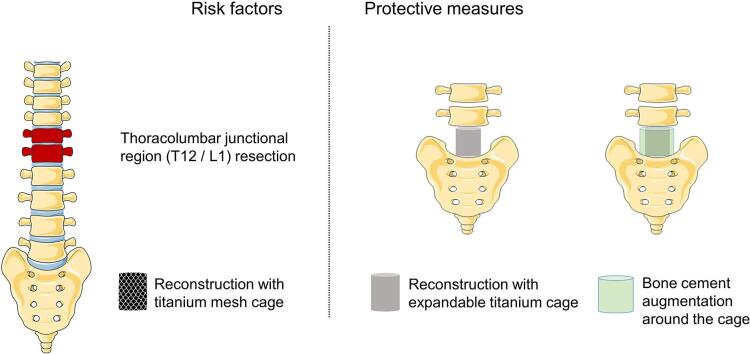
Graphical abstract
Keywords: Spinal tumor, Implant failure, Total spondylectomy, Revision surgery, 3D-printing
Highlights
-
•
Patients undergoing T12/L1 resection are more likely to develop implant failure.
-
•
Bone cement augmentation around the cage can protect against implant failure.
-
•
3D-printed customized artificial vertebral body is a new option in revision surgery.
Abstract
Background
Although there have been several risk factors reported for implant failure (IF), little consensus exists. Potential applicable measures to protect patients from IF are relatively few. This study aimed to discover new risk factors for IF and explore potential protective measures from IF after total spondylectomy for spinal tumors.
Methods
A total of 145 patients undergoing total spondylectomy for thoracic and lumbar spinal tumors between 2010 and 2021 were included from three tertiary university hospitals. Patient demographic and surgical characteristics and follow-up outcomes were collected.
Results
During a mean follow-up of 53.77 months (range, 12 to 149 months), 22 of 145 patients (15.17%) developed IF. Patients undergoing thoracolumbar junctional region (T12/L1) resection were more likely to develop IF compared to those undergoing surgery at other vertebral levels (HR = 21.622, 95% CI = 3.567–131.084, P = 0.001). Patients undergoing titanium mesh cage reconstruction were more likely to develop IF compared to patients undergoing expandable titanium cage reconstruction (HR = 8.315, 95% CI = 1.482–46.645, P = 0.016). Patients with bone cement augmentation around the cage were less likely to develop IF compared to those not receiving bone cement augmentation (HR = 0.015, 95% CI = 0.002–0.107, P < 0.001). Of the 22 patients with IF, 14 (63.63%) accepted personalized revision surgery.
Conclusion
The use of an expandable cage and the use of bone cement augmentation around the anterior column support cage are protective measures against IF after total spondylectomy.
1. Introduction
Primary and metastatic spinal tumors frequently require surgical intervention, whether as a measure to improve quality – and/or quantity – of life. Spinal tumors may cause nerve root and/or spinal cord compression as well as bony osteolysis, leading to back pain, radiculopathy, myelopathy, spinal instability or even paraplegia [1], [2], [3]. Currently, total en bloc spondylectomy (TES) is the surgical treatment of choice for primary malignant, benign aggressive, and solitary metastatic tumors of the spine [4], [5], [6]. Complete removal of tumor-involved portions of the spine improves local recurrence rates in many cases, but also by design creates structural deficits in the spine that necessitate complex reconstruction techniques. Recently, conventional titanium mesh cage and expandable titanium cage have gained popularity over autogenous or allogeneic bone grafts for anterior column reconstruction [7], [8]. Nonunion between these cages and the surrounding bone, however, poses risk and may lead to implant failure (IF) and revision surgeries.
The reported incidence of IF in the literature ranges from 9.55% to 42.6% [9], [10]. A number of studies have reported that factors such as perioperative radiation, cage subsidence, oblique cage position, multi-level spondylectomy and spondylectomy at a lumbar level are independent risk factors for IF after TES [10], [11], [12], [13], [14], [15]. Patient-specific factors such as osteoporosis and sarcopenia have also been reported to play a role [16], [17], [18], but little consensus exists. In this study, we aimed to: (1) uncover new risk factors of IF after spondylectomy and potential protective measures; (2) share our revision surgery strategies for IF after total spondylectomy.
2. Patients and methods
2.1. Patient selection
This clinical retrospective cohort study consecutively collected patients from three tertiary care university hospitals, including Fudan University Shanghai Cancer Center, Naval Medical Center of PLA, Naval Medical University, and The First Affiliated Hospital of Zhengzhou University. STROCSS 2021 Guideline for the reporting of cohort study was followed [19]. Inclusion criteria were: (1) patients who underwent total spondylectomy for thoracic and/or lumbar spinal tumors; (2) patients followed for at least one year. Exclusion criteria were: (1) patient age <14 years old; (2) patients missing baseline demographic and clinical data. A total of 145 patients undergoing total spondylectomy between January 2010 and June 2021 were included. Patients’ age, sex, and body mass index (BMI) were collected via chart review. No patients underwent neoadjuvant radiation before surgery. All patients signed informed consent for surgery. This retrospective observational study was approved by our institutional review board; ethical approval was obtained.
2.2. Surgical intervention and follow-up
Spondylectomy was performed in this series for patients with primary malignant, benign aggressive, and solitary metastatic tumors of the spine. Preoperative spinal X-ray, computed tomography (CT), magnetic resonance imaging (MRI) or Positron Emission Tomography-CT (PET-CT) were performed. Senior spinal surgeons evaluated each patient’s surgical candidacy based on individual medical history, symptoms, physical exam findings, imaging characteristics, American Spinal Injury Association score [20], and Eastern Cooperative Oncology Group performance score [21]. TES was predominantly performed, while total piecemeal spondylectomy was allowed for some solitary metastatic and benign aggressive spine tumors. T12 and/or L1 were defined as the thoracolumbar junctional region. Anterior spinal reconstruction was performed using titanium mesh cage or expandable titanium cage filled with autogenous (ilium and rib) and/or allogeneic bone grain graft. After cage placement, bone cement augmentation around the cage was performed when deemed appropriate. A diagram displaying the method and effect of bone cement augmentation around the expandable titanium cage is shown in Fig. 1. The effect of bone cement augmentation around the titanium mesh cage is shown in Fig. 2. Bone cement augmentation around the anterior column support cage was performed by experienced senior surgeons in our three centers and demonstrated sufficient feasibility. No physical compression and thermal damage was caused by bone cement when applying this technique. Posterior spinal reconstruction was performed using pedicle screws and titanium rods. Patients had no post-operative radiation after total spondylectomy of spinal tumors.
