Summary
Disorders of folic acid metabolism during pregnancy lead to fetal neural tube defects (NTDs). However, the mechanisms still require further investigation. Here, we aim to analyze the brain metabolic profiles of 30 NTDs and 30 healthy fetuses. Our results indicated that low-folate diet during early life played a causal role in cerebral metabolism, especially in lipometabolic disturbance, highlighting the importance of folate in modulating brain development and metabolism. Next, we established a mouse model of NTDs. Interestingly, the differential metabolites are mainly involved in glycerophospholipid metabolism and biosynthesis of unsaturated fatty acids both in human and mice fetal brain. Since intestinal microbes could critically regulate neurofunction via the intestinal-brain axis, we further found the abundances of Firmicutes and Bacteroidetes in the gut of pregnant mice were correlated with the abundances of lipid metabolism related metabolites in the fetal brain. This finding probably reflects the intergenerational microbial-metabolism biomarkers of NTDs.
Subject areas: Developmental neuroscience, Microbiome
Graphical abstract
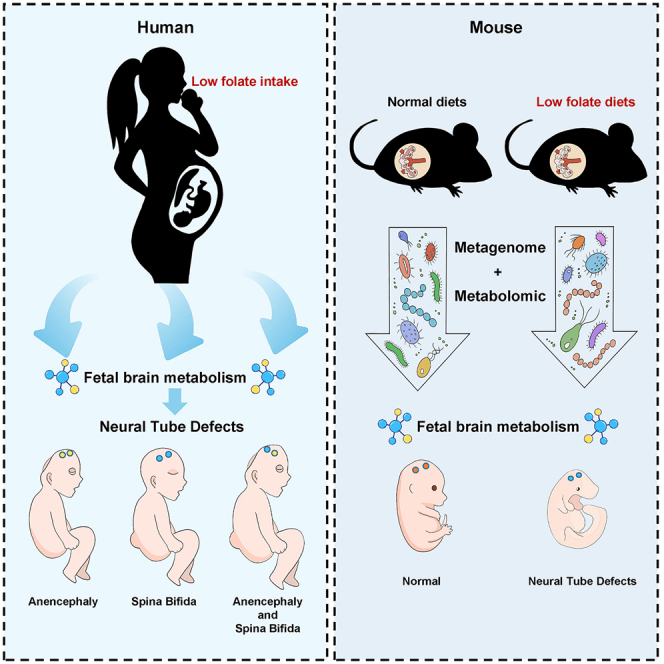
Highlights
-
•
Maternal folate intake is crucial for fetal brain metabolism and neural tube closure
-
•
Maternal folate intake modulated human fetal brain glycerophospholipid metabolism
-
•
The gut microbiota in pregnant mice is associated with fetal brain lipid metabolism
Developmental neuroscience; Microbiome
Introduction
Neural tube defects (NTDs) are life-threatening congenital malformations caused by abnormal tube closure during the third to fourth weeks of pregnancy.1 The survival time of 75% of cases of NTDs-affected live births was less than 5 years, which causes heavy economic burdens to family and society.2 Currently, most of relevant researches mainly emphasize the neural tube closure is an exquisitely coordinated process arising from both genetic and environmental factors. Environmental factors mainly include nutritional status and metabolic diseases during pregnancy, for example, folate intake,2,3 diabetic pregnancy,4 and maternal prepregnant obesity.5 Among these factors, folic acid is the most significant and well-known. In 2019, a systematic review and meta-analysis of the efficacy of folic acid intake and effect on NTDs in fetuses showed a strong correlation between supplementation with folic acid and a decreased prevalence of total NTDs and NTDs subtypes, including spina bifida, anencephaly, and cephalocele.6
Folic acid (vitamin B9) functions as a carrier in one-carbon units after being reduced, and activates the one-carbon metabolism, including the three key reactions in the folate cycle, methionine cycle, and trans-sulphuration pathway, participates in diverse physiological processes involving amino acid biosynthesis (purines and thymidine) and homeostasis (glycine, serine, and methionine).7 Folic acid and one-carbon unit metabolism are associated with many diverse diseases, including the occurrence of birth defects such as NTDs,8 adverse fetal outcomes,9 vascular diseases,10 neuropsychiatric diseases,11 and tumors.12 Disruption of carbon metabolism caused by vitamin deficiency has been shown to be a risk factor for adverse outcomes in pregnancy, including NTDs and miscarriage.13
Metabolic disturbance during pregnancy has the potential to significantly influence embryonic neurodevelopment, especially maternal lipid metabolism disorders. Previous studies have shown that diet can affect lipid metabolism in mouse brain tissues (hippocampus and prefrontal lobe). Lipids account for more than 50% of brain dry weight, and phospholipid metabolism is very important for brain function.14 Glycerophospholipids, a type of phospholipid, are key components of neuronal membranes and myelin sheaths and the major regulators of synaptic function. Four major classes of glycerophospholipids are found in neural membranes.15 Changes in the glycerophospholipid composition of the nerve membrane occur in acute and chronic nerve disease. These changes accompanied by changes in ion homeostasis, free radical accumulation, and damage to energy status, are the causes of irreversible neuronal damage during cerebral ischemia, spinal cord or brain injury. Nonetheless, some crucial questions have not been clearly demonstrated yet. However, how the changes in maternal folate lackage affect fetal brain metabolism and development remains unknown.
Gut microbiota is not only involved in nutrient absorption and energy metabolism, but also in the regulation of brain development through the production of short-chain fatty acids and bioactive substances. At the same time, the gut microbiota, as an important regulator of lipid metabolism, significantly affect the peripheral and central lipid metabolism of the host. The absence of gut microbiota mainly affected the contents of glycerolipids, sphingolipids, and glycolipids in the prefrontal cortex of mice. And the levels of 25 important lipid metabolites were restored after restoration of gut microbiota colonization. These reversed lipid metabolites were glycerophospholipids and glycerolipids.16 Furthermore, folic acid or vitamin B6 deficiency leads to changes in the composition of host gut microbiota, reduced levels of metabolites, such as propionic acid and butyric acid, and impaired amino acid metabolism.17,18 In a colitis model, vitamin B12 imbalance was also shown to be closely related to disrupted intestinal flora.19 Disturbances of maternal intestinal flora and carbon metabolism, to a certain extent, determine the adverse pregnancy outcome, metabolic status and neurodevelopment of offspring.20 Metabolites of gut microbiota in pregnant mothers may be transmitted through the placenta and cord to their offspring.21 Maternal gut bacteria composition is related to early embryonic brain development.22 A porcine animal study showed that lipid levels and intestinal microflora in offspring were influenced by the maternal diet, and their cognitive function and hippocampal neurogenesis were altered through the gut-brain axis.23 Mice fed a high-salt diet had significantly altered gut microbiota, which caused behavioral changes (modeling the Autism spectrum) in their offspring.24 Although the connection between the gut-microbiome and brain has been established, the regulatory mechanisms and/or causality of maternal gut microbiota and fetal neurodevelopment remain unclear.
In this study, we found that the differential metabolites are mainly involved in glycerophospholipid metabolism and biosynthesis of unsaturated fatty acids both in human and mice with low maternal folate intake-associated NTDs brain. It indicates that low-folate diet plays a causal role for the lipometabolic disturbance in early life. We further found a correlation between gut microbiota of pregnant mice and brain glycerophospholipid metabolism of fetal mice with NTDs. This finding probably reflects the intergenerational microbial-metabolism biomarkers of NTDs. Our findings suggest that perinatal microecological factors shape fetal neural tube development.
