Abstract
Temporomandibular disorders (TMDs) affect a high percentage of children and adults worldwide. Surgery may be indicated in severe or recalcitrant cases. Several recent advancements in TMD and temporomandibular joint (TMJ) surgery have elevated understanding and the ability to treat affected patients. We discuss recent advances in TMD epidemiology, juvenile idiopathic arthritis (JIA) of the TMJ, and surgical techniques and technologies. Technical advancements have been identified in TMJ arthroscopy, the treatment of TMJ subluxation and dislocation, and extended prosthetic total TMJ reconstruction (eTMJR). Overall, this review provides valuable insights into significant recent advancements in TMJ disorders and their surgical management.
Keywords: temporomandibular joint, TMJ, surgery, arthroscopy, arthroplasty, dislocation, eTMJR
1. Introduction
Temporomandibular disorders of all varieties affect people in every culture on every continent, affecting up to 11% of children and even as high as 31% of adults [1]. While most patients experience relief or at least a significant improvement in symptoms with noninvasive treatments, surgical care may be warranted in refractory cases or frank arthritic disease, among other scenarios.
A surgeon’s management of temporomandibular disorders has progressed over the last several years. This is due, in large part, to advances in understanding pathophysiology, epidemiology, and both surgical techniques and technologies. We may often look to new technology in innovation for improved outcomes for patients. While this is the case for TMJ surgery, advances in the foundational understanding of the disease state have also brought significant benefits to patients and surgeons alike, leading to better outcomes through better patient selection for surgical intervention, among other modes of care. The purpose of this article is to review recent major advances that will assist the practicing TMJ surgeon in providing high-quality, current, and informed care.
A basic understanding of TMD-related epidemiology has helped surgeons appreciate and apply more holistic and collaborative care models as well as recognize patient cohorts who may be more likely to experience favorable outcomes through surgical intervention. Similarly, the last several years have seen a renewed understanding of TMJ involvement in patients with juvenile idiopathic arthritis (JIA). Through improved understanding, consensus diagnostics, imaging, and treatment protocols aim to improve patient outcomes. On the technical side, arthroscopic TMJ surgery has also seen a proliferation of advocates and techniques, particularly with regard to arthroscopic disc repositioning. Additionally, recent investigations have helped identify additional treatments for recurrent TMJ dislocation. Finally, while prosthetic TMJ reconstruction (TMJR) has been firmly established as a safe and effective technique for the treatment of many severe conditions [2], new frontiers of the application of this technology have been opened through the use of extended TMJR prostheses.
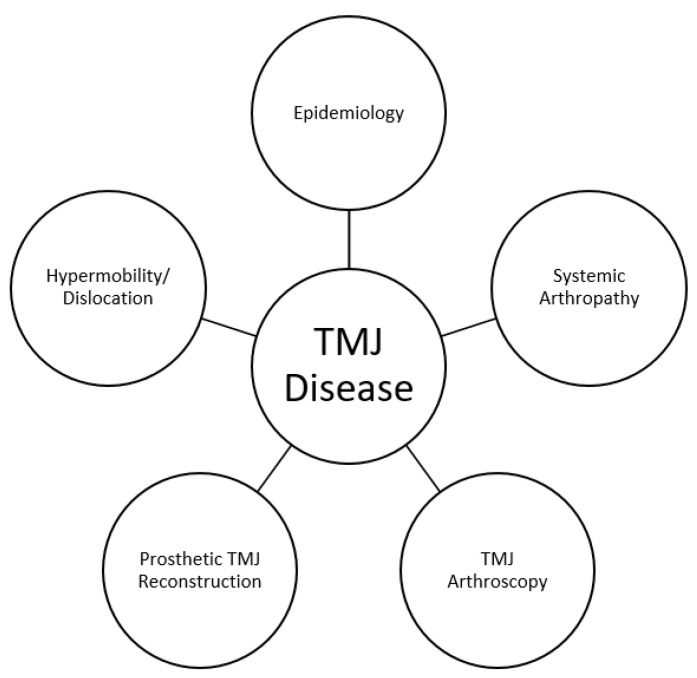
While not exhaustive, these areas of recent progress represent important advances for surgeons treating the TMJ, about which they should certainly be aware to provide current and optimal care for their patients (Figure 1).
Figure 1.
Areas of recent advances impacting surgical management of TMD.
2. TMD Epidemiology: The OPPERA Study
The Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study is a landmark longitudinal study of temporomandibular disorders (TMDs), with its first publication beginning in 2011 [3]. It aimed to improve the understanding of orofacial pain and TMD by identifying risk factors, clarifying pain mechanisms, assessing treatment outcomes, and examining psychosocial contributions to the disease, among other metrics. Unlike previous studies that primarily used cross-sectional designs and convenience samples, OPPERA overcame this limitation by employing a prospective cohort study with a multi-site approach. It has provided valuable insights into TMD prevalence, demographic trends, the role of jaw headaches and injury as risk factors, and the differentiation between primary headaches and TMD-related secondary headaches, among other findings. Furthermore, the study has facilitated the identification of phenotypical clusters, allowing for a more targeted and comprehensive approach to TMD management in clinical practice. While well known among those involved in headache and facial pain treatment, the authors believe these studies are vastly underrecognized and underutilized in the surgical community.
The OPPERA-I study, conducted between May 2006 and May 2013, included a total of 4346 subjects with an age range of 18–44 living in Baltimore, MD; Buffalo, NY; Chapel Hill, NC; and Gainsville, FL, who were studied between 2006 and 2013. There were three study designs utilized: a prospective cohort study, a case-control study, and a nested case-control study. Between December 2014 and May 2016, the OPPERA-2 study was conducted, connecting with subjects who had not previously withdrawn from OPPERA-1. For individuals who provided consent and participated in the research clinics during OPPERA-2, data was gathered through clinical examinations, quantitative sensory testing, blood samples, and self-reported questionnaires.
2.1. Demographics
One of OPPERA’s major strengths is its study design. For example, in 1993, Bush et al. identified that TMD has a higher prevalence in females than in males [4], with LeResche further characterizing TMD as affecting young and middle-aged adults in 1997 [5]. However, the initial findings of the OPPERA studies revealed that TMD is prevalent in the US, with 4% of the population developing TMD on an annual basis [6]. Slade et al. also published the initial findings of the OPPERA study in 2011 revealing the key demographic data [3]. TMD is highest in patients aged 35–44 (7%) and lowest in patients aged 18–24 (3%). Females are four times more likely to suffer from TMD than men, while African Americans and Hispanics had one-fifth odds of developing TMD compared to non-Hispanic Whites. However, Kim et al. performed a cross-sectional study utilizing the OPPERA database, showing that for both Asians and African Americans compared to non-Hispanic Whites, pain catastrophizing played a significant role in mediating the association between race and pain-related measures [7]. Further, compared to healthy individuals, patients with TMD have higher levels of pain-related disability, and it is well established that catastrophizing is a strong predictor of TMD, as are psychosocial stress, affective distress, and somatic symptoms overall [8]. It is crucial to consider these factors appropriately when treatment planning potential surgical candidates.
2.2. Role of Injury
The OPPERA study has played a pivotal role in our understanding of the development and exacerbation of TMD. Sharma et al. in 2019 sought to elucidate jaw injury as a risk factor for TMD [9]. The initial findings of the prospective cohort study showed that individuals who reported a jaw injury had a fourfold higher risk of developing TMD compared to those without an injury, either extrinsic or intrinsic. Intrinsic was defined as injury attributed to yawning or prolonged mouth opening, whereas extrinsic included tooth extraction or dental treatments; oral intubation; sports injury (including falls, bumps, and blows); motor vehicle accidents; accidents resulting in whiplash; and injuries to the shoulder, neck, and head region. Sharma et al. would expand upon their initial study to show that jaw injury significantly increased the odds of developing TMD by a factor of five after adjusting for confounding variables [10]. In both studies performed, intrinsic injuries were more prevalent than extrinsic. Despite most injuries being intrinsic, the risk of TMD increased to a similar extent irrespective of whether the injury was intrinsic or extrinsic.
2.3. Headache and Chronic Pain Conditions
The OPPERA studies shed light on the association between headaches and their role as a risk factor for TMD. Headaches that arise as a secondary symptom of TMD are distinct from primary headaches that can subsequently contribute to the development of TMD. Headaches attributed to TMD (HATMD) are well established and part of Schiffman’s diagnostic criteria for TMD [11] and was recently included in the finalized version of the International Classification of Headache Disorders—third edition (ICHD-3) [12]. In contrast, primary headaches, such as migraines or tension headaches, are known to have an independent etiology but are thought to be comorbid with TMD. Tchivileva et al. utilized the OPPERA data to show that baseline reports of migraine, as well as frequent headaches, served as risk factors for developing TMD [13]. The association between migraines and TMD is unsurprising when considering the shared anatomical pathway of the trigeminal nerve between migraines and TMD [14]. Tchivileva et al. later showed that not only is the baseline presentation of a migraine a risk factor but that HATMD typically presents as migraine dominating. Sonia et al. sought to define characteristics that distinguish a headache that is comorbid with temporomandibular disorder from a HATMD. Utilizing the data from the OPPERA II study, they showed that the more severe the masticatory system pain with a patient, the more likely the headache was secondary to HATMD versus a primary headache [15].
