Highlights
-
•
Aroma property of wampee fruit was evaluated by E-nose, GC–MS, and GC–IMS.
-
•
Taste property of wampee fruit was evaluated by E-tongue and HPLC.
-
•
11 Volatile compounds and 12 taste substances were identified as crucial compounds.
-
•
The seed possessed the most intense and pungent aroma but the lightest taste.
-
•
The peel had a citrus aroma similar to pulp, but is sourer and more astringent.
Chemical compounds studied in this article: Linalool (PubChem CID: 6549), Benzeneacetaldehyde (PubChem CID: 998), (E)-γ-Bisabolene (PubChem CID: 5352437), Succinic acid (PubChem CID: 1110), Malic acid (PubChem CID: 525), Acetic acid (PubChem CID: 176), Alanine (PubChem CID: 5950), Aspartic acid (PubChem CID: 5960), Glutamic acid (PubChem CID: 33032)
Keywords: Wampee, Relative odor activity value, Taste activity value, Principal components analysis, Partial least squares discriminant analysis
Abstract
Wampee is a tropical fruit having high medicinal value. To fully realize the fruit’s potential, it is essential to reveal the flavor characteristics. In this study, a comprehensive analysis of the aroma and taste profiles of different parts from the wampee fruit was conducted. The aroma profile was analyzed by E-nose, and 67 volatile components were identified through HS–SPME–GC–MS. Among them, 11 were considered as crucial compounds. Additionally, 42 volatile components were identified by HS–GC–IMS, with 22 compounds showing a variable importance in projection scores greater than 1.0. Moreover, the taste profile and representative compounds were analyzed by E-tongue and HPLC, and 12 compounds were considered as important taste contributors based on taste activity value. These findings shed light on the various compounds responsible for the unique aroma and taste of the wampee fruit, providing theoretical foundation for exploring ways for its comprehensive utilization and development.
1. Introduction
Wampee (Clausena lansium (Lour.) Skeels), belonging to the family Rutaceae, is an evergreen small tree with great economic value (Fan et al., 2018). This particular species, indigenous to Southern China, now is extensively grown in China, Vietnam, the Philippines, and other Asian nations, and has been introduced to Queensland, Hawaii and Florida, not only for its unique flavor but also for its remarkable nutritional value and pharmacological benefits (Liu et al., 2019). The root and leaves can be exploited to treat cough, asthma, viral hepatitis, and gastrointestinal disorders (Fan et al., 2021). Generally, wampee is classified into sweet or sweet–sour cultivar, depending on the taste of its fruit, the sweet fruit is usually fresh-eating, while the sweet–sour fruit is widely used as a raw material for processing (Yin et al., 2022).
Since all parts of the wampee (seed, pulp, peel, leaf, and stem) have different components with great biological activity values, recent studies on this plant have been mainly focused on the identification, extraction, and utilization of those bioactive substances (Ao et al., 2022, Liu et al., 2012, Peng et al., 2022, Prasad et al., 2010, Sciarrone et al., 2013, Wu et al., 2013, Yan et al., 2018). For instance, researchers have investigated the antifungal activity of amides and the antioxidant activities of acidic polysaccharides isolated from the wampee seed (Wu et al., 2013, Yan et al., 2018), the antioxidant activities of a new benzofuran glycoside from the pulp and peel (Ao et al., 2022), the antioxidant and anticancer activities of 8-hydroxypsoralen as well as the physicochemical, structural, and functional properties of pectin extracted from the peel (Peng et al., 2022, Prasad et al., 2010), a rapid collection and identification of (2E,6E)-2-methyl-6-(4-methylcyclohex-3-enylidene)hept-2-enal from the leaf (Sciarrone et al., 2013), and the neuroprotective activities of carbazole alkaloids from the stem (Liu et al., 2012). The seed and peel of the wampee fruit are frequently treated as waste due to varying dietary habits; however, all parts of the wampee fruit with various flavors are edible (Xu et al., 2014). So far, only few studies have been conducted on the flavor of the wampee fruit. In a study conducted by Chokeprasert et al. (2007), a total of 42 volatile components (VOCs) were identified in the wampee fruit (including the seed, pulp, and peel) using GC–MS. Among these VOCs, terpenes were found to be the most dominant (Chokeprasert et al., 2007). Furthermore, Yin et al. identified multiple key taste components in the wampee juice derived from the pulp, such as d-galactose, d-mannose, and l-citramalic acid, based on widely targeted metabolomics (Yin et al., 2022). Although some VOCs and key taste substances in the wampee fruit have been well characterized in previous studies, a comprehensive analysis encompassing the two distinct dimensions of aroma and taste in various parts of the fruit is still lacking.
The aim of this study was to comprehensively and systematically investigate the aroma and taste properties of the wampee fruit’s seed, pulp, and peel, through more diverse instrumental chemistry analyses to identify richer key flavor substances. For the first time, the aroma properties of the fruit were thoroughly analyzed by using an E-nose, HS-SPME-GC–MS coupled with HS-GC-IMS, and the taste characteristics were studied by employing an E-tongue and based on the composition analyses of the main sugars, organic acids, and amino acids. More importantly, to discern the differences among various parts of the wampee fruit, principal components analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were utilized. Furthermore, in order to identify the crucial flavor components specific to each part of the wampee fruit, relative odor activity values (rOAVs), variable importance in projection (VIP) scores and taste activity values (TAVs) were employed.
2. Materials and methods
2.1. Preparation of wampee fruits and chemicals
Fresh Yongxing Dajixin wampee fruits, officially recognized as a national geographical indication agricultural product in China, were ordered and procured in July 2021 from a local fruit market in Hainan, China and delivered to the laboratory within an hour of purchase. These fruits were all collected from the same batch of five-year-old trees grown at the Yongxing Plantation Base in Hainan Province (19.8754 N, 110.2786 E), with a maturity of 90% (102 ± 5 days after flowering). To form biological samples, 100 plump and undamaged fruits (783.24 ± 12.36 g) were meticulously selected, and the seeds, pulp, and peel were manually separated from each fruit. They were then immediately dried using a freeze-dryer (LGJ-10, Beijing Songyuanhuaxing Technology Develop Co., Ltd, Beijing, China), and subsequently crushed and mixed continuously for 2 min using a mixer mill (MM400, Verder Shanghai Instruments and Equipment Co., Ltd., Shanghai, China) at 30 Hz. This process was repeated three times, yielding 3 sets of biological samples, each consisting of even powders from three different fruit parts of the fruits (the seeds, pulp, and peel). These samples, 3 samples for each of the three parts, were then stored in a dry environment at −80 °C until their use in subsequent analyses.
Standards, including n-alkanes (C4-C25) (chromatographic purity), all amino acids (chromatographic purity), o-phthalaldehyde (OPA, analytical purity) and 9-fluorenylmethyl chloroformate (FmocCl, analytical purity), were obtained from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). All the standards of fructose, glucose, saccharose, galacturonic acid, oxalic acid, malic acid, lactic acid, acetic acid, succinic acid and fumaric acid were of chromatographic purity and were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Acetonitrile and methanol (chromatographic purity) were obtained from CNW Technologies GmbH (Dusseldorf, Germany). All erchloric acid solution (70–72%), sodium dihydrogen phosphate dihydrate and sodium phosphate dibasic dodecahydrate were of analytical purity and were purchased from Guangzhou Chemical Reagent Factory (Guangdong, China).
2.2. E-nose analysis
The general aroma profile was analyzed using a PEN 3 E-nose (Airsense Analytics Co., Ltd., Schwerin, Germany) following to the methodology outlined in the literature (Shen et al., 2021, Zhang et al., 2021) with minor adjustment. To elaborate, 6.0 g of each sample was combined with 18 mL of distilled water in a headspace bottle. The mixture was then balanced in a water bath at 35 °C for 30 min before measurement. The determination conditions were as follows: the cleaning time was set at 60 s, the sample preparation time at 5 s, and the sample measurement time at 200 s.
2.3. HS-SPME-GC–MS analysis
2.3.1. HS-SPME conditions
A sample (5.0 g) was placed into a 100 mL headspace bottle. Subsequently, 6.0 g of NaCl and 15 mL of distilled water (50 °C) were added into the bottle. The headspace bottle was immediately sealed and balanced at 70 °C and 400 rpm for 10 min. Next, the SPME (Solid Phase Microextraction) probe with DVB/CAR/PDMS fiber was inserted into the headspace bottle for 60 min for adsorption, and it was inserted into the GC injection port (250 °C) for desorption for 3 min.
2.3.2. GC–MS conditions
The carrier gas (helium, 99.999% purity) passed through the chromatographic column (HP-5MS, 30 m × 0.25 mm × 0.25 μm) at a flow rate of 1 mL/min, and the injection port was maintained at 250 °C. The GC temperature program was set as follows: the initial temperature was 60 °C; it was then raised to 180 °C at a rate of 2 °C/min; next it was further increased to 250 °C at a rate of 10 °C/min; finally, it was maintained at 250 °C for 3 min. MS parameters were set as follows: an ionization mode of EI mode, a temperature of the ion source of 230 °C, an ionization energy of 70 eV, a temperature of the MS quadrupole of 150 °C, an ion mass scanning ranging from 40 to 600 m/z, and an emission current of 35 μA. To calculate retention indices (RIs), the n-alkanes (C9-C25) were used as external references. The VOCs were identified based on their RIs and comparison of the mass spectra with the NIST 20.L library.
