Abstract
Tritrichomonas foetus is the causative agent of bovine tritrichomonosis, a sexually transmitted disease leading to infertility and abortion. Diagnosis is hampered by putative contamination of samples with intestinal or coprophilic trichomonadid protozoa which might be mistaken for T. foetus. Therefore, we developed a PCR test optimized for applicability in routine diagnosis. Amplification is based upon primers TFR3 and TFR4 directed to the rRNA gene units of T. foetus. In order to avoid potential carryover contamination by products of previous amplification reactions, conditions were adapted to the use of the uracil DNA glycosylase system. Furthermore, documentation and interpretation of results were facilitated by including a DNA enzyme immunoassay for the detection of amplification products. Specificity was confirmed with genomic material from different related trichomonadid protozoa. The high sensitivity of the test allowed the detection of a single T. foetus organism in diagnostic culture medium or about 50 parasites per ml of preputial washing fluid. The present methods are thus proposed as (i) confirmatory tests for microscopic diagnosis following diagnostic in vitro cultivation and (ii) a direct T. foetus screening test with diagnostic samples.
Tritrichomonas foetus is the causative agent of bovine tritrichomonosis, a sexually transmitted disease leading to infertility and abortion (17). Infection is transferred from asymptomatic bulls to heifers or cows at the time of coitus.
Due to the widespread use of artificial insemination (AI) and intensive testing of breeding animals, effective control of tritrichomonosis has been achieved in many areas of central Europe. In Switzerland, for example, until 1994, no cases of T. foetus infection had been reported for a period of 20 years (11), and in the United Kingdom only two cases of bovine tritrichomonosis were reported in the same period (29). However, prevalence is higher under natural breeding conditions. In northwestern Spain, for example, a prevalence of 2.9% was recently reported in herds in which natural mating and AI are alternately used (20); in Saskatchewan, Canada, 6% of tested bulls were infected with T. foetus (25); infected bulls were found in 15.8% of beef herds in California (2) and in 26.7 to 44.1% of ranches in Nevada (15). Comparable results were obtained in Australia (7) and Costa Rica (23). The annual losses to the U.S. beef industry were estimated to be $650 million (28).
To date, the diagnosis of bovine tritrichomonosis is mainly based upon light microscopic examination of preputial washings from bulls and cervicovaginal secretions from female cattle. Previous in vitro cultivation of parasites from diagnostic samples increases sensitivity (1, 3, 27, 30), but diagnosis is hampered by contamination of samples with intestinal or coprophilic trichomonadid protozoa which might be mistaken for T. foetus (9, 18, 29). Consequently, especially when only low parasite numbers are present, these techniques can be time-consuming and laborious, and special experience in the identification of the parasite is necessary for an accurate diagnosis. A sensitive and specific test for confirmation of the identity of the flagellates propagated by in vitro cultivation and for the direct detection of the tritrichomonads in clinical or diagnostic samples would be desirable. Therefore, efforts have been made to establish molecular biology-based diagnostic methods for the detection of T. foetus (13, 28). However, the sensitivity of the first reported DNA probe (28) turned out to be limited (1), and a PCR test that was supposed to be specific for T. foetus (13) seemed to be hampered by unspecific PCR products that occasionally occurred with samples used for diagnostic tests (16).
In a recent publication, Felleisen described the comparative sequence analysis of the 5.8S rRNA gene and the flanking internal transcribed spacer regions ITS1 and ITS2 of various T. foetus isolates originating from different geographic regions (10). The sequences proved to be highly conserved in all strains analyzed. In parallel, other related trichomonads were investigated: Trichomonas vaginalis, Trichomonas gallinae, Trichomonas tenax, and Pentatrichomonas hominis could be clearly discriminated from T. foetus on the basis of rRNA gene sequences (10). Copy number analysis revealed the presence of 12 copies of the rRNA gene unit in the T. foetus genome (4). Therefore, these sequences represent a highly suitable target for amplification by PCR.
In the present publication, we describe the development of a PCR for the sensitive detection of Tritrichomonas spp. and a complementary DNA enzyme immunoassay (DEIA) based upon primers delineated from rRNA gene unit sequences. The subsequent assessment of their diagnostic parameters demonstrated performance improvements for the diagnosis of bovine tritrichomonosis.
MATERIALS AND METHODS
Protozoa.
Different isolates of trichomonadid protozoa were obtained from the American Type Culture Collection (ATCC), Rockville, Md. The Swiss reference strain of T. foetus and a human Trichomonas vaginalis isolate were from the Institute of Veterinary Bacteriology, University of Bern, and Mai Nguyen, Institute of Medical Microbiology, University of Bern, respectively. For detailed documentation of the isolates of trichomonadid organisms, see Table 1.
TABLE 1.
