Abstract
Loss of sweat glands (SwGs) commonly associated with extensive skin defects is a leading cause of hyperthermia and heat stroke. In vivo tissue engineering possesses the potential to take use of the body natural ability to regenerate SwGs, making it more conducive to clinical translation. Despite recent advances in regenerative medicine, reconstructing SwG tissue with the same structure and function as native tissue remains challenging. Elucidating the SwG generation mechanism and developing biomaterials for in vivo tissue engineering is essential for understanding and developing in vivo SwG regenerative strategies. Here, we outline the cell biology associated with functional wound healing and the characteristics of bioactive materials. We critically summarize the recent progress in bioactive material-based cell modulation approaches for in vivo SwG regeneration, including the recruitment of endogenous cells to the skin lesion for SwG regeneration and in vivo cellular reprogramming for SwG regeneration. We discussed the re-establishment of microenvironment via bioactive material-mediated regulators. Besides, we offer promising perspectives for directing in situ SwG regeneration via bioactive material-based cell-free strategy, which is a simple and effective approach to regenerate SwG tissue with both fidelity of structure and function. Finally, we discuss the opportunities and challenges of in vivo SwG regeneration in detail. The molecular mechanisms and cell fate modulation of in vivo SwG regeneration will provide further insights into the regeneration of patient-specific SwGs and the development of potential intervention strategies for gland-derived diseases.
Keywords: Bioactive material, Regeneration, Reprogramming, Sweat gland, Microenvironment
Graphical abstract

Highlights
-
•
Precise control over the inherent and modulable properties of bioactive materials facilitates the in vivo or in situ SwG regeneration.
-
•
Bioactive materials are used for epigenetic modifications and delivery systems to trigger cellular reprogramming for SwG regeneration.
-
•
Bioactive materials can be specifically engineered to modulate microenvironment for innervation, vascularisation and SwG regeneration.
-
•
Bioactive materials contribute to the patient-specific SwG regeneration and intervention strategy development for gland-derived diseases.
Abbreviations
- SwG
sweat gland
- SA
sodium alginate
- BG
bioactive glass
- ECM
extracellular matrix
- TGF-α
transforming growth factor-α
- TGF-β
transforming growth factor-β
- PDGF
platelet-derived growth factor
- H2O2
hydrogen peroxide
- DAMPs
damage-associated molecular patterns
- KGF-1,2
keratinocyte growth factor-1,2
- IGF-1
insulin growth factor-1
- SGLCs
sweat gland-like cells
- EPSCs
epidermal stem cells
- BM-MSC
bone marrow mesenchymal stem cells
- IL
interleukin
- TNF-α
tumor necrosis factor-α
- 3D
three-dimension
- iSGC
induced sweat gland cells
- α-SMA
α-smooth muscle actin
- MMPs
matrix metalloproteinases
- TIMPs
tissue inhibitors of metalloproteinases
- NGF
nerve growth factor
- NT
neurotensin
- α-MSH
α-melanocorticotropin releasing hormone
- CGRP
calcitonin gene-related peptide
- FGF
fibroblast growth factor
- VEGF
vascular endothelial growth factor
- MSCs
mesenchymal stem cells
- MAPS
microporous annealed particle scaffolds
- HA
hyaluronic acid
- IEP
iso-electric point
- Cap
calcium phosphate
- β-TCP
β-tricalcium phosphate
- YAP
yes-associated protein
- WH
whitlockite
- TLR
toll-like receptor
- NF-κB
nuclear factor-κB
- NIR
near-infrared
- EMFs
external magnetic fields
- AMPs
antimicrobial peptides
- SF
silk fibroin
- AaSF
A. assama SF
- BmSF
B. mori SF
- CSMP-PF
chitosan microparticle-pluronic F127
- SDF-1
stromal cell-derived factor 1
- CTH/antimiR-138 NPs
chitosan/tripolyphosphate/hyaluronic acid/antimiRNA-138 nanoparticles
- CS/GP
chitosan/β-sodium glycerol phosphate
- PA
propanoic acid
- KGN
kartogenin
- GelMA
gelatin methacrylate
- TFs
transcription factors
- FOXM1
forkhead box M1
- FOXF1
forkhead box F1
- SNP
supramolecular nanoparticle
- TF·DNA⊂SNPs
TF-encapsulated SNPs
- En-1
Engrailed-1
- EDA
ectodysplasin A
- BMP
bone morphogenetic protein
- SHH
sonic hedgehog
- Foxa1
forkhead box a1
- ECs
epidermal cells
- miRNAs
microRNAs
- siRNA
small interfering RNAs
- RNAi
RNA interference
- BLs
bubble liposomes
- SFP
silk fibroin patch
- CTGF
connective tissue growth factor
- saRNA
self-amplifying mRNA
- CRISPR
clustered regularly interspaced short palindromic repeats
- Cas9
CRISPR-associated protein 9
- dCas9-E
dCas9-effector
- sgRNA
single-guide RNA
- RNPs
ribonucleoprotein complexes
- pDOPA
polyDOPA-melanin
- iPSC
induced pluripotent stem cells
- HDAC
histone deacetylase
- TSA
trichostatin A
- BCP
biphasic calcium phosphate
- Ap-PLGA
apatite-coated poly (lactic-co-glycolic acid)
- PLG
poly (lactic-co-glycolic) acid
- pSmad
(phosphorylated small mother against decapentaplegic)
- P(LLA-CL)
poly(l-lactide-co-caprolactone)
- Fn
fibronectin
- DFO
des-ferrioxamine
- ADSCs
adipose-derived mesenchymal stem cells
- PEG
poly (ethylene glycol)
- CNCs
cellulose nanocrystals
- EMs
exosomes
- HUMSCs
human umbilical cord mesenchymal stem cells
1. Introduction
Sweat glands (SwGs) are coiled tubular skin appendages that derive from embryonic ectoderm and consist of secretory coils and ducts [1,2]. They are responsible for the thermoregulation, fluid, and electrolyte homeostasis of the body [3]. They also harbor stem cells that have the ability to reconstruct both epidermal compartments and SwGs in response to wound healing signals [4]. However, after extensive skin damage, such as full-thickness burns, residual stem cells and progenitors of SwGs exhibit limited regenerative capability [5], partly due to a decrease in functional endogenous stem cells and a hijack of stem cells niche or microenvironment by disruptive signals. Meanwhile, unlike some invertebrates with an extraordinary ability to regenerate functional tissues throughout their lifetime, tissue regeneration is largely limited to gestation in mammals [6]. Patients with large skin defects are vulnerable to scarring healing without regeneration of SwGs. The inability to reconstruct the original tissue structure disrupts skin integrity and functionality, which might dramatically impair the quality of life of survivors [7,8]. For the lack of SwGs even with skin grafts and flap reconstruction, the body may have difficulty excreting sweat, wastes, and toxicants, causing organismal disorders [3]. To achieve functional healing with SwG regeneration, kinds of strategies such as stem cell-based therapies, biomaterial-based gene and biomolecule delivery have been used to modulate the repair process and prepare implantable skin substitutes. Recent advances in SwG regeneration are leveraging the natural potential of tissues to regenerate SwGs with intact structure and function in vivo.
Biomaterials are defined as materials intended to interface with biological systems to evaluate, treat, augment or replace any tissue, organ or function of the body [9]. Bioactive materials represent a new generation of biomaterials, which can induce and conduct responses to biological systems upon interacting [10]. They can be developed to stimulate endogenous cell infiltration, proliferation, and differentiation, as well as release bioactive molecules to elicit specific biological responses for replacing the damaged tissue structurally and functionally [11]. Besides, the negative effects of materials are usually confined to the peri-material area that can be easily detected in preclinical research, rendering them more attractive for tissue reconstruction. Sodium alginate/bioactive glass (SA/BG) composite hydrogel delivered bioactive molecules sequentially into diabetic skin damage to meet the bioactivity requirement of each wound healing stage, achieving inhibition of host inflammation and fibrosis formation, accelerating wound healing and enhancing skin regeneration [12]. In addition to the skin field, bioactive materials are widely used in drug delivery systems, gene therapy, and tissue engineering.
Tissue engineering is an interdisciplinary field that applies the principles of biology and engineering to develop functional biological substitutes to restore, maintain, or improve tissue function [13], which has been adopted for the regeneration of SwGs. Traditional in vitro tissue engineering relies on the in vitro conditioning of the cell-laden constructs used for implantation to produce functional tissues that are equivalent to natural SwGs. However, the ability of in vitro approach to reconstruct in vivo SwG microenvironment is limited. Due to the difficulty of in vitro engineering to maintain cell viability or phenotype, the potential for immune rejection and tumorigenesis [14], in vivo tissue regeneration is becoming increasingly attractive, which takes use of the body's natural capacity for tissue regeneration [15,16]. This process is aided by the exploitation of bioactive materials that can recruit host stem/progenitor cells to the wound site to guide the structural and functional restoration of injured tissues [17]. In this approach, the living body is utilized as an efficient bioreactor that can make full use of in vivo microenvironment to create a more physiological niche for SwG regeneration [18]. Additionally, the approach is relatively simple and limits excessive manipulation of cells in vitro, thus reducing the time and resources for SwG regeneration and facing fewer regulatory hurdles. Therefore, the in vivo SwG regeneration approaches are more conducive than the in vitro approaches to clinical translation. Due to the histocompatibility, effects on gene expression, signaling, and local microenvironments, as well as degradation products of bioactive materials may lead to alterations in vivo microenvironment, in vivo tissue regeneration also require more verification and validation tests to ensure their safety and performance [19].
Cell types, microenvironment cues, properties of bioactive materials and their dynamical interaction are fundamental for perfect skin repair and functional SwG regeneration. This review is concerned with the current advances for in vivo SwG regeneration based on bioactive materials. First, we discuss SwG regeneration in functional wound healing, including biological processes of functional wound healing and the factors affecting the SwG regeneration, and the inherent and modulable properties of bioactive materials. Then, we address the in vivo SwG regeneration based on the bioactive materials. We pay attention to the recruitment of endogenous cells via bioactive materials, the generation of regenerative cells via the delivery of reprogramming factors by bioactive materials, and the reestablishment of microenvironment mediated by bioactive materials. The bioactive materials-driven approaches for in situ SwG regeneration are also discussed. Finally, the opportunities and challenges of bioactive material-based in vivo SwG regeneration are summarized.
2. SwG regeneration in functional wound healing
2.1. Biological processes of functional wound healing with SwG regeneration
Functional wound healing is a complicated and vital process that involves the recovery of skin appendages, including sebaceous glands, hair follicles and SwGs, with the aim of perfectly reconstructing the skin after an injury. From skin damage to SwG regeneration contains four successive but overlapping stages (Fig. 1A): hemostasis, inflammation, proliferation, and remodeling [20]. As the first stage of wound healing and SwG regeneration, hemostasis involves 5- to 10-min vasoconstriction, platelets aggregation, and clotting and complements cascade [21]. Platelets release transforming growth factor-α (TGF-α), transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) [[22], [23], [24], [25]]. Which will trigger a cascade of events for SwG regeneration. Thrombus serves as a provisional framework for the incoming reparative cells [26,27]. Within 24 h after wounding, the inflammatory responses begin to be enhanced, which lays the foundation for SwG regeneration. Hydrogen peroxide (H2O2), calcium waves, damage-associated molecular patterns (DAMPs) and chemotactic molecules initiate the migration of neutrophils towards the wound bed as a first line of defense against infection [23,[28], [29], [30], [31]]. Then, monocytes are recruited to the wound and transform into tissue-activated macrophages to promote SwG regeneration [32], which become the predominant cell population within 48–72 h after injury [21,33]. Later, lymphocytes, Langerhans cells, mast cells, and dendritic cells are involved in the immune responses [34,35]. The proliferative stage is the active period of SwG regeneration, which starts approximately 3–10 days after the injury and is characterized by angiogenesis, granulation tissue formation, collagen deposition, early wound contraction, and re-epithelialization [36]. Angiogenesis peaks on the fifth day of healing, which is a “sprouting” process [21,23]. The establishment of a vascular network enhances the activity of reparative cells, promoting SwG regeneration. Once new blood vessels appear, granulation tissue is formed by fibroblasts to fill the wound area [37,38], which then differentiate into myofibroblasts to bring the wound margins together [39]. Unlike rodents whose wound closure is mainly by construction, 80% of human wounds closure depends on re-epithelialization [40]. Remodeling is essential for SwG regeneration, which takes place on day 21 following the injury and persists for a year or more [36], and is characterized by extracellular matrix (ECM) reorganization, strength increase, cells and blood vessels decrease. Stronger collagen type Ⅰ replaces collagen type III, despite this, the wound strength will only recover to around 80% of the normal skin [34,41]. Once epithelialization is completed, myofibroblasts and remaining cells undergo apoptosis, and newly formed capillaries regress [42,43]. Despite the detailed understanding of the stages of wound healing, the time window for SwG regeneration requires further investigation. Therefore, it is of great significance to gain insight into the factors that influence SwG regeneration during wound healing.
Fig. 1.
The skin wound healing process and the morphology and repair of mouse SwG. (A) The schematic representation of skin wound healing. The healing of skin wounds can be divided into four main stages: hemostasis involving platelet aggregation, inflammation involving immune cells debriding the wound, proliferation involving endogenous cell recruitment, and remodeling of newly formed ECM. (B) The morphology and repair of mouse SwG. (B, Left) Mouse SwG consists of secretory coils deep in the dermis and a relatively straight duct leading to the surface of the skin. The secretion coil contains luminal cells and myoepithelial cells. The sweat duct consists of suprabasal and basal layers. (B, Right) In the epidermal injury, neighboring cells in the sweat duct proliferate and migrate to repair the damaged site, while the gland cells remain quiescent. In glandular injury, neighboring luminal cells proliferate to repair the damaged luminal cells (as shown by the black curved arrow) and neighboring myoepithelial cells proliferate to repair the damaged myoepithelial cells (as shown by the purple curved arrow). ECM, extracellular matrix; PDGF, platelet-derived growth factor; TGF-α,β, transforming growth factor-α,β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; VEGF, vascular endothelial growth factor; α-SMA, α-smooth muscle actin; KGF-1,2, keratinocyte growth factor-1,2; IGF-1, insulin growth factor-1; MMP, matrix metalloproteinases; TIMP, tissue inhibitors of metalloproteinases; DAMPs, damage-associated molecular patterns. Created with BioRender.com.
2.2. The factors affecting the SwG regeneration in wound healing
2.2.1. The effect of reparative cell behavior on SwG regeneration
Skin injuries destroy the tissue structure, leading to gross disruption of the cells and microenvironment, which poses a challenge to SwG regeneration. Synergetic development of various cells, factors leads to the development, repair and regeneration of SwGs. Human SwG formation begins with epidermal buds of pluripotent K14+ progenitors, followed by transient but proliferative K14low/K18+ suprabasal layer of progenitors. These K14+ basal and K14low/K18+ suprabasal ductal progenitors generate myoepithelial and luminal cells of SwGs, respectively [44]. Sweat duct cells are able to continuously proliferate and the luminal layer of the secretory coils has some capacity for self-renewal [45,46], but they are both damaged following injuries, affecting the homeostasis of the SwGs. Although myoepithelial cells remain in a quiescent state in the mature SwGs [47], they retain their pluripotent potential and are able to participate in SwG regeneration after injuries [48]. The multipotent stem cells surrounding the SwG secretory unit are able to differentiate into cycling Lgr6-expressing stem cells after injury to maintain the entire SwG [49]. The human SwG stroma contains Nestin-expressing stem cells, which are capable of multilineage differentiation and potentially involved in SwG regeneration [50]. Therefore, various cells of SwGs function coordinately during development to enable the downward growth of SwG germs to generate SwGs, whereas after injury, some cells that remain relatively quiescent in mature SwGs, such as myoepithelial cells and sweat gland cells, are activated to be involved in the SwG regeneration process. In mouse SwGs, when the epidermis is damaged, the adjacent healthy basal cells of the sweat duct and epidermis proliferate, migrate, and differentiate rapidly to repair the damaged site, while cells in the sweat gland remain quiescent during epidermal wound repair [48]. When gland cells are damaged, nearby healthy cells can be activated for local repair (Fig. 1B).
In addition to tissue-resident cells of SwGs, other cells involved in wound healing are activated to repair the SwGs. The activity of various cells is greatly enhanced during the proliferative phase. Fibroblasts proliferate and differentiate into contractile myofibroblasts to deposit ECM [39,51]. Once myofibroblasts fail to undergo apoptosis, excessive deposition of myofibroblasts may end up with fibrosis or scaring. The fibrotic microenvironment will be detrimental to SwG regeneration. Keratinocytes are modulated by keratinocyte growth factor-1,2 (KGF-1,2) and insulin growth factor-1 (IGF-1) released from cutaneous T cells, contributing to skin re-epithelialization [52,53]. Abnormal behavior of keratinocytes may lead to delayed wound healing, which may result in fibrosis, abnormal repair and consequently affect SwG regeneration. The promising approaches is to convert human epidermal keratinocytes and human dermal fibroblasts into sweat gland-like cells (SGLCs), which subsequently form SwG tissue [54,55]. The approach not only directly facilitates SwG regeneration, but also alleviates fibrosis and scarring, which improves the microenvironment of wound healing that further promotes SwG regeneration. Homing factors are molecular factors that recruit stem cells to the wound site during stem cell homing. Stem cells can respond to gradients of chemo-attractants to be recruited to distant sites under the influence of homing factors to participate in wound healing [14,56]. Therefore, epidermal stem cells (EPSCs), myoepithelial stem cells, hair follicle stem cells, bone marrow mesenchymal stem cells (BM-MSCs) and other stem cells can potentially be recruited to the wound site to participate in SwG regeneration [47,49,50,57,58]. However, under a large area of severe burns, especially full-thickness burns, skin tissues are seriously damaged, causing a shortage of these cells that is insufficient to regenerate the SwGs. Furthermore, Macrophages release pro-inflammatory cytokines to fight against infection, including interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) [59], and recruit other reparative cells in a paracrine manner [40,60]. Macrophage populations are heterogeneous. The transition of macrophage phenotype M1 to M2 contributes to neovascularization and ECM deposition, facilitating the transition from the inflammatory to the proliferative phase [61]. Th2 CD4+ T cell activation is correlated with scarring, involving IL-4, 5 and 13 [23]. Disturbances in the inflammatory responses will lead to chronic non-healing wounds and thus disrupt the process of SwG regeneration [62]. Therefore, supplementing reparative cells with the ability to regenerate the SwGs is one of the most important strategies to achieve SwG regeneration.
2.2.2. The effect of microenvironment state on SwG regeneration
Microenvironment is comprised of mechanical forces, cell contacts, secreted factors, small molecules, substrate or ECM, and three-dimension (3D) architecture [4]. Studies on myoepithelial cells generating different glandular morphologies in different microenvironments demonstrate the significance of microenvironmental cues for SwG regeneration [44]. Because of that, induced sweat gland cells (iSGCs) showed different repair effects in burns with distinct degrees of niche damage [63]. ECM reorganization and fibrosis, angiogenesis and innervation are the primary microenvironmental factors affecting SwG regeneration (Fig. 1B).
2.2.2.1. ECM reorganization and fibrosis
ECM is undergoing cell-mediated reorganization throughout wound healing. ECM is composed of a variety of proteins, including collagen, elastin, and a small number of structural proteins, which contribute to cell signaling, recruitment, and adhesion, as well as tissue anchoring [64]. Dynamic bidirectional interactions between ECM and cells exhibit great significance for wound healing and SwG regeneration. Fibronectin and other ECM protein fragments at the site of injury attract monocytes, which then bind to ECM proteins leading to further breakdown of ECM fragments. This binding also upregulates the production of growth factors, including PDGF-B and TGF-α, affecting the synthesis of ECM components such as proteoglycans [25,65]. Besides, the fibrin-fibronectin provisional substrate functions as a scaffold for cells to adhere and migrate and to be substituted by granulation tissue that is rich in fibronectin, providing a network of vascularization to deposit collagen subsequently [66]. This dynamic change advances the process of SwG regeneration.
Regeneration and fibrosis share a common cascade of injury-inducing events [67]. In the later proliferation stage of wound healing, fibroblasts differentiate into myofibroblasts characterized by the de novo expression of α-smooth muscle actin (α-SMA) via the impact of TGF-β [59]. Excessive myofibroblasts contract and deposit excessive collagen in the dermis, leading to skin fibrosis and scar formation, which disrupts the healing microenvironment and impairs the re-establishment of SwGs. The crosslink and improvement of collagen fiber alignment lead to the remodeling of ECM to increase fibrosis and form a fully matured scar with increased tensile strength and decreased number of cells and blood vessels [30], which is highly detrimental to SwG regeneration. Disruption of the balance between synthesis and degradation of new tissue mediated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) will lead to abnormal healing [21,37,68], affecting SwG regeneration. Besides, inadequate ECM degradation and remodeling owing to imbalanced MMP expression or over-accumulation of ECM due to increased fibroblast and myofibroblast activity will result in hypertrophic scar without SwGs [69].
