reduced the likelihood of making type-I errors and performed a similar function to sensitivity analyses used in clinical trials (Frey & Patil, 2002; Tortorelli & Michaleris, 1994). We included overlapping samples rather than independent samples in order to improve the power to detect effects in each sample. We also include results using independent samples in the Supplementary Material that are generally consistent with the current findings (see Supplementary material online, Tables S14 and S15). Participants taking the gradCPT were also excluded for “tune outs” or extended periods without responses, indicating poor task compliance, as done in previous studies (n = 13; 30 s without a correct commission, see Cognitive Performance; Fortenbaugh et al., 2015). Sample demographics, clinical, and cognitive characteristics can be found in Tables 1–4.
Table 1.
Demographics in full samples
| Variable | Full CWI | Full gradCPT | Full Overlapping |
|---|---|---|---|
| Mean or % | |||
| n | 582 | 297 | 183 |
| Gender (M:F) | 524:58 | 270:27 | 165:18 |
| Age | 34.21 (8.90) | 37.11 (8.59) | 37.61 (8.57) |
| Race | |||
| Black | 14% | 16% | 17% |
| White | 72% | 69% | 69% |
| Other | 14% | 15% | 14% |
| Education (years) | 14.30 (2.12) | 14.66 (2.33) | 14.84 (2.30) |
| Estimated premorbid IQ (WTAR) | 104.65 (11.62) | 106.48 (11.61) | 106.88 (11.40) |
| PTSD symptoms severity (CAPS-IV) | 45.38 (27.88) | 45.41 (28.78) | 42.20 (27.44) |
| Depression symptoms severity (DASS) | 9.00 (9.46) | 10.18 (9.71) | 9.49 (9.48) |
| Current PTSD diagnosis (%) | 51% | 49% | 47% |
| Current mood disorder (%) | 26% | 29% | 28% |
| Current anxiety disorder (%) | 17% | 14% | 15% |
| Current substance use disorder (%) | 16% | 18% | 19% |
| Military mTBI (%) | 50% | 58% | 55% |
| Lifetime mTBI (%) | 69% | 75% | 73% |
| Overall daily life functioning (WHODAS II) | 19.68 (16.36) | 22.75 (17.09) | 22.83 (17.17) |
Functional Disability Measure
The World Health Organization Disability Assessment Schedule II (WHODAS II, Federici et al., 2009) is a validated self-reported functional outcome measure that has been highly recommended to assess functioning in trauma-exposed post-9/11 Veterans (Bovin et al., 2019). The WHODAS II has 36-items with six subscales: Understanding and Communicating, Getting Around, Self-Care, Getting Along with People, Life Activities, and Participation in Society. The simple scoring method was applied, such that each subscale score contributes equally to the WHODAS II total score. We focused our analyses on the total scores, which ranged from 0 to 100, with higher scores indicating greater disability. We also include analyses of the six separate subscales in the supplementary material. After participants report their functional difficulties across the six subscales, participants are separately asked to indicate how much their functioning interferes with their daily life on a scale of 0 (none) to 4 (extreme). The interference subgroups included participants with overall interference scores greater than 0.
Psychiatric Assessments
The Clinician-Administered PTSD Scale (CAPS-IV) structured interview was used to measure continuous PTSD symptoms (Blake et al., 1995). The current CAPS score was used as a summation of all PTSD symptoms, with total scores ranging from 0 to 136. The Depression subscale from the Depression, Anxiety and Stress Scale (DASS) was used to measure continuous depressive symptoms, with total scores ranging from 0 to 42 (Lovibond & Lovibond, 1995). Data regarding other clinical measures were collected for descriptive purposes, including the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) to measure TBI history (Fortier et al., 2014), and the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) as an assessment of current mood disorder, anxiety disorder, and substance use disorder. In addition to these psychiatric assessments, participants were given the Neurobehavioral Symptom Inventory (NSI) and the Validity-10 subscale, an embedded measure (Vanderploeg et al., 2014) used to identify potential symptom exaggeration. It is composed of NSI items that are most strongly correlated with atypical symptoms as well as the items that were most infrequently endorsed in similar populations. A failure on the Validity-10 Scale, indicating possible symptom over-reporting, was defined based on an a priori cutoff score of 23 or higher (range 0–40, Lange, Brickell, Lippa, & French, 2015).
Table 3.
Executive functioning measures in full samples
| Measure | Full CWI | Full gradCPT | Full combined | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| CWI Composite Inhibition and Inhibition/Switching | 581 | 10.14 (2.49) | — | — | 183 | 10.04 (2.65) |
| CWI Inhibition | 582 | 10.36 (2.79) | — | — | 183 | 10.18 (3.24) |
| CWI Inhibition/Switching | 581 | 9.92 (2.81) | — | — | 183 | 9.89 (2.71) |
| gradCPT | — | — | 297 | 3.08 (0.78) | 183 | 3.08 (0.79) |
| FAS: Letter Fluency total score | 454 | 11.04 (3.59) | 188 | 11.28 (3.93) | 100 | 11.21 (4.15) |
| Trails: Number-Letter Switching total time | 572 | 10.23 (2.58) | 290 | 10.29 (2.44) | 179 | 10.34 (2.26) |
| Digit Span total | 458 | 10.03 (2.91) | 192 | 10.26 (2.79) | 100 | 9.96 (2.99) |
| Intra/Extra Dimensional Set Shift: stages completed | 425 | −0.16 (1.00) | 159 | −0.02 (0.75) | 80 | −0.09 (0.77) |
Cognitive Assessments
Participants completed a battery of validated neuropsychological measures to assess multiple facets of executive functions, including inhibitory control (Esterman et al., 2019; Riley et al., 2019). Dependent on the sample, participants completed either one or both measures of inhibitory control: the Color-Word Interference (CWI) test of the Delis-Kaplan Executive Function System (Delis et al., 2001; Delis, Kramer, Kaplan, & Holdnack, 2004) and the gradual-onset Continuous Performance Task (gradCPT) (Esterman et al., 2013b; Fortenbaugh et al., 2015).
The Color-Word Inhibition (CWI) test is a version of the classic Stroop task that involves trying to, quickly and accurately, perceive and say the color of a word (e.g., printed in green ink) while overcoming interference caused by the mismatched name of the word (e.g., BLUE). CWI indexes both distractor suppression and response inhibition aspects of inhibitory control. Age-scaled total time to complete the CWI was used as the outcome measure. The CWI Inhibition/Switching task is similar to the standard CWI but participants must switch between reading the word, an automatic response, and inhibiting word reading to identify the color of the word. The CWI Inhibition and Inhibition/Switching age-scaled total time were used rather than difference scores (i.e., difference between interference trials and color naming or word reading trials) given that difference scores often have low reliability (Peter, Churchill Jr, & Brown, 1993). CWI Inhibition and Inhibition/Switching both measure inhibitory control, though Inhibition/Switching may have greater inhibitory control demands (Bohnen, Jolles, & Twijnstra, 1992; Delis et al., 2001). This study also used the z-transformed average of the Inhibition and Inhibition/Switching scores, or the CWI Composite, to reduce the number of statistical tests and to boost reliability. We also report results of CWI Inhibition and Inhibition/Switching scores separately in the Supplementary Material, which were generally consistent with the CWI composite.
The gradual-onset continuous performance task (gradCPT) is a validated measure of sustained attention and inhibitory control. The gradCPT has been used in a variety of populations, including trauma-exposed individuals (Esterman et al., 2019; Evans et al., 2021; Fortenbaugh et al., 2017; Jagger-Rickels et al., 2022) and older adults (Fortenbaugh et al., 2015; Park et al., 2021; Wooten et al., 2019). The 4-min version of the task (Fortenbaugh et al., 2015) was used in the current study. A series of gray-scale images are shown to participants, transitioning from one image to the next every 800 ms using pixel-by-pixel interpolation for a total of 299 trials. The images show either a city (90% of stimuli) or a mountain (10% of stimuli), and the task requires response inhibition to mountains while maintaining sustained response initiation to cities. In go/no-go tests in which the go task performance is at ceiling, commission errors are the preferred measure. However, in the gradCPT, participants often make errors of omission and to better account for this, we used d’. Although this measure is not a pure measure of inhibitory control, it reflects the participant’s ability to inhibit rare responses while taking into account their ability to maintain consistent response initiation. D’ on the gradCPT has been shown to be reliable (split-half d′ = 0.78, Fortenbaugh et al., 2015; and test–retest r = .67, Wu et al., 2020), and individual differences in d’ correlate moderately with stop-signal performance, a classic response inhibition task (Jun & Lee, 2021). Consistent with previous gradCPT studies, participants were removed if they had any “tune outs,” or periods of no responses for at least 30 s (see Fortenbaugh et al., 2017). Participants completed three 30-s practice sessions before testing.