Headache was not the only pain condition studied within the OPPERA data set. Other chronic overlapping pain conditions (COPCs) often coexist with TMD and exhibit shared biopsychosocial characteristics, symptoms, and risk factors [16]. Research has shown that the overlapping of disease symptoms of COPC can be attributed to central sensitization, a mechanism involving increased synaptic efficiency that affects sensory and nociceptive stimuli [17]. Slade et al. utilized the OPPERA II data set to compare the degree of overlap between TMD and other COPCs, revealing a greater overlap between musculoskeletal conditions such as fibromyalgia and chronic back pain compared to headaches and IBS [18].
2.4. Phenotypical Clusters
While the OPPERA studies have shed light on the heterogenous etiology of TMD with an anatomical focus, they have also statistically validated several risk factors seen among all chronic pain patients allowing for the development of “phenotypical clusters.” Bair et al. were the first to describe these clusters by identifying statistically distinguishable risk factor profiles that patients shared [16]. The three phenotypical clusters described were the adaptive cluster, pain-sensitive cluster (PS), and global symptoms (GS) cluster based on four validated variables: the pressure pain threshold of the trapezius muscle and its association with the subscales of anxiety, depression, and somatization, as measured by the SCL-90R questionnaire [19].
In this study, 33% belonged to the adaptive cluster, which showed minimal hypersensitivity and psychological distress. Individuals in this particular group experience milder pain, minimal psychological distress, and exhibit the lowest sensitivity to muscle pain. The pain they experience is primarily confined to the temporomandibular joint and the surrounding muscles, and they report few comorbidities or COPCs. Additionally, this group has a higher representation of men compared to women. The pain-sensitive cluster comprised 48% of participants. Individuals in this group exhibit the highest sensitivity to muscle pain, slightly elevated psychological distress, and a greater number of associated chronic pain conditions compared to the adaptive group. This group also has a slightly higher number of women compared to men. The remaining cluster, known as global symptoms, accounted for 18% of the participants in Bair’s study. Individuals in this group who have TMD experience the most intense pain and dysfunction. They exhibit a higher number of tender muscle sites and a significantly greater number of associated COPCs. Moreover, they encounter the highest levels of psychological distress among the three groups. Individuals in this group tend to have a higher incidence of jaw injuries and have experienced more traumatic events in their lives. A larger percentage of patients in this group have a history of smoking [19]. Lastly, there is a notable imbalance in gender representation, with a considerably higher proportion of women than men falling into this group.
OPPERA has improved our understanding of the epidemiology of TMD as well as the risk factors associated with the condition. By clarifying the risk factors, such as psychosocial stress, affective distress, somatic symptoms, trauma, and headaches, surgeons can better identify individuals at higher risk and develop targeted prevention strategies. The utilization of the phenotypical clustering method holds great promise for influencing clinical practice in a meaningful way as well. OPPERA has shown that, for individuals in either the PS or GS clusters, it is crucial to initiate a thorough screening for concurrent pain conditions. While there may be clear anatomic abnormalities in a potential TMJ surgical candidate, understanding and applying the findings of the OPPERA studies will help surgeons to make more clear-eyed clinical decisions, seek multidisciplinary treatment, and more thoroughly educate patients to help them understand the implications of their condition. The authors recommend a full review of the OPPERA studies for a more in-depth understanding of the subject and its application.
3. Juvenile Idiopathic Arthritis of the Temporomandibular Joint
Understanding and treatment of juvenile idiopathic arthritis (JIA) and its effects, specifically on the temporomandibular joint, have advanced significantly over the last decade. Once noted to be “the forgotten joint”, [20] due to its omission from diagnosis and management protocols for JIA, the last 10–15 years have hosted a dramatic increase in our understanding about TMJ involvement in sufferers of JIA [21,22,23]. This includes improvements in detection and diagnosis, generalized increased awareness, recognition of morbidity of untreated cases, and established medical and surgical treatments in this patient population.
JIA affects 1 in 1000 children [24] and has seven subtypes, according to the International League of Associations for Rheumatology [25]. It appears that TMJ involvement in these cases is very common (up to 87% [26], but more likely close to 40% [27,28]), albeit frequently undetected due to a lack of symptoms or screening.
3.1. Imaging and Diagnosis
Physical examination is still important, as is a thorough history, in order to identify potential TMJ involvement [29]. However, it is clear that the exam alone is insufficient for screening in the JIA population. A straightforward and efficient six-point examination protocol has been developed by the TMJaw Working Group [30] but should be supplemented with imaging. It is recommended that patients undergo regular TMJ-focused exams as part of initial rheumatologic/JIA evaluations as well as at the time of annual follow-up in order to determine the presence or progression of disease [31].
Imaging these patients becomes critical in light of the frequent lack of symptoms in the presence of early or even destructive late disease. Fortunately, the development of recent guidelines and protocols [32,33] and resources [34,35] reduces the number of patients missed or undertreated. For proper screening to evaluate synovitis or early joint disease, Gadolinium contrast-enhanced MRI is ideal [36,37]. In addition, the wider availability of 3.0-T magnets in recent years has also enhanced diagnostic ability.
It is important for the treating surgeon to communicate clearly with the radiology team to ensure proper imaging is acquired, especially in the case of children who may require sedation, or in whom an abbreviated exam is desirable. A “minimal required protocol” includes several sequences (sagittal oblique fat-suppressed T2 or STIR, sagittal oblique T1 TSE, coronal T1 TSE, and contrast-enhanced T1 FS-weighted), while a longer “ideal protocol” adds sagittal oblique fat-suppressed T1, proton density, and gradient echo sequences [32]. The use of a scoring system for joint health based on these protocols helps stage disease or monitor progression/quiescence [34,38,39]. Further improvements in imaging techniques, such as dynamic contrast enhancement MRI [40] and black bone MRI [41], potentiate earlier detection and discrimination of disease to allow for more complete and timely treatment of this patient population.
Other means of diagnosis remain unsettled or controversial in the scientific literature at this point. This includes the use of panorex, sonography, and synovial fluid analysis, among other methods [42]. In addition, MRI is helpful to detect inflammation and osseous changes but is not necessarily specific for JIA as the etiology of these findings [43]. CT or cone beam CT may assist in detecting osseous changes or evaluating craniofacial changes in affected individuals, but will be unhelpful in monitoring soft tissue inflammation [44].
3.2. Disease Course and Treatment
With greater publicity of TMJ involvement in JIA patients, the late sequelae of un- or undertreated disease have become more visible [22]. Likewise, treatment aimed at reducing the incidence and burden of late-stage disease has advanced.
Since the TMJ is a growth area for the mandible and influences craniofacial growth and development beyond just the condyle itself, these disruptions can cause significant hypoplasia of the mandible, malpositioning of the maxilla, and retrognathia-associated airway compromise or sleep-disordered breathing [23,45,46]. The degree and type of dentofacial deformity are related to the timing and severity of the disease during growth and development [47].
Overall, the optimization of medical treatments for JIA seems to benefit affected temporomandibular joints. Advances in systemic treatments are encouraging; however, in many instances, the TMJ seems to respond less well to medical treatments than other joints [20,28]. However, a recent study by Bollhalder et al. showed hopeful TMJ-related outcomes with methotrexate (and, in many cases, combined with biologics) over several years of treatment [48]. Encouraging outcomes included both symptom control and mandibular growth. Fortunately, more studies are forthcoming to better understand the effects of systemic therapy on the TMJ, in particular, including clinical trials [49]. It is unclear if this has more to do with the overall incidence of temporomandibular disorders (TMDs) casting a confounding shadow over concurrent JIA or if there is something different about the TMJ that causes the TMJ to be less responsive to systemic treatment.
Intra-articular injection of steroids has been advocated for a long time (Stoll 2015), but there is some controversy regarding repeated use and the potential risk of heterotopic bone formation or problematic growth [50,51]. It may be helpful for symptom control (not disease progression), but repeated injections of steroids after an unsuccessful injection should not be encouraged [52]. Interestingly, arthrocentesis without steroid injection was shown to be as effective for symptom control as arthrocentesis with steroid injection [53]. Alternative injections, such as inflixamab, have been explored for systemic arthropathy [54] and the TMJ in particular with mixed results [55,56].
For late-stage disease, with or without extra-articular dentofacial deformity, major operative intervention can be quite successful [57]. For the treatment of the joint itself, orthognathic surgery, costochondral grafting, distraction osteogenesis [58], and prosthetic joint reconstruction have been advocated. Costochondral grafting (CCG)—a successful treatment [59]—seems to be less favored recently, as alloplastic TMJ reconstruction is often viewed by surgeons as more predictable biologically and functionally [60]. Early reports that have shown encouraging success in prosthetic joint reconstruction are encouraging in terms of an improved range of motion, occlusal and masticatory function, and even improvement in obstructive sleep apnea (OSA) [61]. This is a particularly valuable treatment when inflammation and synovitis persist despite medical interventions, or when the counterclockwise rotation of the jaws is planned and autogenous grafts may not be as robust mechanically. In cases involving dentofacial deformity, an interdisciplinary approach (involving orthodontists, oral and maxillofacial surgeons, and pediatric rheumatologists) may be especially helpful [62].
