Abstract
The effect of neuroprotective placental protein composition (NPPC) on the suppression of tinnitus and the restoration of the auditory brainstem response (ABR) characteristics was explored in tinnitus-induced rats. The animals were placed into two groups: (1) the study group, rats received sodium salicylate (SS) at the dose of 200 mg/kg twice a day for two weeks, and then 0.4 mg of the NPPC per day, between the 14th and 28th days, (2) the placebo group, rats received saline for two weeks, and then the NPPC alone between the 14th and 28th days. The gap pre-pulse inhibition of the acoustic startle (GPIAS), the pre-pulse inhibition (PPI), and the ABR assessments were performed on animals in both groups three times (baseline, day 14, and 28). The GPIAS value declined after 14 consecutive days of the SS injection, while NPPC treatment augmented the GPIAS score in the study group on the 28th day. The PPI outcomes revealed no significant changes, indicating hearing preservation after the SS and NPPC administrations. Moreover, some changes in ABR characteristics were observed following SS injection, including (1) higher ABR thresholds, (2) lowered waves I and II amplitudes at the frequencies of 6, 12, and 24 kHz and wave III at the 12 kHz, (3) elevated amplitude ratios, and (4) prolongation in brainstem transmission time (BTT). All the mentioned variables returned to their normal values after applying the NPPC. The NPPC use could exert positive therapeutic effects on the tinnitus-induced rats and improve their ABR parameters.
Keywords: Tinnitus, Evoked potentials, Auditory, Auditory brain stem response, Placental extract
Graphical abstract
The graphical summary of this study.
Highlights
-
•
The behavioral test scores indicate tinnitus reduction following NPPC-treated rats.
-
•
Significant improvement in the ABR thresholds and an enhancement of the amplitude of waves I and II occurred after NPPC administration.
-
•
Prolongation of the BTT was observed after SS injection, which significantly decreased following NPPC-treated rats.
-
•
Improving the ABR's characteristics in NPPC-treated rats indicates the impact of neuromodulation in the recovery of auditory deafferentation.
-
•
The application of NPPC could improve tinnitus and exerts positive therapeutic effects on ABR characteristic.
Abbreviation
Full Name
- NPPC
Neuroprotective Placental Protein Composition
- ABR
Auditory Brainstem Response
- SS
Sodium Salicylate
- GPIAS
Gap Pre-Pulse Inhibition Of The Acoustic Startle
- PPI
Pre-Pulse Inhibition
- BTT
Brainstem Transmission Time
- AEPs
Auditory Evoked Potentials
- PVCN
Posterior Ventral Cochlear Nucleus
- AVCN
Anterior Ventral Cochlear Nucleus
- LL
Lateral Lemniscus
- IC
Inferior Colliculus
- AN
Acoustic Nerve
- HPE
Human Placental Extract
- XP
X-Proteins
- CAP
Compound Action Potential
- NIH
National Institutes Of Health
- IP
Intraperitoneal
- SR
Startle Reflex
- SPL
Sound Pressure Level
- BBN
Broadband Noise
- kΩ
Kiloohm
- SPSS
Statistical Package for Social Sciences
- SD
Standard Deviation
- BTT
Brainstem Transmission Time
- NMDA
N-Methyl-d-Aspartate
- GABA
Gamma-Aminobutyric Acid
1. Introduction
Tinnitus represents one of the common disorders of the auditory system, which can be defined as auditory perceptions without external audit sources, but expressed as phantom ones [1,2]. Tinnitus significantly burdens the healthcare system by affecting 5.1% up to 42.7% of the adult population. The exact pathophysiology of the subjective idiopathic tinnitus generation has remained unclear, and no particular effective treatment has been so far specified [[3], [4], [5], [6]]. Previous research has further demonstrated the importance of the central auditory pathways, spanning the cortical layers and the subcortical structures, such as the hypothalamus, the hippocampus, and the amygdala in the brain [[7], [8], [9], [10]].
One of the commonly approved behavioral methods to identify tinnitus as well as estimate hearing impairment in experimental studies would be the use of the gap-prepulse inhibition model of acoustic startle reflex (GPIAS) and pre-pulse inhibition (PPI) of the acoustic startle reflex (ASR) methods [11]. The GPIAS paradigm can be performed for detecting tinnitus caused by drug-induced ototoxicities such as sodium salicylate or loud noise exposure. GPIAS consists of two phases, with and without a silent gap presented before the start pulse under the background noise environment. In normal conditions, a silent gap inhibits the startle reflex. In animals with tinnitus, disinhibition of silent gaps indicates the presence of tinnitus due to the filling of the gaps with the tinnitus signal. In parallel to GPIAS, Pre-pulse Inhibition (PPI) is performed to evaluate if the rats can detect and hear the background noise in the GPIAS test. The PPI consists of two random phases, single or by pre-pulses before the startle stimulus. When the startle stimulus is presented in a silent environment, the Startle's response increases. The startle response is reduced when a pre-pulse is given before the startle stimulus. Tinnitus is considered when the GPIAS result is unsuccessful, but the PPI test is positive [12]. The auditory brainstem response (ABR) test, also known as the auditory evoked potentials (AEPs), has been accordingly documented for measuring hearing threshold as an evoked potential recording method, generally practiced to reflect on the auditory functions under either pathological or healthy conditions. In this respect, the high temporal resolution (<1 ms) of the AEPs provides a suitable method to take track of the electrical brain activities (viz., the waves), time-locked to the auditory events. The majority of previous studies on rodent ABRs have reported that the ABR wave I originates from the proximal portion of the cranial nerve VIII, wave II is generated by the posterior ventral cochlear nucleus (PVCN), wave III arises from the anterior ventral cochlear nucleus (AVCN) and the trapezoid body, wave IV comes from the superior olivary complex (SOC), and wave V is attributed to the lateral lemniscus (LL) and the inferior colliculus (IC) [13,14].
On the other hand, the ABR amplitudes for waves II, III, and V may produce data concerning the ABR generators or modulators across the auditory brainstem areas [15]. In contrast, the ABR latencies for waves I–V provide evidence of auditory brainstem function [16]. Research animal studies have further presented the ABR test as an objective technique to show the auditory system alterations, which can represent the existence of tinnitus, causing lower sensory transmission and offsetting the changes in the central structures [17,18].
Nonetheless, tinnitus induction is typically associated with pathological activities in one or more cranial nerves, especially the auditory ones. In this line [19], found behavioral evidence suggesting that the damage to rats' acoustic nerve (AN) fibers could be related to tinnitus perceptions. Other studies have correspondingly shown that tinnitus is correlated with cochlear synaptopathy, the non-appearance of any evident damage to the cochlear function in subjects without peripheral hearing loss, and the AN feedback depletion due to different high-threshold AN fibers. Furthermore, neural plasticity in response to masking hearing loss can lead to patterns of pathological activity in the auditory brainstem that potentially cause tinnitus [20,21].
Salicylate is one of the metabolites of Aspirin (Acetylsalicylic acid) and has been shown to cause auditory abnormalities in humans [22] and animals [23]. It is stated that a high dose of Salicylate creates tinnitus and hearing loss [24]. According to reported studies, chronic tinnitus induced by Salicylate could be associated with extensive changes in electrophysiological, histological, and molecular levels leading to other impairments such as degeneration, necrosis, apoptosis, etc., in neurons and brain tissue. Given the above information, if any agent can help decrease these impairments, it would also improve tinnitus [25,26].
Given the existing literature on the pathophysiology of tinnitus and the ineffectiveness of current treatments for this condition, it is of utmost importance to provide evidence-based, rational therapeutic interventions.
The placenta is an organ that temporarily connects the developing fetus to the mother and is rich in various bioactive substances, including proteins, minerals, amino acids, nucleic acids, growth factors, cytokines, enzymes, vitamins, and trace elements [27,28]. The animal and human placental extract (HPE) preparations have been accordingly exploited in different parts of the world as traditional, complementary, and recently, regenerative medicines for prophylactic and therapeutic purposes, such as wound healing, aging protection, organ protection, radioprotection, neuroprotection, neuro-rehabilitation, etc. [29,30]. Although studies on the specific functions of HPE components have been scarce, findings suggest that various HPE proteins and peptides are involved in the processes of the oxidative stress response, resolution of inflammation, and tissue regeneration, which can be further used in modern medicine [[31], [32], [33]].
Some studies have thus reported the beneficial neuroprotective effects of the HPE and the HPE protein components in the animal models of chronic stress [34], hypoxia/ischemia [27], and Alzheimer's disease [35], leading to cognitive and memory function improvements.
Similarly, the protein fraction of the porcine placental extract was introduced by Alexander Anikin to have prominent neuroprotective properties [36]. They further reported the evident positive outcomes of the preparation, initially named X-proteins (XP), on several brain stroke and trauma patients with lasting severe neurological defects, for whom the daily intranasal XP administration during a 12-to-14-day treatment period had reduced the patients' motor, mental, and speech deficits, and improved their emotional states. They concluded that XP might induce the regenerative/reparative processes in the reversibly damaged nervous tissues [36].
According to the possible neuroprotective effects of the HPE mentioned above, and due to the various mechanisms involved in tinnitus, there is still no proven treatment for it [37]. Therefore, this multi-component composite may be effective in treating tinnitus. To the best of our knowledge, the effect of HPE on tinnitus treatment has not been reported before.
Based on the scarcity of the recommended effective medications for the management of tinnitus and the biological activity of the HPE-derived XP, termed the neuroprotective placental protein composition (NPPC), was described earlier, this study aimed to characterize the Sodium Salicylate (SS)-induced tinnitus alterations of the auditory behaviors and the ABR parameters along with the effects of the NPPC administration on tinnitus suppression in a rodent model of SS-induced ototoxicity.
2. Materials and methods
2.1. NPPC preparation
The human placenta for the NPPC preparation was procured from SinaCell Company, officially certified by the Food and Drug Agency of Iran, for ethically distributing the donated human placenta and placental derivatives for medical and research use. The non-hydrolyzed protein extraction and the NPPC preparation from the human placenta were also performed according to the methodology described by Ref. [36] with minor modifications, including the replacement of the DE-52 cellulose resin (CA. b45059) with the DEAE-Sepharose Fast Flow resin (CA.17070901, Cytiva, the United States) without further size-based fractionation of the obtained protein composition. The final total protein concentration (measured by the QuBit 3.0 fluorometer, CA. Q33216, Life Technologies, the United States) and pH were thus set at ∼1 mg/ml and ∼7.0, respectively.
2.2. Animal handling and experimental design
In total, 16 male Wistar rats weighing 200–350 g were recruited in this study. The animals were thus housed based on two to three rats per cage with 12-h light/dark cycles and standard food and water ad libitum freely. Room temperature and humidity were controlled at the favorable ranges of 21–22 °C and 50–55%, respectively. All the procedures were also performed under the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978) and then approved by the Vice-Chancellor's Ethical Committee for Medical Research, Iran University of Medical Sciences, Tehran, Iran (Ethical approval no. IR.IUMS.REC.1398.233).