In addition to the CWI and gradCPT, participants were administered other executive function measures that primarily assessed working memory and cognitive flexibility/task switching. This included, as a measure of working memory, the Digit Span subtest from the Wechsler Adult Intelligence Scale-IV (total score including forward, backwards, and sequencing, Weschler, 1997). We also administered measures of cognitive flexibility/task switching, including the Trail Making Test Number-Letter Switching (total time) from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) and the CANTAB Intra-Extra Dimensional Set Shift Task (IED; number of stages completed; De Luca et al., 2003). We also included the Letter Fluency condition of the Verbal Fluency Test (FAS total scores) from the D-KEFS as a mixed executive function measure (cognitive flexibility and inhibition, Delis et al., 2001). For the executive function battery, all scores were age-adjusted except for the IED, where scaled-scores were not provided and z-scores of the total stages were used. Finally, we assessed reading ability and estimated premorbid IQ by administering the Wechsler Test of Adult Reading (WTAR, Wechsler, 1997).
It should be noted that though previous TRACTS studies have examined clinical associations with functional disability (Bernstein et al., 2022; Fortenbaugh et al., 2020), this is the first study to examine how aspects of cognitive performance are related to functional disability.
Analyses
We performed independent sample t-tests between the functionally impaired and unimpaired subgroups across demographic, clinical, and cognitive variables. All t-tests were FDR-corrected within the sample to control for type I errors.
Next, to determine if neuropsychological performance was associated with disability, we performed Spearman correlations, since global functional disability in the full sample was non-normally distributed and exhibited floor effects (see Fig. 1). To control for type I errors, we used the largest sample, the full CWI sample (n = 582), and employed FDR correction at 0.05 to all EF measures. We focused primarily on the full CWI sample for FDR correction since it provides more power to discover EF/disability associations than any of the other samples. To assess the significance of the gradCPT/WHODAS II association, we used the full gradCPT sample and performed FDR correction using all EFs as separate measures within that sample.
Next, in order to determine whether inhibitory control uniquely predicted disability above and beyond depression and PTSD, a separate hierarchical linear regression model was run examining the contributions of the two inhibitory control measures to disability1. Depression and PTSD were entered in step 1 of this model, and CWI Composite and gradCPT were entered simultaneously into step 2 to determine whether each accounted for additional variance in disability. Depression and PTSD were included in this model given their strong associations with disability in prior work with this sample (Bernstein et al., 2022; DeGutis et al., 2015; Fortenbaugh et al., 2020). In the overlapping sample, both inhibitory measures were included in step 2 of the same model to determine if they capture unique variance in disability. Other psychiatric and somatic symptoms/conditions (i.e., mTBI, alcohol/substance abuse, and impaired sleep) were not included in the model because, in a previous study of this cohort, they did not account for unique variance in functional disability once PTSD and depression were controlled for (Fortenbaugh et al., 2020). However, anxiety and pain did previously explain additional variance in WHODAS disability above depression and PTSD, and so we ran a follow-up model including these variables in step 1 and inhibitory control in step 2. Though linear regressions are often robust to deviations from normality, it should be noted that the interference subgroups deviated less from normality than the full samples and, because of this, the interference subgroup linear regression results may be more valid than the full sample regressions. It should be noted that there was very little missing data for the variables of interest (<3%) and they were excluded case-wise. Also, in all regressions we checked for multicollinearity and found no instances between independent variables (e.g., ρ > .70).
To determine whether inhibitory control moderated the association between depression or PTSD and disability, Model 1 of the PROCESS Macro was run separately for depression and PTSD across all samples (Hayes, 2012). Model 1 employs boot-strapped confidence intervals to determine significance and decreases type-I errors. To determine whether inhibitory control mediated the association between psychological distress (PTSD, depression) and disability, as well as PTSD and depression mediating the association between inhibitory control and disability, we first confirmed significant relationships between the predictors and mediators (e.g., PTSD and depression with CWI Composite), before performing a simple mediation analysis using PROCESS (Model 4; Hayes, 2012).
Transparency and Openness
Raw data files are available following standard data sharing protocols at the Boston VA. Computer syntax is available through SPSS at request. The study design and analysis plan were not preregistered.
Sample Size Justification
The current study is part of an ongoing longitudinal project (TRACTS) that has consistently found significant associations between PTSD/depressive symptoms and functional disability (Bernstein et al., 2022) as well as inhibitory control and PTSD (DeGutis et al., 2015, 2022). Previous studies have found significant associations between inhibitory control tasks and functional behaviors (e.g., anger management, N = 67, Tang & Schmeichel, 2014; impulsivity, N = 31, Enticott et al., 2006; risky driving N = 53, Jongen et al., 2011; N = 117, Sani et al., 2017). Relevant to the current investigation, previous studies relating the Stroop and go-no/go (a similar paradigm to the gradCPT) tasks to risky driving behaviors (Sani et al., 2017) found effect sizes of f2 = 0.21 and f2 = 0.22, respectively. Thus, with α = 0.05 and 1 − β = 0.80, it should only require 40 participants to adequately detect these associations. However, in the current study we wanted additional sensitivity to detect these inhibitory control/disability associations after controlling for PTSD and depression. Given our minimum sample size (n = 145), if PTSD and depressive symptoms accounted for a liberal 2/3rds of inhibitory control’s variance in its association with disability, we would still have a power of 0.89 to detect an effect size 1/3rd as large as the previous literature (f2 ≥ 0.07, where a small effect is 0.2).
Results
Full Sample Demographic, Clinical, And Cognitive Characteristics
The demographics and clinical characteristics of the three samples and their respective interference subgroups, that is, those with self-reported functional difficulties on the WHODAS II, can be found in Table 2. All samples were representative of the post-9/11 US military, with comparable demographic characteristics (Oni et al., 2020). The full CWI sample was the largest sample (n = 582), 90% male, 72% white, with a mean age of 34.2 years (SD = 8.9), and 14.3 years of education (SD = 2.1). The gradCPT sample and overlapping sample were not significantly different in demographics, nor were any of the corresponding interference subgroups. Regarding clinical characteristics, overall daily life functioning on the WHODAS II differed between those reporting and not reporting functional interference, with differences in functioning being >5 times what is considered a clinically meaningful difference (M difference across groups = 21.71, where a change of ~4 is clinically meaningful, Katajapuu, Heinonen, & Saltychev, 2020). The no-interference participants, compared to participants reporting interference, also had significantly lower current depressive and PTSD symptoms, and fewer depression and PTSD diagnoses across all samples after FDR corrections within samples (See Table 4).
Before examining our cognitive measures of interest, we sought to replicate that greater depression and PTSD symptoms were related to increased WHODAS II disability. In the CWI sample, both PTSD and depression were strongly correlated with disability (full sample ρ = .64 and ρ = .74, respectively, both p < .001; interference subgroup ρ = .52 and .64, respectively, both p < .001). This was also observed in the gradCPT and overlapping samples (PTSD ρ = .52–.66; depression ρ = .61–.72).
