Graphical abstract
Keywords: β-Conglycinin, Emulsion-filled gel, Structure, β-Carotene, Release behavior
Highlights
-
•
7S modification and emulsion concentration influenced the structure of the emulsion-filled gels.
-
•
The formation of the gel network was dominated by hydrogen and disulfide bonds, and water migration occurred.
-
•
7S emulsion-filled gel demonstrated a slow-release effect on β-carotene.
-
•
M−15 samples had the best β-carotene bioaccessibility and stability.
Abstract
In this work, emulsion-filled gels were prepared from natural and pH-shifting combined with ultrasound β-conglycinin (7S) as emulsifiers. The emulsifier modification and emulsion concentrations (5, 10, 15, 20 wt%) were evaluated on the structural and β-carotene release properties of the gels. Compared to the 7S hydrogel, the emulsion-filled gels exhibited better water-holding and textural properties. The 7S modification and the increase in emulsion concentration resulted in altered water distribution and improved microstructure and rheological properties of the emulsion-filled gels. The dense and homogeneous gel network was formed at an emulsion content of 15 wt%. The gels were regulated by different release kinetics in a simulated gastrointestinal environment. M−15 showed the highest bioaccessibility and chemical stability (72.25% and 89.87%) with good slow-release properties of β-carotene. These results will guide the development of encapsulated delivery systems for gel food products.
1. Introduction
β-Carotene is widely found in fruits and plants and is the most essential carotenoid. As a precursor to vitamin A, β-carotene plays an essential role in human visual health and cancer prevention, among others [1]. However, β-carotene is poorly water soluble and unstable to oxygen, light, and heat, resulting in a high susceptibility to degradation during application [2]. In addition, the poor bioaccessibility of β-carotene in gastrointestinal digestion further limits the scope of its application [3]. Therefore, the construction of various delivery systems to enhance the stability and bioaccessibility of β-carotene is becoming increasingly popular. A promising strategy is the encapsulation of oil in gel with a three-dimensional network structure [4]. Depending on the preparation method, emulsion gels are divided into two kinds, emulsion-filled gels and emulsion-particle gels [5]. Compared to particle gels, emulsion-filled gels have the advantage of adjustability of particle composition, structure and size [6].
Typically, emulsions are applied as active fillers to strengthen the structural properties of the emulsion-filled gels. The surface activity for the preparation of oil-in-water (O/W) emulsions is influenced by the proteins. The use of natural globulins as gel-filling particles is limited due to their compact structure and relatively low emulsification capacity [7]. β-Conglycinin (7S) is a trimeric form of glycoprotein that stabilizes the protein structure through non-covalent bonds [8]. Tang et al. [9] revealed that 7S had better emulsification activity and could be rapidly absorbed to form a highly viscoelastic interfacial layer on the surface of oil droplets. Peng and Tang [10] found that the use of 7S as an emulsifier and stabilizer could form HIPE gels, however, the freeze–thaw stability was relatively low. In addition, it was also found that 7S formed gels with a looser and more disordered structure compared to soybean protein isolate and 11S [11]. Therefore, further modifications of the emulsification and gelling properties of 7S are needed to expand its application.
Protein structure modification techniques are receiving increasing attention for improving functional properties. pH-Shifting is the unfolding of proteins at extremly pH conditions and then adjusting them into a neutral environment for refolding. Protein functional properties are improved by polar group exposure and enhanced conformational flexibility [12]. In addition, ultrasound treatment breaks down proteins by cavitation and shear stress, thus changing the spatial conformation of the protein [13]. Previous studies by the team demonstrated that pH-shifting combined with ultrasound treatment could enhance the emulsification properties and stability of emulsions [8]. Prior work has concentrated on elucidating the interactions between the emulsion filler and the gel matrix [14], [15]. To achieve the best performances of emulsion-filled gels, the investigators tried to change the conformational of the matrix and filler particles [4]. Feng et al. [16] used beet pectin/soy protein isolate as an emulsion-filled gel matrix and found that the textural, rheological and thermal stability of emulsion-filled gels increased with the addition of beet pectin. Lu et al. [17] changed the gel filler conformation by adding modified whey protein and found that the higher content of modified protein contributed to the higher mechanical properties of the gels. Ma et al. [7] found that the use of ultrasound-treated pea protein emulsion as filler significantly improved the hardness, water binding capacity and cooking loss of emulsion-filled gels with natural pea protein as the matrix. However, there are fewer studies on the effect of rheological properties and mechanical properties of emulsion-filled gels on their release regularity.