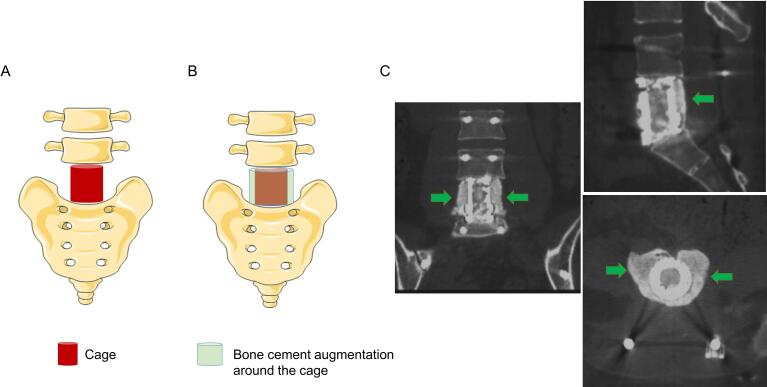
Fig. 1.
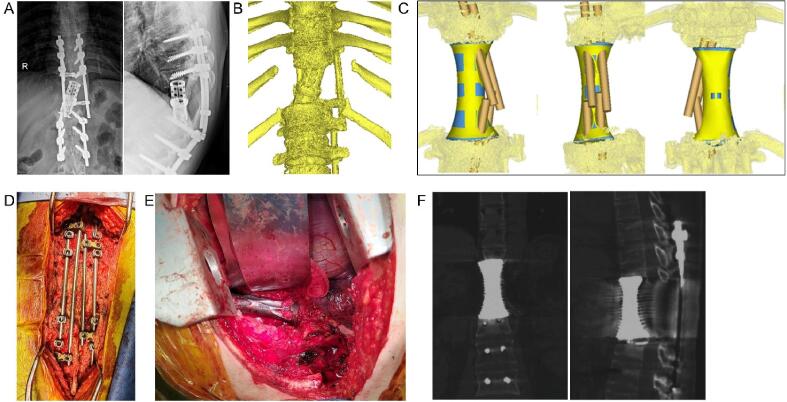
Diagram showing bone cement augmentation around the expandable titanium cage. A: Cage implant after total spondylectomy of L5; B: Bone cement augmentation around the cage; C: Postoperative CT scan showing the effect of bone cement augmentation (green arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
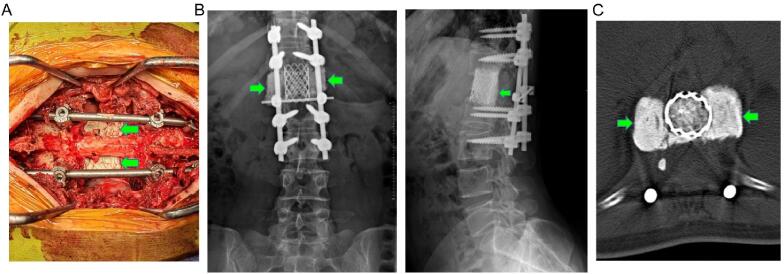
Fig. 2.
Diagram showing bone cement augmentation around the titanium mesh cage. A: Intraoperative image showing the bone cement; B: Postoperative anteroposterior and lateral X-ray showing the effect of bone cement augmentation (green arrow) around the titanium mesh cage after L1 spondylectomy; C: Postoperative CT scan showing the effect of bone cement augmentation (green arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the follow-up, X-rays were performed at 1, 3, 6, 12, 18 and 24 months after total spondylectomy and thereafter once a year. Spinal CT was performed at six months post-operatively to evaluate for bony fusion. The primary study endpoint was occurrence of IF, both symptomatic and radiographic, which was defined as worsening back pain accompanied by imaging findings of cage subsidence, translocation or fracture with or without rod fracture. All patients were alive with disease or without disease at the last follow-up.
2.3. Statistical analysis
Continuous variables were presented as means with standard deviations (SD) or as a range. Categorical variables were presented as a number with corresponding percentage. Independent Student’s t test or Mann-Whitney U test was performed for continuous variables. Chi-square test or Fisher exact probability method was utilized for categorical variables. Univariable Cox regression analysis was performed to identify factors associated with IF. All potentially clinically influencing factors for IF were entered into a multivariable Cox regression model to detect the independent risk factors. Kaplan-Meier curves were created to estimate IF-free survival rate. Gehan-Breslow-Wilcoxon test was used to examine curve difference in the short-term and log-rank test was used to examine curve difference in the long-term. Statistical analysis was performed using IBM SPSS 26.0 and Graph-Pad Prism 7.0 software. A two-sided P value < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Patient characteristics are presented in Table 1. Of 145 tumors resected, 79 (54.5%) were primary and 66 (45.5%) were metastatic. Specific tumor histological type is presented in Table 2.
Table 1.
Demographic and clinical characteristics of patients (n = 145).