Associations Between Executive Functions/Inhibitory Control And Disability
We first compared cognitive performance between those reporting WHODAS II interference in daily life compared to those reporting no interference (see Table 4). Though there was a consistent trend of Digit Span Total performance being worse in interference groups across samples, notably, only gradCPT performance was significantly worse in the interference compared to the no-interference sample after FDR correction (gradCPT sample: no-interference n = 58, M = 3.32, SD = 0.65; interference n = 235, M = 3.02, SD = 0.80, p = .010, q = .050, Cohen’s d = 0.38). In the overlapping sample, there was a similar gradCPT effect, though it did not survive FDR correction (no-interference n = 36, M = 3.33, SD = 0.68; interference n = 145, M = 3.01, SD = 0.80, p = .029, q = 0.116, Cohen’s d = 0.41; See Table 4). Beyond the gradCPT, no other EF measures significantly differed between the interference and no-interference groups after FDR correction. This indicates that those who report their disability interferes with daily functioning have both more severe psychiatric symptoms and worse inhibitory control on the gradCPT.
Next we examined the continuous relationships between EF measures and global functional disability. The full CWI sample with the largest number of participants was used for FDR corrections of all EF measures except the gradCPT (see Methods). Table 5 summarizes the inhibitory control variables across all samples. All samples’ full correlations can be found in the Supplementary material online, Tables S1–S6.
Table 5.
Spearman’s correlations between inhibitory control measures and functional disability (WHODAS II) across samples
| EF Measure | Full CWI | Interference CWI | Full gradCPT | Interference gradCPT | Full overlapping | Interference overlapping |
|---|---|---|---|---|---|---|
| WHODAS-II Overall Total | ||||||
| CWI Composite of Inhibition and Inhibition/Switching | −0.13** | −0.21** | — | — | −0.18* | −0.22** |
| CWI Inhibition | −0.11** | −0.19** | — | — | −0.13 | −0.19* |
| CWI Inhibition/Switching | −0.13** | −0.17** | — | — | −0.16* | −0.17* |
| gradCPT | — | — | −0.13* | −0.07 | −0.17* | −0.14 |
* p < .05.
** p < .01.
In the CWI sample, better performance on CWI inhibitory control measures was significantly correlated with lower disability on the WHODAS II global score (CWI Composite: ρ = −.13, p = .002, q = 0.007; Inhibition: ρ = −.11, p = .006, q = 0.021; Inhibition/Switching: ρ = −.13, p = .002, q = 0.014)2. The same patterns were observed in the interference CWI subgroup (CWI Composite: ρ = −.21, p < .001; Inhibition: ρ = −.19, p < .001; Inhibition/Switching: ρ = −.17, p < .001). For a full analysis of WHODAS II subscales, see the Supplementary Material. In general, the subscales Understanding and Communicating, Getting Around, Getting Along with People, and Participating in Society were most related to CWI and gradCPT. Measures of other facets of EF were not correlated with WHODAS II global or subscales in either CWI sample after FDR-correction (all ps > .05). In the full overlapping sample, we observed similar associations between CWI and WHODAS II (CWI Composite: ρ = −.18, p = .018 and Inhibition/Switching: ρ = −.16, p = .031), except CWI Inhibition alone showed only a trend (ρ = −.13, p = .081). All CWI measures were significantly correlated with disability in the interference overlapping subgroup (CWI Composite: ρ = −.22, p = .009; Inhibition: ρ = −.19, p = .022; Inhibition/Switching: ρ = −.17, p = .045; see Supplementary material online, Tables S5 and S6).
In the full gradCPT sample, higher gradCPT accuracy was significantly associated with decreased disability (ρ = −.13, p = .026), but not in the interference subgroup (ρ = −.07 p = .301). In the full overlapping sample, higher gradCPT accuracy was significantly associated with reduced disability (ρ = −.17, p = .022), with a similar effect size in the interference subgroup, albeit with reduced power (ρ = −.14, p = .097)3.
It should be noted that these significant associations between CWI/gradCPT and WHODAS II were consistent but small and may require an appreciable change (e.g., CWI z-score change of 0.97 for interference overlapping subgroup) to produce a clinically meaningful WHODAS II changes (see Discussion).
Do Color-Word Interference And gradCPT Inhibitory Control Predict Disability Beyond PTSD And Depression?
Better CWI and gradCPT performance were significantly associated with decreased WHODAS II disability and as expected, we also observed significant associations between gradCPT d’ and CWI performance (e.g., gradCPT/CWI Composite ρ = .33, p < .001, see Supplementary Material), as well as associations between depression and PTSD symptoms (e.g., full CWI sample ρ = .60, p < .001). We next sought to determine whether gradCPT and CWI performance explained unique variance in WHODAS II above and beyond depression and PTSD. We did not include other measures of EF in the joint model because they did not demonstrate significant zero-order correlations with disability. We ran a series of regression models by entering PTSD and depression in step 1 in the regression model predicting WHODAS II, and our inhibitory control measure(s) of interest entered in step 2 (see Table 6, Model 2). In the full CWI sample, after accounting for PTSD and depressive symptoms in the first step, the CWI Composite score significantly explained additional variance in disability (F(3, 567) = 287.63, p < .001, R2 = 0.60, R2 change = 0.02, beta = −0.12, p < .001)4, which showed a similar, though larger, effect in the interference subgroup (F(3, 412) = 143.56, p < .001, R2 = 0.51, R2 change = 0.04, beta = −0.19, p < .001)5. In the full gradCPT sample, gradCPT only explained 0.5% of the variance above and beyond depression and PTSD symptoms (F(3, 288) = 124.22, p < .001, R2 = 0.56, R2 change = 0.01, beta = −0.07, p = .064), which showed a similar effect in the interference subgroup (F(3, 228) = 65.33, p < .001, R2 = 0.46, R2 change = 0.01, beta = −0.08, p = .123).
Table 6.
Inhibitory control association with disability after accounting for PTSD and depression
| Measures | Full CWI sample | Full gradCPT sample | Full overlapping sample | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| CWI composite | — | −0.12** | — | — | — | −0.16* |
| gradCPT | — | — | — | −0.07 | — | −0.06 |
| Depressive symptoms | 0.56** | 0.57** | 0.51** | 0.52** | 0.48** | 0.51** |
| PTSD symptoms | 0.29** | 0.28** | 0.32** | 0.30** | 0.32** | 0.27** |
| Model R 2 | 0.59** | 0.60** | 0.56** | 0.56** | 0.52** | 0.55** |
| Model R 2 change | — | 0.02** | — | 0.01 | — | 0.03* |
| Measures | Interference CWI sample | Interference gradCPT sample | Interference overlapping sample | |||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| CWI composite | — | −0.19** | — | — | — | −0.22** |
| gradCPT | — | — | — | −0.08 | — | −0.05 |
| Depressive symptoms | 0.53** | 0.54** | 0.50** | 0.50** | 0.45** | 0.49** |
| PTSD symptoms | 0.26** | 0.23** | 0.25** | 0.25** | 0.27** | 0.22** |
| Model R 2 | 0.48** | 0.51** | 0.46** | 0.46** | 0.41** | 0.55** |
| Model R 2 change | — | 0.04** | — | 0.01 | — | 0.06* |
Note. Figures represent standardized betas. Model 2 controls for depressive symptoms and PTSD symptoms in step 1. CWI = Color-Word Interference, gradCPT = Gradual-Onset Continuous Performance Task.
* p < .05.
** p < .01.
In the overlapping sample and associated interference subgroup, we similarly entered PTSD and depression in step 1 and CWI Composite and gradCPT together in step 2 of a linear regression model predicting WHODAS II (see Table 6, Model 1). In both cases, better CWI Composite but not gradCPT performance significantly predicted decreased WHODAS II disability above and beyond PTSD and depression (full F(4, 174) = 53.86, p = .001, R2 = 0.55, R2 change = 0.03; betas = −0.16 and −0.06, p = .004 and .249, respectively; interference F(4, 140) = 29.77, p = .001, R2 = 0.41, R2 change = 0.06; betas = −0.22 and −0.04, p = .002 and .470, respectively).
Do PTSD/Depression Partially Mediate The Effects of Inhibitory Control on Disability or Vice Versa?