2.3.3. Identification of crucial volatile compounds
The relative odor activity value (rOAV) has been used to assess the contribution of individual VOCs to the overall aroma profile, based on their relative concentrations (Su et al., 2022, Wei et al., 2019). rOAVmax of 100 is defined as the VOC that contributes the most to the overall aroma (Hou et al., 2022). The rOAV for each VOC was determined by calculating the ratio of the odor activity value (OAV) to the highest odor activity value (OAVmax) (Su et al., 2022). In this study, the crucial VOCs in wampee fruit were identified with an rOAV greater than 1.
2.4. HS-GC-IMS analysis
The HS-GC-IMS (FlavourSpec, G.A.S., Dortmund, Germany) was used to conduct further measurements of VOCs. 1.0 g of each sample was placed into a 20 mL headspace bottle and incubated at 500 rpm and 50 °C for 20 min. The temperature of the GC injector was set at 85 °C, and 500 μL of the headspace gas was pre-separated by a chromatographic column (MXT-5, 15 m × 0.53 mm × 1 μm) at 60 °C. The runtime of the GC unit was 30 min, and the programmed flow of the carrier gas (nitrogen, 99.999% purity) was set as follows: the initial flow rate was 2 mL/min, which was then increased to 10 mL/min for 10 min, followed by a further increase to 100 mL/min for another 10 min, and finally raised to 150 mL/min and stopped after 10 min. The IMS was coupled to the GC and maintained at 45 °C, with the speed of the drift gas (nitrogen, 99.999% purity) was set at 150 mL/min.
n-Alkanes (C4-C9) were used as external references to calculate the RIs. To qualitatively determine the VOCs, their RIs and drift times were compared with those of standards in the GC-IMS library Search (G.A.S., Dortmund, Germany). Each sample was tested three times.
2.5. E-tongue analysis
The Taste Sensing System (Intelligent Sensor Technology, Inc., Kanagawa, Japan) was utilized to assess the intensities of eight tastes: sourness, umami, saltiness, bitterness, astringency, aftertaste umami, aftertaste bitterness, and aftertaste astringency. In brief, the samples were rehydrated according to their respective moisture content in fresh fruit (58.7% for the seed, 80.6% for the pulp, 75.9% for the peel), and then were diluted with distilled water at a material - liquid ratio of 1:5 (w/v). The mixture was thoroughly stirred for 2 h and then filtered to obtain the filtrate, which was used to measure the intensity of taste. All these operations were conducted at room temperature.
2.6. Sugars, organic acids and amino acids
2.6.1. HPLC conditions
HPLC was used to analyze the contents of main flavored sugars, organic acids, and amino acids in different parts of the wampee fruit. For the determination of the main flavored sugars, the Aglient 1200 system (Agilent Technologies, Santa Clara, USA) with a column (ZORBAX Original NH2, 250 mm × 4.6 mm × 5 μm) and a refractive index detector (RID, 190–810 nm) were employed. The mobile phase consisted of a mixture of acetonitrile and water in a ratio of 5:1 (v/v), with a flow rate set as 0.7 mL/min. To assess the organic acids composition, an Aglient 1260 infinity Ⅱ system (Agilent Technologies, Santa Clara, USA) equipped with a column (ZORBAX SB-Aq, 30 mm × 4.6 mm × 1.8 μm) and a variable wavelength detector (VWD) set at 210 nm was employed to determine the organic acids composition. The mobile phase consisted of perchloric acid solution (pH = 2.5) and methanol in a ratio of 98:2 (v/v) with the flow rate of 0.6 mL/min.
In order to analyze the amino acids composition, both primary and secondary amino acids were initially derivatized using Agilent's automated on-line derivatization method with OPA and FmocCl respectively. Subsequently, the derivatives of the amino acids were determined using the Aglient 1100 system (Agilent Technologies, Santa Clara, USA) with a column (ZORBAX Eclipse AAA, 150 mm × 4.6 mm × 3.5 μm) and a VWD detector set at 388 nm and 266 nm. Sodium dihydrogen phosphate solution (40 mM, pH = 7.8) and the mixture of acetonitrile, methanol, and water in a ratio of 45:45:10 (v/v/v) were used as solution A and solution B in the solvent system, respectively. The samples were subjected to a decreasing gradient of solution A for 41 min, with a flow rate of 1.0 mL/min. The gradient elution program was as follows: starting with 0–1 min at 100% A, gradually decreasing to 46% A from 1 to 23 min, and further decreasing to 0% A from 23 to 27 min, followed by a steady 0% A from 27 to 34 min. The gradient then increased again to 100% A from 34 to 40 min, and followed by a steady 100% A from 40 to 41 min.
2.6.2. Identification of key contributors to taste
Taste activity values (TAVs) can be applied to assess the contribution of individual components to taste (Hou et al., 2022). A compound with TAV exceeding 1 can be considered an important contributor to the overall taste. TAV is calculated according to the following formula (Chen et al., 2021b):
2.7. Statistical analysis
The data were analyzed using SPSS 26.0 (IBM, New York, US) and the results are expressed as the mean ± standard deviation. To evaluate statistically significant differences, a one-way analysis of variance (ANOVA) and Tukey's multiple comparison test were conducted. For further analysis, both PCA and PLS-DA were performed using SIMCA 14.1 (MKS Umetrics, Sweden), with unit variance (UV) used as the data preprocessing method.
3. Results and discussion
3.1. Aroma profile
The E-nose sensors are either nonspecific or semi-specific for classes of compounds, and their response values are related to the chemical composition and content of VOCs (Cai et al., 2020). On the E-nose, there are 10 different sensors, each designed to detect specific types of substances. The sensors and their corresponding sensitive substances are as follows: the W1C is sensitive to aromatic benzene; the W5S to nitrogen oxides; the W3C to aromatic ammonia; the W6S to hydrogen; the W5C to alkane aromatic compounds; the W1S to short-chain alkanes; the W1W to sulfides and terpenes; the W2S to alcohols, aldehydes, and ketones; the W2W to organic sulfides and aromatic components; and the W3S to long-chain alkanes.
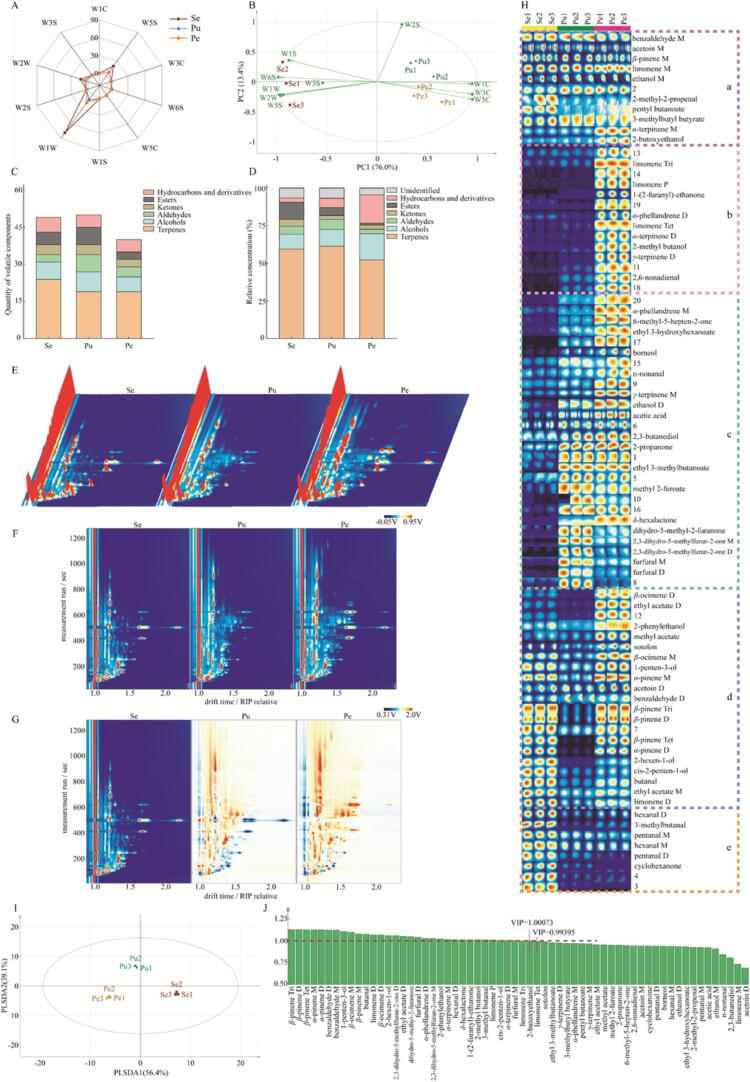
As shown in Fig. 1A, the radar chart revealed that the pulp and peel exhibited a similar shape in their response profiles. However, the total response value for the seed was notably much higher than that of the former two. This observation suggested that the aroma profiles of the pulp and peel were broadly similar, while the aroma of the seed was more intense. Moreover, it was observed that the W1W, W5S, and W2W sensors demonstrated stronger responses to VOCs in each part, with the W1W sensor showing the strongest response. This indicated that the wampee fruit was rich in terpenes, sulfides, organic sulfides, and nitrogen oxides, with terpenes being the most abundant among them.