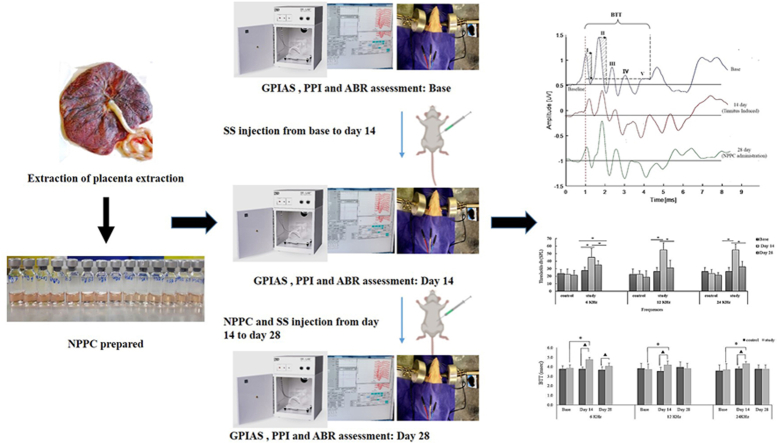
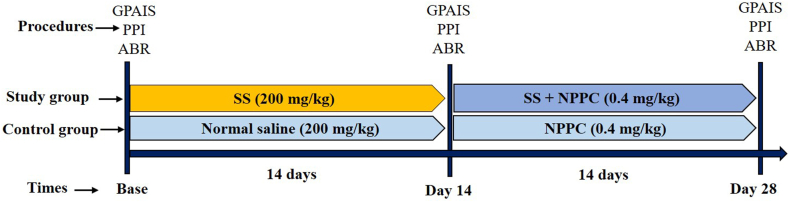
The given animals were randomly placed into two groups: the chronic study group and the control group. Fig. 1 shows the course and the timeline of the studies. The chronic study group first underwent the behavioral and ABR tests (Sections 2, 2.4.5) for the baseline evaluations before receiving SS. The tests were then repeated on day 14. From the 14th to 28th days, all rats received the NPPC treatment daily, and SS was given simultaneously for the stability of tinnitus in the animals. On the 28th day, the behavioral and ABR tests were further repeated to evaluate the therapeutic effects of the NPPC (n = 8 rats). The control group also received normal saline for the first two weeks and the NPPC for the second two weeks (n = 8 rats).
Fig. 1.
Experimental design and timelines for the course of the experiment on two animal groups SS stands for sodium salicylate.
2.3. Animal treatment regimens
SS (CAS 54-21-7, Merck, Darmstadt, Germany) was dissolved in saline with a 200 mg/ml concentration. On the first 14 days, the chronic study group received SS (200 mg/kg), twice a day, at 8:00 a.m. and 4:00 p.m., via an intraperitoneal (IP) injection. From the 14th to 28th days, they also received 0.4 mg/kg of the NPPC once a day in addition to SS. The control group then received normal saline (IP, 200 mg/kg), twice a day, for 14 consecutive days, and then received the IP injections of NPPC (instead of the normal saline) once a day from the 14th to 28th days. Both the chronic study and control groups also underwent behavioral tests and ABR.
2.4. Behavioral tests for tinnitus
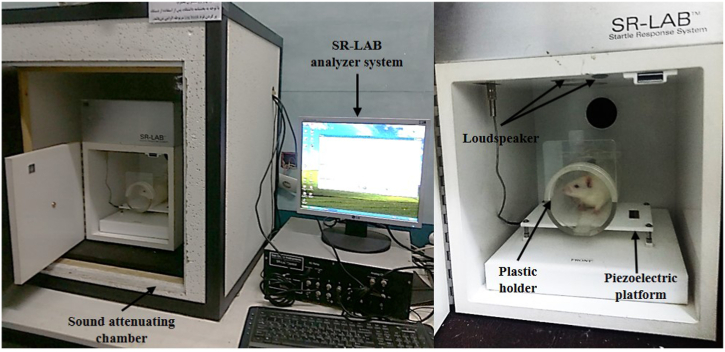
The GPIAS and the PPI behavioral tests evaluate tinnitus in animals [38]. The present study used an acoustic Startle Reflex system (SR-LAB system - San Diego Instruments, San Diego, Canada) to assess tinnitus. The PPI test and the GPIAS were further considered to determine hearing impairment. The GPIAS and PPI tests were accordingly completed at three-time stages, viz.
(1) one day before the SS injection (at the onset of the study), (2) 14 consecutive days after the SS injection (on the 14th day), and (3) 14 consecutive days after the NPPC and SS injections (on the 28th day). The rats were placed in a sound-attenuating chamber on a piezoelectric platform and kept inside a plastic holder. The downward force created by the movement of the rats on the piezoelectric plate and the analogue-to-digital conversion was also started using the SR-LAB software (San Diego Instruments, San Diego, Canada) to calculate the GPIAS and PPI parameters (Fig. 2).
Fig. 2.
Acoustic Startle Response (ASR) setup: A behavioral test to assess GPIAS and PPI in rats. The rats were placed in a sound-attenuating chamber on a piezoelectric platform, which was kept inside a plastic holder. The sounds were delivered through two loudspeakers placed on the ceiling of the room, one for background noise and the other for the presentation of the startle stimuli. The arrows show different parts of the setup.
The peak-to-peak startle amplitude response values were then calculated offline. Two speakers located on the chamber's ceiling ultimately generated the acoustic startle stimuli and the white noise background.
The GPIAS test was accordingly administered using the 60 dB sound pressure level (SPL) background broadband noise (BBN), consisting of 16 trials with a gap and 16 trials without a gap. A 20-ms main pulse burst of the white noise was then performed at the intensity level of 115 dB to get a startle response. The trials with a gap started at 100 ms before the onset of the startle bursts and lasted for 50 ms with a 1-ms rise and fall time. The interval between each startling noise was considered 20 s, and each test took approximately 25 min.
The PPI of the acoustic startle reflex method comprised 15 pulse trials, eight no-stimulus trials, and ten pre-pulse trials. The startle stimuli were presented randomly [namely, single or by a 60-dB SPL BBN burst pre-pulses with 50-ms duration, 1-ms rise/fall time]. The frequency and intensity levels exploited in the PPI test were similar to those in the GPIAS test parameters. Besides, there was a time interval of 100 ms between the noise burst pre-pulse onset and that of the startle stimulus [28].
In the present study, the GPIAS values were calculated based on the following formula, which was by Turner et al., 2006 [28]:
[(AvgTnogap–AvgTgap)/AvgTnogap × 100%]
The AvgTgap represents the average amplitude during the gap trials, and the AvgTnogap indicates the average amplitude of the no-gap test trials. Moreover, the PPI values were computed based on the following formula:
[(AvgTstartle–AvgTpre-pulse)/AvgTstartle × 100%]
The AvgTpre-pulse and the AvgTstartle refer to the average amplitude during the pre-pulse trials and the average amplitude of the startle trials, respectively [28].
2.5. ABR measurements
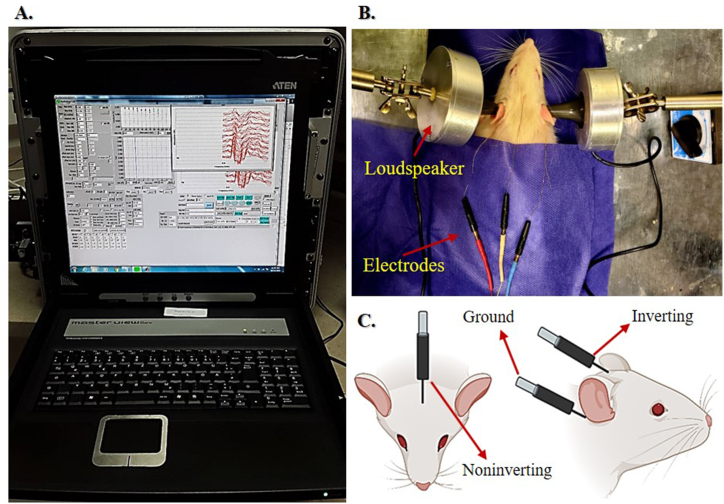
The ABR was recorded in a soundproof booth in response to the ipsilaterally presented pure tone bursts of 6, 12, and 24 kHz (4-ms duration, 2-ms rise/fall time) at a rate of 21.1 stimuli/s, using an Audiology Lab system (Otoconsult, Frankfurt a. M., Germany). A calibrated loudspeaker (DT48, Beyer Dynamic, Heilbronn, Germany) accordingly presented the acoustic stimuli via a plastic cone in the outer ear canal. The ABR waveforms were thus obtained (n = 500 waves) within a time window of 10 ms. Three subdermal needle electrodes were also located at the vertex (noninverting) and under the left mastoid (inverting) and the right (ground) ear [39,40] (Fig. 3). As well, electrode impedances ranged from 1 to 3 kΩ for the electrode pairs. The sampling rate setting was 60 kHz, and the filtering of the evoked potentials was 0.3–3.0 kHz, stored for offline analysis. Besides, a custom-written MATLAB (2016a, The MathWorks Inc.) program autonomously detected the ABR characteristics based on the peak amplitude, the prominence of the ABR waves, and the time gap between every two consecutive peaks. The ABR characteristics evaluated in the present study included hearing thresholds, absolute latencies, peak amplitudes related to the I, II, III, IV, and V waveforms, the amplitude ratios of II/I, IV/I, V/I, and the BTT. The time interval between the wave I peak (viz., auditory-nerve response) and the wave valley following the wave V (namely, the wave V descending part endpoint) was further measured as the auditory BTT (Fig. 4). Likewise, the electrophysiological hearing threshold levels were automatically determined, and then validated by a neuroscientist at the 10-dB steps descending from the maximum stimulus intensity of 90-dB SPL until no response was observed by tracking the repeatable and reproducible wave II.
Fig. 3.
Auditory Brainstem Response (ABR) Recording Setup. (A) Auditory Lab System, (B) The figure indicates how to perform the ABR test protocol in a rat. The loudspeakers and electrons are shown with arrows. (C) Three subdermal needle electrodes were placed at the vertex (noninverting) and under the left mastoid (inverting) and the right (ground) ear.
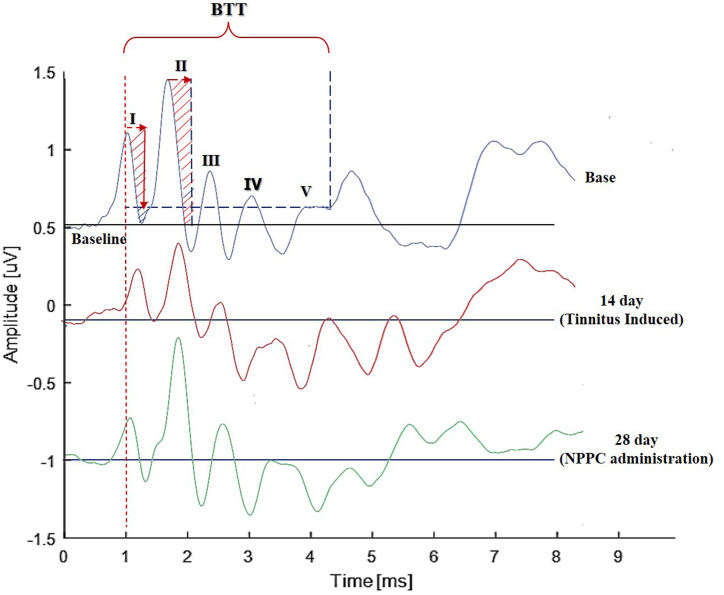
Fig. 4.
