Abstract
A prospective, multicenter study was carried out over a period of 10 months. All patients with clinically significant bacteremia caused by Enterococcus spp. were included. The epidemiological, microbiological, clinical, and prognostic features and the relationship of these features to the presence of high-level resistance to gentamicin (HLRG) were studied. Ninety-three patients with enterococcal bacteremia were included, and 31 of these cases were caused by HLRG (33%). The multivariate analysis selected chronic renal failure, intensive care unit stay, previous use of antimicrobial agents, and Enterococcus faecalis species as the independent risk factors that influenced the development of HLRG. The strains with HLRG showed lower levels of susceptibility to penicillin and ciprofloxacin. Clinical features (except for chronic renal failure) were similar in both groups of patients. HLRG did not influence the prognosis for patients with enterococcal bacteremia in terms of either the crude mortality rate (29% for patients with bacteremia caused by enterococci with HLRG and 28% for patients not infected with strains with HLRG) or the hospital stay after the acquisition of enterococcal bacteremia. Hemodynamic compromise, inappropriate antimicrobial therapy, and mechanical ventilation were revealed in the multivariate analysis to be the independent risk factors for mortality. Prolonged hospitalization was associated with the nosocomial acquisition of bacteremia and polymicrobial infections.
Nosocomial infections caused by Enterococcus spp. are growing in importance. In Spain, nosocomial infections produced by these organisms constitute 9.4% of all infections, and nosocomial enterococcal infections are fourth in frequency after those caused by Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus (31). The frequency of nosocomial enterococcal bacteremia rose from 4.6% in 1990 to 7.1% in 1994, and 70.4% of the cases of bacteremia were caused by Enterococcus faecalis (31). According to data from the National Nosocomial Infections System of the Centers for Disease Control and Prevention, in the United States Enterococcus spp. are responsible for 12% of all nosocomial infections and 8% of all bacteremias acquired nosocomially (30).
Enterococcus spp. have a great capacity for acquiring resistance to antimicrobial agents. The resistance to penicillins or vancomycin and high-level resistance to aminoglycosides are the most important. For most enterococcal infections, the use of a penicillin (or a glycopeptide agent if the organism is resistant to penicillin or the patient is allergic to penicillin has been shown to be sufficient treatment (19). In patients with severe infections, in which it is necessary to obtain bactericidal activity at the site of the infection, the use of a combination of an antimicrobial agent with activity against the bacterial cell wall (penicillin or glycopeptide) and an aminoglycoside is recommended (18, 19). When the strain causing the infection presents high-level resistance to the aminoglycosides, use of the latter combination is no longer possible. Therefore, the study of the characteristics of infections caused by enterococcal strains with this phenotype is of interest, because the management of or the prognosis for patients infected with such strains may need to be modified. The objective of the present study was to compare the epidemiological, microbiological, clinical, and prognostic characteristics of bacteremias caused by Enterococcus spp. with and without high-level resistance to gentamicin (HLRG).
MATERIALS AND METHODS
Design of the study.
A prospective, multicenter study was carried out. Thirteen community and university hospitals in Andalusia, Spain, participated in the study from June 1993 to March 1994. All patients whose blood was found to be positive for Enterococcus spp. on culture were included. Those patients whose positive blood cultures were not considered to be of clinical significance were excluded. A clinical follow-up until the discharge or the death of the patient was carried out for each patient. The following information was collected for each patient: epidemiological data (personal data, age, sex, hospitalization ward, means of acquisition, risk factors for the acquisition of bacteremia, underlying chronic diseases, and the severity of disease), microbiological data (monomicrobial or polymicrobial etiology, isolation of an enterococcal species, and susceptibility of the infecting organism to penicillin, ampicillin, imipenem, ciprofloxacin, vancomycin, teicoplanin, gentamicin, and streptomycin), clinical features (source of the bacteremia, severity of illness, and antimicrobial treatment used), and prognostic features (hospital stay after acquiring the enterococcal bacteremia and crude mortality).
The predisposing factors assessed to determine the means of acquisition of bacteremia were the use of an intravascular catheter, urinary catheter, nasogastric tube, mechanical ventilation, and parenteral nutrition. These are possible predisposing factors for the development of the bacteremia when they are present at the onset of the bacteremia or in the 72 h before its onset. This period of assessment of the presence of predisposing factors was expanded to 14 days for the previous use of antimicrobial agents and surgical treatment.
Definitions.
The isolation and identification of Enterococcus spp. were carried out by customary methods (5). Study of the susceptibilities of the organisms to different antimicrobial agents and the detection of HLRG and high-level resistance to streptomycin were performed according to the criteria of the National Committee for Clinical Laboratory Standards (21).
The acquisition and the source of the bacteremia were established by previously defined criteria (7). Clinically significant bacteremia was defined as the isolation of one or more pathogens from one or more cultures of blood (in a period of 48 h) from the same patient in whom clinical evidence of infection existed (28). Data for only the first episode of bacteremia was included in the analysis.
