Abstract
In two patients with acute salpingitis, C. trachomatis was isolated from the cervix. In one of the patients, the organism was also recovered from the Fallopian tubes, and in the other, chlamydial inclusions were found in Giemsa-stained tubal epithelial cells. A significant change in micro-immunofluorescence antibodies to C. trachomatis occurred in both patients during the course of the disease. The Fallopian tubes of both patients were removed and studied by conventional histological techniques and, in the case of one of them, by transmission electron microscopy.
Full text
PDF

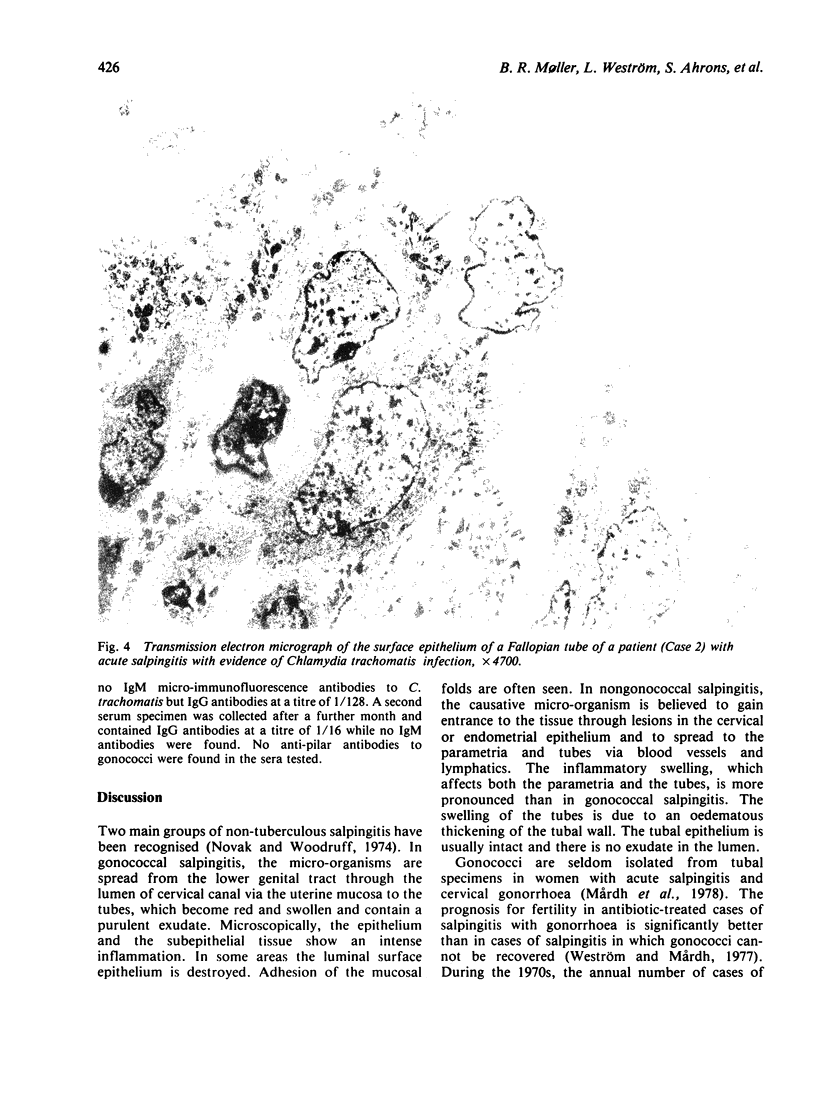


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Gästrin B., Kallings L. O., Marcetic A. The survival time for different bacteria in various transport media. Acta Pathol Microbiol Scand. 1968;74(3):371–380. doi: 10.1111/j.1699-0463.1968.tb03490.x. [DOI] [PubMed] [Google Scholar]
- Hilton A. L., Richmond S. J., Milne J. D., Hindley F., Clarke S. K. Chlamydia A in the female genital tract. Br J Vener Dis. 1974 Feb;50(1):1–10. doi: 10.1136/sti.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Jensen A. Indirect hemagglutination with Mycoplasma antigens: effects of pH on antigen sensitization of tanned fresh and formalinized sheep erythrocytes. Appl Microbiol. 1971 Nov;22(5):756–759. doi: 10.1128/am.22.5.756-759.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Ripa T., Svensson L., Weström L. Chilamydia trachomatis infection in patients with acute salpingitis. N Engl J Med. 1977 Jun 16;296(24):1377–1379. doi: 10.1056/NEJM197706162962403. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Weström L. Tubal and cervical cultures in acute salpingitis with special reference to Mycoplasma hominis and T-strain mycoplasmas. Br J Vener Dis. 1970 Jun;46(3):179–186. [PMC free article] [PubMed] [Google Scholar]
- Møller B. R., Freundt E. A., Black F. T., Frederiksen P. Experimental infection of the genital tract of female grivet monkeys by Mycoplasma hominis. Infect Immun. 1978 Apr;20(1):248–257. doi: 10.1128/iai.20.1.248-257.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriel J. D., Johnson A. L., Barlow D., Thomas B. J., Nayyar K., Reeve P. Infection of the uterine cervix with Chlamydia trachomatis. J Infect Dis. 1978 Apr;137(4):443–451. doi: 10.1093/infdis/137.4.443. [DOI] [PubMed] [Google Scholar]
- Reimann K., Lind I. An indirect haemagglutination test for demonstration of gonococcal antibodies using gonococcal pili as antigen. I. Methodology and preliminary results. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):115–122. doi: 10.1111/j.1699-0463.1977.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Mårdh P. A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol. 1977 Oct;6(4):328–331. doi: 10.1128/jcm.6.4.328-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa K. T., Møller B. R., Mårdh P. A., Freundt E. A., Melsen F. Experimental acute salpingitis in grivet monkeys provoked by Chlamydia trachomatis. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):65–70. doi: 10.1111/j.1699-0463.1979.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Svensson L., Mårdh P. A., Weström L. Chlamydia trachomatis cervicitis in gynecologic outpatients. Obstet Gynecol. 1978 Dec;52(6):698–702. [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (first of three parts). N Engl J Med. 1978 Feb 23;298(8):428–435. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- Treharne J. D., Darougar S., Jones B. R. Modification of the microimmunofluorescence test to provide a routine serodiagnostic test for chlamydial infection. J Clin Pathol. 1977 Jun;30(6):510–517. doi: 10.1136/jcp.30.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne J. D., Ripa K. T., Mårdh P. A., Svensson L., Weström L., Darougar S. Antibodies to Chlamydia trachomatis in acute salpingitis. Br J Vener Dis. 1979 Feb;55(1):26–29. doi: 10.1136/sti.55.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]