An example of the baseline, post-SS (day 14), and post-NPPC (day 28) administration ABR recordings. Note the decrease in the amplitude responses compared to the baseline recordings and after the NPPC. The BTT is also considered as the interval between the peak waves I–V.
2.6. Statistical analysis
Based on the “equation of resources” approach [41], a sample size of 8 rats was considered in each group. All statistical analyses were performed by the Statistical Package for Social Sciences (SPSS V.16; Chicago, the United States). Based on the normal distribution of the data (assessed by Kolmogorov-Smirnov) and homogeneity of variance (evaluated by Levene's test), in both groups (Chronic study group and control group), repeated-measures analysis of variance was conducted on the GPIAS the PPI, and the ABR characteristics. The Bonferroni post-hoc test was performed for multiple comparisons.
All results were presented as the mean ± standard deviation (SD). The p-value <0.05 was considered statistically significant.
3. Results
3.1. Gap pre-pulse inhibition of the acoustic startle (GPIAS) and pre-pulse inhibition (PPI) assessments
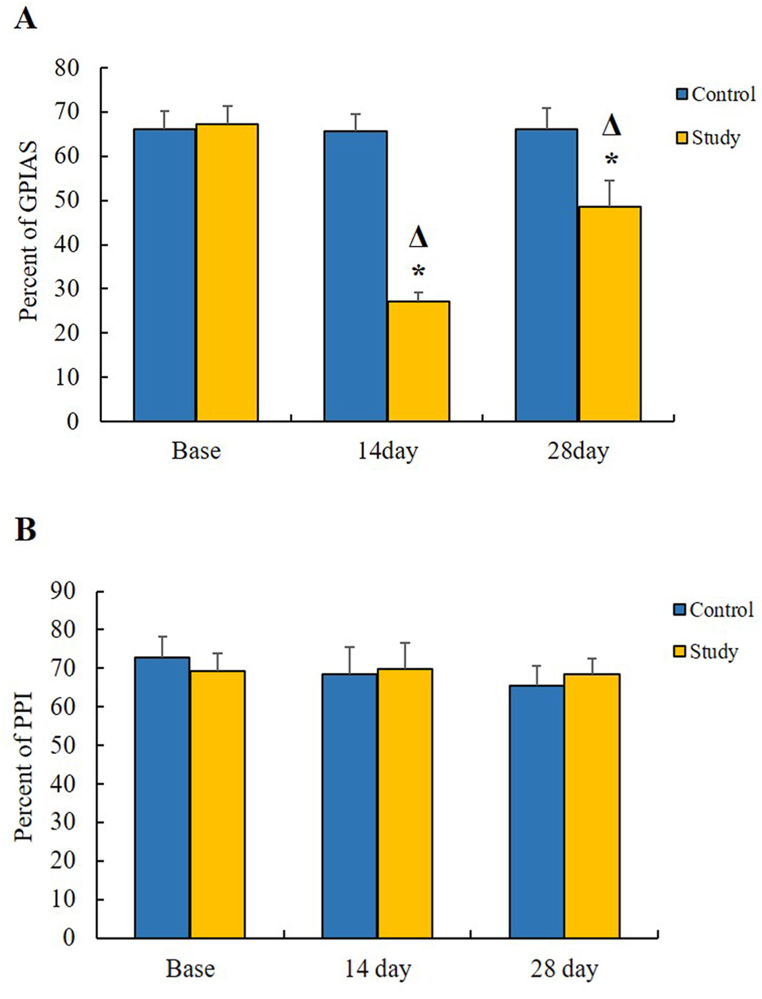
Under the baseline conditions, the discrepancies in the GPIAS and PPI mean values in percentage were not statistically significant between the control (66.21 ± 3.99 and 72.92 ± 5.27, respectively) and chronic study (67.34 ± 4.08 and 69.38 ± 4.37, respectively) groups. These results indicated that the animals in both groups had normal hearing conditions at the baseline (Fig. 5A).
Fig. 5.
GPIAS and PPI measurement results (A) The effects of the SS and the NPPC on the GPIAS values. The chronic study group indicated a significant decrease in the GPIAS values compared with the baseline and the control group. Asterisks (*) represent the significance between the control and chronic study groups, while the triangles (Δ) show significant value differences within each group. (B) The effects of the SS on the PPI values. No significant differences were seen in the PPI values between both study groups (p > 0.05).
Compared with the baseline, the GPIAS mean score in the chronic study group decreased drastically (27.19 ± 1.91) after 14 consecutive days of the SS injections (p < 0.0011). In contrast, NPPC-treated rats showed significant (P < 0.0011) enhancement in the GPIAS mean value (48.50 ± 6.05) compared to the 14 days. The GPIAS results thus indicated a significant suppressive effect of NPPC on SS-induced tinnitus. The PPI values in the chronic study (69.78 ± 6.82) and control (68.58 ± 6.88) groups also did not show any significant differences (p > 0.05) (Fig. 5B).
3.2. Differences in ABR characteristics
3.2.1. ABR threshold differences between and within chronic study and control groups
The ABR results showed significant differences in the hearing thresholds between the chronic study and control groups at the frequencies of 6 (p < 0.001), 12 (p < 0.001), and 24 kHz (p < 0.001) (Table 1).
Table 1.
Mean ± SD for the ABR thresholds at three times: before (baseline), after the SS administration [day 14], and following the NPPC injection (day 28).
| Frequency KHz | Baseline [Mean ± SD] | Day 14 [Mean ± SD] | Day 28 [Mean ± SD] | P.value [Sig.] | |
|---|---|---|---|---|---|
| Control Group | 6 | 23.75 ± 5.17 | 22.50 ± 7.07 | 21.25 ± 6.40 | 0.59 |
| 12 | 22.50 ± 7.07 | 22.50 ± 4.62 | 18.75 ± 8.34 | 0.39 | |
| 24 | 26.25 ± 5.17 | 23.75 ± 5.17 | 21.25 ± 3.53 | 0.14 | |
| Study Group | 6 | 27.50 ± 4.62 | 45.00 ± 11.95 | 35.00 ± 5.34 | <0.001 |
| 12 | 26.25 ± 5.17 | 55.50 ± 9.25 | 31.25 ± 9.91 | <0.001 | |
| 24 | 26.25 ± 5.17 | 55.00 ± 7.55 | 32.50 ± 7.07 | <0.001 |
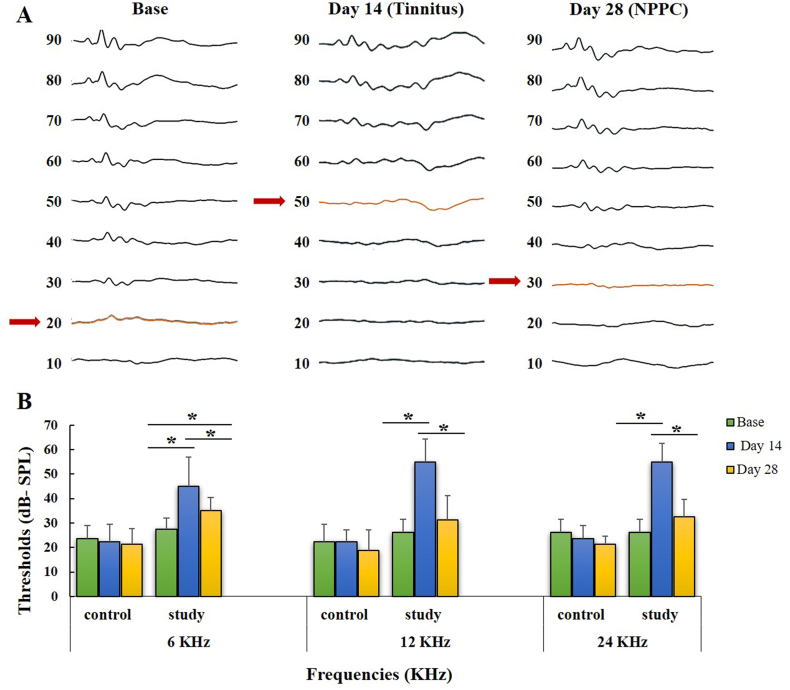
A significant increase in ABR threshold was recorded after SS injection (day 14) compared to baseline in the chronic study group (p < 0.001). However, NPPC significantly reduced ABR thresholds (day 28) at 6 kHz (p = 0.04) as well as at 12 and 24 kHz (p < 0.001), which indicates better treatment outcomes for the higher frequency. Furthermore, baseline and day 28 stage differences were insignificant at 12 and 24 kHz, suggesting a relative recovery of hearing conditions similar to the normal baseline after NPPC treatment. Post hoc analyses in the control group did not show significant differences in ABR thresholds at any of the three frequencies (p > 0.05 for each pair). These results indicate that NPPC has no significant effect on hearing threshold in normal rats (viz., control group). Fig. 6A shows examples of the ABR threshold waveforms from the baseline, tinnitus, and NPPC-treated rats, and Fig. 6B illustrates the summaries of the ABR threshold levels.
Fig. 6.
The effects of the SS and the NPPC on the ABR hearing threshold shift in both study groups (A) The waveforms of the ABRs compared at the baseline, tinnitus (day 14), and NPPC administration (day 28). The arrows indicate the hearing threshold. (B) The summary of the averaged ABR thresholds at various frequencies of 6, 12, and 24 KHz. The asterisks (*) represent the pairwise comparisons within the control and chronic study groups (p < 0.05).
3.2.2. Amplitudes of waves
No significant difference was observed between the chronic and control study groups in all waves’ amplitudes at all frequencies tested at the baseline (p > 0.05). However, data analysis between both groups showed a significant decrease in wave I amplitude (p < 0.001) and wave II amplitude (p = 0.002) on day 14 in the study group compared to the control group at all frequencies (Table 2).
Table 2.