Though inhibitory control explained unique variance in WHODAS II from PTSD and depressive symptoms, it still could be that some part of the relationship between inhibitory control and WHODAS II is mediated by depressive/PTSD symptoms or rather the relationships between depressive/PTSD symptoms and WHODAS II are partially mediated by inhibitory control. Notably, we only observed significant inhibitory control relationships with PTSD symptoms, so we exclusively examined PTSD symptoms for potential mediation effects. In the full CWI sample, CWI Composite was significantly correlated with PTSD (ρ = −.10, p = .012) but not depressive symptoms (ρ = −.05, p = .251). In the full gradCPT sample, better gradCPT performance was associated with decreased PTSD symptoms (ρ = −.14, p = .013), though not depressive symptoms (ρ = −.05, p = .395). Across all samples, mediation analyses revealed no significant indirect effects of PTSD symptoms mediating the effects of inhibitory control on disability, or inhibitory control mediating the effects of PTSD’s effects on disability in all samples (all p’s > .10).
Inhibitory Control Moderates The Association Between Depression And Disability
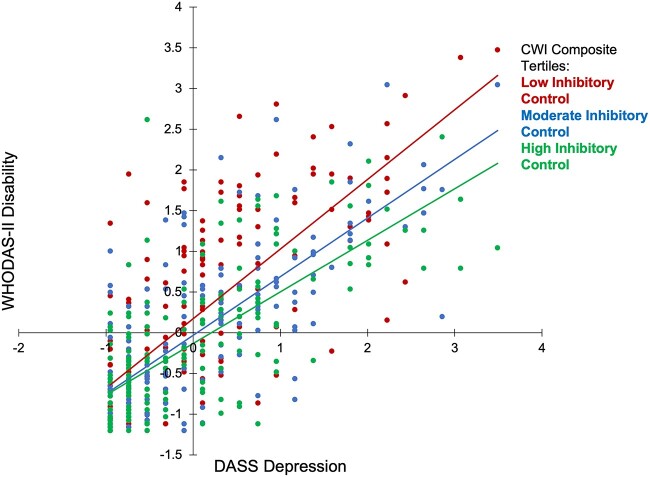
Finally, we sought to determine if inhibitory control moderates the associations between PTSD/depressive symptoms and disability. Based on previous work by Lee and Chao (2012) suggesting that inhibitory control may influence the relationship between depressive symptoms and well-being, we hypothesized that better inhibitory control would result in a reduced depression/disability association. We used the PROCESS macro (Hayes, 2012) to examine the moderation effects of CWI Composite and gradCPT with either depression or PTSD symptoms (see Supplementary material online, Table S13). In the full CWI sample, CWI Composite performance significantly moderated the relationship between depressive symptoms and disability (F(3, 569) = 11.85, p < .001, beta = −0.12, p = .006; see Table 7). Specifically, the positive relationship between increased depressive symptoms and greater disability was stronger in participants with worse CWI Composite scores than in participants with better CWI Composite scores. This suggests that better inhibitory control decreases the influence of depressive symptoms on overall disability. In Fig. 2, for illustrative purposes, we first separated CWI Composite scores into tertiles, grouping the data into low, moderate, and high inhibitory control abilities. We then plotted total WHODAS II scores and depressive symptoms separately for each of the three inhibitory control groups. Lines of best fit further revealed the weakening association between depression and disability at higher levels of inhibitory control (“Low CWI Composite”: slope = 0.86, R2 = 0.57; “Moderate CWI Composite”: slope = 0.72, R2 = 0.56; “High CWI Composite”: slope = 0.63, R2 = 0.51).
Table 7.
Moderating relationship of CWI composite on depressive symptoms and PTSD associations with disability (WHODAS II)
| Depression model | PTSD model | ||
|---|---|---|---|
| Full CWI sample (DV = WHODAS-II) | |||
| Depressive symptoms | 0.74** | PTSD symptoms | 0.60** |
| CWI composite | −0.17** | CWI composite | −0.11* |
| Depression × CWI inhibition | −0.12** | PTSD × CWI inhibition | 0.02 |
| Model R 2 | 0.56** | Model R 2 | 0.39 |
| Interference CWI sample (DV = WHODAS-II) | |||
| Depressive symptoms | 0.66** | PTSD symptoms | 0.49** |
| CWI composite | −0.24** | CWI composite | −0.19* |
| Depression × CWI inhibition | −0.09* | PTSD × CWI inhibition | 0.05 |
| Model R 2 | 0.48** | Model R 2 | 0.29 |
Note. Figures represent standardized betas. CWI = Color-Word Interference, gradCPT = Gradual-Onset Continuous Performance Task.
* p < .05.
** p < .01.
Fig. 2.
Scatterplot of WHODAS II disability and depression by CWI composite in full CWI sample.
Consistent with these results, in the interference CWI subgroup, CWI Composite performance significantly moderated the relationship between depressive symptoms and disability (F(3, 414) = 126.07, p < .001, beta = −0.09, p = .0483; see Table 7). The interference subgroup showed a similarly decelerating slope at high inhibition scores (“Low”: slope = 0.72, R2 = 0.44; “Moderate”: slope = 0.72, R2 = 0.53; “High”: slope = 0.58, R2 = 0.43; see Supplementary material online, Fig. S2).
In the overlapping samples, we found comparable moderating effect sizes of CWI Composite on depressive symptoms predicting disability, though these analyses lacked the power to achieve significance (full: F(3, 177) = 62.56, p < .001, beta = −0.10, p = .170; F(3, 140) = 36.60, p < .001, beta = −0.10, p = .214; see Supplementary material online, Table S13).
In the gradCPT samples, we found no moderation effect of gradCPT with depressive symptoms on disability (full F(3, 290) = 66.88, p < .001, beta = −0.02, p = .712; interference F(3, 230) = 56.63, p < .001, beta = −0.03, p = .556). Across all samples, inhibition measures did not moderate the effects of PTSD symptoms on disability (all p’s > .05; see Supplementary material online, Table S13).
Discussion
The present study found that inhibitory control performance on the Color-Word Interference (CWI) test and the go/no-go gradual onset Continuous Performance Task (gradCPT), but not other aspects of executive functions (EFs), were associated with self-reported disability in trauma-exposed post-9/11 Veterans. This inhibitory control/disability relationship was observed across three partially overlapping samples of Veterans and interference subgroups within these samples, attesting to the robustness of these findings. We found that gradCPT performance significantly differentiated between those with and without self-reported WHODAS II functional interference and that CWI was associated with greater WHODAS II disability, even after controlling for PTSD and depressive symptoms. We additionally found a moderation effect, such that greater CWI Composite inhibitory control weakened the relationship between depressive symptoms and WHODAS II (see Fig. 2). Together, the current results suggest that inhibitory control plays a unique role in functional disability among trauma-exposed post-9/11 Veterans and has both clinical and treatment implications.
The current results replicate and extend prior work demonstrating the importance of inhibitory control processes in trauma-exposed samples and other populations with functional disabilities. We replicated that, in a trauma-exposed Veteran population, greater PTSD symptoms are associated with poorer inhibitory control (Esterman et al., 2013a, Esterman et al., 2013b; Esterman et al., 2019; Dutra et al., 2018), and that this association is not found with other EFs (similar to DeGutis et al., 2015). The novel contribution of the current study is that it demonstrated that inhibitory control (as measured by CWI), and not other executive functions, predicted self-reported disability and that this relationship was present even after controlling for PTSD and depression. This is notable and consistent with studies in other clinical populations, which have frequently found that inhibitory control is more strongly tied to aspects of disability (e.g., understanding and communicating, mobility) than other aspects of cognitive function, including other EF sub-domains (e.g., cardiovascular disease, Jefferson et al., 2006; stroke, Shea-Shumsky et al., 2019). The current study extends these studies by demonstrating an inhibitory control/disability association in a younger and overall less disabled population. Further, these previous studies did not control for depressive or PTSD symptoms, which may be related to both poorer inhibitory control (Aupperle et al., 2012) and greater disability.