A patient was considered to have diabetes mellitus when concentration of glucose in plasma higher than 140 mg/dl was noted on more than one occasion on an empty stomach. Chronic renal failure was defined as having a creatinine level in plasma of more than 2 mg/dl and data suggesting that it was a chronic disturbance (i.e., confirmation of its previous existence, normocytic normochromic anemia, renal atrophy, and renal osteodystrophy). A patient was considered to have cirrhosis when that patient had a defined diagnosis (i.e., with diagnostic liver biopsy) or a probable diagnosis (i.e., with clinical and analytical data suggesting chronic liver disease, hepatocellular dysfunction, and portal hypertension). Neutropenia was defined as a polymorphonuclear leukocyte count of less than 1,000/μl. The severities of the underlying chronic diseases were classified according to the criteria of McCabe and Jackson (16). The severity of illness was classified according to the definitions recommended by the consensus conference of the American College of Chest Physicians and the Society of Critical Care Medicine (1) (sepsis, severe sepsis, and septic shock). In the present work, the state of sepsis (patients not severely ill) was contrasted with severe sepsis or septic shock (severely ill patients). This, in practice, differentiated those patients without hemodynamic compromise and those with hypoperfusion, dysfunction of organs, or hypotension induced by sepsis (with or without a response to adequate fluid resuscitation).
The antimicrobial treatment was considered appropriate when the patients received for at least 48 h intravenous doses of one of the antimicrobial agents active in vitro against the isolate(s) from the blood culture, including penicillins, ureidopenicillins, carbapenems, glycopeptides, and quinolones as monotherapy or associated with aminoglycosides for Enterococcus spp. In the remaining circumstances the antimicrobial therapy was considered inappropriate.
The hospital stay after acquiring the enterococcal bacteremia was defined as the time from the date of the first positive blood culture until discharge (deceased patients were excluded).
Statistical analysis.
The level of resistance to gentamicin (the presence versus the absence of high-level resistance) was chosen as a dependent variable, and its possible association with the epidemiological, microbiological, clinical, and prognostic variables described previously was analyzed. In a first analysis, we studied the influence of the predisposing factors on the appearance of strains with HLRG (epidemiological and microbiological variables) for all strains collected. A univariate analysis was carried out by the chi-square test for qualitative variables (or two-tailed Fisher’s exact test when some expected values were less than 5) and the Mann-Whitney U test for quantitative variables. Those variables for which an association with the appearance of HLRG was obtained were introduced in a multivariate analysis through a forward stepwise multiple logistic regression model, by means of the Wald test, for the determination of those variables that were presented as independent associations (11).
With respect to the susceptibility to the antimicrobial agents, the rates of resistance and the distribution of the MICs of each antimicrobial agent for the strains with and without HLRG were analyzed. The chi-square test (or two-tailed Fisher’s exact test if it was proper) and the Mann-Whitney U test were used, respectively.
Next, the clinical characteristics of the bacteremias in both groups of patients (those with and those without infections caused by strains with HLRG) were analyzed by the chi-square test. Mono- or polymicrobial infection was considered by a stratified analysis. Crude relative risks and relative risks adjusted by the Mantel-Haenszel method were used to detect an interaction or confounding (11).
Finally, the influence of the presence of HLRG on the prognosis for the patients (crude mortality rate and hospital stay after acquiring the enterococcal bacteremia) was studied. Patients with endocarditis were excluded from this study. Given the need for the use of an aminoglycoside associated with a β-lactam or glycopeptide agent in patients with endocarditis, the prognosis could not be the same in those patients with bacteremia caused by strains with HLRG as in those with bacteremia caused by strains without HLRG (2, 6, 20).
For the analysis of mortality, mono- or polymicrobial infection was also considered here, and a stratified analysis was performed in the same way as described above for clinical characteristics. A posterior multivariate analysis was carried out through a logistic regression model in a manner similar to that described above.
With respect to the hospital stay after acquiring the enterococcal bacteremia, in a preliminary study we performed a multiple linear regression analysis whose covariates were those factors with statistical significance in a previous univariate study. In this way, a model with scarce validity (adjusted R2 = 0.209) was obtained. For this reason, a cluster analysis was carried out. Two different groups were identified both for the hospital stay and for the remaining factors introduced in the model. With this breakpoint facilitated by that analysis, the hospital stay after acquiring the enterococcal bacteremia was converted to a dichotomous qualitative variable that was used as a dependent variable in a subsequent logistic regression model. In this way, the breakpoint for the conversion of this variable to a qualitative variable was not arbitrary. Again, the covariates were those factors with statistical significance in the univariate analysis.
The significance level used in the statistical calculations was 5%. SPSS/PC (version 5.01) software was used for all these calculations.
RESULTS
Risk factors associated with HLRG.
A total of 93 patients with bacteremia caused by Enterococcus spp. were included in the study; 31 of them were infected with strains with HLRG (33%) and 62 were infected with strains without HLRG (67%). Twenty-one patients were from community hospitals and 72 patients were from university hospitals, with no differences with respect to the rate of HLRG among patients from the two types of hospitals (P = 0.29).