2.2.2.2. Angiogenesis and innervation
SwGs are innervated by the sympathetic nervous system, mainly cholinergic fibers and a few are adrenergic fibers, which regulate the secretion of sweat [[70], [71], [72]]. Therefore, the ingrowth of the nerves to restore the innervation of the SwGs after an injury is of great value for the regeneration of functional SwGs. Nerve growth factor (NGF) maintains neuronal function and modulates neuroplasticity in wound healing. Keratinocytes are able to release NGF to increase α1-AR expression on peripheral nerve fibers, and catecholamines can increase the migration of keratinocytes by binding to α1-AR [73]. In addition, NGF can lead to neuronal survival via the PI3K/Akt pathway 51 [74], contributing to the maintenance of the peripheral nervous system and the innervation of nascent SwGs. Diabetes is an important factor in chronic wounds and studies have found that the innervation of the SwGs in the dermis is reduced in diabetic patients, with the innervation of cholinergic fibers being even more markedly reduced [75]. Diabetic skin cells lack NGF and other neurotrophic factors, so the ability to induce neurite outgrowth is reduced [76]. Thus, with a lack of nerve-skin interaction, wound healing is delayed and chronic non-healing wounds without SwG regeneration may occur [77]. Recent studies suggest that neuropeptides (such as neurotensin (NT), SP, α-melanocorticotropin releasing hormone (α-MSH) and calcitonin gene-related peptide (CGRP), which act as intercommunication messengers between nerve afferents and skin cells [78]. They are capable of binding to receptors on some skin cells, including fibroblasts, keratinocytes, dermal vascular endothelial cells, Langerhans cells and mast cells, to promote skin repair and regeneration. Thus, there is a dynamic interaction between cytokines, skin cells, and skin nerves that is essential to promote nerve ingrowth to optimize the microenvironment, facilitating SwG regeneration. Modulating the temporal cascade of events in wound healing contributes to the restoration of innervation of the SwGs and thus facilitates SwG reconstruction.
Besides, functional SwG regeneration relies on the restoration of angiogenesis after injury. Angiogenesis is triggered by the release of TGF-β, PDGF and fibroblast growth factor (FGF) from platelets. Vascular endothelial cells line all blood vessels in vivo and interact with matrix adhesive proteins to keep mature vessels in a stable state while remodeling the matrix by sprouting and forming new blood vessels during vascularization or wound repair [79]. During the growth stage of healing, macrophages signal to endothelial cells and activate angiogenesis via releasing PDGF and vascular endothelial growth factor (VEGF). The migration, growth, and angiogenesis capacities are mediated by TNF-α, TGF-β, and VEGF [21]. The vascular network formed in the wound healing transports oxygen and nutrients to the site of SwG regeneration and excretes metabolic waste, accelerating the regeneration of the SwGs.
Angiogenesis, nerve ingrowth, fibrosis and scarring in wound healing affect the microenvironmental state for SwG regeneration. The interaction of various components in the microenvironment, such as cell-cell and cell-ECM, provides an inducible microenvironment for the regeneration of hair follicles, SwGs, and sebaceous glands during the re-epithelialization [23]. Especially, neighboring microenvironments facilitate SwG regeneration after injury via integrated signals relayed to cells involved in wound healing [63]. Once the complex interactions among the microenvironment are disorganized, chronic non-healing wounds will happen, disrupting the SwG regeneration. Based on the understanding of the functional wound healing process, it is expected that bioactive materials can be utilized to supplement endogenous regenerative cells and modulate microenvironmental cues, enhancing in vivo SwG regeneration with structural and functional integrity.
3. Characteristics of bioactive materials for SwG regeneration
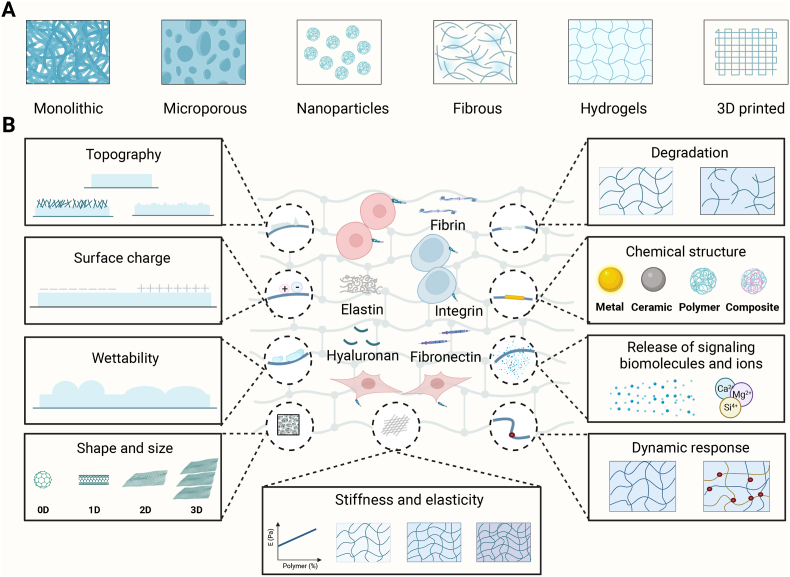
SwG regeneration involves a series of endogenous cells that could perceive and respond to the properties of bioactive materials via transmembrane receptors that include cell-adhesion molecules, integrins and cytoskeleton components. These bioactive materials used for SwG regeneration commonly include monolithic, microporous, nanoparticles, fibrous, hydrogels, and 3D-printed scaffolds (Fig. 2A). Bioactive materials are supposed to react to microenvironmental bio-signals of SwG regeneration and interplay with a range of endogenous cells such as immune cells via inherent and modulable properties (Fig. 2B) to alter the local tissue microenvironment for SwG regeneration.
Fig. 2.
Bioactive materials for in vivo SwG regeneration. (A) Scaffold types. Biomaterials including monolithic, microporous, nanoparticles, fibrous, hydrogels, and 3D-printed scaffolds have been developed to leverage the regenerative potential of the endogenous cells and tissues to regenerate SwGs. (B) Inherent and modulable properties of bioactive materials. Inherent properties include biophysical characteristics such as stiffness and elasticity, surface structure, and degradation, as well as biochemical characteristics such as the chemical structure of biomaterials and the release of signaling biomolecules. Modulable properties can regulate cellular behaviors via dynamic responses to internal and external stimuli. 3D, three-dimension. Created with BioRender.com.
3.1. Inherent properties of bioactive materials for SwG regeneration
3.1.1. Biophysical properties of bioactive materials for SwG regeneration
3.1.1.1. Stiffness and elasticity inspire SwG regeneration
SwGs can be regenerated by stem cells, such as mesenchymal stem cells (MSCs), that can perceive mechanical cues from the microenvironment to promote cytoskeletal re-arrangement. This shows the potential to regulate the differentiation of stem cells via mechanotransduction and thus promote SwG regeneration [14]. Therefore, the stiffness and elasticity of bioactive materials should be well designed to modulate the behavior of cells with the potential to regenerate SwGs and guide their fate determination to promote SwG regeneration. The substrate stiffness affects the adhesion, differentiation, migration and proliferation of stem cells. For example, in soft matrices, MSCs exhibit neurogenic differentiation, intermediate stiffness matrices lead to myogenic differentiation, whereas rigid matrices promote osteogenic differentiation in 2D culture conditions [80]. Similarly, under 3D-bioprinted culture conditions, soft matrices and stiff matrices induce different differentiation states of MSCs [81]. However, these inductive effects of matrix stiffness might be displayed only during the initiation, which may gradually be weakened through the biochemical effects of the culture medium [82]. Therefore, the stiffness of the bioactive materials is supposed to be rationally designed to direct the fate of the stem cells toward the regeneration of SwGs. Besides, substrate stiffness and elasticity affect the migration, proliferation and stratification of keratinocytes to facilitate the restoration of epidermal organization and function [83].
Moreover, SwG regeneration and wound healing are closely linked to the inflammatory response, and thus, the modulation of the inflammatory response by bioactive materials is of great importance to achieve SwG regeneration. Soft substrates are prone to exhibit anti-inflammatory responses, while stiff substrates are prone to pro-inflammatory responses [84]. Especially, macrophages among immunocytes are susceptible to biomaterial stiffness, thereby regulating their adhesion and secretion. For example, human macrophages preferentially adhere to stiffer substrates than soft substrates. But the secretion of TNF-α by adherent human macrophages was inversely proportional to the substrate stiffness: the maximum TNF-α was secreted by cells on 1.4 kPa and the least on 348 kPa [85]. Therefore, inflammatory cells such as macrophages are able to respond to the stiffness of the bioactive materials and optimize the inflammatory response to promote SwG regeneration. Furthermore, it has been found that elastin is a significant functional component of the ECM of the dermis and is important for skin wound repair and SwG regeneration [86]. Therefore, regulating the elasticity of biomaterials may promote the restoration of SwGs by influencing cellular behaviors in wound healing. Tian et al. developed a bioactive elastin-based hydrogel that mimics the skin microenvironment and has a human skin-wide modulus [87]. The hydrogel can recruit immune cells such as M2 macrophages and neutrophils to injury sites, resulting in increased angiogenesis and collagen deposition. Enhanced microcirculation and optimized immune microenvironment provide a favorable background for SwG regeneration. The above studies suggest that rationally designing the stiffness and elasticity of bioactive materials can regenerate SwGs via directing cell lineage commitment and immune response.
3.1.1.2. Surface structure motivate SwG regeneration
One of the key points of SwG regeneration is the recruitment, adhesion and differentiation of endogenous cells, which are influenced by the surface structure of the bioactive materials, including topography, surface charge, wettability, shape and size. For example, the flat surface and the grooved substrate promote different differentiation of MSCs [88], suggesting that modulating the surface structure is of great significance in facilitating SwG regeneration. Besides, the surface topography modification guide the behaviors of endothelial cells, epidermal keratinocytes and dermal fibroblasts and encourage the secretion of growth factors for skin wound healing and SwG regeneration [89]. Hu et al. developed electrospun membranes possessing three different surface topographies (aligned, latticed, and random) and applied them to dorsal skin excisional wounds in mice and rats. The researchers discovered that when an aligned scaffold was present, fibrotic response was reduced and the regeneration of cutaneous appendages was enhanced compared to the other two scaffolds, mainly involving the regulatory effects of T cells on wound healing [90]. Scaffold-mediated cross-talks between cutaneous and immune cells are complex, which have potential applications to the designs and selections of biomaterial for SwG regeneration in clinical settings. However, the mechanisms of modulating the SwG regeneration by immune microenvironments around bioactive materials remain to be further investigated [90].
Cell infiltration and fate determination involved in SwG regeneration are affected by the porosity of the bioactive materials. Micropores can enhance the delivery of nutrients, oxygen, and chemical cues, thus contributing to the differentiation of progenitors and the rate of SwG regeneration, while macropores with several hundreds of micrometers facilitate in vivo regenerative cell migration and neo-vascularization, promoting SwG regeneration [91,92]. Interestingly, porous scaffolds facilitate the inflammatory cell infiltration and blood vessel ingrowth, to create a favorable microenvironment conducive to SwG regeneration. Microporous annealed particle scaffolds (MAPS) are applied to induce macrophage M2 polarization, easing inflammation and transfer to regeneration [93]. Liu and colleagues used microgel-based MAPS of three distinct diameters (40 μm, 70 μm, and 130 μm) to culture primary murine macrophages, founding that the scaffolds with pore size similar to that of cells led to cell morphology and motility-associated changes of macrophages in M1/M2 response. Macrophage polarization towards M2 contributed to the formation of a regenerative microenvironment, which in turn promoted SwG regeneration. MAPS composed of microgels with 130 μm diameter least restricted cell motility characterized by median velocity and maximum travel distance. Increases in the rate of cell migration can modulate the efficiency of SwG regeneration. The study indicated that the spatial constraints caused by the void size within the multi-porous scaffold significantly affected the responses of inflammatory cells in 3D culture. Therefore, appropriate regulation of the porosity of bioactive materials would contribute to the availability of nutrients, vascular networks and a favorable immune microenvironment to enhance SwG regeneration.
Similarly, SwG regeneration is regulated by the surface charge, wettability, shape, and size of the bioactive materials. Surface charge is essential for regenerative cells attached to biomaterials through focal adhesion, especially in the initial phase [94]. Generally, positively charged surface will attract more proteins that influence the attachment of cells and positive charges activate an immune system signaling cascade that enhances tissue regeneration [94]. Thus, bioactive materials loaded with this charge are promising for SwG regeneration. However, some studies about negatively charged biomaterials, such as alginate and hyaluronic acid (HA) showed opposite results: negatively charged surfaces highly activated inflammatory signals, while positively charged surfaces induced lower levels of IL-1β [95]. The founding highlights the necessity of more detailed research to utilize surface charge and biomaterial formulation in improving SwG regeneration outcomes. Besides, the biological process of SwG regeneration involves cell adhesion, which can be achieved by adjusting the surface wettability of bioactive materials, just as platelet adhesion can be reduced by superhydrophobic treatment of vascular stents [96], thus providing an optimized microenvironment for SwG regeneration. Hao and colleagues cultured mouse BM-MSCs using substrates coated with alkanethiol solutions of diverse functional groups, including -OEG, -CH3, -PO3H2, –OH, -NH2, and –COOH, which imparted a wide range of wettability and charge. They found that intermediate wettability and high iso-electric point (IEP) enhanced the adhesion, proliferation and differentiation of mouse BM-MSCs, which is probably related to αv and β1 integrin signaling [97]. Thus, by optimizing the surface wettability of bioactive materials, the behavior of MSCs could be modulated and thus the SwG regeneration process would be enhanced. Because cells of living tissues commonly live in a complex 3D environment, the cultured SwG cells may exhibit limited behaviors in 1D fibrils, and 2D flat surfaces [98]. Therefore, engineered techniques have been improved to reconstruct an in vivo 3D microenvironment for cell infiltration and release growth factors to produce a dynamically organized ECM for ideal cellular behaviors and SwG regeneration outcomes. Recently, Fu and colleagues created a 3D-ECM using 3D bioprinting, which can mimic highly organized biological constructs and stimulate cellular accurate responses to successfully achieve in vitro differentiation of specific cells and in vivo regeneration of functional skin with appendages, including SwGs [99]. However, the fabrication of bioprinted skin with blood vessels and innervation is difficult. Although gelatin hydrogels are commonly employed as bioink, the printed structures contract severely, degrade rapidly and have a limited lifespan [99]. Knowledges from bioengineering, materials science, and cell biology and so forth are needed comprehensively utilize to develop functional 3D bioprinting skin substitutes possessing skin appendages such as SwGs. Overall, the specific design of the surface structure of bioactive materials and the utilization of engineered techniques will advance the process of SwG regeneration.
3.1.1.3. Mechanical performance and degradation stimulate SwG regeneration
In vivo SwG regeneration requires an optimized balance between mechanical properties and biodegradability. The mechanical stability of the scaffold provides a 3D framework for the infiltration of cells involved in SwG regeneration after implantation, and the scaffold should then degrade to enable the ingrowth of neo-SwG tissue. As the degradation of biomaterials, a variety of reparative cells and immunocytes from the site of skin wound infiltrate the scaffold and synergistically promote SwG regeneration. The mechanical properties and biodegradation of wollastonite bio-ceramics are simultaneously improved by precisely controlling dilute concentrations of Mg dopant introduced (Mg/Ca molar ratio: 1.2–2.1%) [100]. The benefits of biocompatibility, corrosion resistance, and bioactivity make biodegradable ceramics attractive for in vivo tissue healing [101]. However, rapid degradation does not provide enough time for newly formed SwG tissue to infiltrate and remodel, while slow degradation prolonged structural support that assists fibrosis to further impair the SwG regeneration process. Therefore, the importance should be the match between degradation rate and neo-tissue formation for structural and functional regeneration of SwGs. Mixing less degradable hydroxyapatite and highly degradable β-tricalcium phosphate (β-TCP), or incorporating other biocompatible Calcium phosphate (Cap) phases give Caps structural stability and degradability at the same time, which would better facilitate tissue regeneration [102]. Hence, the successful manufacture of biomaterials with appropriate biodegradable properties provides insights into the design of bioactive materials with both mechanical properties and biodegradability for enhancing SwG regeneration.
During SwG regeneration, the early stages of stem cell differentiation are mechanosensitive. Biophysical properties such as stiffness of the biomaterial may lead to lineage commitment through mechanotransduction effects, which is possibly mediated by transcriptional co-activator yes-associated protein (YAP) [103]. These effects can further influence the intracellular and intercellular signaling pathways and modulate SwG regeneration signaling networks for the structural and functional integrity of the SwGs. Besides, immune responses are crucial in SwG regeneration, and therefore, dynamic modulation of the biophysical properties of biomaterials to alter the local microenvironment, including the immune microenvironment, and the fate of endogenous cells is of great relevance to promote SwG regeneration. However, the mechanisms by which mechanical cues from bioactive materials influence cell behaviors and the immunoregulatory capacity of bioactive materials require further elucidation.
3.1.2. Biochemical properties of bioactive materials for SwG regeneration
Endogenous cells involved in SwG regeneration are recruited to the wound area under the guidance of biological signals and reconstruct SwG tissues under the influence of the immune microenvironment. Bioactive materials with biochemical properties can affect host immune responses and the recruitment and fate of endogenous cells to facilitate SwG regeneration through the release of signal biomolecules and the degradation of bioactive materials. These biochemical cues can activate specific signal pathways or a set of genes to modulate cell behaviors [19]. For example, sequestering pH-controllable H2S donor, JK1, within biomimetic HA hydrogels induces M2 phenotypic polarization of macrophages in vivo dermal wounds, enhancing angiogenesis and improving wound remodeling effects [104]. Encapsulating cytokines, such as TGF-β1 and IL-10, into polyethylene glycol hydrogels can suppress the maturation of dendritic cells and alleviate the adaptive immune response [105]. These approaches improve the pro-regenerative microenvironment and wound microcirculation, contributing to SwG repair and regeneration. Interestingly, while TGF-β1 can induce scar formation, injection of another isoform of TGF-β, TGF-β3, into incisional wounds is able to accelerate regeneration and reduce post-operative scarring [105]. Inhibition of scar formation would contribute to SwG regeneration. Taken together, these data suggest that bioactive materials incorporating signal molecules can guide and control endogenous cell responses for desirable SwG regeneration. Moreover, the local microenvironment of SwG regeneration can be altered by the degradation by-products of bioactive materials via releasing signaling ions. For example, synthetic whitlockite (WH) nanoparticles can continuously release magnesium and phosphate ions to achieve tissue regeneration through controlling cell differentiation [106,107]. The spatiotemporally controlled release of active ions from BGs during degradation can convert macrophages phenotype from M1 to M2 and alter the secretion of anti-inflammatory and pro-inflammatory cytokines [108], which may be probably related to the toll-like receptor (TLR) pathway and the activation of nuclear factor-κB (NF-κB). This transition towards an anti-inflammatory and pro-regenerative orientation is essential for SwG regeneration. Taken together, bioactive materials could potentially be engineered to encapsulate signal biomolecules or release degradation-by products to modulate endogenous cellular behaviors and the tissue immune microenvironment to further facilitate SwG regeneration.
3.2. Modulable properties of bioactive materials for SwG regeneration
SwG regeneration is a dynamic biological process and therefore dynamic modulation of the properties of biomaterials to participate in the temporal-spatial events of SwG regeneration is of great importance. Properties of some biomaterials can be modulated by environmental stimuli to release target biomolecules or tune the ECM to further control cellular behaviors and immune responses in a user-defined manner for in vivo regeneration. These biomaterials are also called dynamically responsive biomaterials, whose major functions are on-demand release biomolecules and direct cellular responses via modulating properties [19]. The physiochemical characteristics of photo-responsive biomaterials used for SwG regeneration and functional wound healing can be dynamically modulated by exposure to light. Photothermal agents on biomaterials will produce local high temperature under specific wavelengths of light to breakdown the bacterial integrity to fight infection and provide on-demand drug release [109]. Besides, increased blood flow will promote oxygenation, which is highly beneficial for SwG regeneration. For example, in a photosensitive nanoparticle, when irradiated at 310 nm, the drug-carrier bond was irreversibly broken, resulting in drug release [110]. Because the small average size of these nanoparticles is small enough to penetrate cells, it is possible to control intracellular drug release remotely from the outside. However, nano-systems carry an unintended risk of toxicity and their application to SwG regeneration remains challenging. Fortunately, hydrogels have the potential to overcome this obstacle with their tunable mechanical strength, and stability, as well as biocompatibility and biodegradability. Photosensitive hydrogels provide a 3D network to mimic native tissues that is more conducive to SwG regeneration while combining the benefits of photosensitive therapy. A near-infrared (NIR)-responsive hydrogel promotes more angiogenesis and regeneration of skin appendages and less infiltration of inflammatory cells when irradiated by NIR [109]. External electric and magnetic fields can also influence the properties of biomaterials to better interact with the SwG regeneration process. Under the action of external magnetic fields (EMFs), polysaccharide-based magnetic-responsive hydrogels that mimic ECM could improve cell biological activity by increasing cytoskeletal channel activity [111]. Moreover, pH, temperature, pressure, enzymes, and small molecules of the microenvironment can alter the characteristics of biomaterials. A pH-Sensitive HA-based composite hydrogel, with antimicrobial peptides (AMPs) as a cross-linking agent via forming Schiff's base, exhibiting good biostability [112]. This hydrogel showed acidity-triggered on-demand release of loads in specific pathologically acidic environments and accelerated full-thickness wound healing in infected mouse models. As HA is a natural component of skin ECM, HA-based hydrogels can regulate inflammation and promote angiogenesis [113], which is also of great benefit for SwG regeneration. Based on the available studies, the fabrication of bioactive materials with stimuli-responsive capacity to promote SwG regeneration is promising.