Therefore, this research focused on the effect of the concentration in natural and modified protein-stabilized emulsions on the structural properties and release mechanisms of filled gels. The mechanical properties, water holding capacity, interaction forces, water distribution, microstructure and rheological properties of the gels were studied. The release behavior as well as the digestive properties were further evaluated with gel encapsulated β-carotene. This will support the study of emulsion gel systems and the application of bioactive substance delivery systems.
2. Materials and methods
2.1. Materials
Defatted soybeans were obtained from High-Tech Co., Ltd. (Harbin, China). β-carotene (>98%, purity) from Yuanye Biotechnology Co., Ltd (Shanghai, China), and corn oil was bought from a local store (Harbin, China). TGase (for soy protein isolate, 100 U/g) was bought from Solarbio Bio-Technology Co., Ltd. (Beijing, China). All other chemicals were obtained from supply stores as analytical-grade reagents.
2.2. Preparation of β-conglycinin (7S)
7S was extracted based on our previous report [8]. The obtained 7S protein content was 98.02% ± 0.50% tested by the Kjeldahl nitrogen method.
2.3. Modification and emulsion preparation of 7S
In our previous study, it was found that pH 12 combined with ultrasound treatment modified the 7S conformation and emulsification properties significantly higher than other control samples [8]. Therefore, it was chosen for the preparation of filled emulsions in this study.
pH-Shifting combined with ultrasound treatment: The 7S (1%, w/v) was dissolved in deionized (DI) water and stirred at room temperature for 2 h. The pH of solution was conditioned to 12 with 2 M NaOH and incubated for 1 h. After ultrasound at 400 W for 10 min. The pH of solution was adjusted to 7.0 with 2 M HCl, then freeze-dried and used.
Preparation of natural and modified protein-stabilized emulsions: Emulsions were prepared using natural (designated as “N”) and modified 7S (designated as “M”). The 7S suspension (1%, w/v) and corn oil (with/without 0.5 wt% β-carotene) were mixed at 9:1 (v/v) and 10,000 g for 2 min, then homogenized for three cycles at 80 MPa using a high-pressure homogenizer (Microfluidizer, M−110P, Microfluidics, Newton, UK) to obtain the emulsion. In addition, corn oil containing β-carotene was stirred continuously at 50 ℃ until it was completely dissolved.
2.4. Preparation of emulsion-filled gels
The 7S dispersion (10 wt%) was obtained by dissolving 7S in DI water. Different concentrations of emulsions were added to the 7S dispersion and mixed for 30 min at 25 ℃, then, TGase (10 U/g) was added and stirred well, and finally, the gel was formed at 37 ℃ for 2 h [18]. For convenience, the gels containing different types of emulsions were labeled. Gels consisting of natural 7S emulsions (5, 10, 15, 20, wt%) and 10 wt% 7S solutions were referred to as “N-5″, ”N-10″, “N-15″, and ”N-20″. Emulsion-filled gels consisting of modified 7S emulsions (5, 10, 15, 20 wt%) and 10 wt% 7S solutions were referred to as “M−5″, ”M−10″, “M−15″, and ”M−20″. The 7S hydrogel without emulsion was named “7S” as a control group.
2.5. Determination of gel water-holding capacity (WHC)
The WHC was evaluated based on a slight modification reported by Wang et al. [19]. Samples were centrifuged at high speed (10,000 g for 20 min) at 4 °C to remove water. WHC was calculated according to equation (1):
| (1) |
where W1 and W2 are the masses of the gels before and after centrifugation, respectively.