| Characteristics | Total (n = 145) |
Non-IF (n = 123) |
IF (n = 22) |
|---|---|---|---|
| Age, years | |||
| Mean ± standard deviation | 46.87 ± 15.19 | 47.28 ± 14.86 | 44.55 ± 17.14 |
| Median (range) | 50 (17–74) | 50 (18–74) | 50 (17–74) |
| Sex | |||
| Female | 73 (50.3) | 62 (50.4) | 11 (50.0) |
| Male | 72 (49.7) | 61 (49.6) | 11 (50.0) |
| BMI, Kg/m2 | |||
| Mean ± standard deviation | 22.53 ± 2.94 | 22.57 ± 3.02 | 22.32 ± 2.53 |
| Tumor histological type | |||
| Primary | 79 (54.5) | 63 (51.2) | 16 (72.7) |
| Metastasis | 66 (45.5) | 60 (48.8) | 6 (27.3) |
| Surgical type | |||
| Total en bloc spondylectomy | 123 (84.8) | 104 (84.6) | 19 (86.4) |
| Total piecemeal spondylectomy | 22 (15.2) | 19 (15.4) | 3 (13.6) |
| Resected vertebral level | |||
| Thoracic | 92 (63.4) | 79 (64.2) | 13 (59.1) |
| Lumbar | 50 (34.5) | 43 (35.0) | 7 (31.8) |
| Both thoracic and lumbar | 3 (2.1) | 1 (0.8) | 2 (9.1) |
| Resection of thoracolumbar junctional region (T12 and/or L1) | |||
| No | 120 (82.8) | 105 (85.4) | 15 (68.2) |
| Yes | 25 (17.2) | 18 (14.6) | 7 (31.8) |
| Number of resected vertebrae | |||
| 1 | 117 (80.7) | 99 (80.5) | 18 (81.8) |
| ≥2 | 28 (19.3) | 24 (19.5) | 4 (18.2) |
| Mean (range) | 1.25 (1–4) | 1.24 (1–4) | 1.32 (1–3) |
| Number of instrumented vertebrae | |||
| ≤4 | 128 (88.3) | 111 (90.2) | 17 (77.3) |
| ≥5 | 17 (11.7) | 12 (9.8) | 5 (22.7) |
| Mean (range) | 4.16 (3–6) | 4.12 (3–6) | 4.36 (3–6) |
| Instrumentation spanning junctional region | |||
| No | 75 (51.7) | 65 (52.8) | 10 (45.5) |
| Yes | 70 (48.3) | 58 (47.2) | 12 (54.5) |
| Implant type | |||
| Expandable titanium cage | 75 (51.7) | 71 (57.7) | 4 (18.2) |
| Titanium mesh cage | 70 (48.3) | 52 (42.3) | 18 (81.8) |
| Bone cement augmentation around the cage | |||
| No | 65 (44.8) | 45 (36.6) | 20 (90.9) |
| Yes | 80 (55.2) | 78 (63.4) | 2 (9.1) |
IF: Implant failure.
Table 2.
Specific tumor histological type.
| Specific tumor histological type | Number (n = 145) |
|---|---|
| Primary (n = 79) | |
| Giant cell tumor of bone | 40 |
| Aggressive hemangioma | 9 |
| Solitary plasmacytoma of bone | 8 |
| Chondrosarcoma | 5 |
| Osteosarcoma | 4 |
| Chordoma | 2 |
| Epithelioid hemangioendothelioma | 2 |
| Malignant peripheral nerve sheath tumor | 2 |
| Solitary fibrous tumor | 2 |
| Chondroblastoma | 1 |
| Inflammatory myofibroblastic tumor | 1 |
| Langerhans cell histiocytosis | 1 |
| Leiomyosarcoma | 1 |
| Aggressive chondrosteoma | 1 |
| Metastatic (n = 66) | |
| Kidney | 14 |
| Lung | 10 |
| Breast | 10 |
| Unknown origin | 8 |
| Thyroid | 5 |
| Liver | 4 |
| Colorectal | 3 |
| Stomach | 2 |
| Meningioma | 2 |
| Hemangiosarcoma | 2 |
| Lymphoma | 1 |
| Bladder | 1 |
| Nasopharynx | 1 |
| Melanoma | 1 |
| Laryngocarcinoma | 1 |
| Esophagus | 1 |
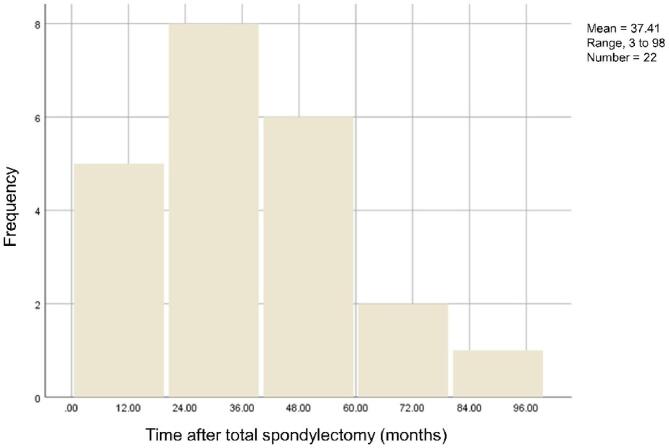
Of 145 patients, 22 (15.17%) developed IF during a mean follow-up of 53.61 months (range, 12 to 149 months). IF occurrence peak was seen between 24 and 36 months. The mean time from total spondylectomy to IF was 37.41 months (range, 3 to 98) (Fig. 3). Patients in the IF group were more likely to have undergone titanium mesh cage reconstruction compared to patients in non-IF group (81.8% vs 42.3%, P = 0.001). Patients in the IF group had lower utilization of bone cement augmentation compared to patients in the non-IF group (9.1% vs 63.4%, P < 0.001). (Table 1).
Fig. 3.
Chart of IF frequency distribution in relation to follow-up time. IF: Implant failure.
3.2. Factors associated with IF
Multivariable analysis demonstrated that patients undergoing T12 and/or L1 resection were more likely to develop IF compared to patients undergoing other vertebral level resection (HR = 21.622, 95% CI = 3.567–131.084, P = 0.001). Patients undergoing titanium mesh cage reconstruction were more likely to develop IF compared with patients undergoing expandable titanium cage reconstruction (HR = 8.315, 95% CI = 1.482–46.645, P = 0.016). Patients who underwent bone cement augmentation around the cage were less likely to develop IF compared to those not receiving bone cement augmentation (HR = 0.015, 95% CI = 0.002–0.107, P < 0.001) (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis identifying factors associated with IF.
