Abstract
Background and Aims
The genus Buxus has high levels of endemism in the Caribbean flora, with ~50 taxa. In Cuba, 82 % grow on ultramafic substrates and 59 % are nickel (Ni) accumulators or Ni hyperaccumulators. Hence it is an ideal model group to study if this diversification could be related to adaptation to ultramafic substrates and to Ni hyperaccumulation.
Methods
We generated a well-resolved molecular phylogeny, including nearly all of the Neotropical and Caribbean Buxus taxa. To obtain robust divergence times we tested for the effects of different calibration scenarios, and we reconstructed ancestral areas and ancestral character states. Phylogenetic trees were examined for trait-independent shifts in diversification rates and we used multi-state models to test for state-dependent speciation and extinction rates. Storms could have contributed to Cuba acting as a species pump and to Buxus reaching other Caribbean islands and northern South America’.
Key Results
We found a Caribbean Buxus clade with Mexican ancestors, encompassing three major subclades, which started to radiate during the middle Miocene (13.25 Mya). Other Caribbean islands and northern South America were reached from ~3 Mya onwards.
Conclusions
An evolutionary scenario is evident in which Buxus plants able to grow on ultramafic substrates by exaptation became ultramafic substrate endemics and evolved stepwise from Ni tolerance through Ni accumulation to Ni hyperaccumulation, which has triggered species diversification of Buxus in Cuba. Storms could have contributed to Cuba acting as a species pump and to Buxus reaching other Caribbean islands and northern South America’.
Keywords: BAMM, Caribbean biogeography, endemic species, fossil calibration, MuSSE, nickel (Ni) accumulation and hyperaccumulation, species diversification, trait-dependent speciation
INTRODUCTION
The Caribbean region is a global biodiversity hotspot (Myers et al., 2000). The Caribbean islands harbour ~10 400 indigenous taxa of seed plants, of which 71 % are endemic (Acevedo-Rodríguez and Strong, 2012). The genus Buxus (Buxaceae) is one of the outstanding lineages in terms of endemism in this area. From the ~50 endemic Caribbean taxa, only 35 species and 7 subspecies are unique to Cuba (Köhler, 2014). Considering that Buxus has a nearly global distribution ranging from tropical America, Africa and throughout Eurasia with 107 taxa (100 species and 7 subspecies), it is remarkable that about half of its species have evolved in a relatively small area.
Buxus is one of the genera with a large number of species restricted to ultramafic substrates in Cuba and other territories (Köhler, 2014; Mizuno and Kirihata, 2015). There are also a number of other genera with significant numbers of obligate ultramafic taxa in Cuba and the Caribbean, such as Leucocroton (Euphorbiaceae) (Jestrow et al., 2012; Cervantes et al., 2016), Phyllanthus (Phyllanthaceae) (Falcón et al., 2020) and Casearia (Salicaceae) (Mestier et al., 2022). A phylogenetic picture is emerging for these genera, which in many cases have revealed endemic Caribbean clades and indicated in-site diversification on the Caribbean islands, mostly since the Miocene (Cervantes et al., 2016; Roncal et al., 2020). However, species-level relationships within these Caribbean clades have mostly not yet been resolved and trait-based speciation rates have so far not been addressed. Altogether, a third of the Cuban endemic flora is restricted to areas with ultramafic soils, which therefore are considered to be important sites of plant speciation and diversification (Borhidi, 1996). A better understanding of the possible effects on species diversification by adaptation of plants to ultramafic soils including specializations like Ni accumulation and hyperaccumulation is thus of wider interest to better understand the evolution of the flora of Cuba and the Caribbean. The genus Buxus can serve as model.
Cuba is the biggest island of the Caribbean and has a complex geological history, like the other Antillean islands (Iturralde-Vinent, 1982, 2006; Hedges, 2001). Ultramafic substrates (soils derived from ultramafic bedrocks) cover 7 % of Cuba, distributed as an archipelago of isolated outcrops (Berazaín-Iturralde, 2001) across the island. The time when the Cuban ultramafic outcrops became exposed to the surface and available to colonization by plants ranged from ~30 to ~1 Mya. The ultramafic outcrops of Cajálbana in the west and Nipe-Cristal-Moa-Baracoa in the east of Cuba are estimated to have an age of 10–30 Mya, whereas those located in central Cuba are just about 1 Mya or even younger (Borhidi, 1996). This distribution of ultramafic habitats like ‘an archipelago of islands’ on the island of Cuba (Jestrow et al., 2012) could constitute a further important factor for isolation and speciation of plants.
Plants growing on ultramafic substrates have developed mechanisms to adapt to the toxicity caused by the high metal content either by exclusion or accumulation (Brooks et al., 1977; Baker, 1981; Kazakou et al., 2008). Brooks et al. (1977) showed that the concentration of Ni in plants growing on ultramafic substrates could be measured in the leaves of herbarium specimens. Elevated concentrations of Ni in the leaves indicate Ni accumulators or, above a certain threshold, even hyperaccumulators (Boyd, 2004; Reeves et al., 2017). This phenomenon of Ni hyperaccumulation occurs in ~600 plant species adapted to ultramafic substrates worldwide. Reeves et al. (2017) cited 532 species and data for 28 species were reported by van der Ent et al. (2019) for the flora of Sabah. Recently, Belloeil et al. (2021) published measurements for a further 30 Cuban taxa of hitherto unexamined species. The largest number of Ni hyperaccumulators, ~170 species, is found in Cuba in the genera Buxus, Phyllanthus, Leucocroton, several genera of Asteraceae and Rubiaceae (Reeves et al., 1996, 1999; Jaffré et al., 2018; Belloeil et al., 2021). Among the Cuban Buxus, 82 % are endemic to ultramafic substrates and 59 % have been identified as Ni accumulators or hyperaccumulators (Reeves et al., 1996; updated here). Based on the assumption that Ni tolerance led to accumulation, and further to hyperaccumulation, we hypothesized that character state transformations occurred in this direction during species diversification.
The flora of the Caribbean including Cuba has a complex history, with ancestors potentially having arrived from South and Central America as well as Mexico and North America (Santiago-Valentin and Olmstead, 2004). Among the different scenarios for the origin of the Caribbean flora, explicit spatio-temporal assumptions were made by the Cretaceous vicariance hypothesis (Rosen, 1975), and the GAARlandia hypothesis (Iturralde-Vinent and MacPhee, 1999), which assumed a land bridge between north-east South America and the Antilles in the Eocene. Other authors hypothesized long-distance dispersal (from continental land masses) and over-water dispersal, for example facilitated by hurricanes (Presley and Willig, 2008). Whereas dated phylogenies have largely rejected vicariance in plants, the GAARlandia hypothesis has received considerable attention more recently. Cervantes et al. (2016) concluded from the broad sampling of the subfamily Acalyphoideae (Euphorbiaceae) with several endemic Caribbean island lineages, but also many species on all adjacent mainlands, that migrations from Central America and Mexico are frequent and have led to ongoing species radiations for ~8–12 Mya. Nieto-Blásquez et al. (2017) and Roncal et al. (2020) analysed published data and found that the Antilles were colonized repeatedly over the last 60 Mya, with arrivals from both Central and South America, depending on the plant group. However, there were still too few comprehensive phylogenetic studies to reliably assess the relative roles of in situ speciation versus recurrent immigration on Caribbean islands. In situ speciation may be predominant in older and larger islands (Warren et al., 2015), such as Cuba, but on the other hand specific traits may also have considerably fuelled speciation.
Here we present a densely sampled phylogeny of Buxus, as one of the major lineages with species-level endemism occurring in Cuba but also Caribbean islands and adjacent mainlands. We use this phylogeny to illuminate patterns of in situ speciation in Cuba and the role Cuba played in the diversification of Caribbean Buxus. We then examine the role of ultramafic habitats and Ni accumulation and hyperaccumulation as traits that potentially affected the speciation of Buxus. Specifically, we test if the diversification of Caribbean Buxus originated from Cuba as the biggest island or, on the contrary, the diversity in Cuba could be explained by multiple arrivals from neighbouring islands. We also specifically test our hypothesis that the evolution of Buxus species occurred in a stepwise process from tolerance to ultramafic soils through Ni accumulation to Ni hyperaccumulation. This hypothesis is based on the assumption that the adaptation to the specific ultramafic habitats predominantly in Cuba led to elevated speciation rates while extinction rates in a rapid radiation were still minimal and thus boosted the evolution of endemism in this genus. If this was the case, the ability to tolerate and adapt to ultramafic soils would be a major factor in the evolution of the rich Cuban and Caribbean flora.
MATERIALS AND METHODS
Taxon sampling and plant material
Our dataset included 81 samples of Buxus including nearly all the Neotropical and Caribbean taxa: 32 species and 5 subspecies endemic to Cuba, 1 species distributed in Cuba and Hispaniola, another in Cuba and the Bahamas, 1 species from Jamaica, 2 from Puerto Rico, 4 from Mexico, and 1 from Panama. At this point our dataset only misses one species from Mexico (B. lancifolia), five Cuban taxa, some of them infraspecific (B. cubana, B. excisa subsp. costata, B. muelleriana, B. retusa subsp. microphylla and B. vaccinioides), two species from Jamaica (B. laevigata and B. macrophylla) and B. subcolumnaris from the Lesser Antilles. Thus our representation of Neotropical taxa including infraspecific entities is 84 %. Our dataset also encompasses four species from Eurasia and six from Africa, Madagascar and adjacent islands. Moreover, we included sequences of all other genera of Buxales and representatives of Trochodendrales, Proteales, Sabiales and Ranunculales to allow for multiple fossil calibration points (Supplementary DataTable S1).