2.6. Texture profile analysis (TPA)
TPA is used as a texture analyzer (FTC, Rockville, MD, USA) [20]. Parameters: P/36R probe, 5 g trigger force, 10.0 mm puncture distance, and 1.0 mm/s determination speed.
2.7. Determination of gel forces
The interaction forces between the molecules of the gels were assayed based on the records of Jiang et al. [21] with slight modifications. The 2 g emulsion gel was crushed and dissolved in 18 mL of the following four different solvents: (A) 50 mM sodium phosphate buffer (pH 7.0); (B) solvent A containing 8 M urea (for determination of hydrogen bonding); (C) solvent A containing 0.5% SDS (for determination of total non-covalent forces); (D) solvent A containing 0.25% β-mercaptoethanol solution (determination of disulfide bonds). The gels and different solvents were thoroughly mixed using Ultra-Turrax (IKA T18, Germany) and centrifuged at 5,000 g for 30 min. The intermolecular forces were represented as the solubility in different solvents.
2.8. Scanning electron microscopy (SEM)
First, the gel samples were cut and fixed with glutaraldehyde. Next, the samples were dehydrated with different concentrations of ethanol. Finally, freeze-drying, sputtering plating and observation were performed (SU8010, Hitachi, Japan) [22].
2.9. Confocal laser scanning microscopy (CLSM)
The emulsion-filled gel samples were cut into very thin slices and labeled with Nile Blue and Nile Red for proteins and lipids, respectively. The samples were then placed in the center of the slides and carefully covered with coverslips and observed using a CLSM (Leica Microsystems, Heidelberg GmbH, Germany) [23]. Excitation wavelength: 633 nm for protein, 488 nm for oil.
2.10. Low-field nuclear magnetic resonance (LF-NMR)
The samples were placed in a 15 mm diameter sample vial. The moisture distribution and state of the gels were tested using an LF-NMR analyzer (Bruker Optik GmbH Ettlingen, Germany) with reference to the method of Shao et al. [24].
2.11. Rheological measurements
The Sample’s apparent viscosity and dynamic viscoelasticity were determined using a DHR-1 rheometer (TA, Crawley, UK). Parameters: parallel shift 35 mm, gap 1 mm, equilibration 120 s, shear rate 0.1 to 100 s−1, frequency 0.1 to 10 rad/s.
2.12. Release kinetics
The release of β-carotene from gels was analyzed as described by Liao et al. [25]. Briefly, the β-carotene content was extracted and determined by simulating the gastrointestinal environment by treating the samples in Tris-HCl at pH 2.0 and PBS at pH 7.5 and evaluating the degree of release according to equation (2):
| (2) |
Next, the release kinetics of β-carotene from the emulsion-filled gel was simulated using the Ritger-Peppas model in equation (3) [26]:
| (3) |
where Q represents the cumulative β-carotene content at t hours, K0 indicates the release velocity, and n represents the release index.
2.13. In vitro simulation digestion model
Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) was prepared as described by Zou et al. [27] and all digestive fluids were preheated to 37 °C for use.
Gastric phase: 15 mL of sample was added to 15 mL of SGF, pH was adjusted to 2.5, and shaken continuously at 100 rpm to simulate gastric digestion for 2 h.
Small intestine phase: To 30 mL of the gastric phase digestion sample, 1.5 mL of SIF, 3.5 mL of bile salt solution (54 mg/mL) and 2.5 mL of lipase solution (24 mg/mL) were added. Then, the pH of the system was adapted to 7.0 and the simulated intestinal digestion was continued for 5 h.
2.14. Bioaccessibility and stability of β-carotene
The simulated digested samples were centrifuged (12,000 g, 30 min) and the micellar phase was collected for β-carotene solubilization. Then, the micellar phase was mixed with acetone, ethanol, and hexane at 1:1:1:2 (v/v) and centrifuged (2,500 g, 15 min). The free content of β-carotene was assessed according to the absorbance at 450 nm [28]. The bioaccessibility and stability of β-carotene were calculated according to equations (4), (5):
| (4) |
| (5) |
where AI, AM, and AD represent the β-carotene concentrations in the small intestine, in the micelle phase, and the small intestinal digest, respectively.
