Abstract
To understand the biological roles of Pediococcus pentosaceus strains as probiotics isolated from the traditional Korean fermented food, Jangajji, Pediococcus pentosaceus was selected based on its high cinnamoyl esterase (CE) and antioxidant activities. The acid and bile stability, intestinal adhesion, antagonistic activity against human pathogens, cholesterol-lowering effects, and immune system stimulation without inflammatory effects were evaluated. Nitric oxide (NO) levels were measured in co-culture with various bacterial stimulants. Fermentation ability was measured by using a broccoli matrix and the sulforaphane levels were measured. Resistance to acidic and bilious conditions and 8% adherence to Caco-2 cells were observed. Cholesterol levels were lowered by 51% by assimilation. Moreover, these strains exhibited immunomodulatory properties with induction of macrophage TNF-α and IL-6 and had microstatic effects on various pathogens. Co-culture with various bacterial stimulants resulted in increased NO production. Fermentation activity was increased with the strains, and higher sulforaphane levels were observed. Therefore, in the future, the applicability of the selected strain to broccoli matrix-based fermented functional foods should be confirmed.
Keywords: Jangajji, lactic acid bacteria, cinnamoyl esterase, probiotic, immunomodulatory, broccoli fermentation
1. Introduction
Pediococcus pentosaceus, a member of the genus Latilactobacillus (phylum Firmicutes, class Bacilli) and a representative homofermentative Gram-positive strain, is an emerging probiotic candidate. It has attracted much attention due to pediocin that can be used in food bio-preservation as well as its anti-inflammation [1,2], anticancer [3,4,5], antioxidant [6,7,8], detoxification [9,10], and lipid-lowering abilities [11,12]. To date, many strains have been isolated from fermented foods [13,14], animals [15], plants [16], and fecal sources [17]; however, there is still insufficient information and a lack of knowledge regarding their probiotic and fermentative properties. Although P. pentosaceus and its pediocin are generally considered safe for use in foods (due to their ‘generally recognized as safe (GRAS) status), it is essential to evaluate their probiotic safety aspects in the human intestine, such as antimicrobial resistance and toxicological safety, through cytotoxicity assays [18,19].
Broccoli (Brassica oleracea L. var. Italica Plenk) is a vegetable belonging to the genus Brassica (family Brassicaceae or Cruciferae) and includes cauliflower, Brussels sprouts, kohlrabi, cabbage, and mustard [20]. It is an important source of various nutrients, including dietary fiber, vitamins, minerals, and phytochemicals such as glucosinolates and phenolic compounds. Recently, interest in broccoli and its bioactive compounds has increased, as many studies have demonstrated the beneficial effects of broccoli glucosinolates and their breakdown products, particularly sulforaphane, on antioxidant, anti-inflammatory, anti-cancer, metabolic syndrome, and neuroprotective properties [21]. The most abundant glucosinolate in broccoli is glucoraphanin [22], which can be hydrolyzed by the endogenous enzyme myrosinase when broccoli is cut or chewed to form isothiocyanate, sulforaphane, a potent anti-cancer substance that works by increasing the level of enzymes in the liver [23]. Based on research that improved the yield and stability of sulforaphane in broccoli and increased the potential health benefits by improving the antioxidant ability through Lactobacillus fermentation [24], broccoli was selected as a raw material for examining the effectiveness of the selected P. pentosaceus strain, because it is likely to be a functional fermented food preparation containing various antioxidants and anticancer substances.
The aim of this study was to evaluate the probiotic properties of an isolate of P. pentosaceus JBCC 106 originating from the traditional Korean fermented food, Jangajji, and its viability and functionality in broccoli juice.
2. Materials and Methods
2.1. Lactic Acid Bacteria Strain Isolated from Jangajji
Lactic acid bacteria (LAB) were isolated from the traditional Korean fermented pickle, Jangajji, a high caffeic acid producer, by Baik et al. (2022, patent no. KACC92458P). The strains were routinely prepared in MRS broth (Difco Laboratories, Detroit, MI, USA) and grown at 37 °C for 24 h. L. rhamnosus GG (ATCC 53103, LGG), a representative probiotic strain, was used as the reference control to evaluate the probiotic properties of the isolated strains.
2.2. Identification of the Isolated Strains
16S rDNA sequencing was performed as described previously [25]. Sequence homology analysis was performed by comparing the obtained sequences with LAB sequences in the DNA database (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 13 April 2022).
2.3. CE Activity
CE activity was measured quantitatively and qualitatively using thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) as previously described [26]. LAB strains (1%, v/v) were inoculated with 5 mL of MRS broth and incubated at 37 °C for 72 h in the presence of a 0.5% (w/v) chlorogenic acid (Sigma, St. Louis, MO, USA). Subsequently, 0.1% (w/v) ascorbic acid was added to the culture and extracted with 2.5 mL ethyl acetate. TLC was performed on silica gel 60 F254 plates (Merck, Darmstadt, Germany) using toluene/ethyl acetate/formic acid (5:4:1, v/v/v) as the solvent system, as described by Kim et al. [26]. HPLC analysis was performed on an Accela (Thermo Scientific, Waltham, MA, USA) equipped with an Inertsil ODS-3 V column (150 × 4.0 mm i.d., 5 m). The mobile phase was 0.5% formic acid and acetonitrile (v/v, 75:25) at a flow rate of 1.0 mL for 10 min at 40 °C. Each sample was analyzed in triplicate.
2.4. Antioxidant Activity
Antioxidant activity was measured by 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging activity, and superoxide dismutase (SOD) activity as follows. ABTS radical scavenging activity was determined as described by Lee et al. [27], with some modifications. The ABTS solution (1 mL) was mixed with 3 mL LAB samples and reacted for six minutes. After the reaction, absorbance was measured at 734 nm. The DPPH radical-scavenging activity of the LAB was determined according to the method described by Li et al. [28]. The SOD activity of the LAB was measured spectrophotometrically according to the method described by Song et al. [29] with some modifications. Briefly, the culture supernatant of LABs was mixed with reaction solution (0.5 mM, Tris-HCl buffer, pH 8.2, and 7.2 mM pyrogallol) at the ratio of 1:3:0.2 and incubated for 10 min at 25 °C. After incubation, the reaction was terminated by adding 1 N HCl (0.5 mL) and the absorbance was measured at 420 nm.