Results
Low folic acid during pregnancy effect on the cerebral metabolic profiles of human NTDs subgroups fetuses
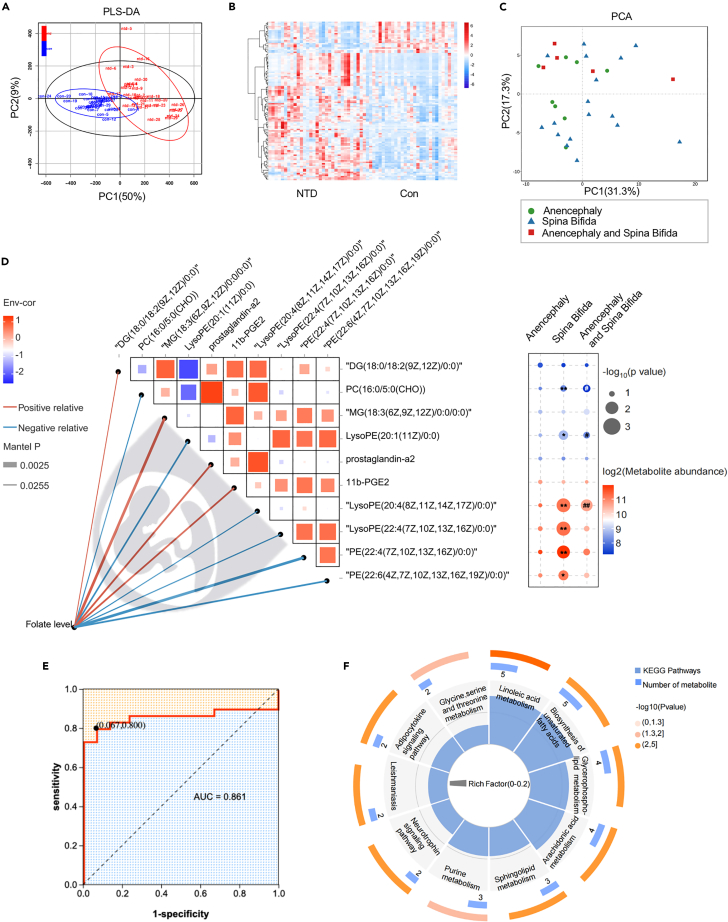
A total of 60 participants were enrolled (detailed in the “Experimental model and study participant details” section) and were further divided into the following two groups based on NTDs diagnosis: control group (n = 30) and NTDs group (n = 30). The characteristics of the pregnant women and fetuses were summarized in Table 1, including gender, gestational weeks, folate level (brain tissue, ng/mg), and malformation type. Liquid chromatograph-mass spectrometer(LC-MS) analysis was used to obtain the cerebral metabolic profiles of all 60 participants. We identified differentially abundant metabolites between the NTDs and the normal fetal brain tissue. Better separation of brain metabolic in NTDs fetuses and normal fetuses was observed and a total of 111 metabolites with significant differences were identified (Figures 1A and 1B; Table S1). A principal component analysis (PCA) of taxonomic compositions of malformation type does not show a clear separation by metabolites (Figure 1C), despite showing on significant differences at the global partial least squares-discriminant analysis (PLS-DA) scores between the NTDs and Con group (Figure 1A). We next analyzed whether cerebral folate level co-vary with specific metabolites and showed the 10 metabolites most correlated with folate concentration by using Spearman’s correlation analysis (Figure 1D left). The high abundance of MG (18:3 (6Z,9Z,12Z)/0:0/0:0), prostaglandin-a2, and 11b-PGE2 was positively correlated with the folate concentration. While the LysoPE (20:1(11Z)/0:0), LysoPE (22:4 (7Z,10Z,13Z,16Z)/0:0), PE (22:4 (7Z,10Z,13Z,16Z)/0:0), and PE(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) showed the opposite effect. We analyzed the enrichment of these 10 differential metabolites related to folate concentration in the types of NTDs malformations (Figure 1D right; Table S2). Surprisingly, there were significant differences in specific metabolites in “Anencephaly and Spina Bifida” with “Anencephaly” or “Spina Bifida”. Subsequently, we could accurately distinguish NTDs from healthy controls, as indicated by the area under the receiver operating curve (AUC), which had a value up to 0.861 (Figure 1E). Functionally, the differential metabolites are mainly involved in linoleic acid metabolism, biosynthesis of unsaturated fatty acids, and glycerophospholipid metabolism (Figure 1F; Table S3). Taken together, the above results indicated that low-folate diet in early life played a causal role in the cerebral metabolism, especially for lipometabolic disturbance, highlighting the importance of folate in modulating brain development and metabolism.
Table 1.
Characteristics of the study cohort
| Control (n = 30) | NTD (n = 30) | p value | |
|---|---|---|---|
| Gendera | 0.791 | ||
| Female | 19(63) | 18(60) | \ |
| Male | 11(37) | 12(40) | \ |
| Gestational weeksb | 20(18, 22) | 24.5(20, 31.50) | <0.001 |
| Folate level (ng/mg)b | 0.123(0.119, 0.128) | 0.078(0.059, 0.110) | <0.001 |
| Malformation typea | |||
| Anencephaly | \ | 8(27.0) | \ |
| Spina Bifida | \ | 17(56) | \ |
| Anencephaly and Spina Bifida | \ | 5(17.0) | \ |
Continuous, not normally distributed variables between two groups were analyzed by the Mann-Whitney U test. Categorical variables were compared by the X2 test.
n(%).
Median (IQR).
Figure 1.
Normal and NTDs human fetal brain metabolome
(A) PLS-DA to distinguish the global metabolic differences between groups and identify the differential metabolites between NTDs and Con group (n = 30).
(B) Heatmap of all differential 111 metabolites between the NTDs and Con fetal brain tissue (n = 30, all p < 0.05, the p values are described in Table S1).
(C) PCA analysis was performed to visually explore the similarity and variations between NTDs subgroups samples’metabolic composition. The percentages in parentheses refer to the proportions of variation explained by each ordination axis. N = 8 in Anencephaly subgroup. N = 17 in Spina Bifida subgroup. N = 5 in Anencephaly and Spina Bifida subgroup. (D, left) Brain folate concentration was related to brain metabolites by Mantel tests. Edge width corresponds to the Mantel’s P statistic, and edge color red/blue connections indicate a positive/negative correlation. Top10 metabolites that strongly correlate with brain folate concentrations are shown. Pairwise comparisons of these brain metabolites are shown, with a color gradient denoting Spearman’s correlation coefficients. (D, right) Bubble plots of metabolite abundance in each NTDs subgroup,∗<0.05,∗∗<0.01 “Spina Bifida” compared to “Anencephaly and Spina Bifida” subgroup; #<0.05, ##<0.01 “Anencephaly”compared to “Anencephaly and Spina Bifida” subgroup, the p values are described in Table S2.
(E) Diagnostic outcomes are shown via receiver operating characteristic (ROC) curves for brain folate concentration among 60 subjects.
(F) Functional pathways significantly regulated by brain metabolite of NTDs and controls (all p value < 0.05, the p values are described in Table S3).