Additionally, proper integration of separate responsive biomaterials wound enhance SwG regeneration. For example, the surface of MXene can be functionalized using γ-Methacryloxypropyltrimethoxysilane (KH570) to augment the interfacial compatibility between the temperature-sensitive PNIPAm polymer and conductive MXene nanosheets to further form a novel smart response hydrogel [114]. The hydrogel showed a highly strain-sensitive and can achieve light-controlled drug release at the same time, illustrating a unique approach to functionalize and release loads spatio-temporally. Although the in vivo efficiency of these emerging methods yet to be demonstrated, they have shown considerable potential to facilitate SwG regeneration. More approaches will be integrated to control the wound repair process to accomplish functional healing with SwG regeneration. Inherent and modulable properties of bioactive materials can enhance in vivo SwG regeneration through the alteration of the microenvironment and the modulation of endogenous cell behaviors. However, SwG regeneration involved a serious of timed events: cell proliferation, migration, and differentiation, as well as microenvironment remodeling. Given that, the properties of biomaterials can be precisely designed to interact with the dynamic process of SwG regeneration to improve in vivo SwG regeneration outcomes.
4. Bioactive material-direct cell modulation for SwG regeneration
4.1. Bioactive material-mediated recruitment of reparative cells for SwG regeneration
Bioactive materials facilitate the rapid migration of endogenous cells to the wound site after injury to promote wound healing and in vivo SwG regeneration. Studies have reported some bioactive materials that are able to recruit endogenous regenerative cells to facilitate tissue regeneration. Bioactive hydrogels containing BG and SA are capable of releasing ions, e.g. Ca and Si ions [115]. The hydrogel-induced macrophages to polarize towards M2 phenotype, thereby recruiting reparative cells, including endothelial cells and fibroblasts, by secreting some specific chemokines and cytokines like TGF-β, VEGF, and bFGF. As a result, ECM synthesis and vascularization were enhanced, demonstrating the capacity of bioactive hydrogel to improve microenvironment for SwG regeneration by recruiting endogenous cells. In another approach, silk fibroin (SF) hydrogel was fabricated through blending A. assama SF (AaSF) and B. mori SF (BmSF) [116]. AaSF protein possessed inherent RGD motifs (3 RGD motifs per heavy chain 230 kDa) that could promote the recruitment and migration of cells. Therefore, the hydrogel facilitated the migration of cells residing the edge of the wound, such as keratinocytes, towards the wound bed, enhancing tissue regeneration in full-thickness skin burn wounds. These results demonstrated that bioactive materials can be designed to recruit endogenous regenerative cells to provide an inductive microenvironment for SwG regeneration.
Additionally, designing bioactive materials incorporated with homing factors is promising to recruit endogenous regenerative cells for in vivo SwG regeneration (Table 1). For example, a chitosan microparticle-pluronic F127 (CSMP-PF) hydrogel complex incorporating SP and TGF-β1 increased the density of skin appendages following the wound healing [117]. Since stromal cell-derived factor-1 (SDF-1) is able to be recognized by CXCR4 receptors, SDF-1 can promote stem/progenitor cells expressing CXCR4 to migrate to the site of injury, such as MSCs and EPSCs [118,119]. In a study, an alginate hydrogel patch delivered SDF-1 increased the homing of stem cells, accelerated wound healing, and reduced scarring [120], which is conducive to SwG regeneration. However, developing bioactive materials that can controllably release bioactive factors in a spatiotemporal sequence to facilitate in vivo SwG regeneration is a challenge. To address this challenge, a nanoparticle/hydrogel composite system was developed [121]. In this approach, SDF-1α and chitosan/tripolyphosphate/hyaluronic acid/antimiRNA-138 nanoparticles (CTH/antimiR-138 NPs) were incorporated into chitosan/β-sodium glycerol phosphate (CS/GP) hydrogel. Compared with the blank group, SDF-1α and antimiR-138 were released in a temporal sequence, promoting MSCs migration and tissue regeneration. Similarly, an injectable chitosan/silk fibroin hydrogel system was developed to recruit endogenous MSCs spatiotemporally [122]. The hydrogel was modified with a p-hydroxybenzene propanoic acid (PA) and contained SDF-1 and kartogenin (KGN)-loaded microspheres, which achieved sequential release of both SDF-1 and KGN. Thus, the design approaches of these biomaterials make the sequential release of the loads possible, which has promising applications for SwG regeneration. Besides, a liposome/gelatin methacrylate (GelMA) nanocomposite hydrogel system was developed [123]. SDF-1α was incorporated into anionic liposomes and then further embedded in Type B GelMA hydrogels with negative charges. The nanocomposite hydrogel system controllably released SDF-1α over time at physiologically relevant levels to recruit macrophages with an anti-inflammatory phenotype, inducing a regenerative microenvironment to facilitate skin tissue healing and SwG regeneration. These promising studies demonstrated the feasibility of the strategies that use bioactive materials to recruit endogenous cells for tissue regeneration, which can be potentially used for in vivo SwG regeneration. Further testing and validation are also needed to ensure the safety and efficacy of the strategies for clinical translation.
Table 1.
The homing factors with potential for in vivo regeneration.
| Homing/migration factors | Stem/Progenitor cells | Functions | Study models | Ref. |
|---|---|---|---|---|
| SDF-1 | EPSC | Skin wound healing | Sprague–Dawley rats with 6-mm full-thickness punch biopsy wounds | [118] |
| MSC | Brain tissue repair | Neonatal rats with HIBD | [214] | |
| Bone defect healing | Wistar rats of the BD-IM and BG models | [215] | ||
| Skeletal muscle regeneration | BALB/c mice with injured gastrocnemius muscles | [216] | ||
| Liver injury repair | Sprague-Dawley rats with liver injury | [217] | ||
| Liver injury repair | Sprague-Dawley rats with acute liver injury | [218] | ||
| Myocardial repair | Lewis rats with ischaemic cardiomyopathy | [219] | ||
| ESC | Production of hematopoietic progenitor cells | E14 and CCE ESC lines | [220] | |
| EPC | Skin wound healing | FVB/NJ mice with 8 mm diameter, full thickness, circular skin wounds | [221] | |
| Angiogenesis | C57BL/6 mice with ischemic hind limb | [222] | ||
| HPSC | HPSCs mobilization | Plasminogen (Plg)−/− and MMP-9−/− C57BL/6 J mice | [223] | |
| NPC | Nerve repair | Murine NPCs | [224] | |
| ASC | Angiogenesis | Murine ASCs and C57/BL6 mice with ischemic dorsal soft tissue | [225] | |
| Muscle satellite cell (muscle stem cell) | Skeletal muscle regeneration | WAG rats with injured soleus muscles | [226] | |
| PGC | Guide the migration of PGCs | Zebrafish PGCs and embryos | [227] | |
| SP | MSC | Calvarial defect repair | Mice with calvarial defects | [228] |
| HPSC | Vascularization | Patients and mice with type 2 diabetes | [229] | |
| Proangiogenic progenitor cell | Angiogenesis | Mice with limb ischemia, patients with acute myocardial infarction | [230] | |
| CD29+stromal-like cell | Wound healing | Mice, rats and rabbits, human MSCs | [231] | |
| TGF-β | ASC, MSC, SSC | Chondrogenesis | Primary human ASCs, MSCs, and SSCs | [232] |
| MSC | Cartilage regeneration | New Zealand rabbits with osteochondral defects | [233] | |
| Myocardial injury repair | Mice with heart I/R injury | [234] | ||
| MSCs migration | Human and murine BM-MSCs | [235] | ||
| Wound healing | Balb/C mice with 5 mm full-thickness skin wound | [236] | ||
| HPSC | HPSC homing | LNT-229 glioma cells, HPCs, nude mice | [237] | |
| G-CSF | EPC | Vascular healing | Hypercholesterolemic rabbits with iliac artery injury | [238] |
| HPSC | Mobilize HPSCs into blood | C57BL/6 J mice | [239] | |
| MSC | Mobilize MSCs into peripheral blood | Sprague–Dawley rats | [240] | |
| MCP | MSC | Improve the cardiac function and decrease the myocardial fibrosis | C57/BL6 mouse MSCs and C57/BL6 mice with dilated cardiomyopathy | [241] |
| Galanin | MSC | MSCs homing | Galanin transgenic mice and eGFP transgenic mice | [242] |
| HGF | MSC | Wound healing | Murine MSCs | [243] |
| CSC | Cardiac injury repair | Dogs with MI | [244] | |
| IGF-1 | MSC | Renal injury repair | Mice with AKI | [245] |
| MSCs migration | Lewis rat MSCs | [246] | ||
| PDGF | MSC | Wound healing | GFP+/FVB or Tie2-LacZ+/FVB transgenic mouse MSCs | [247] |
| Cartilage injury repair | Human MSCs | [248] | ||
| bFGF | MSC | MSCs migration | Human MSCs | [249] |
| Cardiac injury repair | Dogs with MI | [250] |
SDF-1, stromal-derived factor 1; EPSC, epidermal stem cell; MSC, mesenchymal stem cell; ESC, embryonic stem cell; EPC, endothelial progenitor cell; HIBD, hypoxic-ischemic brain damage; BD-IM, bone defect-induced mem-brane; BG, bone-graft; HPSC, hematopoietic progenitor and stem cell; NPC, neural progenitor cell; ASC, adipose stromal cell; WAG, Wistar Albino Glaxo; PGC, primordial germ cell; SSC, synovium stem cell; I/R, ischemia/reperfusion; SP, substance P; TGF-β, transforming growth factor β; G-CSF, granulocyte-macrophage colony-stimulating factor; MCP, monocyte chemotactic protein; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; PDGF, platelet-derived growth factor; bFGF, basic fibroblast growth factor; CSC, cardiac stem cell; MI, myocardial infarction; AKI, acute kidney injury.
4.2. Bioactive material-guided cellular reprogramming for SwG regeneration
An alternative approach to supplement endogenous regenerative cells for SwG regeneration is to integrate the bioactive material utilization and cellular reprogramming technology. Cell fate can be altered under appropriate conditions and cues, which can be depicted using the revised Waddington model (Fig. 3A) [124]. Genes exercise rigorous control over the fate of cells. Thus, cellular reprogramming could favor the conversion of cell fate, which can be achieved in vivo through the utilization of a single or several of following six factors (Fig. 3B): (1) lineage-determining transcription factors (TFs); (2) microRNAs (miRNAs) and small interfering RNAs (siRNAs); (3) mRNA; (4) CRISPR-Cas9; (5) epigenetic modifiers; (6) chemical compounds. In this section, we discuss these approaches based on bioactive materials (Table 2) in detail and discuss the current status and development of these techniques as applied to SwG reprogramming.
Fig. 3.
The strategies of in vivo reprogramming for SwG regeneration. (A) The revised Waddington model for cellular reprogramming. Cells can be reprogrammed from one type to others by the expression of pioneer transcription factors. (B) In vivo cellular reprogramming via the delivery of factors. Endogenous cells can be reprogrammed in vivo by using one or several of the following approaches: leveraging TFs, RNAi molecules, mRNA, CRISPR-Cas9, epigenetic modifiers and chemical compounds. TF, transcription factor; siRNA, small interfering RNA; miRNA, microRNA; CRISPR-Cas9, clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9. Created with BioRender.com.
Table 2.
Comparison of bioactive materials-based approaches for in vivo cellular reprogramming.
| Approaches | Bioactive materials | Loading payloads | Targeting cells | Advantages | Disadvantages | Refs |
|---|---|---|---|---|---|---|
| The delivery of TFs | Nanoparticle | FOXM1 or FOXF1 | Alveolar endothelial cells and myofibroblasts |
|
|
[129,130,209,[251], [252], [253], [254], [255], [256], [257]] |
| TF·DNA⊂SNP | OCT4, SOX2, KLF4, and c-MYC | HeLa cells | ||||
| PEG nanoparticle | MYOD1 | Myoblast cells | ||||
| AuNPs | Gata 4, Mef2c, and Tbx5 | Cardiac cell | ||||
| DARTs | Nrf2 | HepG2 cells | ||||
| TNT platform | ABM, EFF | Skin cells | ||||
| Transgenic expression | ASCL1, TSA | Müller glial cell | ||||
| Injection | Transient OCT4, SOX2, KLF4, cMYC | Skeletal muscle cell | ||||
| Electroporation | OLIG2, SOX10 | Neuroblast | ||||
| The delivery of miRNAs and siRNAs | BL | miR-126 | HUVECs |
|
|
[[144], [145], [146],[258], [259], [260], [261], [262], [263], [264], [265]] |
| Nanoparticle | miR-21 mimic | Cardiac macrophages | ||||
| HA-based nanoparticle | miR-223–5p mimic | Macrophages | ||||
| HP-based hydrogel | miR-26a | BM-MSC | ||||
| PLA-DX-PEG hydrogel | siRNA/Noggin | Dorsal muscle pouch | ||||
| PEG hydrogel | Noggin siRNA and miRNA-20a | MSCs | ||||
| Cationic sterosome | Noggin siRNA | MSCs | ||||
| Injection | miR-1, miR-133, miR-208 and miR-499 | Cardiac fibroblasts | ||||
| The delivery of mRNAs | LNP | Chemically modified mRNA | Retinal cells |
|
|
[152,154,266] |
| LNP | Cre mRNA | Hepatic endothelial cells and Kupffer cells | ||||
| The delivery of CRISPR-Cas9 system | Gold nanoparticle | Cas9 protein, guide RNA and donor DNA | Muscle cells |
|
|
[161,[267], [268], [269], [270], [271], [272], [273]] |
| LNP | CRISPR/Cas9 components | Hepatocytes | ||||
| Exosome-liposome hybrid nanoparticle | CRISPR/Cas9 system | MSCs | ||||
| RNP | Purified recombinant Cas9 protein and guide RNA | Human cells (including ESCs and fibroblasts) | ||||
| Lipid-encapsulated gold nanoparticle | Cas9-sgPlk-1 plasmids | Tumor cells | ||||
| Cationic lipid-assisted PEG-b-PLGA nanoparticle | Macrophage-specific promoter-driven Cas9 expression plasmids (pM458 and pM330) | Macrophages | ||||
| Microinjection | Cas9 mRNA and sgRNAs | One-cell-stage embryos | ||||
| The delivery of epigenetic modifiers | Parallel microgrooves | Topographical cues | Cardiac progenitors |
|
|
[170,171,[274], [275], [276], [277], [278]] |
| Parallel microgrooves or nanofibrous scaffolds with aligned fibre orientation | Topographical cues | Fibroblasts | ||||
| Nanograted surfaces with different features size | Topographical cues | ESCs | ||||
| Soft and rigid matrix | Matrix elastic cues | MSCs | ||||
| Stiffness of biomaterials | Mechanical cues | MSCs | ||||
| α calcium sulfate | Sodium butyrate and TSA | Osteoblasts | ||||
| Chitosan-based scaffold | TSA | hPDLCs | ||||
| The delivery of chemical compounds | 3D porous silk fibrous scaffold | CFLSSVY (CHIR99021, Forskolin, LDN193189, SB431542, SP600125, VPA, and Y27632) | Dermal fibroblasts |
|
|
[172,175,177,[279], [280], [281], [282], [283], [284], [285]] |
| Cationic sterosome | Osteoinductive small molecule phenamil and noggin siRNA | MSCs | ||||
| Ap-PLGA scaffold | Phenamil | MSCs | ||||
| PLGA scaffold | Phenamil | MC3T3-E1 cells | ||||
| Collagen sponge | FK506 | Osteocytes | ||||
| Collagen sponge | KM11073 | Osteocytes | ||||
| Injection | CRFVPTM chemical cocktail | Cardiac fibroblast |
FOXM1, forkhead box M1; FOXF1, forkhead box F1; TF·DNA⊂SNPs, TF-encapsulated SNPs; PEG, poly (ethylene glycol); MYOD1, myoblast determination protein 1; AuNPs, cationic gold nanoparticles; DARTs, DNA assembled recombinant transcription factors; Nrf2, nuclear erythroid 2-related factor 2; TNT, tissue nano-transfection; ABM, Ascl1/Brn2/Myt1l; EFF, Etv2, Foxc2 and Fli 1; HUVECs, human umbilical vein endothelial cells; HP, hyaluronan-heparin; PLA-DX-PEG, Poly-D, l-lactic acid with randomly inserted dioxanone and polyethylene glycol; LNP, lipid nanoparticle; miRNAs, microRNAs; siRNA, small interfering RNAs; MSCs, mesenchymal stem cells; CRISPR, clustered regularly interspaced short palindromic repeats; Cas9, CRISPR-associated protein 9; RNP, RNA-guided engineered nuclease ribonucleoprotein; ESCs, embryonic stem cells; PLGA, poly (lactic-co-glycolic acid); TSA, trichostatin A; hPDLCs, primary human periodontal ligament cells; Ap-PLGA, apatite-coated poly (lactic-co-glycolic acid).
4.2.1. Bioactive material-based delivery of lineage-determining transcription factors for SwG reprogramming
The identity of cells involved in SwG regeneration is regulated by gene transcription so that optimizing bioactive material-mediated transcriptional modulation can promote SwG regeneration. TFs are proteins that bind to DNA sequences and in charge of modulating gene transcription [125,126]. The delivery of lineage-determining TFs via biomaterials can modulate cell identity and induce the lineage-specific differentiation for in vivo SwG regeneration. However, the challenge of this approach is to maintain the stability and functionality of these proteins over the delivery process. Traditional approaches commonly use retroviral, lentiviral and adenoviruses as delivery systems for the introduction of TFs into target cells [127]. However, The ability of the vector to integrate raises insertional mutagenicity and oncogenicity risks, leading to concerns about its safety for the utilization in clinical studies [128]. Given that, some alternative strategies are emerging, including nanoparticles, hydrogels, and microspheres. Craig et al. utilized a newly developed polyethylenimine-(5) myristic acid/polyethylene glycol oleic acid/cholesterol (PEI600-MA5/PEG-OA/Cho) nanoparticles were able to effectively deliver pro-angiogenic transcription factor FOXM1 (forkhead box M1) or FOXF1 (forkhead box F1), improving angiogenesis and tissue regeneration via the VEGF/Flk 1 signaling pathway [129]. Thus, delivery of TFs may promote SwG regeneration through vascular niche. Nevertheless, the uptake efficiency of TFs in target cells is low. To address this limitation, Liu et al. formed supramolecular nanoparticles (SNPs) encapsulated with TFs (TF·DNA⊂SNPs) that can deliver intact TFs with better efficiency than existing approaches [130]. In this approach, anion properties were introduced into the TF to facilitate the encapsulation of the TF into a cationic SNP carrier. A DNA plasmid with a TF-specific matching recognition sequence is used for the formation of an anionic TF·DNA complex. This delivery system enhanced the cellular uptake of TFs, exhibiting dramatically improved delivery performance. Previous studies have demonstrated that several TFs, such as Engrailed-1 (En-1), ectodysplasin A (EDA), sonic hedgehog (SHH), WNT, bone morphogenetic protein (BMP), forkhead box a1 (Foxa1), and NF-κB, are closely related to SwG formation [[131], [132], [133], [134]]. Yao et al. further demonstrated that FoxC1 can directly reprogram epidermal cells (ECs) to functional iSGCs with increased efficiency and recovered sweating both in vitro and in vivo, which may relate to the activation of BMP5, WNT10a, NF-κB, SHH, and EDA transcription [135]. Besides, human fibroblasts during wound healing can be transdifferentiated to macrophages, Sertoli cells, neural stem cells, and hair cell lineage via the delivery of TF cocktails [136,137], thereby reducing tissue fibrosis for SwG regeneration. These studies have shown that the use of TFs is vital for scarless wound healing and in vivo SwG regeneration. However, selecting appropriate TFs for cellular reprogramming is challenging due to trial-and-error approaches. The development of genome-scale studies and so forth will contribute to fine outcomes. Furthermore, since the collaborative effects of lineage-determining TFs will be more efficient than a single one, biomaterials need to be crafted to deliver multiple TFs to modulate the SwG regeneration process to achieve desired SwG regeneration outcomes.