The ages (58.3 ± 13.3 versus 56.4 ± 18.7 years; P > 0.05), and genders (19 of 31 males [61%] versus 42 of 62 [68%]; P > 0.05) were similar in both groups of patients with bacteremia. The frequency of enterococcal bacteremia caused by enterococci with HLRG was higher in intensive care units (ICUs) (21 of 35; 60%) than in medical wards (8 of 51; 16%) and surgical wards (2 of 7 [29%]; P < 0.001).
Previous use of antimicrobial agents was highly associated with the appearance of HLRG. Each group of patients who received antimicrobial agents was compared with the group of patients who had not previously received antimicrobial agents. The antimicrobial agents related to the appearance of enterococcal strains with HLRG were glycopeptides (relative risk [RR] = 7.4; 95% confidence interval [CI] = 3.3 to 16.7), quinolones (RR = 5.9, 95% CI = 2.4 to 15), imipenem (RR = 4; 95% CI = 1.7 to 11.6), and cephalosporins (RR = 3.1, 95% CI = 1.3 to 7.6). The factors predisposing patients to the acquisition of bacteremia related to the presence of HLRG are listed in Table 1.
TABLE 1.
Risk factors related to the acquisition of bacteremias caused by enterococcal strains with HLRG
| Risk factor | No. (%) of patients infected with the following types of strains:
|
RR (95% CI) | |
|---|---|---|---|
| With HLRG | Without HLRG | ||
| Nosocomial acquisition | 27 (87.1)a | 38 (61.3)b | 2.9 (1.1–7.6)c |
| ICU admission | 20 (64.5)d | 15 (24.2)e | 3 (1.8–5)f |
| Mechanical ventilation in ICU | 13 (65) | 8 (53.3) | 1.2 (0.7–2.3) |
| Intravascular catheter | 27 (87.1) | 34 (64.5) | 2.7 (1.02–6.8) |
| Urinary catheter | 22 (71) | 24 (38.7) | 2.5 (1.3–4.8) |
| Nasogastric tube | 14 (45.2) | 15 (24.2) | 1.8 (1.04–3.2) |
| Surgery | 11 (35.5) | 15 (24.2) | 1.4 (0.8–2.5) |
| Parenteral nutrition | 8 (25.8) | 10 (16.1) | 1.5 (0.8–2.7) |
| Previous use of antimicrobial agents | 26 (83.9) | 30 (48.4) | 3.4 (1.5–8.1) |
Previous stay, 23 ± 6 days.
Previous stay, 12 ± 16 days.
P = 0.14.
ICU stay, 13.2 ± 9.4 days.
ICU stay, 8.1 ± 9.7 days.
P = 0.09.
Among the underlying chronic diseases, only chronic renal failure was associated with HLRG. We did not find a relationship between HLRG and the severity of the underlying diseases (Table 2).
TABLE 2.
Diseases, underlying conditions, and sources of bacteremia
| Characteristic | No. (%) of patients infected with the following types of strains:
|
RR (95% CI) | |
|---|---|---|---|
| With HLRG | Without HLRG | ||
| Underlying disease | |||
| Diabetes mellitus | 6 (19.4) | 13 (21) | 0.9 (0.5–2) |
| Chronic renal failure | 12 (38.7) | 6 (9.7) | 2.6 (1.6–4.4) |
| Cirrhosis of the liver | 4 (12.9) | 5 (8) | 1.4 (0.6–3) |
| Malignancies | 6 (19.4) | 18 (29) | 0.7 (0.3–1.5) |
| AIDS | 0 | 4 (6.5) | NSa |
| McCabe classification | |||
| Nonfatal underlying disease | 17 (55) | 29 (47) | 1.2 (0.7–2.2) |
| Ultimately fatal underlying disease | 14 (45) | 26 (42) | 1 (0.6–1.9) |
| Rapidly fatal underlying disease | 0 | 7 (11) | NS |
| Other conditions | |||
| Neutropenia | 1 (3.2) | 8 (12.9) | 0.3 (0.1–2) |
| Valvular heart disease | 1 (3.2) | 4 (6.5) | 0.6 (0.1–3.5) |
| Transplantation | 2 (6.4) | 6 (9.7) | 0.7 (0.2–2.5) |
| Source | |||
| Urinary | 5 (16.1) | 13 (20.9) | 0.8 (0.4–1.8) |
| Intravascular catheter | 6 (19.4) | 7 (11.2) | 1.5 (0.8–2.9) |
| Abdominal | 5 (16.1) | 17 (27.3) | 0.6 (0.3–1.4) |
| Soft tissues | 4 (12.9) | 2 (3.1) | 2.2 (1.1–4.1) |
| Respiratory | 2 (6.5) | 5 (7.9) | 0.9 (0.3–2.8) |
| Endocardium | 0 | 5 (7.9) | NS |
| Genital | 0 | 1 (1.5) | NS |
| Unknown | 9 (29) | 13 (20.9) | 1.3 (0.7–2.4) |
NS, not statistically significant (two-tailed Fisher exact test; P > 0.05).
A relationship between the source of the bacteremia and HLRG was not demonstrated (Table 2). The cases of endocarditis in five patients were caused by enterococcal strains without HLRG and were community acquired.