Interestingly, we found that although the gradCPT and CWI were significantly correlated, they had complementary effects with regard to disability. GradCPT significantly differed between those with and without any WHODAS II self-reported interference in everyday life, suggesting sensitivity to the presence of disability. In contrast, CWI did not significantly differ between the presence or absence of self-reported interference but was associated with continuous disability differences on the WHODAS II. Notably, only CWI significantly predicted disability in the joint model with gradCPT. Further, rerunning analyses in a reduced, independent sample from the overlapping sample, gradCPT had no significant association with disability while CWI similarly predicted disability (see Supplementary Material). One reason why the CWI measures may have performed better overall is that while gradCPT may primarily measure response inhibition (Esterman et al., 2019; Jun & Lee, 2021), CWI measures aspects of response inhibition (e.g., inhibit saying the text) while also requiring distractor suppression (e.g., ignoring the text and attending to the color; Tiego, Testa, Bellgrove, Pantelis, & Whittle, 2018). CWI plausibly requires multiple facets of inhibitory control that better capture deficits related to functional disability.
In addition to inhibitory control uniquely predicting functional disability, we also found that inhibitory control significantly moderated the relationship between depression and disability. The relationship between depression and disability was weaker in those with better inhibitory control abilities on the CWI. In contrast, inhibitory control did not moderate the relationship between PTSD and disability. These findings suggest that better inhibitory control abilities may play a protective role in depressive symptoms leading to disability. For example, better inhibitory control abilities may make it so depressive symptoms (e.g., repetitive negative thoughts, rumination, Zetsche, D’Avanzato, & Joormann, 2012) interfere less with everyday activities, such as providing the ability to better disengage from repetitive thoughts and focus on current task goals. Notably, inhibitory control did not mediate the relationship between depressive or PTSD symptoms and disability, nor did depression or PTSD mediate inhibitory control’s relationship with disability. Together with the mixed results concerning moderation, this pattern of findings suggests that while inhibitory control may buffer the effects of depressive symptoms on disability, it does not explain the relationship between depression or PTSD and disability.
Why were inhibitory control deficits specifically associated with greater functional disability and why did they moderate the depression/WHODAS II association? One possibility is that better inhibitory control could improve general task engagement by reducing distractibility and improving impulse control (Enticott et al., 2006; Tiego et al., 2018). This could lead to an enhanced ability to accomplish goal-oriented behaviors in everyday life in a range of settings such as work, school, and social situations (Bessette et al., 2020). Inhibitory control may also be particularly important for emotion regulation (Tang & Schmeichel, 2014) such as disengaging from ruminative or traumatic thoughts (Aupperle et al., 2012), thus moderating the degree to which clinical symptoms impact functioning. Future studies comparing cool versus hot aspects of inhibitory control (i.e., tasks with non-emotional vs. emotional or trauma-related stimuli, respectively) would be important to better understand the relative contributions to improved functioning.
The current findings have mechanistic and clinical implications. Past work suggests that depression and PTSD account for much of the variance in disability in this population (Bernstein et al., 2022; Fortenbaugh et al., 2020). It may be that PTSD and depression lead to or are associated with poorer inhibitory control performance, but notably the current findings suggest that aspects of inhibitory control are associated with disability beyond their association with PTSD and depression. Clinically, our results suggest the possibility that reductions in PTSD and depressive symptoms following successful treatment (e.g., SSRIs, cognitive processing therapy for PTSD) may not fully remediate functional disability or, as a recent study by Crocker et al. (2022) suggests, functional improvements may be greater in those with better baseline inhibitory control. As such, efforts to improve post-9/11 Veterans’ inhibitory control, such as through cognitive training (Allom, Mullan, & Hagger, 2016) or pharmacological approaches (McAllister et al., 2016) might be beneficial in improving daily functioning as well as PTSD and depressive symptoms (e.g., attentional control training, Badura-Brack et al., 2015). Notably, Lazarov et al. (2019) found that in 24 civilians with PTSD, attentional control training for four weeks was associated with large reductions in both PTSD and depression symptoms between timepoints (Cohen’s d = 1.64 and 0.91, respectively). Veterans may specifically benefit from improving inhibitory control rather than targeting EF improvements more generally.
Limitations and Constraints on Generality
While these results offer important clinical and mechanistic implications, there are several limitations of the current study. The study consisted of post-9/11 Veterans, which were predominantly white, male, and middle aged. Additionally, compared to trauma-exposed civilian populations, Veterans have unique trauma experiences related to combat exposure and it would be important for future studies to replicate these effects in more diverse, trauma-exposed civilian populations. Also, because we introduced the gradCPT to a longitudinal study already in progress, our battery was inconsistent (e.g., the first time participants took the gradCPT was often the second time participants took the rest of the battery) and created multiple subsamples. However, a strength of the current findings is that the results replicated across subsamples. Additionally, though the WHODAS II is a well-validated measure of disability, it had a floor effect in this population. While our results replicated when examining individuals reporting WHODAS II interference in daily life where floor effects were less of an issue, it will be important to replicate these findings using more objective and potentially more sensitive functional assessments, such as clinician-rated measures or ecological momentary assessments (Gromatsky et al., 2020). A final limitation is that inhibitory control abilities explained a modest amount of variance in disability. This effect size is in line with previous cognition-disability studies (Jongen et al., 2011; Sani et al., 2017) and could potentially be increased in future studies by more thoroughly characterizing inhibitory control abilities (e.g., including inhibitory control tasks with emotional stimuli such as the emotional Stroop). Additionally, studies suggest that cognition-disability relationships become stronger with age (Lee & Shinkai, 2005), suggesting that older Veterans may demonstrate more pronounced inhibitory control-disability effects.
In sum, the current study showed that, in a trauma-exposed population of post-9/11 Veterans, inhibitory control was more strongly associated with functional disability than other aspects of executive functioning, and uniquely contributed to functional disability independent of PTSD and depressive symptoms. Results suggest that, in trauma-exposed populations, inhibitory control may be a transdiagnostic target and improvements in inhibitory control may yield benefits for daily life functioning above and beyond efforts to treat depression and PTSD.
Funding
This work was supported by the US Department of Veterans Affairs through the Translational Research Center for TBI and Stress Disorders, a VA Rehabilitation Research & Development TBI National Research Center (B3001-C). ME was supported by a VA Clinical Science Research & Development Merit Review (I01CX001653), AR was supported by a T32 postdoctoral training award from the National Institute of Health (2T32MH01983621), and WM was supported by a National Institute of Health NCCIH grant (R21 AT009430-01).
Conflicts of Interest
None of the authors have conflicts of interest.
Author Contributions
JD and JB conceived of the study. SA performed the data analysis and interpretation as well as data visualization under the supervision of JD and JB. JD, JB, and SA drafted the paper, and ME, AJ, TE, WM, CF, and RM provided critical revisions. RM and WM contributed funding. All authors approved of the final version of the paper for submission.
Supplementary Material
Acknowledgements
The authors report no conflict of interest.
Footnotes
We considered empirically deriving an inhibitory control measure via factor analysis. However, previous factor analyses in large normative samples have already identified the Stroop and go/no-go as clear measures of inhibitory control (Friedman & Miyake, 2004; Miyake et al., 2000). Further, we thought it would be better to focus on these assessments to make it clear how inhibitory control was operationalized and so that future studies could replicate our results by including just the Stroop and go/no-go tasks rather than having to include our entire EF battery.
We also examined CWI color naming and word reading trials and found weak non-significant correlations with total disability in either CWI sample (full ρ = −.04, −.08; p = .294, .056, respectively; interference ρ = −.08, −.09; p = .079, .061, respectively) and no difference between those with or without interference (t = 0.53, 1.06; p = .593, .289, respectively).
We examined whether mTBI was related to CWI and gradCPT in each respective full and interference sample. There was no relationship between military or lifetime TBI and CWI, while worse gradCPT performance was worse in those with compared to without a mTBI (e.g., full sample military mTBI t = −2.28, p = .023). However, in all cases mTBI was no longer significant in a regression controlling for PTSD and depressive symptoms. We also examined if current or lifetime substance use diagnosis were related to inhibitory control and found no significant relationship across both CWI and gradCPT except for current substance use in the interference gradCPT sample, where the presence of a substance use disorder was associated with worse gradCPT (t = −1.98; p = 0.049), though this was no longer significant after controlling for PTSD and depressive symptoms (F = 3.16, p = 0.077). Finally, an ADHD diagnosis was related to worse performance in only the full CWI sample (t = −2.37, p = 0.018). However, it was no longer significant after controlling for PTSD and depressive symptoms (F = 0.342, p = 0.559; see Supplementary Material).