4.2.2. Bioactive material-based delivery of miRNAs and siRNAs for SwG reprogramming
SwG regeneration is a biological process regulated by multiple genes, therefore, silencing of specific genes through bioactive materials is vital for modulating SwG regeneration. MiRNAs are classes of non-coding RNAs that bind to mRNA by Watson-Crick base pairing, thereby silencing genes by degradation of the mRNA and repression of translation [138]. SiRNA are double-stranded RNA molecules that are typically used to transiently silence target genes [139]. Intracellularly delivering miRNAs or siRNAs has been used extensively in regenerative medicine, which can silence specific gene expression to modulate various biological processes via RNA interference (RNAi). However, effective delivery of RNAi molecules is difficult because these nucleic-acid biomolecules degrade easily in the circulation due to their susceptibility to nucleases, while the large anionic size limits the entrance into the cytoplasm or nucleus of target cells for nucleic-acid biomolecules [140]. In this regard, viral vectors, dendrimers, liposomes, hydrogels, polymeric nanoparticles, and metal nanoparticles have all been used as RNAi molecule delivery systems to facilitate SwG regeneration.
MiRNA is more vital for SwG regeneration involving plenty of gene disorders due to the ability to target multiple genes compared to siRNA. The target delivery of miRNAs is a bottleneck in developing miRNA-based therapies to achieve SwG regeneration, owing to effects caused by miRNAs that might vary significantly across cell types and pathophysiological settings [[141], [142], [143]]. SwG regeneration involves a wide range of cells, therefore the development of bioactive materials for targeted delivery of miRNA is crucial to obtain the desired SwG regeneration outcomes. Takahashi et al. injected polyethylene glycol-modified liposomes (Bubble liposomes (BLs)) into the body to reach the target tissue under the monitor of ultrasound and then delivered miRNA, which successfully induced angiogenesis factors and improved blood flow [144]. Enhanced angiogenesis facilitates the delivery of loads and oxygen to the site of SwG regeneration, while metabolic waste is removed, thus promoting SwG repair and reconstruction. In recent studies, nanoparticles encapsulated miRNAs to induce polarization of immune cells in a direction that promotes wound regeneration to facilitate in vivo wound healing and SwG regeneration. For example, nanoparticles encapsulated miRNA-21 mimic elicited a switch in macrophage phenotype from pro-inflammatory to reparative [145], and the miRNA-223–5p mimic loaded hydrogels promoted a polarization of macrophages towards M2 phenotype [146]. The transition from the inflammatory phase to the regenerative phase is necessary to achieve SwG regeneration. Besides, Li et al. attached engineered exosomes loaded with miR146a to a silk fibroin patch (SFP), thereby constructing an efficient miRNA delivery system. The wound dressing suppressed inflammation, increased vascularization and re-epithelialization, while promoting the regeneration of skin appendages [147]. These studies demonstrate the potentials for using biomaterials to deliver miRNAs to achieve in vivo skin wound healing and SwG regeneration. The focus should be placed on designing biomaterials loaded with various miRNAs, combined with antibacterial strategies to prevent the growth of drug-resistant microbial infections, thereby achieving scarless wound healing with SwGs.
SiRNA-based therapeutics have opened a novel avenue to improve the repair and regeneration processes of SwGs. Studies have reported that the use of siRNA can knockdown scarring genes, which is certainly attractive for SwG regeneration. However, these approaches use unencapsulated siRNA directly, making it susceptible to rapid degradation and have poor bioavailability [148,149]. Fortunately, Steven et al. reported an ultrathin polymer film coating delivering siRNAs sustainedly into third-degree burn wounds, which can silence connective tissue growth factor (CTGF) and reduce fibrotic responses [150]. This approach encapsulates the siRNAs in an ultrathin coating, avoiding rapid clearance of siRNAs from the target tissue, reducing tissue fibrosis that can provide a favorable microenvironment for SwG regeneration after injury. Based on this understanding of the use of siRNA to suppress the expression of CTGF, a pharmaceutical for skin repair, RXI-109, has been developed and is under evaluation in clinical trials [151]. RXI-109 is able to reduce skin tissue scar formation and alleviate fibrosis, so it could also potentially be used to facilitate SwG regeneration. The above studies suggest that biomaterial-mediated miRNAs/siRNAs-based therapies have great potential in scarless wound healing with SwG regeneration. The approach is simpler than gene editing as miRNA and siRNA are delivered into the cytoplasm via biomaterials. However, employing biomaterials to deliver miRNAs/siRNAs to improve SwG regeneration is still in its early stages, and its clinical application still faces kinds of obstacles that must be resolved, such as the possibility of initiating immune reactions and vector degradation in circulation.
4.2.3. Bioactive material-based delivery of mRNA for SwG reprogramming
mRNA can be delivered into the cytoplasm instead of the nucleus of target cells involved in SwG regeneration by bioactive materials to efficiently express functional proteins. mRNA delivery greatly reduces the risk of integration or insertional mutagenesis, gendering it to be growingly attractive for in vivo SwG regeneration. A range of mRNA carriers are developed, such as polymers, cationic peptides and lipid nanoparticles [152]. Blakney et al. synthesized mannosylated poly (ethylene imine) copolymers to deliver self-amplifying mRNA (saRNA), enhancing saRNA uptake and protein expression in human skin epithelial cells [153]. In another study, lipid nanoparticles containing oxidized cholesterol were used to deliver mRNA to immunocytes with high efficiency [154]. These studies demonstrated the potential of delivering mRNA via biomaterials to regulate cell behaviors involved in wound healing to promote SwG regeneration. However, reaching a good balance between biocompatibility and expression efficiency is a concern. Herein, a pH-responsive DNA nano-hydrogel system is fabricated to deliver mRNA [155]. In this approach, the nano-hydrogel was compacted into a nanosphere via the crosslinking by “X”-shaped DNA scaffolds and DNA linkers to facilitate cell endocytosis, and a pH-responsive i-motif structure was incorporated into the nano-hydrogel for smart release of the mRNA. As a result, this nano-hydrogel system can efficiently deliver and smartly release mRNA. Besides, the system has high protein expression efficiency comparable to commercial liposomes but possesses much better biocompatibility. Thus, this mRNA delivery system is expected to interact with the microenvironment of SwG regeneration and thus promote SwG regeneration. However, mRNA-based therapeutics face challenges of immunogenicity and stability. Fortunately, introducing new chemical modifications into the mRNA holds promise for overcoming these limitations. A modified mRNA encoding VEGF-A (AZD8601) reduced immune responses and increased protein expression [156], which could orient the biological process toward the SwG regeneration. The application of AZD in some diabetic patients has also achieved good results and has been well-tolerated. Fibroblasts-based delivery of synthetic VEGF-modified mRNA improved vascular density in the wound site [157]. It improved vascularization and thus this approach is very attractive for SwG regeneration. These studies illustrated that the in vivo delivery of therapeutic mRNA can stimulate the production of endogenous proteins, providing an idea for the in vivo restoration of SwGs. By constantly overcoming remaining obstacles, such as targeted delivery and long-term control of protein expression levels in cells without a severe immune response, bioactive materials-based mRNA delivery will further facilitate in vivo SwG regeneration.
4.2.4. Bioactive material-based delivery of CRISPR-Cas9 components for SwG reprogramming
SwG regeneration involves a series of genetic events, and therefore bioactive material-mediated gene editing is regarded as another effective means of promoting SwG regeneration. The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system is a powerful in vivo gene editing technology. Due to the merits of the uncomplex design, high efficiency, broad application, and low off-target rate, CRISPR-Cas9 holds promising for facilitating localized gene editing to control cell fate precisely, thus contributing to in vivo SwG regeneration. Viral-based approaches are common methods for both in vitro and in vivo genome editing. Sun and colleagues employed lentiviral-based vectors to deliver CRISPR/dCas9-effector (dCas9-E) and single-guide RNAs (sgRNAs) to transform BM-MSCs into SGLCs by inducing the overexpression of EDA gene [158]. Hematoxylin staining, eosin staining, and immunofluorescence staining showed the formation of sweat ducts and the expression of sweat gland markers (CEA and CK19). Despite their advantages as delivery vectors, virus-based delivery system has limitations of small insertion size and high carcinogenic risk [159]. Alternatively, lipid-based nanoparticles have been used as non-viral delivery systems for the delivery of CRISPR/Cas9 components. The classic ionizable lipid nanoparticle formulations were incorporated with a permanently cationic lipid for efficient delivery of ribonucleoprotein complexes (RNPs) [160]. The supplemental lipid component preserved RNP activity and redirected DNA editing to targeted tissues. And dendrimer lipid nanoparticles modified in this approach can be used to deliver RNPs to Duchenne muscular dystrophy mice to restore dystrophin expression, indicating that this approach is universal to some extent. Therefore, this approach is very promising for SwG repair and regeneration. The in vivo delivery of CRISPR-Cas9 components has also been done using other kinds of synthetic nanoparticles, such as gold-based nanoparticles, polymeric-based nanoparticles and ligand-coupled nanoparticles [161,162]. However, nanoparticle-based delivery strategies face challenges of low efficiency and the possibility of off-target effects. In this regard, biomimicking fiber scaffolds coated with polyDOPA-melanin (pDOPA) were developed for the local and non-viral delivery of Cas9 protein and sgRNA complexes. The pDOPA coating facilitates the adhesion of the CRISPR/Cas9 component to the scaffolds, while the laminin coating helps maintain cell viability and proliferation. These scaffolds eliminated undesirable off-target effects caused by using micro-/nano-particles via injection or systemic delivery while allowing for effective gene editing [163]. The combination of CRISPR/Cas9 systems and effective delivery methods hold significant promise for in vivo SwG regeneration. The discovery and design of new biomaterials and systems will help overcome delivery barriers to facilitate in vivo genome editing, promoting in vivo SwG regeneration.
4.2.5. Bioactive material-based modulation of epigenetic modifiers for SwG reprogramming
SwG regeneration is modulated by epigenetic pathways and the properties of bioactive materials are supposed to be specifically designed to influence the epigenetic state to enhance SwG regeneration. Epigenetic modification modulates the expression of manipulating genes without alteration in the DNA sequence, typically including DNA methylation and histone modification. Biomaterials are already being used for the induction of epigenetic modifications under the catalyzation of enzymes, which are reversible through another group of enzymes [19]. The modulation of epigenetic status via the use of biomaterial-derived cues is considered a promising strategy to modulate the behavior of resident cells for in vivo SwG regeneration.
Biophysical properties of biomaterials, involving stiffness, elasticity, topography, and so forth can affect epigenetic states [164,165]. In a study, increased matrix stiffness reduced DNA methylation in the promoter region of the mechanosensitive YAP, while in situ softening of the matrix restored DNA methylation [166]. In other studies, soft matrix and stiff matrix promoted the differentiation of MSCs to different lineages [80,167]. These studies have shown that the interaction between cells and biomaterials is vital for regulating cell fate. Cell-substrate interactions result in cytoskeletal rearrangement, the forces are transmitted to the nucleus and chromatin through the cytoskeleton [168]. This mechanical conduction from the cell membrane to the nucleus affects the packaging and release of DNA and histones, which in turn affects cell behavior and stem cell differentiation to further influence the SwG regeneration. In addition, biophysical cues affect reprogramming efficiency via the delivery of epigenetic modifiers. Micro-grooved or nanofibrous scaffolds displayed improved cellular reprogramming efficiency over smooth surfaces [169]. This has been attributed to the micro-grooved substrate provoking an increase in histone H3 acetylation, which had been shown to increase the efficiency of reprogramming into induced pluripotent stem cells (iPSCs). Similarly, microgrooves improved the reprogramming efficiency of fibroblasts by inducing histone H3 acetylation via reducing histone deacetylase (HDAC) activity [170]. Hence, properly engineering the biophysical properties of biomaterials can contribute to control the fate of cells during SwG regeneration and improve reprogramming efficiency. Moreover, biochemical cues of biomaterials, such as drugs, are able to modulate cell behaviors for in vivo tissue regeneration via affecting the epigenetic state. Teerawat et al. developed a chitosan-based scaffold incorporated with an epigenetic modifier molecule, trichostatin A (TSA) [171]. Biphasic calcium phosphate (BCP) was added to the scaffold, which improved the mechanical properties and delayed the degradation rate. TSA inhibited HDAC to induce cell differentiation, thereby promoting tissue regeneration. SwG regeneration is controlled by interdependent epigenetic pathways, the ability to regulate the epigenetic landscape with relatively simple biomaterials cues suggests that biomaterials can potentially be utilized to elicit epigenetic modifications to facilitate in vivo SwG regeneration.
4.2.6. Bioactive material-based drug delivery of chemical compounds for SwG reprogramming
Bioactive material-mediated chemical approaches are increasingly attractive for manipulating cell fate to achieve SwG regeneration. Compared with transgenic methods, chemical methods have the advantages of non-integrative to the genome, reversible function, low cost, easier control and standardization [172]. Mouse somatic cells and human skin dermal fibroblasts were successfully reprogrammed into pluripotent stem cells by chemical compounds [173,174]. Thus, fabricating biomaterials loaded with these chemicals is promising for in vivo SwG regeneration by modulating cell fate. Although chemical molecules can be delivered via biomaterials such as ceramic, polymeric carriers, collagen sponge, and apatite-coated poly (lactic-co-glycolic acid) (Ap-PLGA) scaffolds, developing effective delivery strategies for chemical compound remains a challenge [[175], [176], [177]]. The unique properties of gold nanoparticles, such as high surface area, adjustable stability and easy functionalization and fabrication, make them promising carriers for small molecule delivery. For example, gold nanoparticles effectively delivered and released NO in mild acidic environments such as inflammatory and tumor tissues by forming acid labile structures with NO [178]. Thus, this approach holds promise for using the immune microenvironment at the wound site to deliver the loads so as to promote SwG regeneration. However, the elimination and long-term toxicity of metal nanoparticles remain an obstacle to clinical application [179]. Therefore, a systematic and comprehensive construction and screening of the material library is compulsory to optimize the material design. Hydrogels are widely used for the delivery of chemical compounds due to their adjustable physical characteristics and controllable degradability. They can modulate the phenotype, function and fate of cells by spatially and temporally controlling the release of payloads to facilitate SwG regeneration. Reprogrammed stem cells are capable of expressing high-level stemness genes, such as Sox 2, Oct 3/4, and Nanog [180]. Nevertheless, due to the complexities of cellular microenvironments, the long-term efficiency of in vivo treatment of hydrogels remains unknown, which requires further research and review. Accumulating evidence indicates that the fate of the SwGs are vulnerable to a series of signaling pathways, such as EDA, BMP, Wnt. Shh. Ji et al. found that small-molecule cocktails treatment combined with EDA transfection via retroviral vector can directly reprogram human dermal fibroblasts into functional iSGCs expressed SwG-specific markers like CK15, CK18 and in vivo tests showed the ability of full restoration of SwG function [55]. Interestingly, human dermal fibroblasts were directly converted into SwG cells via chemical components without going through the intermediate pluripotent stage. The delivery of chemical molecules using biomaterials represents a promising strategy to modulate endogenous cell fate for in vivo tissue regeneration. With advances in biomaterial-based delivery approaches for chemical molecules and a deeper understanding of the molecular mechanism of SwG regeneration, the cell fate and plasticity of endogenous cells can be better modulated for in vivo SwG regeneration.
5. Bioactive biomaterial-based microenvironmental re-establishment for SwG regeneration
The interaction of biomaterials with the microenvironment provides specific cues for cell behaviors, which can be conducive to promote the optimal regenerative responses of target cells. Herein, we will focus on using biomaterials as regulators of microenvironmental cues for functional wound healing with SwGs.
5.1. Bioactive material-based fibrosis inhibition strategy for SwG regeneration
Bioactive material-based strategies are expected to optimize the microenvironment by modulating myofibroblasts differentiation to reduce tissue fibrosis and scarring, thereby facilitating in vivo SwG regeneration. A novel poly (lactic-co-glycolic) acid (PLG) nanoparticle has been used to regulate TGF-β/phosphorylated small mother against decapentaplegic (pSmad) signal transduction involved in tissue fibrosis [181]. The nanoparticle constitutes a biodegradable, carboxylated, FDA-approved polymer and was negatively charged, which selectively suppressed activated MARCO+ inflammatory monocytes and decrease profibrotic signaling directly to alleviate skin fibrosis, which could potentially promote SwG regeneration. Besides, PLG nanoparticles strongly down-regulated α-SMA generation in the fibroblasts isolated from patients but not from healthy individuals, suggesting safe usage for PLG nanoparticles. Although the inhibition of TGF-β signaling is an effective strategy to mitigate tissue fibrosis for SwG regeneration, TGF-β also exerts essential anti-inflammatory effects in immunomodulatory response. Therefore, the regulation of TGF-β may induce undesirable immune responses. To address this limitation, a small molecule, CBR-096-4, was identified that can overcome the immune response concerns related to long-term inhibition of TGF-β signaling, while inhibiting myofibroblast differentiation [182]. The delivery of CBR-096-4 is promising for optimizing the microenvironment directly in the wound area for in vivo SwG regeneration. Growing evidence indicates that the Wnt/β‐catenin signaling pathway associated with TGF‐β/Smad, Hedgehog, and Hippo fibrotic pathways participates in the formation of skin fibrosis [[183], [184], [185]], which represents a potentially attractive target for biomaterial-based scar-free healing with SwGs. Zhang et al. created a collagen/poly (l-lactide-co-caprolactone) (P (LLA-CL)) scaffold loaded with an inhibitor of Wnt pathway, i.e. ICG-001 [186]. In this approach, P (LLA-CL) and collagen were combined and ICG-001 was loaded into the scaffold by co-axial electrospinning technology, which improved the biocompatibility, mechanical strength and drug release properties of the scaffold. In vitro use of the scaffold reduced the inflammatory responses and inhibited the production of ECM-related proteins to prevent fibrosis, exhibiting application potential for SwG regeneration. However, Wnt signaling has also been shown to have anti-fibrosis and improved wound healing effects [187]. Therefore, further evidence is needed to gain insight into the underlying mechanisms of regenerative and fibrotic responses after skin injury. Emerging evidence indicates that Hippo/YAP signaling is involved in the pathophysiology of skin fibrosis [188]. Studies have shown that YAP-dependent collagen deposition is promoted by matrix stiffness [189], suggesting that modifying biomaterials to modulate the YAP signaling pathway is a promising strategy for alleviating tissue fibrosis and thus promoting SwG regeneration. Biomaterials loaded with YAP inhibitors such as dimethyl fumarate (DMF, Tecfidera®) [190] can also be potentially used for the treatment of fibrosis. Due to the redundancy of the signaling pathways related to fibrosis, the emphasis should be put on developing multi-targeted therapies that moderate skin fibrosis. Further revealing of different profibrotic pathways in skin scar formation and their crosstalk will provide new targets. Advances in biomaterials will help to modulate the fibrosis and scarring process, thereby promoting SwG regeneration. Complementary organotypic cultures and in silico models can be used to elucidate the dynamics that control human regeneration and fibrosis to further facilitate in vivo SwG regeneration [67].
5.2. Bioactive material-based neo-vascularization strategy for SwG regeneration
Bioactive materials can provide a permissive microenvironment for endothelial, pericytes, or vascular smooth muscle cells to attach, grow and migrate, stimulating angiogenesis in vivo or in vitro. The preparation conditions of biomaterials are not stringent, the implantation damage is small, and the performance is adjustable, which allows them to be used as carriers of growth factors, vasoactive or radical scavenging drugs to speed up local vascularization. Besides, the degradation rate of biomaterials can be adjusted to accommodate the vascularization process [191]. Given that, the use of biomaterials to modulate vascular endothelial cells to promote vascularization for in vivo SwG regeneration is a promising strategy.
Modifying surface properties of bioactive materials have been shown to induce the adsorption of proteins and the subsequent adhesion of cells. For example, cells on stiff surfaces formed a more organized cytoskeleton, actin stress fibers, and increased focal adhesion, resulting in higher adhesion strength than on soft surfaces [192]. The results indicate the potential of the approach for promoting the adhesion of vascular endothelial cells for vascularization to facilitate SwG regeneration. Besides, bioactive materials can be loaded with bioactive molecules to regulate vascular endothelial cell behaviors. For example, the in vivo delivery of VEGF, FGF, and PDGF promoted vascularization and increased blood flow [193,194]. Besides, several ECM components, including fibronectin (Fn), type I and Type IV collagen, were used to coat the surface of biomaterials to enhance the adhesion and migration of endothelial cells by interacting with their receptors, such as integrin α5β1, αvβ3, α1β1, α2β1 [192]. These bioactive molecules are expected to be used to modify biomaterials and thus promote SwG regeneration. However, these bioactive molecules have high prices, harsh transport and storage conditions, and short half-life [195]. Alternatively, an angiogenetic drug des-ferrioxamine (DFO) was grafted to a porous sponge via an amide bond, which enhanced the vascularization of the damaged skin by stimulating the secretion of HIF-1α [195]. Interestingly, the regeneration of skin appendages was observed after 28 days of treatment with the sponge in rats with skin injury. Jirigala et al. fabricated MSC-loaded GelMA hydrogels that mimic the microenvironment of SwG regeneration, promoting angiogenesis and SwG formation (Fig. 4) [196]. In another approach, 3D-bioprinted specific ECM microenvironments directed the SwG differentiation of adipose-derived mesenchymal stem cells (ADSCs), then SwG cell spheroids were cultured in angiogenic ECM scaffolds (AES), which enhanced the SwG-vasculature interactions to promote the vascularized glandular morphogenesis in vitro and in vivo (Fig. 5) [197]. These studies demonstrate that biomaterials can enhance vascularization to optimize the regenerative microenvironment for functional SwG regeneration. Although biomaterials containing specific cues (e.g., pro-angiogenesis) are able to enhance vascularization via the recruitment of endogenous cells, the integration of these vascularized structures formed through scaffolds into the vascular network of the host is limited. Fortunately, incorporating specific biochemical cues into 3D-printed structures like adhesion ligands or signaling biomolecules, are able to facilitate the integration with the host blood vessels [19]. Overall, biomaterials provide an artificial network for cells to attach and recruit to further enhance angiogenesis. Focus on biomaterial-based vascularization may supply instructive cues to facilitate the reconstruction of SwGs that more closely resemble the native ones.