2.5. Probiotic Properties
2.5.1. Acid Resistance and Bile Tolerance
The acid resistance of the selected LAB was determined according to the method described by Liong and Shah [30] with some modifications. After cultivation in MRS broth at 37 °C, the recovered cell was resuspended in an equal volume of MRS broth (final pH 1.5 and 2.0) and incubated at 37 °C for 2 h. For bile tolerance, 100 μL culture was dropped onto MRS agar plates containing 0%, 0.5%, 1%, and 3% (w/v) of bile (Sigma Chemical Co., St. Louis, MO, USA), and incubated at 37 °C for 24–48 h [31]. Acid and bile tolerance were determined based on the survival rate. The survival rate was calculated according to the following:
| (1) |
A1: Total viable count of probiotic strains after treatment, A0: Total viable count of probiotic strains before treatment.
2.5.2. Bile Salt Hydrolase (BSH) Activity
Sterile discs were placed on a BSH screening medium, and 100 μL of the selected LAB strain culture was added. BSH screening medium was prepared using MRS agar supplemented with 0.5% (w/v) sodium salt, taurodeoxycholic acid (Sigma Aldrich, St Louis, MO, USA), and 0.37 g/L of CaCl2 [32].
2.5.3. Antimicrobial Activity
Antimicrobial activity was determined using a paper disc according to the method described by Chiu et al. [33] with minor modifications. A total of 50 μL of supernatants of LAB strain supernatant was dropped onto the discs on nutrient broth agar plates. Each indicator pathogenic strain was grown in nutrient broth at 37 °C for 24 h and sterile 8 mm paper discs with LABs were placed on the agar. After the plates were incubated for 24 h at 37 °C, the diameter of the clear zone was measured.
2.5.4. Antibiotic Resistance
Ampicillin and vancomycin as inhibitors of cell wall synthesis and kanamycin and chloramphenicol as inhibitors of protein synthesis (Sigma) were used. All antibiotic powders were dissolved in appropriate diluents and filter-sterilized before addition to MRS medium. To test antibiotic resistance, LABs were inoculated (1%, v/v) in MRS broth supplemented with antibiotics at various final concentrations (from 2 to 1024 mg/mL) and measured the growth at 580 nm after 24 h incubation at 37 °C. The MIC was defined as the lowest concentration of antibiotics that completely inhibited visible growth in comparison with an antibiotic-free control well.
2.5.5. Cholesterol Assimilation
The ability of lactobacilli to assimilate cholesterol was measured according to the methods described by Ren et al. [34] and Liong et al. [30]. Cholesterol assimilation was calculated using the following equation:
| (2) |
A1: Cholesterol presents MRS at time 0 h, A0: Cholesterol presents MRS at time 24 h.
2.5.6. Bacterial Adhesion Capacity
Cell Surface Hydrophobicity
Bacterial adhesion to hydrocarbons was determined following the method of Kaushik et al. [35] Surface hydrophobicity was calculated as the percentage decrease in the absorbance of the aqueous phase after mixing and phase separation relative to that of the original suspension (AbsInitial) using the following equation:
| (3) |
Cell Aggregation
The auto-aggregation assay was performed according to the method described by Kaushik et al. [35]. The percentage difference between the initial and final absorbances provides an index of cellular auto-aggregation, which can be expressed by the following equation:
| (4) |
| (5) |
Probiotic Adhesion to Caco-2 Cells
An in vitro adhesion assay was performed using the method described by Ren et al. [34]. Adhesion to Caco-2 cells was calculated using the following equation:
| (6) |
| (7) |
2.6. Cell Cytotoxicity
Cytotoxicity was assessed using the methylthiazolyltetrazolium (MTT) assay, as described by Levy and Simon [36]. RAW 264.7 cells were seeded at a density of 5 × 104 CFU/mL in 96-well plates (Nunc, Roskilde, Denmark) for 24 h. Absorbance at 450 nm was measured using a microplate spectrophotometer. The results were expressed as absorbance values measured at 450 nm with the respective controls.
2.7. NO and Cytokine (TNF-α and IL-6) Measurement
NO production was determined based on the level of nitrite formed in the supernatant of the cultured RAW 264.7 cells. The cells were seeded at 5 × 104 CFU/mL on 96-well culture plates for 2 h. Either viable or heat-inactivated bacteria at representing bacteria (100 μL) were added to the wells. After 24 h incubation (37 °C, 5% CO2), the supernatant was collected and NO and cytokines were measured. NO production was measured using the Griess reaction [37].
2.8. Lactic Acid Fermentation on Broccoli Juice
Broccoli (Brassica oleracea var. italica) used in this experiment was purchased from a local market in Jeonju, Korea. Broccoli juice was prepared as described by Xu et al. [38] with several modifications. After washing the broccoli with distilled water, the leaves, and stalks were removed, and the florets were homogenized in sterile distilled water at a ratio of 1:2 (broccoli/distilled water, g/mL). The prepared broccoli juice was filtered through a cloth to separate the solid components, transferred to an Erlenmeyer flask, and pasteurized at 60 °C for 5 min. P. pentosaceus JBCC 106 was pre-cultured in MRS broth medium at 37 °C for 24 h and inoculated into the broccoli juice and fermented at 37 °C for 36 h. During fermentation, 30 mL samples were collected at 0, 3, 6, 12, 24, and 36 h and stored at −20 °C until analysis. The pH of the stored samples was determined using a pH meter (Starter3100, Ohaus, Parsippany, NS, USA) and viable cell count was measured by dispensing each sample diluted in sterilized distilled water onto an MRS agar plate and incubating at 37 °C for 24 h. All experiments were conducted in triplicate.