Orthognathic surgery, with or without arthroplasty, has a place in the treatment of this patient population. Reporting on combined orthognathic and prosthetic joint reconstruction, Trivedi et al. evaluated 40 JIA-affected patients in a case-control model and showed dramatic improvements in function and a reduction in pain [63]. Regarding mandibular orthognathic surgery, Raffaini et al. reviewed 13 cases retrospectively, noting stable findings and quiescent disease 1 year post-operatively [64]. All of these patients had some condylar changes and reduced ramus height (12 bilateral), but disease was quiescent in the TMJ at the time of surgery. In addition, all underwent medical treatment in the form of etanercept for at least one year prior to surgery. Other reports of orthognathic surgery in this population seem to indicate stable outcomes in the quiescent joint [65,66,67]. Similarly, distraction osteogenesis may be considered in the same population [62].
Another concern for JIA-affected individuals, particularly with TMJ involvement, is the risk of developing obstructive sleep apnea (OSA). Recent studies indicate the significant risk of OSA in this population as compared to non-JIA individuals [68,69]. The condylar resorption and rotation of the mandible that causes retrognathia seem to be a significant factor in developing these symptoms in the young adult years (age 18–30) [68]. This is simply another reason for early detection and intervention in this patient population in order to minimize the risk of dentofacial deformity and all its problematic sequelae.
Finally, treatments commonly used for temporomandibular disorders, such as physical therapy or occlusal appliances, may be implemented for symptom relief [70,71]. If a patient responds to an occlusal appliance alone, for example, it is less likely that the underlying source of symptoms is inflammatory arthritis.
4. Technical Advances in TMJ Surgery
4.1. Advanced TMJ Arthroscopy
TMJ arthroscopy is a treatment modality with a rich history (Figure 2), and there are several areas within arthroscopy that have seen advancement recently. One of the most notable developments in advanced TMJ arthroscopy in the last 10–15 years has been the development of discopexy for the repositioning and fixation of an anteriorly displaced TMJ disc [72,73]. First reported in English by Israel in 1989 [74], important variations soon followed [75,76,77,78,79]. The technique documented in 1992 by McCain et al. [80], involving 11 temporomandibular joints (8 patients), was subsequently modified by Yang et al. in 2012 [81]. The technique described by McCain et al. involves the release of the anterior portion of the disc from its attachment to the synovium. Once the disc is reduced, it is sutured posterolaterally. With this technique, the suture is passed through the posterior margin of the disc using a spinal needle, while the Meniscus Mender II uses a lasso-type suture retriever. McCain’s technique involves a small incision within the preauricular crease adjacent to the suture exit point to facilitate tying the suture within the extracapsular fatty tissue.
Figure 2.
Some highlights of evolution of TMJ arthroscopic discopexy [74,79,80,81,82,83,84,85].
Yang and colleagues’ main modifications to this suture discopexy technique is in the suturing technique and instruments used [81]. Most notably, a horizontal mattress pattern is used with two sutures and the sutures are tied such that the knots are beneath the cartilage of the external auditory canal (EAC). This method of suturing not only prevents dimpling of the skin, but also minimizes the risk of entrapment of the frontal branch of the facial nerve and allows for a vector of traction on the disc that is directed along the anterior–posterior long axis of the disc, as opposed to the posterolateral traction of prior techniques. Yang’s technique also involves an exchangeable, custom-designed lasso-type and hook-type gripper.
A follow-up study by Yang et al. that used MRI to evaluate the efficacy of their arthroscopic suture discopexy technique to reposition anteriorly displaced discs in 764 joints found a success rate of 98.56%. However, the post-operative MRI was obtained only between 1 and 7 days post-operatively [86]. More recently, Jerez et al. describe a modification to Yang’s suture discopexy technique that utilizes more commonly available suture equipment consisting of two patented lasso grippers, and two Meniscus Mender II curved and straight spinal needles. However, this technique requires five to six puncture sites compared to three puncture sites with Yang’s technique [84].
Alternative techniques for discopexy have also arisen. Martinez-Gimeno has described a single portal discopexy technique, fixing the disc to the tragal cartilage without an anterior release [87]. A 1-year follow-up seemed favorable for a limited number of subjects, most with anterior disc displacement with reduction. Alternatively, arthroscopic discopexy using resorbable pins in lieu of sutures has also recently received more long-term appraisal, showing efficacy [88]. Using the technique initially published in 2016 [89], and similar to Goizueta-Adame in 2014 [90], resorbable pin use appears to offer at least five years of benefit, in terms of range of motion and pain reduction. Although the sample consisted of 33 subjects and only 23 made it to 5-year follow-up, the findings are encouraging, and larger case numbers will further solidify this technique as viable for a Wilkes III patient. Lastly, a separate study reported arthroscopic use of a titanium anchor for disc repositioning and 6 months of follow-up with patient benefits in a Wilkes II-III population [91].
Multiple studies have assessed the effectiveness of disc repositioning and suturing in arthroscopic vs. open techniques. A study by Abdelrehem et al. evaluated the outcomes of TMJ arthroscopic versus open disc repositioning for the management of anterior disc displacement in 277 joints (177 patients) [92]. This study found that while there was an improvement in pain score, clicking, diet, and MIO, in both the arthroscopic and open groups, the clinical improvements occurred earlier in the arthroscopic group (1 month) versus the open group (6 months.) Additionally, the success rate in the arthroscopic group was slightly higher than the open group at 98.1% versus 97.3%. Condylar remodeling occurred in 70.2% of patients in the arthroscopy group versus 30.1% of patients in the open group. Recent systematic reviews by Askar et al. and Santos et al. have found that arthroscopic and open disc repositioning reduced pain and improved MIO. However, both studies indicated that the number of studies and evidence was limited, with Askar et al. noting that the heterogeneous nature of the study designs and data reporting was such that the studies could not be directly compared, and quantitative analysis could not be performed [93,94].
The authors also noted the lack of large subject numbers as well as medium and, especially, long-term follow-up for either open or arthroscopic disc repositioning studies. Because of these deficiencies, it is difficult to advocate for one technique over another, and more data is needed. While remaining optimistic that this treatment benefits many patients, the current volume and quality of data are consistent with the controversial view this surgery may at times garner. One possible virtue of the arthroscopic technique is the avoidance of open surgery, the accumulation of which often renders a later total joint arthroplasty less successful. However, we also have no helpful data on outcomes upon conversion of arthroscopic disc repositioning to total joint arthroplasty. While less invasive than an open technique, it is unclear if this advanced arthroscopic technique “counts against” the tally of prior TMJ surgeries that would decrease the success of a later prosthetic reconstruction.
The Wilkes classification of TMJ internal derangement has been shown to predict the likelihood of a successful arthroscopic discopexy. McCain et al. found that Wilkes stages II and III had a successful primary outcome (the absence of joint pain at 12 months post-operatively) of 86.7% compared to 25% for Wilkes stages IV and V [85]. This contrasts an older study by Murakami et al. that reported a 92% and 93% success rate for Wilkes stages IV and V, respectively [95]. However, this study utilized arthroscopic lysis and lavage for Wilkes stage IV and advanced arthroscopic procedures (synovectomy, discoplasty, and debridement) for Wilkes stage V, as opposed to discopexy.
Some of the findings regarding Wilkes staging and success in arthroscopic discopexy were further assessed in a very recent study by Sah et al. [96]. This retrospective study assessed whether certain prognostic factors impacted the success of Yang’s arthroscopic suture discopexy technique in the treatment of TMJ closed lock. It was found that age, duration of illness, Wilkes classification, and prior orthodontic treatment all impacted surgical outcomes. Specifically, younger age, Wilkes stage III, shorter duration of illness, and current orthodontic treatment were all associated with positive surgical outcomes. On the contrary, older age, Wilkes stage IV, longer duration of illness, and previous orthodontic treatment were associated with poor surgical outcomes.
When considering the efficacy of TMJ disc repositioning in cases of anterior disc displacement, it is also important to consider the ability of disc repositioning to prevent complications that could arise secondary to a lack of treatment of disc displacement. In the adolescent population, untreated unilateral TMJ anterior disc displacement may result in mandibular asymmetry, while bilateral anterior disc displacement may result in mandibular retrusion. Prior to recently, there were very few studies that evaluated condylar bone remodeling following arthroscopic TMJ surgery. Condylar bone remodeling following the treatment of disc displacement would be beneficial in preventing complications regarding mandibular symmetry and, thus, occlusion in patients with disc displacement. Dong and colleagues recently performed a study to evaluate condylar remodeling following Yang’s arthroscopic surgery in patients with anterior disc displacement both with and without reduction [83]. While the specifics of the arthroscopic surgery patients underwent were not discussed in detail, they found that 70.3% of the 229 patients had new condylar bone formation when evaluated with MRI at 1 year following their arthroscopic surgery. The youngest age group (10–15 years old) had the greatest percentage of patients with new condylar bone formation (94.33%), while the oldest age group (above 30 years old) had the lowest percentage (25%). The percentage of new condylar bone formation was found to decrease as patient age increased [83]. More than half of the patients (53.53%) had bone formation on the posterior slope of the condyle. Meanwhile, the area of the condyle with the smallest amount of bone formation was the anterior slope of the condyle, with only 10.37% of patients having new bone formation in this area. This study is important in showing that the condyle still has the propensity to form new bone, especially in younger patients, after repositioning of the TMJ disc. This may ultimately represent a protective factor in preventing mandibular asymmetry and retrusion in cases of anterior disc displacement.