Fetal brain lipid changes after low-folate diets combined with MTX-induced during pregnancy
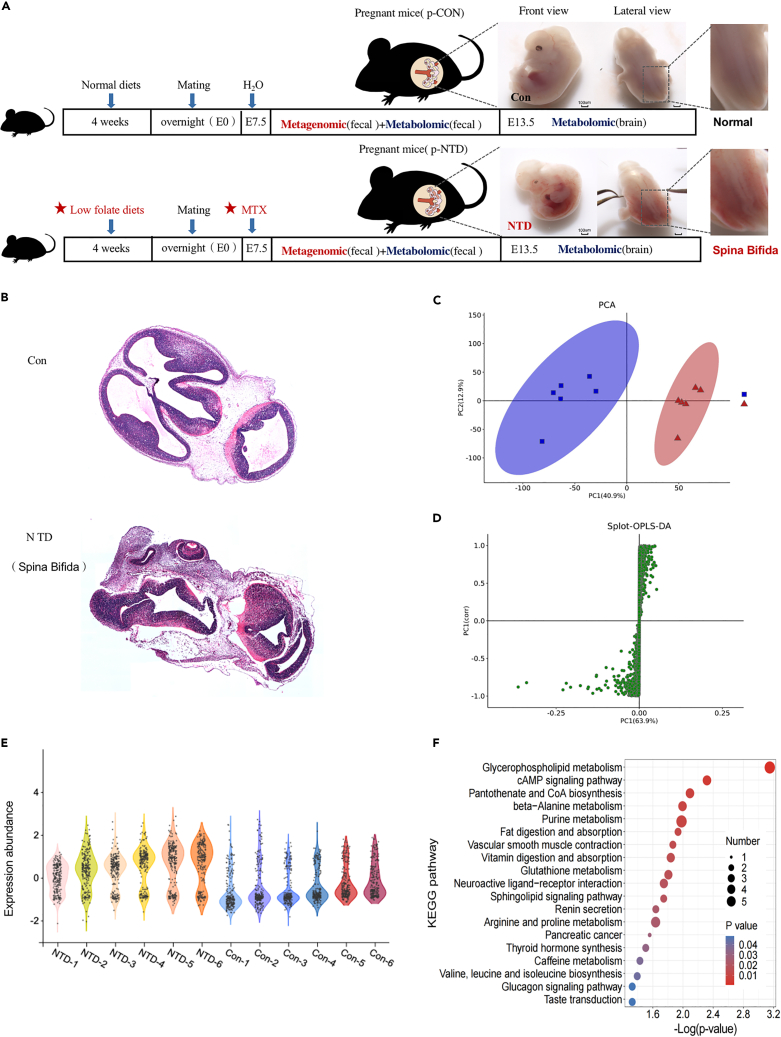
We established a mouse model of NTDs via intraperitoneal injection of MTX (1.5 mg/kg) at 7.5 days of gestation under low-folate diet conditions.25,26 MTX, a folate antagonist, exerts its effect by inhibiting the enzyme dihydrofolate reductase, thereby impeding the conversion of folate to its active form and hindering nucleotide biosynthesis. As shown in Figure 2A, normal mice appeared full and rounded, and the neural tube was completely closed (E13.5). Malformed embryos had different degrees of developmental delay, embryos were small, and the main phenotype was NTDs (spina bifida). The results of HE staining at 13.5 days of embryo development showed that neural cells in the Con group were closely arranged, without nucleolysis and pyknosis. In the NTDs group, neural tube structure was abnormal and neural cells were loosely arranged, because the neural tube was curved, the fourth ventricle was undeveloped completely (Figure 2B). Mouse brains were harvested from NTDs and Con group for metabolome through LC-MS. Five fetal whole-brain tissues were combined to form one experimental sample, and six samples were tested in each group. The current PCA and orthogonal partial least-squares discriminant analysis (OPLS-DA) analysis revealed a significant difference in metabolites between NTDs fetal brain and Con groups (Figures 2C and 2D). The different characteristics of metabolites between the two groups were visualized by violin maps of the different samples (Figure 2E). The KEGG pathway enrichment of the brain metabolites in the two groups showed most significant differences in glycerophospholipid metabolism pathway (Figure 2F).
Figure 2.
Normal and NTDs mouse fetal brain metabolome
(A) Experimental group and time lines. Low-folate diets combined with MTX-induced NTDs (Spina Bifida) in C57BL/6 mouse embryos.
(B) H&E staining of brain tissue pathological changes of mice.
(C and D) PCA analysis to the overall distribution and stability of each sample, and spolt-OPLS-DA to distinguish the global metabolic differences between groups and identify the differential metabolites between NTDs and Con group (n = 6 per group).
(E) The violin maps of each sample in the two groups.
(F) The bubble diagram of KEGG pathway enrichment in the two groups.
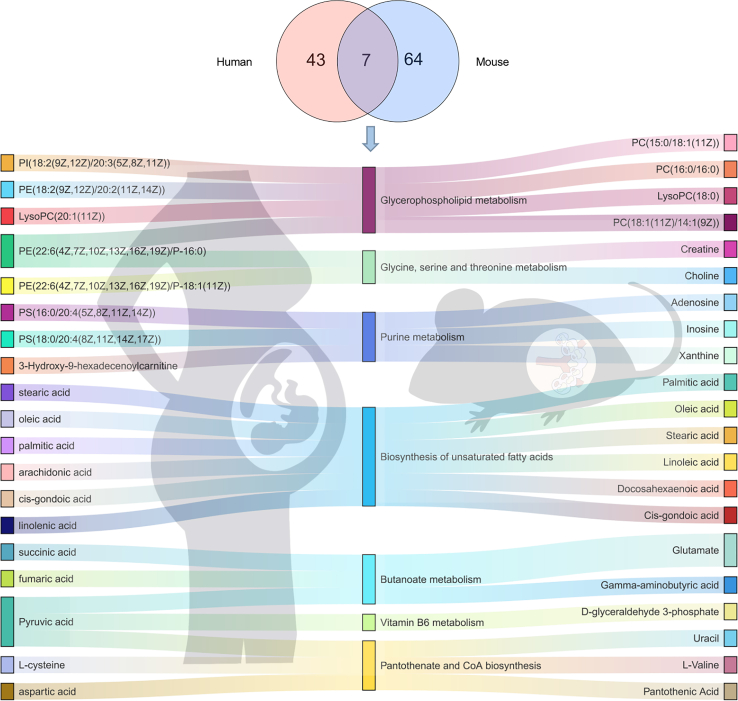
To provide strong evidence of low-folate diet on fetal brain metabolism, the metabolomic sequencing were further explored to identify the low folate-altered metabolism in both humans and mice fetus brain. Seven common differential metabolic pathways shared were related to metabolic activity, including glycerophospholipid metabolism, glycine, serine, and threonine metabolism, purine metabolism, biosynthesis of unsaturated fatty acids, butanoate metabolism, vitamin B6 metabolism, and pantothenate and CoA biosynthesis (Figure 3). Of particular interest is lipids, and lipid-like substances account for a large part of differential metabolites, especially the molecules belonging to glycerophospholipids. Glycerophospholipids, which are highly enriched in the brain, were found to be key components of the neural membrane and involved in dendrite branching and outgrowth. Evidence has shown that polyunsaturated fatty acids which used in brain for glycerophospholipid synthesis are transported from the gastrointestinal tract. Thus, we explored the effect of low-folate diets combined with MTX-induced NTDs on the fecal metabolic profiles of pregnant mice.
Figure 3.
Venn diagram depicting the number of functional pathways from brain metabolites in human and mice
The differential metabolites in both humans and mice fetus brain are mainly involved in seven common functional pathways (middle). Differential metabolites associated with functional pathways in mouse (right) and human (left) brains are shown on both sides.