DNA extraction, amplification and sequencing
Total DNA was isolated from silica-gel-dried leaves or herbarium specimens using a triple CTAB extraction method (Borsch et al., 2003) or the NucleoSpin Plant II extraction kit (Macherey Nagel, Düren, Germany). We used a combined data set of plastid introns and spacers with known high levels of hierarchical phylogenetic signal at species level and alignability across early branching eudicots (Barniske et al., 2012). Sequences of the trnK intron including matK, the trnL intron and trnL-F spacer were generated as described in González Gutiérrez et al. (2013) and for the petD intron and petD-petB spacer following Löhne and Borsch (2005). For difficult samples internal primers were designed (BxpetD500F, 5ʹ-ATTCATTTCCTCTGCATCG-3ʹ; BxpetD556R, 5ʹ-GTTACTAATATAGTCTAGCC-3ʹ) for petD. Sanger sequencing was performed at Macrogen Inc., South Korea. Chromatograms were inspected by eye to correct erroneous base-calls and final sequences were assembled in PhyDE v. 0.9971 (Müller et al., 2005).
Sequence alignment and phylogenetic analyses
We employed a motif-alignment approach using PhyDE following the criteria of Löhne and Borsch (2005). Regions of uncertain homology were removed from the matrices prior to phylogenetic analyses. Microstructural mutations were coded using simple indel coding (Simmons and Ochoterena, 2000) implemented in SeqState 1.40 (Müller, 2005a). An inversion of 37 nucleotides in the 3ʹ part of the trnK intron in nine samples was reverse-complemented and coded as present in the indel matrix.
Phylogenetic analyses
Maximum parsimony, maximum likelihood (ML) and Bayesian inference were calculated using a concatenated matrix. The maximum parsimony trees were inferred using the parsimony ratchet (Nixon, 1999) as implemented in PRAP (Müller, 2004) in combination with PAUP v. 4.0b10 (Swofford, 1998). Settings were: 200 ratchet iterations with 25 % of the positions randomly up-weighted (weight = 2) during each replicate and ten random addition cycles. The command files generated with PRAP were then run in PAUP, using heuristic searches with all characters given equal weight, gaps treated as missing, TBR branch swapping, initial swapping on one tree already in memory, and branches collapsed actively when branch length was zero. Jackknife values were calculated in PAUP with 10 000 replicates, 36.788 % of the characters were deleted and one tree was held during each replicate (Müller, 2005b). GTR + G was obtained for all partitions based on the Akaike information criterion (AIC) implemented in Modeltest 2.3 (Posada and Crandall, 1998). A binary model was applied for the indel partition.
Maximum likelihood analysis was performed using RAxML v. 8 on XSEDE (Stamatakis, 2006) on CIPRES. We implemented an independent general time-reversible model (GTR) and γ distribution for site rates for each data partition. Rapid bootstrap support was estimated based on the majority-rule consensus tree from 1000 replicates.
Bayesian trees were calculated using MrBayes 3.2.6 (Ronquist and Huelsenbeck, 2003) on CIPRES. The analysis was performed with four independent runs of Monte Carlo Markov chains (MCMCs), each with four parallel chains. Each chain was performed for 5 000 000 generations, saving one random tree every 1000th generation. The results of the four chains were analysed in Tracer v. 1.6 (Rambaut and Drummond, 2007), to calculate the convergence value in our data. Then, the burn-in was set to 10 % in the commands of MrBayes and a majority consensus tree was computed with the remaining trees.
Fossil calibration and divergence time estimation
Divergence times were estimated with BEAST v. 1.8.4 (Drummond and Rambaut, 2007). Considering that the minimum age of the fossil, the prior for the expected occurrence through time, the maximum age of the root and the selection and placement of fossils as well as potential uncertainty in the original age estimates used as secondary calibration points could affect divergence times within Buxus (Bell et al., 2010; Foster et al., 2017), six calibration scenarios were tested (Supplementary Data Tables S2 and S3).
We used an older age of angiosperm diversification [145 Mya sensuFoster et al. (2017) for the eudicot crown set as maximum age compared with 129 Mya sensuBell et al. (2010)]. The flowers of Spanomera from the Mid-Cretaceous (Drinnan et al., 1991) and leaves of Sapindopsis from the Early Cretaceous (Crane et al., 1993) served as calibration points in line with the phylogenetic relationships inferred with Buxaceae and Platanaceae, respectively, by Doyle and Endress (2010). We also included the oldest known Buxus fossil from the Oligocene (Fujun et al., 2015). All fossils were used as minimum age constraints for respective stem or crown nodes (Supplementary Data Table S3). Additionally, we defined two secondary calibration points using age estimates of Buxales and Proteales (~135 and 137 Mya, respectively) from Foster et al. (2017). Ages of the geological periods are according to The Geologic Time Scale v. 5.0 (Walker et al., 2018).
To select the appropriate branching process (speciation tree), we conducted a marginal likelihood estimation (MLE) using stepping-stone sampling with 150 path steps in BEAST (Xie et al., 2011), following Canal et al. (2018). Based on the MLE results, a birth–death model best fitted the data [Bayes factor (BF) = 163.88] (Supplementary Data Table S4). An uncorrelated relaxed clock and a lognormal distribution of rate changes were assumed for all scenarios (Drummond et al., 2006). MCMC runs were performed with 100 million generations each, which was evaluated via effective sample size (ESS) values in Tracer v. 1.4 (Rambaut and Drummond, 2007). Mean values and 95 % highest posterior density (HPD) intervals of ages were then calculated from post-burn-in trees using TreeAnnotator v. 1.8.4 (Drummond and Rambaut, 2007).
Ancestral range estimation
Ancestral area probabilities were inferred within Buxales using the maximum clade credibility (MCC) tree from the BEAST analysis, pruned to 89 taxa with the R package ‘phyloch’ v. 1.5–5 (Heibl, 2008). Considering that limits of some species are not yet clear and that distribution areas depend on currently accepted names with which specimens are identified, we coded all samples by the respective areas from which they were collected. The estimation was done with BioGeoBears implemented in RASP v. 4.2 (Yu et al., 2015, 2020). When a taxon occurred in distant geographical localities, respective terminals were included for different geographical locations as this was needed for the analysis of ancestral migration routes in the Caribbean. We coded six distribution areas: A, Cuba; B, other Caribbean islands; C, South America (including Panama); D, Mexico–Mesoamerica (until Costa Rica); E, Eurasia; and F, Africa and neighbouring islands (Supplementary Data Table S5). We evaluated the models Bayarealike, DIVA and DEC, compared the AIC values and performed likelihood ratio tests.
Selection and pruning of trees for further analyses
Using Mesquite v. 3.70 (Maddison and Maddison, 2021), we randomly extracted 500 trees from the post-burn-in tree distribution obtained with BEAST. For the diversification rate and character evolution analyses, the trees were pruned to include only one terminal per taxon (species or subspecies) of Buxus in order not to introduce any bias, using the R package ‘phyloch’ v. 1.5–5 (Heibl, 2008). With the R package ‘phangorn’ v2.5.5 (Schliep, 2011) we then computed the MCC tree.
Diversification rates across trees
We evaluated possible rate shifts across the entire Buxales. Rate heterogeneity of speciation and extinction was calculated with BAMM v. 2.5.0 (Rabosky, 2014). We defined sampling fractions for all main clades (Supplementary Data Table S6). In four independent runs analyses were performed with 10 000 000 MCMC generations each, and a sampling frequency of 1000. With the R package BAMMtools v. 2.1.5 (Rabosky et al., 2014) we subsequently analysed and visualized the results and with the R package CODA v. 0.19–1 (Plummer et al., 2006) we examined run convergence. We removed 10 % of all sampled MCMC generations as burn-in, inferred posterior probabilities of rate-shift configurations under different numbers of rate shifts and calculated BFs for evaluation. All rate-shift configurations with prior and posterior probabilities greater than zero were compared with a zero-shift model, considering BF > 20 as substantial evidence for one model over another, and BF > 50 as very strong evidence in support of the model under analysis. The 95 % credible set of distinct rate-shift configurations and the single best-shift configuration with the highest posterior probabilities were generated as final results.
Definition and coding of traits
Trait 1 records the tolerance to ultramafic soils (1 = grows only on limestone and other non-ultramafic substrates; 2 = occurs on ultramafic substrates and on non-ultramafic substrates; 3 = occurs only on ultramafic substrates). The states of the Cuban taxa were taken from field observations (herbarium labels) or were assessed from the treatment of Buxus for Cuba (Köhler, 2014). For the other species we obtained information from publications, herbarium labels and personal communications with regional experts (Supplementary Data Table S7). Trait 2 entails the ability to accumulate Ni with three states defined following the criteria of Brooks et al. (1977), Reeves (1992) and Reeves et al. (1996): 1 = no Ni accumulation, i.e. Ni content in dry leaf tissue <100 μg g−1 and plants growing on substrates without Ni or with very low concentrations of it; 2 = Ni accumulator, i.e. reported Ni content between 100 and 1000 μg g−1; 3 = Ni hyperaccumulator, i.e. at least one sample with Ni concentration >1000 μg g−1 known. Data concerning the Cuban taxa were taken from Reeves et al. (1996) and from our own sources (Supplementary Data Table S8). Middle-aged leaves from 32 specimens of 22 Buxus taxa [mostly described by E. Köhler after the publication of Reeves et al. (1996)] were removed from herbarium specimens. The samples were vacuum-dried for 2 days before homogenization with plastic and ceramic instruments to avoid metal contamination, and after that they were pulverized using a ceramic mortar and pestle. Samples were digested in a closed vessel microwave system (MARS5, CEM, Matthews, USA) using nitric and hydrochloric acids following the procedure described by Brackhage et al. (2014). The limit of detection (LOD) was calculated as the 3-fold standard deviation of instrument blank (acidified water). According to DIN (2004) the elemental analysis was performed with an inductively coupled plasma mass spectrometer (PQ exCell, Thermo Fisher Scientific, UK). Calibrations were performed with mixed calibration samples which consisted of single and multi-element solutions (Bernd Kraft, Duisburg, Germany). Calibration validity was confirmed with digests of standard reference material (GBW7604, poplar leaves, China). The resulting Ni concentration was converted from micrograms per litre to micrograms per gram of dry mass.