2.15. Statistical analysis
SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and Origin 2023 (Origin-Lab Co., Northampton, MA, USA) were used for statistical analysis and graphical drawing. All indicators were measured in three replicates. p < 0.05 represented significant differences.
3. Results and discussion
3.1. TPA
TPA, as an important indicator to assess the suitability of a gel as a delivery system, is often used to simulate the palatability of emulsion gels and to analyze their structural properties [29]. From Table 1, the hardness, resilience, cohesiveness, springiness, and gumminess of 7S emulsion-filled gels were significantly stronger (p < 0.05) than those of 7S hydrogel. The mechanical properties of the emulsion-filled gels enhanced with the increase of emulsion concentration (0 to 15 wt%). This was probably attributed to the interaction of the emulsion droplets as active fillers with the gel matrix, forming a highly integrated gel network [30], with the interaction enhancing as the emulsion concentration increased. When the emulsion concentration was 20 wt%, the textural properties started to deteriorate. The cross-linking of molecules in the gel matrix might be reduced at too high emulsion content, which was not favorable for the formation of the gel [31]. Moreover, the textural characteristics of the emulsion-filled gels treated with pH-shifting combined with ultrasound were superior to the natural emulsion-filled gels. The modified protein was amphiphilic by unfolding at the oil droplet interface structure, which may result in the stronger chemical affinity of the emulsion particles for the 7S matrix [5]. M−15 treated with pH-shifting and ultrasound treatment showed better textural properties compared to the other samples.
Table 1.
Textural properties of the control (7S hydrogel) and natural or modified emulsion-filled gels with different emulsion concentrations (5, 10, 15, 20 wt%). Different letters (a-i) indicate significant differences (p < 0.05) within the same group.
| Samples | Hardness (g) | Resilience (%) | Cohesiveness | Springiness (mm) | Gumminess |
|---|---|---|---|---|---|
| 7S | 195.41 ± 2.05 h | 0.13 ± 0.05ab | 0.32 ± 0.01e | 0.78 ± 0.02i | 78.32 ± 1.00i |
| N-5 | 267.23 ± 2.38f | 0.12 ± 0.01b | 0.38 ± 0.01d | 0.98 ± 0.01 h | 86.94 ± 1.00 h |
| M−5 | 343.81 ± 2.06b | 0.17 ± 0.02a | 0.52 ± 0.01b | 1.02 ± 0.06 g | 123.50 ± 1.03e |
| N-10 | 273.42 ± 0.67e | 0.14 ± 0.03ab | 0.48 ± 0.01c | 1.67 ± 0.02d | 137.58 ± 0.65d |
| M−10 | 303.33 ± 2.24c | 0.13 ± 0.03ab | 0.52 ± 0.02b | 1.89 ± 0.01b | 147.42 ± 0.63b |
| N-15 | 294.34 ± 2.01d | 0.15 ± 0.02ab | 0.53 ± 0.02ab | 1.77 ± 0.02c | 144.68 ± 0.87c |
| M−15 | 461.65 ± 3.21a | 0.15 ± 0.02ab | 0.54 ± 0.03ab | 1.97 ± 0.02a | 174.47 ± 0.81a |
| N-20 | 227.90 ± 2.03 g | 0.12 ± 0.01b | 0.48 ± 0.01c | 1.53 ± 0.01f | 103.85 ± 1.15 g |
| M−20 | 230.00 ± 1.56 g | 0.15 ± 0.01ab | 0.56 ± 0.02a | 1.64 ± 0.01e | 111.48 ± 1.26f |
3.2. WHC
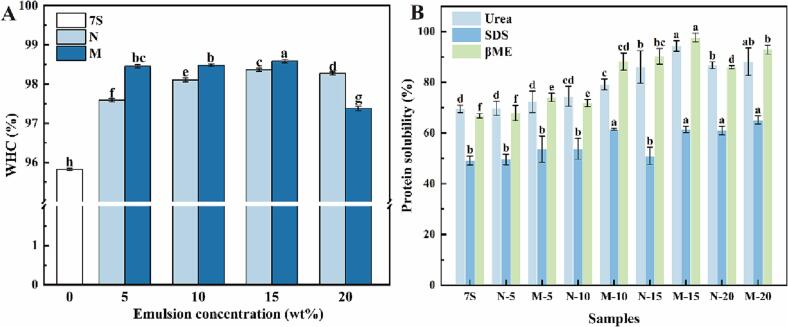