Fecal metabolites of pregnant mice may be associated with fetal brain lipid metabolism
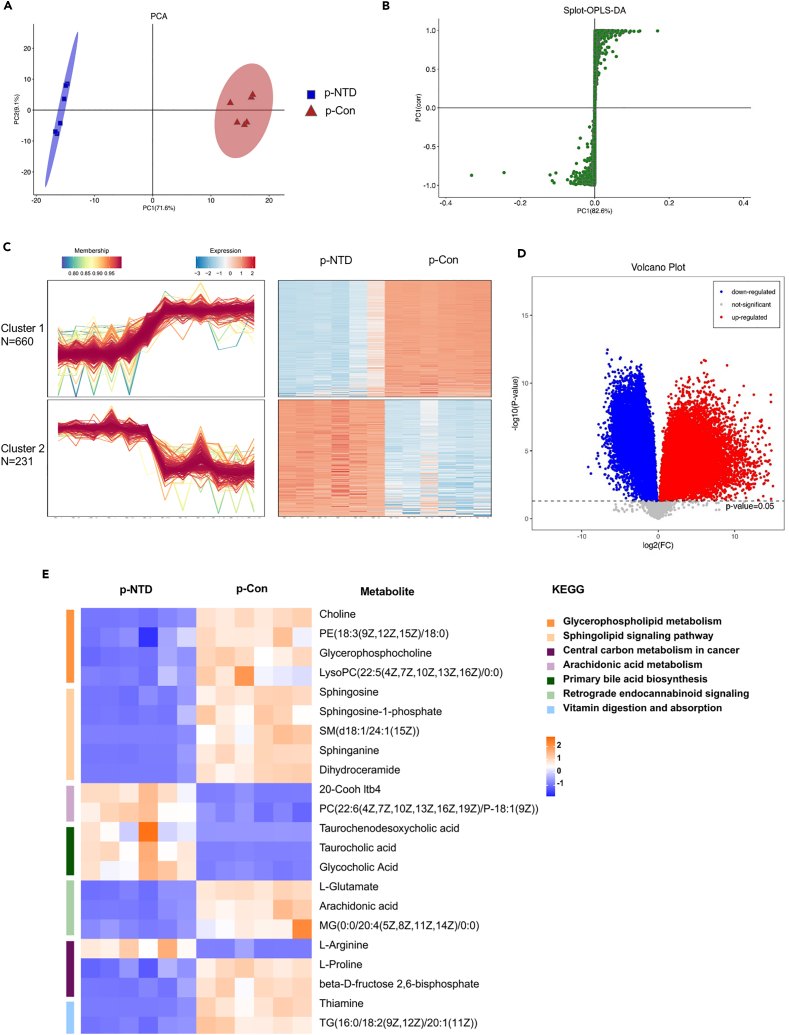
We defined p-NTDs and p-Con as maternal mice with their offerings under NTDs and normal phenotype after a low-folate diet combined with MTX-induction. Maternal diet and offspring neurodevelopment reflected through a lens of metabolic activity, in which fecal metabolic activity may play a role in between.40 LC-MS analysis was used to obtain the fecal metabolic profiles of pregnant mice. PCA showed differences in multidimensional data as a two-dimensional coordinate graph. The more similar the sample composition, the closer the distance in a PCA graph. In this study, PCA analysis revealed robust differences between the p-NTDs and p-Con groups, as shown in Figure 4A. There were significant differences in the OPLS-DA score between the two groups (Figures 4B, S1A, and S1B for gas chromatography-mass spectrometry,GC-MS analysis). We performed cluster analysis on the expression of differential metabolites by the Mfuzz method. Those decreased metabolites in the p-NTDs group compared with the p-Con group were identified as cluster 1 (n = 660), and those increased in the p-NTDs group versus the p-Con group were identified as cluster 2 (n = 231) (Figure 4C). The volcanic map of differential metabolites is shown in Figure 4D (Table S4; Figure S1C for GC-MS analysis). A total of 256 metabolites were identified in the GC-MS analysis, among which 157 were down-regulated and 218 were up-regulated in the p-NTDs group compared with the p-Con group (Figure S1D). We then examined pathways with significant differences in KEGG (Figure S1E) between the two groups of pregnant mice. Several pathways and pathway-related metabolites related to NTDs and folic acid metabolism disorders may be involved in the mechanism of NTDs, including glycerophospholipid metabolism, sphingolipid signaling, central carbon metabolism in cancer, and vitamin digestion and absorption pathway (Figure 4E; Table S5). The glycerophospholipid metabolism pathway and related metabolites in our study are shown in Figure S2. In total, these results indicated that low-folate diets combined with MTX-induction significantly changed the gut microbiota and the metabolite phenotype of pregnant mice.
Figure 4.
LC-MS of fecal in pregnant mice
(A and B) PCA analysis to the overall distribution and stability of each sample, and spolt-OPLS-DA to distinguish the global metabolic differences between groups and identify the differential metabolites between NTDs and Con group(n = 6 per group).
(C) Cluster heatmap of differential metabolites (p < 0.05).
(D) Volcano plots of all differential metabolites between two groups, the p values are described in Table S4.
(E) Representative differential metabolites in the two group were screened out, the KEGG terms shown on the right side (n = 6, the p values are described in Table S5).
Metagenomic analysis and functional pathways in pregnant mice
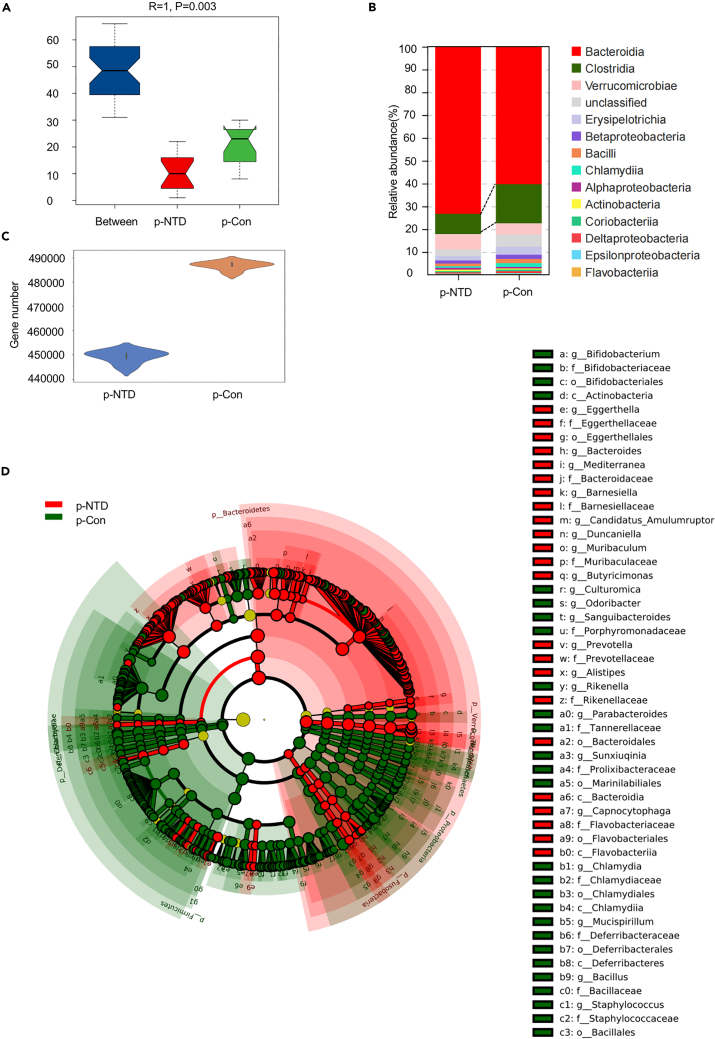
Increasing evidence points to a mutualistic relationship between the health status (short- and long-term) of offspring and gut microbiota during pregnancy. Metagenomic analysis of fecal samples collected from p-NTDs and p-Con mice investigated the composition and function of gut microbiota. ANOSIM (analysis of similarity) showed that the difference between the two groups was significantly greater than the differences within the two groups (Figure 5A). According to the distribution of gene numbers in p-Con and p-NTDs groups, a violin diagram of numbers of genes between groups was drawn, indicating a significant reduction of gene expression in the p-NTDs group compared with the p-Con group, as shown in Figure 5C. At the class classification level, the histogram of the TOP 15 species were classified in the abundance order of the species annotation was determined. In particular, Clostridia were significantly decreased and Bacteroidia were significantly increased in the p-NTDs group compared with the p-Con group (Figure 5B). A total of 40 differentially abundant taxons (from phylum to genus level) were identified by LEfSe between the p-Con and p-NTDs groups (Figure 5D). At the phylum level, p-NTDs mice featured a decrease of Firmicutes and an increase of Bacteroidetes. At the family level, p-NTDs mice featured a decrease of Lachnospiraceae and Ruminococcaceae (Figure S3A). The different functional terms of Cellular Component, Molecular Function, and Biological Process in gene ontologies (GO) between two groups are shown in Figure S3B. Through GO platform analysis, we found that Biological Process was mainly involved in the metabolic process and cellular process, Cellular Component was mainly involved in the cell part and membrane part, and Molecular Function was mainly involved in binding, catalytic activity, and transporter activity. Our data suggested that folic acid metabolism disorders during pregnancy may be associated with the composition and function of gut microbiota in pregnant mice.
Figure 5.
Metagenome of fecal in pregnant mice
(A) The difference between the p-NTDs and p-Con groups was significantly greater than that within each group by Anosim analysis.
(B) Taxonomic summary of the gut microbiota of two groups at class level. The abscissa represents the group and the ordinate represents the relative abundance (n = 6).
(C) A violin diagram of the number of genes between groups.