2.9. Analysis of Sulforaphane from Broccoli Juice
Sulforaphane was extracted from fermented broccoli samples as described by Ghawi et al. [39] and Abukhabta et al. [40] with some modifications. A total of 2 mL of the sample was placed in a tube and incubated for 5 h at 30 °C to ensure complete hydrolysis of glucosinolates by myrosinase. Samples were then centrifuged at 13,000× g for 10 min, the pellet was dissolved in 2 mL of distilled water, and the previous process was repeated. To extract sulforaphane from the supernatant, 10 mL of dichloromethane was added to the collected supernatant in a conical tube, vortexed for 15 min, and centrifuged at 13,000× g for 10 min. The organic phase was then collected, and 10 mL of dichloromethane was added to the pellet for re-extraction. The collected organic phase was concentrated using a rotary evaporator (EYELA N-1110, Tokyo, Japan) and suspended in 6 mL of acetonitrile to prepare a sample for HPLC analysis.
Sulforaphane levels were analyzed by HPLC (Thermo Fisher Scientific Inc., Waltham, MA, USA), which consisted of an accela 600 pump (San Jose, CA, USA) equipped with an accela PDA 80 hz detector (San Jose, CA, USA) using an Exsil 100 ODS column (250 × 4.6 mm, 5 µm; Exmere LTD., Lancashire, UK). The sample was filtered through a 0.45 μm membrane before analysis. The mobile phase was acetonitrile/water (30:70) at a flow rate of 0.6 mL/min and the injection volume was 10 µL.
2.10. Statistical Analysis
All experiments were performed in triplicate. The error bars in the graphs indicate standard deviation. Data were analyzed using analysis of variance (ANOVA) and Student’s t-test. Differences were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Bacterial Strains
The LAB used in this study were isolated from five traditional Korean fermented foods, Jangajji and were identified as P. pentosaceus JBCC 106 using full-length 16S rDNA sequences. (GenBank accession number: NR_042058; Figure S1).
3.2. Physicochemical Properties of P. pentosaceus JBCC 106
When this isolate was examined for in vitro CE activity using chlorogenic acid as substrate, CA was produced up to 30 µM with a conversion ratio of 16.4% (Table 1), which was slightly lower than that of previous LAB strains [41]. To the best of our knowledge, there have been few studies on P. pentosaceus related to CE activity. The antioxidant capacity of the isolated strain was explained through SOD activity, ABTS, and DPPH radical scavenging activities, which were 39%, 41%, and 45%, respectively, which had better antioxidant abilities than strains isolated from Jangajji and Jeotgal in other studies [42].
Table 1.
Identification and physicochemical properties of strains isolated from the traditional Korean fermented food, Jangajji.
| LAB Isolates | 16s rDNA Analysis | CE Activity | Antioxidant Activities (%) | ||||
|---|---|---|---|---|---|---|---|
| Strains | Homology (%) | Generated CA (mM) | Conversion Rate (%) | ABTS | DPPH | SOD | |
| JBCC 106 |
Pediococcus
pentosaceus |
99 | 0.03 | 16.39 | 41.1 ± 0.09 | 45.5 ± 0.18 | ±0.01 |
3.3. Probiotic Properties of P. pentosaceus JBCC 106
3.3.1. Resistance in Artificial Gastrointestinal Conditions
As shown in Table 2, P. pentosaceus JBCC 106 exhibited moderate survival of 79.4% at pH 3.0, which was lower survivability than L. rhamnosus GG, but showed higher acid tolerance at pH 2.0. P. pentosaceus JBCC 106 was tolerant to even 3% (w/v) oxgall, as evidenced by survival rates greater than 97% even after 24 h exposure (Table 2). However, P. pentosaceus JBCC 106 showed moderate BSH activity but a good survival rate at a reduced pH of 1.5. Bile salt tolerance is closely related to both BSH and osmotic stress tolerance. Because BSH activity is not common among bacteria isolated in non-bilious environments, such as cheese or fermented vegetables [43,44,45], it is not surprising that most of the isolates showed low BSH activity. We measured BSH activity in strains treated with simulated gastric juice for 2 h because LAB is in a state of acid stress that must resist exposure to bile secreted into the small intestine. Although it is not clear whether BSH activity is a desirable trait for the selection of promising probiotic strains, our results suggest that this activity could maximize the intestinal survival of P. pentosaceus JBCC 106, increasing their overall beneficial effects related to P. pentosaceus JBCC 106 [46].
Table 2.
Basic probiotic properties of the strain isolated from Jangajji and comparison with the control strain, L. rhamnosus GG.
| Probiotic Properties | Strains | ||
|---|---|---|---|
| L. rhamnosus GG | P. pentosaceus JBCC 106 | ||
| Acid tolerance (Survival rate, %) |
pH 3 | 95.4 | 79.4 |
| pH 2 | 29.9 | 39.9 | |
| pH 1.5 | 38.6 | 53.3 | |
| Bile salt tolerance (Survival rate, %) |
0.5% bile salt | 97.7 | 98 |
| 1% bile salt | 94.9 | 97.5 | |
| 3% bile salt | 88.7 | 99.5 | |
| Pathogens a | S. aureus | ++ | ++++ |
| S. epidermidis | ++ | ++ | |
| P. aeruginosa | ++ | ++ | |
| Pro. acnes | - | + | |
| P. putida | ++ | ± | |
| S. xylosus | +++++ | ++++ | |
| E. coli | ++ | - | |
| B. cereus | +++ | + | |
| B. vallismortis | ++ | ++ | |
| MICs b (μg/mL) |
Ampicillin (A) | <2 | <2 |
| Chloramphenicol (C) | <2 | 4 | |
| Vancomycin (V) | 64 | 64 | |
| Kanamycin (K) | ≥1024 R | 64 | |
a Antimicrobial activity (zone size): - not clear zone; ± 0–1 mm; + 1–2 mm; ++ 2–3 mm; +++ 3–4 mm; ++++ 4–5 mm; +++++ >5 mm. b MIC: minimum inhibitory concentration. R Resistant according to the EFSA’s breakpoints (EFSA, 2012).