State-dependent speciation and extinction rates
To examine the impact of traits on diversification rates we conducted multi-state speciation and extinction (MuSSE) analyses as implemented in the R package ‘diversitree’ v. 0.9-15 (FitzJohn, 2012) for two traits. We specified a global sampling fraction for the MuSSE analyses (Supplementary Data Tables S9 and S10). We are aware of the current discussion on theoretical backgrounds regarding methods to analyse diversification rates. Here we concur with the views expressed by Helmstetter et al. (2022) rather than by Louca and Pennell (2020), who advocate using a deterministic lineage through time plot (LTT). Also, LTTs summarize the overall accumulation of lineages over time within trees, whereas our approach was to test specific hypotheses on diversification rates depending on state transformation of a multi-state character by calculating the fit of specific models, focusing at a shallow terminal subclade of Buxus. According to our hypotheses, we defined 42 models for trait 1 (tolerance to ultramafic soils) and 30 models for trait 2 (ability to accumulate Ni) with nine parameters each (i.e. the rates of speciation, extinction and character transition under character states 1, 2 and 3). The number of constraints applied to each model was varied and rates were either set as equal or zero across character states (Supplementary Data Tables S11 and S12). We evaluated each model compared with the unconstrained model: with likelihood ratio tests we determined if any model was significantly different from the unconstrained model, and by ML optimization we examined relative model fit and identified the best-fitting model for each character via the AIC as well as the AICc (AIC corrected for small sample size; Hurvich and Tsai, 1989). After selecting the appropriate model for trait 1 (tolerance to ultramafic soils) and trait 2 (ability to accumulate Ni) via ML optimization, we applied MCMC sampling to estimate marginal posterior probability densities for the diversification rate parameters of the two best-fitting models. To integrate over phylogenetic uncertainty, parameter estimates were calculated over a post-burn-in tree distribution of 100 trees derived from the 500-tree file. MCMC searches were conducted for 10 000 generations and 95 % HPD intervals calculated for each parameter.
Ancestral character state reconstruction
We reconstructed the ancestral states of traits under two different models: (1) with the function ‘asr’ of the R package diversitree v. 0.9-15 (FitzJohn, 2012) using the best-fitting model found in MuSSE; and (2) with BayesTraits v. 2 (Pagel et al., 2004; Pagel and Meade, 2006). Probabilities of ancestral states were visualized on the MCC tree. BayesTraits analyses employed a post-burn-in tree random sample of 500 trees. Commands were generated with TreeGraph2 v. 2.14.0-771 (Stöver and Müller, 2010) for both MCMC and ML, using reverse jump hyper-prior and with restricted transition rates. Probabilities of ancestral states were then visualized on the MCC tree in TreeGraph2.
RESULTS
Overall relationships within Buxaceae: Buxus relationships depict a Caribbean clade
The genus Buxus was highly supported as monophyletic and sister to a clade of Pachysandra, Stylocereus and Sarcococca, in line with results of Balthazar et al. (2000). The genus Sarcococca is not recovered as monophyletic since S. conzattii from Mexico and Guatemala appears as more related to Pachysandra and Styloceras than to other species of Sarcococca from Asia (Fig. 1; Supplementary Data Fig. S1). The inclusion of S. conzattii within Sarcococca was questioned earlier (Sealy, 1986).
Fig. 1.

Bayesian majority rule tree based on the concatenated matrix of trnK-matK + petD + trnL-F and indels of Buxus. Supports are given as: Bayesian inference posterior probabilities (shown above branches, left), ML bootstrap (bootstrap support, shown above branches, right) and maximum parsimony jackknife values (shown below branches). Highlighted in violet is the Gonoclada subclade and B. revoluta, which belongs to it (upper left photograph by Kurt Zoglauer), in yellow is the Shaferi subclade and B. foliosa, which belongs to it (middle left photograph by John Clark), and in green is the Glomerata subclade and B. rotundifolia, which belongs to it (lower left photograph by Pedro A. González Gutiérrez). Scale bars in photographs = 1 cm.
Within Buxus, our trees resolve three clades: the Eurasian clade, the African clade and the American clade corresponding to the sections Eubuxus, Tricera and Probuxus originally proposed by Mathou (1940). In the American clade, the Mexican species appear in two successive lineages, the second of which includes the west Cuban B. brevipes. Such affinities were already suggested by anatomical data (Köhler and Schirarend, 1989). Most species are depicted in a Caribbean clade in which B. jaucoensis, an endemic from cliffs of south-eastern Cuba (Fig. 1; Supplementary DataFig. S1) branches first. The Caribbean Buxus clade entails three subclades differing in distribution and ecology. The Gonoclada subclade includes taxa from the east and central phytogeographic subprovinces of Cuba (Borhidi, 1996), and all of them are restricted to ultramafic substrates and are Ni hyperaccumulators. The Shaferi subclade includes species from north-eastern Cuba, which grow in the understorey of rainforests or along streams and rivers. Apart from B. yunquensis, they are limited to ultramafic substrates. The Glomerata subclade is distributed in all three phytogeographic subprovinces of Cuba and extends to four other Caribbean islands and to northern South America up to Panama. Its species inhabit a wide range of ecological conditions on limestone or ultramafic soils from the coast to the mountains. The almost complete sampling of Buxus for the Neotropics in our study allows us to draw a representative picture of phylogenetic relationships (Fig. 1), which is essential for understanding the evolutionary diversification of this group in time and space. However, some of the currently recognized subspecies (e.g. B. gonoclada) does not apparently include vicariant subentities of the species they are currently assigned to , which is in line with morphological differences . We have therefore regarded all taxa with the same weight in our diversification analysis, irrespective of specific or subspecific rank.
Caribbean Buxus diversification started in the Miocene
Divergence times estimated in all six scenarios are very similar for the Caribbean Buxus clade and subclades (Supplementary Data Fig. S2, Supplementary Data Table S13). The assumption of an older maximum age of the eudicot crown (145 Mya; scenarios 1 and 2) leads to marginally older deep nodes in Buxus (in the range of ~1 Mya) and the crown groups of the Eurasian, African and American clades. Also, the confidence intervals of node ages in all six scenarios are in the same range. We therefore consider the divergence times inferred here as robust for the subsequent analyses of evolutionary diversification. For further analyses we chose scenario 3 (Fig. 2). The crown of the Caribbean Buxus clade dates to the middle to late Miocene (13.25 Mya, 95 % HPD 10.07–17.39). The crowns of the Glomerata subclade (5.4 Mya, 95 % HPD 3.86–7.27), Shaferi subclade (4.52 Mya, 95 % HPD 2.85–6.63) and Gonoclada subclade (3.19 Mya, 95 % HPD 1.82–4.88), then diverged during the late Miocene and Pliocene.
Fig. 2.

MCC tree of BEAST scenario 3 showing the times of divergence of the Caribbean Buxus clade and subclades. The ages (in million years) of the Caribbean Buxus clade and the subclades are indicated with bold black numbers. Plotted pies are the BioGeoBears ancestral areas estimation under the DEC + J model. Highlighted in violet are the Gonoclada subclades and its distribution (upper left), in yellow are the Shaferi subclade and its distribution (middle left), and in green are the Glomerata subclade and its distribution (lower left).
Ancestral distributions of Caribbean Buxus
The DEC + J model scored the highest relative probabilities (Supplementary Data Table S14), based on AICc values and their weights. Under this model Cuba is likely the most ancestral area of the Caribbean clade of Buxus. The ancestors of the Gonoclada, Shaferi and Glomerata subclades also originated in Cuba (Fig. 2; Supplementary DataFig. S3).
Shifts in diversification rates
Our analyses in BAMM indicated that the two-shift configuration had the highest posterior probability (0.32) (Supplementary Data Fig. S4) with convergence assessed in four independent runs via ESS values (ESS log-likelihood, 1676.60–2469.17; ESS number of shifts, 3154.11–3563.72). Model comparison via BFs showed very strong evidence in support of a model with one or more shifts relative to the null model, as BFs for all models with one or more shifts were >50, ranging from 68.2 to 601.3. Computing the 95 % credible set of macroevolutionary rate shifts yielded a set of four distinct shift configurations, out of which two accounted for 93 % of the posterior probability (Fig. 3).
Fig. 3.
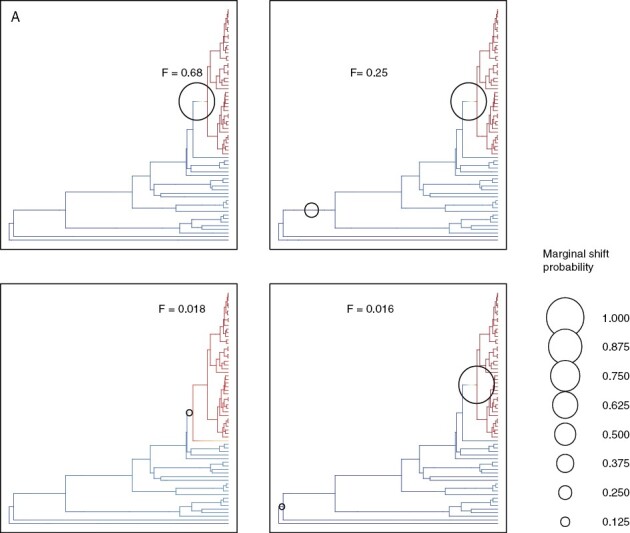
BAMM shift configurations with the highest posterior probabilities out of the 95 % credible set of distinct shift configurations. The plots display model-averaged mean rate parameters across all samples of a given configuration. The top left plot highlights the plot that displays the most frequently sampled configuration out of the credible shift. Blue represents low rates and red represents high rates. The circles mark the positions of the most probable speciation shifts.