(D) Significantly discriminative taxa among the various groups determined using linear discriminant analysis effect size (LDA effect size). The overall representation of bacteria composition in NTDs and Con groups by cladogram (n = 6). Taxa were sorted by degree of difference. Only the taxa meeting a significant LDA threshold value of >2.0 are shown.
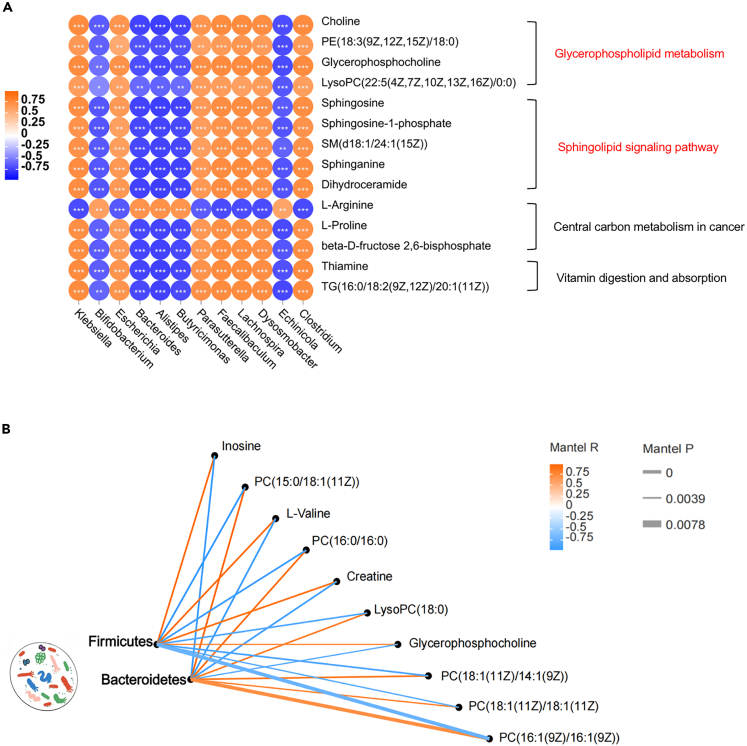
Correlation analysis between microbiota and metabolites in fecal samples of pregnant mice
Malnutrition leads to changes in short-chain fatty acids, amino acids, and various microbiota dependent metabolites suggesting that the microbiome impacts metabolic responses to dietary malnutrition. Correlation analysis explored the relationship between fecal microbiota and metabolites of the pregnant mice. The resulting metabolic association heatmap indicated positive and negative correlations between the levels of metabolites and identified bacterial groups in pregnant mice (Figure 6A). Clostridium, Lachnospira, Faecalibaculum, and Parasutterella displayed strong positive correlations with glycerophospholipid metabolism and sphingolipid signaling pathway. While the Butyricimonas, Alistipes, Bacteroides, and Bifidobacterium showed the opposite effect.
Figure 6.
Correlation between mouse brain metabolites and maternal microbiota
(A) Association heatmap of metabolism and gut microbiota in pregnant mice (genera level), the KEGG terms shown on the right side (n = 6, all p < 0.05).
(B) Fetal mice brain metabolites was related to maternal microbiota (Firmicutes and Bacteroidetes) by Mantel tests, the p values are described in Table S6. Edge width corresponds to the Mantel’s P statistic, and edge color corresponds to the Mantel’s R statistic.
Brain lipometabolic disturbance correlate to maternal gut microbiota metabolism in NTDs mice
Pearson’s correlation analysis determined whether the distribution of gut microbiota in pregnant mice and the metabolic profiles of brain samples from fetal mice with NTDs were correlated. In the phylum level, according to the correlation coefficient and p value, the metabolites with strong correlation with Firmicutes and Bacteroidetes were screened out (Figure 6B; Table S6). It could be seen that these two phyla have opposite correlations with lipid metabolism related metabolites. In the order level, the abundances of Bacteroidales, Bifidobacteriales, Fusobacteriales, and Rhodospirillales were positively correlated with the abundances of lipid metabolites, such as LysoPC(0:0/16:0), PA(16:1(9Z)/21:0), and PE(18:0/20:4(8Z,11Z,14Z,17Z)). The abundances of Enterobacterales, Bacillales, Erysipelotrichales, and Clostridiales were negatively correlated with these lipid metabolites (Figure S4A). A metabolomic Venn diagram of maternal and fetal mice showed 11 metabolites were synchronously down-regulated in feces of pregnant mice and fetal NTDs mice, including inosine, uridine, L-carnitine, and glycerophosphocholine. Furthermore, four metabolites were synchronously up-regulated in pregnant mice feces and fetal NTDs brain, including PE(18:0/0:0) and PC(0:0/18:0) (Figure S4B). In summary, low-folate diets combined with MTX-induction led to changes in the structure/composition of gut microbiota and substantially altered the fecal metabolic phenotype of pregnant mice and the brain metabolic phenotype of fetal mice with NTDs. This result probably reflects the intergenerational microecological mechanism of NTDs.
Discussion
Previous studies found that there is no definitive pathogenic genetic mutation underlying NTDs, indicating that neural tube closure disorder is caused by the mutual effects of genetic and environmental factors, such as folic acid and vitamin deficiency. We used LC-MS to obtain the human cerebral metabolic profiles of fetal neural tube deficits which caused by low maternal folate intake. Functionally, the differential metabolites are mainly involved in glycerophospholipid metabolism and biosynthesis of unsaturated fatty acids. Perinatal factors, including maternal nutritional status, alter the maternal gut microbiota during pregnancy. Increasing evidence indicates that there is a reciprocal relationship between development and function of the nervous system in early life and the maternal gut microbiota. However, the mechanism and causality remain unclear. The present study showed that perinatal malnutrition alters fetal brain development and found a metabolic link between maternal gut microbiota and NTDs by establishing a mouse model of NTDs. To our knowledge, this is the first evidence of an intergenerational association between gut microbiota in disrupted folate metabolism of maternal mice and fetal brain metabolites in mice with NTDs.
Folic acid is the main carrier of one-carbon units in the body and its absorption and metabolism play an important role in NTDs. Disruption of carbon metabolism caused by folic acid deficiency has been shown to be a risk factor for adverse outcomes in pregnancy, including NTDs and miscarriage.13 More importantly, although human and mouse brain metabolomics are markedly different, they share some differential metabolites and cluster into the same metabolic pathways. To provide strong evidence of low-folate diet on fetal brain metabolism, we identified the low folate-altered metabolic pathways in both humans and mice brain, including glycerophospholipid metabolism, glycine, serine, and threonine metabolism, purine metabolism, and biosynthesis of unsaturated fatty acids (Figure 3). Interestingly, lipid metabolites were mainly enriched glycerophospholipids, and many of them are highly permeable to the blood-brain barrier, and may have effects on neurological function. The KEGG pathway enrichment of brain metabolites between the NTDs and Con mice groups showed most significant differences in glycerophospholipid metabolism pathway (Figure 2F). Glycerophospholipids, a type of phospholipid, were key components of neuronal membranes and myelin sheaths and major regulators of synaptic function. Changes in the glycerophospholipid composition of the nerve membrane occurred in acute nerve injury and chronic nerve disease. These alterations accompanied by changes in ion homeostasis and impairment of energy status may be responsible for irreversible neuronal damage during spinal cord or brain injury,15,27 the same as we observed in spina bifida fetal mice.