3.3.2. Antimicrobial Activity and Antibiotic Susceptibility of P. pentosaceus JBCC 106
As shown in Table 2, P. pentosaceus JBCC 106 exhibited diverse antimicrobial activities against different Gram-positive and Gram-negative pathogens, such as S. aureus, S. epidermidis, and S. xylosus, and Gram-negative strains of P. aeruginosa, P. putida, Pro. acnes, B. cereus, and B. vallismortis, except Escherichia coli. P. pentosaceus has played an increasingly pivotal role in the LAB industry in recent years. Many strains of P. pentosaceus have been isolated from various sources, such as fermented food, aquatic products, raw animal meats, plant products, and feces. Additionally, links to the human gastrointestinal tract have finally been proven. This is in good agreement with P. pentosaceus, which generally produces a variety of functional compounds such as pediocin, exopolysaccharides, bacteriocin-like inhibitory substances (BLISs), and 3-phenyllactic acid, resulting in broad-spectrum antibacterial properties against a variety of food-spoiling bacteria and fungi. We also determined the MIC, which is the lowest antibiotic concentration that can inhibit bacterial growth [47]. MICs ≥ 8 μg/mL are considered “moderately resistant”; above 32 μg/mL is considered “clinically resistant” [48]. As shown in Table 2, P. pentosaceus JBCC 106 was resistant to both vancomycin and kanamycin with a significantly high MIC of 64 μg/mL. According to EFSA (2012), the resistance to vancomycin and kanamycin of P. pentosaceus strains and even L. rhamnosus GG as a control can be considered native non-transferable to other species because of embedded chromosomal genetic features which lack transportable properties [49].
3.3.3. Cholesterol-Lowering Effect of P. pentosaceus JBCC 106
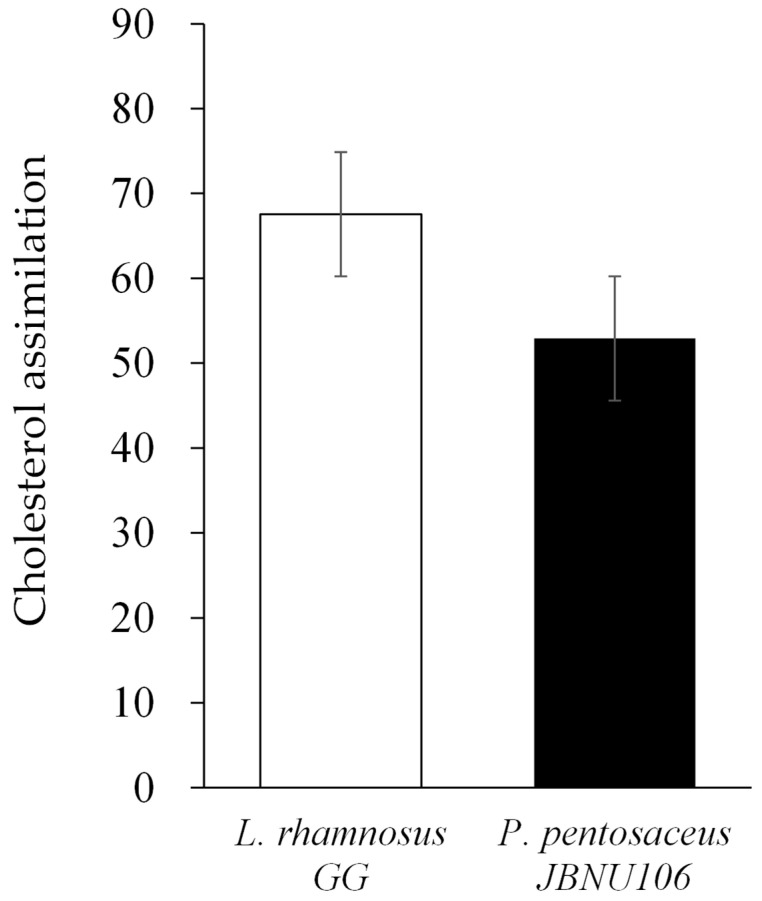
As shown in Figure 1, P. pentosaceus JBCC 106 showed no significant difference in cholesterol-lowering effects compared to the control strain L. rhamnosus GG (p > 0.05). It has been known that the presence of BSH activity in a probiotic strain has always been linked to cholesterol-lowering potential and crucial indicator for the selection of probiotic strain adjuncts to manage hypercholesterolemia. The presence of BSH in probiotics renders them more tolerant to bile salts, which helps reduce host blood cholesterol levels [50].
Figure 1.
Comparison of the cholesterol-lowering ability between the strain isolated from Jangajji and the control strain, L. rhamnosus GG.
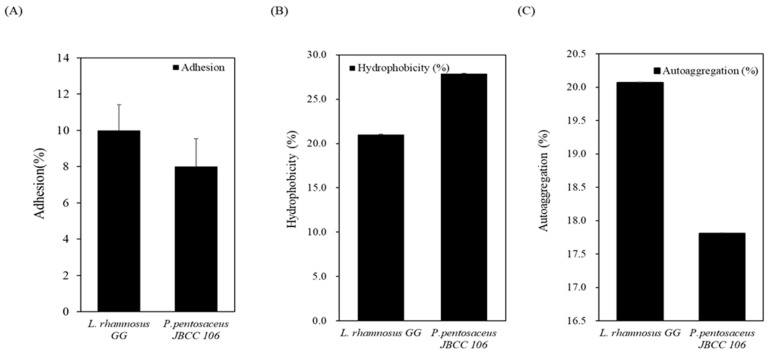
3.3.4. Cell Surface Hydrophobicity, Auto-Aggregation, and Bacterial Adhesion to Caco-2 Cells of P. pentosaceus JBCC 106
Mucosal surface adhesion ability is another commonly encountered criterion for probiotic strain selection because it is directly related to colonization and persistence in the GI tract [51]. As shown in Figure 2A, heat-inactivated P. pentosaceus JBCC 106 cells showed an adhesion percentage of 8%, which was not significantly different from that of the reference probiotic strain of L. rhamnosus GG (p > 0.05). P. pentosaceus JBCC 106 exhibited a good hydrophobic cell surface, with a high percentage of 28%, but a low auto-aggregation rate of 18% (Figure 2B). P. pentosaceus JBCC 106 strain had higher hydrophobicity and auto-aggregation rates than the control L. rhamnosus GG.