Fig. 4.
MSC-loaded GelMA hydrogels with PD for vascularization and SwG regeneration. (A) Schematic illustration of the whole process of in vivo GelMA hydrogel transplantation for SwG regeneration. (B) The shape of 3D-bioprinted constructs (circle, triangle, square) in vitro and in vivo. (C) MSC-loaded GelMA hydrogels combined with PD promote SwG regeneration and vascularization identified with immunofluorescence staining (reprinted with permission from Ref. [196]). GelMA, methacrylated gelatin; MSC, mesenchymal stem cell; PD, SG specific ECM-plantar dermis.
Fig. 5.
Angiogenic ECM scaffolds for vascularized SwG regeneration. (A) 3D-bioprinted ADSC-loaded constructs using PD ECM solution. (B) 3D-bioprinted constructs contribute to SwG differentiation. (C) The macroscopic morphology (optical images) and microstructure (SEM micrographs) of angiogenic ECM scaffolds. (D) Schematic illustration of the in vitro establishment of a biomimetic SwG-vasculature interaction model. (E) Angiogenic ECM scaffolds promote the vascularized SwG regeneration identified with immunofluorescence staining and Sweat test (reprinted with permission from Ref. [197]). ADSC, adipose-derived mesenchymal stem cell.
5.3. Bioactive material-based re-innervation strategy for SwG regeneration
Bioactive materials hold promise for restoring the innervation of SwGs, in which neuropeptides play an important role. Coating the surface of nanoparticles with poly (ethylene glycol) (PEG) can reduce undesired phagocytic clearance and thus prolongs the circulation time [198]. Given that, PLGA nanoparticle modified with PEG was used for the delivery and in vivo sustained release of the CGRP [199]. Notably, a novel biomaterials, PLGA and cellulose nanocrystals (CNCs) (PLGA/CNC) nanofiber membranes was used for the delivery of NTs into the full-thickness cutaneous injuries of spontaneously diabetic mice [200]. Results of the study showed that NT was released from PLGA/CNC composite nanofiber membranes for 2 weeks, which promoted epidermal and dermal regeneration, while also reducing inflammatory cytokines IL-1b and IL-6 expression. The attenuated inflammatory response and enhanced regenerative response provided a favorable microenvironment for SwG regeneration. Besides, topically instilling the COOH-terminal tripeptide α-MSH11-13 (KPV), which is an effective message sequence of α-MSH, has been shown to promote wound healing [201]. These studies indicated the significant potential of biomaterial-based strategies to develop innervation for functional in vivo SwG regeneration via the modulation of neuropeptides. However, scRNA-seq data of human dorsal root ganglia indicated differences in peripheral afferents subsets between the mouse and human [202], which may lead to unexpected side effects during clinical trials. Therefore, the focus should be on using human samples to clarify the underlying molecular mechanisms during wound healing and SwG regeneration and the potential therapeutic targets. Fortunately, the current 3D printed skin model has complex human skin structure, including epidermis, dermis, and subcutaneous tissue [203], providing a favorable research model. And 3D reconstructed SwGs have been demonstrated to be innervated by both cholinergic and adrenergic fibers [204]. Overall, the regeneration of SwGs is a dynamic procedure that involves a number of molecular events. Pioneering research on the restoration of innervation provided insights into accelerating the recovery of SwGs with functional integrity. Optimizing the design of biomaterials to promote innervation could provide a favorable microenvironment to facilitate in vivo regeneration of SwGs with structural and functional integrity.
6. Bioactive material-based cell-free strategy for in situ SwG regeneration
The key to in situ SwG regeneration is to recruit sufficient stem cells with the potential to regenerate SwGs to the wound sites and induce them to differentiate into SwGs (Fig. 6), avoiding the introduction of exogenous cells as far as possible. In addition to inducing the proliferation and differentiation of autologous SwG-derived progenitors into functional glands through wound-healing mechanisms, the recruited stem cells can also be transformed into SwG cells via reprogramming and further develop into functional SwG tissues, providing a novel strategy for in situ SwG regeneration. By avoiding the necessity to remove cells from patients and culture them in vitro, in situ regeneration prevents the impairment of cellular function and/or the delivery of unintended signals. As the recovery of SwGs is highly dependent on their local microenvironment, the properties of bioactive materials can be designed to direct and modulate cell behaviors via microenvironment cues to reduce scar formation and reconstruct blood vessels and nerves for functional wound recovery. Heparinized-decellularized adipose tissue hydrogel incorporated with VEGF increases collagen deposition and new blood vessels formation of skin wound, significantly accelerating wound healing compared with the other groups [193], which also provided a favorable microenvironment for SwG regeneration. To further facilitate SwG regeneration, components containing a specific microenvironment of SwGs were added to the hydrogel, which promoted the differentiation of SwG cells and the formation of SwGs [196]. Biomimetic exosomes (EMs) enriched TGF-β1 were obtained from human umbilical cord mesenchymal stem cells (HUMSCs), which endowed the epidermal keratinocytes with stem cell-like properties and enhanced the migration of keratinocytes to accelerate the in situ restoration of SwGs after injury (Fig. 7) [205]. Engineered scaffolds have also been shown to promote in situ vascularization, facilitating differentiation of the SwG lineage by optimizing the microenvironment [197]. The fabrication of a vascularized microenvironment enhances the interaction between SwGs and blood vessels thereby enhancing SwG regeneration. Moreover, dynamical-responsive bioactive materials are capable of responding to internal and external stimuli, such as pressure, temperature, pH, enzymes, electricity, and magnetism. Future improvement of the dynamical changes of biomaterial structures will control cellular behaviors by regulating biophysical and biochemical characteristics on demand and sustainable and spatio-temporal release of biomolecules to facilitate in situ SwG regeneration. Biomaterials that can form a pore to recruit endogenous cells and control the migration, proliferation and differentiation of endogenous stem/progenitor cells by controlling the pore formation rate will better fit the cellular morphologies and behaviors during SwG regeneration to accelerate in situ SwG regeneration.
Fig. 6.
The recruitment of endogenous cells for in situ SwG regeneration. Recruitment of endogenous cells for in situ SwG regeneration. Specific bio-signaling cues, such as cytokines, can be loaded onto bioactive materials and continuously released in vivo to recruit endogenous stem/progenitor cells or endogenous immune cells and further promote their proliferation and differentiation, thus facilitating wound healing and regeneration. Created with BioRender.com.
Fig. 7.
Biomimetic EMs for functional SwG restoration. (A) Schematic illustration of the development of bioactive EMs for SwG restoration. (B) Characterization of EMs. (C) EMs endow the epidermal keratinocytes with stem cell-like properties. (D) EMs facilitate the restoration of SwG function identified with Starch-iodine sweat tests and H&E staining (reprinted with permission from Ref. [205]). EMs, exosomes; TEM, transmission electron microscopy.
7. Summary and outlook
As an important appendage of the skin, which is the largest organ of the body, SwGs are of great significance to maintain skin homeostasis and physiological function. The feasibility and possibility of clinical application of SwG regeneration have always been a concern. The understanding of SwG stem/progenitor cell lineages has been considerably improved by identifying the specific molecular and cellular components required for SwG formation. These stem/progenitor cells can be recruited to the wound site under the impact of specific signal biomolecules to facilitate in vivo SwG regeneration. Emerged evidence has suggested that cell fate is not immutable and can be reprogrammed directly, which supports the hypothesis that in vivo SwG regeneration can be achieved via in vivo reprogramming, and delivering reprogramming factors through bioactive materials may pave the way for the needed breakthroughs. Further molecular biological studies of functional wound healing indicated that the interactions among microenvironmental components closely modulate SwG regeneration, involving various cells, signaling molecules, ECMs. This novel finding provides insight into activating the SwG regenerative response during the injury-induced regenerative response. The considerable progress of various regeneration strategies provides encouraging evidence that in vivo regeneration of SwG is feasible. Clinical translation of in vivo SwG regeneration strategies is promising to solve diseases associated with dyshidrosis. Given that, in vivo SwG regeneration approaches will be boomed within the next decade.
Research on the proliferative potential within the sweat ducts and glands dates back to the early 1950s. Subsequent studies have concentrated on further exploration of the identification, characterization, and regenerative potential of SwG stem/progenitor cells. As a component involved in tissue homeostasis, SwG stem cells may exhibit benefits in delaying skin aging. They would contribute to epidermal repair and reduce scarring. However, the composition, function, and distribution of SwG stem/progenitor cell populations are heterogeneous, so techniques such as the identification of cell-specific markers and microenvironment-response cues are needed to facilitate the transition from the laboratory to the clinic. Natural or synthetic biomaterials can be incorporated signaling biomolecules to provoke endogenous stem/progenitor cells homing through immune-mediated or contact-guided pathways, and further induce the proliferation and differentiation of these cells for in situ regeneration of SwGs. The approach directly leverages the endogenous cells of patients, opening up opportunities for patient-individualized therapy. Especially, pluripotent glandular stem cells can trigger critical wound healing mechanisms that are missing in chronic or burn wounds, rendering them potential pharmacological targets for in vivo SwG regeneration. Recent studies have revealed that ECM-derived biomaterials prepared by decellularization of mammalian tissues are essential for the homing of endogenous cells [206]. However, decellularization approaches require a balance between the maintenance of the native ECM structure and the removal of cellular components like mitochondria, DNA, and membrane lipids. A combination of many variables such as matrix density, thickness, and morphology will serve to produce an ideal decellularized scaffold to facilitate in vivo SwG regeneration. A further understanding of the characteristics of bioactive materials and the heterogeneity and roles of SwG stem cells during SwG development and homeostasis will provide insights into the cues of the preparation of more complex tissue-engineered skin models. Reconstructed skin models that introduce skin appendages will better serve as an alternative for in vivo studies. Besides, the bioactive materials-based regulatory mechanisms of SwG stem cell fate may to some extent establish a paradigm for other glandular stem cells, including mammary glands, pancreas, and salivary glands. Improved knowledge of biomaterial-mediated glandular stem cell homeostasis dynamics and post-injury molecular biology is essential for the treatment of gland-derived diseases, including cancers, via biomaterials.
Cellular fate and identity are tightly controlled by a sequence of precisely timed events affecting the activation or suppression of genes. Cell fate reprogramming represents a highly attractive approach for tissue regeneration, particularly for diseased tissues with a large number of cells in terminal differentiation or lacking proliferation. The pioneering study demonstrated the power of cellular reprogramming and the therapeutic potential of direct reprogramming [207,208]. Biomaterials have been available for various in vivo reprogramming approaches to elicit the modification of genes or epigenetics. Major existing approaches based on biomaterials include the delivery of TFs, RNAs, Cas9 system, and chemical components, as well as epigenetic modification via the biophysical or biochemical cues of biomaterials, which can be used to precisely modulate gene expression profiles for in vivo SwG regeneration. Nanoparticle-based platforms are commonly used for in vivo cellular reprogramming. Negatively charged nucleic acids are often loaded to cationic gold nanoparticles to translocate across the negatively charged cell membranes to improve the delivery efficiency [209]. However, off-target tissue uptake limits the in vivo gene modulation capability of cationic carriers [210]. Fortunately, modification of nanoparticles by molecules such as PEG reduces the off-target tissue uptake and increases the circulating time by reducing aggregation and clearance by the immune system, thereby facilitating in vivo cellular reprogramming. To further ensure the targeting of nanocarriers, sequential targeted nanoparticles have been developed to precisely deliver the payloads to target cells at the site of injury using a biomimetic strategy to enable safe and effective in vivo reprogramming [211]. Additionally, future delivery platforms for SwG regeneration must be non-toxic, well-pharmacokinetic, and have “stealth” capabilities to avoid immune system responses [140]. Since the 3D microenvironment is more conducive to the modulation of cell behaviors, hydrogels have been introduced into cellular reprogramming. Nevertheless, little is known about the precise molecular mechanisms of the reprogramming. The advance of single-cell omics technology and machine learning may provide the opportunity to better understand the conversion of cell fate [212]. Reprogramming factors for desired lineages can be predicted and then experimentally verified via interdisciplinary cooperation, including computational modeling and CRISPR-Cas9 screening [213], thus facilitating in vivo reprogramming for in vivo SwG regeneration. The inefficiency of in vivo reprogramming and the immaturity and heterogeneity of reprogrammed cells also require consideration to accomplish the desired effect for clinical treatment. Besides, delivery systems need to be developed or optimized for favorable in vivo reprogramming outcomes. The development of targeting and controlled release technologies would help to reduce the risk of unintended ectopic reprogramming caused by the local delivery of cell-specific cues to target cells. Microfluidic platforms may facilitate the incorporation of biophysical and biochemical cues into delivery systems to promote SwG regeneration. 3D printing is holding considerable promise toward generating complex 3D niches for cellular reprogramming to enhance the performance of reprogramming in vitro and in vivo. An emerging area of biomaterials is to design stimuli-responsive and smart delivery systems for efficiently delivering reprogramming factors in situ in a target-oriented manner. Overview, the comprehensive utilization of biochemical and biophysical cues during the cellular reprogramming process will speed up the reprogramming technology translation to facilitate the development of clinical therapies for in vivo SwG regeneration.
Moreover, the microenvironment is of considerable importance for cell-fate manipulation to facilitate in vivo SwG regeneration. Bioactive biomaterials elicit a specific biological response and are able to interact with the microenvironment. Once implanted, bioactive materials interact with cells to modulate the local microenvironment to direct endogenous cell behaviors via direct-contact guidance or immune-mediated pathways for in vivo SwG regeneration. The biophysical and biochemical properties of bioactive materials are crucial to reconstruct a suitable microenvironment, which is scar-free and has favorable circulation and innervation to support cellular adhesion, proliferation, and differentiation for functional SwG recovery. Importantly, responsive biomaterials can on-demand modulate cell behaviors and synchronize the release of signaling biomolecules with the continuing phases of the morphogenesis and involution of SwGs. Future design of bioactive materials for SwG regeneration should focus on the integration of the independent and different responsive properties into the same system. The development of new approaches to control the dynamic scaffold-tissue interfaces is essential for in vivo SwG regeneration. Due to the need for in situ regeneration for biomimetic matrix systems based on the composition and structure of the native tissue, the future design of biomaterials could consider additive-manufacturing approaches that take into account the actual complex nature of ECM and the environment necessary for regeneration. Especially 4D printing, which involves the production of 3D objects with specific shapes while applying external stimuli like temperature, pH, electric fields, and magnetic fields to cause a transformation of the materials for regeneration [18]. In addition, although the biocompatibility and degradability of bioactive materials have been improved, precisely designing biomaterials to adapt to specific injury sites and to meet the personalized needs of specific patients and injuries are also challenging. The development of future biomaterials is expected to concentrate on the integration of different and separate properties into the same system to fabricate a more complex system to exploit innate regenerative capacities of the injured skin tissue for functional wound healing with the regeneration of skin appendages. Furthermore, collective efforts will contribute to further advances, including minimally invasive delivery, omics-based approaches, and mineral-based biomaterials [19]. Based on the promising results of these preclinical studies, further scaled-up research of these therapeutic strategies and the development of predictive modeling simulating clinical settings to ensure the safety and efficacy of these therapies prior to their use in clinical treatment are needed to be focused on in the future. Advances and integration of regenerative medicine and tissue engineering will offer these interdisciplinary frontiers inspiring opportunities for both research and translation. It is reasonable to expect that the development and optimization of biomaterials will enhance the outcome of in vivo SwG regeneration and accelerate its clinical translation.
Author Contributions
X.Y., M.X., X.F., and X.S.: the conceptualization and design of this study. X.Y. and M.X.: the investigation and methodology of this study. X.Y. and M.X.: writing – original draft. X.Y. and M.X.: Figures and tables of the manuscript. X.Y., M.X., X.F., and X.S.: writing – review & editing of the manuscript. X.F., and X.S.: the funding acquisition. All authors have read and approved the final manuscript.
Declaration of competing Interest
The authors declare no competing interests.
Ethics approval and consent to participate
This review is not concerned with ethical issues.
Acknowledgements
This work was supported in part by the National Nature Science Foundation of China [92268206, 81830064]; the CAMS Innovation Fund for Medical Sciences [CIFMS, 2019-I2M-5–059]; the Military Medical Research Projects [145AKJ260015000X; 2022-JCJQ-ZB-09600, 2020-JCJQ-ZD-256-021]; the Military Medical Research and Development Projects [AWS17J005, 2019–126]; and the Specific Research Fund of The Innovation Platform for Academicians of Hainan Province [YSPTZX202317].
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Xiaobing Fu, Email: fuxiaobing@vip.sina.com.
Xiaoyan Sun, Email: yanzisun1979@sina.com.