Evidence has shown that most of the polyunsaturated fatty acids utilized for glycerophospholipids synthesis required for brain development in the neural membrane are not produced in the central nervous system (CNS) but are transported from the gastrointestinal tract to the CNS.28 They either come from the diet or were produced by the liver.28 Previous studies have found that gut microbiota deficiency could hindered the metabolism of glycerophospholipids and sphingolipids in the prefrontal cortex of mice, and microbiota transplantation from specific pathogen-free to bacteria-free mice restored some of these lipids.29 It has been recognized that abnormal changes during pregnancy may impact the health of the fetus after birth. An important underlying factor is the matenal gut microbiota. Nutrients supply and gut microbiota metabolites to the fetus through the umbilical cord may affect their development. To provide strong evidence of the associations of host brain metabolites with gut microbiota, the metagenomic sequencing was further explored to identify the lipids metabolism-related species at strain level. A total of 40 differentially abundant taxons (from phylum to genus level) were identified by LEfSe between the p-Con and p-NTDs groups (Figure 5D). Sequencing of the gut microbiota showed a decrease in the relative abundance of c-Actinobacteria and f-Ruminococcaceae in the low-folate diets combined with the MTX-induced maternal group compared with the Con group. Consistent with this finding, the abundance of Actinobacteria was reported to be positively correlated with head circumference in malnourished and stunted children in Mumbai.30 The present study found an increased abundance of Lactobacillales in the low-folate diet combined with the MTX-induced maternal group. Similarly, a reduction of lactobacillus in the maternal vaginal and infant gut microbiota was associated with changes in infant brain amino acid metabolism in a prenatal stress mouse model.31 The microbiome is involved in a stress signaling pathway between the mother and fetus. Studies have shown that the stress response induced by maternal malnutrition may interact with the microbiome, thus inducing neurodevelopmental changes in offspring.32,33 A study of germ-free brain development in mice also showed that the microbiome influences microglia colonization in the fetal brain during the perinatal period.34 It was found that 240 genes were significantly and differentially expressed between the brain tissues of offspring of normal versus germ-free pregnant mice, suggesting that the maternal microbiome may direct the maturation of microglia during prenatal development. Consistent with this finding, 19 differentially expressed genes were detected in the microglia of E14.5 fetal mice.35 These findings support our conclusion that maternal nutrition affects offspring neurodevelopment and gut microbiota is involved.
Critically, the influence of gut microbiota on folic acid was involved in their conversion and metabolism. Biosynthesis of folic acid and vitamin B12 is a major biochemical feature of gut microbiota.36 Low folic acid diets alter the composition and function of gut microbiota.37 Folic acid deficiency leads to the changes in methylation patterns of specific genes and affects the progression of NTDs.38 We hypothesize that the maternal gut microbiota-derived metabolites were transmitted across the placenta to the fetus and were involved in the regulation of fetal epigenetic mechanisms, such as DNA methylation and histone modification, to further influence the process of neural tube closure.
The current study explored the correlation between the gut microbiota of pregnant mice and the brain metabolic profiles of fetal mice with NTDs (Figure 6B). Coincidentally, the specific changes of such bacteria have been reported in patients with major depressive disorder, autism, and memory deficit. For example, the Alistipes was reported to be elevated in patients with anxiety and depression;39 the Alistipes and Butyricimonas were found to be negatively correlated with cognition ability;40 the Bacteroides increased significantly in patients and mice with autism.41 These associations provide further evidence that specific bacteria may help to regulate neurological metabolism. According to our data, the abundances of gut Enterobacterales and Clostridiales were negatively correlated with the abundances of lipid metabolites, such as LysoPC (0:0/16:0), PA(16:1(9Z)/21:0), and PE(18:0/20:4(8Z,11Z,14Z,17Z)) in the fetal brain (Figure S2A). A clinical study found an increased abundance of Enterobacteriaceae and increased metabolites relating to fatty acid oxidation and lipolysis in the intestinal tract of low-birth-weight infants.42 In the present study, 11 metabolites were synchronously down-regulated in feces of maternal mice and the mice NTDs fetal brain, including glycerophosphocholine, inosine, and L-carnitine (Figure S4B). Previous studies have reported a prophylactic effect of L-carnitine on NTDs rat fetuses, possibly due to its antioxidant activity.43 In addition, another study reported that inosine protect the nervous system by regulating the gut-brain axis.44
Maternal health, including maternal metabolic health, nutritional status, and current nutrient intake, are crucial for the development of offspring.45 The complex and diverse microbial ecosystem residing within a host contributes critically to these intergenerational impacts. These factors shape the fetal nervous system by altering the composition and diversity of maternal gut microbes. Gut microbiota and metabolites are potential therapeutic targets for neurological diseases and potential biomarkers for disease screening in clinical practice. In the past, prenatal imaging examinations were the standard method for diagnosing NTDs. This study confirms an intergenerational correlation between metabolic disorders of maternal gut microbiota caused by folic acid deficiency and fetal brain metabolites in NTDs. Accordingly, the findings of this study have potential implications for the development of a diagnostic kit that can detect maternal intestinal microbial flora and metabolites to accurately predict potential NTDs in the fetus at an earlier stage. This study also suggests that the prevention of neurologic defects in offspring should start from the establishment of a health gut microbiota environment during pregnancy.
Limitations of the study
This study primarily investigates the intergenerational correlation between maternal intestinal microbiota metabolism disorder caused by folate deficiency in a mouse model and fetal brain metabolites in NTDs offspring. Moreover, the same metabolic pathways were identified in the brain tissue of human NTDs fetuses induced by low maternal folate levels during pregnancy. Future work needs to further examine the microbial communities in maternal amniotic fluid and fetal meconium affected by low folate levels, in order to gain a deeper understanding of this intergenerational transmission mechanism.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| The human neural tube defects fetal brain tissues | Lvliang area of Shanxi Province in northern China | N/A |
| Healthy fetal brain tissues | Lvliang area of Shanxi Province in northern China | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Methotrexate | Sigma | Cat#06563 |
| Critical commercial assays | ||
| TruSeq Nano DNA LT Sample Prepararion Kit | Illumina | Cat#FC-121-4001 |
| KAPA Library Quantification Kits | Kapa Biosystems | Cat#KK4824 |
| Deposited data | ||
| Metagenomics data | NCBI | BioProject PRJNA896594 |
| Mass spectrometry data | Metabolights | MTBLS4893 and MTBLS4894 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Charles River Laboratories (CRL) | Cat#213 |
| Software and algorithms | ||
| Prism 9.0 | Graphpad | https://www.graphpad.com/scientific-software/prism |
| R | The R Foundation | https://www.r-project.org/about.html |
| R Studio | R Studio | https://rstudio.com/ |
| SPSS | IBM SPSS Statistics | https://www.ibm.com/spss |
| Other | ||
| Low folate diet chow | Beijing Keao Xieli Feed Co.,Ltd | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shan Wang(wsaquarius@sina.com).
Materials availability
This study did not generate new unique reagent. Information on reagents used in this study is available in the key resources table.
Experimental model and study participant details
Human fetus sample
The human NTDs and matched normal tissues were approved by department of Lvliang area of Shanxi Province in northern China. The classification of NTDs is based on the International Classification of Diseases (ICD-10), which mainly includes anencephaly, spina bifida, brain or spinal membrane expansion. NTDs group samples: Pregnant women with NTDs fetus detected by B-ultrasound in the hospital and undergoing therapeutic induction of labor, fetal samples were collected after informed consent. The fetal samples were pathologically dissected, and the brain tissue samples were frozen and buried. Samples of control group: From the same area as the case, no abnormalities were found by B-ultrasound examination, but labor was induced for non-pathological reasons, and their blood samples and fetal tissue samples from pathological anatomy were also collected after informed consent. Informed consent was obtained from all subjects or their relatives. Human fetus samples were collected and analyzed in accordance with Capital Institute of Pediatrics approval (SHERLLM2021024). The Ethics Board of Capital Institute of Pediatrics approved the study protocol.
Animal
8-weeks-old male and female C57BL/6 mice (weight 18-20g) were allowed to have an adjustable feeding of 2 days before the conduction of experiments. Male and female mice were sectionalized on the basis of table of random number, bred and maintained under specific pathogen-free housing conditions. Female mice were fed on low-folate diet or normal diet for 4 weeks from the beginning of the experiment. Human interference which may interfere with experimental results was minimized during breeding. Water, fodders and beddings were changed at a fixed time every week. The environment was kept in quiet. Sexually matured individuals were mated overnight, vaginal plug was detected at 8:00 am in the following morning and was designated as E0.5 day. NTDs mouse models were induced by intraperitoneal injection with 1.5 mg/kg of methotrexate (MTX) on E7.5. MTX was provided by Sigma in the United States and the concentration of the stock solution was 500 mg/20 ml. Pregnant mice were killed by cervical dislocation on E13.5. The Ethics Committee of Capital Institute of Pediatrics approved all protocols used in this study (DWLL2021011), and procedures involving animal handling complied with institutional guidelines for the care of laboratory animals.