Outside of arthroscopic discopexy, arthroscopic management of a painful or problematic alloplastic TMJ prosthesis has been recently reported [97]. This is an important advancement in technique, as TMJ prostheses are gaining traction and becoming more widely used. The method described involves altered access points and the opportunity to examine the prosthesis and potentially remove areas of synovial impingement or fibrosis. It represents an opportunity for less-invasive diagnosis and treatment of symptomatic prostheses, but is also in its infancy as a technique, with a higher risk for damaging the prosthesis or neighboring structures due to different access and the instruments required.
4.2. Treatment of TMJ Subluxation and Dislocation
TMJ subluxation, or forward displacement of the mandibular condyle past the articular eminence which reduces spontaneously or can be self-reduced, can have multiple different etiologies [98]. It is most commonly an acute event that may be spontaneous or secondary to trauma, a congenital condition, prior dental or otorhinolaryngological procedure, or an underlying psychiatric condition. Rarely, it may become a recurrent or habitual event. It is important to distinguish TMJ subluxation from dislocation, where the condyle is displaced out of the glenoid fossa and usually must be reduced by someone else [98]. Over the years, less invasive procedures have been added to the arsenal of oral and maxillofacial surgeons for recurrent subluxation and dislocation [99].
Botulinum toxin type A, which traditionally has been used to treat focal dystonia or other conditions involving involuntary muscle activity, has recently been shown to be an option for the treatment of recurrent TMJ dislocation. A study by Fu et al. explored the long-term efficacy of botulinum toxin type A for recurrent TMJ dislocation [100]. They found that injections of 25–50 units of BTX-A into the lateral pterygoid muscle were successful in preventing any additional TMJ dislocations during a follow-up spanning 3 months to 2 years, without needing additional injections. However, the sample size of this study was small (n = 5), and CTs were obtained to determine the position of the lateral pterygoid muscles. This can be performed with EMG guidance [101] or alternatively could be combined with another procedure with direct or arthroscopic visualization.
Other conservative treatments for habitual TMJ subluxation that have been a focus of research are autologous blood injection (ABI) and dextrose prolotherapy [102]. Studies on ABI indicate that it is effective, although it will occasionally require multiple injections [103,104,105], and has good long-term success [106]. It appears to be more effective when injected in the pericapsular tissues and not just the superior joint space alone [107,108], and the technique may be combined successfully with arthrocentesis or arthroscopy [109,110]. The European Society of TMJ Surgeons (ESTMJS) has recently published a consensus on the treatment of condylar dislocation, finding the best level of evidence for the use of autologous blood as a minimally invasive technique [98]. Following autologous blood prolotherapy, there may be a benefit in limiting the MIO of the jaws. However, intermaxillary fixation alone is currently not recommended [98]. It does appear that a combination of a course of IMF and ABI is more effective at reducing recurrence than ABI alone [105].
Dextrose prolotherapy may have similar effectiveness in the long-term management of symptoms associated with TMJ subluxation. A study by Refai, in which 10% dextrose prolotherapy was administered to 61 patients with symptomatic TMJ subluxation, found a significant reduction and pain, clicking, and frequency of locking in those with symptomatic TMJ subluxation [111]. It is important to note that only three patients in this study had recurrent TMJ dislocation, but they all reported improvement after one treatment. A few systematic reviews evaluating dextrose prolotherapy versus placebo have been performed in recent years. These reviews have found that dextrose prolotherapy significantly reduced pain. However, there were differing findings regarding a significant reduction in MMO and functional scores [112,113]. Additionally, a systematic review by Nagori et al. found that there was no significant difference in the frequency of TMJ subluxation or dislocation [112]. It is important to note that the literature regarding the efficacy of dextrose prolotherapy is not very robust currently, with each systematic review comprising only 3 and 10 randomized controlled trials [112,113]. Thus, it appears that further studies using dextrose prolotherapy would be beneficial in determining its efficacy. A more recent study by Pandey et al. compared autologous blood and 25% dextrose prolotherapy for the treatment of recurrent TMJ dislocation [114]. This retrospective study found that autologous blood prolotherapy was more effective in reducing MMO and improving lateral and protrusive mandibular movements, while dextrose prolotherapy was more effective in reducing pain intensity.
4.3. Extended Total Temporomandibular Joint Reconstruction Prostheses (eTMJR)
The first total temporomandibular joint reconstruction procedure was first described in the 1970s. By the early 2000s, multiple companies had developed a total temporomandibular joint prosthesis that consisted of a titanium mandibular condyle and a polyethylene mandibular fossa implant [115]. Although there have been advances in the workflow of conventional total temporomandibular joint reconstruction (TJR) prostheses over the last 20 years, the overall design of the prosthesis replacing the glenoid fossa and the mandibular condyle has largely remained the same. Generally, the mandibular component of the TJR prosthesis does not extend beyond the area of the angle of the mandible. However, in patients with extensive pathologies or deformities involving the TMJ, the conventional prosthesis design cannot be used. For these purposes, extended TJR (eTMJR) prostheses have been designed and used in situations in which defects in the mandible or base of the skull must be reconstructed in addition to the TMJ complex [116,117].
A classification system for the eTMJR prosthesis has recently been described to aid in communication and clinical decision making. There is a separate classification for both the fossa component and the condyle/mandible component. The fossa component classification ranges from F0 (the standard fossa component) up to F5 (fossa prosthesis covering a temporal defect that extends to the jugular foramen). Similarly, the condyle/mandible classification ranges from M0 (standard condyle–ramus component), up to M4 (total alloplastic mandible prosthesis that includes both condyles). This initial classification was based on a review of 19 patients/prostheses from the manufacturer TMJ Concepts (Ventura, CA, USA) [118]. This classification system was recently validated by the same authors by sending a survey to 64 high-volume alloplastic TJR surgeons. It was found that the mandibular component of the classification system had good inter-rater agreement. This was not the case with the fossa classification [119]. This study proposed a revision of the original classification for the fossa component to a simpler three-tier classification system. This classification system ranged from F0 (standard fossa component) to FA (extended fossa component confined to zygomatic arch) to FT (extended fossa component that includes a temporal bone defect). Unfortunately, there were multiple limitations in this study, including the respondent size (n = 17) and survey protocol, which made it difficult to view and score the fossa components. The modified fossa component classification was again validated by the same group of authors [120]. This study found better inter-rater agreement with the three-tier fossa component classification system. Again, the study was limited by the small number of respondents (n = 24).
Overall, data regarding the efficacy of the various eTMJR prostheses have been limited to primarily case reports and case series [121]. A recent review by Khattak et al. looked to evaluate the effectiveness of eTMJRs based on functional and esthetic variables, and the post-operative complications associated with these prostheses [116]. The variables reported and analyzed included maximum incisal opening, occlusion, symmetry, pain, and diet. The authors found that overall there was an improvement across these variables with the use of eTMJR prostheses. Yet, this study also revealed the significant gaps in information regarding eTMJR prostheses such as the prevalence of post-operative complications (nerve palsy, infection, etc.). Lastly, it was noted that only one of the studies analyzed utilized the eTMJR prosthesis classification system, making it difficult to perform comparative analyses. This highlights the importance of utilizing a classification system and more comprehensive data reporting in future studies on eTMJR prostheses.
5. Conclusions
Our knowledge both clinically and surgically regarding the temporomandibular joint has vastly increased over the last 15 years. Landmark studies in basic science, epidemiology, and surgical technique have significantly advanced not only how we treatment plan patients with orofacial pain and TMJ disorders but also the techniques employed to perform procedures that provide the most benefit to the patient. The field continues to evolve as technology improves with novel techniques and prostheses, such as the extended TMJ prosthesis. These advances are allowing TMJ surgeons to not only directly treat the temporomandibular joint, but also the surrounding structures. From patients with JIA to patients with large craniofacial defects, these advancements in knowledge, techniques, and technology are pushing into exciting new territories of TMJ surgery that will continue to revolutionize patient care and quality of life.
Acknowledgments
Leslie C. Hassett, MLS, is gratefully acknowledged for assistance with the literature review.