Documentation for isolates of trichomonadid protozoa
| Isolate no. | Species | ATCC strain no. | Strain, origin |
|---|---|---|---|
| 1 | Tritrichomonas foetus | Reference, Switzerland | |
| 2 | Tritrichomonas foetus | 30003 | BP-4, United States |
| 3 | Tritrichomonas foetus | 30166 | Belfast, Ireland |
| 4 | Tritrichomonas foetus | 30231 | DK-2, United States |
| 5 | Tritrichomonas foetus | 30232 | UT, United States |
| 6 | Tritrichomonas foetus | 30924 | KV-1, Czech Republic |
| 7 | Tritrichomonas foetus | 50151 | KV1/M-100, Austria |
| 8 | Tritrichomonas foetus | 50152 | KV1-1MR-100, Czech Republic |
| 9 | Tritrichomonas suis | 30167 | 1/N, United States |
| 10 | Tritrichomonas suis | 30168 | 11/S, United States |
| 11 | Tritrichomonas suis | 30169 | C19F, United States |
| 12 | Tritrichomonas mobilensis | 50116 | USA:M776, Bolivia |
| 13 | Tritrichomonas gallinarum | 30097 | TP-79, United States |
| 14 | Pentatrichomonas hominis | 30098 | R51, United States |
| 15 | Trichomonas vaginalis | 30240 | JH37A-2, United States |
| 16 | Trichomonas vaginalis | 30241 | JH37A-4, United States |
| 17 | Trichomonas vaginalis | Human isolate, Switzerland | |
| 18 | Trichomonas gallinae | 30230 | TG |
| 19 | Trichomonas tenax | 30207 | Hs-4:NIH |
In vitro cultivation of trichomonads.
Trichomonads were cultivated in vitro either in the respective medium recommended by ATCC or in Diamond’s medium (8) supplemented with 5% heat-inactivated horse serum at 37°C. The InPouchTF test was obtained from Biomed Diagnostics, San Jose, Calif.
Samples and animals.
Sampling techniques are described below. The following groups of samples were collected.
(i) Preputial wash fluids and in vitro culture sediments were obtained during the period from September 1994 to September 1997 from seven different bulls with suspected T. foetus infection. This diagnostic suspicion was based upon microscopic detection of flagellated trichomonad-like organisms after in vitro cultivation in the InPouchTF test. The numbers of organisms in in vitro cultures ranged from a few cells per milliliter of medium to densely grown cultures. These clinical isolates originated from a total of more than 10,000 specimens routinely investigated by microscopic examination and cultivation over a period of 15 years at the Institute for Veterinary Bacteriology of the University of Bern and represent the first cases of T. foetus infection documented in Switzerland since 1972.
(ii) Diagnostic mucus and vaginal washing fluids were obtained from 71 cows kept on farms with frequent sterility or abortion problems.
(iii) In order to obtain standardized control samples for comparative testing by PCR and in vitro cultivation, preputial washings were obtained by the same investigator from 123 bulls in quarantine at the Swiss Association for Artificial Insemination.
(iv) Vaginal mucus was collected from 20 healthy cows at the Clinic for Large Animals, University of Bern.
Methods of sampling of material used for diagnostic tests and isolation of genomic DNA.
Preputial washings from bulls were obtained with a 20-cm-long catheter fitted to a rubber bulb. The preputial cavities were rinsed several times with 50 ml of sterile physiological sodium chloride solution (prewarmed to 30 to 37°C). The washings (10 ml) were centrifuged at 800 × g, and supernatants were discarded. One-third of each sample of pelleted material was used for in vitro cultivation in Diamond’s medium (8) and the InPouchTF test (Biomed Diagnostics) according to the manufacturer’s instructions. The remaining one-third of the material was washed once with 10 ml of phosphate-buffered saline (PBS; pH 7.2) and was subsequently subjected to DNA isolation either by a modified proteinase K digestion procedure described previously (10) or by using a PCR template preparation kit (Boehringer, Mannheim, Germany) according to the manufacturer’s protocols.
Vaginal mucus was collected with a long pipette fitted to a syringe. Aliquots of 1 ml of the samples were used for in vitro cultivation in Diamond’s medium (8) and the InPouchTF test. Prior to DNA purification, samples were repeatedly passed through a needle with a 0.5-mm diameter by using a syringe to reduce the high viscosity of the mucus. An aliquot of 1 ml was centrifuged at 3,000 × g, and the supernatant was discarded. The pelleted material was processed as described above.
Preparation of genomic DNA from in vitro-cultivated parasites in the logarithmic phase of growth was performed by a proteinase K digestion method as described recently (10). Bacterial DNA was prepared by standard methods (26).
Nucleic acids.
Primers TFR3 and TFR4, used for PCR, and TFR3pK and TFR4pK, used for the generation of an internal positive control, were synthesized by MWG, Münchenstein, Switzerland. Biotinylated oligonucleotide TFR8-Bio was used as a capture probe and was obtained from Gibco-BRL, Basel, Switzerland. For documentation of the primer sequences see Table 2. As a DNA molecular weight standard, bacteriophage φX174 DNA digested with the restriction enzyme HaeIII was used.
TABLE 2.
Primer sequences
| Primer | Sequence (5′ to 3′) |
|---|---|
| TFR3 | CGGGTCTTCCTATATGAGACAGAACC |
| TFR4 | CCTGCCGTTGGATCAGTTTCGTTAA |
| TFR3pK | CGGGTCTTCCTATATGAGACAGAACCGGAGCTGAATG |
| TFR4pK | CCTGCCGTTGGATCAGTTTCGTTAAGGGATTTTGGT |
| TFR8-Bio | Biotin-CCGTTTTAGCTTGCTAGAACAC |
PCR.