The first configuration accounted for 68 % of the probability of the data, the second for 25 %, the third for 1.8 %, and the fourth for 1.6 %. The model with one shift had the highest posterior probability (0.68). A phylorate plot that displays the most frequently sampled configuration out of the credible shift set is given in Fig. 3.
State-dependent speciation and extinction
In our MuSSE analyses of trait 1 (tolerance to ultramafic soils) we found 26 of the 42 models to be significantly different from the unconstrained model: 25 models were significantly worse and only one model was significantly better compared with the unconstrained model. Comparisons of relative model fit via the AICc showed that model 41, with transition rates constrained to be stepwise between character states forward and reverse and extinction rates set to 0 in states 1 and 3 (q31 ~ 0, q13 ~ 0, μ1 ~ 0, μ3 ~ 0), was the best-fitting model (Supplementary Data Table S11). Accordingly, we calculated the parameter values for speciation, extinction and character state transitions under model 41 (Supplementary Data Table S15). Rates of speciation were found to be elevated stepwise between character states (λ1 < λ 2 < λ3). Trait transitions appeared to be highest for reversals from state 2 to state 1, while in general reversals appeared more often than transitions in the other direction.
MuSSE analyses of trait 2 (ability to accumulate Ni) revealed 20 of the 30 models to be significantly different from the unconstrained model: 15 models were significantly worse and 5 models were significantly better compared with the unconstrained model. Comparisons of relative model fit via the AICc showed that model 11, with transition rates constrained to be stepwise between character states forward and reverse (q31 ~ 0, q13 ~ 0), was the best fitting model (Supplementary Data Table S12). Accordingly, we calculated the parameter values for speciation, extinction and character state transitions under model 11 (Supplementary DataTable S16). Rates of speciation were found to be similar with states 1 and 2 but strongly elevated with state 3. Extinction rates were generally low, resulting in strongly elevated net diversification (= speciation minus extinction) rates for character state 3 compared with both other states.
Accumulation of Ni in extant Buxus taxa
The first broad assessment of Ni concentration in Buxus was conducted by Reeves et al. (1996). More recently, Belloeil et al. (2021) added data on two further Cuban species of Buxus. However, these authors could not cover all currently accepted species, because many were not known to science at that time in the case of Reeves et al. (1996), or due to lack of material in the case of Belloeil et al. (2021). Here we report Ni concentrations of further species (B. cristalensis, B. ekmanii, B. glomerata, B. nipensis and B. yunquensis) and subspecies (B. ekmanii ssp. woodfredensis, B. gonoclada ssp. orientensis, B. gonoclada ssp. toldoensis and B. pilosula ssp. cacuminis), mostly from Cuba, and a sample of B. glomerata from Dominican Republic (Supplementary Data Table S8). From the Cuban Buxus species and subspecies accepted by Köhler (2014), 22 taxa are Ni hyperaccumulators and four Ni accumulators. Within Buxus, only Cuban taxa and a population of B. microphylla growing on ultramafic substrates in Japan have been identified as Ni accumulators (Mizuno and Kirihata, 2015). From other genera of Buxales only Sarcococca saligna from Pakistan was shown to accumulate modest amounts of Ni (Muhammad et al., 2013) (Supplementary Data Table S8). This updated assessment supported our MuSSE analyses (Supplementary Data Table S10).
Ancestral character state reconstruction of Buxus on ultramafic substrates
Under model 41, found to be the best MuSSE model (Supplementary Data Tables S11 and S15), the ancestral character state reconstruction indicates that the ancestor of the Caribbean Buxus was able to grow on ultramafic and non-ultramafic substrates, whereas the ancestors of the three subclades occurred on ultramafic substrates. Within the Glomerata subclade the specialization of being able to grow on ultramafic substrates was lost three times (growth on ultramafic and non-ultramafic substrates) and seven extant species reversed to only grow on non-ultramafic substrates (Fig. 4; Supplementary Data Fig. S5). The ancestral character state reconstruction conducted in BayesTraits shows that the ancestor of the Caribbean clade probably was a plant specialized for ultramafic substrates as well as the ancestors of the three internal subclades (Fig. 4; Supplementary Data Fig. S6).
Fig. 4.

(A) Phylorate plot of the single best overall shift configuration taken from the 95 % credible set of distinct shift configurations. (B) Reconstruction of ancestral character state of Buxus with BayesTraits (pies above the branches) and MuSSe (pies under the branches). For both methods trait 1 [tolerance to ultramafic soils (S)] is indicated by the pies on the left and trait 2 [ability to accumulate Ni (Ni)] by the pies on the right. The red star highlights the most probable speciation shift reconstructed by BAMM and shown in the phylorate plot.
Ancestral character state reconstruction of Ni accumulation and hyperaccumulation in Buxus
Model 11 was inferred as the best MuSSE model (Supplementary Data Tables S12 and S16). Under this model the ancestor of the Caribbean Buxus was not an Ni accumulator like the ancestor of the Glomerata subclade. In contrast, the ancestors of the Gonoclada and Shaferi subclades are shown as Ni hyperaccumulators. Moreover, two species within the Shaferi subclade shifted back from Ni hyperaccumulation to Ni accumulation (Fig. 4; Supplementary Data Fig. S7). The BayesTraits results converge in that the ancestors of the Caribbean and of the Glomerata subclade were not Ni accumulators. Within the latter clade only B. marginalis evolved the ability to accumulate Ni. According to this analysis the ancestor of the Shaferi subclade was an Ni accumulator, and most species later developed Ni hyperaccumulation, except B. yunquensis and B. leivae, which are Ni accumulators. The ancestor of the Gonoclada subclade was also found to be an Ni hyperaccumulator and this state has been maintained during the evolution of this clade (Fig. 4; Supplementary Data Fig. S8).
DISCUSSION
Arrival of Buxus in Cuba and its dispersal on the Cuban archipelago of ultramafic outcrops and in other Caribbean territories
Cuba is the ancestral area for the diversification of Caribbean Buxus. The close relationship of Cuban with Mexican Buxus (Fig. 1) is consistent with the geological hypothesis that since the Eocene–Oligocene boundary (≈35–33 Mya) Cuba has been closest to the Mexican mainland out of all Antillean islands (Iturralde-Vinent, 2006). Our results indicate the ancestors of the Caribbean Buxus arrived from Mexico in the Miocene (13.25 Mya; Fig. 2). However, it is remarkable that B. jaucoensis as the sister to all other Cuban and Caribbean Buxus is now a local endemic growing in the south-east of Cuba, at present at the greatest distance from Mexico. Considering that the known extant populations of B. mexicana are in the highlands far away from the coast, thus limiting dispersal, it could be that the ranges of their ancestors were more widespread. On the contrary, B. brevipes may result from an independent, arrival in western Cuba (the current Pinar del Rio province) over relatively short geographical distances (Figs 1 and 2).
The crown group age of the Caribbean Buxus (13.25 Mya) is comparable to that of other Caribbean clades, within genera such as Casearia (Mestier et al., 2022), or within Euphorbiaceae (Cervantes et al., 2016) or Rutaceae [Caribbean endemic genus Spathelia (Appelhans et al., 2012)]. The genus Buxus therefore provides another example of a plant lineage that arrived in the Miocene and diversified in the Caribbean archipelago (see also Nieto-Blásquez et al., 2017). However, previous studies have provided only little phylogenetic resolution within Caribbean clades or have not included species-rich genera with many obligate ultramafic taxa.
Our ancestral area reconstruction shows that the diversification of Caribbean Buxus originated in Cuba, from where several dispersals took place: one within Cuba, a second to the other Caribbean islands and a third to northern South America (Fig. 2). The Glomerata subclade (5.4 Mya) is the oldest among the three internal subclades and it includes taxa distributed in Cuba, in other Caribbean islands (B. arborea, B. portoricensis, B. vahlii) and in northern South America (B. citrifolia). Our ancestral area reconstruction indicates that Cuba acted as a species pump for the Caribbean area. Considering that the islands were separated, the migration from Cuba to these islands must have involved over-water long-distance dispersal, probably facilitated by hurricanes (Nathan et al., 2008; Presley and Willig, 2008). This also applies to B. citrifolia, the Cuban ancestor of which reached northern South America. Fruits of Buxus are typically dehiscent three-carpelar (very rarely two-carpelar) capsules. The seeds are primarily dispersed passively, ballistically and occasionally by ants (Lázaro et al., 2006). There are no records of Buxus seed dispersal by birds or bats. Because the seeds and capsules of Buxus are too heavy to be transported by regular winds, the most likely mode of colonization in the Caribbean area was extreme meteorological events like hurricanes.
The Shaferi subclade, although being only slightly younger (4.52 Mya, Fig. 2) than the Glomerata subclade, just diversified in the mountainous area of north-eastern Cuba. This may be explained by specific habitat requirements as all species are confined to riverine vegetation or wet rainforests on ultramafic soils (Köhler, 2014). This area has the highest amounts of rainfall on the island (Nuevo Atlas Nacional de Cuba, 1989; Borhidi, 1996). The members of the even younger Gonoclada subclade (3.19 Mya) appear to have spread to central Cuba (e.g. Villa Clara), growing in mesophytic to xerophytic vegetation on ultramafic soils, thus being isolated on the Cuban archipelago of ultramafic outcrops.