There were no differences between the groups with respect to hemodynamic compromise (13 of 31 [42%] versus 18 of 62 [29%]; P = 0.21). The frequency of polymicrobial bacteremias was 42% in the group infected with strains with HLRG and 34% in the group infected with strains without HLRG (P = 0.45). There were no differences between the two groups with respect to hemodynamic compromise when the infections were stratified by mono- or polymicrobial infections. Four of 18 patients with monomicrobial bacteremia caused by strains with HLRG and 9 of 41 with monomicrobial bacteremia caused by strains without HLRG developed hemodynamic compromise (22% for both groups; P = 1). In the group with polymicrobial cases of bacteremia, 9 of 13 patients with bacteremia caused by strains with HLRG (69%) versus 9 of 21 patients with bacteremia caused by strains without HLRG developed hemodynamic compromise (42%; P = 0.14). Six patients with enterococcal bacteremia caused by strains with HLRG (19%) and 11 patients with bacteremia caused by strains without HLRG (18%) received inappropriate antimicrobial therapy (P = 0.87).
E. faecalis was the species that showed HLRG more frequently (97% of all strains with HLRG). No Enterococcus faecium strain and only one Enterococcus durans strain (3%) showed HLRG. Strains without HLRG included 47 strains of E. faecalis (76%), 1 strain of E. durans (2%), and 14 strains of E. faecium (23%) (P = 0.003).
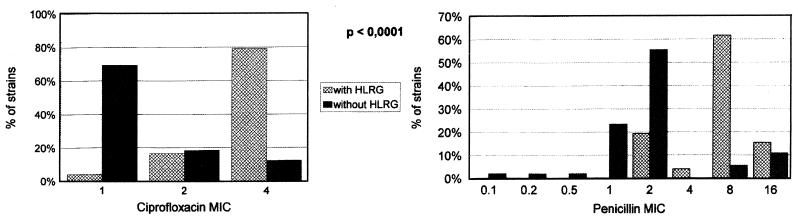
Ninety-six percent of the enterococcal strains with HLRG were also highly resistant to streptomycin. Four percent of E. faecalis strains with HLRG and none of the strains without HLRG were resistant to ampicillin (P > 0.05). Only 36% of the E. faecium strains were resistant to ampicillin. Only one E. faecalis strain was resistant to ampicillin and presented high-level resistance to both aminoglycosides. We have not detected any isolate resistant to vancomycin. The rates of resistance to ciprofloxacin were higher among the strains with HLRG (35.8 versus 30.6%; P < 0.001). Furthermore, the highest MICs of ciprofloxacin and penicillin were detected for the strains with HLRG (P < 0.001) (Fig. 1).
FIG. 1.
Penicillin and ciprofloxacin MICs for strains with and without HLRG.
The following independent risk factors were associated with the presence of HLRG: E. faecalis species, chronic renal failure, previous ICU admission, and previous use of antimicrobial agents (Table 3).
TABLE 3.
Multivariate analysis of predisposing risk factors for acquiring bacteramia caused by enterococcal strains with HLRGa
| Variable | B | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| Chronic renal failure | 2.628 | 0.757 | 13.85 | 3.13–61.17 | <0.001 |
| Previous ICU admission | 2.083 | 0.654 | 8.03 | 2.22–28.94 | 0.001 |
| E. faecalis species | 3.225 | 1.300 | 25.17 | 1.96–321.97 | 0.013 |
| Previous use of antimicrobial agents | 1.593 | 0.701 | 4.91 | 1.24–19.46 | 0.023 |
| Constant | −1.394 | 0.660 | 0.034 |
χ2 = 45.151; degree of freedom = 4; P < 0.0001; goodness of fit = 86.2%. Correlation matrix did not demonstrate colinearity among variables. B, regression coefficients; SE, standard error; OR, odds ratio.
Influence of HLRG on prognosis.
Eighty-eight patients without endocarditis were included in the crude mortality analysis. HLRG did not influence the outcome (9 of 31 patients with bacteremia caused by strains with HLRG [29%] versus 16 of 57 patients with bacteremia caused by strains without HLRG [28%]). An analysis of the probable influence of the poly or monomicrobial infection on mortality is presented in Table 4. It seems that different groups of factors are related to death in the groups of patients with mono- and polymicrobial infections, and previous ICU admission could not actually be related to death. Infection with a strain with HLRG was not a risk factor for death. The independent factors that were associated with excess of crude mortality were hemodynamic compromise, inappropriate antimicrobial therapy, and mechanical ventilation (Table 5).
TABLE 4.