The amplitude values of the ABR waves at three frequencies and three-time stages in the chronic study and control groups.
| Group | Amplitude | Frequency [KHz] | Base [Mean ± SD] | Day 14 [Mean ± SD] | Day 28 [Mean ± SD] | P.value [Sig.] |
|---|---|---|---|---|---|---|
| Control | Wave I | 6 | 0.84 ± 0.11 | 0.87 ± 0.12 | 0.88 ± 0.12 | 0.437 |
| 12 | 0.83 ± 0.07 | 0.82 ± 0.08 | 0.84 ± 0.07 | 0.625 | ||
| 24 | 0.85 ± 0.08 | 0.82 ± 0.07 | 0.86 ± 0.07 | 0.053 | ||
| Wave II | 6 | 1.02 ± 0.08 | 1.01 ± 0.06 | 1.05 ± 0.05 | 0.785 | |
| 12 | 1.09 ± 0.08 | 1.08 ± 0.11 | 1.06 ± 0.04 | 0.825 | ||
| 24 | 1.05 ± 0.08 | 1.02 ± 0.05 | 1.02 ± 0.04 | 0.542 | ||
| Wave III | 6 | 0.36 ± 0.14 | 0.33 ± 0.14 | 0.34 ± 0.15 | 0.833 | |
| 12 | 0.50 ± 0.31 | 0.54 ± 0.13 | 0.51 ± 0.28 | 0.974 | ||
| 24 | 0.33 ± 0.05 | 0.36 ± 0.04 | 0.36 ± 0.03 | 0.681 | ||
| Wave IV | 6 | 0.33 ± 0.05 | 0.33 ± 0.03 | 0.35 ± 0.05 | 0.620 | |
| 12 | 0.33 ± 0.05 | 0.30 ± 0.05 | 0.33 ± 0.03 | 0.647 | ||
| 24 | 0.33 ± 0.08 | 0.35 ± 0.11 | 0.37 ± 0.11 | 0.301 | ||
| Wave V | 6 | 0.23 ± 0.09 | 0.25 ± 0.10 | 0.27 ± 0.10 | 0.158 | |
| 12 | 0.26 ± 0.06 | 0.25 ± 0.06 | 0.26 ± 0.08 | 0.749 | ||
| 24 | 0.24 ± 0.06 | 0.24 ± 0.05 | 0.23 ± 0.05 | 0.564 | ||
| Study | Wave I | 6 | 0.80 ± 0.13 | 0.32 ± 0.09 | 0.61 ± 0.04 | < 0.001 |
| 12 | 0.84 ± 0.07 | 0.39 ± 0.06 | 0.58 ± 0.05 | < 0.001 | ||
| 24 | 0.88 ± 0.07 | 0.41 ± 0.04 | 0.57 ± 0.03 | < 0.001 | ||
| Wave II | 6 | 1.01 ± 0.20 | 0.43 ± 0.14 | 0.90 ± 0.25 | < 0.001 | |
| 12 | 1.02 ± 0.05 | 0.61 ± 0.10 | 0.84 ± 0.15 | 0.025 | ||
| 24 | 1.00 ± 0.05 | 0.53 ± 0.08 | 0.68 ± 0.09 | < 0.001 | ||
| Wave III | 6 | 0.38 ± 0.11 | 0.28 ± 0.12 | 0.44 ± 0.12 | 0.014 | |
| 12 | 0.55 ± 0.27 | 0.32 ± 0.12 | 0.50 ± 0.22 | 0.022 | ||
| 24 | 0.36 ± 0.07 | 0.28 ± 0.10 | 0.28 ± 0.04 | 0.040 | ||
| Wave IV | 6 | 0.27 ± 0.12 | 0.25 ± 0.21 | 0.32 ± 0.10 | 0.309 | |
| 12 | 0.28 ± 0.21 | 0.28 ± 0.20 | 0.20 ± 0.08 | 0.334 | ||
| 24 | 0.33 ± 0.07 | 0.27 ± 0.10 | 0.30 ± 0.06 | 0.293 | ||
| Wave V | 6 | 0.26 ± 0.10 | 0.23 ± 0.04 | 0.24 ± 0.08 | 0.490 | |
| 12 | 0.25 ± 0.06 | 0.17 ± 0.06 | 0.25 ± 0.06 | < 0.001 | ||
| 24 | 0.23 ± 0.06 | 0.19 ± 0.08 | 0.20 ± 0.05 | 0.106 |
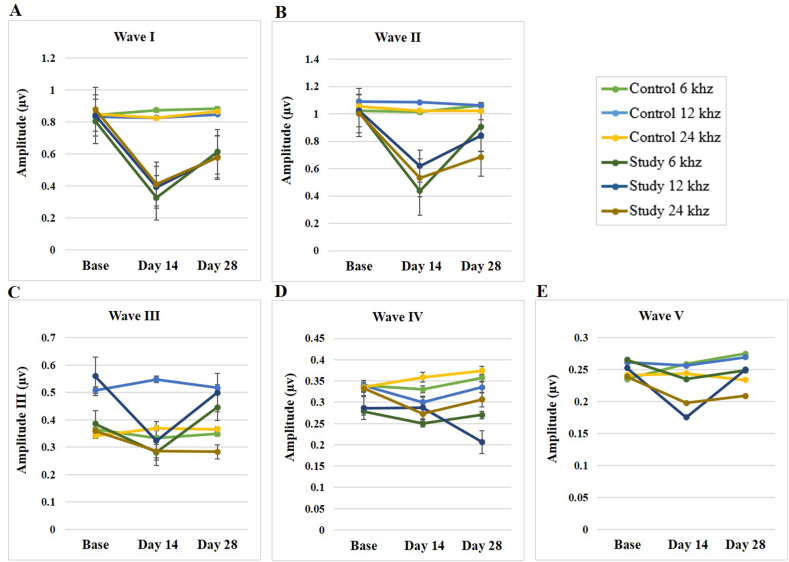
On the 28th day, the waves I and II amplitude significantly dropped at 12 and 24 kHz (p < 0.001). In addition, the comparative analyses exhibited no significant differences for the amplitudes of waves III, IV, and V at the frequency of 6 kHz and waves IV and V at 24 kHz at any of the three-time stages (p > 0.05). Despite this, significant differences were observed between the chronic study and control groups for the ABR wave III at the frequency of 24 kHz on day 28 (p = 0.02), wave IV on day 28 (p = 0.002), and the wave V on day 14 (p = 0.032) at the frequency of 12 kHz.
Post hoc analysis in the chronic study group showed a significant decrease in the amplitudes of waves I and II on day 14 compared to baseline, while a significant recovery of amplitudes of waves I and II (p < 0.001) was observed after NPPC treatment. (namely, day 28).
The statistical results within the chronic study group also showed a significant difference in the paired comparisons of wave III at the frequency of 12 kHz between the baseline and day 14, as well as between day 14 and day 28 (Table 2, Fig. 7). No notable difference was observed in the wave IV amplitude at all frequencies and any of the three-time stages (p > 0.05). The exact intra-group comparisons of the wave V amplitude also showed no significant differences at the frequencies 6 and 24 kHz (p > 0.05). In contrast, a considerable difference was mentioned between the baseline and day 14 (p < 0.001), as well as between day 14 and day 28 (p = 0.002) at the frequency of 12 kHz. No significant differences were evident between the amplitudes of any ABR waves in the control group (p > 0.05).
Fig. 7.
The effects of the SS and the NPPC on the ABR amplitude shift in the rats. The amplitude shift were determined using 6, 12 and 24 kHz tone burst stimulations (n = 8), calculated before the SS administration (baseline), the day 14 after the SS administration (the day 14), and day 14 after the SS + NPPC administration (the day 28). (A) Amplitude of wave I (B) amplitude of wave II (C) amplitude of wave III (D) amplitude of wave IV (E) amplitude of wave V. The statistical significance level was considered as (p < 0.05).
3.2.3. Absolute latencies
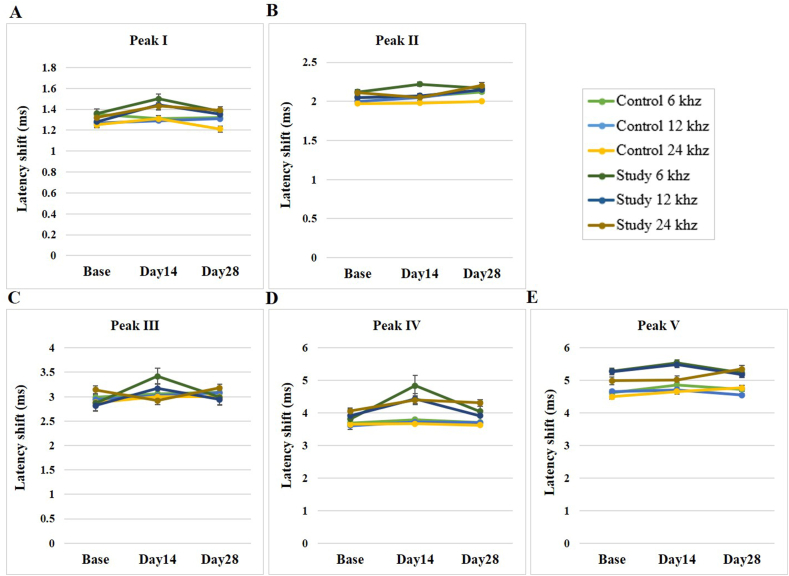
Analysis of the absolute peak latencies of the chronic study group at the three-time stages indicated significant differences in the peak I latency at the frequencies of 6 and 24 kHz (p = 0.01 and p = 0.03, respectively). The chronic study group also had significant differences in the ABR wave peak III and IV latencies at 6 kHz. However, no significant differences were seen for other absolute peak latencies. Post hoc analysis also confirmed significant increases in pairwise comparisons of absolute peak latencies of wave I at 6, 12, and 24 kHz and waves III and IV at 6 kHz after SS injection. However, after NPPC treatment, a significant decrease in peak wave latencies was observed. Accordingly, as shown in Table 3, a significant recovery was evident after the NPPC treatment for the frequencies mentioned above (Fig. 8).
Table 3.