WHC represents the ability of the gel under external pressure (centrifugation or compression) to prevent internal water loss [32]. Fig. 1 shows the WHC of different emulsion-filled gel samples. The WHC of emulsion-filled gels ranged from 97.37 to 98.58%, which was significantly higher (p < 0.05) than the control 7S hydrogel (95.82%). The M−15 showed the highest WHC (98.58%), which was consistent with the TPA (Section 3.1). The moderate emulsion addition could improve the strength and densification of the gel network, reduce the porosity, and thus improve WHC [31]. However, the addition of excess emulsion, on the contrary, can loosen the gel structure and increase the porosity of the gel network. Compared to natural emulsion-filled gels, there were more interaction forces in the emulsion gel network with the pH-shifting combined with ultrasound. This was attributed to the covalent linkage of the emulsion particles to the 7S protein, resulting in greater resistance to the applied external pressure and higher WHC [33].
Fig. 1.
WHC (A) and protein solubility (B) of control (7S hydrogel) and natural or modified emulsion-filled gels with different emulsion concentrations (5, 10, 15, 20 wt%). Different letters (a-h) indicate significant differences (p < 0.05) with the same group.
3.3. Microstructure
The SEM images of the gels are shown in Fig. 2A. The 7S hydrogel shows an inhomogeneous structure and the pores of the gel vary with increasing emulsion concentration. Compared to the 7S hydrogel, the natural and modified emulsion-filled gels showed smaller pores at lower emulsion concentrations, however, when emulsion concentrations reached 20 wt%, excessive porosity was induced The high emulsion content caused the gel network to become loose, the roughness to increase, and the pores to enlarge [34]. Furthermore, the modified emulsion-filled gels exhibited more regular and uniform, with finer and denser pores compared to these natural emulsion-filled gels. This might be attributed to the unfolding of protein structure, subunit dissociation and exposure of active sites by pH-shifting combined with ultrasound treatment, which facilitated the formation of densely structured gels with strong interactions [33].
Fig. 2.
SEM images (A) and CLSM images (B) of control (7S hydrogel) and natural or modified emulsion-filled gels with different emulsion concentrations (5, 10, 15, 20 wt%).
CLSM was used to better analyze the filling state and distribution of emulsion droplets in gels [18]. As shown in Fig. 2B, the distribution of oil droplets in natural and modified emulsion-filled gels was found to be similar, while the modified emulsion-filled gels formed smaller aggregates. The protein electrostatic repulsion was enhanced after the modification treatment, which had a strong spatial site resistance effect [8]. Meanwhile, the interaction between the proteins at the oil droplet interface was enhanced, resulting in the formation of smaller aggregates [23]. With the increase of emulsion concentration, the emulsion particles in the gel become smaller and more uniformly distributed, with the optimal distribution of M−15. This indicated that the emulsion droplets as active fillers interacted with the gel matrix increased, forming a more dense and homogeneous gel network [30].
The microstructural changes brought about by the protein modification and emulsion concentration further explained the improvement in TPA and WHC of the gels (3.1, 3.2), which was consistent with the findings of Lee and Lucey [35]. Overall, pH-shifting combined with ultrasound treatment and emulsion concentration could change the microstructure of emulsion-filled gels to form a more dense and homogeneous network structure.