References
- 1.Cui C.-Y., Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp. Dermatol. 2015;24:644–650. doi: 10.1111/exd.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev. Physiol. Biochem. Pharmacol. 1977;79:51–131. doi: 10.1007/BFb0037089. [DOI] [PubMed] [Google Scholar]
- 3.Lb B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temp. Austin Tex. 2019;6 doi: 10.1080/23328940.2019.1632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R., Zhu Z., Ji S., Geng Z., Hou Q., Sun X., Fu X. Sweat gland regeneration: current strategies and future opportunities. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120201. [DOI] [PubMed] [Google Scholar]
- 5.Lu C.P., Polak L., Rocha A.S., Pasolli H.A., Chen S.-C., Sharma N., Blanpain C., Fuchs E. Identification of stem cell populations in sweat glands and ducts: roles in homeostasis and wound repair. Cell. 2012;150:136. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konieczny P., Naik S. Healing without scarring. Science. 2021;372:346–347. doi: 10.1126/science.abi5770. [DOI] [PubMed] [Google Scholar]
- 7.O B., G S.O., P M., U M. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006;297 doi: 10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 8.M M., S K., S M., E J. Regenerative scar-free skin wound healing. Tissue Eng., Part B. 2019;25 doi: 10.1089/ten.TEB.2018.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John A.A., Subramanian A.P., Vellayappan M.V., Balaji A., Jaganathan S.K., Mohandas H., Paramalinggam T., Supriyanto E., Yusof M. Review: physico-chemical modification as a versatile strategy for the biocompatibility enhancement of biomaterials. RSC Adv. 2015;5:39232–39244. doi: 10.1039/C5RA03018H. [DOI] [Google Scholar]
- 10.Zhao X. In: Bioact. Mater. Med. Zhao X., Courtney J.M., Qian H., editors. Woodhead Publishing; 2011. 1 - introduction to bioactive materials in medicine; pp. 1–13. [DOI] [Google Scholar]
- 11.Chandra P., Yoo J.J., Lee S.J. In: Transl. Regen. Med. Atala A., Allickson J.G., editors. Academic Press; Boston: 2015. Chapter 13 - biomaterials in regenerative medicine: challenges in technology transfer from science to process development; pp. 151–167. [DOI] [Google Scholar]
- 12.Ma Z., Song W., He Y., Li H. Multilayer injectable hydrogel system sequentially delivers bioactive substances for each wound healing stage. ACS Appl. Mater. Interfaces. 2020;12:29787–29806. doi: 10.1021/acsami.0c06360. [DOI] [PubMed] [Google Scholar]
- 13.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 14.Safina I., Embree M.C. Biomaterials for recruiting and activating endogenous stem cells in situ tissue regeneration. Acta Biomater. 2022;143:26–38. doi: 10.1016/j.actbio.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.J., Yoo J.J., Atala A. In: Situ Tissue Regen. Lee S.J., Yoo J.J., Atala A., editors. Academic Press; Boston: 2016. Chapter 1 - fundamentals of in situ tissue regeneration; pp. 3–17. [DOI] [Google Scholar]
- 16.McCullen S.D., Chow A.G., Stevens M.M. In vivo tissue engineering of musculoskeletal tissues. Curr. Opin. Biotechnol. 2011;22:715–720. doi: 10.1016/j.copbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Li Q., Ma L., Gao C. Biomaterials for in situ tissue regeneration: development and perspectives. J. Mater. Chem. B. 2015;3:8921–8938. doi: 10.1039/c5tb01863c. [DOI] [PubMed] [Google Scholar]
- 18.S A., Gr M. Biomaterials for in situ tissue regeneration: a review. Biomolecules. 2019;9 doi: 10.3390/biom9110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5:686–705. doi: 10.1038/s41578-020-0209-x. [DOI] [Google Scholar]
- 20.Le L., O S., I P., M T.C. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 2016;138 doi: 10.1097/PRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uluer E.T., Vatansever H.S., Kurt F.Ő. In: Wound Heal. Turksen K., editor. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2017. Wound healing and microenvironment; pp. 67–77. [DOI] [Google Scholar]
- 22.Nieswandt B., Varga-szabo D., Elvers M. Integrins in platelet activation. J. Thromb. Haemostasis. 2009;7:206–209. doi: 10.1111/j.1538-7836.2009.03370.x. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound healing: a cellular perspective. Physiol. Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adib Y., Bensussan A., Michel L. Cutaneous wound healing: a review about innate immune response and current therapeutic applications. Mediat. Inflamm. 2022:2022. doi: 10.1155/2022/5344085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz G.S., Davidson J.M., Kirsner R.S., Bornstein P., Herman I.M. Dynamic reciprocity in the wound microenvironment, wound repair regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sm A., Fd F., Se H., K E., Rz M. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Mol. Basel Switz. 2021;26 doi: 10.3390/molecules26164917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soliman A.M., Barreda D.R. Acute inflammation in tissue healing. Int. J. Mol. Sci. 2022;24:641. doi: 10.3390/ijms24010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dovi J.V., Szpaderska A.M., DiPietro L.A. Neutrophil function in the healing wound: adding insult to injury? Thromb. Haemostasis. 2004;92:275–280. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 29.Gillitzer R., Goebeler M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 30.Nc N., Dm M., R M., As P. Cutaneous innervation in impaired diabetic wound healing. Transl. Res. J. Lab. Clin. Med. 2021;236 doi: 10.1016/j.trsl.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 32.Tsirogianni A.K., Moutsopoulos N.M., Moutsopoulos H.M. Wound healing: immunological aspects. Injury. 2006;37(Suppl 1):S5–S12. doi: 10.1016/j.injury.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Portou M.J., Baker D., Abraham D., Tsui J. The innate immune system, toll-like receptors and dermal wound healing: a review. Vasc. Pharmacol. 2015;71:31–36. doi: 10.1016/j.vph.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Velnar T., Bailey T., Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 35.Jameson J.M., Cauvi G., Witherden D.A., Havran W.L. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J. Immunol. Baltim. Md 1950. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 36.Reinke J.M., Sorg H. Wound repair and regeneration. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 37.Kangal M.K.O., Regan J.-P. StatPearls Publishing; 2022. Wound Healing.https://www.ncbi.nlm.nih.gov/books/NBK535406/ [PubMed] [Google Scholar]
- 38.Young A., McNaught C.-E. The physiology of wound healing. Surg. Oxf. 2011;29:475–479. doi: 10.1016/j.mpsur.2011.06.011. [DOI] [Google Scholar]
- 39.P B. Wound healing and the role of fibroblasts. J. Wound Care. 2013;22 doi: 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- 40.H S., Dj T., S H., J H., U M. Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2017;58 doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- 41.Broughton G., Janis J.E., Attinger C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 42.A D., M R., I D., G G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995;146 https://pubmed.ncbi.nlm.nih.gov/7856739/ [PMC free article] [PubMed] [Google Scholar]
- 43.Greenhalgh D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998;30:1019–1030. doi: 10.1016/s1357-2725(98)00058-2. [DOI] [PubMed] [Google Scholar]
- 44.Cp L., L P., As R., Ha P., Sc C., N S., C B., E F. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150 doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lobitz W.C., Holyoke J.B., Montagna W. The epidermal eccrine sweat duct unit; a morphologic and biologic entity. J. Invest. Dermatol. 1954;22:157–158. doi: 10.1038/jid.1954.20. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto Y., Saga K. Proliferating cells in human eccrine and apocrine sweat glands. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1995;43:1217–1221. doi: 10.1177/43.12.8537637. [DOI] [PubMed] [Google Scholar]
- 47.M B., C K. Heterogeneity of sweat gland stem cells. Adv. Exp. Med. Biol. 2019;1169 doi: 10.1007/978-3-030-24108-7_3. [DOI] [PubMed] [Google Scholar]
- 48.Lu C., Fuchs E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb. Perspect. Med. 2014;4:a015222. doi: 10.1101/cshperspect.a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagel S., Rohr F., Weber C., Kier J., Siemers F., Kruse C., Danner S., Brandenburger M., Matthiessen A.E. Multipotent nestin-positive stem cells reside in the stroma of human eccrine and apocrine sweat glands and can be propagated robustly in vitro. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danner S., Kremer M., Petschnik A.E., Nagel S., Zhang Z., Hopfner U., Reckhenrich A.K., Weber C., Schenck T.L., Becker T., Kruse C., Machens H.-G., Egaña J.T. The use of human sweat gland-derived stem cells for enhancing vascularization during dermal regeneration. J. Invest. Dermatol. 2012;132:1707–1716. doi: 10.1038/jid.2012.31. [DOI] [PubMed] [Google Scholar]
- 51.Hata S., Okamura K., Hatta M., Ishikawa H., Yamazaki J. Proteolytic and non-proteolytic activation of keratinocyte-derived latent TGF-β1 induces fibroblast differentiation in a wound-healing model using rat skin. J. Pharmacol. Sci. 2014;124:230–243. doi: 10.1254/jphs.13209fp. [DOI] [PubMed] [Google Scholar]
- 52.Gantwerker E.A., Hom D.B. Skin: histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011;19:441–453. doi: 10.1016/j.fsc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Havran W.L., Jameson J.M. Epidermal T cells and wound healing. J. Immunol. Baltim. Md 1950. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shikiji T., Minami M., Inoue T., Hirose K., Oura H., Arase S. Keratinocytes can differentiate into eccrine sweat ducts in vitro: involvement of epidermal growth factor and fetal bovine serum. J. Dermatol. Sci. 2003;33:141–150. doi: 10.1016/j.jdermsci.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Ji S.-F., Zhou L.-X., Sun Z.-F., Xiang J.-B., Cui S.-Y., Li Y., Chen H.-T., Liu Y.-Q., Gao H.-H., Fu X.-B., Sun X.-Y. Small molecules facilitate single factor-mediated sweat gland cell reprogramming. Mil. Med. Res. 2022;9:13. doi: 10.1186/s40779-022-00372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ik K., Sj L., A A., Jj Y. In situ tissue regeneration through host stem cell recruitment. Exp. Mol. Med. 2013;45 doi: 10.1038/emm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Liu Z.-Y., Zhao Q., Sun T.-Z., Ma K., Fu X.-B. Future application of hair follicle stem cells: capable in differentiation into sweat gland cells. Chin. Med. J. (Engl.). 2013;126:3545–3552. [PubMed] [Google Scholar]
- 58.Chen Y., Li Q., Tan Z., Zhang C., Fu X. MicroRNA-mediated regulation of BM-MSCs differentiation into sweat gland-like cells: targeting NF-κB. J. Mol. Histol. 2019;50:155–166. doi: 10.1007/s10735-019-09814-2. [DOI] [PubMed] [Google Scholar]
- 59.A D., M S., S D., M A., S C., Ck S., S R. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015;185 doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Je P., A B. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004;187 doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 61.Sy K., Mg N. Macrophages in wound healing: activation and plasticity. Immunol. Cell Biol. 2019;97 doi: 10.1111/imcb.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falanga V., Isseroff R.R., Soulika A.M., Romanelli M., Margolis D., Kapp S., Granick M., Harding K. Chronic wounds. Nat. Rev. Dis. Prim. 2022;8:50. doi: 10.1038/s41572-022-00377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.B Y., W S., Z L., T H., R W., Y W., S H., X F. Irf6 directs glandular lineage differentiation of epidermal progenitors and promotes limited sweat gland regeneration in a mouse burn model. Stem Cell Res. Ther. 2018;9 doi: 10.1186/s13287-018-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diller R.B., Tabor A.J. The role of the extracellular matrix (ECM) in wound healing: a review. Biomim. Basel Switz. 2022;7:87. doi: 10.3390/biomimetics7030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schultz G.S., Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 66.Midwood K.S., Williams L.V., Schwarzbauer J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004;36:1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Cordero-Espinoza L., Huch M. The balancing act of the liver: tissue regeneration versus fibrosis. J. Clin. Invest. 2018;128:85–96. doi: 10.1172/JCI93562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demidova-Rice T.N., Hamblin M.R., Herman I.M. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv. Skin Wound Care. 2012;25:304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Z Z., J D., Ee T. The molecular basis of hypertrophic scars. Burns Trauma. 2016;4 doi: 10.1186/s41038-015-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.G T., A V. Autonomic nerve dysfunction and impaired diabetic wound healing: the role of neuropeptides. Auton. Neurosci. Basic Clin. 2020;223 doi: 10.1016/j.autneu.2019.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog. Histochem. Cytochem. 2002;37:323–386. doi: 10.1016/S0079-6336(02)80005-5. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L., Zhang X., Du L., Zhang C., Li H. Cholinergic- rather than adrenergic-induced sweating play a role in developing and developed rat eccrine sweat glands. Exp. Anim. 2021;70:218–224. doi: 10.1538/expanim.20-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El Baassiri M.G., Dosh L., Haidar H., Gerges A., Baassiri S., Leone A., Rappa F., Jurjus A. Nerve growth factor and burn wound healing: update of molecular interactions with skin cells. Burns. 2023;49:989–1002. doi: 10.1016/j.burns.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Tls A., Jr de S., Asc F., Lfm F., Jas Q. Nerve growth factor and pathogenesis of leprosy: review and update. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Billiet J., Ebenezer G.J., Pan B., Hauer P., Wei J., Polydefkis M. Factors influencing sweat gland innervation in diabetes. Neurology. 2015;84:1652–1659. doi: 10.1212/WNL.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 76.O R., T F., G N., M S., H G., V K., H W., F S., L T., L K., D R. Impaired glyoxalase activity is associated with reduced expression of neurotrophic factors and pro-inflammatory processes in diabetic skin cells. Exp. Dermatol. 2017;26 doi: 10.1111/exd.13118. [DOI] [PubMed] [Google Scholar]
- 77.C R., Ho R., K R., Pm V., Jd K. Paracrine loop of keratinocyte proliferation and directed neuritic outgrowth in a neuroepithelial coculture. Ann. Plast. Surg. 2013;70 doi: 10.1097/SPA.0b013e318276d946. [DOI] [PubMed] [Google Scholar]
- 78.Jc A., Ca A., I S., Kl Q., Je O., Sw C., Nw B. Interactions of the skin and nervous system. J. Invest. Dermatol. Symp. Proc. 1997;2 doi: 10.1038/jidsymp.1997.6. [DOI] [PubMed] [Google Scholar]
- 79.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 80.Lv H., Wang H., Zhang Z., Yang W., Liu W., Li Y., Li L. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42–48. doi: 10.1016/j.lfs.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 81.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., Rivera-Feliciano J., Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y., Li Z., Li J., Yang S., Zhang Y., Yao B., Song W., Fu X., Huang S. Stiffness-mediated mesenchymal stem cell fate decision in 3D-bioprinted hydrogels. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa029. tkaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zarkoob H., Bodduluri S., Ponnaluri S.V., Selby J.C., Sander E.A. Substrate stiffness affects human keratinocyte colony formation. Cell. Mol. Bioeng. 2015;8:32–50. doi: 10.1007/s12195-015-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.M C., K C., A S., Xs R., X Z., Mj G., S K., M L., C H., M A., F B., Yt C., H L., Nk K., J J., C P., M K., C G., E M., X C.C., Ps O., Eg E., S W., I J., S T., Da W. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108609. [DOI] [PubMed] [Google Scholar]
- 85.McWhorter F.Y., Davis C.T., Liu W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015;72:1303–1316. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Q W., Sm M., As W. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020;38 doi: 10.1016/j.tibtech.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Dm T., Hh W., Jr C., Yb Y., Y H., Y L., Ly T., Zy H., Kz L., Cj G., Sl L., Q X., Z Y., C L., Xj X., Cs R., Ys X., C Z., L L., Lp Y. In-situ formed elastin-based hydrogels enhance wound healing via promoting innate immune cells recruitment and angiogenesis. Mater. Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang P.-Y., Li W.-T., Yu J., Tsai W.-B. Modulation of osteogenic, adipogenic and myogenic differentiation of mesenchymal stem cells by submicron grooved topography. J. Mater. Sci. Mater. Med. 2012;23:3015–3028. doi: 10.1007/s10856-012-4748-6. [DOI] [PubMed] [Google Scholar]
- 89.Cui L., Yao Y., Yim E.K.F. The effects of surface topography modification on hydrogel properties. APL Bioeng. 2021;5 doi: 10.1063/5.0046076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.C H., C C., L L., C W., S J., R Y., S R., J L., Y Q., Y M. Dissecting the microenvironment around biosynthetic scaffolds in murine skin wound healing. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.X W., T L., W Z., G S., C L., G C. The effect of fiber size and pore size on cell proliferation and infiltration in PLLA scaffolds on bone tissue engineering. J. Biomater. Appl. 2016;30 doi: 10.1177/0885328216636320. [DOI] [PubMed] [Google Scholar]
- 92.Rl Y., Jp S., Kr L., Ld S. Microporous scaffolds support assembly and differentiation of pancreatic progenitors into β-cell clusters. Acta Biomater. 2019;96 doi: 10.1016/j.actbio.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y., Suarez-Arnedo A., Riley L., Miley T., Segura T. 2022. Spatial Confinement Modulates Macrophage Response in Microporous Scaffolds; p. 2022. 08.16.504156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.S M., U S. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng., C. 2019;104 doi: 10.1016/j.msec.2019.109883. [DOI] [PubMed] [Google Scholar]
- 95.Mariani E., Lisignoli G., Borzì R.M., Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019;20:636. doi: 10.3390/ijms20030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.H F., Z G. Bioinspired surfaces with wettability: biomolecule adhesion behaviors. Biomater. Sci. 2020;8 doi: 10.1039/c9bm01729a. [DOI] [PubMed] [Google Scholar]
- 97.Hao L., Fu X., Li T., Zhao N., Shi X., Cui F., Du C., Wang Y. Surface chemistry from wettability and charge for the control of mesenchymal stem cell fate through self-assembled monolayers. Colloids Surf. B Biointerfaces. 2016;148:549–556. doi: 10.1016/j.colsurfb.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 98.Km Y., M S. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019;20 doi: 10.1038/s41580-019-0172-9. [DOI] [PubMed] [Google Scholar]
- 99.R W., Y W., B Y., T H., Z L., S H., X F. Beyond 2D: 3D bioprinting for skin regeneration. Int. Wound J. 2019;16 doi: 10.1111/iwj.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie J., Yang X., Shao H., Ye J., He Y., Fu J., Gao C., Gou Z. Simultaneous mechanical property and biodegradation improvement of wollastonite bioceramic through magnesium dilute doping. J. Mech. Behav. Biomed. Mater. 2016;54:60–71. doi: 10.1016/j.jmbbm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Wei S., Ma J.-X., Xu L., Gu X.-S., Ma X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020;7:54. doi: 10.1186/s40779-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davison N.L., Barrère-de Groot F., Grijpma D.W. In: Tissue Eng. second ed. Blitterswijk C.A.V., De Boer J., editors. Academic Press; Oxford: 2014. Chapter 6 - degradation of biomaterials; pp. 177–215. [DOI] [Google Scholar]
- 103.Rammensee S., Kang M.S., Georgiou K., Kumar S., Schaffer D.V. Dynamics of mechanosensitive neural stem cell differentiation. Stem Cells Dayt. Ohio. 2017;35:497–506. doi: 10.1002/stem.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.J W., A C., Y Z., S Z., Y Y., Y A., K X., H H., J K., Ja L., M X., J X., Q W. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials. 2019:222. doi: 10.1016/j.biomaterials.2019.119398. [DOI] [PubMed] [Google Scholar]
- 105.Wu P., Liang Y., Sun G. Null Pw, null Gs, null Gs, null Pw, null Yl, null Yl, Engineering immune-responsive biomaterials for skin regeneration, Biomater. Transl. 2021;2:61–71. doi: 10.3877/cma.j.issn.2096-112X.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hd K., Hl J., Hy A., Hk L., J P., Es L., Ea L., Yh J., Dg K., Kt N., Ns H. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials. 2017;112 doi: 10.1016/j.biomaterials.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 107.Jeong J., Kim J.H., Shim J.H., Hwang N.S., Heo C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019;23:4. doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng K., Niu W., Lei B., Boccaccini A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021;133:168–186. doi: 10.1016/j.actbio.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 109.A M., J H., S B., V N., Ma S., B G. Multifunctional photoactive hydrogels for wound healing acceleration. ACS Nano. 2021;15 doi: 10.1021/acsnano.1c08334. [DOI] [PubMed] [Google Scholar]
- 110.C A.-L., L B., A C. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009;85 doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 111.Eh F., Vh F., Ep S., At P., Ec da S.F., Mr M., R S., Af R., Ec M. Magnetic-responsive polysaccharide hydrogels as smart biomaterials: synthesis, properties, and biomedical applications. Carbohydr. Polym. 2022;292 doi: 10.1016/j.carbpol.2022.119665. [DOI] [PubMed] [Google Scholar]
- 112.H S., M H., H W., N Z., J T., H J., J Z. Injectable and pH-sensitive hyaluronic acid-based hydrogels with on-demand release of antimicrobial peptides for infected wound healing. Biomacromolecules. 2021;22 doi: 10.1021/acs.biomac.1c00502. [DOI] [PubMed] [Google Scholar]