Univariate analysis of crude mortality (stratified by poly- or monomicrobial infections)
| Factor | Crude RR (95% CI) | RR (95% CI)
|
MHRRa (95% CI) | |
|---|---|---|---|---|
| Monomicrobial RR (95% CI) | Polymicrobial RR (95% CI) | |||
| Intensive medicine areas | 2.8 (1.4–5.7) | 5.7 (1.7–19.3) | 1.3 (0.6–2.7) | 2.2 (1.2–4) |
| Nasogastric tube | 2.2 (1.2–4.2) | 4 (1.3–12.2) | 1.2 (0.6–2.5) | 1.9 (1–3.4) |
| Mechanical ventilation | 4 (2.2–7.5) | 10.5 (3.3–33.7) | 1.8 (0.9–3.6) | 3.2 (1.9–5.7) |
| Previous ICU admission | 2.3 (1.2–4.5) | 5.2 (1.5–17.8) | 1 (0.5–2) | 1.8 (1–3.3) |
| Parenteral nutrition | 2.6 (1.4–4.8) | 5.9 (2.2–15.8) | 1.2 (0.5–2.5) | 2.1 (1.2–3.6) |
| Respiratory source | 2.9 (1.6–5.3) | 1.9 (0.4–10.6) | 2.6 (1.7–4.2) | 2.5 (1.4–4.2) |
| Hemodynamic compromise | 9.7 (3.6–25.6) | 8.4 (2.5–27.5) | 10.3 (1.5–69.5) | 9.3 (3–28.4) |
| Inappropriate antimicrobial therapy | 3 (1.7–5.2) | 2.7 (1.1–7.2) | 2.6 (1.4–4.9) | 2.7 (1.6–4.6) |
| HLRG | 1 (0.5–2) | 0.9 (0.3–3) | 1 (0.5–2.2) | 1 (0.5–1.9) |
| Polymicrobial infection | 2.5 (1.3–4.9) | |||
MHRR, Mantel-Hanzel RR adjusted by polymicrobial infection.
TABLE 5.
Multivariate analysis of mortality risk factorsa
| Variable | B | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| Hemodynamic compromise | 3.19 | 0.82 | 24.50 | 4.9–121.2 | <0.001 |
| Inappropriate treatment | 3.09 | 0.93 | 21.90 | 3.6–136 | 0.001 |
| Mechanical ventilation | 1.91 | 0.73 | 6.70 | 1.6–28.2 | 0.009 |
| Constant | 0.37 | 0.42 | 0.380 |
χ2 = 50.91; degree of freedom = 3; P < 0.0001; goodness of fit = 86%. Correlation matrix did not demonstrate colinearity among variables. B, regression coefficients; SE, standard error; OR, odds ratio. Interactions between polymicrobial infections and each factor were taken into account.
Finally, the 63 patients without endocarditis who did not die were included in the analysis of the hospital stay after acquiring the enterococcal bacteremia. Patients with bacteremia caused by enterococci with HLRG had longer hospital stays (an average of 10 days longer). Other variables that were associated with longer hospital stays are presented in Table 6.
TABLE 6.
Variables associated with extended hospital stay after the acquisition of enterococcal bacteremia
| Variable | Hospital stay (days)
|
P | |
|---|---|---|---|
| Condition present | Condition absent | ||
| Nosocomial acquisition | 28.9 ± 22.3 | 10.8 ± 6 | 0.0001 |
| Risk factors | |||
| Intravascular catheter | 28.4 ± 22.3 | 11.3 ± 6.7 | 0.00098 |
| Nasogastric tube | 36.8 ± 30 | 20.1 ± 15.1 | 0.013 |
| ICU stay | 36.6 ± 29.2 | 18.6 ± 12.6 | 0.01 |
| Parenteral nutrition | 36.1 ± 17.4 | 22.6 ± 21 | 0.03 |
| Previous use of antimicrobial agents | 30.9 ± 23.7 | 15.9 ± 13.09 | 0.0013 |
| Source of bacteremia | |||
| Urinary | 12 ± 7 | 27.8 ± 22.3 | 0.003 |
| Unknown | 33.8 ± 29.2 | 21.1 ± 16.6 | 0.03 |
| Diabetes mellitus | 30.5 ± 16.3 | 22.4 ± 22 | 0.02 |
| Hemodynamic compromise | 35 ± 18.5 | 22.5 ± 20.9 | 0.024 |
| Polymicrobial etiology | 34.1 ± 28.6 | 20.4 ± 15.8 | 0.01 |
| HLRG | 31.1 ± 26.6 | 20.6 ± 16.5 | 0.047 |
In the multivariate analysis described in the Materials and Methods section, two groups of patients were determined through an analysis of clusters. The first group had a short hospital stay after acquiring the enterococcal bacteremia (average stay, 10.3 days; range, 2 to 17 days), and the second one had an extended hospital stay (average stay, 33.5 days; range, 20 to 54 days). Three patients with very extended hospital stays were also identified. Since these three patients could distort the analysis of the factors that could influence the stays of the remaining patients, data for these three patients were eliminated from the subsequent analysis. With this conversion of the hospital stay after the acquisition of the enterococcal bacteremia, a logistic regression analysis was carried out. In that analysis the dependent variable was the hospital stay after the acquisition of the enterococcal bacteremia (short versus extended), and the covariates were those that appeared in Table 6. By this analysis HLRG was not selected as an independent risk factor (Table 7).
TABLE 7.