The absolute latency values of the ABR waves at three frequencies and three time stages in the chronic study and control groups.
| Group | Absolute Latency | Frequency [KHz] | Baseline [Mean ± SD] | Day 14 [Mean ± SD] | Day 28 [Mean ± SD] | P.value [Sig.] |
|---|---|---|---|---|---|---|
| Control | Wave I | 6 | 1.35 ± 0.05 | 1.31 ± 0.04 | 1.32 ± 0.08 | 0.485 |
| 12 | 1.27 ± 0.03 | 1.29 ± 0.06 | 1.24 ± 0.06 | 0.566 | ||
| 24 | 1.25 ± 0.07 | 1.31 ± 0.08 | 1.21 ± 0.09 | 0.098 | ||
| Wave II | 6 | 2.05 ± 0.08 | 2.06 ± 0.06 | 2.12 ± 0.06 | 0.314 | |
| 12 | 2.00 ± 0.10 | 2.05 ± 0.03 | 2.15 ± 0.13 | 0.764 | ||
| 24 | 1.97 ± 0.09 | 1.98 ± 0.08 | 2.00 ± 0.08 | 0.742 | ||
| Wave III | 6 | 2.99 ± 0.22 | 3.06 ± 0.29 | 3.09 ± 0.27 | 0.308 | |
| 12 | 2.94 ± 0.22 | 3.02 ± 0.16 | 3.08 ± 0.07 | 0.231 | ||
| 24 | 2.88 ± 0.23 | 3.00 ± 0.17 | 3.00 ± 0.24 | 0.741 | ||
| Wave IV | 6 | 3.69 ± 0.15 | 3.79 ± 0.25 | 3.71 ± 0.25 | 0.937 | |
| 12 | 3.61 ± 0.15 | 3.72 ± 0.19 | 3.70 ± 0.20 | 0.778 | ||
| 24 | 3.65 ± 0.20 | 3.67 ± 0.16 | 3.62 ± 0.11 | 0.990 | ||
| Wave V | 6 | 4.62 ± 0.32 | 4.86 ± 0.25 | 4.73 ± 0.30 | 0.738 | |
| 12 | 4.66 ± 0.29 | 4.71 ± 0.28 | 4.55 ± 0.32 | 0.685 | ||
| 24 | 4.50 ± 0.14 | 4.66 ± 0.25 | 4.77 ± 0.27 | 0.605 | ||
| Study | Wave I | 6 | 1.36 ± 0.05 | 1.50 ± 0.14 | 1.38 ± 0.06 | 0.015 |
| 12 | 1.28 ± 0.04 | 1.44 ± 0.18 | 1.35 ± 0.08 | 0.050 | ||
| 24 | 1.32 ± 0.02 | 1.43 ± 0.17 | 1.39 ± 0.07 | 0.039 | ||
| Wave II | 6 | 2.12 ± 0.09 | 2.22 ± 0.15 | 2.17 ± 0.11 | 0.178 | |
| 12 | 2.05 ± 0.06 | 2.07 ± 0.23 | 2.15 ± 0.11 | 0.086 | ||
| 24 | 2.11 ± 0.04 | 2.05 ± 0.26 | 2.20 ± 0.10 | 0.076 | ||
| Wave III | 6 | 2.87 ± 0.12 | 3.42 ± 0.40 | 2.99 ± 0.15 | 0.008 | |
| 12 | 2.82 ± 0.08 | 3.17 ± 0.41 | 2.94 ± 0.16 | 0.065 | ||
| 24 | 3.14 ± 0.46 | 2.92 ± 0.41 | 3.18 ± 0.47 | 0.396 | ||
| Wave IV | 6 | 3.81 ± 0.20 | 4.84 ± 1.14 | 4.05 ± 0.32 | 0.006 | |
| 12 | 3.91 ± 0.47 | 4.43 ± 0.70 | 3.91 ± 0.18 | 0.054 | ||
| 24 | 4.06 ± 0.48 | 4.40 ± 1.01 | 4.31 ± 0.65 | 0.470 | ||
| Wave V | 6 | 5.29 ± 0.53 | 5.54 ± .088 | 5.23 ± 0.22 | 0.261 | |
| 12 | 5.27 ± 0.69 | 5.48 ± 0.66 | 5.18 ± 0.36 | 0.314 | ||
| 24 | 4.99 ± 0.55 | 5.02 ± 0.62 | 5.35 ± 0.68 | 0.359 |
Fig. 8.
The effects of the SS and the NPPC on the ABR absolute latency shift in the rats (n = 8). The absolute latency shifts were determined using 6, 12 and 24 kHz tone burst stimulations, calculated before the SS administration (baseline), after the SS administration (day 14), and the day 14 after the SS + NPPC administration (day 28). (A) Absolute latency shift of peak I (B) absolute latency shift of peak II (C) absolute latency shift of peak III (D) absolute latency shift of peak IV (E) absolute latency shift of peak V.
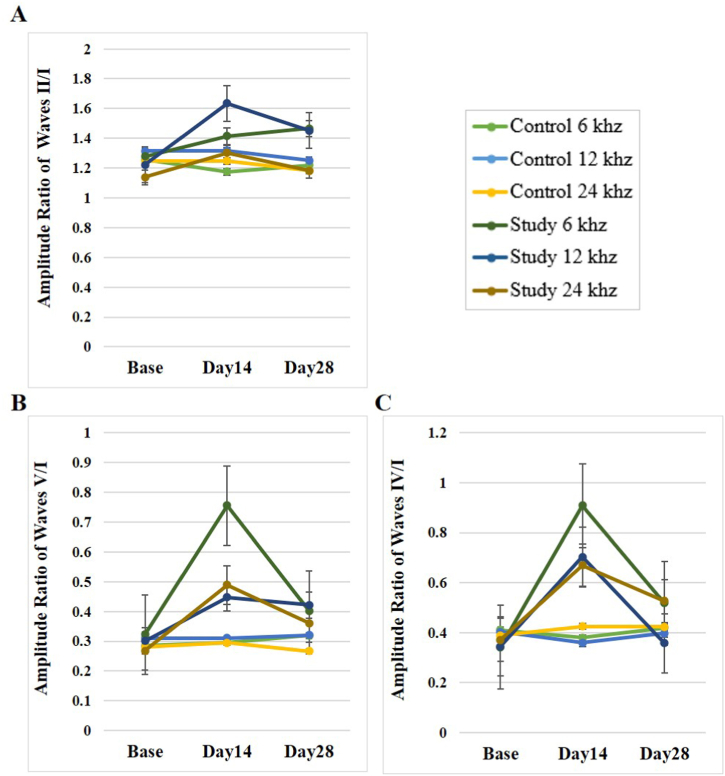
3.2.4. Amplitude ratios
No significant difference was observed between the chronic study and control groups in the wave amplitude ratios of II/I, IV/I, and V/I at all frequencies tested at the baseline on day 28 (p > 0.05).
However, the same comparison on the 14th day showed significant differences in the wave amplitude ratio of IV/I at the frequency of 24 kHz (p = 0.029) and the wave amplitude ratio of V/I at the frequency of 6 kHz (p < 0.001).
Post hoc Bonferroni analysis within the chronic study group, comparing day 14 and day 28 with the baseline state, revealed significant differences for the amplitude wave ratio of II/I at the frequency of 12 kHz (p = 0.02 and p = 0.033, respectively), as well as between the baseline and the day-14 stages at the frequency of 24 kHz (p = 0.038).
The same analysis of the wave amplitude ratio of IV/I on days 14 and 28 compared to the baseline showed significant differences at 24 kHz (p = 0.004 and p = 0.001, respectively), and on day 28 at 6 kHz (p = 0.014, respectively).
The amplitude ratio of IV/I at the frequency of 12 kHz showed significant differences between the baseline and the 14th day (p = 0.009) as well as between day 14 and day 28 (p = 0.044, respectively). The amplitude ratio of V/I showed significant differences on days 14 and 28 in comparison to the baseline at 6 kHz (p < 0.001 and p = 0.009, respectively), 12 kHz (p = 0.001 and p = 0.001, respectively) and 24 kHz (p = 0.008 and p = 0.004, respectively). Also, in the comparison between days 14 and 28, a significant difference was observed in the frequency of 6 (p < 0.001) and 24 kHz (p < 0.03). Overall, all the wave amplitude ratios of II/I, IV/I, and V/I were elevated after the SS injections, but the administration of NPPCN improved the effects of salicylate. Of note, the post hoc Bonferroni analysis within the control group did not show statistically significant differences between any of the three-time stages and all frequencies (p > 0.05) (see Table 4) (Fig. 9).
Table 4.
The amplitude ratio values of the ABR waves at three frequencies and three time stages in the chronic study and control groups.
| Group | Amp. Ratio | Frequency [KHz] | Base |
Day 14 |
Day 28 |
p.value |
|---|---|---|---|---|---|---|
| [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | ||||
| Control | Wave II/I | 6 | 1.25 ± 0.18 | 1.17 ± 0.19 | 1.21 ± 0.26 | 0.784 |
| 12 | 1.31 ± 0.13 | 1.31 ± 0.17 | 1.25 ± 0.08 | 0.736 | ||
| 24 | 1.24 ± 0.15 | 1.24 ± 0.15 | 1.18 ± 0.11 | 0.328 | ||
| Wave IV/I | 6 | 0.41 ± 0.10 | 0.38 ± 0.07 | 0.41 ± 0.08 | 0.978 | |
| 12 | 0.40 ± 0.06 | 0.35 ± 0.06 | 0.39 ± 0.05 | 0.91 | ||
| 24 | 0.38 ± 0.08 | 0.42 ± 0.11 | 0.42 ± 0.11 | 0.546 | ||
| Wave V/I | 6 | 0.28 ± 0.14 | 0.29 ± 0.13 | 0.31 ± 0.14 | 0.37 | |
| 12 | 0.31 ± 0.07 | 0.31 ± 0.09 | 0.32 ± 0.12 | 0.932 | ||
| 24 | 0.28 ± 0.07 | 0.29 ± 0.08 | 0.26 ± 0.06 | 0.385 | ||
| Study | Wave II/I | 6 | 1.27 ± 0.34 | 1.41 ± 0.62 | 1.46 ± 0.39 | 0.176 |
| 12 | 1.22 ± 0.13 | 1.63 ± 0.52 | 1.45 ± 0.28 | 0.006 | ||
| 24 | 1.13 ± 0.10 | 1.30 ± 0.23 | 1.18 ± 0.17 | 0.033 | ||
| Wave IV/I | 6 | 0.34 ± 0.18 | 0.90 ± 0.97 | 0.51 ± 0.16 | 0.004 | |
| 12 | 0.34 ± 0.26 | 0.70 ± 0.50 | 0.35 ± 0.16 | 0.014 | ||
| 24 | 0.37 ± 0.07 | 0.67 ± 0.26 | 0.52 ± 0.10 | < 0.001 | ||
| Wave V/I | 6 | 0.32 ± 0.12 | 0.75 ± 0.21 | 0.40 ± 0.14 | < 0.001 | |
| 12 | 0.30 ± 0.10 | 0.44 ± 0.17 | 0.42 ± 0.09 | < 0.001 | ||
| 24 | 0.26 ± 0.08 | 0.48 ± 0.25 | 0.36 ± 0.11 | 0.006 |
Fig. 9.
The effects of the SS and the NPPC on the ABR amplitude ratios in the rats (n = 8). The amplitude ratios were determined using 6, 12 and 24 kHz tone burst stimulations, calculated before the SS administration (baseline), after the SS administration (day 14), and day 14 after the SS + NPPC administration (day 28). (A) Amplitude ratio of waves II/I (B) Amplitude ratio of waves V/I (C) Amplitude ratio of waves IV/I.