Fig. 1.
Aroma profiles and VOCs of different parts of the wampee fruit: (A) the radar chart and (B) PCA biplot of E-nose response values; (C) the quantity and (D) relative concentration of each class of VOCs detected by HS–SPME–GC–MS; (E) the 3D topographic plot, (F) top view, (G) comparison plot, (H) fingerprint plot of HS-GC-IMS; (I) the PLS-DA score plot and (J) VIP chart of VOCs detected by HS-GC-IMS. “Se”, “Pu”, and “Pe” indicate seed, pulp, and peel, respectively.
PCA, an unconstrained dimension reduction method, was employed to achieve a more comprehensive understanding of the aroma properties in different parts of the wampee fruit. To visualize the contributions of the ten sensors in discriminating different wampee parts, a biplot of scores and loadings was generated (Fig. 1B). The first two principal components (PC1 and PC2) explained a total variance of 89.4%, with PC1 and PC2 accounting for 76.0% and 13.4% of the variance, respectively. The biplot exhibited two distinct clusters, the seed formed a cluster on the left, while the pulp and peel formed another cluster on the right. The observation aligns with the findings from the radar chart, confirming that the aroma profiles of the pulp and peel were indeed similar. Furthermore, measurements from most E-nose sensors clustered to the left, including W1S, W3S, W6S, W1W, W5S, and W2W, implying positive correlations between these sensors and the seed. On the other hand, W1C, W3C, and W5C clustered to the right, signifying strong positive correlations with the pulp and peel. And the W2S sensor showed a weak correlation with all of the samples and the other sensors.
3.2. Assessment of VOCs detected by HS-SPME-GC–MS
The VOCs in the different parts of the wampee fruit were identified and relatively quantified using HS-SPME-GC–MS, and the detailed results are presented in Table 1. In total, 67 VOCs were detected, belonging to 6 categories, including terpenes, alcohols, aldehydes, ketones, esters, and hydrocarbons and derivatives.
Table 1.
The relative contents of VOCs identified by HS–SPME–GC–MS.
| RI1 | RI2 | VOCs | Relative concentration (%) |
||
|---|---|---|---|---|---|
| Seed | Pulp | Peel | |||
| Terpenes (28) | 59.57 | 61.48 | 52.39 | ||
| 968 | 966 | β-Thujene | 3.81 | – | – |
| 989 | 991 | β-Myrcene | 0.22 | – | – |
| 1003 | 1007 | α-Phellandrene | 0.20 | 0.40 | 0.78 |
| 1013 | 1014 | δ-Carene | 0.45 | 0.79 | 0.58 |
| 1054 | 1056 | γ-Terpinene | 0.81 | 1.32 | 1.46 |
| 1345 | 1347 | α-Cubebene | 2.33 | 1.21 | – |
| 1405 | 1417 | Sesquithujene | 1.37 | 0.90 | 1.24 |
| 1415 | 1407 | Isocaryophyllene | 5.23 | 8.49 | – |
| 1417 | 1420 | α-Santalene | 1.33 | 2.69 | 2.17 |
| 1432 | 1422 | α-Cedrene | – | – | 1.69 |
| 1458 | 1459 | (E)-β-Farnesene | 1.98 | 4.30 | 5.11 |
| 1472 | 1491 | γ-Cadinene | 1.28 | – | – |
| 1474 | – | 1-Epibicyclosesquiphellandrene | – | 3.28 | 5.86 |
| 1477 | 1478 | γ-Curcumene | 1.36 | – | 8.69 |
| 1481 | 1482 | α-Curcumene | 1.86 | 2.49 | 2.24 |
| 1495 | 1495 | α-Zingiberene | – | – | 5.70 |
| 1502 | 1519 | δ-Cadinene | 0.35 | – | – |
| 1502 | 1501 | Epizonarene | – | 0.39 | – |
| 1528 | 1524 | β-Sesquiphellanderene | 16.98 | 2.96 | 0.47 |
| 1540 | 1534 | (E)-γ-Bisabolene | 14.50 | 28.10 | 11.89 |
| 1541 | 1542 | α-Calacorene | 0.27 | – | 0.62 |
| 1542 | 1544 | α-Cadinene | 0.34 | 0.66 | 0.59 |
| 1544 | 1543 | β-Calacorene | 1.01 | 0.73 | – |
| 1545 | 1545 | (E)-α-Bisabolene | 0.56 | 1.08 | 1.95 |
| 1098 | 1098 | Linalool | 0.31 | 0.36 | 0.26 |
| 1174 | 1182 | 4-Terpineol | 0.97 | – | 0.71 |
| 1187 | 1187 | α-Terpineol | 1.58 | 1.02 | 0.38 |
| 1253 | 1254 | Geraniol | 0.47 | 0.31 | – |
| Alcohols (9) | 9.80 | 11.04 | 17.29 | ||
| 1546 | 1559 | Hedycaryol | 0.41 | – | – |
| 1569 | 1566 | Nerolidol | 2.21 | 4.42 | 2.25 |
| 1572 | 1578 | Spathulenol | 2.44 | 0.52 | 8.53 |
| 1596 | 1595 | Rosifoliol | – | 0.36 | – |
| 1610 | 1605 | Junenol | 2.46 | 0.60 | – |
| 1623 | 1618 | cis-Cubenol | 1.08 | 0.64 | 0.72 |
| 1650 | 1653 | α-Cadinol | – | 1.12 | 1.18 |
| 1686 | 1685 | α-Bisabolol | 0.60 | 1.50 | 3.11 |
| 1695 | 1683 | α-Santalol | 0.60 | 1.88 | 1.50 |
| Aldehydes (8) | 5.28 | 6.71 | 3.00 | ||
| 815 | 820 | 3-Furaldehyde | – | 0.74 | – |
| 953 | 961 | Benzaldehyde | 4.11 | 0.54 | 0.63 |
| 1038 | 1039 | Benzeneacetaldehyde | 0.99 | 0.29 | 0.31 |
| 1204 | 1200 | Decanal | 0.18 | – | – |
| 1211 | 1217 | P-Menth-1-en-9-al | – | 0.30 | – |
| 1269 | 1276 | Phellandral | – | 3.82 | 0.24 |
| 1278 | 1282 | 1,3-p-Menthadien-7-al | – | 0.26 | – |
| 1752 | 1758 | α-Sinensal | – | 0.76 | 1.82 |
| Ketones (5) | 4.61 | 2.52 | 2.71 | ||
| 982 | 987 | 6-Methylhept-5-en-2-one | 0.37 | 0.32 | 0.49 |
| 1179 | 1183 | 4-methylacetophenone | 1.12 | 0.56 | 1.82 |
| 1380 | 1384 | Damascenone | – | 0.73 | – |
| 1395 | 1396 | Jasmone | 2.35 | 0.91 | 0.40 |
| 1687 | 1698 | 4-(1,5-Dimethylhex-4-enyl)cyclohex-2-enone | 0.77 | – | – |
| Esters (9) | 11.26 | 5.31 | 1.31 | ||
| 1068 | 1090 | Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl)propan-2-yl carbonate | – | 0.39 | – |
| 1213 | – | Fumaric acid, di(cyclohex-3-enylmethyl) ester | 0.29 | 0.32 | – |
| 1364 | 1368 | Neryl acetate | 0.23 | 0.38 | 0.33 |
| 1384 | 1386 | Geranyl acetate | 0.58 | 1.23 | 0.72 |
| 1865 | – | Phthalic acid, isobutyl 4-octyl ester | – | 1.55 | – |
| 1959 | – | Phthalic acid, 6-ethyl-3-octyl butyl ester | – | 0.33 | – |
| 1995 | 1994 | Ethyl palmitate | – | 1.11 | 0.26 |
| 2221 | 2198 | Ethyl linolenate | 0.61 | – | – |
| 2385 | – | Vinyl cinnamate | 9.55 | – | – |
| Hydrocarbons and derivatives (8) | 2.99 | 6.22 | 18.84 | ||
| 1020 | 1018 | o-Cymene | 0.19 | 0.33 | 0.37 |
| 1062 | 1068 | trans-4-Thujanol | 0.39 | – | – |
| 1071 | 1075 | p-Cresol | – | – | 0.62 |
| 1284 | 1285 | β-Methylnaphthalene | 0.29 | 0.60 | 0.18 |
| 1440 | 1458 | Naphthalene | 0.24 | – | – |
| 1469 | 1481 | Isocadinene | 0.27 | 0.99 | 1.93 |
| 1476 | – | 2-phenyl-N-(1-phenylpropan-2-yl) acetamide | – | 3.72 | 15.74 |
| 1601 | – | 6-ethylundecane | 1.61 | 0.58 | – |
| Unidentified components | 6.49 | 6.72 | 4.46 | ||
“RI1”, retention index on HP-5MS column.