Author Contributions
All authors contributed to conceptualization, literature review, and writing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Valesan L.F., Da-Cas C.D., Reus J.C., Denardin A.C.S., Garanhani R.R., Bonotto D., Januzzi E., de Souza B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021;25:441–453. doi: 10.1007/s00784-020-03710-w. [DOI] [PubMed] [Google Scholar]
- 2.Mercuri L.G., Neto M.Q., Pourzal R. Alloplastic temporomandibular joint replacement: Present status and future perspectives of the elements of embodiment. Int. J. Oral Maxillofac. Surg. 2022;51:1573–1578. doi: 10.1016/j.ijom.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Slade G.D., Bair E., By K., Mulkey F., Baraian C., Rothwell R., Reynolds M., Miller V., Gonzalez Y., Gordon S., et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J. Pain. 2011;12:T12–T26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush F.M., Harkins S.W., Harrington W.G., Price D.D. Analysis of gender effects on pain perception and symptom presentation in temporomandibular pain. Pain. 1993;53:73–80. doi: 10.1016/0304-3959(93)90058-W. [DOI] [PubMed] [Google Scholar]
- 5.LeResche L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit. Rev. Oral Biol. Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 6.Slade G.D., Fillingim R.B., Sanders A.E., Bair E., Greenspan J.D., Ohrbach R., Dubner R., Diatchenko L., Smith S.B., Knott C., et al. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: Implications and future directions. J. Pain. 2013;14:T116–T124. doi: 10.1016/j.jpain.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H.J., Greenspan J.D., Ohrbach R., Fillingim R.B., Maixner W., Renn C.L., Johantgen M., Zhu S., Dorsey S.G. Racial/ethnic differences in experimental pain sensitivity and associated factors-Cardiovascular responsiveness and psychological status. PLoS ONE. 2019;14:e0215534. doi: 10.1371/journal.pone.0215534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willassen L., Johansson A.A., Kvinnsland S., Staniszewski K., Berge T., Rosén A. Catastrophizing Has a Better Prediction for TMD Than Other Psychometric and Experimental Pain Variable. Pain Res. Manag. 2020;12:7893023. doi: 10.1155/2020/7893023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S., Wactawski-Wende J., LaMonte M.J., Zhao J., Slade G.D., Bair E., Greenspan J.D., Fillingim R.B., Maixner W., Ohrbach R. Incident injury is strongly associated with subsequent incident temporomandibular disorder: Results from the OPPERA study. Pain. 2019;160:1551–1561. doi: 10.1097/j.pain.0000000000001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S., Ohrbach R., Fillingim R.B., Greenspan J.D., Slade G. Pain Sensitivity Modifies Risk of Injury-Related Temporomandibular Disorder. J. Dent. Res. 2020;99:530–536. doi: 10.1177/0022034520913247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 13.Tchivileva I.E., Ohrbach R., Fillingim R.B., Greenspan J.D., Maixner W., Slade G.D. Temporal change in headache and its contribution to the risk of developing first-onset temporomandibular disorder in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study. Pain. 2017;158:120–129. doi: 10.1097/j.pain.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chichorro J.G., Porreca F., Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37:613–626. doi: 10.1177/0333102417704187. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S., Slade G.D., Fillingim R.B., Ohrbach R. A rose by another name? Characteristics that distinguish headache secondary to temporomandibular disorder from headache that is comorbid with temporomandibular disorder. Pain. 2023;164:820–830. doi: 10.1097/j.pain.0000000000002770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bair E., Gaynor S., Slade G.D., Ohrbach R., Fillingim R.B., Greenspan J.D., Dubner R., Smith S.B., Diatchenko L., Maixner W. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: The OPPERA study. Pain. 2016;157:1266–1278. doi: 10.1097/j.pain.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolf C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slade G.D., Greenspan J.D., Fillingim R.B., Maixner W., Sharma S., Ohrbach R. Overlap of Five Chronic Pain Conditions: Temporomandibular Disorders, Headache, Back Pain, Irritable Bowel Syndrome, and Fibromyalgia. J. Oral Facial Pain Headache. 2020;34:s15–s28. doi: 10.11607/ofph.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaynor S.M., Bortsov A., Bair E., Fillingim R.B., Greenspan J.D., Ohrbach R., Diatchenko L., Nackley A., Tchivileva I.E., Whitehead W., et al. Phenotypic profile clustering pragmatically identifies diagnostically and mechanistically informative subgroups of chronic pain patients. Pain. 2021;162:1528–1538. doi: 10.1097/j.pain.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arabshahi B., Cron R.Q. Temporomandibular joint arthritis in juvenile idiopathic arthritis: The forgotten joint. Curr. Opin. Rheumatol. 2006;18:490–495. doi: 10.1097/01.bor.0000240360.24465.4c. [DOI] [PubMed] [Google Scholar]
- 21.Ma K.S., Thota E., Huang J.Y., Wei J.C., Resnick C.M. Increased risk of temporomandibular joint disorders and craniofacial deformities in patients with juvenile idiopathic arthritis: A population-based cohort study. Int. J. Oral Maxillofac. Surg. 2022;51:1482–1487. doi: 10.1016/j.ijom.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Resnick C.M., Dang R., Henderson L.A., Zander D.A., Daniels K.M., Nigrovic P.A., Kaban L.B. Frequency and Morbidity of Temporomandibular Joint Involvement in Adult Patients With a History of Juvenile Idiopathic Arthritis. J. Oral Maxillofac. Surg. 2017;75:1191–1200. doi: 10.1016/j.joms.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Patel K., Gerber B., Bailey K., Saeed N.R. Juvenile idiopathic arthritis of the temporomandibular joint-no longer the forgotten joint. Br. J. Oral Maxillofac. Surg. 2022;60:247–256. doi: 10.1016/j.bjoms.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Niibo P., Pruunsild C., Voog-Oras U., Nikopensius T., Jagomagi T., Saag M. Contemporary management of TMJ involvement in JIA patients and its orofacial consequences. EPMA J. 2016;7:12. doi: 10.1186/s13167-016-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petty R.E., Southwood T.R., Manners P., Baum J., Glass D.N., Goldenberg J., He X., Maldonado-Cocco J., Orozco-Alcala J., Prieur A.M., et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 26.Kovalko I., Stoustrup P., Twilt M. Temporomandibular joint involvement in juvenile idiopathic arthritis: Challenges in diagnosis, treatment, and outcomes. Curr. Treat. Options Rheumatol. 2018;4:44–54. doi: 10.1007/s40674-018-0086-2. [DOI] [Google Scholar]
- 27.Cannizzaro E., Schroeder S., Muller L.M., Kellenberger C.J., Saurenmann R.K. Temporomandibular joint involvement in children with juvenile idiopathic arthritis. J. Rheumatol. 2011;38:510–515. doi: 10.3899/jrheum.100325. [DOI] [PubMed] [Google Scholar]
- 28.Stoll M.L., Sharpe T., Beukelman T., Good J., Young D., Cron R.Q. Risk factors for temporomandibular joint arthritis in children with juvenile idiopathic arthritis. J. Rheumatol. 2012;39:1880–1887. doi: 10.3899/jrheum.111441. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen T.K., Kuseler A., Gelineck J., Herlin T. A prospective study of magnetic resonance and radiographic imaging in relation to symptoms and clinical findings of the temporomandibular joint in children with juvenile idiopathic arthritis. J. Rheumatol. 2008;35:1668–1675. [PubMed] [Google Scholar]
- 30.Stoustrup P., Herlin T., Spiegel L., Rahimi H., Koos B., Pedersen T.K., Twilt M. Temporomandibular Joint Juvenile Arthritis Working, G. Standardizing the Clinical Orofacial Examination in Juvenile Idiopathic Arthritis: An Interdisciplinary, Consensus-based, Short Screening Protocol. J. Rheumatol. 2020;47:1397–1404. doi: 10.3899/jrheum.190661. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt C., Ertel T., Arbogast M., Hugle B., Kalle T.V., Neff A. The Diagnosis and Treatment of Rheumatoid and Juvenile Idiopathic Arthritis of the Temporomandibular Joint. Dtsch. Arztebl. Int. 2022;119:47–54. doi: 10.3238/arztebl.m2021.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inarejos Clemente E.J., Tolend M., Navallas M., Doria A.S., Meyers A.B. MRI of the temporomandibular joint in children with juvenile idiopathic arthritis: Protocol and findings. Pediatr. Radiol. 2023;53:1498–1512. doi: 10.1007/s00247-023-05616-7. [DOI] [PubMed] [Google Scholar]
- 33.Miller E., Inarejos Clemente E.J., Tzaribachev N., Guleria S., Tolend M., Meyers A.B., von Kalle T., Stimec J., Koos B., Appenzeller S., et al. Imaging of temporomandibular joint abnormalities in juvenile idiopathic arthritis with a focus on developing a magnetic resonance imaging protocol. Pediatr. Radiol. 2018;48:792–800. doi: 10.1007/s00247-017-4005-8. [DOI] [PubMed] [Google Scholar]