Standard PCR was carried out in a 50-μl reaction volume of 1× PCR buffer (Perkin-Elmer, Rotkreuz, Switzerland) with 2 U of AmpliTaq DNA polymerase (Perkin-Elmer). For genomic DNAs isolated from samples used in diagnostic tests, different amounts from a serial dilution (e.g., 10, 50, and 100 ng) were used as templates. For cultured isolates, approximately 50 ng of genomic DNA was routinely assessed. The final concentrations of the TFR primers and each deoxynucleoside triphosphate were 1 and 200 μM, respectively. Samples were overlaid with mineral oil and amplified in a Perkin-Elmer thermal cycler (model 480) by using the following temperature profile: denaturation at 94°C for 30 s, annealing at 67°C for 30 s, and extension at 72°C for 90 s. Following 40 cycles, a final extension step of 15 min at 72°C was added. Aliquots of the amplification reactions were then analyzed by gel electrophoresis.
Modified conditions were used for the application of the uracil DNA glycosylase (UDG) system to prevent carryover contamination (19): amplification was done in a total volume of 50 μl of 1× PCR buffer supplemented with MgCl2 to give a final magnesium concentration of 2.5 mM. As nucleotides, the deoxynucleoside triphosphate mixture of Boehringer was used, resulting in final concentrations of 200 μM for dATP, dGTP, and dCTP and 400 μM for dUTP. Primers and polymerase were used as described above. UDG (0.5 U; Life Technologies, Basel, Switzerland) was added, and the samples were incubated for 5 min at 20°C and 2 min at 50°C for the UDG reaction. Inactivation of the enzyme and cleavage of DNA at the abasic sites was done at 95°C for 5 min. Then, amplification was done by using the profile described above for 40 cycles. After PCR, the tubes were held at 72°C and 100 μl of CHCl3 was added to prevent any degradation of dU-containing products by residual UDG activity.
Gel electrophoresis of DNA samples.
Aliquots of the PCR amplification products were separated either on 2% agarose gels or on 10% polyacrylamide gels by standard methods (26). For some experiments, acrylamide gels were stained with silver following electrophoresis. Briefly, the gels were fixed in fixation solution (10% ethanol, 0.5% acetic acid) and were subsequently stained with 35 mM AgNO3 (dissolved in fixation solution). After brief washing in H2O, the bands were visualized in development solution (3% NaOH, 0.25% formaldehyde) and were fixed again. This simple staining method displayed an approximately threefold increased sensitivity compared to that of ethidium bromide staining.
DEIA.
Detection of PCR amplification products was performed as described recently by Müller and coworkers (21) by using a GEN-ETI-K DEIA kit (Sorin Biomedica, Saluggia, Italy). Briefly, streptavidin-coated microplates were sensitized with 5′-biotinylated oligonucleotide TFR8-Bio. To each well, 100 μl of a 0.5-μg/ml solution of TFR8-Bio in TE (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA) was applied overnight at 4°C. Unbound oligonucleotides were removed by washing with PBS containing 0.3% Tween 20. Ten-microliter aliquots of the PCR products were heat denaturated for 5 min in a boiling water bath, chilled in an ice bath, and subsequently hybridized with TFR8-Bio for 1 h at 45°C in 100 μl of the hybridization buffer supplied with the kit. After a subsequent washing, the remaining hybrids were detected with a double-strand-specific mouse monoclonal antibody provided with the kit; hybrid detection with the monoclonal antibody was performed according to the instructions of the manufacturer. Primary monoclonal antibody reactivity was assessed colorimetrically with an alkaline phosphatase-labelled goat anti-mouse immunoglobulin (Promega, Catalys AG, Wallisellen, Switzerland) at a 1:500 dilution and the corresponding substrate solution as described previously (21).
RESULTS
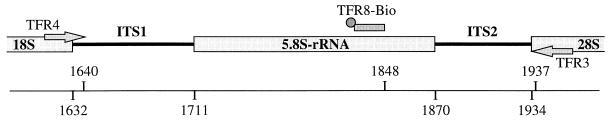
Very recently, comparative sequence analysis of the 5.8S rRNA gene and the internal transcribed spacer regions (ITS1 and ITS2) from several different isolates of T. foetus demonstrated the strict intraspecies conservation of these sequences and their distinctness from the respective sequences from other trichomonadid protozoa (10). This sequence information was applied to the delineation of primers TFR3 and TFR4 for the development of a PCR amplification system. TFR3 is complementary to the ultimate 5′ end of the 28S rRNA gene, and TFR4 is located at the border of the 18S rRNA gene and ITS1 (Fig. 1). The sequences of both areas are different from those of other related trichomonadid flagellates (10). Sufficient lengths of both primers were chosen for use in the UDG system for the prevention of carryover PCR contaminants (19). PCR with primers TFR3 and TFR4 and genomic DNA of T. foetus as a template resulted in a major amplification product of 347 bp (Fig. 2). All eight T. foetus strains obtained from ATCC and analyzed in this study gave rise to an amplification product of the same size.
FIG. 1.
Schematic representation of the primer sites used for PCR amplification and as a capture probe with respect to the rRNA gene unit of T. foetus. The positions of the boundaries between rRNA genes and internal transcribed spacer regions (numbers below the line) and the 3′ ends of primers TFR3, TFR4, and TFR8-Bio (numbers above line) are indicated in reference to T. foetus UT-1 (GenBank M81842) (4). The figure is drawn approximately to scale.
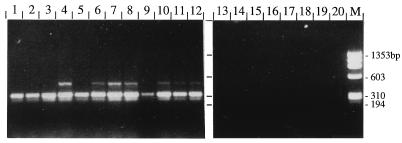
FIG. 2.