“RI2”, retention index recorded in the literature.
–, undetected.
As can be seen from Table 1, (E)-γ-bisabolene and β-sesquiphellanderene were the most in the seed, with relative concentrations of 14.50% and 16.98%, respectively; (E)-γ-bisabolene (28.10%) was the most in the pulp; (E)-γ-bisabolene and 2-phenyl-N-(1-phenylpropan-2-yl) acetamide were the most in the peel, with 11.89% and 15.74%, respectively. Remarkably, (E)-γ-bisabolene was abundantly present in all parts of the wampee fruit, emerging as the most prevalent VOC. This compound emits a spicy and soapy odor. Despite the higher odor threshold of this sesquiterpene, it still demonstrated a negative correlation with the fruity aroma and a positive correlation with a harsh odor (Fukuda et al., 2013, Muchlinski et al., 2020). Moreover, β-Sesquiphellanderene in the seed was much more abundant than that in the pulp and peel (2.96% and 0.47%, respectively). Conversely, 2-phenyl-N-(1-phenylpropan-2-yl) acetamide was found at a reasonably high level in the peel, a relatively low level in the pulp (3.72%), and was not detected in the seed.
Furthermore, the concentrations of β-thujene, benzaldehyde, and vinyl trans-cinnamate in the seed were 3.81%, 4.11%, and 9.55%, respectively. However, they were either present at extremely low levels or even not detected in the pulp and peel. α-Zingiberene with a high concentration (5.7%) was found only in the peel and was not detected in the seed or pulp. Phellandral in the pulp was in a high concentration (3.82%), but in a very low concentration or even undetected in the seed and peel. In addition, both the seed and pulp were rich in isocaryophyllene (5.23% and 8.49%, respectively), whereas it was not detected in the peel. γ-Curcumene and spathulenol were relatively abundant in the peel (8.69% and 8.53%, respectively), less in the seed (1.36% and 2.44%, respectively), and almost absent in the pulp. 1-Epibicyclosesquiphellandrene was present in both the pulp and peel at high concentrations (3.28% and 5.86%, respectively), but was not detected in the seed.
To further illustrate the differences in VOCs among the different parts of the wampee fruit, the numbers and relative concentrations of each category of VOCs were presented in Fig. 1C and Fig. 1D. In Fig. 1C, the number of identified VOCs for each class is displayed and distinguished by 6 colors: orange (terpenes), blue (alcohols), green (aldehydes), brown (ketones), black (esters), and pink (hydrocarbons and derivatives). The Y-axis represents the number, with longer color bars indicating a higher number of compounds in the corresponding category. In total, 67 VOCs were detected with 49 identified in the seed (24 terpenes, 7 alcohols, 3 aldehydes, 4 ketones, 5 esters, 6 hydrocarbons and derivatives), 50 in the pulp (19 terpenes, 8 alcohols, 7 aldehydes, 4 ketones, 7 esters, 5 hydrocarbons and derivatives), and 40 in the peel (19 terpenes, 6 alcohols, 4 aldehydes, 3 ketones, 3 esters, and 5 hydrocarbons and derivatives). Thus, it can be observed that the seed and pulp exhibited a richer variety of VOCs, resulting in more complex aromas, compared to the peel.
In Fig. 1D, the relative concentrations of VOCs for each class are displayed and distinguished by 7 colors, including the previously mentioned 6 colors and gray (unidentified compounds). The Y-axis represents the relative concentration, with longer color bars indicating a higher relative content of the corresponding category of compounds. Numerically, terpenes were the most predominant compounds in all parts of the wampee fruit, accounting for 59.57% in the seed, 61.48% in the pulp, and 52.39% in the peel (Fig. 1D). This finding was consistent with the results from the E-nose analysis, reinforcing terpenes being the key contributors to the fruit’s overall aroma. Moreover, the relative concentrations of alcohols, aldehydes, and ketones did not differ dramatically among the different parts of the wampee fruit, but esters in the seed were much more abundant (11.26%) compared to the peel (1.31%), and hydrocarbons and derivatives in the peel were much more abundant (18.84%) than in the seed (2.99%). Meanwhile, the pulp exhibited relatively moderate contents of these two classes of VOCs with 5.31% esters and 6.22% hydrocarbons and derivatives.
The odor threshold refers to the lowest concentration of a specific VOC that a person can perceive. If the concentration of a VOC is constant, the lower its aroma threshold, the greater its aroma contribution. rOAV has been widely used to quantify the extent of aroma contribution from a compound (Su et al., 2022).
In general, a VOC with an rOAV ≥ 1 is considered as a critical VOC, while a VOC with 0.1 ≤ rOAV < 1 is regarded as a modified component (Su et al., 2022). Table 2 lists all the compounds whose thresholds were able to be obtained and whose rOAVs were greater than 0.1. Notably, linalool (rOAV = 100 in the seed, pulp, and peel), endowing with a floral and citrus-like aroma, was the most prominent contributor to the entire wampee fruit. Benzeneacetaldehyde with a honey and sweet aroma (rOAV = 11.15 in the seed, 2.81 in the pulp, and 4.16 in the peel), β-methylnaphthalene with a sweet and floral aroma (rOAV = 6.86 in the seed, 12.22 in the pulp, and 5.08 in the peel) and 4-methylacetophenone with an almond-like aroma (rOAV = 3.78 in the seed, 1.63 in the pulp, and 7.33 in the peel) emerged as essential VOCs in all parts of the wampee fruit. Isocaryophyllene (rOAV = 18.56 in the seed, 25.94 in the pulp) and geraniol (rOAV = 5.05 in the seed, 2.87 in the pulp) were critical VOCs in the seed and pulp, contributing citrus-like and rose-like aromas. β-myrcene (rOAV = 13.01) with a woody and resinous odor, decanal (rOAV = 4.26) with a fresh and plastic-like odor, and naphthalene (rOAV = 2.84) with a pungent and tarry odor were critical VOCs only in the seed. α-Phellandrene (rOAV = 1.65) with a citrus-like and herbal aroma as well as p-cresol (rOAV = 13.45) with a muddy and animal odor were critical VOCs just in the peel. Additionally, benzaldehyde, 6-methylhept-5-en-2-one, geranyl acetate, nerolidol, γ-terpinene, and α-sinensal played modifying roles in shaping the aroma of the different parts of the wampee fruit. Overall, the pulp has a citrus-like and floral aroma, making it particularly popular among consumers. Moreover, despite the presence of a certain muddy odor in the peel due to p-cresol, it still presents a relatively satisfactory aroma, complemented by the influence of other contributing compounds.
Table 2.
The odor descriptions and rOAVs of VOCs identified by HS–SPME–GC–MS.
| VOCs | Odor descriptions | Thresholda (mg/kg) | rOAV |
||
|---|---|---|---|---|---|
| Seed | Pulp | Peel | |||
| Linalool | Floral, citrus-like, fruity | 0.00022 | 100.00 | 100.00 | 100.00 |
| Isocaryophyllene | Citrus-like | 0.02 | 18.56 | 25.94 | 0.00 |
| β-Myrcene | Woody, resinous, musty | 0.0012 | 13.01 | 0.00 | 0.00 |
| Benzeneacetaldehyde | Honey, sweet | 0.0063 | 11.15 | 2.81 | 4.16 |
| β-methylnaphthalene | Sweet, floral | 0.003 | 6.86 | 12.22 | 5.08 |
| Geraniol | Rose-like, citrus-like | 0.0066 | 5.05 | 2.87 | 0.00 |
| Decanal | Fresh, plastic-like | 0.003 | 4.26 | 0.00 | 0.00 |
| 4-methylacetophenone | Almond-like | 0.021 | 3.78 | 1.63 | 7.33 |
| Naphthalene | Pungent, tarry | 0.006 | 2.84 | 0.00 | 0.00 |
| Benzaldehyde | Honey, floral, almond-like | 0.75089 | 0.39 | 0.04 | 0.07 |
| 6-Methylhept-5-en-2-one | Citrus-like | 0.068 | 0.39 | 0.29 | 0.61 |
| α-Phellandrene | Citrus-like, terpenic, herbal, pine-like | 0.04 | 0.35 | 0.61 | 1.65 |
| Geranyl acetate | Floral, rose-like, fruity | 0.15 | 0.27 | 0.50 | 0.41 |
| Nerolidol | Floral | 2.25 | 0.07 | 0.12 | 0.08 |
| γ-Terpinene | Terpenic, oily, woody, lemon-like | 1 | 0.06 | 0.08 | 0.12 |
| α-Sinensal | Citrus-like | 0.22 | 0.00 | 0.21 | 0.70 |
| p-Cresol | Muddy | 0.0039 | 0.00 | 0.00 | 13.45 |
The thresholds were from the literature, measured in water (Gemert, 2011, Zhai and Granvogl, 2019).