- 34.Kellenberger C.J., Abramowicz S., Arvidsson L.Z., Kirkhus E., Tzaribachev N., Larheim T.A. Recommendations for a Standard Magnetic Resonance Imaging Protocol of Temporomandibular Joints in Juvenile Idiopathic Arthritis. J. Oral Maxillofac. Surg. 2018;76:2463–2465. doi: 10.1016/j.joms.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Vaid Y.N., Dunnavant F.D., Royal S.A., Beukelman T., Stoll M.L., Cron R.Q. Imaging of the temporomandibular joint in juvenile idiopathic arthritis. Arthritis Care Res. 2014;66:47–54. doi: 10.1002/acr.22177. [DOI] [PubMed] [Google Scholar]
- 36.Navallas M., Inarejos E.J., Iglesias E., Cho Lee G.Y., Rodríguez N., Antón J. MR Imaging of the Temporomandibular Joint in Juvenile Idiopathic Arthritis: Technique and Findings. Radiographics. 2017;37:595–612. doi: 10.1148/rg.2017160078. [DOI] [PubMed] [Google Scholar]
- 37.Muller L., Kellenberger C.J., Cannizzaro E., Ettlin D., Schraner T., Bolt I.B., Peltomaki T., Saurenmann R.K. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: A pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology. 2009;48:680–685. doi: 10.1093/rheumatology/kep068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maxwell L.J., Beaton D.E., Boers M., d’Agostino M.A., Conaghan P.G., Grosskleg S., Shea B.J., Bingham Iii C.O., Boonen A., Christensen R., et al. The evolution of instrument selection for inclusion in core outcome sets at OMERACT: Filter 2.2. Semin. Arthritis Rheum. 2021;51:1320–1330. doi: 10.1016/j.semarthrit.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Tolend M.A., Twilt M., Cron R.Q., Tzaribachev N., Guleria S., von Kalle T., Koos B., Miller E., Stimec J., Vaid Y., et al. Toward Establishing a Standardized Magnetic Resonance Imaging Scoring System for Temporomandibular Joints in Juvenile Idiopathic Arthritis. Arthritis Care Res. 2018;70:758–767. doi: 10.1002/acr.23340. [DOI] [PubMed] [Google Scholar]
- 40.Buch K., Peacock Z.S., Resnick C.M., Rothermel H., Kaban L.B., Caruso P. Regional diferences in temporomandibular joint infammation in patients with juvenile idiopathic arthritis: A dynamic post-contrast magnetic resonance imaging study. Int. J. Oral Maxillofac. Surg. 2020;49:1210–1216. doi: 10.1016/j.ijom.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Kupka M.J., Aguet J., Wagner M.M., Callaghan F.M., Goudy S.L., Abramowicz S., Kellenberger C.J. Preliminary experience with black bone magnetic resonance imaging for morphometry of the mandible and visualisation of the facial skeleton. Pediatr. Radiol. 2022;52:951–958. doi: 10.1007/s00247-021-05257-8. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt C., Reich R., Koos B., Ertel T., Ahlers M.O., Arbogast M., Feurer I., Habermann-Krebs M., Hilgenfeld T., Hirsch C., et al. Controversial Aspects of Diagnostics and Therapy of Arthritis of the Temporomandibular Joint in Rheumatoid and Juvenile Idiopathic Arthritis-An Analysis of Evidence- and Consensus-Based Recommendations Based on an Interdisciplinary Guideline Project. J. Clin. Med. 2022;11:1761. doi: 10.3390/jcm11071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bousquet B., Kellenberger C.J., Caprio R.M., Jindal S., Resnick C.M. Does Magnetic Resonance Imaging Distinguish Juvenile Idiopathic Arthritis From Other Causes of Progressive Temporomandibular Joint Destruction? J. Oral Maxillofac. Surg. 2023;81:820–830. doi: 10.1016/j.joms.2023.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Bag A.K., Gaddikeri S., Singhal A., Hardin S., Tran B.D., Medina J.A., Cure J.K. Imaging of the temporomandibular joint: An update. World J. Radiol. 2014;6:567–582. doi: 10.4329/wjr.v6.i8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Assar de la Fuente S., Angenete O., Jellestad S., Tzaribachev N., Koos B., Rosendahl K. Juvenile idiopathic arthritis and the temporomandibular joint: A comprehensive review. J. Cranio-Maxillofac. Surg. 2016;44:597–607. doi: 10.1016/j.jcms.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Stoustrup P., Lerman M.A., Twilt M. The Temporomandibular Joint in Juvenile Idiopathic Arthritis. Rheum. Dis. Clin. N. Am. 2021;47:607–617. doi: 10.1016/j.rdc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Stoustrup P., Twilt M. Improving treatment of the temporomandibular joint in juvenile idiopathic arthritis: Let’s face it. Expert Rev. Clin. Immunol. 2019;15:1119–1121. doi: 10.1080/1744666x.2020.1676151. [DOI] [PubMed] [Google Scholar]
- 48.Bollhalder A., Patcas R., Eichenberger M., Müller L., Schroeder-Kohler S., Saurenmann R.K., Kellenberger C.J. Magnetic Resonance Imaging Followup of Temporomandibular Joint Inflammation, Deformation, and Mandibular Growth in Juvenile Idiopathic Arthritis Patients Receiving Systemic Treatment. J. Rheumatol. 2020;47:909–916. doi: 10.3899/jrheum.190168. [DOI] [PubMed] [Google Scholar]
- 49.Kinard B., Goldberg B., Kau C., Abramowicz S. Clinical trials of temporomandibular joint involvement of juvenile idiopathic arthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;131:617–619. doi: 10.1016/j.oooo.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Stoll M.L., Amin D., Powell K.K., Poholek C.H., Strait R.H., Aban I., Beukelman T., Young D.W., Cron R.Q., Waite P.D. Risk Factors for Intraarticular Heterotopic Bone Formation in the Temporomandibular Joint in Juvenile Idiopathic Arthritis. J. Rheumatol. 2018;45:1301–1307. doi: 10.3899/jrheum.171306. [DOI] [PubMed] [Google Scholar]
- 51.Lochbuhler N., Saurenmann R.K., Muller L., Kellenberger C.J. Magnetic Resonance Imaging Assessment of Temporomandibular Joint Involvement and Mandibular Growth Following Corticosteroid Injection in Juvenile Idiopathic Arthritis. J. Rheumatol. 2015;42:1514–1522. doi: 10.3899/jrheum.141502. [DOI] [PubMed] [Google Scholar]
- 52.Resnick C.M., Pedersen T.K., Abramowicz S., Twilt M., Stoustrup P.B. Time to Reconsider Management of the Temporomandibular Joint in Juvenile Idiopathic Arthritis. J. Oral Maxillofac. Surg. 2018;76:1145–1146. doi: 10.1016/j.joms.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Olsen-Bergem H., Bjornland T. A cohort study of patients with juvenile idiopathic arthritis and arthritis of the temporomandibular joint: Outcome of arthrocentesis with and without the use of steroids. Int. J. Oral Maxillofac. Surg. 2014;43:990–995. doi: 10.1016/j.ijom.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Alstergren P., Larsson P.T., Kopp S. Successful treatment with multipleintra-articular injections of infliximab in a patient with psoriatic arthritis. Scand. J. Rheumatol. 2008;37:155–157. doi: 10.1080/03009740701675635. [DOI] [PubMed] [Google Scholar]
- 55.Stoll M.L., Cron R.Q., Saurenmann R.K. Systemic and intraarticular antiinflammatory therapy of temporomandibular joint arthritis in children with juvenile idiopathic arthritis. Semin. Orthod. 2015;21:125–133. doi: 10.1053/j.sodo.2015.02.009. [DOI] [Google Scholar]
- 56.Stoll M.L., Morlandt A.B., Teerawattanapong S., Young D., Waite P.D., Cron R.Q. Safety and efficacy of intra-articular infliximab therapy for treatment-resistant temporomandibular joint arthritis in children: A retrospective study. Rheumatology. 2013;52:554–559. doi: 10.1093/rheumatology/kes318. [DOI] [PubMed] [Google Scholar]
- 57.Frid P., Resnick C., Abramowicz S., Stoustrup P., Nørholt S.E. Surgical correction of dentofacial deformities in juvenile idiopathic arthritis: A systematic literature review. Int. J. Oral Maxillofac. Surg. 2019;48:1032–1042. doi: 10.1016/j.ijom.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Norholt S.E., Pedersen T.K., Herlin T. Functional changes following distraction osteogenesis treatment of asymmetric mandibular growth deviation in unilateral juvenile idiopathic arthritis: A prospective study with long-term follow-up. Int. J. Oral Maxillofac. Surg. 2013;42:329–336. doi: 10.1016/j.ijom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Svensson B., Adell R. Costochondral grafts to replace mandibular condyles in juvenile chronic arthritis patients: Long-term effects on facial growth. J. Craniomaxillofac. Surg. 1998;26:275–285. doi: 10.1016/S1010-5182(98)80055-3. [DOI] [PubMed] [Google Scholar]