Analysis of PCR amplification products with primers TFR3 and TFR4 by 2% agarose gel electrophoresis. Purified genomic DNAs from Tritrichomonas spp. (lanes 1 to 12) and other trichomonads (lanes 13 to 19) were used as templates. For documentation regarding numbering of lanes and respective species and strains, see Table 1. Lane 20, negative control; lane M, molecular size standard.
The specificity of PCR was evaluated with genomic DNAs purified from closely related Tritrichomonas spp. Using genomic DNAs of Tritrichomonas suis and Tritrichomonas mobilensis as a template, we obtained an amplification product of the same size as that obtained with T. foetus (Fig. 2). With genomic DNAs from several other trichomonads (T. vaginalis, T. gallinae, T. tenax, Tetratrichomonas gallinarum, P. hominis) no amplification was observed (Fig. 2). Furthermore, no amplification was observed with bacterial DNA (isolated from Escherichia coli XL1-Blue) or purified genomic bovine DNA (data not shown).
For establishing the UDG system for the prevention of carryover contaminants (19), PCR was performed with primers TFR3 and TFR4 in the presence of UDG and with the incorporation of dUTP instead of dTTP as a nucleotide. However, even when large amounts of T. foetus genomic DNA were used as a template, no amplification products were observed by using standard PCR buffer. This problem could be overcome by optimizing the MgCl2 concentration. Raising the MgCl2 concentration in the PCR mixture resulted in an increased yield of amplification products. Maximum yield was obtained with MgCl2 concentrations of between 2.5 and 4.5 mM (data not shown). Higher concentrations of MgCl2 caused a decrease in the product yields. Consequently, 2.5 mM MgCl2 was used as a standard concentration for further PCR experiments with the UDG system.
In order to avoid false-negative results potentially related to inhibition of our test by constituents of the diagnostic sample, an internal positive control was established by using an artificial DNA template molecule unrelated to the diagnostic target. This control DNA was developed with the composite primers TFR3pK and TFR4pK, which contain sequences derived from pBluescript KS+ flanked by the TFR3 and TFR4 primer sequences. PCR with these composite primers and pBluescript KS+ DNA as a template generated a 528-bp fragment including plasmid sequences (from bases 1862 to 2339) and the T. foetus primer sequences located at the very ends. This artificial fragment was then purified, and serial dilutions were used as templates for amplification by PCR with TFR3 and TFR4. The limiting amount of template DNA giving rise to an amplification product representing the equivalent of a few template molecules was determined and was used as an inhibition control by adding it to test samples.
The sensitivity of the PCR test was assessed with (i) purified genomic T. foetus DNA and (ii) samples spiked with T. foetus organisms, as follows.
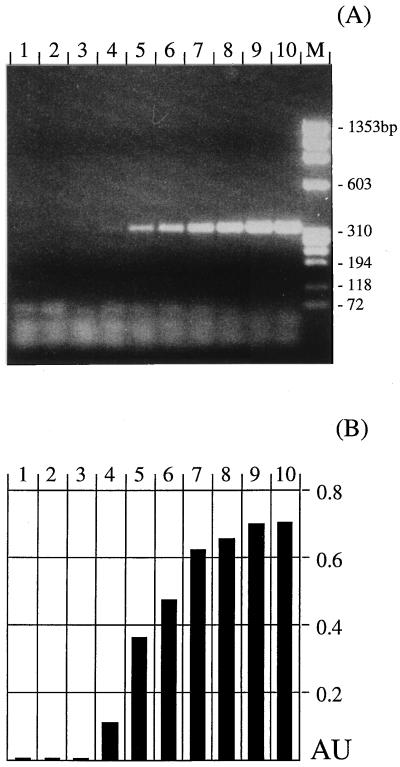
(i) Serial dilutions (1:10) of genomic DNA purified from in vitro-cultivated T. foetus were used as a template for PCR. Amplification was done by the conventional PCR protocol as well as with the UDG system. By using the conditions optimized for the UDG system, a minimum of 0.03 pg of purified T. foetus DNA could be detected (Fig. 3A). Similar results were obtained by using standard conditions without the addition of UDG (data not shown).
FIG. 3.
Sensitivity of PCR amplification with primers TFR3-TFR4 and the UDG system. (A) Analysis of the products was by 2% agarose gel electrophoresis. (B) Detection of products by DEIA with biotinylated oligonucleotide TFR8-Bio. Tenfold serial dilutions of purified T. foetus genomic DNA were used as templates. Amounts of template were 0.3 fg (lane 2), 3 fg (lane 3), 30 fg (lane 4), 0.3 pg (lane 5), 3 pg (lane 6), 30 pg (lane 7), 0.3 ng (lane 8), 3 ng (lane 9), and 30 ng (lane 10). Lane 1, negative control; lane M, molecular size standard. The absorbance units (AU) presented in panel B are A630 values subtracted from A450 values.