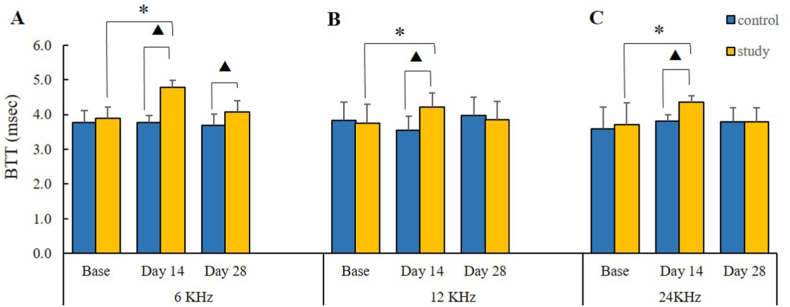
3.2.5. Brainstem transmission time (BTT)
The time interval between the peak of wave I (the distal portion of the auditory nerve) and the endpoint of wave V descending part was considered as an auditory brain stem transmission time (BTT) (Fig. 4). The comparison of BTT between the chronic study and control groups showed statistically significant differences at three frequencies of 6, 12, and 24 kHz only on day 14 (p = 0.02, p = 0.002, and p = 0.02, respectively). Post hoc Bonferroni analysis within the chronic study group indicated that the mean BTT was significantly different at all frequencies between the baseline and the day-14 stages (p = 0.04, p = 0.04, and p = 0.01, respectively). These results revealed that chronic high doses of sodium salicylate administration cause BTT prolongation. The comparison between baseline and day 28, which was insignificant, indicated that NPPC could have positive ameliorating effects on the BTT. The post hoc Bonferroni analysis did not display any statistically significant differences between the three-time stages and the frequencies in the control group (p > 0.05) (Fig. 10).
Fig. 10.
BTT results. The effects of the SS and the NPPC on the BTT values. The chronic study group indicated a significant increase in the BTT values on day 14 (the SS injection) compared with the baseline, while the BTT value dropped after the NPPC administration on day 28 compared to that on day 14. (A) BTT of 6 KHz (B) BTT of 12 KHz (C) BTT of 24 KHz. Asterisks (*) represent the significance between the control and chronic study groups, whereas the triangles (Δ) show significant value differences within each group (p < 0.05).
4. Discussion
For the first time, the results of the present study showed that placental neuroprotective protein compounds (NPPC) could have a therapeutic effect against salicylate-related ototoxic effects and could suppress SS-induced tinnitus. This study describes electrophysiological changes in the underlying pathophysiology of tinnitus and subsequent improvement after NPPC administration.
Our results showed that significant changes in ABR characteristics, such as an increase in ABR threshold, decrease in amplitude, especially waves I and II, enhancement of the amplitude ratios of II/I, IV/I, and V/I, and prolongation of BTT were evident in SS-treated rats compared to the control group. Also, the behavioral evidence of tinnitus was reflected in the behavioral findings indexed by the auditory startle response after salicylate administration. As well as a significant improvement in some critical characteristics of ABR recordings as well as tinnitus suppression is evident after NPPC administration.
Tinnitus is primarily associated with sensorineural hearing loss caused by acoustic trauma, traumatic brain injury, and ototoxicity drugs. Among the list of drug-related ototoxicity, salicylate, the active ingredient of aspirin, is a non-steroidal anti-inflammatory drug that causes reversible hearing loss and tinnitus [42]. The investigations on the pathophysiological changes in SS-induced ototoxicity have proven that the central and peripheral auditory systems play critical roles in tinnitus generation [43]. Therefore, tinnitus caused by salicylate interacts with the auditory system in both the cochlea and the central auditory system. Salicylate has several independent effects on the cochlear function, which include the down-regulation of prestin, transmembrane protein in the outer hair cells, increased activity of NMDA receptors following interaction with the arachidonic acid cycle, and ultimately increased spontaneous firing rate in auditory nerve fibers [44,45].
In the central auditory system, salicylate reduces the activity of GABA and serotonin in the auditory cortex. Salicylates also increase the sensitivity of the more central parts of the auditory system to sound, which is reflected in increased startle responses, potentially causing hyperacusis. Chronic salicylate administration reduces spontaneous firing rates in the primary auditory cortex [44,45].
A hearing loss of cochlear origin, which is associated with a lowered level of activity in cochlear hair cells, may trigger the tinnitus-related neural alterations that could extend from the periphery (the inner ear) to the central auditory cortex and perhaps broader cortical and subcortical regions in the brain as shown by brain mapping studies [42,46]. This relation between cochlear hearing loss and central tinnitus is significant since recent studies have shown that in NIHL incidence of tinnitus and associated synaptic imbalance is mediated by neuroinflammation in the auditory cortex, the resolution of which may prevent tinnitus development [47]. More recently, a study using a rodent model of salicylate-induced tinnitus showed that pro-inflammatory activation of astrocytes and microglia (neuroglia) in the auditory cortex played a significant role in tinnitus development [48]. On the other hand, previous research has confirmed the association of salicylate toxicity with oxidative stress [49]. Human placenta extracts (HPEs) are obtained by lysing human placental tissues collected at full-term delivery. According to the data obtained from various research studies, HPEs possess anti-inflammatory, analgesic [50], antioxidant, antiapoptotic [51,52], cytoprotective and radioprotective [53], and anti-allergic properties and express hormonal activity [54], as well as stimulate proliferation and regenerative processes [55]. Based on such studies that showed the multi-potent features of HPEs, and the pathophysiology of tinnitus, as a complex medical condition with no standard treatment, we hypothesized that placenta extract might be able to reverse salicylate-induced changes. To the best of our knowledge, this is the first study that evaluated the effect of HPE in, tinnitus treatment.
The ABR data analysis indicated that the wave I amplitude decreased (representing the peripheral input loss and hidden hearing loss) and the wave amplitude ratios of V/I and IV/I elevated (denoting the increased central neuronal gain) after the long-term SS administration. The literature on the pharmacological treatment and the electrophysiological assessment of blast-induced tinnitus mainly indicates that the growth in the ABR wave amplitude ratios of V/I and IV/I might serve as a reliable parameter to identify tinnitus objectively [20,56,57]. The present study on SS-induced tinnitus, in agreement with previous research, accordingly revealed a correlation between the reduction in the ABR wave I amplitude and the enhancement in the wave amplitude ratios of IV/I and V/I, followed by compensatory plasticity changes in the central auditory pathway. Furthermore, the results of the present study provided physiological evidence that the NPPC could counteract this apparent CNS dysfunction in response to the reduced cochlear neural output. SS can traverse the blood-brain barrier [BBB] and directly act on the central auditory system [58]. In vitro, studies suggested SS probably diminishes the inhibitory post-synaptic currents [59,60], increasing spontaneous activity [61]. Besides, in vivo investigation revealed that SS may affect cochlear fast synaptic transmission through the activation of the N-methyl-d-aspartate (NMDA) glutamate receptors, which can, in turn, cause the occurrence of tinnitus [62]. Once administered systemically, SS can directly impact the functions of the auditory cortex by decreasing the inhibitory effects of the gamma-aminobutyric acid GABA-ergic neurons [60]. Moreover, another study confirmed that the cochlea-originated tinnitus and the associated synaptic imbalance had been mediated by the auditory cortex neuroinflammation, which is a resolution that might prevent tinnitus development [47]. Therefore, the underlying pathophysiology of tinnitus includes neuroinflammatory activities and synaptopathy in the auditory nervous system, which requires further investigation in this field.
The NPPC as an HPE contains diverse multifunctional proteins involved in oxidant detoxification and oxidative stress response, cytoprotection, immunity, and inflammatory response regulation, with neuroprotection, tissue regeneration, and wound healing properties [29,30]. Therefore, NPPC, through this remarkable diversity, may provide a synergistic biological function that can address different pathological aspects of tinnitus-manifesting neuro-otological disorders. Abd-Elhakim et al. (2022) recorded an increase in malondialdehyde (MDA) and a decrease in glutathione levels (GSH) and catalase activity after the administration of salicylate. Exposure to salicylate significantly induces pathological changes in cochlear and vestibular tissue and increases the expression of apoptotic factors like caspase-3 and the inflammatory indicator nuclear factor kappa (NF-κB) [63]. Several studies have revealed the anti-inflammatory and anti-oxidative activities of HPE in different models [64,65]. These studies show that HPE can decrease the level of MDA, increase GSH levels, and markedly enhance the antioxidant enzyme activities of catalase (CAT) and superoxide dismutase (SOD). Therefore, it can be said that one of the NPPC mechanisms that probably suppresses salicylate-induced tinnitus is its antioxidant effect by increasing non-enzymatic defense systems such as GSH and the activity of antioxidant enzymes such as SOD and CAT.
The results of the present study indicated the reduction of amplitudes of the ABR wave peaks I and II after the SS injection at the three frequencies of 6, 12, and 24 kHz, while the ABR wave III amplitude showed a reduction at the frequency of 12 kHz, following this procedure. A previous study in mice with SS-induced tinnitus exhibited decreased ABR wave peak amplitudes and increased amplitude ratios at the frequency range of 6–24 kHz [66]. Another investigation on high-dose SS-injected rats had similarly indicated that the ABR wave peak amplitudes could be enhanced, followed by forwarding acoustic masking at high frequencies [67]. Also, the results of the present study showed that chronic administration of SS increased hearing thresholds in all frequencies, and after administration of NPPC for two weeks, ABR thresholds relatively returned to normal values. These findings are in line with numerous investigations on the animal models of noise-induced tinnitus or SS toxicity that had identified the ABR changes associated with tinnitus [18,66]. It is hypothesized that the SS-induced ototoxicity reduced cochlear output and the auditory threshold increased as a result [68]. In the present study, it was shown that along with the increase in hearing threshold, the amplitude of wave I decreased. It has been further reported that the reduced wave I amplitude and the enhanced auditory threshold might show the diminished sensory input or the synaptopathy condition of the cochlea found in tinnitus, which is consistent with the hidden hearing loss [69]. As previous studies have proposed neural alterations regarding the ABR characteristics in tinnitus [70], the BTT measurement in this study was utilized as a considerably more stable parameter to assess the tinnitus phenomenon [71] objectively. The BTT neuro-index also increased following the SS administration on day 14 at 6, 12, and 24 kHz (Fig. 10). However, this index decreased on the 28th day after the NPPC treatment, compared to the 14th day. According to these results, the NPPC might lessen the tinnitus-related activity, as confirmed by the GPIAS behavioral assessments. It can be due to the enhanced neural synchrony process in the auditory pathway and homeostasis improvement associated with the NPPC administration.
It is well known that another substance in the placenta is estrogen [72]. Estrogens have diverse neuromodulation functions that exert their neuromodulatory activities by inducing the expression of neurotrophic factors (NTs) such as glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factors (BDNF) [34]. It is widely accepted that BDNF can exert various functions in both the central and peripheral nervous systems by modulating synaptic plasticity, differentiation, neuronal survival, and the development of neurons [73]. Many studies in rat models have demonstrated that BDNF can reduce auditory thresholds, rescue spiral ganglion cells (SGCs) from degeneration, and repair damaged auditory nerve fibers [74,75]. Clinical improvement also has been found in patients with tinnitus and Sensorineural Hearing Loss (SNHL) after the treatment with NTs [76,77]. Therefore, NPPC may collectively induce other neurotrophic factors, such as BDNF, via its potential multifunctional effects, leading to improved ABR and auditory properties. This new therapy may represent a new promise for treating tinnitus and hearing disorders. Future studies need to address these mechanistic implications experimentally.