Multivariate analysis of factors which determine hospital stay after the acquisition of enterococcal bacteremiaa
| Variable | B | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| Polymicrobial etiology | 2.273 | 0.839 | 9.71 | 1.87–50.31 | 0.006 |
| Nosocomial acquisition | 2.768 | 0.968 | 15.93 | 2.38–106.21 | 0.004 |
| Constant | −0.391 | 0.455 | 0.389 |
χ2 = 21.26; degree of freedom = 2; P < 0.0001; goodness of fit = 71.67%. Correlation matrix did not demonstrate colinearity among variables. B, regression coefficients; SE, standard error; OR, odds ratio.
DISCUSSION
The frequency of bacteremia caused by enterococci with HLRG is variable (7 to 63%), according to different investigators, and is related to the sources of specimens, the species considered, and the hospital (3, 8, 17, 23, 25, 34). Furthermore, the prevalence has been increasing in the last 15 years, as has been demonstrated in some long-term epidemiological follow-up studies (29, 34). In our series, one-third of the patients with enterococcal bacteremia were infected with strains with HLRG. This figure is similar to that found by other investigators in Spain in past years (4, 12, 15, 27, 33).
We have not found differences among patients with enterococcal bacteremias caused by strains with and without HLRG with respect to age, sex, or severity of the chronic underlying diseases. Disparity exists on this point in the literature, and while, as in our series, investigators have not found differences (23, 29), other investigators have isolated greater numbers of enterococcal strains with HLRG from those patients with more severe underlying diseases (34).
Chronic renal failure has been the only underlying disease or condition associated in the series with a greater frequency of bacteremias caused by strains with HLRG. This relationship has been described previously in relation to vancomycin-resistant strains (9, 32, 36).
Patients with bacteremia caused by strains with HLRG were grouped in the ICUs, while those in whom this characteristic was not found were more frequently grouped in medical wards. The results of other studies are disparate on this point, according to the different characteristics of the patients included in the study and the participating hospitals (3, 29). This makes a comparison between those studies and our series impossible.
Multiple studies support a relationship between the nosocomial acquisition and the development of enterococcal bacteremia caused by strains with HLRG (54 to 94% of all enterococcal bacteremias) (3, 8, 9, 23, 34, 35, 37). This relationship was also found in the present series and was independent of the duration of the previous hospital stay. However, according to the results of the multivariate analysis, it does not seem to be a risk factor by itself. It could be an expression of the fact that in this environment several factors present at the same time facilitate the appearance of strains with this characteristic (HLRG).
Those patients who had previously received antimicrobial agents had a five times greater risk of acquiring an enterococcal bacteremia caused by a strain with HLRG. Glycopeptides, quinolones, imipenem, and cephalosporins have been the antimicrobial agents related to the appearance of HLRG. This antecedent has been considered to be a risk factor or not to be a risk factor in different studies (3, 9, 23, 37). In a recent case-control study and by means of a multivariate analysis, the previous use of antimicrobial agents (and, more concretely, cephalosporins and aminoglycosides) was an independent risk factor for the acquisition of infections caused by β-lactamase-producing, gentamicin-resistant E. faecalis strains (35).
Although one might think that previous ICU admission and previous administration of antimicrobial agents might be directly related, the multivariate analysis discards this possibility. Regarding previous ICU admission, the relationship found in our series did not depend on the previous duration of the stay in these units or the need for mechanical ventilation. Factors such as the presence of an intravascular catheter, urinary catheter, and nasogastric tube are not independent risk factors, and they may have been related to the grouping of the patients with enterococcal bacteremias caused by strains with HLRG in the ICUs.
Although we have not found any study in the literature that supports this fact, several investigators have demonstrated that ICUs have been the starting points of different epidemic outbreaks of infections caused by enterococcal strains with HLRG (alone or in association with resistance to other antimicrobial agents) (9, 33, 35).
Previous studies found that surgery was a risk factor for the acquisition of enterococcal bacteremias caused by strains with HLRG (35). We have not found a relationship between HLRG and a stay on surgical hospitalization wards or previous surgery. Again, local differences could explain this fact.
Although there was a higher proportion of severely ill patients among the patients with enterococcal bacteremias caused by strains with HLRG, this was not significant, and no other clinical feature differentiated the groups of patients. Other studies have identified an APACHE II index score of more than 6 points as a factor predicting infection with enterococcal strains with HLRG (35).
The frequencies of the sources of bacteremia in both groups were somewhat different, but they did not reach significance (bacteremias caused by strains with HLRG were more frequently of an unknown origin or were the result of the presence of an intravascular catheter). As in previous studies, we also found that endocarditis had a monomicrobial etiology and that the patients had community-acquired infections (13, 14, 25).
Contrary to other investigators who found a higher frequency of strains with HLRG among patients with polymicrobial bacteremia (34), we, among others, have not found such an association (3). The species most associated with HLRG was E. faecalis, and the total absence of E. faecium strains with HLRG is worth noting. There is a large range of HLRG according to the different enterococcal species (4 to 63% for E. faecalis and 2 to 24% for E. faecium) (3, 8, 17, 23, 33, 34), and the predominant species also differs. In some studies it is E. faecalis species (8, 33, 34), and in other studies it is E. faecium species (17). This special characteristic of our series makes E. faecalis species the most important independent risk factor for the development of HLRG. This is a very important epidemiological datum in our environment, although it may not be applicable to other geographical areas.