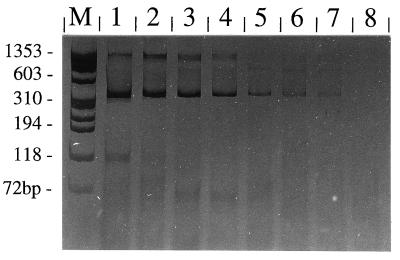
(ii) In order to determine the sensitivity of PCR with clinical samples used for diagnostic tests, serial dilutions of in vitro-cultivated T. foetus were added to 1 ml of a pool of preputial washings (from 11 uninfected bulls) or 1 ml of a pool of vaginal mucus (from 10 uninfected cows), and genomic DNA was subsequently purified. In parallel, genomic DNA was isolated from defined numbers of in vitro-cultivated parasites and analyzed by PCR for comparison. DNAs from low numbers of T. foetus parasites from in vitro cultures could successfully be amplified by PCR. In repeated experiments, a weak albeit clearly detectable amplification product was obtained by using an amount of genomic DNA equivalent to that in a single parasite cell (Fig. 4). However, by using genomic DNA isolated by the standard method from pooled preputial washings or vaginal mucus samples which were spiked with in vitro-cultivated T. foetus, the sensitivity was lower: only about 50 parasites could successfully be detected in each sample by PCR (data not shown). Lowered sensitivity was due to inhibition of the amplification reaction, as shown by the addition of the internal inhibition control. Consequently, as an alternative to the conventional DNA preparation method, we evaluated a PCR template preparation kit (Boehringer). However, the sensitivity of the test with preputial washings spiked with parasites was slightly decreased compared to that of the standard proteinase K method (data not shown).
FIG. 4.
Sensitivity of PCR amplification with primers TFR3-TFR4 and the UDG system. Analysis of the products was by 10% acrylamide gel electrophoresis and silver staining. Genomic DNAs from serial dilutions of T. foetus organisms were used as templates. The numbers of T. foetus organisms were 1,000 (lane 1), 100 (lane 2), 50 (lane 3), 10 (lane 4), and 5 (lane 5). Lanes 6 and 7, 1 parasite; lane 8, negative control; lane M, molecular size standard.
For an improvement and simplification of detection and documentation of amplification results, we established a DEIA as an alternative to conventional analysis of amplification products by gel electrophoresis with biotinylated oligonucleotide TFR8-Bio hybridizing to the 5.8S rRNA gene of T. foetus. The performance of DEIA was evaluated with products derived from PCR amplifications of different amounts of purified genomic T. foetus DNA with primers TFR3 and TFR4 as described above (Fig. 3B). The sensitivity proved to be comparable to that obtained by gel electrophoresis analysis. By DEIA PCR products from an amplification with 0.03 pg of template could be detected. PCR product that was present in an amount that was clearly visible on a gel gave clear-cut results by DEIA, hardly visible bands gave borderline results, and lanes scoring negative showed absorbance values of about zero (Fig. 3A and B). While all TFR3 and TFR4 PCR products derived from Tritrichomonas spp. were clearly detectable by DEIA, none of the samples derived from other trichomonads, bacteria, protozoa, or bovine DNA reacted in this test (data not shown).
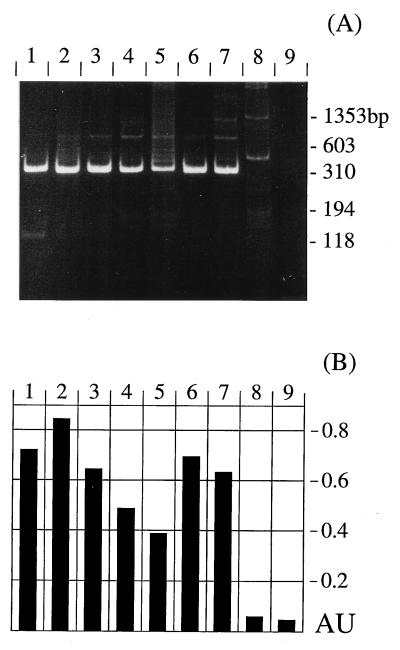
In order to evaluate the applicability of the PCR with TFR3 and TFR4 under routine test conditions for diagnostic purposes, bovine samples originating from different sources were analyzed by in vitro cultivation and PCR (the results are summarized in Table 3). (i) Routine samples represented by preputial washings from seven different bulls with suspected T. foetus infection were analyzed. The samples from six of the seven bulls used for diagnostic tests gave rise to an amplification product of approximately 347 bp in the PCR (Fig. 5A). For samples from the one remaining bull, a slightly larger amplification product was observed (Fig. 5A, lane 8). Therefore, amplification products were subsequently tested by DEIA with TFR8-Bio. Amplification products apparently of the correct size could unambiguously be identified by DEIA (Fig. 5B). The slightly larger products, however, did not react in this test. Thus, DEIA allows the correct discrimination between positive and negative samples for samples with unclear results because of the presence of unspecific amplicons.
TABLE 3.
Evaluation of PCR with TFR primers for routine diagnostic application
| Animals, samples | No. of samples
|
|||||
|---|---|---|---|---|---|---|
| InPouchTF test
|
TFR PCR
|
Total | ||||
| Negative | Positive | Negative | Positive | Inhibited | ||
| Bulls, diagnostic | 0 | 7 | 1a | 6 | 0 | 7 |
| Bulls, quarantine AI | 123 | 0 | 121 | 0 | 2 | 123 |
| Cows, diagnostic | 71 | 0 | 63 | 7b | 1 | 71 |
| Cows, control | 20 | 0 | 20 | 0 | 0 | 20 |
Slightly larger PCR product; negative by DEIA (see Fig. 5).
All animals reacting positively by the PCR originated from the same farm.
FIG. 5.
Application of PCR with TFR primers to diagnostic samples. (A) Analysis of products by 10% acrylamide gel electrophoresis. (B) Detection of products by DEIA with biotinylated oligonucleotide TFR8-Bio. Lanes 2 to 8, diagnostic samples from seven different bulls with suspected T. foetus infection; lane 1, T. foetus-positive control; lane 9, negative control. The absorbance units (AU) presented in panel B are A630 values subtracted from A450 values. Animals were retested at different times; results for only one sample per bull are shown.