4.1. Study limitations
This animal study showed that NPPC may improve tinnitus and its associated electrophysiological characteristic but some limitations need further work to address some important questions: the potential mechanisms that contribute to tinnitus suppression need to be evaluated. As salicylate–induced ototoxicity leads to cochlear dysfunctions, assessment of cochlear structure and function after HPE (NPPC) administration can be helpful. The current study provides a novel line of evidence from an animal research perspective for more animal and clinical studies in the field of tinnitus management.
5. Conclusion
Our results revealed that human placental extract (NPPC) could alleviate the behavioral and electrophysiological changes in salicylate–induced tinnitus. The observed neuroelectrical alterations are characterized by a significantly decreased BTT and the enhanced amplitude of I and II waves and related thresholds that may indicate tinnitus reduction, manifesting as modulation of auditory deafferentation following NPPC-treated rats. The application of NPPC could improve tinnitus and exerts positive therapeutic effects on ABR characteristic. Additional research is thus needed to reveal the mechanism of action of this new potential therapeutic approach.
Author contribution statement
Saeid Mahmoudian, Zeinab Akbarnejad: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mohammad Farhadi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ali Gorji: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Marjan Mirsalehi: Performed the experiments; Wrote the paper.
Alexander Borisovich Poletaev, Fereidoun Mahboudi, Mohammad Ebrahimi, Mohaddeseh Beiranvand, Mohaddeseh Dehghani Khaftari: Contributed reagents, materials, analysis tools or data, Wrote the paper.
Marcus Mülle, Abdoreza Asadpour: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Also, all authors agree to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved.
Funding statement
This study was financially supported by the Iran National Science Foundation (INSF, Synergy Grant Code no. insf-98020383-1400/03/23), and ENT and Head & Neck Research Center, Hazrate Rasoul Akram Hospital, the Five Senses Institute, School of Medicine, Iran University of Medical Sciences, Tehran, Iran (Ethical approval no. IR.IUMS.REC.1398.233).
Data availability statement
Data included in article material/referenced in the article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Mohammad Farhadi reports financial support was provided by Iran National Science Foundation. Dr. Saeid Mahmoudian reports financial support and administrative support were provided by ENT and Head and Neck Research Center, The Five Senses Health Institute, Iran University of Medical Sciences.
Acknowledgements
The support of the Iran National Science Foundation (INSF), ENT and Head & Neck Research Center, Iran University of Medical Sciences to assign credit to the research line auditory neuroscience is gratefully acknowledged. Also, all authors would like to appreciate the late Professor Alexander Borisovich Poletaev (*06 November 1951 – † March 6th, 2021). He was the first to report the evident positive effects of the protein fraction of porcine placental extract introduced by Alexander Anikin, in terms of having prominent neuroprotective properties, initially named X-proteins (XP). He also generously provided the placenta extract extraction method to this research group. Moreover, the authors would like to extend special thanks to Professor Marcus Müller (*06 November 1957 – †14 June 2020), Associate Professor of Physiology, Department of Otolaryngology, Head, and Neck Surgery of the University of Tübingen, Tübingen, Germany, for his unwavering support to set up the ABR system and record appropriate waveforms. He was a highly recognized auditory physiologist, tireless researcher, and dedicated teacher. The authors further thank all colleagues as well as administrative personnel at Shefa Neuroscience Institute, Tehran, Iran, who contributed to data collection.
Contributor Information
Zeinab Akbarnejad, Email: akbarnejad.z@iums.ac.ir, zeinab.akbarnejad8@gmail.com.
Saeid Mahmoudian, Email: s-mahmoudian@iums.ac.ir, saeid.mahmoudian@gmail.com.
References
- 1.Haider H.F., Hoare D.J., Costa R.F.P., Potgieter I., Kikidis D., Lapira A., et al. Pathophysiology, diagnosis and treatment of somatosensory tinnitus: a scoping review. Front. Neurosci. 2017;11:207. doi: 10.3389/fnins.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messina A., Corvaia A., Marino C. Definition of tinnitus. Audiol Res. 2022;12(3):281–289. doi: 10.3390/audiolres12030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landry E.C., Sandoval X.C.R., Simeone C.N., Tidball G., Lea J., Westerberg B.D. Systematic review and network meta-analysis of cognitive and/or behavioral therapies [CBT] for tinnitus. Otol. Neurotol. 2020;41(2):153–166. doi: 10.1097/MAO.0000000000002472. [DOI] [PubMed] [Google Scholar]
- 4.Rosing S.N., Schmidt J.H., Wedderkopp N., Baguley D.M. Prevalence of tinnitus and hyperacusis in children and adolescents: a systematic review. BMJ Open. 2016 Jun;6(6) doi: 10.1136/bmjopen-2015-010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack A., Edmondson-Jones M., Somerset S., Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 2016 Jul;337:70–79. doi: 10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudian S., Farhadi M., Najafi-Koopaie M., Darestani-Farahani E., Mohebbi M., Dengler R., et al. Central auditory processing during chronic tinnitus as indexed by topographical maps of the mismatch negativity obtained with the multi-feature paradigm. Brain Res. 2013;1527:161–173. doi: 10.1016/j.brainres.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 7.De Ridder D., Elgoyhen A.B., Romo R., Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. USA. 2011;108(20):8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer M., Neff P., Liem F., Kleinjung T., Weidt S., Langguth B., et al. Differential tinnitus-related neuroplastic alterations of cortical thickness and surface area. Hear. Res. 2016;342:1–12. doi: 10.1016/j.heares.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Tae W.-S., Yakunina N., Lee W.H., Ryu Y.-J., Ham H., Pyun S.-B., et al. Changes in the regional shape and volume of subcortical nuclei in patients with tinnitus comorbid with mild hearing loss. Neuroradiology. 2018;60(11):1203–1211. doi: 10.1007/s00234-018-2093-2. [DOI] [PubMed] [Google Scholar]
- 10.Henry J.A., Roberts L.E., Caspary D.M., Theodoroff S.M., Salvi R.J. Underlying mechanisms of tinnitus: review and clinical implications. J. Am. Acad. Audiol. 2014;25(1):5–22. doi: 10.3766/jaaa.25.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H., Vikhe Patil K., Han C., Fabella B., Canlon B., Someya S., et al. GLAST deficiency in mice exacerbates gap detection deficits in a model of salicylate-induced tinnitus. Front. Behav. Neurosci. 2016;10:158. doi: 10.3389/fnbeh.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galazyuk A., Hebert S. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Front. Neurol. 2015;6:88. doi: 10.3389/fneur.2015.00088. 2015. Epub 2015/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parham K., Sun X.-M., Kim D.O. Handbook of Mouse Auditory Research. CRC Press; 2001. Noninvasive assessment of auditory function in mice: auditory brainstem response and distortion product otoacoustic emissions; pp. 51–72. [Google Scholar]
- 14.Reichmuth C., Mulsow J., Finneran J.J., Houser D.S., Supin A.Y. Measurement and response characteristics of auditory brainstem responses in pinnipeds. Aquat. Mamm. 2007;33(1):132–150. [Google Scholar]
- 15.Szczepek A.J., Dietz G.P.H., Reich U., Hegend O., Olze H., Mazurek B. Differences in Stress-induced modulation of the auditory system between Wistar and Lewis rats. Front. Neurosci. 2018;12:828. doi: 10.3389/fnins.2018.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabiani M., Sohmer H., Tait C., Gafni M., Kinarti R. A functional measure of brain activity: brain stem transmission time. Electroencephalogr. Clin. Neurophysiol. 1979;47(4):483–491. doi: 10.1016/0013-4694(79)90164-0. [DOI] [PubMed] [Google Scholar]
- 17.Gu J.W., Herrmann B.S., Levine R.A., Melcher J.R. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol. 2012;13(6):819–833. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X.-P., Chen L. Forward acoustic masking enhances the auditory brainstem response in a diotic, but not dichotic, paradigm in salicylate-induced tinnitus. Hear. Res. 2015;323:51–60. doi: 10.1016/j.heares.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Bauer C.A., Brozoski T.J., Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J. Neurosci. Res. 2007;85(7):1489–1498. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- 20.Schaette R., McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J. Neurosci. 2011;31(38):13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baysal E. 2017. Masking Treatment and its Effect on Tinnitus Parameters. [DOI] [PubMed] [Google Scholar]
- 22.Halla J.T., Hardin J.G. Salicylate ototoxicity in patients with rheumatoid arthritis: a controlled study. Ann. Rheum. Dis. 1988;47(2):134–137. doi: 10.1136/ard.47.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolzberg D., Salvi R.J., Allman B.L. Salicylate toxicity model of tinnitus. Front. Syst. Neurosci. 2012;6:28. doi: 10.3389/fnsys.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T., Kunze C., Dunlop M.J. Salicylate increases fitness cost associated with MarA-mediated antibiotic resistance. Biophys. J. 2019;117(3):563–571. doi: 10.1016/j.bpj.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh K.-W., Qian T., Brenner D.A., Lemasters J.J. Salicylate enhances necrosis and apoptosis mediated by the mitochondrial permeability transition. Toxicol. Sci. 2003;73(1):44–52. doi: 10.1093/toxsci/kfg045. [DOI] [PubMed] [Google Scholar]
- 26.Abd-Elhakim Y.M., Abdel-Motal S.M., Malhat S.M., Mostafa H.I., Moselhy A.A.A., Beheiry R.R., et al. Curcumin mitigates neurotoxic and neurobehavioral changes of gentamicin and sodium salicylate in rats by adjusting oxidative stress and apoptosis. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118824. [DOI] [PubMed] [Google Scholar]