Most strains with HLRG have also been highly resistant to streptomycin. This is an interesting feature that is scarcely reported in Spain (4, 15) (rates from 7 to 59%, generally [27, 33]) but that is frequently found worldwide (41 to 82%) (3, 8, 17, 23, 26). It calls our attention to the possibility of a lack of alternative treatment for severe enterococcal infections in our environment. It is also important to emphasize that we have only found one strain resistant at the same time to ampicillin and to both aminoglycosides and that we have not found vancomycin-resistant strains. Enterococcal strains with HLRG were also less susceptible to penicillin and ciprofloxacin. This lower degree of susceptibility of enterococcal strains with HLRG to quinolones has been described previously (4, 22).
The crude mortality rate for the patients with enterococcal bacteremia without endocarditis has not been influenced by the susceptibility to gentamicin and is intermediate in comparison with that reported in the literature (from 18 to 61%) (24). This absence of an influence continued even after analyzing only the monomicrobial bacteremias (Table 4). This fact has also been recently reported by Patterson et al. (25), who found that only two factors were independent predictors of the outcomes for patients with enterococcal infection: resistance to ampicillin and the APACHE II index score. Factors which were determinants of the outcomes for patients in our series were hemodynamic compromise, inappropriate antimicrobial therapy, and the need for mechanical ventilation during the ICU stay. So, monotherapy with an active antimicrobial agent (penicillin or a glycopeptide) is probably sufficient for the treatment of enterococcal bacteremias without endocarditis.
The management of patients with enterococcal bacteremia without endocarditis has been discussed over the past 15 years in relationship to the clinical significance of such isolations. The mortality among these patients could be more related to the underlying conditions than to the enterococcal bacteremia (20). However, subsequent studies have outlined the need for an appropriate antimicrobial treatment for these patients to achieve a lower mortality rate (10, 13, 24, 25). The present study stresses this need and indicates that appropriate antimicrobial treatment is a very important factor in the patient’s outcome. Patterson et al. (25) reported a mortality rate of 71% for patients who did not receive appropriate antimicrobial treatment, compared to a mortality rate of 53% for patients who did, although this difference did not reach statistical significance.
A polymicrobial etiology and the nosocomial acquisition of enterococcal bacteremia have been the independent risk factors for a long hospital stay after the acquisition of enterococcal bacteremia.
REFERENCES
- 1.ACCP/SCCM Consensus Conference Committee. Definitions of sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Almirante B, Tornos M P, Gurgui M, Pujol M, Miró J M. Prognosis of enterococcal endocarditis. Rev Infect Dis. 1991;13:1248–1249. doi: 10.1093/clinids/13.6.1248. [DOI] [PubMed] [Google Scholar]
- 3.Antalek M D, Mylotte J M, Lesse A J, Sellick J A., Jr Clinical and molecular epidemiology of Enterococcus faecalis bacteremia, with special reference to strains with high-level resistance to gentamicin. Clin Infect Dis. 1995;20:103–109. doi: 10.1093/clinids/20.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Balas D, Alós J I, Carvajal R, Gómez-Garcés J L. Enterococos aislados en sangre (1989–1993): evolución de la susceptibilidad a los antibióticos. Enferm Infecc Microbiol Clin. 1995;13:455–459. [PubMed] [Google Scholar]
- 5.Facklam R R, Carey R B. Streptococci and aerococci. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H G, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 154–175. [Google Scholar]
- 6.Fernández-Guerrero, M. L., and A. Núñez García. 1995. La endocarditis enterocócica, modelo de dificultad terapéutica. Rev. Clin. Esp. 195(Suppl. 4):41–45. [PubMed]
- 7.Garner J S, Jarvis W R, Emori T G, Horan T C, Hughes J M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S, Swenson J M, Hill B C, Pigott N E, Facklam R R, Cooksey R C, Thornsberry C, Jarvis W R, Tenover F C. Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. J Clin Microbiol. 1992;30:2373–2378. doi: 10.1128/jcm.30.9.2373-2378.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh K V, Murray B E, Wolff J, Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 10.Hoge C W, Adams J, Buchanan B, Sears S D. Enterococcal bacteremia: to treat or not to treat, a reappraisal. Rev Infect Dis. 1991;13:600–605. doi: 10.1093/clinids/13.4.600. [DOI] [PubMed] [Google Scholar]
- 11.Kleinbaum D G, Kupper L L, Morgenstern H. Epidemiologic research. Principles and quantitative methods. New York, N.Y: Van Nostrand Reinhold; 1982. [Google Scholar]
- 12.Liñares, J., J. Ayats, and T. Alonso. 1995. Resistencia de los enterococos a los antibiticos betalactámicos y aminoglucósidos. Rev. Clin. Esp. 195(Suppl. 4):16–21. [PubMed]