- 113.Zhao X., Wang L., Gao J., Chen X., Wang K. Hyaluronic acid/lysozyme self-assembled coacervate to promote cutaneous wound healing. Biomater. Sci. 2020;8:1702–1710. doi: 10.1039/c9bm01886g. [DOI] [PubMed] [Google Scholar]
- 114.F H., L W., B C., L Q., J N., G M. Bifunctional smart hydrogel dressing with strain sensitivity and NIR-responsive performance. ACS Appl. Mater. Interfaces. 2021;13 doi: 10.1021/acsami.1c15312. [DOI] [PubMed] [Google Scholar]
- 115.Zhu Y., Ma Z., Kong L., He Y., Chan H.F., Li H. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials. 2020;256 doi: 10.1016/j.biomaterials.2020.120216. [DOI] [PubMed] [Google Scholar]
- 116.Chouhan D., Lohe T.-U., Samudrala P.K., Mandal B.B. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv. Healthcare Mater. 2018;7 doi: 10.1002/adhm.201801092. [DOI] [PubMed] [Google Scholar]
- 117.Sj P., W C., Ms K., Bk G., Cm K., G K., Ch K. Substance-P and transforming growth factor-β in chitosan microparticle-pluronic hydrogel accelerates regenerative wound repair of skin injury by local ionizing radiation. J. Tissue Eng. Regen. Med. 2018;12 doi: 10.1002/term.2445. [DOI] [PubMed] [Google Scholar]
- 118.R G., L C., L C., W C., L G., X L., H L., S L., C C. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells, in Vitro Cell. Dev. Biol. Anim. 2015;51 doi: 10.1007/s11626-014-9862-y. [DOI] [PubMed] [Google Scholar]
- 119.Chen P., Tao J., Zhu S., Cai Y., Mao Q., Yu D., Dai J., Ouyang H. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials. 2015;39:114–123. doi: 10.1016/j.biomaterials.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 120.Rabbany S.Y., Pastore J., Yamamoto M., Miller T., Rafii S., Aras R., Penn M. Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant. 2010;19:399–408. doi: 10.3727/096368909X481782. [DOI] [PubMed] [Google Scholar]
- 121.G W., C F., J Q., Z W., W W., S Z., S K., G H., X C., Q W. In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr. Polym. 2018;182 doi: 10.1016/j.carbpol.2017.10.090. [DOI] [PubMed] [Google Scholar]
- 122.Dong Y., Liu Y., Chen Y., Sun X., Zhang L., Zhang Z., Wang Y., Qi C., Wang S., Yang Q. Spatiotemporal regulation of endogenous MSCs using a functional injectable hydrogel system for cartilage regeneration. NPG Asia Mater. 2021;13:1–17. doi: 10.1038/s41427-021-00339-3. [DOI] [Google Scholar]
- 123.Yu J.R. 2021. A NANOCOMPOSITE HYDROGEL FOR STROMAL CELL-DERIVED FACTOR-1 ALPHA DELIVERY AND MODULATION OF MACROPHAGE PHENOTYPE FOR SKIN TISSUE REGENERATION. [DOI] [Google Scholar]
- 124.Ladewig J., Koch P., Brüstle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat. Rev. Mol. Cell Biol. 2013;14:225–236. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- 125.Lambert S.A., Jolma A., Campitelli L.F., Das P.K., Yin Y., Albu M., Chen X., Taipale J., Hughes T.R., Weirauch M.T. The human transcription factors. Cell. 2018;172:650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 126.Fletcher M. Genome-scale characterization of transcription factors. Nat. Genet. 2023;55 doi: 10.1038/s41588-023-01351-9. 357–357. [DOI] [PubMed] [Google Scholar]
- 127.Sepehrimanesh M., Ding B. Generation and optimization of highly pure motor neurons from human induced pluripotent stem cells via lentiviral delivery of transcription factors. Am. J. Physiol. Cell Physiol. 2020;319:C771–C780. doi: 10.1152/ajpcell.00279.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kantor B., Chiba-Falek O. In: Methods IPSC Technol. Birbrair A., editor. Academic Press; 2021. Chapter 4 - lentiviral vectors as the delivery vehicles for transduction into iPSCs: shortcomings and benefits; pp. 79–100. [DOI] [Google Scholar]
- 129.Bolte C., Ustiyan V., Ren X., Dunn A.W., Pradhan A., Wang G., Kolesnichenko O.A., Deng Z., Zhang Y., Shi D., Greenberg J.M., Jobe A.H., Kalin T.V., Kalinichenko V.V. Nanoparticle delivery of proangiogenic transcription factors into the neonatal circulation inhibits alveolar simplification caused by hyperoxia. Am. J. Respir. Crit. Care Med. 2020;202:100–111. doi: 10.1164/rccm.201906-1232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y., Wang H., Kamei K., Yan M., Chen K.-J., Yuan Q., Shi L., Lu Y., Tseng H.-R. Delivery of intact transcription factor by using self-assembled supramolecular nanoparticles. Angew. Chem., Int. Ed. Engl. 2011;50:3058–3062. doi: 10.1002/anie.201005740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen R., Zhu Z., Ji S., Geng Z., Hou Q., Sun X., Fu X. Sweat gland regeneration: current strategies and future opportunities. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120201. [DOI] [PubMed] [Google Scholar]
- 132.Cy C., M Y., J S., V C., M M., Y P., D S. Involvement of Wnt, Eda and Shh at defined stages of sweat gland development. Dev. Camb. Engl. 2014;141 doi: 10.1242/dev.109231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.J Z., L Z., L D., Z C., Y T., L C., X L., L Y., Y Z., X F., H L. Foxa1 mediates eccrine sweat gland development through transcriptional regulation of Na-K-ATPase expression. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2022;55 doi: 10.1590/1414-431X2022e12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.D A., B K., C L., A A., Pr A., Yg K. The transcription factor Deaf 1 modulates engrailed-1 expression to regulate skin appendage fate. J. Invest. Dermatol. 2019;139 doi: 10.1016/j.jid.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 135.B Y., J X., N L., T H., W S., S H., X F. Direct reprogramming of epidermal cells toward sweat gland-like cells by defined factors. Cell Death Dis. 2019;10 doi: 10.1038/s41419-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Daniel M.G., Lemischka I.R., Moore K. Converting cell fates: generating hematopoietic stem cells de novo via transcription factor reprogramming. Ann. N. Y. Acad. Sci. 2016;1370:24–35. doi: 10.1111/nyas.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duran Alonso M.B., Lopez Hernandez I., de la Fuente M.A., Garcia-Sancho J., Giraldez F., Schimmang T. Transcription factor induced conversion of human fibroblasts towards the hair cell lineage. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Banerjee J., Sen C.K. microRNA and wound healing. Adv. Exp. Med. Biol. 2015;888:291–305. doi: 10.1007/978-3-319-22671-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kumar M., DeVaux R.S., Herschkowitz J.I. In: Prog. Mol. Biol. Transl. Sci. Pruitt K., editor. Academic Press; 2016. Chapter thirteen - molecular and cellular changes in breast cancer and new roles of lncRNAs in breast cancer initiation and progression; pp. 563–586. [DOI] [PubMed] [Google Scholar]
- 140.P S., A K., T A., Ad D., Fd M., I R., M G., S Y., A B., A M., Vr Y., O M., E S. Nucleic acid-based therapeutics for dermal wound healing. Int. J. Biol. Macromol. 2022;220 doi: 10.1016/j.ijbiomac.2022.08.099. [DOI] [PubMed] [Google Scholar]
- 141.Cao Z., Liao Q., Su M., Huang K., Jin J., Cao D. AKT and ERK dual inhibitors: the way forward? Cancer Lett. 2019;459:30–40. doi: 10.1016/j.canlet.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 142.Qu M., Pan J., Wang L., Zhou P., Song Y., Wang S., Jiang L., Geng J., Zhang Z., Wang Y., Tang Y., Yang G.-Y. MicroRNA-126 regulates angiogenesis and neurogenesis in a mouse model of focal cerebral ischemia. Mol. Ther. Nucleic Acids. 2019;16:15–25. doi: 10.1016/j.omtn.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sasahira T., Kurihara M., Bhawal U.K., Ueda N., Shimomoto T., Yamamoto K., Kirita T., Kuniyasu H. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br. J. Cancer. 2012;107:700–706. doi: 10.1038/bjc.2012.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Y E.-T., Y N., A N., S U., K O., Y O., K S., F M., N T., R S., K M., Y A. Systemic delivery of miR-126 by miRNA-loaded Bubble liposomes for the treatment of hindlimb ischemia. Sci. Rep. 2014;4 doi: 10.1038/srep03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bejerano T., Etzion S., Elyagon S., Etzion Y., Cohen S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 2018;18:5885–5891. doi: 10.1021/acs.nanolett.8b02578. [DOI] [PubMed] [Google Scholar]
- 146.B S., Hk D., R P.-L., E S.S., R A., Mm A., N A. Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small Weinh. Bergstr. Ger. 2019;15 doi: 10.1002/smll.201902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Q., Hu W., Huang Q., Yang J., Li B., Ma K., Wei Q., Wang Y., Su J., Sun M., Cui S., Yang R., Li H., Fu X., Zhang C. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing by targeting IRAK1. Signal Transduct. Targeted Ther. 2023;8:62. doi: 10.1038/s41392-022-01263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sriram S., Robinson P., Pi L., Lewin A.S., Schultz G. Triple combination of siRNAs targeting TGFβ1, TGFβR2, and CTGF enhances reduction of collagen I and smooth muscle actin in corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2013;54:8214. doi: 10.1167/iovs.13-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.G L., Q X., Y S., D L., M Z., S J., H Z., H L., Y J. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J. Gene Med. 2006;8 doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- 150.Sa C., A G., Ma S., S K., Bd A., Wg A., Ml Y., Pt H. Nanolayered siRNA delivery platforms for local silencing of CTGF reduce cutaneous scar contraction in third-degree burns. Biomaterials. 2016;95 doi: 10.1016/j.biomaterials.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mottaghitalab F., Rastegari A., Farokhi M., Dinarvand R., Hosseinkhani H., Ou K.-L., Pack D.W., Mao C., Dinarvand M., Fatahi Y., Atyabi F. Prospects of siRNA applications in regenerative medicine. Int. J. Pharm. 2017;524:312–329. doi: 10.1016/j.ijpharm.2017.03.092. [DOI] [PubMed] [Google Scholar]
- 152.Fang H., Chen Q. Applications and challenges of biomaterial mediated mRNA delivery. Explor Target Antitumor Ther. 2022;3:428–444. doi: 10.37349/etat.2022.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blakney A.K., Abdouni Y., Yilmaz G., Liu R., McKay P.F., Bouton C.R., Shattock R.J., Becer C.R. Mannosylated poly(ethylene imine) copolymers enhance saRNA uptake and expression in human skin explants. Biomacromolecules. 2020;21:2482–2492. doi: 10.1021/acs.biomac.0c00445. [DOI] [PubMed] [Google Scholar]
- 154.Paunovska K., Da Silva Sanchez A.J., Sago C.D., Gan Z., Lokugamage M.P., Islam F.Z., Kalathoor S., Krupczak B.R., Dahlman J.E. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. Deerfield Beach Fla. 2019;31 doi: 10.1002/adma.201807748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fu X., Chen T., Song Y., Feng C., Chen H., Zhang Q., Chen G., Zhu X. mRNA delivery by a pH-responsive DNA nano-hydrogel. Small Weinh. Bergstr. Ger. 2021;17 doi: 10.1002/smll.202101224. [DOI] [PubMed] [Google Scholar]
- 156.Delivery of synthetic mRNAs for tissue regeneration. Adv. Drug Deliv. Rev. 2021;179 doi: 10.1016/j.addr.2021.114007. [DOI] [PubMed] [Google Scholar]
- 157.Yu Z., Witman N., Wang W., Li D., Yan B., Deng M., Wang X., Wang H., Zhou G., Liu W., Sahara M., Cao Y., Fritsche-Danielson R., Zhang W., Fu W., Chien K.R. Cell-mediated delivery of VEGF modified mRNA enhances blood vessel regeneration and ameliorates murine critical limb ischemia. J. Control. Release Off. J. Control. Release Soc. 2019;310:103–114. doi: 10.1016/j.jconrel.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 158.S S., J X., J H., Z G., K M., X S., X F. Targeting ectodysplasin promotor by CRISPR/dCas9-effector effectively induces the reprogramming of human bone marrow-derived mesenchymal stem cells into sweat gland-like cells. Stem Cell Res. Ther. 2018;9 doi: 10.1186/s13287-017-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seifinejad A., Tabebordbar M., Baharvand H., Boyer L.A., Salekdeh G.H. Progress and promise towards safe induced pluripotent stem cells for therapy. Stem Cell Rev. Rep. 2010;6:297–306. doi: 10.1007/s12015-010-9121-x. [DOI] [PubMed] [Google Scholar]
- 160.Wei T., Cheng Q., Min Y.-L., Olson E.N., Siegwart D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee K., Conboy M., Park H.M., Jiang F., Kim H.J., Dewitt M.A., Mackley V.A., Chang K., Rao A., Skinner C., Shobha T., Mehdipour M., Liu H., Huang W.-C., Lan F., Bray N.L., Li S., Corn J.E., Kataoka K., Doudna J.A., Conboy I., Murthy N. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat. Biomed. Eng. 2017;1:889–901. doi: 10.1038/s41551-017-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aghamiri S., Talaei S., Ghavidel A.A., Zandsalimi F., Masoumi S., Hafshejani N.H., Jajarmi V. Nanoparticles-mediated CRISPR/Cas9 delivery: recent advances in cancer treatment. J. Drug Deliv. Sci. Technol. 2020;56 doi: 10.1016/j.jddst.2020.101533. [DOI] [Google Scholar]
- 163.Chin J.S., Chooi W.H., Wang H., Ong W., Leong K.W., Chew S.Y. Scaffold-mediated non-viral delivery platform for CRISPR/Cas9-based genome editing. Acta Biomater. 2019;90:60–70. doi: 10.1016/j.actbio.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 164.Crowder S.W., Leonardo V., Whittaker T., Papathanasiou P., Stevens M.M. Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell. 2016;18:39–52. doi: 10.1016/j.stem.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.LvLongwei TangYiman, ZhangPing LiuYunsong, BaiXiangsong ZhouYongsheng. Biomaterial cues regulate epigenetic state and cell functions—a systematic review. Tissue Eng., Part B. 2018 doi: 10.1089/ten.teb.2017.0287. [DOI] [PubMed] [Google Scholar]
- 166.M J., J A., Sw O., Jy L., J K., Jk C., Jh C., P K. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat. Biomed. Eng. 2021;5 doi: 10.1038/s41551-020-00657-x. [DOI] [PubMed] [Google Scholar]
- 167.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 168.L L., Y T., P Z., Y L., X B., Y Z. Biomaterial cues regulate epigenetic state and cell functions-A systematic review. Tissue Eng., Part B. 2018;24 doi: 10.1089/ten.teb.2017.0287. [DOI] [PubMed] [Google Scholar]
- 169.C M., M N., Ma P., E B., Md S., Mm S. Enhanced efficiency of genetic programming toward cardiomyocyte creation through topographical cues. Biomaterials. 2015;70 doi: 10.1016/j.biomaterials.2015.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Downing T.L., Soto J., Morez C., Houssin T., Fritz A., Yuan F., Chu J., Patel S., Schaffer D.V., Li S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sukpaita T., Chirachanchai S., Chanamuangkon T., Nampuksa K., Monmaturapoj N., Sumrejkanchanakij P., Pimkhaokham A., Ampornaramveth R.S. Novel epigenetic modulation chitosan-based scaffold as a promising bone regenerative material. Cells. 2022;11:3217. doi: 10.3390/cells11203217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Y Z. Chemically induced cell fate reprogramming and the acquisition of plasticity in somatic cells. Curr. Opin. Chem. Biol. 2019;51 doi: 10.1016/j.cbpa.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 173.P H., Y L., X Z., C L., J G., H L., T Z., J Y., W Y., K L., J G., J X., Q Z., Y Z., H D. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341 doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 174.Guan J., Wang G., Wang J., Zhang Z., Fu Y., Cheng L., Meng G., Lyu Y., Zhu J., Li Y., Wang Y., Liuyang S., Liu B., Yang Z., He H., Zhong X., Chen Q., Zhang X., Sun S., Lai W., Shi Y., Liu L., Wang L., Li C., Lu S., Deng H. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature. 2022;605:325–331. doi: 10.1038/s41586-022-04593-5. [DOI] [PubMed] [Google Scholar]
- 175.Baek S., Choi S.-W., Park S.-J., Lee S.-H., Chun H.-S., Kim S.H. Quinoline compound KM11073 enhances BMP-2-dependent osteogenic differentiation of C2C12 cells via activation of p38 signaling and exhibits in vivo bone forming activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laurencin C.T., Ashe K.M., Henry N., Kan H.M., Lo K.W.-H. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov. Today. 2014;19:794–800. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fan J., Im C.S., Guo M., Cui Z.-K., Fartash A., Kim S., Patel N., Bezouglaia O., Wu B.M., Wang C.-Y., Aghaloo T.L., Lee M. Enhanced osteogenesis of adipose-derived stem cells by regulating bone morphogenetic protein signaling antagonists and agonists, stem cells transl. Méd. 2016;5:539–551. doi: 10.5966/sctm.2015-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ghosh P., Han G., De M., Kim C.K., Rotello V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 179.Jahangirian H., Kalantari K., Izadiyan Z., Rafiee-Moghaddam R., Shameli K., Webster T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019;14:1633–1657. doi: 10.2147/IJN.S184723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Suzuka J., Tsuda M., Wang L., Kohsaka S., Kishida K., Semba S., Sugino H., Aburatani S., Frauenlob M., Kurokawa T., Kojima S., Ueno T., Ohmiya Y., Mano H., Yasuda K., Gong J.P., Tanaka S. Rapid reprogramming of tumour cells into cancer stem cells on double-network hydrogels. Nat. Biomed. Eng. 2021;5:914–925. doi: 10.1038/s41551-021-00692-2. [DOI] [PubMed] [Google Scholar]
- 181.D X., S B., W W., I I., Mac W., D P., A Y., S B., Rg M., C H., Sd M., J V. PLG nanoparticles target fibroblasts and MARCO+ monocytes to reverse multiorgan fibrosis. JCI Insight. 2022;7 doi: 10.1172/jci.insight.151037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bollong M.J., Yang B., Vergani N., Beyer B.A., Chin E.N., Zambaldo C., Wang D., Chatterjee A.K., Lairson L.L., Schultz P.G. Small molecule-mediated inhibition of myofibroblast transdifferentiation for the treatment of fibrosis. Proc. Natl. Acad. Sci. U.S.A. 2017;114:4679–4684. doi: 10.1073/pnas.1702750114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mastrogiannaki M., Lichtenberger B.M., Reimer A., Collins C.A., Driskell R.R., Watt F.M. β-Catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J. Invest. Dermatol. 2016;136:1130–1142. doi: 10.1016/j.jid.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mf G., J H., Fj E., N Q., Mt L. The role of Wnt signaling in skin fibrosis. Med. Res. Rev. 2022;42 doi: 10.1002/med.21853. [DOI] [PubMed] [Google Scholar]
- 185.Liu J., Zhao B., Zhu H., Pan Q., Cai M., Bai X., Li X., Hu X., Zhang M., Shi J., Zheng Z., Yang A., Hu D. Wnt 4 negatively regulates the TGF-β1-induced human dermal fibroblast-to-myofibroblast transition via targeting Smad 3 and ERK. Cell Tissue Res. 2020;379:537–548. doi: 10.1007/s00441-019-03110-x. [DOI] [PubMed] [Google Scholar]
- 186.Zhang K., Guo X., Zhao W., Niu G., Mo X., Fu Q. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: in vitro and in vivo study. Int. J. Mol. Sci. 2015;16:27659–27676. doi: 10.3390/ijms161126050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kur-Piotrowska A., Kopcewicz M., Kozak L.P., Sachadyn P., Grabowska A., Gawronska-Kozak B. Neotenic phenomenon in gene expression in the skin of Foxn1- deficient (nude) mice - a projection for regenerative skin wound healing. BMC Genom. 2017;18:56. doi: 10.1186/s12864-016-3401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.B P., S de R., Pm W., S B., B H., Mm van B., Ra B. YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am. J. Pathol. 2015;185 doi: 10.1016/j.ajpath.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 189.Panciera T., Azzolin L., Cordenonsi M., Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Toyama T., Looney A.P., Baker B.M., Stawski L., Haines P., Simms R., Szymaniak A.D., Varelas X., Trojanowska M. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J. Invest. Dermatol. 2018;138:78. doi: 10.1016/j.jid.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pei Y., Zhang L., Mao X., Liu Z., Cui W., Sun X., Zhang Y. Biomaterial scaffolds for improving vascularization during skin flap regeneration. Chin. J. Plast. Reconstr. Surg. 2020;2:109–119. doi: 10.1016/S2096-6911(21)00021-2. [DOI] [Google Scholar]
- 192.E M., P V. Effects of surface properties and bioactivation of biomaterials on endothelial cells. Front. Biosci. Sch. Ed. 2010;2 doi: 10.2741/s61. [DOI] [PubMed] [Google Scholar]
- 193.K L., M Z., Y L., L L., D H. VEGF loaded porcine decellularized adipose tissue derived hydrogel could enhance angiogenesis in vitro and in vivo. J. Biomater. Sci. Polym. Ed. 2022;33 doi: 10.1080/09205063.2021.2002235. [DOI] [PubMed] [Google Scholar]
- 194.Cao R., Bråkenhielm E., Pawliuk R., Wariaro D., Post M.J., Wahlberg E., Leboulch P., Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 195.J B., L B., X G., X W., Q Z., J H., Z X., Ff H., Z G., W C. Bacteria-engineered porous sponge for hemostasis and vascularization. J. Nanobiotechnol. 2022;20 doi: 10.1186/s12951-022-01254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jirigala E., Yao B., Li Z., Zhang Y.-J., Zhang C., Liang L.-T., Zhang F.-L., Yuan X.-Y., Duan X.-L., Song W., Zhang M.-D., Kong Y., Fu X.-B., Huang S. In situ forming injectable MSC-loaded GelMA hydrogels combined with PD for vascularized sweat gland regeneration. Mil. Med. Res. 2023;10:17. doi: 10.1186/s40779-023-00456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.X Y., X D., None E., Z L., B Y., W S., Y W., Y K., S Z., F Z., L L., M Z., C Z., D K., M Z., S H., X F. Reciprocal interaction between vascular niche and sweat gland promotes sweat gland regeneration. Bioact. Mater. 2022;21 doi: 10.1016/j.bioactmat.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang F., Deng Y., Wang J., Yu L., Ding F., Lian W., Liu Q., Lin X. The PLGA nanoparticles for sustainable release of CGRP to ameliorate the inflammatory and vascular disorders in the lung of CGRP-deficient rats. Drug Deliv. 2021;28:865–872. doi: 10.1080/10717544.2021.1902021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zheng Z., Liu Y., Huang W., Mo Y., Lan Y., Guo R., Cheng B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells, Nanomed. Biotechnol. 2018;46:493–501. doi: 10.1080/21691401.2018.1460372. [DOI] [PubMed] [Google Scholar]