Diversification rates in Buxus are connected to increased adaptation to ultramafic habitats
This is the first analysis of diversification rates (speciation minus extinction) of a Caribbean clade of flowering plants. Our trait-independent diversification rate analysis with BAMM detected an overall increase in diversification rates for the Caribbean Buxus clade (Fig. 3) compared with the other clades of Buxus. This upshift of diversification rates corresponds to the core clade of Caribbean Buxus after the divergence of B. jaucoensis, for which the ancestor was reconstructed with high probabilities as an ultramafic substrate endemic and Ni hyperaccumulator with MuSSE and BayesTraits (Fig. 4). Since the best model explaining the evolution of character states assumes a stepwise acquisition of the ability to grow on ultramafic substrates, becoming an ultramafic endemic (character 1) and then accumulating Ni (character 2) the stepwise adaptation to ultramafic soils during the evolution of Buxus is evident. With the elevated speciation rates in particular depending on the acquisition of the ability to hyperaccumulate Ni, at the same time extinction rates being low (Supplementary Data Tables S2 and S3), our results provide evidence for further increased diversification rates in the Gonoclada and Shaferi subclades. Their ability to accumulate and hyperaccumulate Ni is therefore likely to have triggered the speciation rates in Cuban Buxus.
Ultramafic substrates can contribute to speciation in two ways: (1) they can be a barrier to the reproductive process that genetically isolates ultramafic populations from non-ultramafic relatives; and (2) the patchy distribution of ultramafic substrates can contribute to the geographical isolation of populations (Rajakaruna, 2004; Kay et al., 2011). In Cuban Buxus both factors could have influenced speciation, considering the archipelago-like distribution of Cuban ultramafic outcrops (Reeves et al., 1996; Berazaín-Iturralde, 2001). However, the vast majority of species in the Ni-hyperaccumulating Gonoclada clade are centred in eastern Cuba (Fig. 2), with only few migration events to distant central Cuban ultramafic outcrops. A deeper understanding of speciation patterns will therefore require population-level analyses across the ranges of the respective species.
Divergence times of Ni-adapted Cuban and Caribbean plant lineages
The age of the ultramafic outcrops and thus the time when ultramafic substrates first became available, their size and the richness of the surrounding flora have been pointed to as factors influencing the evolution of their floras (Borhidi, 1996; Kazakou et al., 2008 and references therein). The largest ultramafic outcrops of Cuba are in the mountain ranges of Nipe, Cristal and Moa-Baracoa (north-eastern Cuba) and they are also the oldest Cuban ultramafic outcrops (~10–30 Mya; Borhidi, 1996). The diversification of Buxus adapted to ultramafic substrates within the Caribbean Buxus clade appears to be slightly younger than those of other Caribbean clades adapted to such substrates. For example, the crown group of Spathelia with 11 species dates to 8.7 Mya (Appelhans et al., 2012) and the Leucocroton–Lasiocroton–Garciadelia and Acidoton–Platygyna within Acalyphoideae that are exclusively or mostly constituted by obligate ultramafic species (Cervantes et al., 2016) started to diversify 9.1 and 9.3 Mya, respectively. Interestingly, all >26 species of Leucocroton in its monophyletic circumscription (Jestrow et al., 2012) are Ni hyperaccumulators, whereas this is not the case in the less diverse successive sisters Lasiocoroton (six species) and Garciadelia (three species). The Cuban subclade within Casearia, with obligate ultramafic species, is younger (6.1 Mya crown group age; Mestier et al., 2022) and comprises five or six species, of which only two are reported as Ni-accumulating (Reeves et al., 1999). The many Ni-hyperaccumulating species of Phyllanthus all belong to the large Caribbean clade (Falcón et al., 2020), whereas an independently evolved small Caribbean clade has no obligate ultramafic species. However, speciation and extinction rates have not yet been determined for any of these lineages and their species-level relationships are still insufficiently known.
Evolution of Ni accumulation and hyperaccumulation in Cuban Buxus
The ancestor of the Caribbean Buxus clade was able to grow on ultramafic substrates (under MuSSE and BayesTraits ancestral character state reconstruction), whereas there is no strong indication that it already accumulated Ni (Fig. 4). The closest relatives of the Caribbean Buxus clade are species recorded in non-ultramafic substrates of Mexico and Central America (Belize, Guatemala, El Salvador). Also B. jaucoensis as the south-eastern Cuban sister to the core Caribbean Buxus clade has an exclusive limestone ecology, which further suggests that Ni tolerance evolved on Cuba. Some African and Eurasian species also grow on ultramafic substrates (Schatz and Lowry, 2002; Mizuno and Kirihata, 2015), which may indicate that Buxus possesses an ancestral fortuitous pre-adaptation, known as ‘exaptation’ (Pillon et al., 2010; Jaffré et al., 2013). Such exaptation to grow on extreme environments like ultramafic substrates, if understood as a pre-adaptation, could have facilitated the adaptation of the ancestors of the core Caribbean Buxus clade to the ultramafic Cuban outcrops.
The ancestral character state reconstructions reveal that the ancestor of the Caribbean Buxus clade, like the ancestor of the Glomerata clade, probably did not accumulate Ni (Fig. 4). Low concentrations of Ni in the leaf tissues of plants of the Glomerata subclade (Fig. 4; Supplementary Data Table S8) growing on ultramafic substrates are in line with exclusion or limitation of Ni uptake, as this could have been the initial strategy of Buxus adapting to Cuban ultramafic soils.
To the contrary, the ancestors of the Shaferi and Gonoclada subclades were ultramafic endemics and Ni hyperaccumulators by MuSSE (Fig. 4), although the BayesTraits reconstruction under a less specific model found the ancestor of the Shaferi subclade to most probably have been a Ni-accumulating plant. This indicates a stepwise evolution of Ni hyperaccumulation from simply tolerant and non-accumulating plants via Ni accumulators to highly specialized Ni hyperaccumulators. And even the extent of Ni hyperaccumulation shows an increase from plants belonging to the Shaferi subclade (reaching a maximum of 1940 µg g−1 in B. imbricata) compared with taxa belonging to the Gonoclada subclade (up to 30 759 µg g−1 in B. nipensis) (Reeves et al., 1996 and Supplementary Data Table S8). The Gonoclada subclade as the most recent lineage (crown group age of 3.19 Mya) also shows the highest specialization in Ni hyperaccumulation (Fig. 4). On the other hand, B. leivae as well as B. yunquensis reversed from Ni hyperaccumulation to Ni accumulation (Fig. 4), but this could also result from an environmental effect of lower Ni concentrations in the respective substrates (Van der Pass and Ingle, 2019). Similar reversals were observed by Mengoni et al. (2003) in Odontarrhena (= Alyssum).
Although investigations on the evolution of Ni accumulation and hyperaccumulation are still scarce, the available publications provide some insights on the direction of the evolution of Ni accumulation and Ni hyperaccumulation in plant groups occurring in geographical areas other than the Caribbean and without detailed biogeographic and speciation rate analyses, as in the case of Buxus reported here. Cecchi et al. (2010) pointed out that the ability to accumulate Ni in Alysseae (Brassicaceae) has been lost or gained over different south European ultramafic areas through independent events based on nrITS trees, but further phylogenetic research and analyses of species limits will be needed in that case to evaluate how far hybridization and reticulation as well as adaptive ecological speciation played a role. In the Australian genus Stackhousia only one species from ultramafic soils was identified as an Ni hyperaccumulator, representing probably an early stage of the evolution of the trait during the diversification of Stackhousia (Burge and Barker, 2010). Although the evolution of Ni hyperaccumulation in the Cuban endemic genus Leucocroton was not an objective of Jestrow et al. (2012), the detected monophyly of this plant group and the fact that all species are Ni hyperaccumulators indicate that this ability was acquired once in a common ancestor. In the New Caledonian species of the genus Geissois the ability to hyperaccumulate Ni appeared in G. hirsuta, a species occurring on ultramafic and on non-ultramafic substrates (Pillon et al., 2014).
The genetic bases of Ni accumulation and hyperaccumulation are not well understood (Seregin and Kozhevnikova, 2006) and only recently García de la Torre et al. (2021) provided evidence for a convergent role of IREG/ferroportin transporters in the evolution of Ni hyperaccumulation across angiosperms. Boyd and Martens (1992) hypothesized Ni hyperaccumulation to be a defence mechanism against herbivores and pathogens (Boyd, 2004), and also a protection against environmental stresses such as UV irradiation (Berazaín-Iturralde et al., 2007). Moreover, Ni hyperaccumulation in Cuban Buxus could serve as a barrier for avoiding excessive loss of water in the harsh conditions of ultramafic environments. The mesophyll and epidermis cells of Cuban Ni-hyperaccumulating species are considerably darker than in other species, which suggests that Ni could be stored in these cells (Köhler and Schirarend, 1989). Our results underscore that Ni hyperaccumulation is a fixed state at the gene level that was obviously passed on to descendant species in the radiating Buxus Gonoclada subclade (Fig. 4), which then also spread across distant ultramafic outcrops. The ability to accumulate Ni has thus to be seen as a key innovation rather than a consequence of ecological speciation with recurrent shifts from non-accumulating species or populations (Rundle and Nosil, 2005), converging with observations by Sobczyk et al. (2017) in Odontarrhena (= Alyssum). Whereas García de la Torre et al. (2021) compared distant taxa, this investigation in Buxus includes all species of an evolutionary radiation, thus illuminating character state transitions at species level. Buxus could therefore be an interesting model to reconstruct the molecular evolution of the genes involved in increased levels of Ni accumulation, including the identification of mutations leading to higher expression of FPNI/IREG genes, raised by Clemens (2021).