Given previous literature finding an association between education, age, and gender on disability (Fortenbaugh et al., 2020), we ran correlations in the full CWI sample. Greater education was correlated with decreased disability (ρ = −.08, p = .046), but not gender (ρ = .05, p = .202) or age (ρ = .08, p = .061). However, in a regression model, education explained no additional variance in a joint model with CWI Composite and PTSD and depressive symptoms. We further added the NSI total score to the model as it was correlated with CWI (ρ = −.18; p < .001) and gradCPT (ρ = −.15; p = .011) in their respective full samples. Notably, CWI still predicted unique variance in the WHODAS II after controlling for PTSD, depressive symptoms, and NSI total (CWI beta = −0.05, p = .04), though this was not the case for gradCPT.
We ran additional models accounting for PTSD, depression, anxiety, and pain symptoms in step 1 and CWI in step 2 across both the full and interference CWI samples (Bernstein et al., 2022). CWI explained additional variance in both samples (full F(5, 459) = 209.04, p < .001, R2 = 0.70, R2 change = 0.01, beta = −0.10, p < .001; interference F(5, 317) = 97.61, p < .001, R2 = 0.61, R2 change = 0.03, beta = −0.17, p < .001).
Contributor Information
Joseph DeGutis, Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Health Care System, Boston, MA, USA; Boston Attention and Learning (BAL) Lab, VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Sam Agnoli, Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Health Care System, Boston, MA, USA; Boston Attention and Learning (BAL) Lab, VA Boston Health Care System, Boston, MA, USA.
John P K Bernstein, Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Health Care System, Boston, MA, USA.
Audreyana Jagger-Rickels, Boston Attention and Learning (BAL) Lab, VA Boston Health Care System, Boston, MA, USA; National Center for PTSD, VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA.
Travis C Evans, Boston Attention and Learning (BAL) Lab, VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA.
Catherine B Fortier, Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Regina E McGlinchey, Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
William P Milberg, Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Harvard Medical School, Boston, MA, USA.
Michael Esterman, Boston Attention and Learning (BAL) Lab, VA Boston Health Care System, Boston, MA, USA; National Center for PTSD, VA Boston Health Care System, Boston, MA, USA; Department of Psychiatry, Boston University School of Medicine, Boston, MA, USA.
References
- Allom, V., Mullan, B., & Hagger, M. (2016). Does inhibitory control training improve health behaviour? A meta-analysis. Health Psychology Review, 10(2), 168–186. 10.1080/17437199.2015.1051078. [DOI] [PubMed] [Google Scholar]
- Ashley, V., Honzel, N., Larsen, J., Justus, T., & Swick, D. (2013). Attentional bias for trauma-related words: Exaggerated emotional Stroop effect in Afghanistan and Iraq war veterans with PTSD. BMC Psychiatry, 13(1), 1–11. 10.1186/1471-244X-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle, R. L., Melrose, A. J., Stein, M. B., & Paulus, M. P. (2012). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62(2), 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura-Brack, A. S., Naim, R., Ryan, T. J., Levy, O., Abend, R., Khanna, M. M. et al. (2015). Effect of attention training on attention bias variability and PTSD symptoms: Randomized controlled trials in Israeli and US combat veterans. American Journal of Psychiatry, 172(12), 1233–1241. 10.1176/appi.ajp.2015.14121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeen, J. R., & Read, J. P. (2010). Attentional control, trauma, and affect regulation: A preliminary investigation. Traumatology, 16(3), 11–18. 10.1177/1534765610362801. [DOI] [Google Scholar]
- Bell, T., Pope, C., & Stavrinos, D. (2018). Executive function mediates the relation between emotional regulation and pain catastrophizing in older adults. The Journal of Pain, 19(3), S102. 10.1016/j.jpain.2017.12.234. [DOI] [Google Scholar]
- Bell-McGinty, S., Podell, K., Franzen, M., Baird, A. D., & Williams, M. J. (2002). Standard measures of executive function in predicting instrumental activities of daily living in older adults. International Journal of Geriatric Psychiatry, 17(9), 828–834. 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- Bernstein, J. P., Stumps, A., Fortenbaugh, F., Fonda, J. R., McGlinchey, R. E., Milberg, W. P. et al. (2022). Associations between changes in somatic and psychiatric symptoms and disability alterations in recent-era US veterans. Journal of Traumatic Stress, 35(3), 1011–1024. 10.1002/jts.22809. [DOI] [PubMed] [Google Scholar]
- Bessette, K. L., Karstens, A. J., Crane, N. A., Peters, A. T., Stange, J. P., Elverman, K. H. et al. (2020). A lifespan model of interference resolution and inhibitory control: Risk for depression and changes with illness progression. Neuropsychology Review, 30(4), 477–498. 10.1007/s11065-019-09424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S. et al. (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Bohnen, N. I. L. J., Jolles, J., & Twijnstra, A. (1992). Modification of the Stroop color word test improves differentiation between patients with mild head injury and matched controls. The Clinical Neuropsychologist, 6(2), 178–184. 10.1080/13854049208401854. [DOI] [PubMed] [Google Scholar]
- Bovin, M. J., Meyer, E. C., Kimbrel, N. A., Kleiman, S. E., Green, J. D., Morissette, S. B. et al. (2019). Using the World Health Organization disability assessment schedule 2.0 to assess disability in veterans with posttraumatic stress disorder. PLoS One, 14(8), e0220806. 10.1371/journal.pone.0220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino, A., Küpper, C. S., Werner-Seidler, A., Dalgleish, T., & Anderson, M. C. (2015). Failing to forget: Inhibitory-control deficits compromise memory suppression in posttraumatic stress disorder. Psychological Science, 26(5), 604–616. 10.1177/0956797615569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrena, C., Branco, L. D., Shansis, F. M., & Fonseca, R. P. (2016). Executive function impairments in depression and bipolar disorder: Association with functional impairment and quality of life. Journal of Affective Disorders, 190, 744–753. 10.1016/j.jad.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Crocker, L. D., Jurick, S. M., Thomas, K. R., Keller, A. V., Sanderson-Cimino, M., Boyd, B. et al. (2018). Worse baseline executive functioning is associated with dropout and poorer response to trauma-focused treatment for veterans with PTSD and comorbid traumatic brain injury. Behaviour Research and Therapy, 108, 68–77. 10.1016/j.brat.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Crocker, L. D., Sullan, M. J., Jurick, S. M., Thomas, K. R., Davey, D. K., Hoffman, S. N. et al. (2022). Baseline executive functioning moderates treatment-related changes in quality of life in veterans with posttraumatic stress disorder and comorbid traumatic brain injury. Journal of Traumatic Stress. 10.1002/jts.22883. [DOI] [PubMed] [Google Scholar]
- Cunningham, M. L., & Regan, M. A. (2016). The impact of emotion, life stress and mental health issues on driving performance and safety. Road & Transport Research: A Journal of Australian and New Zealand Research and Practice, 25(3), 40–50. [Google Scholar]
- De Luca, C. R., Wood, S. J., Anderson, V., Buchanan, J. A., Proffitt, T. M., Mahony, K. et al. (2003). Normative data from the CANTAB. I: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology, 25(2), 242–254. 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- DeGutis, J., Agnoli, S., Gaudet, C., Stumps, A., Kim, S., Evans, T. C. et al. (2022). Inhibitory control and alcohol use history predict changes in post-traumatic stress disorder symptoms. Psyarxiv. [DOI] [PubMed] [Google Scholar]