- 201.Bonfiglio V., Camillieri G., Avitabile T., Leggio G.M., Drago F. Effects of the COOH-terminal tripeptide alpha-MSH(11-13) on corneal epithelial wound healing: role of nitric oxide. Exp. Eye Res. 2006;83:1366–1372. doi: 10.1016/j.exer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 202.Ray P., Torck A., Quigley L., Wangzhou A., Neiman M., Rao C., Lam T., Kim J.-Y., Kim T.H., Zhang M.Q., Dussor G., Price T.J. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain. 2018;159:1325–1345. doi: 10.1097/j.pain.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim B.S., Gao G., Kim J.Y., Cho D.-W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801019. [DOI] [PubMed] [Google Scholar]
- 204.Zhang M., Li H., Chen L., Fang S., Xie S., Lin C. Three-dimensional reconstructed eccrine sweat glands with vascularization and cholinergic and adrenergic innervation. J. Mol. Histol. 2018;49:339–345. doi: 10.1007/s10735-018-9773-4. [DOI] [PubMed] [Google Scholar]
- 205.Chen H., Liu Y., Liu Y., Ji S., Xiang J., Li Y., Zhou L., Gao H., Deng Z., Li B., Sun S., Cui S., Li G., Sheng W., Liu H., Chen C., Zhao Y., Zhang H., Liu K., Fu X., Sun X. Biomimetic small exosome with outstanding surgical applications for rapid large-scale wound healing and functional sweat gland restoration. Nano Today. 2022;45 doi: 10.1016/j.nantod.2022.101531. [DOI] [Google Scholar]
- 206.Keane T.J., Swinehart I.T., Badylak S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods San Diego Calif. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 207.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 208.Ieda M., Fu J.-D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chang Y., Lee E., Kim J., Kwon Y.-W., Kwon Y., Kim J. Efficient in vivo direct conversion of fibroblasts into cardiomyocytes using a nanoparticle-based gene carrier. Biomaterials. 2019;192:500–509. doi: 10.1016/j.biomaterials.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 210.Elci S.G., Jiang Y., Yan B., Kim S.T., Saha K., Moyano D.F., Yesilbag Tonga G., Jackson L.C., Rotello V.M., Vachet R.W. Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano. 2016;10:5536–5542. doi: 10.1021/acsnano.6b02086. [DOI] [PubMed] [Google Scholar]
- 211.Wang Q., Song Y., Chen J., Li Q., Gao J., Tan H., Zhu Y., Wang Z., Li M., Yang H., Zhang N., Li X., Qian J., Pang Z., Huang Z., Ge J. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121028. [DOI] [PubMed] [Google Scholar]
- 212.Stone N.R., Gifford C.A., Thomas R., Pratt K.J.B., Samse-Knapp K., Mohamed T.M.A., Radzinsky E.M., Schricker A., Ye L., Yu P., van Bemmel J.G., Ivey K.N., Pollard K.S., Srivastava D. Context-specific transcription factor functions regulate epigenomic and transcriptional dynamics during cardiac reprogramming. Cell Stem Cell. 2019;25:87–102. doi: 10.1016/j.stem.2019.06.012. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang H., Yang Y., Liu J., Qian L. Direct cell reprogramming: approaches, mechanisms and progress. Nat. Rev. Mol. Cell Biol. 2021;22:410–424. doi: 10.1038/s41580-021-00335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu Q., Liu L., Lin J., Wang Y., Xuan X., Guo Y., Hu S. SDF-1α/CXCR4 Axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in A rat model. Cell J. 2015;16:440–447. doi: 10.22074/cellj.2015.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang H., Li X., Li J., Zhong L., Chen X., Chen S. SDF-1 mediates mesenchymal stem cell recruitment and migration via the SDF-1/CXCR4 axis in bone defect. J. Bone Miner. Metabol. 2021;39:126–138. doi: 10.1007/s00774-020-01122-0. [DOI] [PubMed] [Google Scholar]
- 216.Kowalski K., Kołodziejczyk A., Sikorska M., Płaczkiewicz J., Cichosz P., Kowalewska M., Stremińska W., Jańczyk-Ilach K., Koblowska M., Fogtman A., Iwanicka-Nowicka R., Ciemerych M.A., Brzoska E. Stem cells migration during skeletal muscle regeneration - the role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adhes. Migrat. 2016;11:384–398. doi: 10.1080/19336918.2016.1227911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xiao Ling K., Peng L., Jian Feng Z., Wei C., Wei Yan Y., Nan S., Cheng Qi G., Zhi Wei W. Stromal derived factor-1/CXCR4 Axis involved in bone marrow mesenchymal stem cells recruitment to injured liver. Stem Cell. Int. 2016 doi: 10.1155/2016/8906945. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xiu G., Li X., Yin Y., Li J., Li B., Chen X., Liu P., Sun J., Ling B. SDF-1/CXCR4 augments the therapeutic effect of bone marrow mesenchymal stem cells in the treatment of lipopolysaccharide-induced liver injury by promoting their migration through PI3K/akt signaling pathway. Cell Transplant. 2020;29 doi: 10.1177/0963689720929992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Askari A.T., Unzek S., Popovic Z.B., Goldman C.K., Forudi F., Kiedrowski M., Rovner A., Ellis S.G., Thomas J.D., DiCorleto P.E., Topol E.J., Penn M.S. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet Lond. Engl. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 220.Y G., G H., H B., Lm P., He B. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells Dayt. Ohio. 2005;23 doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 221.Lm M., Ca K., Sa L., S P., Ar M., Jf V., D A., Sg K., Fy L., Tm C. Characterization of endothelial progenitor cells mobilization following cutaneous wounding, Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2010;18 doi: 10.1111/j.1524-475X.2010.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang X., Jiang H., Guo L., Wang S., Cheng W., Wan L., Zhang Z., Xing L., Zhou Q., Yang X., Han H., Chen X., Wu X. SDF-1 secreted by mesenchymal stem cells promotes the migration of endothelial progenitor cells via CXCR4/PI3K/AKT pathway. J. Mol. Histol. 2021;52:1155–1164. doi: 10.1007/s10735-021-10008-y. [DOI] [PubMed] [Google Scholar]
- 223.Gong Y., Fan Y., Hoover-Plow J. Plasminogen regulates SDF-1/CXCR4-mediated hematopoietic stem cell mobilization by activation of matrix metalloproteinase-9. Arterioscler. Thromb. Vasc. Biol. 2011;31:2035–2043. doi: 10.1161/ATVBAHA.111.229583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.W Y., L M., J Z., K H., Q Y., Yy G., Sb L., Yh L., Ym W. The migration of neural progenitor cell mediated by SDF-1 is NF-κB/HIF-1α dependent upon hypoxia. CNS Neurosci. Ther. 2013;19 doi: 10.1111/cns.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.H T., In V., E C., S E.-F., M J., Ei C., J P., E N., Mt L., Gc G. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells Dayt. Ohio. 2009;27 doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 226.Brzoska E., Kowalewska M., Markowska-Zagrajek A., Kowalski K., Archacka K., Zimowska M., Grabowska I., Czerwińska A.M., Czarnecka-Góra M., Stremińska W., Jańczyk-Ilach K., Ciemerych M.A. Sdf-1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol. Cell. 2012;104:722–737. doi: 10.1111/boc.201200022. [DOI] [PubMed] [Google Scholar]
- 227.M D., M R.-F., J S., M K., J D., D M., Cv E., T L., E R. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111 doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 228.Zhang Y., An S., Hao J., Tian F., Fang X., Wang J. Systemic injection of substance P promotes murine calvarial repair through mobilizing endogenous mesenchymal stem cells. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-31414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Z D., D M., G S., E S., F C., F R., E S., F C., A F., A P., E P., B L., C S., M A., R C., A A., A F., F Q., K O., I R.-A., C E., M S., Gp F., P M. Sensory neuropathy hampers nociception-mediated bone marrow stem cell release in mice and patients with diabetes. Diabetologia. 2015;58 doi: 10.1007/s00125-015-3735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.S A., C R., R K., M M., A O., Ap B., E A., D C., O F., G S., R A., E C., M V., Sp H., C E., P M. Role for substance p-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation. 2012;125 doi: 10.1161/CIRCULATIONAHA.111.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hs H., J L., E L., Ys K., E L., W A., Mh J., Jc K., Y S. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat. Med. 2009;15 doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- 232.A M., E F., C A., E J., M C., W Z., Jj M. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011;25 doi: 10.1096/fj.10-176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.W F., L Y., J L., Z W., J C., C G., X M., Z Y. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater. Sci. Eng., C. 2020;110 doi: 10.1016/j.msec.2020.110705. [DOI] [PubMed] [Google Scholar]
- 234.Zhang S.-J., Song X.-Y., He M., Yu S.-B. Effect of TGF-β1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2016;20:899–905. [PubMed] [Google Scholar]
- 235.Dubon M.J., Yu J., Choi S., Park K.-S. Transforming growth factor β induces bone marrow mesenchymal stem cell migration via noncanonical signals and N-cadherin. J. Cell. Physiol. 2018;233:201–213. doi: 10.1002/jcp.25863. [DOI] [PubMed] [Google Scholar]
- 236.Ghosh D., McGrail D.J., Dawson M.R. TGF-β1 pretreatment improves the function of mesenchymal stem cells in the wound bed. Front. Cell Dev. Biol. 2017;5:28. doi: 10.3389/fcell.2017.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tabatabai G., Frank B., Möhle R., Weller M., Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1 alpha-mediated induction of CXCL12. Brain J. Neurol. 2006;129:2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 238.Hj C., Hs K., Mm L., Dh K., Hj Y., J H., Kk H., S O., Yj C., Ih C., Bh O., Ys C., K W., Yb P. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108 doi: 10.1161/01.CIR.0000097001.79750.78. [DOI] [PubMed] [Google Scholar]
- 239.Y S., C S., E A., T K., H H., T S., N Y., H G., T I., N F., M N. Synergistic effect of FLT-3 ligand on the granulocyte colony-stimulating factor-induced mobilization of hematopoietic stem cells and progenitor cells into blood in mice. Blood. 1997;89 https://pubmed.ncbi.nlm.nih.gov/9129021/ [PubMed] [Google Scholar]
- 240.Kim J., Kim N.K., Park S.R., Choi B.H. GM-CSF enhances mobilization of bone marrow mesenchymal stem cells via a CXCR4-medicated mechanism. Tissue Eng. Regen. Med. 2019;16:59–68. doi: 10.1007/s13770-018-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.J G., H Z., J X., J W., Y Y., Z L., Y Z., X L. Monocyte chemotactic protein-1 promotes the myocardial homing of mesenchymal stem cells in dilated cardiomyopathy. Int. J. Mol. Sci. 2013;14 doi: 10.3390/ijms14048164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Louridas M., Letourneau S., Lautatzis M.-E., Vrontakis M. Galanin is highly expressed in bone marrow mesenchymal stem cells and facilitates migration of cells both in vitro and in vivo. Biochem. Biophys. Res. Commun. 2009;390:867–871. doi: 10.1016/j.bbrc.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 243.Forte G., Minieri M., Cossa P., Antenucci D., Sala M., Gnocchi V., Fiaccavento R., Carotenuto F., De Vito P., Baldini P.M., Prat M., Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells Dayt. Ohio. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 244.Linke A., Müller P., Nurzynska D., Casarsa C., Torella D., Nascimbene A., Castaldo C., Cascapera S., Böhm M., Quaini F., Urbanek K., Leri A., Hintze T.H., Kajstura J., Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xinaris C., Morigi M., Benedetti V., Imberti B., Fabricio A.S., Squarcina E., Benigni A., Gagliardini E., Remuzzi G. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22:423–436. doi: 10.3727/096368912X653246. [DOI] [PubMed] [Google Scholar]
- 246.Li Y., Yu X., Lin S., Li X., Zhang S., Song Y.-H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007;356:780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 247.Nedeau A.E., Bauer R.J., Gallagher K., Chen H., Liu Z.-J., Velazquez O.C. A CXCL5- and bFGF-dependent effect of PDGF-B-activated fibroblasts in promoting trafficking and differentiation of bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 2008;314:2176–2186. doi: 10.1016/j.yexcr.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mishima Y., Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 249.Schmidt A., Ladage D., Schinköthe T., Klausmann U., Ulrichs C., Klinz F.-J., Brixius K., Arnhold S., Desai B., Mehlhorn U., Schwinger R.H.G., Staib P., Addicks K., Bloch W. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells Dayt. Ohio. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 250.Wang X., Zhen L., Miao H., Sun Q., Yang Y., Que B., Lopes Lao E.P., Wu X., Ren H., Shi S., Lau W.B., Ma X., Ma C., Nie S. Concomitant retrograde coronary venous infusion of basic fibroblast growth factor enhances engraftment and differentiation of bone marrow mesenchymal stem cells for cardiac repair after myocardial infarction. Theranostics. 2015;5:995–1006. doi: 10.7150/thno.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Biswas A., Liu Y., Liu T., Fan G., Tang Y. Polyethylene glycol-based protein nanocapsules for functional delivery of a differentiation transcription factor. Biomaterials. 2012;33:5459–5467. doi: 10.1016/j.biomaterials.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 252.K L., M R., X W., K A., X F., C L.S., R T., N L., Hj K., N M. delivery of transcription factors with multifunctional oligonucleotides. Nat. Mater. 2015;14 doi: 10.1038/nmat4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gallego-Perez D., Pal D., Ghatak S., Malkoc V., Higuita-Castro N., Gnyawali S., Chang L., Liao W.-C., Shi J., Sinha M., Singh K., Steen E., Sunyecz A., Stewart R., Moore J., Ziebro T., Northcutt R.G., Homsy M., Bertani P., Lu W., Roy S., Khanna S., Rink C., Sundaresan V.B., Otero J.J., Lee L.J., Sen C.K. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 2017;12:974–979. doi: 10.1038/nnano.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jorstad N.L., Wilken M.S., Grimes W.N., Wohl S.G., VandenBosch L.S., Yoshimatsu T., Wong R.O., Rieke F., Reh T.A. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017;548:103–107. doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.de Lázaro I., Yilmazer A., Nam Y., Qubisi S., Razak F.M.A., Degens H., Cossu G., Kostarelos K. Non-viral, tumor-free induction of transient cell reprogramming in mouse skeletal muscle to enhance tissue regeneration. Mol. Ther. 2019;27:59–75. doi: 10.1016/j.ymthe.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.El Waly B., Cayre M., Durbec P. Promoting myelin repair through in vivo neuroblast reprogramming. Stem Cell Rep. 2018;10:1492–1504. doi: 10.1016/j.stemcr.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Becskei A. Tuning up transcription factors for therapy. Molecules. 2020;25:1902. doi: 10.3390/molecules25081902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li Y., Fan L., Liu S., Liu W., Zhang H., Zhou T., Wu D., Yang P., Shen L., Chen J., Jin Y. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials. 2013;34:5048–5058. doi: 10.1016/j.biomaterials.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 259.Ben-Shushan D., Markovsky E., Gibori H., Tiram G., Scomparin A., Satchi-Fainaro R. Overcoming obstacles in microRNA delivery towards improved cancer therapy. Drug Deliv. Transl. Res. 2014;4:38–49. doi: 10.1007/s13346-013-0160-0. [DOI] [PubMed] [Google Scholar]
- 260.Jayawardena T.M., Egemnazarov B., Finch E.A., Zhang L., Payne J.A., Pandya K., Zhang Z., Rosenberg P., Mirotsou M., Dzau V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Manaka T., Suzuki A., Takayama K., Imai Y., Nakamura H., Takaoka K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials. 2011;32:9642–9648. doi: 10.1016/j.biomaterials.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 262.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X.-J. Therapeutic siRNA: state of the art. Signal Transduct. Targeted Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nguyen M.K., Jeon O., Dang P.N., Huynh C.T., Varghai D., Riazi H., McMillan A., Herberg S., Alsberg E. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 2018;75:105–114. doi: 10.1016/j.actbio.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cui Z.-K., Fan J., Kim S., Bezouglaia O., Fartash A., Wu B.M., Aghaloo T., Lee M. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J. Control. Release Off. J. Control. Release Soc. 2015;217:42–52. doi: 10.1016/j.jconrel.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Setten R.L., Rossi J.J., Han S. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 266.J D., K P., H D., C V., L D.G., L M., Sy Ö., D D., Sc D.S., K R. Non-viral delivery of chemically modified mRNA to the retina: subretinal versus intravitreal administration. J. Control. Release Off. J. Control. Release Soc. 2019;307 doi: 10.1016/j.jconrel.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 267.Finn J.D., Smith A.R., Patel M.C., Shaw L., Youniss M.R., van Heteren J., Dirstine T., Ciullo C., Lescarbeau R., Seitzer J., Shah R.R., Shah A., Ling D., Growe J., Pink M., Rohde E., Wood K.M., Salomon W.E., Harrington W.F., Dombrowski C., Strapps W.R., Chang Y., Morrissey D.V. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22:2227–2235. doi: 10.1016/j.celrep.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 268.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2018;5 doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kim S., Kim D., Cho S.W., Kim J., Kim J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang P., Zhang L., Zheng W., Cong L., Guo Z., Xie Y., Wang L., Tang R., Feng Q., Hamada Y., Gonda K., Hu Z., Wu X., Jiang X. Thermo-triggered release of CRISPR-cas9 system by lipid-encapsulated gold nanoparticles for tumor therapy. Angew. Chem., Int. Ed. Engl. 2018;57:1491–1496. doi: 10.1002/anie.201708689. [DOI] [PubMed] [Google Scholar]
- 271.Luo Y.-L., Xu C.-F., Li H.-J., Cao Z.-T., Liu J., Wang J.-L., Du X.-J., Yang X.-Z., Gu Z., Wang J. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano. 2018;12:994–1005. doi: 10.1021/acsnano.7b07874. [DOI] [PubMed] [Google Scholar]
- 272.Niu Y., Shen B., Cui Y., Chen Y., Wang J., Wang L., Kang Y., Zhao X., Si W., Li W., Xiang A.P., Zhou J., Guo X., Bi Y., Si C., Hu B., Dong G., Wang H., Zhou Z., Li T., Tan T., Pu X., Wang F., Ji S., Zhou Q., Huang X., Ji W., Sha J. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 273.Yin H., Song C.-Q., Dorkin J.R., Zhu L.J., Li Y., Wu Q., Park A., Yang J., Suresh S., Bizhanova A., Gupta A., Bolukbasi M.F., Walsh S., Bogorad R.L., Gao G., Weng Z., Dong Y., Koteliansky V., Wolfe S.A., Langer R., Xue W., Anderson D.G. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 2016;34:328. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Natale C.F., Angrisano T., Pistelli L., Falco G., Calabrò V., Netti P.A., Ventre M. Topographic cues impact on embryonic stem cell zscan4-metastate. Front. Bioeng. Biotechnol. 2020;8:178. doi: 10.3389/fbioe.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schellenberg A., Joussen S., Moser K., Hampe N., Hersch N., Hemeda H., Schnitker J., Denecke B., Lin Q., Pallua N., Zenke M., Merkel R., Hoffmann B., Wagner W. Matrix elasticity, replicative senescence and DNA methylation patterns of mesenchymal stem cells. Biomaterials. 2014;35:6351–6358. doi: 10.1016/j.biomaterials.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 276.Lv H., Wang H., Zhang Z., Yang W., Liu W., Li Y., Li L. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42–48. doi: 10.1016/j.lfs.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 277.Jung H.-M., Song G.-A., Lee Y.-K., Baek J.-H., Ryoo H.-M., Kim G.-S., Choung P.-H., Woo K.M. Modulation of the resorption and osteoconductivity of alpha-calcium sulfate by histone deacetylase inhibitors. Biomaterials. 2010;31:29–37. doi: 10.1016/j.biomaterials.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 278.Nemec S., Kilian K.A. Materials control of the epigenetics underlying cell plasticity. Nat. Rev. Mater. 2021;6:69–83. doi: 10.1038/s41578-020-00238-z. [DOI] [Google Scholar]
- 279.Hu Y., Zhang F., Zhong W., Liu Y., He Q., Yang M., Chen H., Xu X., Bian K., Xu J., Li J., Shen Y., Zhang H. Transplantation of neural scaffolds consisting of dermal fibroblast-reprogrammed neurons and 3D silk fibrous materials promotes the repair of spinal cord injury. J. Mater. Chem. B. 2019;7:7525–7539. doi: 10.1039/c9tb01929d. [DOI] [PubMed] [Google Scholar]
- 280.Balmayor E.R. Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev. 2015;94:13–27. doi: 10.1016/j.addr.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 281.Cui Z.-K., Sun J.A., Baljon J.J., Fan J., Kim S., Wu B.M., Aghaloo T., Lee M. Simultaneous delivery of hydrophobic small molecules and siRNA using Sterosomes to direct mesenchymal stem cell differentiation for bone repair. Acta Biomater. 2017;58:214–224. doi: 10.1016/j.actbio.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fan J., Im C.S., Cui Z.-K., Guo M., Bezouglaia O., Fartash A., Lee J.-Y., Nguyen J., Wu B.M., Aghaloo T., Lee M. Delivery of phenamil enhances BMP-2-induced osteogenic differentiation of adipose-derived stem cells and bone formation in calvarial defects. Tissue Eng. 2015;21:2053–2065. doi: 10.1089/ten.TEA.2014.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lo K.W.-H., Kan H.M., Laurencin C.T. Short-term administration of small molecule phenamil induced a protracted osteogenic effect on osteoblast-like MC3T3-E1 cells. J. Tissue Eng. Regen. Med. 2016;10:518–526. doi: 10.1002/term.1786. [DOI] [PubMed] [Google Scholar]
- 284.Sangadala S., Devereaux E.J., Presciutti S.M., Boden S.D., Willet N.J. FK506 induces ligand-independent activation of the bone morphogenetic protein pathway and osteogenesis. Int. J. Mol. Sci. 2019;20:1900. doi: 10.3390/ijms20081900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Huang C., Tu W., Fu Y., Wang J., Xie X. Chemical-induced cardiac reprogramming in vivo. Cell Res. 2018;28:686–689. doi: 10.1038/s41422-018-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]