Conclusions
Nickel-adapted subclades appear as part of the Caribbean clades, which has become evident also in other flowering plant lineages, such as Casearia (Salicaceae; Mestier et al., 2022), Acalyphoideae (Cervantes et al., 2016), Phyllanthus (Falcón et al., 2020) or Clusia (Cristina Panfet, Jardin Botanico Nacional de Cuba ). This study for the first time demonstrates elevated speciation rates in Caribbean plant radiations, which are connected to traits like tolerance or adaptation to specific ultramafic soils and physiological features (Ni accumulation and Ni hyperaccumulation) rather than to a time-for-speciation effect (Stephens and Wiens, 2003), as was concluded by Roncal et al. (2020) in relation to earlier or later arrivals. Considering the high number of obligate ultramafic taxa in the Cuban and other Caribbean floras, the effect of traits linked to Ni on speciation may override other factors. Further lineages need to be analysed in a detailed way to draw more general conclusions. Our results suggest that Cuba could have acted as a species pump and constituted an ancestral area for the colonization of other islands, primarily fuelled by the high habitat diversity of Cuba. Nevertheless, Cuba may have been colonized more than once by closely related ancestors, as B. brevipes indicates, with different evolutionary success depending on the environmental conditions in the area of arrival. To test this, an almost complete taxon sampling of species-rich lineages on Cuba, adjacent islands and the mainland together with the inference of resolved phylogenies will be needed.
SUPPLEMENTARY DATA
Supplementary Data are available online at https://academic.oup.com/aob and consist of the following.
Figure S1: Bayesian majority rule tree based on the concatenated matrix of trnK-matK + petD + trnL-F and indels of Buxus. Figure S2: MCC trees of BEAST, scenarios 1–6, showing divergence times estimation for Buxus. Figure S3: reconstruction of ancestral areas in Buxus with BioGeoBears. Figure S4: BAMM prior and posterior probability shifts distribution in Buxus. Figure S5: ancestral character state reconstruction of trait 1 with MuSSE. Figure S6: ancestral character state reconstruction of trait 1 with BayesTraits. Figure S7: ancestral character state reconstruction of Ni accumulation trait 2 with MuSSE. Figure S8: ancestral character state reconstruction of Ni accumulation trait 2 with BayesTraits. Table S1: taxa analysed in this study. Table S2: calibration scenarios tested in this study, conditions and priors. Table S3: information about fossils and secondary calibrations consulted for this study. Table S4: values of the marginal likelihood estimation and Bayes factor analyses using stepping-stone sampling. Table S5: distribution areas. Table S6: clade-specific sampling fractions specified for BAMM. Table S7 character states for trait 1. Table S8: character states for trait 2 and update of Ni concentrations in Buxus and other Buxaceae. Table S9: sampling proportions of taxa within Buxales for trait 1. Table S10: sampling proportions of taxa within Buxales for trait 2. Table S11: model fit of different multi-state speciation and extinction models regarding trait 1. Table S12: model fit of different multi-state speciation and extinction models regarding trait 2. Table S13: estimated divergence ages. Table S14: results of the BioGeoBears model test. Table S15: mean and median rates of speciation, extinction and character transition for trait 1 under the best-fitting model. Table S16: mean and median rates of speciation, extinction and character transition for trait 2 under the best-fitting model.
ACKNOWLEDGEMENTS
P.A.G.G. and T.B. designed the research, P.A.G.G., S.F-B. and T.B. conducted field work, P.A.G.G. conducted the laboratory work, and S.F.-B., P.A.G.G. and V.d.V. analysed the data with contributions from T.B. The manuscript was written by P.A.G.G. with contributions by T.B., S.F.-B., V.d.V. and R.B.-I. All authors contributed to editing the manuscript. We thank the following persons and institutions for helping us logistically and by providing samples or information: Rosa Rankin Rodríguez (National Botanical Garden of Cuba), Carmen Galdames (Smithsonian Tropical Research Institute, Panamá), Werner Greuter (Herbarium Mediterraneum at Università degli Studi di Palermo, Italy; Botanical Garden and Botanical Museum of Berlin, Germany), Kurt Zoglauer (Humboldt University of Berlin, Germany), James C. Solomon (Herbarium Missouri Botanical Garden, USA), Lilian Ferrufino (Honduras), Alberto Areces (Puerto Rico), Miguel Cházaro and R. Sánchez (University of Guadalajara, México), Sergio Avendaño (Institute of Xalapa, México), Yocupitzia Ramírez (Institute of Ecology, Michoacán, México), John Clark (University of Alabama, USA), and the curators of herbaria B, HAJB, IEB, MO, NY, TEFH, US, and XAL. Michael Rodewald (Botanical Garden and Botanical Museum of Berlin, Germany) and Susy Fuentes-Bazán (Botanical Garden and Botanical Museum of Berlin, Germany) prepared the figures. The work was technically supported in the laboratory by Gert Dudel (Technical University Dresden, Germany), Ramona Henkel (Humboldt University, Berlin, Germany), Bettina Giesicke (Free University, Berlin, Germany) and Markus Ackermann (Koblenz University, Germany). The Cuban and Panamanian environmental authorities are acknowledged for granting the respective collecting permits. Thanks to Jardín Botánico Nacional de Cuba for its support. This study was in partial fulfilment of the requirements of a PhD thesis of P.A.G.G. at Freie Universität Berlin. We are grateful to two anonymous reviewers for their comments that helped to further improve the original manuscript.
We dedicate this work to Dr Egon Köhler (Institut für Biologie, Humboldt Universität, Berlin, Germany), who studied the Cuban Buxus and described several taxa.
Contributor Information
Pedro A González Gutiérrez, Centro de Investigaciones y Servicios Ambientales de Holguín, Calle 18 s/n, entre 1ª y Maceo, Holguín 80100, Cuba.
Susy Fuentes-Bazan, Institut für Biologie der Freien Universität Berlin. Altensteinstraße 6, 14195 Berlin, Germany; Botanischer Garten und Botanisches Museum Berlin-Dahlem, Königin-Luise-Straße 6-8, 14195 Berlin, Germany.
Vanessa Di Vincenzo, Institut für Biologie der Freien Universität Berlin. Altensteinstraße 6, 14195 Berlin, Germany; Botanischer Garten und Botanisches Museum Berlin-Dahlem, Königin-Luise-Straße 6-8, 14195 Berlin, Germany.
Rosalina Berazaín-Iturralde, Jardín Botánico Nacional de Cuba, Carretera del Rocío Km 3½, Calabazar, Boyeros, La Habana, CP 19230, Cuba.
Thomas Borsch, Institut für Biologie der Freien Universität Berlin. Altensteinstraße 6, 14195 Berlin, Germany; Botanischer Garten und Botanisches Museum Berlin-Dahlem, Königin-Luise-Straße 6-8, 14195 Berlin, Germany.
FUNDING
This work was mainly supported by Verein der Freunde des Botanischen Gartens und Botanischen Museums Berlin-Dahlem e.V., which provided funding for field work in Cuba and scholarships to P.A.G.G. in Berlin. The German Academic Exchange Service (DAAD) partially supported P.A.G.G. for this study, from 1 September 2007 until 29 February 2008 (STIBET program) and from 15 March 2011 until 30 June 2011.
CONFLICT OF INTEREST
The authors declare not to have any conflict of interest.