Method details
LC-MS analysis
ACQUITY UPLC system I-Class coupled with VION IMS Q-Tof Mass spectrometer System (Waters, Milford, MA) was used to analyze the metabolic profiling in both ESI positive and ESI negative ion modes. The capillary voltages, EP, CE were 1.0kV, 40V and 6eV, respectively. Source temperature and desolvation temperature were set at 120°C and 500°C, respectively, with a desolvation gas flow of 900 L/h. Centroid data was collected from 50 to 1,000 m/z with a scan time of 0.1 s and interscan delay of 0.02 s over a 13 min analysis time. An ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters, Milford, MA) was used and the binary gradient elution system consisted of (A) water (containing 0.1 % formic acid, v/v) and (B) acetonitrile (containing 0.1 % formic acid, v/v). The gradient elution program was as follows: 0–2 min (5–20 % B), 2–4 min (20–60 % B), 4–11 min (60–100 % B), 11-13 min (100 % B), 13–13.5 min (100 % -5 % B), 13.5–14.5 min (5 % B). The flow rate was 0.4 mL/min and column temperature was 45°C. All the samples were kept at 4°C during the analysis. The QCs were injected at regular intervals (every 10 samples) throughout the analytical run to provide a set of data from which repeatability can be assessed.
Animal feces sample collection
Feces were freshly collected in a clean catch method from mice, attentions were paid to avoid urine pollution. Samples were collected and stored in a special box and immediately frozen under −80°C for further processing.
Metagenomic analysis
The post-filtered pair-end reads were aligned against the host genome using bowtie2(v2.2.9)and the aligned reads were discarded. Metagenome assembly was performed using MEGAHIT (v1.1.2) after getting valid reads. ORF prediction of assembled scaffolds was performed and translated into amino acid sequences by prodigal (v2.6.3). CDHIT (v4.6.7) was used to conduct non-redundant sets of genes predicted from all samples. Bowtie2 (v2.2.9) was used to compared the sequence of each sample with non-redundant gene sets, and the rich information of genes in their corresponding samples was counted. NR, KEGG, COG, SWISSPROT, GO database were used to annotate the gene set representative sequence (amino acid sequence) with an e-value of 1e-5. We obtained species annotations from the taxonomic information database corresponding to the NR database, and then the abundance of species were calculated using the sum of gene abundance.
Metabolomic analysis
The LC-MS analysis includes sample pretreatment, metabolite extraction, metabolite derivatization, LC-MS detection, data pretreatment and statistical analysis, etc. Qualitative and relative quantitative analyses were performed on raw data based on the untargeted metabolomics of quadrupole mass spectrometry, combined with the qualitative software MS-DIAL for metabolomics data. Metabolomic experiments were performed as previously described.46
Quantification and statistical analysis
Metagenomic analysis
Nonparametric test Anosim analysis is used to test whether the difference between groups is significantly greater than the difference within the group, so as to judge the reasonability of grouping. PERMANOVA (ADONIS analysis.) was used to analyze the explanatory power of different grouping factors for sample differences, and significance statistics were performed using replacement tests. Wilcoxon rank-sum was used to test the hypothesis of species abundance data between groups based on species abundance tables of different levels, and p value was obtained. P < 0.05 was considered as significant difference.
Metabolomic analysis
A two-tailed Student’s T-test was used to verify significance between groups. Spearman’s correlation analysis was used to analyze the association between gut microbiota and metabolites.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82071690 and 81400848), Research Foundation of Capital Institute of Pediatrics (FX-2020-05 and CXYJ-2-21-09), Public service development and reform pilot project of Beijing Medical Research Institute (BMR2019-11 and BMR2021-3). We thank Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
Author contributions
S.W. conceived and designed this study. S.W., P.P., and Y.W. participated in laboratory work. X.H., M.Z., and X.Z. performed the data analysis. S.W. and X.H. participated in the writing of the manuscript. S.W. and T.Z. participated in advising and revising the manuscript critically. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: July 29, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107514.
Contributor Information
Shan Wang, Email: wsaquarius@sina.com.
Min Zhang, Email: mmisagirl@foxmail.com.
Ting Zhang, Email: zhangtingcv@126.com.
Supplemental information
Data and code availability
-
•
The accession number for the metagenomics data reported in this paper is NCBI Sequence Read Archive:BioProject PRJNA896594. The accession number for the metabolomics data reported in this paper is Metabolights:MTBLS4893 and MTBLS4894.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Frey L., Hauser W.A. Epidemiology of neural tube defects. Epilepsia. 2003;44(Suppl 3):4–13. doi: 10.1046/j.1528-1157.44.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilde J.J., Petersen J.R., Niswander L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu. Rev. Genet. 2014;48:583–611. doi: 10.1146/annurev-genet-120213-092208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnell R.H., Caiaffa C.D., Kim S.E., Lei Y., Steele J., Cao X., Tukeman G., Lin Y.L., Cabrera R.M., Wlodarczyk B.J. Gene Environment Interactions in the Etiology of Neural Tube Defects. Front. Genet. 2021;12:659612. doi: 10.3389/fgene.2021.659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine E.L., Horal M., Chang T.I., Fortin G., Loeken M.R. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–2462. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- 5.Shaw G.M., Velie E.M., Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA. 1996;275:1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- 6.Keats E.C., Neufeld L.M., Garrett G.S., Mbuya M.N.N., Bhutta Z.A. Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: evidence from a systematic review and meta-analysis. Am. J. Clin. Nutr. 2019;109:1696–1708. doi: 10.1093/ajcn/nqz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luciano-Mateo F., Hernández-Aguilera A., Cabre N., Camps J., Fernández-Arroyo S., Lopez-Miranda J., Menendez J.A., Joven J. Nutrients in Energy and One-Carbon Metabolism: Learning from Metformin Users. Nutrients. 2017;9:121. doi: 10.3390/nu9020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copp A.J., Stanier P., Greene N.D.E. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. doi: 10.1016/s1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony A.C. In utero physiology: role of folic acid in nutrient delivery and fetal development. Am. J. Clin. Nutr. 2007;85:598s–603s. doi: 10.1093/ajcn/85.2.598S. [DOI] [PubMed] [Google Scholar]
- 10.Raghubeer S., Matsha T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients. 2021;13:4562. doi: 10.3390/nu13124562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fekete K., Berti C., Cetin I., Hermoso M., Koletzko B.V., Decsi T. Perinatal folate supply: relevance in health outcome parameters. Matern. Child Nutr. 2010;6(Suppl 2):23–38. doi: 10.1111/j.1740-8709.2010.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson R., Jain M., Madhusudhan N., Sheppard N.G., Strittmatter L., Kampf C., Huang J., Asplund A., Mootha V.K. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat. Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogne T., Tielemans M.J., Chong M.F.F., Yajnik C.S., Krishnaveni G.V., Poston L., Jaddoe V.W.V., Steegers E.A.P., Joshi S., Chong Y.S., et al. Associations of Maternal Vitamin B12 Concentration in Pregnancy With the Risks of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Individual Participant Data. Am. J. Epidemiol. 2017;185:212–223. doi: 10.1093/aje/kww212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian T., Mao Q., Xie J., Wang Y., Shao W.H., Zhong Q., Chen J.J. Multi-omics data reveals the disturbance of glycerophospholipid metabolism caused by disordered gut microbiota in depressed mice. J. Adv. Res. 2022;39:135–145. doi: 10.1016/j.jare.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.arooqui AA H.L., Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids. 2000;106:101–129. doi: 10.1016/s0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen J.J., Xie J., Zeng B.H., Li W.W., Bai S.J., Zhou C., Chen W., Wei H., Xie P. Absence of gut microbiota affects lipid metabolism in the prefrontal cortex of mice. Neurol. Res. 2019;41:1104–1112. doi: 10.1080/01616412.2019.1675021. [DOI] [PubMed] [Google Scholar]