- DeGutis, J., Esterman, M., McCulloch, B., Rosenblatt, A., Milberg, W., & McGlinchey, R. (2015). Posttraumatic psychological symptoms are associated with reduced inhibitory control, not general executive dysfunction. Journal of the International Neuropsychological Society, 21(5), 342–352. 10.1017/S1355617715000235. [DOI] [PubMed] [Google Scholar]
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001). Delis-Kapan executive function system. Assessment. [DOI] [PubMed]
- Delis, D. C., Kramer, J. H., Kaplan, E., & Holdnack, J. (2004). Reliability and validity of the Delis-Kaplan executive function system: An update. Journal of the International Neuropsychological Society, 10(2), 301–303. 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Dolan, S., Martindale, S., Robinson, J., Kimbrel, N. A., Meyer, E. C., Kruse, M. I. et al. (2012). Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychology Review, 22(1), 21–34. 10.1007/s11065-012-9190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, S. J., Marx, B. P., McGlinchey, R., DeGutis, J., & Esterman, M. (2018). Reward ameliorates posttraumatic stress disorder-related impairment in sustained attention. Chronic Stress, 2, 247054701881240. 10.1177/2470547018812400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, S. J., & Sadeh, N. (2018). Psychological flexibility mitigates effects of PTSD symptoms and negative urgency on aggressive behavior in trauma-exposed veterans. Personality Disorders: Theory, Research, and Treatment, 9(4), 315–323. 10.1037/per0000251. [DOI] [PubMed] [Google Scholar]
- Enticott, P. G., Ogloff, J. R., & Bradshaw, J. L. (2006). Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Personality and Individual Differences, 41(2), 285–294. 10.1016/j.paid.2006.01.011. [DOI] [Google Scholar]
- Esterman, M., DeGutis, J., Mercado, R., Rosenblatt, A., Vasterling, J. J., Milberg, W. et al. (2013a). Stress-related psychological symptoms are associated with increased attentional capture by visually salient distractors. Journal of the International Neuropsychological Society, 19(7), 835–840. 10.1017/S135561771300057X. [DOI] [PubMed] [Google Scholar]
- Esterman, M., Fortenbaugh, F. C., Pierce, M. E., Fonda, J. R., DeGutis, J., Milberg, W. et al. (2019). Trauma-related psychiatric and behavioral conditions are uniquely associated with sustained attention dysfunction. Neuropsychology, 33(5), 711–724. 10.1037/neu0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman, M., Noonan, S. K., Rosenberg, M., & DeGutis, J. (2013b). In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cerebral Cortex, 23(11), 2712–2723. 10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- Evans, T. C., DeGutis, J., Rothlein, D., Jagger-Rickels, A., Yamashita, A., Fortier, C. B., ... & Esterman, M. (2021). Punishment and reward normalize error-related cognitive control in ptsd by modulating salience network activation and connectivity. Cortex, 145, 295–314. [DOI] [PubMed] [Google Scholar]
- Federici, S., Meloni, F., & Presti, A. L. (2009). International literature review on WHODAS II. Life Span and Disability, 12(1), 83–110. [Google Scholar]
- Fortenbaugh, F. C., Corbo, V., Poole, V., McGlinchey, R., Milberg, W., Salat, D. et al. (2017). Interpersonal early-life trauma alters amygdala connectivity and sustained attention performance. Brain and Behavior, 7(5), e00684. 10.1002/brb3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh, F. C., DeGutis, J., Germine, L., Wilmer, J. B., Grosso, M., Russo, K. et al. (2015). Sustained attention across the life span in a sample of 10,000: Dissociating ability and strategy. Psychological Science, 26(9), 1497–1510. 10.1177/0956797615594896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbaugh, F. C., Fonda, J. R., Fortier, C. B., Amick, M. M., Milberg, W. P., & McGlinchey, R. E. (2020). The impact of common psychiatric and Behavioral comorbidities on functional disability across time and individuals in Post-9/11 veterans. Journal of Traumatic Stress, 33(5), 750–761. 10.1002/jts.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier, C. B., Amick, M. M., Grande, L., McGlynn, S., Kenna, A., Morra, L. et al. (2014). The Boston assessment of traumatic brain injury–lifetime (BAT-L) semistructured interview: Evidence of research utility and validity. The Journal of Head Trauma Rehabilitation, 29(1), 89–98. 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey, C. H., & Patil, S. R. (2002). Identification and review of sensitivity analysis methods. Risk Analysis, 22(3), 553–578. 10.1111/0272-4332.00039. [DOI] [PubMed] [Google Scholar]
- Friedman, N. P., & Miyake, A. (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General, 133(1), 101–135. 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Green, P. (2004). Medical symptom validity test (MSVT) for microsoft windows: User’s manual. Paul Green Pubwishing, Edmonton. [Google Scholar]
- Gromatsky, M., Sullivan, S. R., Spears, A. P., Mitchell, E., Walsh, S., Kimbrel, N. A. et al. (2020). Ecological momentary assessment (EMA) of mental health outcomes in veterans and servicemembers: A scoping review. Psychiatry Research, 292, 113359. 10.1016/j.psychres.2020.113359. [DOI] [PubMed] [Google Scholar]
- Haaland, K. Y., Sadek, J. R., Keller, J. E., & Castillo, D. T. (2016). Neurocognitive correlates of successful treatment of PTSD in female veterans. Journal of the International Neuropsychological Society, 22(6), 643–651. 10.1017/S1355617716000424. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. University of Kansas, Lawrence, KS. [Google Scholar]
- Howlett, J. R., Campbell-Sills, L., Jain, S., Heeringa, S. G., Nock, M. K., Sun, X. et al. (2018). Attention deficit hyperactivity disorder and risk of posttraumatic stress and related disorders: A prospective longitudinal evaluation in US army soldiers. Journal of Traumatic Stress, 31(6), 909–918. 10.1002/jts.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger-Rickels, A., Rothlein, D., Stumps, A., Evans, T. C., Bernstein, J., Milberg, W. et al. (2022). An executive function subtype of PTSD with unique neural markers and clinical trajectories. Translational Psychiatry, 12(1), 1–10. 10.1038/s41398-022-02011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, A. L., Paul, R. H., Ozonoff, A., & Cohen, R. A. (2006). Evaluating elements of executive functioning as predictors of instrumental activities of daily living (IADLs). Archives of Clinical Neuropsychology, 21(4), 311–320. 10.1016/j.acn.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen, E. M., Brijs, K., Komlos, M., Brijs, T., & Wets, G. (2011). Inhibitory control and reward predict risky driving in young novice drivers–a simulator study. Procedia-Social and Behavioral Sciences, 20, 604–612. 10.1016/j.sbspro.2011.08.067. [DOI] [Google Scholar]
- Joormann, J. (2010). Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science, 19(3), 161–166. 10.1177/0963721410370293. [DOI] [Google Scholar]
- Joormann, J., & Gotlib, I. H. (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion, 24(2), 281–298. 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun, J., & Lee, V. G. (2021). Perceptual and response factors in the gradual onset continuous performance tasks. Attention, Perception, & Psychophysics, 83(7), 3008–3023. 10.3758/s13414-021-02353-7. [DOI] [PubMed] [Google Scholar]
- Jurick, S. M., McCabe, C. T., Watrous, J. R., Walker, L. E., Stewart, I. J., & Galarneau, M. R. (2022). Prevalence and correlates of self-reported cognitive difficulties in deployment-injured US military personnel. Journal of Traumatic Stress, 35(5), 1343–1356. 10.1002/jts.22833. [DOI] [PubMed] [Google Scholar]
- Katajapuu, N., Heinonen, A., & Saltychev, M. (2020). Minimal clinically important difference and minimal detectable change of the World Health Organization disability assessment schedule 2.0 (WHODAS 2.0) amongst patients with chronic musculoskeletal pain. Clinical Rehabilitation, 34(12), 1506–1511. 10.1177/0269215520942573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, R. T., Brickell, T. A., Lippa, S. M., & French, L. M. (2015). Clinical utility of the neurobehavioral symptom inventory validity scales to screen for symptom exaggeration following traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 37(8), 853–862. 10.1080/13803395.2015.1064864. [DOI] [PubMed] [Google Scholar]