(ii) Preputial washings were obtained from 123 bulls in quarantine at the field station of the Swiss Association for Artificial Insemination. Most of the animals had never been used for natural breeding. By parallel in vitro cultivation in Diamond’s medium (8) and in the commercially available InPouchTF test, no infection with T. foetus was detected parasitologically. However, some of the cultures were contaminated with bacteria or fungi. This did not affect the performance of PCR, as monitored with the inhibition control. Genomic DNA isolated from preputial washings from 121 of these bulls turned out to be negative by PCR with TFR3 and TFR4; no unspecific amplification was observed. Due to inhibition of the PCR, two samples could not be analyzed (Table 3).
(iii) With samples from 71 cows originating from farms with frequent problems of sterility or abortion, no parasite growth was observed in in vitro cultures (using Diamond’s medium [8] and the commercial InPouchTF test). However, seven animals originating from the same farm reacted positively by the PCR assay.
(iv) All mucosal samples from 20 healthy cows were negative by PCR and in vitro cultivation (using Diamond’s medium [8] and the commercial InPouchTF test).
DISCUSSION
Although many efforts have been made to develop a vaccine against T. foetus infection in cattle (for a review, see reference 5), strict surveillance of bulls by sensitive and accurate methods of diagnosis is still the most effective measure for the prevention of bovine tritrichomonosis. However, conventional parasitological techniques are based on the morphological characteristics of the parasite and are thus hampered by potential problems of specificity (9, 18, 29). Furthermore, difficulties with sensitivity may be inherent to these methods.
Recently, comparative sequence analysis of the 5.8S rRNA gene and the flanking internal transcribed spacer regions ITS1 and ITS2 of various T. foetus isolates originating from geographically different regions revealed the conservation of these sequences in all T. foetus isolates studied so far (10). The sequence information was exploited for the delineation of Tritrichomonas-specific primers TFR3 and TFR4. As extrapolated from the sequencing data, genomic DNAs from all T. foetus isolates analyzed in this study yielded amplification products of the expected size of 347 bp by PCR with these primers. Furthermore, an amplification product of the same size was obtained with genomic DNAs from two other Tritrichomonas species analyzed: T. mobilensis and T. suis. T. mobilensis has been described as an intestinal parasite of the squirrel monkey (6, 24) and is thus of lesser importance in a differential diagnosis. T. suis, however, is a parasite of swine, with the nasal cavity and the digestive tract being sites for which the organism has a predilection (14). T. foetus and T. suis displayed sequence identities in their rRNA gene units (10). Furthermore, using a recently described PCR test for the detection of bovine tritrichomonosis (13), we obtained identical PCR products with genomic DNAs of T. suis and T. foetus (our unpublished observations). Consequently, this test also could not discriminate between the two species. These observations thus supported the hypothesis, already formulated by others (12, 14, 17, 22), that the tritrichomonads from cattle and swine represent only strains or variants of the same species, characterized by differences in host range and potentially pathogenicity (10). This hypothesis definitely deserves to be further tested via appropriate experiments with more isolates. Nevertheless, we may postulate that discrimination between T. suis and T. foetus by our novel PCR test is only of secondary importance.
The degrees of homology of the sequences of the 5.8S rRNA gene and ITS1 and ITS2 of Tritrichomonas spp. to the sequences of other trichomonadid protozoa that have been analyzed are only limited (10), and primers TFR3 and TFR4 were delineated from regions displaying differences. Consistently, no amplification by PCR was observed in our study with genomic DNAs from T. vaginalis, T. gallinae, T. tenax, P. hominis, and T. gallinarum. Also, no unspecific amplification was observed by using genomic DNA from bacteria and bovine DNA as templates. We thus conclude that the specificity of our PCR test is very high. The main objective of the present study was to establish a PCR for the molecular biology-based identification of T. foetus-like organisms morphologically detected by in vitro cultivation. Commercially available tests such as the InPouchTF test represent a useful primary diagnostic tool, and the test can be performed relatively easily. Nevertheless, not all isolates exhibit the same growth kinetics, and the actual identification of the parasite thus becomes very difficult when only a few organisms can be seen in the culture medium and when these organisms, for any reason, do not propagate as expected. As little as 0.03 pg of purified T. foetus DNA could be detected with a high degree of sensitivity by our test; thus, we were able to detect as little as a single parasite per PCR in repeated experiments. Consequently, by using in vitro-propagated material as a sample, the combined sensitivity of the tests becomes extremely high.