| Characteristics | Unadjusted HR (95% CI) |
P | Adjusted HR (95% CI) |
P |
|---|---|---|---|---|
| Age, years | ||||
| < 50 | 1.000 | 1.000 | ||
| ≥ 50 | 1.655 (0.688–3.983) | 0.261 | 1.74 (0.545–5.556) | 0.350 |
| Sex | ||||
| Female | 1.000 | 1.000 | ||
| Male | 1.194 (0.513–2.776) | 0.681 | 0.846 (0.239–2.990) | 0.795 |
| BMI, kg/m2 | ||||
| < 24 | 1.000 | 1.000 | ||
| ≥ 24 | 1.171 (0.451–3.041) | 0.745 | 2.525 (0.454–14.039) | 0.290 |
| Tumor histological type | ||||
| Primary | 1.000 | 1.000 | ||
| Metastasis | 0.753 (0.284–1.996) | 0.568 | 2.451 (0.549–10.943) | 0.240 |
| Surgical type | ||||
| Total en bloc spondylectomy | 1.000 | 1.000 | ||
| Total piecemeal spondylectomy | 1.038 (0.303–3.552) | 0.953 | 4.715 (0.705–31.522) | 0.110 |
| Resected vertebral level | ||||
| Thoracic | 1.000 | 1.000 | ||
| Lumbar | 0.817 (0.324–2.061) | 0.817 | 1.912 (0.357–10.243) | 0.449 |
| Resection of thoracolumbar junctional region (T12 and/or L1) | ||||
| No | 1.000 | 1.000 | ||
| Yes | 2.556 (1.039–6.285) | 0.041* | 21.622 (3.567–131.084) | 0.001* |
| Number of resected vertebrae | ||||
| 1 | 1.000 | 1.000 | ||
| ≥2 | 0.914 (0.309–2.705) | 0.871 | 0.205 (0.023–1.836) | 0.157 |
| Number of instrumented vertebrae | ||||
| ≤4 | 1.000 | 1.000 | ||
| ≥5 | 2.605 (0.952–7.127) | 0.062 | 2.816 (0.444–17.869) | 0.272 |
| Instrumentation spanning junctional region | ||||
| No | 1.000 | 1.000 | ||
| Yes | 1.171 (0.505–2.715) | 0.712 | 0.323 (0.062–1.676) | 0.178 |
| Implant type | ||||
| Expandable titanium cage | 1.000 | 1.000 | ||
| Titanium mesh cage | 2.620 (0.867–7.916) | 0.088 | 8.315 (1.482–46.645) | 0.016* |
| Bone cement augmentation around the cage | ||||
| No | 1.000 | 1.000 | ||
| Yes | 0.037 (0.008–0.164) | <0.001* | 0.015 (0.002–0.107) | <0.001* |
IF: implant failure; BMI: body mass index; HR: hazard ratio; CI: confidence interval.
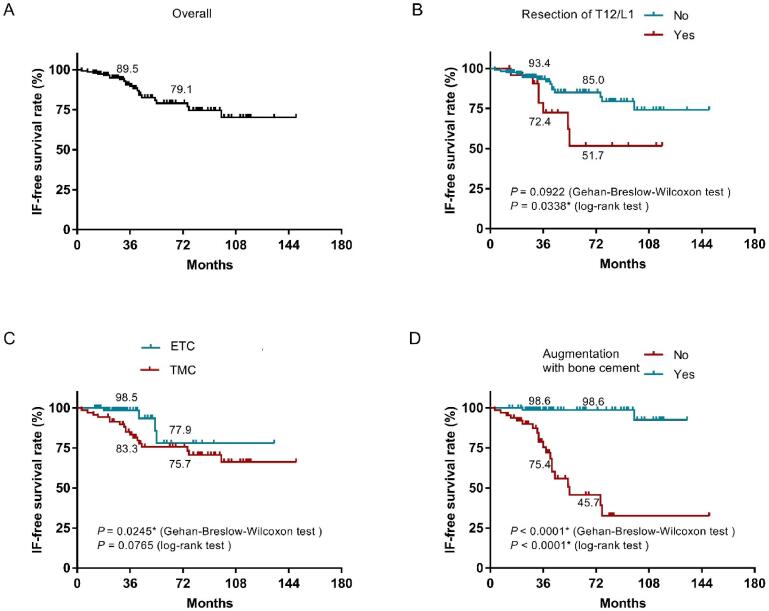
In Kaplan-Meier curve analysis, the three-year IF-free survival rate was 89.5% and six-year IF-free survival rate was 79.1% after total spondylectomy (Fig. 4A). Patients who underwent thoracolumbar junctional region (T12 and/or L1) resection had a shorter IF-free survival compared to patients who didn’t in long-term analysis (log rank test, P = 0.0338) (Fig. 4B). Patients undergoing titanium mesh cage reconstruction had a shorter IF-free survival compared to patients undergoing expandable titanium cage reconstruction in short-term analysis (Gehan-Breslow-Wilcoxon test, P = 0.0245) (Fig. 4C). Patients who received bone cement augmentation around the cage had a longer IF-free survival compared to those without bone cement augmentation both in short- and long-term (Gehan-Breslow-Wilcoxon test, P < 0.0001; log rank test, P < 0.0001) (Fig. 4D).
Fig. 4.
Kaplan-Meier curves showing IF-free survival after total spondylectomy; (A) All cases included, (B) stratified by resection of T12 and/or L1, (C) stratified by implant type, and (D) stratified by use of bone cement augmentation around the cage. ETC: Expandable titanium cage; TMC: Titanium mesh cage. IF: implant failure.
3.3. Revision surgery
Of 22 patients who occurred IF, 14 (63.63%) underwent revision surgery (Table 4). The other 8 patients (36.36%) occurring IF refused revision surgery, because 6 patients had mild symptoms which didn’t have much impact on their daily life and chose close follow-up; and 2 patients concerned about revision surgery risk and chose conservative treatment with brace.
Table 4.
Characteristics of patients undergoing revision surgery.