Chokeprasert et al. (2007) conducted an analysis of VOCs in various parts of a unique wampee cultivar grown in Nan Province, in northern Thailand, using GC–MS. Their study identified more than 60 VOCs in the fruit. Intriguingly, among the three parts of the fruit, the peel was found to have the highest number of VOCs species (30 species), followed by the seed (25 species), while the pulp contained the fewest (16 species). This means that its peel and seed have a more complex aroma, which is not similar to our results. Meanwhile, although terpenes remained the most abundant VOCs in this wampee fruit, their composition differed dramatically from that of our cultivar. For instance, sabinene, which was the compound with the highest content in their wampee fruit, was not even detected in our biological samples. Despite many differences observed between these two wampee cultivars, it is interesting that Chokeprasert et al. also pointed out that their wampee fruit is commonly consumed directly without peeling when fully ripe. Being the part covering the fruit’s surface and directly exposed to the air, the peel generally emits an easily noticeable and fairly attractive aroma and often attracts consumers to taste.
Regarding, the seed of our wampee fruit, compounds like β-myrcene, decanal, and naphthalene exhibit high rOAVs, contributing to a pungent odor like wood, mold, and plastic. Moreover, the overall aroma of the seed is much more intense than that of the pulp and peel, which could result in the pronounced unpleasant odors of these contributors. Therefore, even though the seed is actually edible and has certain health benefits, its intense and pungent aroma might deter consumers from exploring its taste. This could be one of the reasons why a substantial number of seeds are wasted every year.
3.3. Assessment of VOCs detected by HS-GC-IMS
The HS-GC-IMS analysis was used to further identify VOCs. Previous studies have found that when compared to the VOCs identified by HS-SPME-GC–MS, the majority of the VOCs detected by HS-GC-IMS exhibited lower boiling points and RIs (Feng et al., 2022). HS-GC-IMS demonstrated greater sensitivity towards high-volatile compounds, whereas SPME possessed the robust capacity for capturing high-boiling substances and most of the VOCs detected by HS–SPME–GC–MS had lower volatility (Pu et al., 2019, Pu et al., 2020). Therefore, in this study GC-IMS, as a complementary method, was employed to identify other high-volatile compounds in various parts of the wampee fruit.
In Fig. 1E, a three-dimensional (3D) topographic plot displays the comprehensive data on VOCs for each sample. The ion drift time for identification, the retention time of gas chromatograph, and the peak height for quantification are represented by the X, Y, and Z axes, respectively (Xiao et al., 2022). The continuous red peak on the left side of the 3D topographic plot represents the reaction ion peak (RIP), while each red peak distributed on both sides of the RIP represents a specific VOC. The different positions of the peaks indicate the presence of different compounds, and the peak height represents the content of the respective VOC, with higher peaks indicating higher content.
In Fig. 1F, presenting a top view of Fig. 1E, the X-axis represents the normalized ion drift time, while the Y-axis represents the retention time of gas chromatography. The red vertical line at X-axis 1.0 represents the normalized RIP. Each colored dot on both sides of the RIP represents a specific VOC, with white symbolizing a low concentration and red indicating a high concentration. Thus, the darker the color, the higher the concentration. In Fig. 1G, the two-dimensional figure of the seed was used as a reference to demonstrate the color differences among the three parts of the wampee fruit. If the concentrations of VOCs in the peel and pulp were equivalent to those in the seed, the VOCs showed white dots. The blue or red dots, on the other hand, indicated the concentrations of VOCs were lower or higher than those in the seed. The intensity of the blue color indicates the degree of decrease in concentration (that is, the darker the blue, the lower the concentration), while the red color signifies the extent of increase. Overall, as shown in Fig. 1E–G, the composition and content of VOCs in the three parts of the wampee fruit were obviously different.
In order to provide detailed information regarding the variations in the composition of VOCs among the different parts of the wampee fruit, all signal peaks of each sample were collected to form its GC-IMS fingerprint, as shown in Fig. 1H. Each column in the figure represents one sample, while each row represents the signal peaks of a specific VOC.
Out of a total of 82 signal peaks, 62 were identified as 42 different VOCs, while the others remained unidentified. These VOCs were classified into five segments, marked as a-e, based on their distribution in the different parts of the wampee fruit. VOCs in segment a were found abundantly in all parts of the wampee fruit; in segment b, VOCs were rich in the peel; in segment c, VOCs were widely present in both the pulp and peel; in segment d, VOCs were extensively detected in both the seed and peel; lastly, in segment e, VOCs were predominantly abundant in the seed.
PLS-DA, a supervised discriminant analysis statistical method, was used to establish a correlation model between the intensity of VOCs measured by HS-GC-IMS and the sample categories. The results were shown in Fig. 1I. It is evident that the seed, pulp, and peel can be effectively distinguished based on the PLSDA1 (56.4%) and PLSDA2 (39.1%), accounting for a cumulative variance contribution of 95.5%. The VIP score, a weighted sum of squares of the PLS-DA loadings, was employed to quantify the contribution of each variable to the classification. As shown in Fig. 1J, a total of 22 different VOCs were identified as having significant contributions (VIP greater than 1) to the variations among the different parts of the wampee fruit, including β-pinene (M, D, Tri, Tet), α-pinene (M, D), benzaldehyde (M, D), 1-penten-3-ol, β-ocimene (M, D), butanal, limonene (D, Tri, P), 2-hexen-1-ol, 2,3-dihydro-5-methylfuran-2-one (M, D), ethyl acetate (D), dihydro-5-methyl-2-furanone, furfural (M, D), α-phellandrene (D), 2-phenylethanol, α-terpinene (M, D), hexanal (D), δ-hexalactone, 1-(2-furanyl)-ethanone, 2-methyl butanol, 3-methyl butanal, cis-2-penten-1-ol, and 2-butoxyethanol.
3.4. Taste profile
The E-tongue, which has electronic sensors to mimic human taste perception, was utilized to assess the taste profiles of the different parts from the wampee fruit. These sensors are sensitivities to sourness, umami, saltiness, bitterness, astringency, aftertaste umami, aftertaste bitterness, and aftertaste astringency, respectively. The electric potential (EP) value measured by each sensor reflects the intensity of a specific taste. Notably, EP of the sourness sensor exhibits a positive response to sourness, while EP of the other sensors shows a negative response to their corresponding taste. In other words, for sourness, a higher EP indicates a stronger taste intensity, while for the other tastes, a lower EP implies a stronger taste intensity.
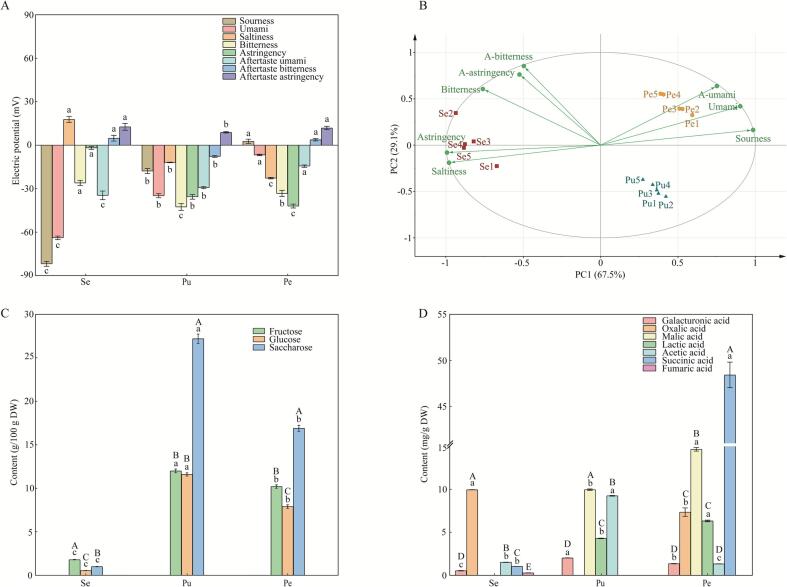
As shown in Fig. 2A, the intensity orders of sourness, saltiness, and astringency were the peel > the pulp > the seed; the orders of umami and aftertaste umami were the seed > the pulp > the peel; the order of bitterness was the pulp > the peel > the seed; aftertaste bitterness and aftertaste astringency were stronger in the pulp but without a significant difference between the seed and peel. In summary, the seed exhibited relatively stronger umami and aftertaste umami, the pulp had relatively stronger bitterness, aftertaste bitterness, and aftertaste astringency, and the peel had stronger sourness, astringency, and saltiness. The main source of sourness is organic acids, and for umami, it may be attributed to certain organic acids and free amino acids. Moreover, the polyphenols and flavonoids that are abundantly present in the pulp and peel may contribute significantly to their bitterness and astringency, as explored in our preliminary work and recent studies (Li et al., 2023, Lin et al., 2022, Soares et al., 2017). Notably, using simulated in vitro digestion process, Chen et al. (2023) conducted an investigation on the characteristics of total phenolic and flavonoid of the wampee fruit from Yongxing, Hainan. Their findings indicated that the total phenolic content and total flavonoid content in the peel were higher than those in the pulp. And interestingly, the phenolics and flavonoids in the peel were mainly released during the oral digestion stage, while those in the pulp were primarily released during the small intestine digestion stage. Such distinct release characteristics could explain the relatively more prominent bitterness and astringency experienced when consuming the peel.