The second objective of the study was to assess the PCR as a direct diagnostic screening test. With preputial washings and cervicovaginal secrections, however, the sensitivity was limited to the detection of about 50 parasites per ml of sample, as we found using samples spiked with defined numbers of parasites. This decrease in sensitivity was related to the presence of inhibitory substances which could not be removed, e.g., by the alternative application of a commercially available PCR template preparation kit, thus emphasizing the usefulness of the artificial control template fragment for the recognition of potentially false-negative results due to inhibition of the PCR. Nevertheless, the overall sensitivity with preputial washings and cervicovaginal secretions exhibited, in our opinion, diagnostic operating characteristics sufficient to justify further assessment of the PCR for the application as a direct screening test. A problem frequently encountered with PCR-based diagnostic tests is the presence of unspecific amplification products. For example, by another diagnostic PCR for bovine tritrichomonosis (13), unspecific amplicons very similar in size to the size of the specific amplicon were obtained for about one-third of the samples (16). Thus, the clear discrimination between positive and negative results was very difficult in some cases, depending upon the composition of the test samples (16). On very rare occasions, such unspecific products of similar size were also observed by our test (e.g., Fig. 5A, lane 8), but the results were clarified by the subsequent DEIA. This DEIA introduces another step that can be used to increase specificity, because TFR8-Bio also represents Tritrichomonas-specific sequences. Therefore, the DEIA with TFR8-Bio is highly suitable as a further confirmation test for samples with unclear or doubtful results when the presence of additional amplification bands that are occasionally observed may complicate the interpretation of the results of analysis by gel electrophoresis. The confirmation of PCR results by DEIA with oligonucleotide TFR8-Bio also has the advantage that it can be applied in a 96-well format in a microplate assay by basically the same methodology used for enzyme-linked immunosorbent assay, which is well established in many diagnostic laboratories. The opportunity to test many samples in parallel could offer a tool for large-scale epidemiological surveys. Furthermore, the UDG system represents an important and helpful tool for technologically improving the performance of routine PCR, in that it prevents carryover contamination with products of previous amplification reactions (19).
We demonstrated the applicability of our test procedure by performing routine diagnostic tests with cervicovaginal secretions from cows and preputial washings from bulls. A considerable number of the samples were contaminated to different extents with bacteria and/or fungi, which hampered the microscopic examination directly after sampling and following in vitro cultivation. However, inhibition of the PCR, detected with the internal control fragment, was observed for only 1.5% of the samples from bulls, i.e., 2 of a total of 130 preputial washings. Because only a few template molecules of the internal control fragment were added to test samples and because very stringent conditions were used, we can presume that the two inhibited samples were also negative. Ninety mucosal samples from cows could be analyzed without problems; only one sample inhibited the PCR. For the seven samples from cows reacting positively in the PCR, no parasite growth was observed in the InPouchTF test, thus pointing toward a potentially higher diagnostic sensitivity of the PCR compared to that of in vitro cultivation. In conclusion, the molecular biology-based methods (PCR, the UDG system, the use of an internal inhibition control, and DEIA) proposed in the present study exhibit several methodological advantages compared to conventional morphological diagnosis of T. foetus infection. However, overall improvement of diagnosis should be confirmed by future studies with larger numbers of diagnostic samples from geographic areas with a higher prevalence of T. foetus infection. In many countries, bovine tritrichomonosis still represents a substantial problem causing relevant economic losses. Other countries, e.g., Switzerland, have already achieved effective control but nevertheless must be well prepared for the eventual reemergence of the parasite and an increasing incidence of infections in the future. In both situations, accurate diagnostic techniques such as the PCR methods presented here are indispensable for the effective control and monitoring of T. foetus.
ACKNOWLEDGMENTS
This work was supported by a grant from the Swiss Federal Veterinary Office (BVET; grant 012.2.94.5) and by the Swiss Association for Artificial Insemination.
We are indebted to Bernadette Connolly for critical reading of the manuscript, Urs Küpfer for providing some of the diagnostic samples, and Carol Suter and Verena Zimmermann for technical assistance.
REFERENCES
- 1.Appell L H, Mickelsen W D, Thomas M W, Harmon W M. A comparison of techniques used for the diagnosis of Tritrichomonas foetus infections in beef bulls. Agri Pract. 1993;14:30–34. [Google Scholar]
- 2.BonDurant R H, Anderson M L, Blanchard P, Hird D, Danaye-Elmi C, Palmer C, Sischo W M, Suther D, Utterback W, Weigler B J. Prevalence of trichomoniasis among California beef herds. J Am Vet Med Assoc. 1990;196:1590–1593. [PubMed] [Google Scholar]
- 3.Borchardt K A, Norman B B, Thomas M W, Harmon W M. Evaluation of a new culture method for diagnosing Tritrichomonas foetus infection. Vet Med. 1992;1992:104–112. [Google Scholar]