| Case number | Age at the time of TS (years) |
Sex | Tumor type | Total spondylectomy |
Implant failure |
Revision surgery type | Follow up |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level of resected vertebra | Reconstruction method | Time after TS (months) | Type | Patient’s symptoms and signs | Time after revision surgery (months) |
Outcome | |||||
| 1 | 56 | F | Aggressive hemangioma | L2 | TMC filled with autogenous and allogeneic bone graft + Screw-rod at T12-L1 and L3-4 |
75 | TMC subsidence + 1 rod broken | Back pain | ETC filled with autogenous bone graft + Screw-rod at T11-L1 and L3-5 |
21 | Alive with no disease and IF recurrence |
| 2 | 54 | M | Chordoma | T11-L1 | TMC filled with autogenous bone graft + Screw-rod at T8-10 and L2-4 |
29 | TMC subsidence and fracture + 1 rod broken | Back pain | First: Autogenous iliac crest bone graft + Connecting rod replacement Second: Allogeneic femur segment filled with autogenous bone graft + Steel plate |
47 | Alive with no disease and IF recurrence |
| 3 | 27 | F | GCTB | T11 | TMC filled with autogenous bone graft + Screw-rod at T9-10 and T12-L1 |
38 | TMC subsidence and fracture + 2 rods broken | Back pain, Abnormal knocking | ETC filled with autogenous bone graft + Screw-rod at T8-10 and T12-L2 |
28 | Alive with no disease and IF recurrence |
| 4 | 23 | F | GCTB | L5 | TMC filled with autogenous bone graft + Screw-rod at L3-4 and S1 |
76 | TMC subsidence + 1 rod broken | Back pain | ETC filled with autogenous bone graft + Screw-rod at L3-4 and S1 |
19 | Alive with disease but no IF recurrence |
| 5 | 34 | M | GCTB | T5 | TMC filled with autogenous bone graft + Screw-rod at T3-4 and T6-7 |
41 | TMC subsidence + 1 rod broken | Back pain, Abnormal knocking | Autogenous bone graft + Connecting rod replacement | 80 | Alive with no disease and IF recurrence |
| 6 | 17 | M | Chondrosarcoma | T12 | TMC filled with allogeneic bone graft + Screw-rod at T10-11 and L1-2 |
14 | TMC subsidence and translocation + 1 rod broken | Back pain | ETC filled with autogenous bone graft + Screw-rod at T10-11 and L1-2 |
92 | Alive with no disease and IF recurrence |
| 7 | 38 | M | GCTB | L1 | TMC filled with allogeneic bone graft + Screw-rod at T11-12 and L2-3 |
33 | TMC subsidence + 1 rod broken | Back pain | ETC filled with autogenous bone graft + Screw-rod at T10-12 and L2-4 |
95 | Alive with no disease and IF recurrence |
| 8 | 39 | M | GCTB | L1 | ETC filled with allogeneic bone graft + Screw-rod at T11-12 and L2-3 |
53 | ETC translocation | Back pain | ETC filled with autogenous bone graft + Screw-rod at T10-11 and L2-4 + Satellite rods |
10 | Alive with no disease and IF recurrence |
| 9 | 55 | M | Hemangioendothelioma | L2 | TMC filled with autogenous and allogeneic bone graft + Screw-rod at T12-L1 and L3-4 |
44 | TMC subsidence and fracture + 1 rod broken | Back pain | ETC filled with autogenous bone graft + Screw-rod at T12-L1 and L3-4 |
33 | Alive with no disease and IF recurrence |
| 10 |
55 | F | Thyroid cancer metastasis |
T4 | TMC filled with bone cement + Screw-rod at T2-3 and T5-6 | 98 | TMC subsidence and translocation + Screw loose | Back pain, lower limb weakness | ETC filled with allogeneic bone graft + Screw-rod at T1-3 and T5-8 |
16 | Alive with disease but no IF recurrence |
| 11 | 51 | F | Thyroid cancer metastasis | T4 | TMC filled with bone cement + Screw-rod at T2-3 and T5-6 | 22 | TMC subsidence and translocation | Back pain | T3-5 trim + 3D-printed AVB + Screw-rod at C7-T2 and T6-8 | 8 | Alive with no disease and IF recurrence |
| 12 | 26 | F | GCTB | T7 | TMC filled with bone cement + Screw-rod at T5-6 and T8-9 | 32 | TMC subsidence + 1 rod broken; Tumor recurrence | Back pain | T6-8 trim + ETC filled with autogenous and allogeneic bone graft + Screw-rod at L3-5 and T9-11 |
15 | Alive with no disease and IF recurrence |
| 13 | 25 | M | Hemangioendothelioma | T11-12 | ETC filled with autogenous and allogeneic bone graft + Screw-rod at T8-10 and L1-3 |
54 | ETC translocation + 1 rod broken | Back pain | 3D-printed AVB + Screw-rod at T8-10 and L1-3 + Satellite rods | 1 | Alive with no disease and IF recurrence |
| 14 | 27 | M | Solitary fibrous tumor | L1 | TMC filled with autogenous and allogeneic bone graft + Screw-rod at T11-12 and L2-3 |
36 | TMC subsidence and translocation + 1 rod broken | Back pain | ETC filled with autogenous bone graft + Screw-rod at T11-12 and L2-3 |
27 | Alive with no disease and IF recurrence |
TMC: Titanium mesh cage; ETC: Expandable titanium cage; AVB: artificial vertebral body; GCTB: Giant cell tumor of bone.
In 12 patients with mesh cage-associated IF, 9 underwent mesh cage replacement with an expandable cage, 1 underwent mesh cage replacement with a 3D-printed patient-customized artificial vertebral body and 2 underwent replacement with autogenous iliac crest bone graft with connecting rod replacement. In 2 patients with expandable cage -associated IF, 1 underwent expandable cage replacement with a 3D-printed patient-customized artificial vertebral body (Fig. 5), and the other patient underwent a new expandable cage replacement. Mean operative time for revision surgery was 272.1 (range, 154 to 425) minutes. Mean estimated blood loss was 1264 (range, 300 to 1900) ml. After the first revision surgery, over a mean 35.14 months of follow-up (range 1 to 95), one patient (Case 2) developed IF recurrence and underwent a second revision surgery (Fig. 6).
Fig. 5.
3D-printed patient-customized artificial vertebral body for revision surgery. (A) X-ray showing IF. (B) 3D imaging displaying IF. (C) computer-aided design of 3D-printed patient-customized artificial vertebral body, planned for replacement of expandable cage in revision surgery. (D) removal of failed expandable cage via posterior approach and posterior column reconstruction with satellite rods. (E) intraoperative placement of 3D-printed artificial vertebral body for anterior column reconstruction via anterior approach. (F) Postoperative CT scan showing position of 3D-printed artificial vertebral body. IF: implant failure.
Fig. 6.