Fig. 2.
Taste profiles and taste substances of different parts of the wampee fruit: (A) the electric potential of each E-tongue sensor. Different letters indicate significant differences among different parts. (B) PCA biplot of E-tongue response values. A-umami, A-bitterness, and A-astringency means aftertaste umami, aftertaste bitterness, and aftertaste astringency, respectively. The contents of (C) the main sugars and (D) the organic acids from different parts of the wampee fruit. Different letters indicate significant differences among different parts, and different capital letters indicate significant differences among different compounds. “Se”, “Pu”, and “Pe” indicate seed, pulp, and peel, respectively.
A deeper understanding of the taste differences among various parts of the wampee fruit was achieved through PCA using the data from the E-tongue measurement. The cumulative variance contribution from PC1 (67.5%) and PC2 (29.1%) reached an impressive 96.6%. As illustrated in Fig. 2B, the seed, pulp, and peel formed distinct clusters, reflecting their different taste profiles. Furthermore, among all the loadings, the astringency and saltiness showed strong positive correlations with the seed; the bitterness, aftertaste astringency, and aftertaste bitterness demonstrated negative correlations with the pulp; lastly, the umami, aftertaste umami, and sourness exhibited positive correlations with the peel.
3.5. Assessment of 29 taste compounds detected by HPLC
Overall, all of the three main sugars were most abundant in the pulp, followed by the peel, and the seed contained the least (Fig. 2C). Specially, in the seed, fructose was the most abundant (1.80 ± 0.03 g/100 g), followed by saccharose (1.00 ± 0.02 g/100 g), and glucose was the least (0.54 ± 0.02 g/100 g). In the pulp, the order of the sugar contents was saccharose (27.20 ± 0.54 g/100 g) > glucose (12.00 ± 0.22 g/100 g) and fructose (11.60 ± 0.19 g/100 g), with no significant difference between the latter two. In the peel, saccharose was the most abundant (16.90 ± 0.35 g/100 g), followed by fructose (10.20 ± 0.20 g/100 g), and glucose was the least (7.90 ± 0.20 g/100 g).
As shown in Fig. 2D, the seed contained 5 types of organic acids, including galacturonic acid, oxalic acid, acetic acid, succinic acid, and fumaric acid; the pulp possessed 4 types (galacturonic acid, malic acid, lactic acid, and acetic acid); and the peel had 6 types (galacturonic acid, oxalic acid, malic acid, lactic acid, acetic acid, and succinic acid). The order of the main organic acid contents in the seed was oxalic acid (9.95 ± 0.01 mg/g) > acetic acid (1.52 ± 0.02 mg/g) > succinic acid (1.04 ± 0.01 mg/g) > galacturonic acid (0.54 ± 0.03 mg/g) > fumaric acid (0.29 ± 0.01 mg/g). In the pulp, malic acid was the most abundant (9.96 ± 0.09 mg/g), followed by acetic acid (9.25 ± 0.04 mg/g), lactic acid (4.29 ± 0.04 mg/g), and galacturonic acid (2.03 ± 0.01 mg/g), respectively. While in the peel, the succinic acid was extremely prominent (48.43 ± 1.39 mg/g) and the malic acid was the second most abundant (14.65 ± 0.21 mg/g). There was no significant difference between oxalic acid (7.35 ± 0.49 mg/g) and lactic acid (6.32 ± 0.09 mg/g), and similarly, no significant difference between galacturonic acid (1.36 ± 0.02 mg/g) and acetic acid (1.34 ± 0.01 mg/g).
The distribution of free amino acids in the wampee fruit is shown in Table 3. Among the 19 measured amino acids, 16 were found in both the seed and pulp, 15 in the peel, and the total content was highest in the pulp, followed by the peel, and the least in the seed. In the seed, asparagine was the most predominant amino acid, followed by norvaline, while methionine, tyrosine, and glutamine were not detected. In the pulp, alanine was the most abundant, followed by asparagine, and methionine, lysine, and citrulline were not found. In the peel, alanine was the most prominent, followed by asparagine, while glutamic acid, phenylalanine, tyrosine, as well as lysine were not detected. Except for the amino acids mentioned above, others were distributed in all parts of the wampee fruit with varying contents.
Table 3.
The contents of amino acids in different parts of the wampee fruit.
| Amino acid (mg/g DW) | Seed | Pulp | Peel |
|---|---|---|---|
| Aspartic acid | 0.22 ± 0.02c | 1.15 ± 0.04 a | 0.90 ± 0.02b |
| Glutamic acid | 0.35 ± 0.02 a | 0.33 ± 0.01 a | – |
| Glycine | 0.02 ± 0.03b | 0.12 ± 0.01 a | 0.07 ± 0.01 ab |
| Threonine | 0.09 ± 0.01b | 0.12 ± 0.01 a | 0.10 ± 0.01 ab |
| Serine | 0.17 ± 0.01c | 0.90 ± 0.02 a | 0.57 ± 0.01b |
| Alanine | 0.48 ± 0.03c | 7.21 ± 0.16 a | 2.91 ± 0.08b |
| Arginine | 0.47 ± 0.03 a | 0.27 ± 0.01 ab | 0.10 ± 0.14b |
| Valine | 0.14 ± 0.01 a | 0.13 ± 0.01 a | 0.09 ± 0.03 a |
| Methionine | – | – | 0.03 ± 0.01 |
| Phenylalanine | 0.21 ± 0.01 a | 0.11 ± 0.01b | – |
| Tyrosine | – | 0.11 ± 0.01 | – |
| Proline | 0.63 ± 0.05b | 2.39 ± 0.22 a | 0.98 ± 0.02b |
| Lysine | 0.08 ± 0.01 | – | – |
| Asparagine | 2.15 ± 0.14b | 3.66 ± 0.08 a | 1.66 ± 0.03c |
| Glutamine | – | 0.15 ± 0.01b | 0.19 ± 0.01 a |
| Citrulline | 0.13 ± 0.01 a | – | 0.12 ± 0.02 a |
| Norvaline | 0.95 ± 0.06 a | 1.07 ± 0.02 a | 0.61 ± 0.02b |
| Hydroxyproline | 0.25 ± 0.02b | 0.47 ± 0.04 a | 0.31 ± 0.01b |
| Sarcosine | 0.34 ± 0.18 a | 0.07 ± 0.04 a | 0.07 ± 0.01 a |
| Total | 6.68 | 18.26 | 8.71 |
Data were expressed as mean ± SD (n = 3), values in the same row with different letters are significantly different (p < 0.05).
–, undetected.
The taste descriptions, thresholds, and TAVs of the 3 sugars, 7 organic acids, and 19 amino acids are displayed in Table 4. Among the 3 flavored sugars, fructose had the highest TAVs in all parts of the wampee fruit, making it the most significant contributor to the sweetness of the whole fruit. Saccharose was also an important contributor to the sweetness of all parts of the wampee fruit, as its TAV exceeded 1 in the seed, pulp, and peel. Additionally, the TAVs of glucose in the pulp and peel were both above 1, while it was<1 in the seed, indicating that glucose was an important contributor to the sweetness only in the pulp and peel.
Table 4.