LITERATURE CITED
- Acevedo-Rodríguez P, Strong MT.. 2012. Catalogue of seed plants of the West Indies. Smithsonian Contributions to Botany 98: 1–1193. [Google Scholar]
- Appelhans MS, Kessler PJA, Smets E, Razafimandimbison SG, Janssens SB.. 2012. Age and historical biogeography of the pantropically distributed Spathelioideae (Rutaceae, Sapindales). Journal of Biogeography 39: 1235–1250. [Google Scholar]
- Baker AJM. 1981. Accumulators and excluders – strategies in response of plants to heavy metals. Journal of Plant Nutrition 3: 643–654. doi: 10.1080/01904168109362867. [DOI] [Google Scholar]
- von Balthazar M, Endress P, Qiu Y-L.. 2000. Phylogenetic relationships in Buxaceae based on nuclear internal transcribed spacers and plastid ndhF sequences. International Journal of Plant Sciences 161: 785–792. [Google Scholar]
- Barniske A-M, Borsch T, Müller K, et al. 2012. Phylogenetics of early branching eudicots: comparing phylogenetic signal across plastid introns, spacers, and genes. Journal of Systematics and Evolution 50: 85–108. doi: 10.1111/j.1759-6831.2012.00181.x. [DOI] [Google Scholar]
- Bell CD, Soltis DE, Soltis PS.. 2010. The age and diversification of the angiosperms re-revisited. American Journal of Botany 97: 1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- Belloeil C, Jouannais P, Malfaisan C, et al. 2021. The X-ray fluorescence screening of multiple elements in herbarium specimens from the Neotropical region reveals new records of metal accumulation in plants. Metallomics 13: mfab045. doi: 10.1093/mtomcs/mfab045. [DOI] [PubMed] [Google Scholar]
- Berazaín-Iturralde R. 2001. The influence of ultramafic soils on plants in Cuba. South African Journal of Science 97: 510–512. [Google Scholar]
- Berazaín-Iturralde R, de la Fuente V, Rufo L, et al. 2007. Nickel localization in tissues of different hyperaccumulator species of Euphorbiaceae from ultramafic areas of Cuba. Plant and Soil 293: 99–106. [DOI] [PubMed] [Google Scholar]
- Borhidi A. 1996. Phytogeography and vegetation ecology of Cuba. Budapest: Akadémiai Kiadó. [Google Scholar]
- Borsch T, Hilu KW, Quandt D, Wilde V, Neinhuis C, Barthlott W.. 2003. Non-coding plastid trnT-trnF sequences reveal a well resolved phylogeny of basal angiosperms. Journal of Evolutionary Biology 16: 558–576. doi: 10.1046/j.1420-9101.2003.00577.x. [DOI] [PubMed] [Google Scholar]
- Boyd RS. 2004. Ecology of metal hyperaccumulation. New Phytologist 162: 563–567. doi: 10.1111/j.1469-8137.2004.01079.x. [DOI] [PubMed] [Google Scholar]
- Boyd RS, Martens SN.. 1992. The raison d’être for metal hyperaccumulation by plants. In: Baker AJM, Proctor J, Reeves RD, eds. The vegetation of ultramafic (serpentine) soils. Andover: Intercept, 279–289. [Google Scholar]
- Brackhage C, Huang J-H, Schaller J, Elzinga EJ, Dudel EG.. 2014. Readily available phosphorous and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.). Scientific Reports 4: 4944. doi: 10.1038/srep04944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks RR, Lee J, Reeves RD, Jaffre T.. 1977. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. Journal of Geochemical Exploration 7: 49–57. doi: 10.1016/0375-6742(77)90074-7. [DOI] [Google Scholar]
- Burge DO, Barker WR.. 2010. Evolution of nickel hyperaccumulation by Stackhousia tryonii (Celastraceae), a serpentinite-endemic plant from Queensland, Australia. Australian Systematic Botany 23: 415–430. doi: 10.1071/sb10029. [DOI] [Google Scholar]
- Canal D, Köster N, Jones KE, Korotkova N, Croat TB, Borsch T.. 2018. Phylogeny and diversification history of the large Neotropical genus Philodendron (Araceae): accelerated speciation in a lineage dominated by epiphytes. American Journal of Botany 105: 1035–1052. doi: 10.1002/ajb2.1111. [DOI] [PubMed] [Google Scholar]
- Cecchi L, Gabbrielli R, Arnetoli M, Gonnelli C, Hasko A, Selvi F.. 2010. Evolutionary lineages of nickel hyperaccumulation and systematics in European Alysseae (Brassicaceae): evidence from nrDNA sequence data. Annals of Botany 106: 751–767. doi: 10.1093/aob/mcq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes A, Fuentes S, Gutiérrez J, Magallón S, Borsch T.. 2016. Successive arrivals since the Miocene shaped the diversity of Caribbean Acalyphoideae (Euphorbiaceae). Journal of Biogeography 43: 1773–1785. doi: 10.1111/jbi.12790. [DOI] [Google Scholar]
- Clemens S. 2021. Casting a wide cross-species transcriptomics net: convergent evolution of nickel hyperaccumulation. New Phytologist 229: 653–655. [DOI] [PubMed] [Google Scholar]
- Crane PR, Pedersen KR, Friis EM, Drinnan AN.. 1993. Early Cretaceous (Early to Middle Albian) platanoid inflorescences associated with Sapindopsis leaves from the Potomac Group of eastern North America. Systematic Botany 18: 328–344. doi: 10.2307/2419407. [DOI] [Google Scholar]
- DIN. 2004. DIN-EN-ISO-17294-2, Wasserbeschaffenheit – Anwendung der induktiv gekoppelten Plasma-Massenspektrometrie (ICP-MS) – Teil 2: Bestimmung von 62 Elementen (ISO 17294-2:2003), Deutsche Fassung EN ISO 17294-2:2004. Berlin: Deutsches Institut für Normung. [Google Scholar]
- Doyle JA, Endress PK.. 2010. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: Magnoliidae and eudicots. Journal of Systematics and Evolution 48: 1–35. doi: 10.1111/j.1759-6831.2009.00058.x. [DOI] [Google Scholar]
- Drinnan AN, Crane PR, Friis EM, Pedersen KR.. 1991. Angiosperm flowers and tricolpate pollen of buxaceous affinity from the Potomac Group (Mid-Cretaceous) of eastern North America. American Journal of Botany 78: 153–176. doi: 10.1002/j.1537-2197.1991.tb15743.x. [DOI] [Google Scholar]
- Drummond AJ, Rambaut A.. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7: 214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Ho SYW, Phyllips MJ, Rambaut A.. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biology 4: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón B, Fuentes S, Berazaín R, Borsch T.. 2020. Phylogenetic relationships and character evolution of Phyllanthus (Phyllanthaceae), with a focus on the Cuban and Caribbean taxa. International Journal of Plant Sciences 181: 284–305. [Google Scholar]
- FitzJohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution 3: 1084–1092. doi: 10.1111/j.2041-210x.2012.00234.x. [DOI] [Google Scholar]
- Foster CSP, Sauquet H, van der Merwe M, McPherson H, Rossetto M, Ho SYW.. 2017. Evaluating the impact of genomic data and priors on Bayesian estimates of the angiosperm evolutionary timescale. Systematic Biology 66: 338–351. doi: 10.1093/sysbio/syw086. [DOI] [PubMed] [Google Scholar]
- Fujun MA, Qiujun W, Junling D, et al. 2015. Buxus leaves from the Oligocene of Guangxi, China and their biogeographical significance. Acta Geologica Sinica 89: 1453–1469. [Google Scholar]
- García de la Torre VS, Majorel-Loulergue C, Rigaill GJ, et al. 2021. Wide cross-species RNA-Seq comparison reveals convergent molecular mechanisms involved in nickel hyperaccumulation across dicotyledons. New Phytologist 229: 994–1006. [DOI] [PubMed] [Google Scholar]
- González Gutiérrez PA, Köhler E, Borsch T.. 2013. New species of Buxus (Buxaceae) from northeastern Cuba based on morphological and molecular characters, including some comments on molecular diagnosis. Willdenowia 43: 125–137. [Google Scholar]
- Hedges SB. 2001. Biogeography of the West Indies: an overview. In: Woods CA, Sergile FE, eds. Biogeography of the West Indies: patterns and perspectives. Boca Raton: CRC Press, 15–33. [Google Scholar]
- Heibl C. 2008. PHYLOCH: R language tree plotting tools and interfaces to diverse phylogenetic software packages. http://www.christophheibl.de/Rpackages.html. accessed November/2021
- Helmstetter AJ, Glemin S, Käfer J, et al. 2022. Pulled diversification rates, lineages-through-time plots, and modern macroevolutionary modeling. Systematic Biology 71: 758–773. doi: 10.1093/sysbio/syab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvich CM, Tsai C-L.. 1989. Regression and time series model selection in small samples. Biometrika 76: 297–307. doi: 10.1093/biomet/76.2.297. [DOI] [Google Scholar]
- Iturralde-Vinent MA. 1982. Aspectos geológicos de la Biogeografía de Cuba. Ciencias de la Tierra y del Espacio 5: 85–100. [Google Scholar]
- Iturralde-Vinent MA. 2006. La Paleogeografía del Caribe y sus implicaciones para la biogeografía histórica. Revista del Jardín Botánico Nacional 25–26: 49–78. [Google Scholar]
- Iturralde-Vinent MA, MacPhee RDE.. 1999. Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bulletin of the American Museum of Natural History 238: 1–95. [Google Scholar]
- Jaffré T, Pillon Y, Thomine S, Merlot S.. 2013. The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants. Frontiers in Plant Science 4: 1–7. doi: 10.3389/fpls.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffré T, Reeves RD, Baker AJM, Schat H, van der Ent A.. 2018. The discovery of nickel hyperaccumulation in the New Caledonian tree Pycnandra acuminata 40 years on: an introduction to a virtual issue. New Phytologist 218: 397–400. [DOI] [PubMed] [Google Scholar]
- Jestrow B, Gutiérrez-Amaro J, Francisco-Ortega J.. 2012. Islands within islands: a molecular phylogenetic study of the Leucocroton alliance (Euphorbiaceae) across the Caribbean islands and within the serpentinite archipelago of Cuba. Journal of Biogeography 39: 452–464. [Google Scholar]
- Kay KM, Ward KL, Watt LR, Schemske DW.. 2011. Plant speciation. In: Harrison S, Rajakaruna N, eds. Serpentine: the evolution and ecology of a model system. Oakland: University of California Press, 71–95. [Google Scholar]
- Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY.. 2008. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: from species to ecosystem level. Biological Reviews 83: 495–508. [DOI] [PubMed] [Google Scholar]
- Köhler E. 2014. Buxaceae. In: Greuter W, Rankin-Rodríguez R, eds. Flora de la República de Cuba. Vol. 19 (1). Königstein: Koeltz Scientific Books, 1–124. [Google Scholar]
- Köhler E, Schirarend C.. 1989. Zur Blattanatomie der neotropischen Buxus-Arten und ihre Bedeutung für die Systematik (Buxaceae). Flora 183: 1–38. [Google Scholar]
- Lázaro A, Traveset A, Castillo A.. 2006. Spatial concordance at a regional scale in the regeneration process of a circum-Mediterranean relict (Buxus balearica): connecting seed dispersal to seedling establishment. Ecography 29: 683–696. doi: 10.1111/j.2006.0906-7590.04667.x. [DOI] [Google Scholar]
- Löhne C, Borsch T.. 2005. Molecular evolution and phylogenetic utility of the petD group II intron: a case study in basal angiosperms. Molecular Biology and Evolution 22: 317–332. doi: 10.1093/molbev/msi019. [DOI] [PubMed] [Google Scholar]
- Louca S, Pennell MW.. 2020. Extant timetrees are consistent with a myriad of diversification histories. Nature 580: 502–505. doi: 10.1038/s41586-020-2176-1. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR.. 2021. Mesquite: a modular system for evolutionary analysis. Version 3.70. http://www.mesquiteproject.org. quizas este analisis se hizo en el propio 2021?, november o diciembre?