Figure 2.
Mucosal surface attachment ability of the strain isolated from Jangajji and the control strain, (A) shows bacterial adhesion to Caco-2 cells, and (B) shows cell surface hydrophobicity and (C) shows auto-aggregation.
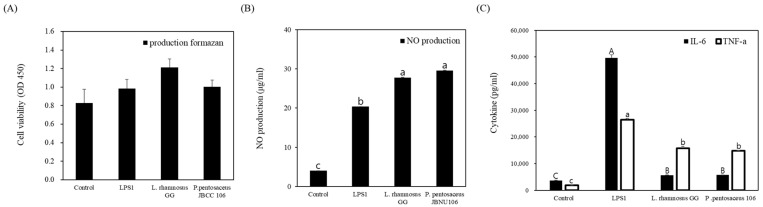
3.4. Immunological Activity of P. pentosaceus JBCC 106
P. pentosaceus JBCC 106 showed no cytotoxic effects on macrophages at up to 8 and 9 when the OD values shown in Figure 3 were converted to log CFU/mL, indicating P. pentosaceus JBCC 106 did not cause cytotoxicity, indicating its toxicological safety for further application as a probiotic. Then, heat-killed whole bacterial cells of the isolated LAB strain were added to RAW 264.7 cell cultures, and NO production was evaluated to examine the anti-inflammatory activity of the LAB isolate in LPS-stimulated RAW 264.7 cells. NO has various biological functions in many types of immune cells, including induction of bactericidal effects in macrophages and signal transduction during inflammation [52]. Additionally, NO is the most important factor in vasodilation, which relaxes the inner muscles of blood vessels, causing them to dilate and increase circulation [53]. As shown in Figure 3B, P. pentosaceus JBCC 106 critically showed increased NO production in the RAW 264.7 cell cultures compared to the LPS induced control, indicating that P. pentosaceus JBCC 106 may be effective as a probiotic. We also examined cytokine induction activity, which contributes to maintaining inflammatory homeostasis in the gut mucosa under normal conditions. As shown in Figure 3C, the production of the pro-inflammatory cytokines TNF-α and IL-6 in macrophages treated with P. pentosaceus JBCC 106 was identical to that of the control strain, L. rhamnosus GG, which showed significantly reduced levels compared to LPS RAW 264.7 cells.
Figure 3.
The immune-related activity of the strain isolated from Jangajji and the control strain. (A) shows the cytotoxic effect of the strain on 264.7 cells, (B,C) show the effect on anti-inflammatory indicators such as nitric oxide and cytokine secretion in 264.7 cells. Data are presented as the means for the three independent replicates (mean ± SD). Values that do not share the same letter are significantly different (p < 0.05).
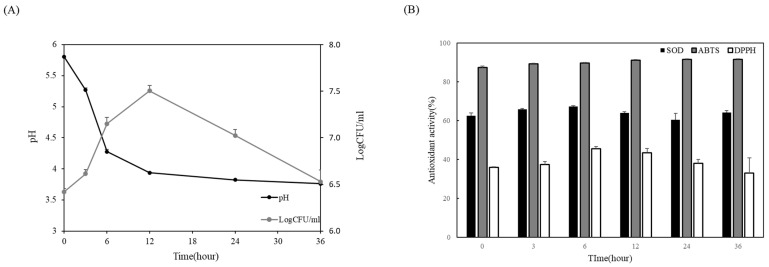
3.5. Fermentation of Broccoli Juice by Pediococcus pentosaceus JBCC 106
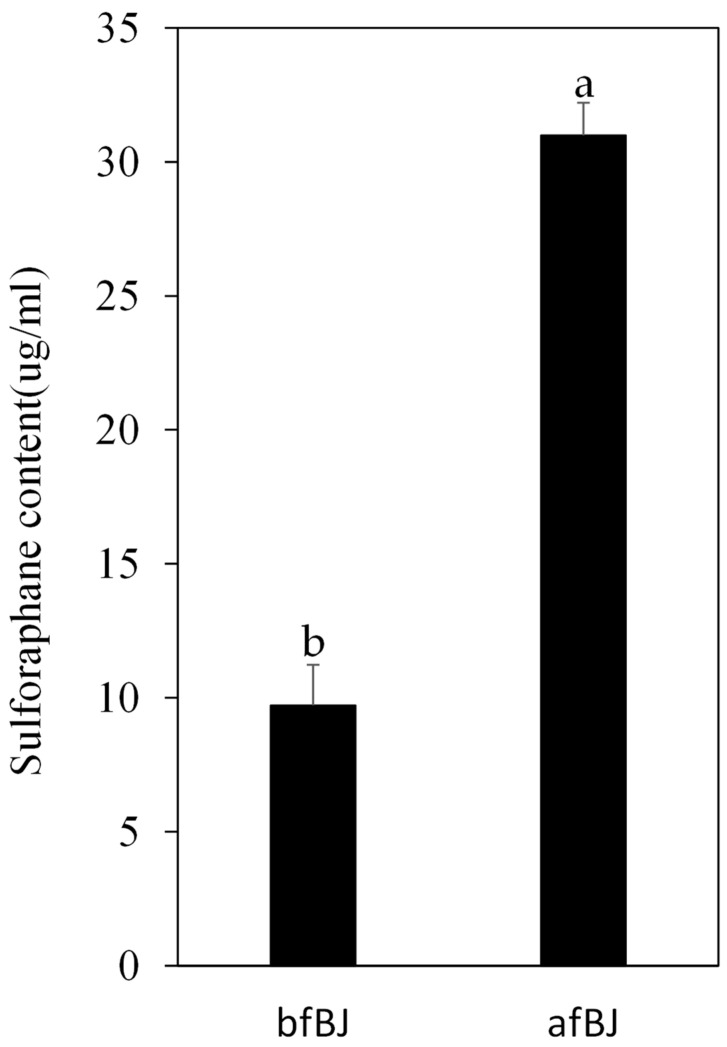
P. pentosaceus JBCC 106 was inoculated into pasteurized broccoli juice and fermented for 36 h to measure the viable cell counts and pH changes to evaluate the fermentation activity in the broccoli matrix. As shown in Figure 4A, P. pentosaceus JBCC 106 grew well from an initial live cell concentration of 6.4 Log CFU/mL to 7.5 Log CFU/mL until 12 h of fermentation and tended to decrease. The pH of the broccoli juice decreased sharply from 5.8 to 3.9 until 12 h, and then gradually decreased to 3.8. During fermentation, the antioxidant activity of the fermented broccoli juice was calculated using SOD, ABTS, and DPPH radical scavenging activities. The SOD activity of broccoli juice increased by about 60% compared to that of raw broccoli juice before fermentation, and the ABTS and DPPH radical scavenging activities increased by about 90% and 40% after fermentation, respectively (Figure 4B). We also examined sulforaphane content change during LAB fermentation on broccoli juice by P. pentosaceus JBCC 106. The sulforaphane content in broccoli juice increased approximately 3.2-fold after LAB fermentation by P. pentosaceus JBCC 106 (Figure 5, 31 g/mL), demonstrating that P. pentosaceus JBCC 106 can enhance antioxidant and sulforaphane contents when fermented as a starter in a broccoli matrix. However, further optimization of the method to enhance sulforaphane content is needed.
Figure 4.
The strain isolated from Jangajji was applied to broccoli juice as a starter for fermentation, (A) shows the growth characteristics of the strain during the fermentation process, and (B) shows the antioxidant activity change of broccoli juice during fermentation time.
Figure 5.
Changes in sulforaphane contents in broccoli juice fermentation using strains isolated from Jangajji as a starter. Different lower-case letters indicate that the values are significantly different (p < 0.05). bfBJ, broccoli juice before fermentation; afBJ, broccoli juice after fermentation.
4. Conclusions