- 4.Chakrabarti D, Dame J B, Gutell R R, Yowell C A. Characterization of the rDNA unit and sequence analysis of the small subunit rRNA and 5.8S rRNA genes from Tritrichomonas foetus. Mol Biochem Parasitol. 1992;52:75–84. doi: 10.1016/0166-6851(92)90037-k. [DOI] [PubMed] [Google Scholar]
- 5.Corbeil L B. Vaccination strategies against Tritrichomonas foetus. Parasitol Today. 1994;10:103–106. doi: 10.1016/0169-4758(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 6.Culberson D E, Pindak F F, Gardner W A, Honigberg B M. Tritrichomonas mobilensis n. sp. (Zoomastigophorea: Trichomonadida) from the Bolivian squirrel monkey Saimiri boliviensis boliviensis. J Protozool. 1986;33:301–304. doi: 10.1111/j.1550-7408.1986.tb05611.x. [DOI] [PubMed] [Google Scholar]
- 7.Dennett D P, Reece R L, Barasa J O, Johnson R H. Observations in the incidence and distribution of serotypes of Tritrichomonas foetus in beef cattle in north-eastern Australia. Aust Vet J. 1974;50:427–431. doi: 10.1111/j.1751-0813.1974.tb06863.x. [DOI] [PubMed] [Google Scholar]
- 8.Diamond L S. The establishment of various trichomonads of animal and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- 9.Eckert J, Kutzer E, Rommel M, Bürger H-J, Körting W. Veterinärmedizinische parasitologie. 4th ed. Berlin, Germany: Verlag Paul Parey; 1992. [Google Scholar]
- 10.Felleisen R. Comparative sequence analysis of 5.8S rRNA genes and internal transcribed spacer (ITS) regions of trichomonadid protozoa. Parasitology. 1997;115:111–119. doi: 10.1017/s0031182097001212. [DOI] [PubMed] [Google Scholar]
- 11.Felleisen R, Müller N, Yamage M, Gottstein B. Diagnostische PCR in der Veterinärparasitologie: Tritrichomonose, Neosporose/Toxoplasmose, Echinokokkose/Zystizerkose. Schweiz Arch Tierheilkd. 1996;138:144–151. [PubMed] [Google Scholar]
- 12.Hibler C P, Hammond D M, Caskey F H, Johnson A E, Fitzgerald P R. The morphology and incidence of the trichomonads of swine, Tritrichomonas suis (Gruby & Delafond), Tritrichomonas rotunda, n. sp. and Trichomonas buttreyi, n. sp. J Protozool. 1960;7:159–171. [Google Scholar]
- 13.Ho M S Y, Conrad P A, Conrad P J, LeFebvre R B, Perez E, BonDurant R H. Detection of bovine trichomoniasis with a specific DNA probe and PCR amplification system. J Clin Microbiol. 1994;32:98–104. doi: 10.1128/jcm.32.1.98-104.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honigberg B M. Parasitic protozoa. II. Intestinal flagellates, histomonads, trichomonads, amoeba, opalinids, and ciliates. New York, N.Y: Academic Press, Inc.; 1978. [Google Scholar]
- 15.Kvasnicka W G, Taylor R E L, Huang J-C, Hanks D, Tronstad R J, Bosomworth A, Hall M R. Investigations of the incidence of bovine trichomoniasis in Nevada and of the efficacy of immunizing cattle with vaccines containing Tritrichomonas foetus. Theriogenology. 1989;31:963–971. doi: 10.1016/0093-691x(89)90479-2. [DOI] [PubMed] [Google Scholar]
- 16.Lambelet N. Tritrichomonas foetus: Etablierung und Validierung molekularer und immunologischer Methoden zur Identifizierung und Charakterisierung des Parasiten. Ph.D. thesis. Bern, Switzerland: Faculty of Veterinary Medicine, University of Bern; 1997. [Google Scholar]
- 17.Levine N D. Protozoan parasites of domestic animals and of man. 2nd ed. Minneapolis, Minn: Burgess Publishing Company; 1973. [Google Scholar]
- 18.Levine N D. Veterinary protozoology. 1st ed. Ames: The Iowa State University Press; 1985. [Google Scholar]
- 19.Longo M C, Beringer M S, Hartley J L. Use of uracil DNA glycosylase to control carry-over contaminations in polymerase chain reaction. Gene. 1990;93:125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- 20.Martín Gómez S, González Paniello R, Pereira Bueno J, Ortega Mora L M. Prevalence of Tritrichomonas foetus infection in beef bulls in Northwestern Spain. Parassitologia. 1996;38:295. doi: 10.1016/s0304-4017(97)00189-1. [DOI] [PubMed] [Google Scholar]
- 21.Müller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol. 1996;34:2850–2852. doi: 10.1128/jcm.34.11.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pakandl M, Grubhofer L. Some properties of sialic-acid binding systems in Tritrichomonas suis and Tritrichomonas foetus. Comp Biochem Physiol B Biochem Mol Biol. 1994;108:529–536. doi: 10.1016/0305-0491(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 23.Perez E, Conrad P A, Hird D, Ortuno A, Chacon J, BonDurant R, Noordhuizen J. Prevalence and risk factors for Tritrichomonas foetus infection in cattle in northeastern Costa Rica. Prev Vet Med. 1992;14:155–165. [Google Scholar]
- 24.Pindak F F, Pindak M M, Abee C R, Gardner W A. Detection and cultivation of intestinal trichomonads of squirrel monkeys (Saimiri sciureus) Am J Primatol. 1985;9:197–205. doi: 10.1002/ajp.1350090305. [DOI] [PubMed] [Google Scholar]
- 25.Riley D E, Wagner B, Polley L, Krieger J N. PCR-based study of conserved and variable DNA sequences of Tritrichomonas foetus isolates from Saskatchewan, Canada. J Clin Microbiol. 1995;33:1308–1313. doi: 10.1128/jcm.33.5.1308-1313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Schönmann M J, BonDurant R H, Gardner I A, Van Hoosear K, Baltzer W, Kachulis C. Comparison of sampling and culture methods for the diagnosis of Tritrichomonas foetus infection in bulls. Vet Rec. 1994;134:620–622. doi: 10.1136/vr.134.24.620. [DOI] [PubMed] [Google Scholar]
- 28.Speer C A, White M W. Bovine trichomoniasis. Large Anim Vet. 1991;46:18–20. [Google Scholar]
- 29.Taylor M A, Marshall R N, Stack M. Morphological differentiation of Tritrichomonas foetus from other protozoa of the bovine reproductive tract. Br Vet J. 1994;150:73–80. doi: 10.1016/S0007-1935(05)80098-3. [DOI] [PubMed] [Google Scholar]
- 30.Thomas M W, Harmon W M, White C. An improved method for the detection of Tritrichomonas foetus infection by culture in bulls. Agric Pract. 1990;11:13–17. [Google Scholar]