- 60.Saeed N., Hensher R., McLeod N., Kent J. Reconstruction of the temporomandibular joint autogenous compared with alloplastic. Br. J. Oral Maxillofac. Surg. 2002;40:296–299. doi: 10.1016/S0266-4356(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 61.Hechler B.L., Matthews N.S. Role of alloplastic reconstruction of the temporomandibular joint in the juvenile idiopathic arthritis population. Br. J. Oral Maxillofac. Surg. 2021;59:21–27. doi: 10.1016/j.bjoms.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 62.Stoustrup P., Pedersen T.K., Nørholt S.E., Resnick C.M., Abramowicz S. Interdisciplinary Management of Dentofacial Deformity in Juvenile Idiopathic Arthritis. Oral Maxillofac. Surg. Clin. N. Am. 2020;32:117–134. doi: 10.1016/j.coms.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Trivedi B., Wolford L.M., Kesterke M.J., Pinto L.P. Does Combined Temporomandibular Joint Reconstruction With Patient Fitted Total Joint Prosthesis and Orthognathic Surgery Reduce Symptoms in Juvenile Idiopathic Arthritis Patients? J. Oral Maxillofac. Surg. 2022;80:267–275. doi: 10.1016/j.joms.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Raffaini M., Arcuri F. Orthognathic surgery for juvenile idiopathic arthritis of the temporomandibular joint: A critical reappraisal based on surgical experience. Int. J. Oral Maxillofac. Surg. 2022;51:799–805. doi: 10.1016/j.ijom.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Oye F., Bjornland T., Store G. Mandibular osteotomies in patients with juvenile rheumatoid arthritic disease. Scand. J. Rheumatol. 2003;32:168–173. doi: 10.1080/03009740310002515. [DOI] [PubMed] [Google Scholar]
- 66.Leshem D., Tompson B., Britto J.A., Forrest C.R., Phillips J.H. Orthognathic surgery in juvenile rheumatoid arthritis patients. Plast Reconstr. Surg. 2006;117:1941–1946. doi: 10.1097/01.prs.0000209922.46292.58. [DOI] [PubMed] [Google Scholar]
- 67.Pagnoni M., Amodeo G., Fadda M.T., Brauner E., Guarino G., Virciglio P., Iannetti G. Juvenile idiopathic/rheumatoid arthritis and orthognatic surgery without mandibular osteotomies in the remittent phase. J. Craniofac. Surg. 2013;24:1940–1945. doi: 10.1097/SCS.0b013e31829a8458. [DOI] [PubMed] [Google Scholar]
- 68.Ma K.S.K., Illescas Ralda M.M., Veeravalli J.J., Wang L.T., Thota E., Huang J.Y., Kao C.T., Wei J.C.C., Resnick C.M. Patients with juvenile idiopathic arthritis are at increased risk for obstructive sleep apnoea: A population-based cohort study. Eur. J. Orthod. 2022;44:222–231. doi: 10.1093/ejo/cjab050. [DOI] [PubMed] [Google Scholar]
- 69.Ward T.M., Archbold K., Lentz M., Ringold S., Wallace C.A., Landis C.A. Sleep disturbance, daytime sleepiness, and neurocognitive performance in children with juvenile idiopathic arthritis. Sleep. 2010;33:252–259. doi: 10.1093/sleep/33.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tegelberg A., Kopp S. Short-term effect of physical training on temporomandibular joint disorder in individuals with rheumatoid arthritis and ankylosing spondylitis. Acta Odontol. Scand. 1988;46:49–56. doi: 10.3109/00016358809004746. [DOI] [PubMed] [Google Scholar]
- 71.Stoustrup P., Kristensen K.D., Küseler A., Verna C., Herlin T., Pedersen T.K. Management of temporomandibular joint arthritis-related orofacial symptoms in juvenile idiopathic arthritis by the use of a stabilization splint. Scand. J. Rheumatol. 2014;43:137–145. doi: 10.3109/03009742.2013.830146. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez-Garcia R., Martin-Granizo R. Arthroscopic Disc Repositioning Techniques of the Temporomandibular Joint: Part 1: Sutures. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2022;30:175–183. doi: 10.1016/j.cxom.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Martin-Granizo R., Gonzalez-Garcia R. Arthroscopic Disc Repositioning Techniques of the Temporomandibular Joint Part 2: Resorbable Pins. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2022;30:185–191. doi: 10.1016/j.cxom.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Israel H.A. Technique for placement of a discal traction suture during temporomandibular joint arthroscopy. J. Oral Maxillofac. Surg. 1989;47:311–313. doi: 10.1016/0278-2391(89)90240-1. [DOI] [PubMed] [Google Scholar]
- 75.Tarro A.W. Arthroscopic treatment of anterior disc displacement: A preliminary report. J. Oral Maxillofac. Surg. 1989;47:353–358. doi: 10.1016/0278-2391(89)90336-4. [DOI] [PubMed] [Google Scholar]
- 76.Ohnishi M. Arthroscopic laser surgery and suturing for temporomandibular joint disorders: Technique and clinical results. Arthroscopy. 1991;7:212–220. doi: 10.1016/0749-8063(91)90110-J. [DOI] [PubMed] [Google Scholar]
- 77.Kondoh T. Arthroscopic traction suturing. Treatment of internal derangement by arthroscopic repositioning and suturing of the disk. In: Clark G.T., Sanders B., Bertolami C.N., editors. Advances in Diagnostic and Surgical Arthroscopy of the Temporomandibular Joint. WB Saunders Company; Philadelphia, PA, USA: 1993. pp. 117–127. [Google Scholar]
- 78.Tarro A.W. A fully visualized arthroscopic disc suturing technique. J. Oral Maxillofac. Surg. 1994;52:362–369. doi: 10.1016/0278-2391(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 79.Goizueta Adame C.C., Munoz-Guerra M.F. The posterior double pass suture in repositioning of the temporomandibular disc during arthroscopic surgery: A report of 16 cases. J. Craniomaxillofac. Surg. 2012;40:86–91. doi: 10.1016/j.jcms.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 80.McCain J.P., Podrasky A.E., Zabiegalski N.A. Arthroscopic disc repositioning and suturing: A preliminary report. J. Oral Maxillofac. Surg. 1992;50:568–579. doi: 10.1016/0278-2391(92)90435-3. [DOI] [PubMed] [Google Scholar]
- 81.Yang C., Cai X.Y., Chen M.J., Zhang S.Y. New arthroscopic disc repositioning and suturing technique for treating an anteriorly displaced disc of the temporomandibular joint: Part I—Technique introduction. Int. J. Oral Maxillofac. Surg. 2012;41:1058–1063. doi: 10.1016/j.ijom.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 82.Onishi M. Arthroscopy of the temporomandibular joint (author’s transl) Kokubyo Gakkai Zasshi. 1975;42:207–213. doi: 10.5357/koubyou.42.207. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S.Y., Liu X.M., Yang C., Cai X.Y., Chen M.J., Haddad M.S., Yun B., Chen Z.Z. New arthroscopic disc repositioning and suturing technique for treating internal derangement of the temporomandibular joint: Part II—Magnetic resonance imaging evaluation. J. Oral Maxillofac. Surg. 2010;68:1813–1817. doi: 10.1016/j.joms.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Jerez D., Laissle G., Fuenzalida C., Uribe S. Modification to Yang’s Arthroscopic Discopexy Technique for Temporomandibular Joint Disc Displacement: A Technical Note. J. Oral Maxillofac. Surg. 2022;80:989–995. doi: 10.1016/j.joms.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Martinez-Gimeno C., Garcia-Hernandez A., Martinez-Martinez R. Single portal arthroscopic temporomandibular joint discopexy: Technique and results. J. Craniomaxillofac. Surg. 2021;49:171–176. doi: 10.1016/j.jcms.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Millon-Cruz A., Martin-Granizo Lopez R. Long-term clinical outcomes of arthroscopic discopexy with resorbable pins. J. Craniomaxillofac. Surg. 2020;48:1074–1079. doi: 10.1016/j.jcms.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Martin-Granizo R., Millon-Cruz A. Discopexy using resorbable pins in temporomandibular joint arthroscopy: Clinical and magnetic resonance imaging medium-term results. J. Craniomaxillofac. Surg. 2016;44:479–486. doi: 10.1016/j.jcms.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Goizueta-Adame C.C., Pastor-Zuazaga D., Orts Banon J.E. Arthroscopic disc fixation to the condylar head. Use of resorbable pins for internal derangement of the temporomandibular joint (stage II-IV). Preliminary report of 34 joints. J Craniomaxillofac. Surg. 2014;42:340–346. doi: 10.1016/j.jcms.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 89.Loureiro Sato F.R., Tralli G. Arthroscopic discopexy technique with anchors for treatment of temporomandibular joint internal derangement: Clinical and magnetic resonance imaging evaluation. J. Craniomaxillofac. Surg. 2020;48:501–507. doi: 10.1016/j.jcms.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Abdelrehem A., Hu Y.K., Yang C., Zheng J.S., Shen P., Shen Q.C. Arthroscopic versus open disc repositioning and suturing techniques for the treatment of temporomandibular joint anterior disc displacement: 3-year follow-up study. Int. J. Oral Maxillofac. Surg. 2021;50:1351–1360. doi: 10.1016/j.ijom.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 91.Askar H., Aronovich S., Christensen B.J., McCain J., Hakim M. Is Arthroscopic Disk Repositioning Equally Efficacious to Open Disk Repositioning? A Systematic Review. J. Oral Maxillofac. Surg. 2021;79:2030–2041. doi: 10.1016/j.joms.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 92.Santos T.S., Pagotto L.E.C., Santos Nascimento E., Rezende da Cunha L., Serra Cassano D., Goncalves J.R. Effectiveness of disk repositioning and suturing comparing open-joint versus arthroscopic techniques: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;132:506–513. doi: 10.1016/j.oooo.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 93.McCain J.P., Hossameldin R.H., Srouji S., Maher A. Arthroscopic discopexy is effective in managing temporomandibular joint internal derangement in patients with Wilkes stage II and III. J. Oral Maxillofac. Surg. 2015;73:391–401. doi: 10.1016/j.joms.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Murakami K.I., Tsuboi Y., Bessho K., Yokoe Y., Nishida M., Iizuka T. Outcome of arthroscopic surgery to the temporomandibular joint correlates with stage of internal derangement: Five-year follow-up study. Br. J. Oral Maxillofac. Surg. 1998;36:30–34. doi: 10.1016/S0266-4356(98)90744-6. [DOI] [PubMed] [Google Scholar]