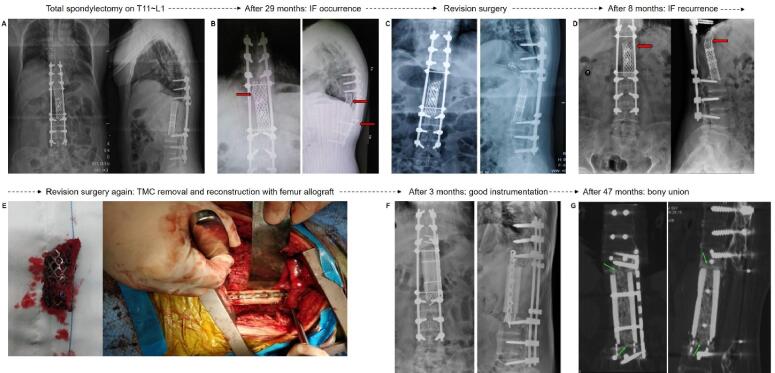
Recurrent IF and second revision surgery. (A) Total en bloc spondylectomy of T11 ∼ L1 and reconstruction with titanium mesh cage. (B) First IF occurred at 29 months. Titanium mesh cage subsidence/fracture and broken rod are seen (red arrow). (C) First revision surgery: Titanium mesh cage filled with autogenous iliac crest bone graft and connecting rod replacement. (D) Second IF occurred at 8 months after first revision surgery (red arrow). (E) Second revision surgery: Titanium mesh cage was removed and spinal reconstruction was performed using allogeneic femur segment filled with autogenous bone graft and steel plate fixation. (F) X-ray at 3 months after second revision surgery showed good implant position and instrumentation; (G) CT scan at 47 months after second revision surgery showed obvious bony fusion (green arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
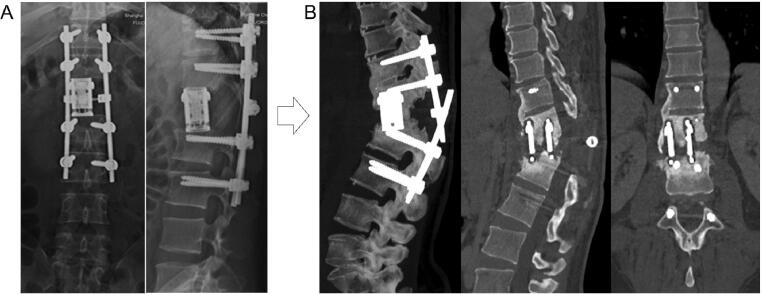
An ideal vertebral body replacement implant should ideally possess the following properties: (1) height similar to the resected vertebral body, allowing for complete filling of the bony defect, (2) hollow structure which can be filled with bone graft to promote fusion, as well as (3) effective support of the anterior column, providing spinal stability immediately after surgery and maintaining this stability over the long-term [22]. In the 1960s, iliac crest autograft was first introduced for spinal reconstruction in a patient who underwent cervical spinal tumor resection [23], and tricortical iliac crest was considered the implant of choice for some time. In the 1970s, however, artificial spinal prosthesis technology reached development with capabilities and outcomes similar to those seen with iliac crest autograft [24]. As materials and manufacturing techniques have continuously improved, titanium mesh cage and expandable titanium cage have become the preferred spinal implants for anterior column support after total spondylectomy for many surgeons. Our study demonstrates that patients undergoing traditional mesh cage reconstruction were more likely to develop IF compared to patients undergoing expandable cage reconstruction. This might be related to the fact that mesh cages lack a height-adjustable function; they must instead be tailored to precisely fit the spondylectomized space, and thus may not offer the same degree of anterior column support as expandable cage. In addition, the endplates of an expandable cage can be modified to better oppose the vertebral endplate, fit with the spinal physiological curvature (e.g. amount of kyphosis/lordosis needed) and are designed with spikes which penetrate into adjacent vertebrae to enhance stability [25], [26]. Expandable cage may thus be a more reliable spinal implant during initial spinal reconstruction and subsequent revision surgery for IF. Nonetheless, in our cohort, two patients (case 8 and 13; Table 4) developed IF after expandable cage reconstruction. In these patients, the expandable cage-associated IF might be because: (1) The spondylectomy involved in thoracolumbar junctional and mobile region (T12 and/or L1) which increased IF risk; (2) expandable cage was not placed at a satisfactory position in the initial surgery (Fig. 7A); (3) lack of end caps for the expandable cage; and (4) excessive expansion of the cage, leading to a relatively insufficient internal filling of bone graft and subsequent bone non-union (Fig. 7B).
Fig. 7.
Expandable cage-related failure. A: X-ray showing a non-ideal cage position after L1 spondylectomy and reconstruction; B: CT scan showing implant failure and insufficient internal filling of bone graft and bone non-union.
This study is the first to demonstrate that spondylectomy involving the thoracolumbar junction (T12 and/or L1) is a risk factor for IF compared to spondylectomy at other sites. This might be because the thoracolumbar junctional region is relatively hypermobile, leading to excessive forces on the anterior column implant which may promote cage translocation. The thoracolumbar junctional region is the transitional point from thoracic kyphosis to lumbar lordosis. It is also the region where the coronally oriented facet joints of the thoracic vertebrae transform to the sagittaly oriented facet joints of the lumbar spine. Together these factors may lead to increased rotational forces on the implant, as well as increased forces in the sagittal and coronal planes, predisposing to implant motion.
This study demonstrates that bone cement augmentation around the cage is an effective measure that can be taken to reduce IF risk after spondylectomy. Bone cement augmentation is a commonly used technique in spine surgery for osteoporotic bone. Pedicle screws in the osteoporotic spine are susceptible to loosening and subsequent failure, and thus pedicle screw augmentation with polymethylmethacrylate is often performed in patients with poor bone quality [27], [28]. Similarly, anterior column support devices in the osteoporotic spine are susceptible to subsidence and subsequent failure, and although pedicle screw augmentation can increase screw stability, it is often insufficient to increase cage stability. It has been reported that vertebroplasty of adjacent vertebral levels can lead to a superior cage stability in patients with osteoporosis [29], [30]. Our study demonstrates that placement of polymethylmethacrylate cement directly around the anterior column support device leads increased cage stability and decreased IF risk. Bone cement augmentation around a cage in this manner can increase the contact area between cage endplates and adjacent vertebrae and provide additional support strength (Fig. 1, Fig. 2).
In our revision surgeries, most of traditional titanium mesh cages were replaced with expandable cages. In addition, 3D-printed patient-specific artificial vertebral bodies were also used in some instances. 3D-printed artificial vertebral bodies are an emerging tool for anterior spinal reconstruction [31]. Personalized 3D-printed artificial vertebral bodies can accurately match spinal bony defects, restore spinal height and physiological curvature, and may offer superior biomechanical function over conventional anterior column support devices. 3D-printed artificial vertebral bodies can incorporate solid and porous structures into a titanium alloy implant which improves bone ingrowth and fusion [32]. It has been demonstrated that 3D-printed artificial vertebral bodies have lower subsidence rates compared to traditional titanium mesh cages [33], [34]. Therefore, 3D-printed patient-customized artificial vertebral bodies represent an attractive solution in revision surgery and complex spinal reconstruction, such as is required after total en bloc spondylectomy.
There are some limitations in our study. First, this study is a retrospective cohort study, which inevitably carried selection bias. Sagittal alignment is a key parameter responsible for the longevity of a construct, also in tumor patients. A thorough evaluation of the sagittal alignment will be worthwhile. Second, as a new created surgical technique to protect against IF, to date, intraoperative bone cement augmentation around the cage is only performed in several national hospitals. It needs more spinal centers to examine its feasibility, advantages and potential risks. Third, although our study had a mean follow-up for more than 4.5 years, the outcome event of IF was relatively few (N = 22), which causes wide CIs in the statistical models. As we previously suggested, this situation is common in those clinical observational studies with a low-incidence of outcome event [35], [36]. A future study including a large population of patients can well address this issue.