This study addressed the CE activity of P. pentosaceus, evaluated the probiotic potential of P. pentosaceus JBCC106, and evaluated its effect on broccoli fermentation using L. rhamnosus GG as a control. L. rhamnosus GG is a representative probiotic with excellent acid resistance and adhesion, and both proved to have similar probiotic properties. In addition, when the isolated strain was inoculated into a broccoli matrix as a starter and fermented, its applicability to fermentation was confirmed, with an increase to antioxidant activity and sulforaphane contents. These experimental results suggest that P. pentosaceus JBCC 106 is a good candidate for use as a probiotic supplement and has the potential to be applied in various functional fermented foods, including broccoli.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms11081920/s1, Figure S1: Phylogenetic tree of 16S rDNA sequences of JBCC106 isolated from Korean traditional fermented food, Jangajji.
Author Contributions
Conceptualization, S.-H.B.; methodology, N.-E.S.; software, H.J.; validation, S.-H.B., N.-E.S., S.-K.P., and H.J.; formal analysis, S.-K.P.; investigation, N.-E.S.; resources, H.J.; data curation, N.-E.S.; writing-original draft preparation, N.-E.S. and S.-K.P.; writing—review and editing, S.-H.B.; visualization, S.-K.P.; supervision, N.-E.S.; project administration, S.-H.B.; funding acquisition, S.-H.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jonganurakkun B., Wang Q., Xu S.H., Tada Y., Minamida K., Yasokawa D., Sugi M., Hara H., Asano K. Pediococcus pentosaceus NB-17 for probiotic use. J. Biosci. Bioeng. 2008;106:69–73. doi: 10.1263/jbb.106.69. [DOI] [PubMed] [Google Scholar]
- 2.Asami K., Kondo A., Suda Y., Shimoyamada M., Kanauchi M. Neutralization of lipopolysaccharide by heat shock protein in Pediococcus pentosaceus AK-23. J. Food Sci. 2017;82:1657–1663. doi: 10.1111/1750-3841.13679. [DOI] [PubMed] [Google Scholar]
- 3.Thirabunyanon M., Hongwittayakorn P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotechnol. 2013;169:511–525. doi: 10.1007/s12010-012-9995-y. [DOI] [PubMed] [Google Scholar]
- 4.Shukla R., Goyal A. Novel dextran from Pediococcus pentosaceus CRAG3 isolated from fermented cucumber with anti-cancer properties. Int. J. Biol. Macromol. 2013;62:352–357. doi: 10.1016/j.ijbiomac.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Ayyash M., Abu-Jdayil B., Olaimat A., Esposito G., Itsaranuwat P., Osaili T., Obaid R., Kizhakkayil J., Liu S.-Q. Physicochemical, bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41. Carbohydr. Polym. 2020;229:115462. doi: 10.1016/j.carbpol.2019.115462. [DOI] [PubMed] [Google Scholar]
- 6.Ilavenil S., Vijayakumar M., Kim D.H., Valan Arasu M., Park H.S., Ravikumar S., Choi K.C. Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J. Sci. Food Agric. 2016;96:593–601. doi: 10.1002/jsfa.7128. [DOI] [PubMed] [Google Scholar]
- 7.Kuda T., Kawahara M., Nemoto M., Takahashi H., Kimura B. In vitro antioxidant and anti-inflammation properties of lactic acid bacteria isolated from fish intestines and fermented fish from the Sanriku Satoumi region in Japan. Food Res. Int. 2014;64:248–255. doi: 10.1016/j.foodres.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto N., Shoji M., Hoshigami H., Watanabe K., Takatsuzu T., Yasuda S., Igoshi K., Kinoshita H. Antioxidant capacity of soymilk yogurt and exopolysaccharides produced by lactic acid bacteria. Biosci. Microbiota Food Health. 2019;38:97–104. doi: 10.12938/bmfh.18-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le B., Yang S.H. Biosorption of cadmium by potential probiotic Pediococcus pentosaceus using in vitro digestion model. Biotechnol. Appl. Biochem. 2019;66:673–680. doi: 10.1002/bab.1783. [DOI] [PubMed] [Google Scholar]
- 10.Bengmark S. Bio-ecological control of chronic liver disease and encephalopathy. Metab. Brain Dis. 2009;24:223–236. doi: 10.1007/s11011-008-9128-z. [DOI] [PubMed] [Google Scholar]
- 11.Jiang S., Cai L., Lv L., Li L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Factories. 2021;20:45. doi: 10.1186/s12934-021-01537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damodharan K., Lee Y.S., Palaniyandi S.A., Yang S.H., Suh J.-W. Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front. Microbiol. 2015;6:768. doi: 10.3389/fmicb.2015.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin M., Han S., Ryu J., Kim K., Lee W. Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2 isolated from Kimchi. J. Appl. Microbiol. 2008;105:331–339. doi: 10.1111/j.1365-2672.2008.03770.x. [DOI] [PubMed] [Google Scholar]
- 14.Shukla R., Goyal A. Probiotic potential of Pediococcus pentosaceus CRAG3: A new isolate from fermented cucumber. Probiotics Antimicrob. Proteins. 2014;6:11–21. doi: 10.1007/s12602-013-9149-8. [DOI] [PubMed] [Google Scholar]