- 95.Sah M.K., Abdelrehem A., Chen S., Shen P., Jiao Z., Hu Y.K., Nie X., Yang C. Prognostic indicators of arthroscopic discopexy for management of temporomandibular joint closed lock. Sci. Rep. 2022;12:3194. doi: 10.1038/s41598-022-07014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dong M., Jiao Z., Sun Q., Tao X., Yang C., Qiu W. The magnetic resonance imaging evaluation of condylar new bone remodeling after Yang’s TMJ arthroscopic surgery. Sci. Rep. 2021;11:5219. doi: 10.1038/s41598-021-84591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis C.M., Hakim M., Choi D.D., Behrman D.A., Israel H., McCain J.P. Early Clinical Outcomes of Arthroscopic Management of the Failing Alloplastic Temporomandibular Joint Prosthesis. J. Oral Maxillofac. Surg. 2020;78:903–907. doi: 10.1016/j.joms.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Neff A., McLeod N., Spijkervet F., Riechmann M., Vieth U., Kolk A., Sidebottom A.J., Bonte B., Speculand B., Saridin C., et al. The ESTMJS (European Society of Temporomandibular Joint Surgeons) Consensus and Evidence-Based Recommendations on Management of Condylar Dislocation. J. Clin. Med. 2021;10:5068. doi: 10.3390/jcm10215068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Renapurkar S.K., Laskin D.M. Injectable Agents Versus Surgery for Recurrent Temporomandibular Joint Dislocation. Oral Maxillofac. Surg. Clin. N. Am. 2018;30:343–349. doi: 10.1016/j.coms.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Fu K.Y., Chen H.M., Sun Z.P., Zhang Z.K., Ma X.C. Long-term efficacy of botulinum toxin type A for the treatment of habitual dislocation of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2010;48:281–284. doi: 10.1016/j.bjoms.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 101.Borghol K., Abdelrahman A., Pigadas N. Guided botulinum toxin injection to the lateral pterygoid muscles for recurrent dislocation of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2021;59:845–846. doi: 10.1016/j.bjoms.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 102.Tocaciu S., McCullough M.J., Dimitroulis G. Surgical management of recurrent TMJ dislocation-a systematic review. Oral Maxillofac. Surg. 2019;23:35–45. doi: 10.1007/s10006-019-00746-5. [DOI] [PubMed] [Google Scholar]
- 103.Machon V., Abramowicz S., Paska J., Dolwick M.F. Autologous blood injection for the treatment of chronic recurrent temporomandibular joint dislocation. J. Oral Maxillofac. Surg. 2009;67:114–119. doi: 10.1016/j.joms.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 104.Coser R., da Silveira H., Medeiros P., Ritto F.G. Autologous blood injection for the treatment of recurrent mandibular dislocation. Int. J. Oral Maxillofac. Surg. 2015;44:1034–1037. doi: 10.1016/j.ijom.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 105.Hegab A.F. Treatment of chronic recurrent dislocation of the temporomandibular joint with injection of autologous blood alone, intermaxillary fixation alone, or both together: A prospective, randomised, controlled clinical trial. Br. J. Oral Maxillofac. Surg. 2013;51:813–817. doi: 10.1016/j.bjoms.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 106.Yoshida H., Nakatani Y.I., Gamoh S., Shimizutani K., Morita S. Clinical outcome after 36 months of treatment with injections of autologous blood for recurrent dislocation of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2018;56:64–66. doi: 10.1016/j.bjoms.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Daif E.T. Autologous blood injection as a new treatment modality for chronic recurrent temporomandibular joint dislocation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;109:31–36. doi: 10.1016/j.tripleo.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Machon V., Levorova J., Hirjak D., Wisniewski M., Drahos M., Sidebottom A., Foltan R. A prospective assessment of outcomes following the use of autologous blood for the management of recurrent temporomandibular joint dislocation. Oral Maxillofac. Surg. 2018;22:53–57. doi: 10.1007/s10006-017-0666-6. [DOI] [PubMed] [Google Scholar]
- 109.Bayoumi A.M., Al-Sebaei M.O., Mohamed K.M., Al-Yamani A.O., Makrami A.M. Arthrocentesis followed by intra-articular autologous blood injection for the treatment of recurrent temporomandibular joint dislocation. Int. J. Oral Maxillofac. Surg. 2014;43:1224–1228. doi: 10.1016/j.ijom.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 110.Sharma V., Anchlia S., Sadhwani B.S., Bhatt U., Rajpoot D. Arthrocentesis Followed by Autologous Blood Injection in the Treatment of Chronic Symptomatic Subluxation of Temporomandibular Joint. J. Maxillofac. Oral Surg. 2022;21:1218–1226. doi: 10.1007/s12663-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Refai H. Long-term therapeutic effects of dextrose prolotherapy in patients with hypermobility of the temporomandibular joint: A single-arm study with 1-4 years’ follow up. Br. J. Oral Maxillofac. Surg. 2017;55:465–470. doi: 10.1016/j.bjoms.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 112.Nagori S.A., Jose A., Gopalakrishnan V., Roy I.D., Chattopadhyay P.K., Roychoudhury A. The efficacy of dextrose prolotherapy over placebo for temporomandibular joint hypermobility: A systematic review and meta-analysis. J. Oral Rehabil. 2018;45:998–1006. doi: 10.1111/joor.12698. [DOI] [PubMed] [Google Scholar]
- 113.Sit R.W., Reeves K.D., Zhong C.C., Wong C.H.L., Wang B., Chung V.C., Wong S.Y., Rabago D. Efficacy of hypertonic dextrose injection (prolotherapy) in temporomandibular joint dysfunction: A systematic review and meta-analysis. Sci. Rep. 2021;11:14638. doi: 10.1038/s41598-021-94119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pandey S., Baidya M., Srivastava A., Garg H. Comparison of autologous blood prolotherapy and 25% dextrose prolotherapy for the treatment of chronic recurrent temporomandibular joint dislocation on the basis of clinical parameters: A retrospective study. Natl. J. Maxillofac. Surg. 2022;13:398. doi: 10.4103/njms.njms_509_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoda T., Ogi N., Yoshitake H., Kawakami T., Takagi R., Murakami K., Yuasa H., Kondoh T., Tei K., Kurita K. Clinical guidelines for total temporomandibular joint replacement. Jpn. Dent. Sci. Rev. 2020;56:77–83. doi: 10.1016/j.jdsr.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khattak Y.R., Arif H., Gull H., Ahmad I. Extended total temporomandibular joint reconstruction prosthesis: A comprehensive analysis. J. Stomatol. Oral Maxillofac. Surg. 2023;124:101404. doi: 10.1016/j.jormas.2023.101404. [DOI] [PubMed] [Google Scholar]
- 117.Al-Qudsi A., Matel D., Mercuri L., Shah B., Emmerling M., Murphy J. Utilization of extended temporomandibular joint replacements in patients with hemifacial microsomia. Int. J. Oral Maxillofac. Surg. 2023;23:133–139. doi: 10.1016/j.ijom.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 118.Elledge R., Mercuri L.G., Speculand B. Extended total temporomandibular joint replacements: A classification system. Br. J. Oral Maxillofac. Surg. 2018;56:578–581. doi: 10.1016/j.bjoms.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Elledge R.O.C., Higginson J., Mercuri L.G., Speculand B. Validation of an extended total joint replacement (eTJR) classification system for the temporomandibular joint (TMJ) Br. J. Oral Maxillofac. Surg. 2021;59:788–791. doi: 10.1016/j.bjoms.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 120.Higginson J., Panayides C., Speculand B., Mercuri L.G., Elledge R.O.C. Modification of an extended total temporomandibular joint replacement (eTMJR) classification system. Br. J. Oral Maxillofac. Surg. 2022;60:983–986. doi: 10.1016/j.bjoms.2022.03.011. [DOI] [PubMed] [Google Scholar]
- 121.Mommaerts M.Y., Nicolescu I., Dorobantu M., De Meurechy N. Extended Total Temporomandibular Joint Replacement with Occlusal Adjustments: Pitfalls, Patient-reported Outcomes, Subclassification, and a New Paradigm. Ann. Maxillofac. Surg. 2020;10:73–79. doi: 10.4103/ams.ams_245_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.