The taste descriptions and TAVs of amino acids in different parts of the wampee fruit.
| Taste components | Taste descriptions | Thresholda (mg/100 g) |
TAVs |
||
|---|---|---|---|---|---|
| Seed | Pulp | Peel | |||
| Sugars | |||||
| Glucose | Sweet | 864.8 | 0.62 | 13.41 | 9.14 |
| Saccharose | Sweet | 478 | 2.09 | 56.90 | 35.36 |
| Fructose | Sweet | 90.1 | 19.98 | 133.19 | 113.21 |
| Organic acids | |||||
| Fumaric acid | Sour/astringent | 40 | 0.73 | – | – |
| Galacturonic acid | Sour | 12.5 | 4.32 | 16.24 | 10.88 |
| Succinic acid | Sour/light umami | 10 | 10.40 | – | 484.30 |
| Acetic acid | Sour/gentle astringent | 12 | 12.67 | 77.08 | 11.17 |
| Oxalic acid | Sour/gentle astringent | 50.4 | 19.74 | – | 14.58 |
| Malic acid | Sour/gentle astringent | 49.6 | – | 20.08 | 29.54 |
| Lactic acid | Sour/gentle astringent | 130 | – | 3.30 | 4.86 |
| Amino acids | |||||
| Phenylalanine | Bitter | 90 | 0.24 | 0.12 | – |
| Citrulline | Bitter | 14 | 0.96 | – | 0.84 |
| Tyrosine | Bitter | 72.5 | – | 0.15 | – |
| Arginine | Bitter/sweet | 50 | 0.93 | 0.53 | 0.20 |
| Methionine | Bitter/sweet/sulfurous | 30 | – | – | 0.11 |
| Glycine | Sweet | 130 | 0.02 | 0.09 | 0.06 |
| Threonine | Sweet | 260 | 0.03 | 0.05 | 0.04 |
| Serine | Sweet | 150 | 0.11 | 0.60 | 0.38 |
| Alanine | Sweet | 60 | 0.81 | 12.02 | 4.86 |
| Lysine | Sweet/bitter | 50 | 0.17 | – | – |
| Proline | Sweet/bitter | 300 | 0.21 | 0.80 | 0.33 |
| Valine | Sweet/bitter | 40 | 0.35 | 0.32 | 0.23 |
| Aspartic acid | Umami | 100 | 0.22 | 1.15 | 0.90 |
| Glutamic acid | Umami | 30 | 1.17 | 1.08 | – |
| Glutamine | Umami | ND | ND | ND | ND |
| Asparagine | Flat/umami | ND | ND | ND | ND |
| Norvaline | ND | ND | ND | ND | ND |
| Hydroxyproline | ND | ND | ND | ND | ND |
| Sarcosine | ND | ND | ND | ND | ND |
–, undetected.
“ND”, the descriptions or thresholds have not been found.
The thresholds were from the literature, measured in water (Chen and Zhang, 2007, Chen et al., 2021a, Ervina et al., 2020, Guo et al., 2019, Hufnagel and Hofmann, 2008, Kubícková and Grosch, 1998, Rotzoll et al., 2006, Zhang et al., 2022).
Except for fumaric acid, all organic acids with TAVs greater than 1 played important roles in the sourness of the wampee fruit. Galacturonic acid contributed to the sourness in the seed, pulp, and peel. Succinic acid (TAV = 10.40/484.30) contributed to both sourness and light umami in the seed and peel, respectively. Acetic acid, oxalic acid, malic acid, and lactic acid contributed not only to the sourness but also to the gentle astringency in different parts of the wampee fruit.
Most of the free amino acids had TAVs < 1, except for alanine, aspartic acid, and glutamic acid, indicating that they generally contributed less to the taste of the wampee fruit compared to the sugars and organic acids. Among the sweet amino acids, alanine (TAV = 12.02/4.86) was an important contributor to the sweetness in the pulp and peel, respectively. Additionally, among the umami amino acids, aspartic acid in the pulp and glutamic acid in the seed and pulp were important sources of umami for the certain parts of the fruit.
Yin et al. (2022) performed widely targeted metabolome analysis on two different wampees originated from Guangdong Province and Hainan Province in China. Their study identified several of important taste contributors, including d-galactose, d-mannose, d-fructose 6-photosphate, l-citramalic acid, 2-hydroxyglutaric acid, 3-methylmalic acid, and so on. This is not consistent with our results, and Yin et al. pointed out that “ascorbate and aldarate metabolism” and “C5-branched dibasic acid metabolism” are the main underlying causes of differences in tastes between the different wampee cultivars.
The TAVs of all important contributors to sweetness were summed to yield a total TAVsweet, and similarly, the total TAVsour and the total TAVumami were calculated. The total TAVsweet for the seed, pulp, or peel was 22.07, 215.52, or 162.55, respectively. The total TAVsour was 47.13 for the seed, 116.7 for the pulp, and 555.33 for the peel, while the total TAVumami was 1.17 for the seed, 2.23 for the pulp, and 0 for the peel. Clearly, the pulp possessed the largest total TAVsweet, and the peel had the largest total TAVsour. This means that under the influence of numerous important contributors, the pulp exhibited the strongest sweetness, and the peel had the strongest sourness, while the seed tasted relatively flat, which aligned with the findings from the E-tongue analysis. However, it was observed that the total TAVumami of the pulp was higher than that of the seed, which was not consistent with the results from the E-tongue analysis. This discrepancy might be due to some taste components that were not analyzed in this study.
Overall, both the peel and pulp of the wampee fruit exhibit prominent flavors. The pulp, with its citrus-like aroma and sweet taste, is highly appealing to consumers. The peel, with its sour taste and moderate sweetness, as well as a noticeable and attractive aroma, making it equally enticing to consumers. On the other hand, the seed’s flat taste and certain pungent odor may not immediately captivate consumers, which could explain why they are often discarded. Nevertheless, the seed still possesses some flavor characteristics that may be appreciated and accepted by certain individuals, such as its light umami and intense and complex aroma.
4. Conclusion
The aroma and taste characteristics of the different parts of the wampee fruit were notably distinct. On the one hand, regarding the aroma, the seed exhibited the highest total response value in the E-nose analysis and contained a rich variety of VOCs, resulting in an intense and complex aroma. Therefore, the seed can potentially be utilized as a valuable raw material for preparing essential oils. On the other hand, in terms of the taste, the overall taste of the seed was much flatter than that of the other two parts, the peel possessed the strongest sour and moderately sweet taste, and the pulp demonstrated the strongest sweetness with moderate sourness. The reason why many consumers tend to eat the pulp and peel rather than the seed could be explained by these results. Moreover, it has been confirmed in recent studies that there were abundant polyphenols and flavonoids with some bioactivities in the peel. Thus, the peel can be processed to improve its taste and also be fully utilized for its nutritional value; for example, it can be considered as a raw material for candied fruit and preserves. Furthermore, the crucial volatile components and the key contributors to the taste of the three parts of the wampee fruit were fully explored through rich instrumental chemistry methods and multivariate statistical methods in this work, which can provide theoretical foundation for the comprehensive utilization and development of the different parts from wampee fruit.
Funding
This work was supported by Hainan Provincial Natural Science Foundation of China (321RC1030 and 321RC1075) and National Natural Science Foundation of China (32060513).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Zhiheng Zhao, Email: 21210832000034@hainanu.edu.cn.
Yaofei Hao, Email: 21220860000045@hainanu.edu.cn.
Yijun Liu, Email: liuyijunmm@163.com.
Yousheng Shi, Email: shiys0418@163.com.
Xue Lin, Email: xuelin@hainanu.edu.cn.
Lu Wang, Email: lwang@hainanu.edu.cn.
Pan Wen, Email: peizhis@hntou.edu.cn.
Xiaoping Hu, Email: huxiaoping03@hainanu.edu.cn.
Jianxun Li, Email: lijianxun@caas.cn.
Data availability
Data will be made available on request.