- Mathou T. 1940. Recherches sur la famille des Buxacées. Toulouse: Les Frères Douladoure Imprimeurs. [Google Scholar]
- Mengoni A, Baker AJM, Bazzicalupo M, et al. 2003. Evolutionary dynamics of nickel hyperaccumulation in Alyssum revealed by ITS nrDNA analysis. New Phytologist 159: 691–699. doi: 10.1046/j.1469-8137.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- Mestier Ade, Brokamp G, Celis M, Falcón B, Gutiérrez J, Borsch T.. 2022. Character evolution and biogeography of Casearia (Salicaceae): evidence for the South American origin of a pantropical genus and for multiple migrations to the Caribbean islands. Taxon 71: 321–347. [Google Scholar]
- Mizuno T, Kirihata Y.. 2015. Elemental composition of plants from the serpentine soil of Sugashima Island, Japan. Australian Journal of Botany 63(4): 252–260. doi: 10.1071/BT14226. [DOI] [Google Scholar]
- Muhammad S, Shah MT, Khan S, et al. 2013. Wild plant assessment for heavy metal phytoremediation potential along the mafic and ultramafic terrain in northern Pakistan. Biomed Research International 2013: 194765. doi: 10.1155/2013/194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K. 2004. PRAP – computation of Bremer support for large data sets. Molecular Phylogenetics and Evolution 31: 780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Müller KF. 2005a. SeqState-primer design and sequence statistics for phylogenetic DNA data sets. Applied Bioinformatics 4: 65–69. [DOI] [PubMed] [Google Scholar]
- Müller KF. 2005b. The efficiency of different search strategies in estimating parsimony jackknife, bootstrap, and Bremer support. BMC Evolutionary Biology 5: 58. doi: 10.1186/1471-2148-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Quandt D, Müller J, Neinhuis C.. 2005. PhyDE, Version 0.92: Phylogenetic data editor. www.phyde.de.
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J.. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A.. 2008. Mechanisms of long-distance seed dispersal. Trends in Ecology and Evolution 23: 638–647. doi: 10.1016/j.tree.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Nieto-Blázquez ME, Antonelli A, Roncal J.. 2017. Historical biogeography of endemic seed plant genera in the Caribbean: did GAARlandia play a role? Ecology and Evolution 7: 10158–10174. doi: 10.1002/ece3.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon KC. 1999. The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics 15: 407–414. doi: 10.1111/j.1096-0031.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Nuevo Atlas Nacional de Cuba. 1989. La Habana: Editorial del Instituto de Geografía y el Instituto Cubano de Geodesia y Cartografía. [Google Scholar]
- Pagel M, Meade A.. 2006. Bayesian analysis of correlated evolution of discrete characters by reversible jump Markov chain Monte Carlo. American Naturalist 167: 808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- Pagel M, Meade A, Barker D.. 2004. Bayesian estimation of ancestral character states on phylogenies. Systematic Biology 53: 673–684. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]
- Van der Pass L, Ingle LA.. 2019. Towards an understanding of the molecular basis of Nickel hyperaccumulation in plants. Plants (Basel) 8: 11. doi: 10.3390/plants8010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon Y, Munzinger J, Amir H, Lebrun M.. 2010. Ultramafic soils and species sorting in the flora of New Caledonia. Journal of Ecology 98: 1108–1116. doi: 10.1111/j.1365-2745.2010.01689.x. [DOI] [Google Scholar]
- Pillon Y, Hopkins HCF, Rigault F, Jaffré T, Stacy EA.. 2014. Cryptic adaptive radiation in tropical forest trees in New Caledonia. New Phytologist 202: 521–530. doi: 10.1111/nph.12677. [DOI] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K, Vines K.. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6: 7–11. [Google Scholar]
- Posada D, Crandall K.. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Presley SJ, Willig MR.. 2008. Composition and structure of Caribbean bat (Chiroptera) assemblages: effects of inter-island distance, area, elevation and hurricane-induced disturbance. Global Ecology and Biogeography 17: 747–757. doi: 10.1111/j.1466-8238.2008.00412.x. [DOI] [Google Scholar]
- Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One 9: e89543. doi: 10.1371/journal.pone.0089543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL, Grundler M, Anderson C, et al. 2014. BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods in Ecology and Evolution 5: 701–707. doi: 10.1111/2041-210x.12199. [DOI] [Google Scholar]
- Rajakaruna N. 2004. The edaphic factor in the origin of plant species. International Geology Review 46: 471–478. doi: 10.2747/0020-6814.46.5.471. [DOI] [Google Scholar]
- Rambaut A, Drummond AJ.. 2007. Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer. [accessed November/2021]
- Reeves RD. 1992. Hyperaccumulation of nickel by serpentine plants. In: Baker AJM, Proctor J, Reeves RD, eds. The vegetation of ultramafic (serpentine) soils. Andover: Intercept, 253–277. [Google Scholar]
- Reeves RD, Baker AJM, Borhidi A, Berazaín-Iturralde R.. 1996. Nickel accumulating plants from the ancient serpentine soils of Cuba. New Phytologist 133: 217–224. [DOI] [PubMed] [Google Scholar]
- Reeves RD, Baker AJM, Borhidi A, Berazaín-Iturralde R.. 1999. Nickel hyperaccumulation in the serpentine flora of Cuba. Annals of Botany 83: 29–38. [Google Scholar]
- Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A.. 2017. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytologist 218: 407–411. [DOI] [PubMed] [Google Scholar]
- Roncal J, Nieto-Blázquez ME, Cardona A, Bacon C.. 2020. Historical biogeography of Caribbean plants revises regional paleogeography. In: Rull V, Carnaval A, eds. Neotropical diversification: patterns and processes. Cham: Springer. doi: 10.1007/978-3-030-31167-4_20. [DOI] [Google Scholar]
- Ronquist F, Huelsenbeck JP.. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rosen DE. 1975. A vicariance model of Caribbean biogeography. Systematic Zoology 24: 431–464. doi: 10.2307/2412905. [DOI] [Google Scholar]
- Rundle HD, Nosil P.. 2005. Ecological speciation. Ecology Letters 8: 336–352. doi: 10.1111/j.1461-0248.2004.00715.x. [DOI] [Google Scholar]
- Santiago-Valentin E, Olmstead RG.. 2004. Historical biogeography of Caribbean plants: introduction to current knowledge and possibilities from a phylogenetic perspective. Taxon 53: 299–319. doi: 10.2307/4135610. [DOI] [Google Scholar]
- Schatz GE, Lowry PP. II. 2002. A synoptic revision of the genus Buxus L. (Buxaceae) in Madagascar and the Comoro Islands. Adansonia 24: 179–196. [Google Scholar]
- Schliep KP. 2011. Phangorn: phylogenetic analysis in R. Bioinformatics 27: 592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy JR. 1986. A revision of the genus Sarcococca (Buxaceae). Botanical Journal of the Linnean Society 92: 117–159. doi: 10.1111/j.1095-8339.1986.tb01828.x. [DOI] [Google Scholar]
- Seregin IV, Kozhevnikova AD.. 2006. Physiological role of nickel and its toxic effects on higher plants. Russian Journal of Plant Physiology 53: 257–277. doi: 10.1134/s1021443706020178. [DOI] [Google Scholar]
- Simmons MP, Ochoterena H.. 2000. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369–381. [PubMed] [Google Scholar]
- Sobczyk MK, Smith JAC, Pollard AJ, Filatov DA.. 2017. Evolution of nickel hyperaccumulation and serpentine adaptation in the Alyssum serpyllifolium species complex. Heredity 118: 31–41. doi: 10.1038/hdy.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2006. Raxml-VI-HPC: maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stephens PR, Wiens JJ.. 2003. Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. American Naturalist 161: 112–128. doi: 10.1086/345091. [DOI] [PubMed] [Google Scholar]
- Stöver B, Müller KM.. 2010. TreeGraph2 vs 2.14.0-771. [accessed November/2021].
- Swofford DL. 1998. PAUP/phylogenetic analysis using parsimony (and other methods). Sunderland: Sinauer. [Google Scholar]
- van der Ent A, Ocenar A, Tisserand R, Sugau JB, Echevarria G, Erskine PD.. 2019. Herbarium X-ray florescence screening for nickel, cobalt and manganese hyperaccumulator plants in the flora of Sabah (Malaysia, Borneo Island). Journal of Geochemical Exploration 202: 49–58. doi: 10.1016/j.gexplo.2019.03.013. [DOI] [Google Scholar]
- Walker JD, Geissman JW, Bowring SA, Babcock LE (compilers) 2018. Geologic Time Scale v. 5.0. Geological Society of America. doi: 10.1130/2018.CTS005R3C. [DOI]
- Warren BH, Simberloff D, Ricklefs RE, et al. 2015. Islands as model systems in ecology and evolution: prospects fifty years after MacArthur-Wilson. Ecology Letters 18: 200–217. doi: 10.1111/ele.12398. [DOI] [PubMed] [Google Scholar]
- Xie W, Lewis PO, Fan Y, Kuo L, Chen M-H.. 2011. Improving marginal likelihood estimation for Bayesian phylogenetic model selection. Systematic Biology 60: 150–160. doi: 10.1093/sysbio/syq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, Blair C, He X.. 2015. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Molecular Phylogenetics and Evolution 87: 46–49. doi: 10.1016/j.ympev.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Yu Y, Blair C, He X.. 2020. RASP 4: ancestral state reconstruction tool for multiple genes and characters. Molecular Biology and Evolution 37: 604–606. doi: 10.1093/molbev/msz257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