- Lazarov, A., Suarez-Jimenez, B., Abend, R., Naim, R., Shvil, E., Helpman, L. et al. (2019). Bias-contingent attention bias modification and attention control training in treatment of PTSD: A randomized control trial. Psychological Medicine, 49(14), 2432–2440. 10.1017/S0033291718003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.-C., & Chao, H.-F. (2012). The role of active inhibitory control in psychological well-being and mindfulness. Personality and Individual Differences, 53(5), 618–621. 10.1016/j.paid.2012.05.001. [DOI] [Google Scholar]
- Lee, Y., & Shinkai, S. (2005). Correlates of cognitive impairment and depressive symptoms among older adults in Korea and Japan. International journal of geriatric psychiatry, 20(6), 576–586. [DOI] [PubMed] [Google Scholar]
- Lippa, S. M., Fonda, J. R., Fortier, C. B., Amick, M. A., Kenna, A., Milberg, W. P. et al. (2015). Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. Journal of Traumatic Stress, 28(1), 25–33. 10.1002/jts.21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond, P. F., & Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behaviour Research and Therapy, 33(3), 335–343. 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- Maraver, M. J., Bajo, M. T., & Gomez-Ariza, C. J. (2016). Training on working memory and inhibitory control in young adults. Frontiers in Human Neuroscience, 10, 588. 10.3389/fnhum.2016.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister, T. W., Zafonte, R., Jain, S., Flashman, L. A., George, M. S., Grant, G. A. et al. (2016). Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology, 41(5), 1191–1198. 10.1038/npp.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey, R. E., Milberg, W. P., Fonda, J. R., & Fortier, C. B. (2017). A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: The TRACTS longitudinal prospective cohort study. International Journal of Methods in Psychiatric Research, 26(3), e1556. 10.1002/mpr.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, R. S., Cha, D. S., Soczynska, J. K., Woldeyohannes, H. O., Gallaugher, L. A., Kudlow, P. et al. (2013). Cognitive deficits and functional outcomes in major depressive disorder: Determinants, substrates, and treatment interventions. Depression and Anxiety, 30(6), 515–527. 10.1002/da.22063. [DOI] [PubMed] [Google Scholar]
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Oni, O., Singh, V., Sharma, R., Sharma, M., Burns, D. M., Sharma, R. et al. (2020). Demographic profile and service-connection trends of posttraumatic stress disorder and traumatic brain injury in US veterans pre-and post-9/11. Federal Practitioner, 37(3), 128. [PMC free article] [PubMed] [Google Scholar]
- Park, H., Aul, C., DeGutis, J., Lo, O. Y., Poole, V. N., McGlinchey, R. et al. (2021). Evidence for a specific association between sustained attention and gait speed in middle-to-older-aged adults. Frontiers in Aging Neuroscience, 13(326), 1–8. 10.3389/fnagi.2021.703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, J. P., Churchill, G. A., Jr., & Brown, T. J. (1993). Caution in the use of difference scores in consumer research. Journal of Consumer Research, 19(4), 655–662. 10.1086/209329. [DOI] [Google Scholar]
- Qureshi, S. U., Long, M. E., Bradshaw, M. R., Pyne, J. M., Magruder, K. M., Kimbrell, T. et al. (2011). Does PTSD impair cognition beyond the effect of trauma? The Journal of Neuropsychiatry and Clinical Neurosciences, 23(1), 16–28. 10.1176/appi.neuropsych.23.1.16. [DOI] [PubMed] [Google Scholar]
- Riley, E., Mitko, A., Stumps, A., Robinson, M., Milberg, W., McGlinchey, R. et al. (2019). Clinically significant cognitive dysfunction in OEF/OIF/OND veterans: Prevalence and clinical associations. Neuropsychology, 33(4), 534–546. 10.1037/neu0000529. [DOI] [PubMed] [Google Scholar]
- Samuelson, K. W., Newman, J., Abu Amara, D., Qian, M., Li, M., Schultebraucks, K. et al. (2020). Predeployment neurocognitive functioning predicts postdeployment posttraumatic stress in Army personnel. Neuropsychology, 34(3), 276–287. 10.1037/neu0000603. [DOI] [PubMed] [Google Scholar]
- Sani, S. R. H., Tabibi, Z., Fadardi, J. S., & Stavrinos, D. (2017). Aggression, emotional self-regulation, attentional bias, and cognitive inhibition predict risky driving behavior. Accident Analysis & Prevention, 109, 78–88. 10.1016/j.aap.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Shea-Shumsky, N. B., Schoeneberger, S., & Grigsby, J. (2019). Executive functioning as a predictor of stroke rehabilitation outcomes. The Clinical Neuropsychologist, 33(5), 854–872. 10.1080/13854046.2018.1546905. [DOI] [PubMed] [Google Scholar]
- Stetz, M. C., Thomas, M. L., Russo, M. B., Stetz, T. A., Wildzunas, R. M., McDonald, J. J. et al. (2007). Stress, mental health, and cognition: A brief review of relationships and countermeasures. Aviation, Space, and Environmental Medicine, 78(5), B252–B260. [PubMed] [Google Scholar]
- Swick, D., Honzel, N., Larsen, J., Ashley, V., & Justus, T. (2012). Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. Journal of the International Neuropsychological Society, 18(5), 917–926. 10.1017/S1355617712000458. [DOI] [PubMed] [Google Scholar]
- Tang, D., & Schmeichel, B. J. (2014). Stopping anger and anxiety: Evidence that inhibitory ability predicts negative emotional responding. Cognition & Emotion, 28(1), 132–142. 10.1080/02699931.2013.799459. [DOI] [PubMed] [Google Scholar]
- Tiego, J., Testa, R., Bellgrove, M. A., Pantelis, C., & Whittle, S. (2018). A hierarchical model of inhibitory control. Frontiers in Psychology, 9, 1339. 10.3389/fpsyg.2018.01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorelli, D. A., & Michaleris, P. (1994). Design sensitivity analysis: Overview and review. Inverse problems in Engineering, 1(1), 71–105. 10.1080/174159794088027573. [DOI] [Google Scholar]
- Vanderploeg, R. D., Cooper, D. B., Belanger, H. G., Donnell, A. J., Kennedy, J. E., Hopewell, C. A. et al. (2014). Screening for postdeployment conditions: Development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. The Journal of Head Trauma Rehabilitation, 29(1), 1–10. 10.1097/HTR.0b013e318281966e. [DOI] [PubMed] [Google Scholar]
- Vasterling, J. J., & Arditte Hall, K. A. (2018). Neurocognitive and information processing biases in posttraumatic stress disorder. Current Psychiatry Reports, 20(11), 1–10. 10.1007/s11920-018-0964-1. [DOI] [PubMed] [Google Scholar]
- Waszak, D. L., & Holmes, A. M. (2017). The unique health needs of post-9/11 US veterans. Workplace Health & Safety, 65(9), 430–444. 10.1177/2165079916682524. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1997). Manual for the Wechsler adult intelligence scale-III. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wooten, T., Ferland, T., Poole, V., Milberg, W., McGlinchey, R., DeGutis, J. et al. (2019). Metabolic risk in older adults is associated with impaired sustained attention. Neuropsychology, 33(7), 947–955. 10.1037/neu0000554. [DOI] [PubMed] [Google Scholar]
- Wu, E. X., Liaw, G. J., Goh, R. Z., Chia, T. T., Chee, A. M., Obana, T. et al. (2020). Overlapping attentional networks yield divergent behavioral predictions across tasks: Neuromarkers for diffuse and focused attention? NeuroImage, 209, 116535. 10.1016/j.neuroimage.2020.116535. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Cao, S., Shields, G. S., Teng, Z., & Liu, Y. (2017). The relationships between rumination and core executive functions: A meta-analysis. Depression and Anxiety, 34(1), 37–50. 10.1002/da.22539. [DOI] [PubMed] [Google Scholar]
- Zetsche, U., D’Avanzato, C., & Joormann, J. (2012). Depression and rumination: Relation to components of inhibition. Cognition & Emotion, 26(4), 758–767. 10.1080/02699931.2011.613919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.