- 17.Mayengbam S., Chleilat F., Reimer R.A. Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats. Biomedicines. 2020;8:469. doi: 10.3390/biomedicines8110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J., Cai D., Yang M., Hao Y., Zhu Y., Chen Z., Aziz T., Sarwar A., Yang Z. Screening of folate-producing lactic acid bacteria and modulatory effects of folate-biofortified yogurt on gut dysbacteriosis of folate-deficient rats. Food Funct. 2020;11:6308–6318. doi: 10.1039/d0fo00480d. [DOI] [PubMed] [Google Scholar]
- 19.Lurz E., Horne R.G., Määttänen P., Wu R.Y., Botts S.R., Li B., Rossi L., Johnson-Henry K.C., Pierro A., Surette M.G., Sherman P.M. Vitamin B12 Deficiency Alters the Gut Microbiota in a Murine Model of Colitis. Front. Nutr. 2020;7:83. doi: 10.3389/fnut.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubini E., Schenkelaars N., Rousian M., Sinclair K.D., Wekema L., Faas M.M., Steegers-Theunissen R.P.M., Schoenmakers S. Maternal obesity during pregnancy leads to derangements in one-carbon metabolism and the gut microbiota: implications for fetal development and offspring wellbeing. Am. J. Obstet. Gynecol. 2022;227:392–400. doi: 10.1016/j.ajog.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Satokari R., Grönroos T., Laitinen K., Salminen S., Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett. Appl. Microbiol. 2009;48:8–12. doi: 10.1111/j.1472-765X.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- 22.Vuong H.E., Pronovost G.N., Williams D.W., Coley E.J.L., Siegler E.L., Qiu A., Kazantsev M., Wilson C.J., Rendon T., Hsiao E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586:281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Val-Laillet D., Besson M., Guérin S., Coquery N., Randuineau G., Kanzari A., Quesnel H., Bonhomme N., Bolhuis J.E., Kemp B., et al. A maternal Western diet during gestation and lactation modifies offspring's microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. Faseb. J. 2017;31:2037–2049. doi: 10.1096/fj.201601015R. [DOI] [PubMed] [Google Scholar]
- 24.Afroz K.F., Reyes N., Young K., Parikh K., Misra V., Alviña K. Altered gut microbiome and autism like behavior are associated with parental high salt diet in male mice. Sci. Rep. 2021;11:8364. doi: 10.1038/s41598-021-87678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Xie Q., Gao J., Wang F., Bao Y., Wu L., Yang L., Liu Z., Guo R., Khan A., et al. Aberrant Gcm1 expression mediates Wnt/beta-catenin pathway activation in folate deficiency involved in neural tube defects. Cell Death Dis. 2021;12:234. doi: 10.1038/s41419-020-03313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei P., Cheng X., Yu J., Shen J., Li X., Wu J., Wang S., Zhang T. Folate deficiency induced H2A ubiquitination to lead to downregulated expression of genes involved in neural tube defects. Epigenet. Chromatin. 2019;12:69. doi: 10.1186/s13072-019-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meikle P.J., Summers S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017;13:79–91. doi: 10.1038/nrendo.2016.169. [DOI] [PubMed] [Google Scholar]
- 28.Liu G., Yu Q., Tan B., Ke X., Zhang C., Li H., Zhang T., Lu Y. Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome. Gut Microb. 2022;14:2104089. doi: 10.1080/19490976.2022.2104089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolopoulou E., Galea G.L., Rolo A., Greene N.D.E., Copp A.J. Neural tube closure: cellular, molecular and biomechanical mechanisms. Development. 2017;144:552–566. doi: 10.1242/dev.145904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huey S.L., Jiang L., Fedarko M.W., McDonald D., Martino C., Ali F., Russell D.G., Udipi S.A., Thorat A., Thakker V., et al. Nutrition and the Gut Microbiota in 10- to 18-Month-Old Children Living in Urban Slums of Mumbai, India. mSphere. 2020;5:e00731-20. doi: 10.1128/mSphere.00731-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jašarević E., Howerton C.L., Howard C.D., Bale T.L. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G., Longman R.S., Honda K., Littman D.R., Choi G.B., Huh J.R. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castillo-Ruiz A., Mosley M., George A.J., Mussaji L.F., Fullerton E.F., Ruszkowski E.M., Jacobs A.J., Gewirtz A.T., Chassaing B., Forger N.G. The microbiota influences cell death and microglial colonization in the perinatal mouse brain. Brain Behav. Immun. 2018;67:218–229. doi: 10.1016/j.bbi.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pronovost G.N., Hsiao E.Y. Perinatal Interactions between the Microbiome, Immunity, and Neurodevelopment. Immunity. 2019;50:18–36. doi: 10.1016/j.immuni.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engevik M.A., Morra C.N., Röth D., Engevik K., Spinler J.K., Devaraj S., Crawford S.E., Estes M.K., Kalkum M., Versalovic J. Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 2019;10:2305. doi: 10.3389/fmicb.2019.02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas C.M., Saulnier D.M.A., Spinler J.K., Hemarajata P., Gao C., Jones S.E., Grimm A., Balderas M.A., Burstein M.D., Morra C., et al. FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiol. 2016;5:802–818. doi: 10.1002/mbo3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J., Wang L., Pei P., Li X., Wu J., Qiu Z., Zhang J., Ao R., Wang S., Zhang T., Xie J. Reduced H3K27me3 leads to abnormal Hox gene expression in neural tube defects. Epigenet. Chromatin. 2019;12:76. doi: 10.1186/s13072-019-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson C.A., Diaz-Arteche C., Eliby D., Schwartz O.S., Simmons J.G., Cowan C.S.M. The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev. 2021;83:101943. doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 40.Ren T., Gao Y., Qiu Y., Jiang S., Zhang Q., Zhang J., Wang L., Zhang Y., Wang L., Nie K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson's Disease. Front. Neurol. 2020;11:137. doi: 10.3389/fneur.2020.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coretti L., Cristiano C., Florio E., Scala G., Lama A., Keller S., Cuomo M., Russo R., Pero R., Paciello O., et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep. 2017;7:45356. doi: 10.1038/srep45356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younge N.E., Newgard C.B., Cotten C.M., Goldberg R.N., Muehlbauer M.J., Bain J.R., Stevens R.D., O'Connell T.M., Rawls J.F., Seed P.C., Ashley P.L. Disrupted Maturation of the Microbiota and Metabolome among Extremely Preterm Infants with Postnatal Growth Failure. Sci. Rep. 2019;9:8167. doi: 10.1038/s41598-019-44547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X., Teng T., Li X., Fan L., Xiang Y., Jiang Y., Du K., Zhang Y., Zhou X., Xie P. Impact of Inosine on Chronic Unpredictable Mild Stress-Induced Depressive and Anxiety-Like Behaviors With the Alteration of Gut Microbiota. Front. Cell. Infect. Microbiol. 2021;11:697640. doi: 10.3389/fcimb.2021.697640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khaksary Mahabady M., Najafzadeh Varzi H., Zareyan Jahromi S. L-Carnitine Protect against Cyclophosphamide Induced Skeletal and Neural Tube Malformations in Rat Fetuses. Acta Med. Iran. 2015;53:703–710. [PubMed] [Google Scholar]
- 45.Coley E.J.L., Hsiao E.Y. Malnutrition and the microbiome as modifiers of early neurodevelopment. Trends Neurosci. 2021;44:753–764. doi: 10.1016/j.tins.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 46.He X., Zhang T., Zeng Y., Pei P., Liu Y., Jia W., Zhao H., Bi M., Wang S. Sodium butyrate mediates histone crotonylation and alleviated neonatal rats hypoxic-ischemic brain injury through gut-brain axis. Front. Microbiol. 2022;13:993146. doi: 10.3389/fmicb.2022.993146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The accession number for the metagenomics data reported in this paper is NCBI Sequence Read Archive:BioProject PRJNA896594. The accession number for the metabolomics data reported in this paper is Metabolights:MTBLS4893 and MTBLS4894.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.