In conclusion, our study identified that spondylectomy at the thoracolumbar junction and titanium mesh cage reconstruction are risk factors for IF after total spondylectomy for spinal tumors. Intraoperative bone cement augmentation around the cage is an applicable and effective measure to lower IF risk. In our experience, expandable cage implants and 3D-printed-customized artificial vertebral bodies are ideal for revision surgery after IF.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Hongjian Liu, Email: hongjianmd@126.com.
Wending Huang, Email: orienthwd@163.com.
Wangjun Yan, Email: yanwj@fudan.edu.cn.
References
- 1.Goodwin M.L., Buchowski J.M., Schwab J.H., Sciubba D.M. Spinal Tumors: diagnosis and treatment. J. Am. Acad. Orthop. Surg. 2022;30(17):e1106–e1121. doi: 10.5435/JAAOS-D-21-00710. [DOI] [PubMed] [Google Scholar]
- 2.Lawton A.J., Lee K.A., Cheville A.L., Ferrone M.L., Rades D., Balboni T.A., Abrahm J.L. Assessment and management of patients with metastatic spinal cord compression: a multidisciplinary review. J. Clin. Oncol. 2019;37(1):61–71. doi: 10.1200/JCO.2018.78.1211. [DOI] [PubMed] [Google Scholar]
- 3.MacLean M.A., Touchette C.J., Georgiopoulos M., Brunette-Clément T., Abduljabbar F.H., Ames C.P., Bettegowda C., Charest-Morin R., Dea N., Fehlings M.G., Gokaslan Z.L., Goodwin C.R., Laufer I., Netzer C., Rhines L.D., Sahgal A., Shin J.H., Sciubba D.M., Stephens B.F., Fourney D.R., Weber M.H. AO Spine Knowledge Forum Tumor. Systemic considerations for the surgical treatment of spinal metastatic disease: a scoping literature review. Lancet Oncol. 2022;23(7):e321–e333. doi: 10.1016/S1470-2045(22)00126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A.A., Paulino Pereira N.R., Pedlow F.X., Wain J.C., Yoon S.S., Hornicek F.J., Schwab J.H. Modified En Bloc Spondylectomy for tumors of the thoracic and lumbar spine: surgical technique and outcomes. J. Bone Joint Surg. Am. 2017 Sep 6;99(17):1476–1484. doi: 10.2106/JBJS.17.00141. [DOI] [PubMed] [Google Scholar]
- 5.Sciubba D.M., De la Garza R.R., Goodwin C.R., Xu R., Bydon A., Witham T.F., Gokaslan Z.L., Wolinsky J.P. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur. Spine J. 2016;25(12):4080–4087. doi: 10.1007/s00586-016-4641-y. [DOI] [PubMed] [Google Scholar]
- 6.Luzzati A.D., Shah S., Gagliano F., Perrucchini G., Scotto G., Alloisio M. Multilevel en bloc spondylectomy for tumors of the thoracic and lumbar spine is challenging but rewarding. Clin. Orthop. Relat. Res. 2015;473(3):858–867. doi: 10.1007/s11999-014-3578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampel G.A., Yilmaz E., Massrey C., Clifton W., Iwanaga J., Loukas M., Tubbs R.S. History of bone grafts in spine surgery. Cureus. 2022;14(5):e24655. doi: 10.7759/cureus.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarhan T., Froemel D., Rickert M., Rauschmann M., Fleege C. Geschichte des Wirbelkörperersatzes [History of vertebral body replacement] Unfallchirurg. 2015;118(S1):73–79. doi: 10.1007/s00113-015-0084-x. [DOI] [PubMed] [Google Scholar]
- 9.Shinmura K., Kato S., Demura S., Yokogawa N., Yonezawa N., Shimizu T., Oku N., Kitagawa R., Handa M., Annen R., Murakami H., Tsuchiya H. Revision surgery for instrumentation failure after total en bloc spondylectomy: a retrospective case series. BMC Musculoskelet. Disord. 2020;21(1):591. doi: 10.1186/s12891-020-03622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao W.D., Jia Q., Wang T., Lou Y., Jiang D.J., Yang C., Yang X., Huang Q., Wei H.F., Xiao J.R. Factors related to instrumentation failure in titanium mesh reconstruction for thoracic and lumbar tumors: retrospective analysis of 178 patients. Cancer Manag. Res. 2021;15(13):3345–3355. doi: 10.2147/CMAR.S294616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Z., Zhao Y., Tang X., Yang R., Yan T., Guo W. Factors associated with spinal fixation mechanical failure after tumor resection: a systematic review and meta-analysis. J. Orthop. Surg. Res. 2022;17(1):110. doi: 10.1186/s13018-022-03007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z., Wei F., Liu Z., Liu X., Jiang L., Yu M., Xu N., Wu F., Dang L., Zhou H., Li Z. Risk factors for instrumentation failure after total En Bloc spondylectomy of thoracic and lumbar spine tumors using titanium mesh cage for anterior reconstruction. World Neurosurg. 2020;135:e106–e115. doi: 10.1016/j.wneu.2019.11.057. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M., Watanabe K., Tsuji T., Ishii K., Nakamura M., Chiba K., Toyama Y. Late instrumentation failure after total en bloc spondylectomy. J. Neurosurg. Spine. 2011;15(3):320–327. doi: 10.3171/2011.5.SPINE10813. [DOI] [PubMed] [Google Scholar]
- 14.Park S.J., Lee C.S., Chang B.S., Kim Y.H., Kim H., Kim S.I., Chang S.Y. Korean Spine Tumor Study Group. Rod fracture and related factors after total en bloc spondylectomy. Spine J. 2019;19(10):1613–1619. doi: 10.1016/j.spinee.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka K., Murakami H., Demura S., Kato S., Yokogawa N., Kawahara N., Tomita K., Tsuchiya H. Risk factors of instrumentation failure after multilevel total en bloc spondylectomy. Spine Surg Relat Res. 2017;1(1):31–39. doi: 10.22603/ssrr.1.2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenzlin H., Schmidt L., Jankovic D., Schulze C., Brockmann M.A., Ringel F., Keric N. Impact of Sarcopenia and bone mineral density on implant failure after dorsal instrumentation in patients with osteoporotic vertebral fractures. Medicina (Kaunas) 2022;58(6):748. doi: 10.3390/medicina58060748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao J.C. Impact of osteoporosis on different type of short-segment posterior instrumentation for thoracolumbar burst fracture-A finite element analysis. World Neurosurg. 2020;139:e643–e651. doi: 10.1016/j.wneu.2020.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A., Cha T., Schwab J., Fogel H., Tobert D., Razi A.E., Hecht A., Bono C.M., Hershman S. Osteoporosis increases the likelihood of revision surgery following a long spinal fusion for adult spinal deformity. Spine J. 2021 Jan;21(1):134–140. doi: 10.1016/j.spinee.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Mathew G., Agha R., Albrecht J., et al. STROCSS Group. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. 106165. [DOI] [PubMed] [Google Scholar]