3.4. Interaction forces
As shown in Fig. 1B, compared to 7S hydrogel (69.5%), protein solubility in the emulsion-filled gels with added urea significantly increased with rising emulsion content (p < 0.05). The addition of emulsion might promote changes in the protein structure, exposing more tyrosine residues and more binding sites to water molecules, leading to increased hydrogen bonding forces in the gel [36]. The protein solubility of the gels in SDS did not change significantly (p > 0.05), indicating that hydrophobic interactions played an auxiliary role in the construction of protein gel networks. However, the protein solubility of the gel filled with modified emulsion was relatively higher. This could be explained that the pH-shifting combined with ultrasound treatment unfolded the protein structure, exposing more hydrophobic groups and enhancing the hydrophobic interactions within the gel [7].
In addition, gels formed by the addition of 5 to 15 wt% emulsion showed a significant increase in protein solubility in β-mercaptoethanol compared to the 7S hydrogel (p < 0.05). These suggested that moderate amount of emulsion added promoted the increase of disulfide bond content during gelation and strengthened the cross-linking of the gels. However, excessive emulsion addition led to droplet aggregation and hindered the formation of disulfide bonds [37]. The protein solubility of N-20 and M−20 in β-mercaptoethanol was reduced to 86.00% and 92.92%. Moreover, compared to natural emulsion-filled gels, modified emulsion-filled gels showed higher solubility in β-mercaptoethanol. This was attributed to the fact that the pH-shifting combined with the ultrasound treatment could promote the oxidation of -SH and enhance the disulfide bonding in the gels [8]. The results suggested that formation of emulsion-filled gel was dominated by intermolecular hydrogen and disulfide bonds and also closely related to hydrophobic interactions.
3.5. LF-NMR
LF-NMR was employed to characterize the distribution state and mobility of water molecules in the gel system [38]. The water of gel is classified into three types depending on the T2 relaxation time: T2b (1–10 ms) is regarded as bound water; T21 (50–500 ms) denotes fixed water in the structure; T22 (1,000–3,000 ms) indicates free water [39]. As shown in Fig. 3A and 3B, with the increase of emulsion content, the T2b, T21 and T22 curves shifted toward lower relaxation times compared to 7S hydrogel. Generally, the longer the relaxation time, the more mobile the water will be in the emulsion-filled gel structure [40]. Therefore, the shortening of the relaxation time of the samples reflected the increased interaction among the water molecules and the gel and between the gel matrix and the emulsion [41]. Ma et al. [39] reported that the more fluid the water in the gel, the weaker the WHC, which was the same as our findings (Section 3.2).
Fig. 3.
Transverse relaxation curves (A), water ratio (B), and transverse relaxation time and peak area (C) of control (7S hydrogel) and natural or modified emulsion-filled gels with different emulsion concentrations (5, 10, 15, 20 wt%). PT2b, PT21, and PT22 denote the percentage of bound water, immobilized water, and free water, respectively. Different letters (a-f) indicate significant differences (p < 0.05) with the same group.
From Fig. 3C, T2b decreased from 2.25 ms to 0.64 ms, and the T2b of the gels filled with the modified protein stabilization emulsion became smaller compared to the natural protein. This indicated that the water molecules in the pH-shifting combined with ultrasound treatment of the emulsion gel were more tightly bound to the proteins or other substances in the gel [22]. The T21 values of the gels filled with pH-shifting combined with ultrasound protein emulsions were all lower than those of the gels filled with natural protein emulsions. This might be attributed to the smaller aperture size of the modified gel structure (Section 3.3). Interestingly, the conversion of immobilized water (PT21) to free water (PT22) in the system started after the addition of the modified emulsion. The reason was that the exposed hydrophobic group of the protein resulted from modification treatment and the increased capillary effect caused by the dense network structure of the gel, which facilitated the transfer of water [7].