- 15.Nanasombat S., Treebavonkusol P., Kittisrisopit S., Jaichalad T., Phunpruch S., Kootmas A., Nualsri I. Lactic acid bacteria isolated from raw and fermented pork products: Identification and characterization of catalase-producing Pediococcus pentosaceus. Food Sci. Biotechnol. 2017;26:173–179. doi: 10.1007/s10068-017-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fugaban J.I.I., Vazquez Bucheli J.E., Park Y.J., Suh D.H., Jung E.S., de Melo Franco B.D.G., Ivanova I.V., Holzapfel W.H., Todorov S.D. Antimicrobial properties of Pediococcus acidilactici and Pediococcus pentosaceus isolated from silage. J. Appl. Microbiol. 2022;132:311–330. doi: 10.1111/jam.15205. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Sun X., Zhang J., Gao F., Yu L., Dong L., Zhang G., Wu C. Isolation and characterisation of pulsatilla radix-utilising bacteria Pediococcus pentosaceus PR-1 from human faeces. FEMS Microbiol. Lett. 2022;369:fnac089. doi: 10.1093/femsle/fnac089. [DOI] [PubMed] [Google Scholar]
- 18.Fontana L., Bermudez-Brito M., Plaza-Diaz J., Munoz-Quezada S., Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013;109:S35–S50. doi: 10.1017/S0007114512004011. [DOI] [PubMed] [Google Scholar]
- 19.Saarela M., Mogensen G., Fonden R., Mättö J., Mattila-Sandholm T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000;84:197–215. doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 20.Latté K.P., Appel K.-E., Lampen A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011;49:3287–3309. doi: 10.1016/j.fct.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Xia Y., Liu H.-Y., Guo H., He X.-Q., Liu Y., Wu D.-T., Mai Y.-H., Li H.-B., Zou L. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022;119:288–308. doi: 10.1016/j.tifs.2021.12.015. [DOI] [Google Scholar]
- 22.Sun J., Wang Y., Pang X., Tian S., Hu Q., Li X., Liu J., Wang J., Lu Y. The effect of processing and cooking on glucoraphanin and sulforaphane in brassica vegetables. Food Chem. 2021;360:130007. doi: 10.1016/j.foodchem.2021.130007. [DOI] [PubMed] [Google Scholar]
- 23.Nandini D., Rao R.S., Deepak B., Reddy P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. JOMFP. 2020;24:405. doi: 10.4103/jomfp.JOMFP_126_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y.X., Wang J.H., McAuley C., Augustin M.A., Terefe N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods. 2019;61:103461. doi: 10.1016/j.jff.2019.103461. [DOI] [Google Scholar]
- 25.Song Y.-R., Shin N.-S., Baik S.-H. Physicochemical and functional characteristics of a novel fermented pepper (Capsiccum annuum L.) leaves-based beverage using lactic acid bacteria. Food Sci. Biotechnol. 2014;23:187–194. doi: 10.1007/s10068-014-0025-4. [DOI] [Google Scholar]
- 26.Kim J.-H., Baik S.-H. Preparation and characterization of fermented dandelion (Taraxacum officinale) beverage using Lactobacillus acidophilus F46 having cinnamoyl esterase activity. Food Sci. Biotechnol. 2015;24:583–593. doi: 10.1007/s10068-015-0076-1. [DOI] [Google Scholar]
- 27.Lee S.-J., Bang W.-S., Hong J.-Y., Kwon O.-J., Shin S.-R., Yoon K.-Y. Antioxidant and antimicrobial activities of black Doraji (Platycodon grandiflorum) Korean J. Food Preserv. 2013;20:510–517. doi: 10.11002/kjfp.2013.20.4.510. [DOI] [Google Scholar]
- 28.Li S., Zhao Y., Zhang L., Zhang X., Huang L., Li D., Niu C., Yang Z., Wang Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012;135:1914–1919. doi: 10.1016/j.foodchem.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Song Y.-R., Kim Y.-E., Kim J.-H., Song N.-E., Jeong D.-Y., Baik S.-H. Preparation of fermented sugar-soaked black soybean snacks (FSBSS) and characterization of their quality changes. Food Sci. Biotechnol. 2011;20:1547–1553. doi: 10.1007/s10068-011-0214-3. [DOI] [Google Scholar]
- 30.Liong M., Shah N. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 2005;88:55–66. doi: 10.3168/jds.S0022-0302(05)72662-X. [DOI] [PubMed] [Google Scholar]
- 31.Vinderola C.G., Reinheimer J.A. Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 2003;36:895–904. doi: 10.1016/S0963-9969(03)00098-X. [DOI] [Google Scholar]
- 32.Choi E.A., Chang H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT—Food Sci. Technol. 2015;62:210–217. doi: 10.1016/j.lwt.2015.01.019. [DOI] [Google Scholar]
- 33.Chiu H.H., Tsai C.C., Hsih H.Y., Tsen H.Y. Screening from pickled vegetables the potential probiotic strains of lactic acid bacteria able to inhibit the Salmonella invasion in mice. J. Appl. Microbiol. 2008;104:605–612. doi: 10.1111/j.1365-2672.2007.03573.x. [DOI] [PubMed] [Google Scholar]