- 20.El Masry W.S., Tsubo M., Katoh S., El Miligui Y.H.S., Khan A. Validation of the American Spinal Injury Association (ASIA) Motor Score and the National Acute Spinal Cord Injury Study (NASCIS) motor score. Spine. 1996;21(5):614–619. doi: 10.1097/00007632-199603010-00015. [DOI] [PubMed] [Google Scholar]
- 21.Giantonio B.J., Forastiere A.A., Comis R.L. Eastern Cooperative Oncology Group. The role of the Eastern Cooperative Oncology Group in establishing standards of cancer care: over 50 years of progress through clinical research. Semin. Oncol. 2008;35(5):494–506. doi: 10.1053/j.seminoncol.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Kasapovic A., Bornemann R., Pflugmacher R., Rommelspacher Y. Implants for vertebral body replacement - which systems are available and have become established. Z. Orthop. Unfall. 2021;159(1):83–90. doi: 10.1055/a-1017-3968. [DOI] [PubMed] [Google Scholar]
- 23.Bailey R.W., Badgley C.E. Stabilization of the Cervical Spine by Anterior Fusion: J. Bone Joint Surg. 1960;42(4):565–624. [PubMed] [Google Scholar]
- 24.Hamdi F.A. Prosthesis for an excised lumbar vertebra: a preliminary report. Can. Med. Assoc. J. 1969;100(12):576–580. PMID: 5775083; PMCID: PMC1945789. [PMC free article] [PubMed] [Google Scholar]
- 25.Lang S., Neumann C., Schwaiger C., Voss A., Alt V., Loibl M., Kerschbaum M. Radiological and mid- to long-term patient-reported outcome after stabilization of traumatic thoraco-lumbar spinal fractures using an expandable vertebral body replacement implant. BMC Musculoskelet. Disord. 2021;22(1):744. doi: 10.1186/s12891-021-04585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin G.X., Kim J.S., Kotheeranurak V., Chen C.M., Hu B.S., Rui G. Does the application of expandable cages in TLIF provide improved clinical and radiological results compared to static cages? A meta-analysis. Front Surg. 2022;10(9) doi: 10.3389/fsurg.2022.949938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuetze K., Eickhoff A., Röderer G., Gebhard F., Richter P.H. Osteoporotic bone: when and how to use augmentation? J. Orthop. Trauma. 2019;33(Suppl 8):S21–S26. doi: 10.1097/BOT.0000000000001643. [DOI] [PubMed] [Google Scholar]
- 28.Hoppe S., Keel M.J. Pedicle screw augmentation in osteoporotic spine: indications, limitations and technical aspects. Eur. J. Trauma Emerg. Surg. 2017;43(1):3–8. doi: 10.1007/s00068-016-0750-x. [DOI] [PubMed] [Google Scholar]
- 29.Oberkircher L., Krüger A., Hörth D., Hack J., Ruchholtz S., Fleege C., Rauschmann M., Arabmotlagh M. Anterior cement augmentation of adjacent levels after vertebral body replacement leads to superior stability of the corpectomy cage under cyclic loading-a biomechanical investigation. Spine J. 2018;18(3):525–531. doi: 10.1016/j.spinee.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 30.Geiger F., Kafchitsas K., Rauschmann M. Anterior vertebroplasty of adjacent levels after vertebral body replacement. Eur. Spine J. 2011;20(8):1385–1392. doi: 10.1007/s00586-011-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lador R., Regev G., Salame K., Khashan M., Lidar Z. Use of 3-dimensional printing technology in complex spine surgeries. World Neurosurg. 2020;133:e327–e341. doi: 10.1016/j.wneu.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Fogel G., Martin N., Lynch K., Pelletier M.H., Wills D., Wang T., Walsh W.R., Williams G.M., Malik J., Peng Y., Jekir M. Subsidence and fusion performance of a 3D-printed porous interbody cage with stress-optimized body lattice and microporous endplates - a comprehensive mechanical and biological analysis. Spine J. 2022;22(6):1028–1037. doi: 10.1016/j.spinee.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Fang T., Zhang M., Yan J., Zhao J., Pan W., Wang X., Zhou Q. Comparative analysis of 3D-printed artificial vertebral body versus titanium mesh cage in repairing bone defects following single-level anterior cervical corpectomy and fusion. Med. Sci. Monit. 2021 Feb;7(27):e928022. doi: 10.12659/MSM.928022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei F., Xu N., Li Z., Cai H., Zhou F., Yang J., Yu M., Liu X., Sun Y., Zhang K., Pan S., Wu F., Liu Z. A prospective randomized cohort study on 3D-printed artificial vertebral body in single-level anterior cervical corpectomy for cervical spondylotic myelopathy. Ann Transl Med. 2020;8(17):1070. doi: 10.21037/atm-19-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veiga L.H.S., Vo J.B., Curtis R.E., Mille M.M., Lee C., Ramin C., Bodelon C., Aiello Bowles E.J., Buist D.S.M., Weinmann S., Feigelson H.S., Gierach G.L., Berrington de Gonzalez A. Treatment-related thoracic soft tissue sarcomas in US breast cancer survivors: a retrospective cohort study. Lancet Oncol. 2022;23(11):1451–1464. doi: 10.1016/S1470-2045(22)00561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu X., Fujiwara T., Sun Y., Huang W., Yan W. Treatment-related thoracic soft tissue sarcomas in survivors of breast cancer. Lancet Oncol. 2023;24(1):e6. doi: 10.1016/S1470-2045(22)00683-0. [DOI] [PubMed] [Google Scholar]