References
- Ao H.T., Jia X.C., Dong L.H., Zhang R.F., Liu L., Huang F.…Zhang M.W. A new benzofuran glycoside from the fruit of Clausena lansium. Natural Product Research. 2022;36(2):501–507. doi: 10.1080/14786419.2020.1788557. [DOI] [PubMed] [Google Scholar]
- Cai W.C., Tang F.X., Guo Z., Guo X., Zhang Q., Zhao X.X.…Shan C.H. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME-GC-MS. Food Chemistry. 2020;330 doi: 10.1016/j.foodchem.2020.127330. [DOI] [PubMed] [Google Scholar]
- Chen D.W., Zhang M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis) Food Chemistry. 2007;104(3):1200–1205. [Google Scholar]
- Chen H., Shi Y.S., Wang L., Hu X.P., Lin X. Phenolic profile and α-glucosidase inhibitory potential of wampee (Clausena lansium (Lour.) Skeels) peel and pulp: In vitro digestion/in silico evaluations. Food Research International. 2023;173 doi: 10.1016/j.foodres.2023.113274. [DOI] [PubMed] [Google Scholar]
- Chen L.H., Zeng W.H., Rong Y.Z., Lou B. Characterisation of taste-active compositions, umami attributes and aroma compounds in Chinese shrimp. International Journal of Food Science and Technology. 2021;56(12):6311–6321. [Google Scholar]
- Chen Z.Q., Gao H.Y., Wu W.J., Chen H.J., Fang X.J., Han Y.C., Mu H.L. Effects of fermentation with different microbial species on the umami taste of Shiitake mushroom (Lentinus edodes) LWT. 2021;141 [Google Scholar]
- Chokeprasert P., Charles A.L., Sue K.H., Huang T.C. Volatile components of the leaves, fruits and seeds of wampee [Clausena lansium (Lour.) Skeels] Journal of Food Composition and Analysis. 2007;20(1):52–56. [Google Scholar]
- Ervina E., Berget I., Almli V.L. Investigating the relationships between basic tastes sensitivities, fattiness sensitivity, and food liking in 11-year-old children. Foods. 2020;9(9):1315. doi: 10.3390/foods9091315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.J., Chen H.Q., Mei W.L., Kong F.D., Li F.X., Chen P.W.…Dai H.F. Nematicidal amide alkaloids from the seeds of Clausena lansium. Fitoterapia. 2018;128:20–25. doi: 10.1016/j.fitote.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Fan Y.N., Sahu S.K., Yang T., Mu W.X., Wei J.P., Cheng L.…Liu H. The Clausena lansium (Wampee) genome reveal new insights into the carbazole alkaloids biosynthesis pathway. Genomics. 2021;113(5):3696–3704. doi: 10.1016/j.ygeno.2021.09.007. [DOI] [PubMed] [Google Scholar]
- Feng X.Y., Wang H.W., Wang Z.R., Huang P.M., Kan J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chemistry. 2022;375 doi: 10.1016/j.foodchem.2021.131671. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Okazaki K., Shinano T. Aroma characteristic and volatile profiling of carrot varieties and quantitative role of terpenoid compounds for carrot sensory attributes. Journal of Food Science. 2013;78(11):S1800–S1806. doi: 10.1111/1750-3841.12292. [DOI] [PubMed] [Google Scholar]
- Gemert L.J.V. 2nd ed. Oliemans Punter & Partners BV; The Netherlands: 2011. Odour Thresholds-Compilations of Odour Threshold Values in Air, Water and Other Media. [Google Scholar]
- Guo Q., Kong X.F., Hu C.J., Zhou B., Wang C.T., Shen Q.W. Fatty acid content, flavor compounds, and sensory quality of pork loin as affected by dietary supplementation with L-arginine and glutamic acid. Journal of Food Science. 2019;84(12):3445–3453. doi: 10.1111/1750-3841.14959. [DOI] [PubMed] [Google Scholar]
- Hou Z.S., Wei Y.Y., Sun L.B., Xia R.R., Xu H.R., Li Y.T.…Xin G. Effects of drying temperature on umami taste and aroma profiles of mushrooms (Suillus granulatus) Journal of Food Science. 2022;87(5):1983–1998. doi: 10.1111/1750-3841.16127. [DOI] [PubMed] [Google Scholar]
- Hufnagel J.C., Hofmann T. Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. Journal of Agricultural and Food Chemistry. 2008;56(19):9190–9199. doi: 10.1021/jf801742w. [DOI] [PubMed] [Google Scholar]
- Kubícková J., Grosch W. Evaluation of flavour compounds of Camembert cheese. International Dairy Journal. 1998;8(1):11–16. [Google Scholar]
- Li L.J., Yan X., Chen F.Y., Zheng L.Y., Hu Y., He F.…Li Q.B. A comprehensive review of the metabolism of citrus flavonoids and their binding to bitter taste receptors. Comprehensive Reviews in Food Science and Food Safety. 2023;22(3):1763–1793. doi: 10.1111/1541-4337.13129. [DOI] [PubMed] [Google Scholar]
- Lin X., Shi Y.S., Wen P., Hu X.P., Wang L. Free, conjugated, and bound phenolics in peel and pulp from four wampee varieties: Relationship between phenolic composition and bio-activities by multivariate analysis. Antioxidants. 2022;11(9):1831. doi: 10.3390/antiox11091831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li C.J., Yang J.Z., Ning N., Si Y.K., Li L.…Zhang D.M. Carbazole alkaloids from the stems of Clausena lansium. Journal of Natural Products. 2012;75(4):677–682. doi: 10.1021/np200919a. [DOI] [PubMed] [Google Scholar]
- Liu Y.P., Guo J.M., Wang X.P., Liu Y.Y., Zhang W., Wang T.…Fu Y.H. Geranylated carbazole alkaloids with potential neuroprotective activities from the stems and leaves of Clausena lansium. Bioorganic Chemistry. 2019;92 doi: 10.1016/j.bioorg.2019.103278. [DOI] [PubMed] [Google Scholar]
- Muchlinski A., Ibdah M., Ellison S., Yahyaa M., Nawade B., Laliberte S.…Tholl D. Diversity and function of terpene synthases in the production of carrot aroma and flavor compounds. Scientific Reports. 2020;10(1):9989. doi: 10.1038/s41598-020-66866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Bu Z.B., Ren H.Y., He Q., Yu Y.S., Xu Y.J.…Li L. Physicochemical, structural, and functional properties of wampee (Clausena lansium (Lour.) Skeels) fruit peel pectin extracted with different organic acids. Food Chemistry. 2022;386 doi: 10.1016/j.foodchem.2022.132834. [DOI] [PubMed] [Google Scholar]
- Prasad K.N., Xie H.H., Hao J., Yang B., Qiu S.X., Wei X.Y.…Jiang Y.M. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chemistry. 2010;118(1):62–66. [Google Scholar]
- Pu D.D., Duan W., Huang Y., Zhang Y.Y., Sun B.G., Ren F.Z.…Tang Y.Z. Characterization of the key odorants contributing to retronasal olfaction during bread consumption. Food Chemistry. 2020;318 doi: 10.1016/j.foodchem.2020.126520. [DOI] [PubMed] [Google Scholar]
- Pu D.D., Zhang H.Y., Zhang Y.Y., Sun B.G., Ren F.Z., Chen H.T., He J.N. Characterization of the aroma release and perception of white bread during oral processing by gas chromatography-ion mobility spectrometry and temporal dominance of sensations analysis. Food Research International. 2019;123:612–622. doi: 10.1016/j.foodres.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Rotzoll N., Dunkel A., Hofmann T. Quantitative studies, taste reconstitution, and omission experiments on the key taste compounds in morel mushrooms (Morchella deliciosa Fr.) Journal of Agricultural and Food Chemistry. 2006;54(7):2705–2711. doi: 10.1021/jf053131y. [DOI] [PubMed] [Google Scholar]
- Sciarrone D., Pantò S., Rotondo A., Tedone L., Tranchida P.Q., Dugo P., Mondello L. Rapid collection and identification of a novel component from Clausena lansium Skeels leaves by means of three-dimensional preparative gas chromatography and nuclear magnetic resonance/infrared/mass spectrometric analysis. Analytica Chimica Acta. 2013;785:119–125. doi: 10.1016/j.aca.2013.04.069. [DOI] [PubMed] [Google Scholar]
- Shen D.Y., Li M.K., Song H.L., Zou T.T., Zhang L., Xiong J. Characterization of aroma in response surface optimized no-salt bovine bone protein extract by switchable GC/GC×GC-olfactometry-mass spectrometry, electronic nose, and sensory evaluation. LWT. 2021;147 [Google Scholar]
- Soares S., Brandão E., Mateus N., Freitas V.D. Sensorial properties of red wine polyphenols: Astringency and bitterness. Critical Reviews in Food Science and Nutrition. 2017;57(5):937–948. doi: 10.1080/10408398.2014.946468. [DOI] [PubMed] [Google Scholar]
- Su D., He J.J., Zhou Y.Z., Li Y.L., Zhou H.J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131587. [DOI] [PubMed] [Google Scholar]
- Wei J.P., Wang S.Y., Zhang Y.X., Yuan Y.H., Yue T.L. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT. 2019;107:191–198. [Google Scholar]
- Wu H., Min T., Li X.F., Li L., Lai F.R., Tang Y.Q., Yang X.H. Physicochemical properties and antioxidant activities of acidic polysaccharides from wampee seeds. International Journal of Biological Macromolecules. 2013;59:90–95. doi: 10.1016/j.ijbiomac.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Xiao N.Y., Xu H.Y., Jiang X., Sun T.T., Luo Y.X., Shi W.Z. Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Research International. 2022;158 doi: 10.1016/j.foodres.2022.111584. [DOI] [PubMed] [Google Scholar]
- Xu X.Y., Xie H.H., Wei X.Y. Jasmonoid glucosides, sesquiterpenes and coumarins from the fruit of Clausena lansium. LWT. 2014;59(1):65–69. [Google Scholar]
- Yan H., Xiong Z., Xie N., Liu S.Z., Zhang L.L., Xu F.…Feng J.T. Bioassay-guided isolation of antifungal amides against Sclerotinia sclerotiorum from the seeds of Clausena lansium. Industrial Crops and Products. 2018;121:352–359. [Google Scholar]
- Yin Q.C., Ji J.B., Zhang R.H., Duan Z.W., Xie H., Chen Z.…Deng H. Identification and verification of key taste components in wampee using widely targeted metabolomics. Food Chemistry: X. 2022;13 doi: 10.1016/j.fochx.2022.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X.T., Granvogl M. Characterization of the key aroma compounds in two differently dried toona sinensis (A. Juss.) roem. by means of the molecular sensory science concept. Journal of Agricultural and Food Chemistry. 2019;67(35):9885–9894. doi: 10.1021/acs.jafc.8b06656. [DOI] [PubMed] [Google Scholar]
- Zhang L., Hu Y.Y., Wang Y., Kong B.H., Chen Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT. 2021;140 [Google Scholar]
- Zhang X., Du X., Li Y.Z., Nie C.N., Wang C.M., Bian J.L., Luo F. Are organic acids really related to the sour taste difference between Chinese black tea and green tea? Food Science & Nutrition. 2022;10(6):2071–2081. doi: 10.1002/fsn3.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.