- 13.Maki D G, Agger W A. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988;67:248–269. [PubMed] [Google Scholar]
- 14.Malone D A, Wagner R A, Myers J P, Watanakunakorn C. Enterococcal bacteremia in two large community teaching hospitals. Am J Med. 1986;81:601–606. doi: 10.1016/0002-9343(86)90544-9. [DOI] [PubMed] [Google Scholar]
- 15.Martínez Martínez L, Torres C, Ortega M C, Suárez A I, Domínguez A. Detección de resistencia de alto grado a aminoglucósidos en Enterococcus sp. con el sistema MicroScan y paneles con caldo de glucosa fosfato. Rev Esp Quimioter. 1993;6:298–300. [Google Scholar]
- 16.McCabe W R, Jackson G G. Gram-negative bacteremia. I. Etiology and ecology. Arch Intern Med. 1962;110:847–855. [Google Scholar]
- 17.McNamara E B, King E M, Smyth E G. A survey of antimicrobial susceptibility of clinical isolates of Enterococcus spp. from Irish hospitals. J Antimicrob Chemother. 1995;35:185–189. doi: 10.1093/jac/35.1.185. [DOI] [PubMed] [Google Scholar]
- 18.Megran D W. Enterococcal endocarditis. Clin Infect Dis. 1992;15:63–71. doi: 10.1093/clinids/15.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Moellering R C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1178. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 20.Murray B E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. Document M7A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 22.Noskin G A, Mehl P, Warren J R. Bactericidal activity of the fluorquinolone WIN 57273 against high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1993;37:2470–2473. doi: 10.1128/aac.37.11.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noskin G A, Till M, Patterson B K, Clarke J T, Warren J R. High-level gentamicin resistance in Enterococcus faecalis bacteremia. J Infect Dis. 1991;164:1212–1215. doi: 10.1093/infdis/164.6.1212. [DOI] [PubMed] [Google Scholar]
- 24.Pallarés, R., M. J. Barberá, and J. Guillamont. 1995. Bacteriemia enterocócica nosocomial. Rev. Clin. Esp. 195(Suppl. 4):12–15. [PubMed]
- 25.Patterson J E, Sweeney A H, Simms M, Carley N, Mangi R, Sabetta J, Lyons R W. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine (Baltimore) 1995;74:191–200. doi: 10.1097/00005792-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Patterson J E, Zervos M J. High-level gentamicin resistance in Enterococcus: microbiology, genetic basis, and epidemiology. Rev Infect Dis. 1990;12:644–652. doi: 10.1093/clinids/12.4.644. [DOI] [PubMed] [Google Scholar]
- 27.Reina J, Llompart I, Gómez J, Borrell N, Serra A. Análisis de los patrones de sensibilidad y detección de alto grado de resistencia a los aminoglucósidos en 360 cepas pertenecientes al género Enterococcus sp. aisladas en muestras clínicas. Rev Esp Quimioterap. 1991;4:62–68. [Google Scholar]
- 28.Roberts F J, Geere I W, Coldman A. A three-year study of positive blood cultures, with emphasis on prognosis. Rev Infect Dis. 1991;13:34–46. doi: 10.1093/clinids/13.1.34. [DOI] [PubMed] [Google Scholar]
- 29.Sáenz González M C, Valero Juan L F. Resistencia de Enterococcus spp., un problema creciente. Estudio epidemiológico (1987–1993) Med Clin (Barcelona) 1994;103:485–489. [PubMed] [Google Scholar]
- 30.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91(Suppl. 3B):72S–75S. [DOI] [PubMed]
- 31.Sociedad Española de Higiene y Medicina Preventiva Hospitalaria y Grupo de Trabajo EPINCAT. Prevalencia de las infecciones nosocomiales en los hospitales españoles. Proyecto EPINE 1990–1994. Madrid, Spain: Sociedad Española de Higiene y Medicina Preventiva Hospitalaria y Grupo de Trabajo EPINCAT; 1995. [Google Scholar]
- 32.Uttley A H C, George R C, Naidoo J, Woodford N, Johnson A P, Collins C H, Morrison D, Gilfillan A J, Fitch L E, Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989;103:173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valle-Ortiz O, Gallés C, Codina G, Cano A. Enterococos: alto nivel de resistencia a aminoglucósidos. Enferm Infecc Microbiol Clin. 1989;7:535–541. [PubMed] [Google Scholar]
- 34.Watanakunakorn C, Patel R. Comparison of patients with enterococcal bacteremia due to strains with and without high-level resistance to gentamicin. Clin Infect Dis. 1993;17:74–78. doi: 10.1093/clinids/17.1.74. [DOI] [PubMed] [Google Scholar]
- 35.Wells V D, Wong E S, Murray B E, Coudron P E, Williams D S, Markowitz S M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992;116:285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- 36.Woodford N, Johnson A P, Morrison D, Chin A T L, Stephenson J R, George R C. Two distinct forms of vancomycin resistance among enterococci in the UK. Lancet. 1990;i:226. doi: 10.1016/0140-6736(90)90317-x. [DOI] [PubMed] [Google Scholar]
- 37.Zervos M J, Kauffman C A, Therasse P M, Bergman A G, Mikesell T S, Schaberg D R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. Ann Intern Med. 1987;106:687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]