- 27.Park J.Y., Byeon J.H., Park S.-W., Eun S.-H., Chae K.Y., Eun B.-L. Neuroprotective effect of human placental extract on hypoxic–ischemic brain injury in neonatal rats. Brain Dev. 2013;35(1):68–74. doi: 10.1016/j.braindev.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Turner J.G., Brozoski T.J., Bauer C.A., Parrish J.L., Myers K., Hughes L.F., et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav. Neurosci. 2006;120(1):188. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 29.Kim H., Kim S., Seo J., Bae G., Kim K., Kang J. Effect of single‐dose, oral enzymatic porcine placental extract on pharmacokinetics of alcohol and liver function in rats. Alcohol Clin. Exp. Res. 2020;44(5):1018–1024. doi: 10.1111/acer.14319. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi A., Kamiyoshi A., Sakurai T., Miyazaki H., Hirano E., Lim H.S., et al. Placental extract suppresses cardiac hypertrophy and fibrosis in an angiotensin II-induced cachexia model in mice. Heliyon. 2019;5(10) doi: 10.1016/j.heliyon.2019.e02655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jazayeri M.H., Barzaman K., Nedaeinia R., Aghaie T., Motallebnezhad M. Human placental extract attenuates neurological symptoms in the experimental autoimmune encephalomyelitis model of multiple sclerosis-a putative approach in MS disease? Autoimmun Highlights. 2020 Dec 4;11(1):14. doi: 10.1186/s13317-020-00137-x. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi H.Y., Kim S.W., Kim B., Lee H.N., Kim S.-J., Song M., et al. In: Exhibits Synergistic Antioxidant Activity in the Presence of Estradiol. Veitia R.A., editor. vol. 9. PLoS One; 2014. Alpha-fetoprotein, identified as a novel marker for the antioxidant effect of placental extract. [Internet] 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muluye R.A., Bian Y., Wang L., Alemu P.N., Cui H., Peng X., et al. Placenta peptide can protect mitochondrial dysfunction through inhibiting ROS and TNF-α generation, by maintaining mitochondrial dynamic network and by increasing IL-6 level during chronic fatigue. Front Pharmacol [Internet] 2016 Sep 27:7. doi: 10.3389/fphar.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takuma K., Mizoguchi H., Funatsu Y., Kitahara Y., Ibi D., Kamei H., et al. Placental extract improves hippocampal neuronal loss and fear memory impairment resulting from chronic restraint stress in ovariectomized mice. J. Pharmacol. Sci. 2012;120(2):89–97. doi: 10.1254/jphs.12115FP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogure C., Tohda C. Human placenta extract ameliorates memory dysfunction and dendritic atrophy in a 5XFAD mouse model of Alzheimer's disease. Tradit Kampo Med. 2017;4(2):94–104. [Google Scholar]
- 36.Poletaev A.B., Arapov N.A. Rehabilitation of patients after stroke: new remedy? Pharmacol Online. 2006;3:73–79. [Google Scholar]
- 37.Hesse G. Evidence and evidence gaps in tinnitus therapy. GMS Curr. Top. Otorhinolaryngol., Head Neck Surg. 2016;15 doi: 10.3205/cto000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi B., Hu S., Zuo C., Jiao F., Lv J., Chen D., et al. Effects of long-term salicylate administration on synaptic ultrastructure and metabolic activity in the rat CNS. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep24428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overbeck G.W., Church M.W. Effects of tone burst frequency and intensity on the auditory brainstem response [ABR] from albino and pigmented rats. Hear. Res. 1992;59(2):129–137. doi: 10.1016/0378-5955(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 40.Church M.W., Hotra J.W., Holmes P.A., Anumba J.I., Jackson D.A., Adams B.R. Auditory brainstem response [ABR] abnormalities across the life span of rats prenatally exposed to alcohol. Alcohol Clin. Exp. Res. 2012;36(1):83–96. doi: 10.1111/j.1530-0277.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arifin W.N., Zahiruddin W.M. Sample size calculation in animal studies using resource equation approach. Malaysian J Med Sci MJMS. 2017;24(5):101. doi: 10.21315/mjms2017.24.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noreña A.J. Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiol Neurotol. 2015;20(Suppl. 1):53–59. doi: 10.1159/000380749. [DOI] [PubMed] [Google Scholar]
- 43.Chen G., Feng L., Liu Z., Sun Y., Chang H., Cui P. Both central and peripheral auditory systems are involved in salicylate-induced tinnitus in rats: a behavioral study. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108659. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greeson J.N., Raphael R.M. Amphipath-induced nanoscale changes in outer hair cell plasma membrane curvature. Biophys. J. 2009;96(2):510–520. doi: 10.1016/j.bpj.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggermont J.J. second ed. Senses A Compr Ref Vol 1-7; 2020. Tinnitus; pp. 896–922. 2.46. 2. [Google Scholar]
- 46.Sedley W., Gander P.E., Kumar S., Oya H., Kovach C.K., Nourski K.V., et al. Intracranial mapping of a cortical tinnitus system using residual inhibition. Curr. Biol. 2015;25(9):1208–1214. doi: 10.1016/j.cub.2015.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W., Zhang L.S., Zinsmaier A.K., Patterson G., Leptich E.J., Shoemaker S.L., et al. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol. 2019;17(6) doi: 10.1371/journal.pbio.3000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia C., Yin M., Wu C., Ji Y., Zhou Y. Neuroglial activation in the auditory cortex and medial geniculate body of salicylate-induced tinnitus rats. Am J Transl Res. 2020;12(10):6043. [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed D.I., Khairy E., Saad S.S.T., Habib E.K., Hamouda M.A. Potential protective effects of Dapagliflozin in gentamicin induced nephrotoxicity rat model via modulation of apoptosis associated miRNAs. Gene. 2019;707:198–204. doi: 10.1016/j.gene.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Lee K.-H., Kim T.-H., Lee W.-C., Kim S.H., Lee S.Y., Lee S.-M. Anti-inflammatory and analgesic effects of human placenta extract. Nat. Prod. Res. 2011;25(11):1090–1100. doi: 10.1080/14786419.2010.489050. [DOI] [PubMed] [Google Scholar]
- 51.Togashi S., Takahashi N., Iwama M., Watanabe S., Tamagawa K., Fukui T. Antioxidative collagen-derived peptides in human-placenta extract. Placenta. 2002;23(6):497–502. doi: 10.1053/plac.2002.0833. [DOI] [PubMed] [Google Scholar]
- 52.Shen L.-H., Fan L., Zhang Y., Zhu Y.-K., Zong X.-L., Peng G.-N., et al. Protective effect and mechanism of placenta extract on liver. Nutrients. 2022;14(23):5071. doi: 10.3390/nu14235071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakatsu M., Urata Y., Goto S., Ono Y., Li T.S. Placental extract protects bone marrow-derived stem/progenitor cells against radiation injury through anti-inflammatory activity. J. Radiat. Res. 2013;54(2):268–276. doi: 10.1093/jrr/rrs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han N.-R., Park C.-L., Kim N.-R., Kim H.-Y., Yoou M.-S., Nam S.-Y., et al. Protective effect of porcine placenta in a menopausal ovariectomized mouse. Reproduction. 2015;150(3):173–181. doi: 10.1530/REP-15-0157. [DOI] [PubMed] [Google Scholar]
- 55.Ma K., Yao H., Zhang M., Guo J.-J., Cheng L., Li J.-H., et al. Effect of human placental extract on proliferation of human umbilical cord blood CD34 (+) cells in vitro. Zhongguo shi yan xue ye xue za zhi. 2012;20(5):1183–1186. [PubMed] [Google Scholar]
- 56.Lu J., West M.B., Du X., Cai Q., Ewert D.L., Cheng W., et al. Electrophysiological assessment and pharmacological treatment of blast-induced tinnitus. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0243903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickox A.E., Liberman M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014;111(3):552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jastreboff P.J., Hansen R., Sasaki P.G., Sasaki C.T. Differential uptake of salicylate in serum, cerebrospinal fluid, and perilymph. Arch Otolaryngol Neck Surg. 1986;112(10):1050–1053. doi: 10.1001/archotol.1986.03780100038004. [DOI] [PubMed] [Google Scholar]
- 59.Wang H.-T., Luo B., Zhou K.-Q., Xu T.-L., Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear. Res. 2006;215(1–2):77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Su Y.-Y., Luo B., Wang H.-T., Chen L. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear. Res. 2009;253(1–2):60–66. doi: 10.1016/j.heares.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Basta D., Ernst A. Effects of salicylate on spontaneous activity in inferior colliculus brain slices. Neurosci. Res. 2004;50(2):237–243. doi: 10.1016/j.neures.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Guitton M.J., Caston J., Ruel J., Johnson R.M., Pujol R., Puel J.-L. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J. Neurosci. 2003;23(9):3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abd-Elhakim Y.M., Abdel-Motal S.M., Malhat S.M., Mostafa H.I., Ibrahim W.M., Beheiry R.R., et al. Curcumin attenuates gentamicin and sodium salicylate ototoxic effects by modulating the nuclear factor-kappaB and apoptotic pathways in rats. Environ. Sci. Pollut. Res. 2022;29(60):89954–89968. doi: 10.1007/s11356-022-21932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park S.Y., Phark S., Lee M., Lim J.Y., Sul D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo [a] pyrene-exposed rats. Placenta. 2010;31(10):873–879. doi: 10.1016/j.placenta.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Liu J., Luo S., Yang J., Ren F., Zhao Y., Luo H., et al. The protective effect of sheep placental extract on concanavalin A-induced liver injury in mice. Molecules. 2018;24(1):28. doi: 10.3390/molecules24010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowe A.S., Walton J.P. Alterations in peripheral and central components of the auditory brainstem response: a neural assay of tinnitus. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0117228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X.-P., Chen L. Auditory brainstem response as a possible objective indicator for salicylate-induced tinnitus in rats. Brain Res. 2012;1485:88–94. doi: 10.1016/j.brainres.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 68.Noreña A.J., Farley B.J. Tinnitus-related neural activity: theories of generation, propagation, and centralization. Hear. Res. 2013;295:161–171. doi: 10.1016/j.heares.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Castañeda R., Natarajan S., Jeong S.Y., Hong B.N., Kang T.H. Electrophysiological changes in auditory evoked potentials in rats with salicylate-induced tinnitus. Brain Res. 2019;1715:235–244. doi: 10.1016/j.brainres.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Gerken G.M., Hesse P.S., Wiorkowski J.J. Auditory evoked responses in control subjects and in patients with problem-tinnitus. Hear. Res. 2001;157(1–2):52–64. doi: 10.1016/s0378-5955(01)00277-5. [DOI] [PubMed] [Google Scholar]
- 71.Fabiani M., Sohmer H., Tait C., Bordieri O. Mathematical expression of relationship between auditory brainstem transmission time and age. Dev. Med. Child Neurol. 1984;26(4):461–465. doi: 10.1111/j.1469-8749.1984.tb04472.x. [DOI] [PubMed] [Google Scholar]
- 72.Simpson E.R., MacDonald P.C. Endocrine physiology of the placenta. Annu. Rev. Physiol. 1981;43(1):163–188. doi: 10.1146/annurev.ph.43.030181.001115. [DOI] [PubMed] [Google Scholar]
- 73.Cocchiaro P., Giorgio C., Novelli R., Aramini A., Allegretti M., Brandolini L. The role of neurotrophins in hearing loss and their implications in developing innovative therapies. J Biotechnol Biomed. 2022;5(2):117–136. [Google Scholar]
- 74.Sly D.J., Hampson A.J., Minter R.L., Heffer L.F., Li J., Millard R.E., et al. Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea. J Assoc Res Otolaryngol. 2012;13:1–16. doi: 10.1007/s10162-011-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agterberg M.J.H., Versnel H., De Groot J.C.M.J., Smoorenburg G.F., Albers F.W.J., Klis S.F.L. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened Guinea pigs. Hear. Res. 2008;244(1–2):25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Pinyon J.L., von Jonquieres G., Crawford E.N., Duxbury M., Al Abed A., Lovell N.H., et al. Neurotrophin gene augmentation by electrotransfer to improve cochlear implant hearing outcomes. Hear. Res. 2019;380:137–149. doi: 10.1016/j.heares.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Salvinelli F., Frari V., Rocco M.L., Rosso P., Aloe L. Enhanced presence of NGF and mast cells number in nasal cavity after autologous stimulation: relation with sensorineural hearing deficit. Eur. Rev. Med. Pharmacol. Sci. 2015;19(3):381–391. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article material/referenced in the article.