- 34.Ren D., Li C., Qin Y., Yin R., Du S., Ye F., Liu C., Liu H., Wang M., Li Y. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe. 2014;30:1–10. doi: 10.1016/j.anaerobe.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Kaushik J.K., Kumar A., Duary R.K., Mohanty A.K., Grover S., Batish V.K. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS ONE. 2009;4:e8099. doi: 10.1371/journal.pone.0008099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy A., Simon O. Six-shogaol inhibits production of tumour necrosis factor alpha, interleukin-1 beta and nitric oxide from lipopolysaccharide-stimulated RAW 264.7 macrophages. West Indian Med. J. 2009;58:295–300. [PubMed] [Google Scholar]
- 37.Chang C.-K., Wang S.-C., Chiu C.-K., Chen S.-Y., Chen Z.-T., Duh P.-D. Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac. J. Trop. Biomed. 2015;5:281–286. doi: 10.1016/S2221-1691(15)30346-4. [DOI] [Google Scholar]
- 38.Xu X., Bi S., Lao F., Chen F., Liao X., Wu J. Induced changes in bioactive compounds of broccoli juices after fermented by animal-and plant-derived Pediococcus pentosaceus. Food Chem. 2021;357:129767. doi: 10.1016/j.foodchem.2021.129767. [DOI] [PubMed] [Google Scholar]
- 39.Ghawi S.K., Methven L., Niranjan K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba) Food Chem. 2013;138:1734–1741. doi: 10.1016/j.foodchem.2012.10.119. [DOI] [PubMed] [Google Scholar]
- 40.Abukhabta S., Khalil Ghawi S., Karatzas K.A., Charalampopoulos D., McDougall G., Allwood J.W., Verrall S., Lavery S., Latimer C., Pourshahidi L.K. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021;60:1263–1276. doi: 10.1007/s00394-020-02322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.-H., Baik S.-H. Probiotic properties of Lactobacillus strains with high cinnamoyl esterase activity isolated from jeot-gal, a high-salt fermented seafood. Ann. Microbiol. 2019;69:407–417. doi: 10.1007/s13213-018-1424-1. [DOI] [Google Scholar]
- 42.Son S.-H., Yang S.-J., Jeon H.-L., Yu H.-S., Lee N.-K., Park Y.-S., Paik H.-D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, Jangajji. Microb. Pathog. 2018;125:486–492. doi: 10.1016/j.micpath.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Solieri L., Bianchi A., Mottolese G., Lemmetti F., Giudici P. Tailoring the probiotic potential of non-starter Lactobacillus strains from ripened Parmigiano Reggiano cheese by in vitro screening and principal component analysis. Food Microbiol. 2014;38:240–249. doi: 10.1016/j.fm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Bautista-Gallego J., Arroyo-López F., Rantsiou K., Jiménez-Díaz R., Garrido-Fernández A., Cocolin L. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential. Food Res. Int. 2013;50:135–142. doi: 10.1016/j.foodres.2012.10.004. [DOI] [Google Scholar]
- 45.Casarotti S.N., Carneiro B.M., Todorov S.D., Nero L.A., Rahal P., Penna A.L.B. In vitro assessment of safety and probiotic potential characteristics of Lactobacillus strains isolated from water buffalo mozzarella cheese. Ann. Microbiol. 2017;67:289–301. doi: 10.1007/s13213-017-1258-2. [DOI] [Google Scholar]
- 46.Begley M., Hill C., Gahan C.G. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson R., Zhanel G., Phillips R., Hoban D. Human serum enhances the postantibiotic effect of fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 1991;35:1261–1263. doi: 10.1128/AAC.35.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh C. Antibiotics: Actions, Origins, Resistance. American Society for Microbiology (ASM); Washington, DC, USA: 2003. [Google Scholar]
- 49.Morrow L.E., Gogineni V., Malesker M.A. Probiotics in the intensive care unit. Nutr. Clin. Pract. 2012;27:235–241. doi: 10.1177/0884533612440290. [DOI] [PubMed] [Google Scholar]
- 50.Noriega L., Cuevas I., Margolles A., De los Reyes-Gavilan C.G. Deconjugation and bile salts hydrolase activity by Bifidobacterium strains with acquired resistance to bile. Int. Dairy J. 2006;16:850–855. doi: 10.1016/j.idairyj.2005.09.008. [DOI] [Google Scholar]
- 51.Morelli L. In vitro assessment of probiotic bacteria: From survival to functionality. Int. Dairy J. 2007;17:1278–1283. doi: 10.1016/j.idairyj.2007.01.015. [DOI] [Google Scholar]
- 52.Jeong I.-Y., Lee H.-J., Jin C.-H., Park Y.-D., Choi D.-S., Kang M.-A. Anti-inflammatory activity of Stevia rebaudiana in LPS-induced RAW 264.7 cells. Prev. Nutr. Food Sci. 2010;15:14–18. doi: 10.3746/jfn.2010.15.1.014. [DOI] [Google Scholar]
- 53.Levine A.B., Punihaole D., Levine T.B. Characterization of the role of nitric oxide and its clinical applications. Cardiology. 2012;122:55–68. doi: 10.1159/000338150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.