3.6. Rheological measurement
The effects of emulsion type and addition amount on the rheological properties of the gel are shown in Fig. 4. From Fig. 4A, as the shear rate rose, the samples sheared thinner and the apparent viscosity kept decreasing. This was attributed to the mechanical shear disrupting the gel network structure, resulting in reduced flow resistance [42]. With the addition of modified emulsion, the viscosity of the samples increased continuously and M−15 showed the maximum apparent viscosity. This could be explained by the higher oil content at higher modified emulsion concentrations, which increased the potential for the oil to be encapsulated by proteins, thus affecting the network structure of the gel matrix and leading to an increase in viscosity [43]. As shown in Fig. 4B and 4C, the dynamic rheological properties (G' and G'') of the emulsion-filled gels were superior to the 7S hydrogel. When the emulsion concentration was raised, the G' and G“ of the emulsion-filled gels tended to increase and then decrease, reaching the optimum at 15 wt%. This indicated that the gel internal interactions were enhanced at appropriate concentrations [44]. The G' was higher than the G” of the gels in the frequency range, and the G' and G“ values varied less with frequency, indicating the high elasticity of the gels [45]. Moreover, the G' of the gels with the addition of modified emulsions were all higher than those with the natural emulsions. On the one hand, the modified protein was more flexible, with increased binding sites for interactions and cross-linking; on the other hand, it formed smaller emulsion droplets that were more effective as active fillers, resulting in a more compact gel structure, leading to higher G' [46]. These results suggested that the pH-shifting combined with ultrasound treatment and emulsion concentration influenced the rheological properties of the emulsion-filled gels by changing the structure and interactions to form a more dense gel network.
Fig. 4.
Apparent viscosity (A), Storage modulus (G') (B) and loss modulus (G'') (C) of control (7S hydrogel) and natural or modified emulsion-filled gels with different emulsion concentrations (5, 10, 15, 20 wt%).
3.7. Release kinetics
Since the control group (7S hydrogel) did not contain β-carotene, only the emulsion-filled gel samples were analyzed. Fig. 5 shows the release behavior of β-carotene from emulsion-filled gels in continuous gastrointestinal simulated digestion. The release was below 29% at 2 h of simulated gastric digestion and accelerated upon entering the intestinal phase. This was attributed to the pH change causing water uptake and swelling of the emulsion-filled gel and increased erosion of the gel matrix, leading to rapid diffusion of β-carotene [47]. The slow-release properties of the emulsion-filled gels improved with increasing emulsion concentration, and the best slow-release was achieved with the 15 wt% emulsion concentration. It could be hypothesized that the addition of a moderate amount of emulsion enhanced the hardness of the emulsion-filled gels and formed a tighter network structure, which effectively resisted the erosion of gastric and intestinal fluids [48]. The modified emulsion-filled gel showed better retardation than the natural emulsion-filled gel. This could be explained that the pH-shifting combined with ultrasound treatment enhanced the hydrophobicity of the protein surface and the structural properties of the gel, inhibiting the diffusion of β-carotene from the oil phase to the external environment [8].
Fig. 5.
Release behavior of β-carotene from emulsion-filled gel in continuous simulated gastrointestinal fluid.
The release mechanism of β-carotene from gels utilizing the Ritger-Peppas model, and the release index (n) and the regression coefficients (R2) were calculated (Table 2). The R2 for all samples was higher than 0.9, suggesting that the model was appropriate for fitting the data. The n can specifically indicate the kinetic release behavior of the nutrient. Among them, n < 0.45 represents Fickian diffusion kinetics; 0.45 < n < 0.81 release behavior belongs to the superposition of Fickian diffusion kinetics and erosion, and n > 0.81 means substrate erosion [26]. The n of β-carotene release during simulated gastric fluid was close to 0.45, indicating that the release behavior was mainly Fickian diffusion. At this time the gel matrix was dehydrated and contracted, and β-carotene diffused from the undegraded matrix. The release mechanism of the emulsion-filled gel in the simulated intestinal phase was combined with Fickian diffusion and erosion. This implied that erosion and swelling of the gel matrix became the main factors affecting the diffusive release of β-carotene.
Table 2.
Release kinetics parameters of β-carotene from the emulsion-filled gel at pH 2.0 to pH 7.5.
| Samples | Simulated gastric fluid |
Simulated intestinal fluid |
||
|---|---|---|---|---|
| n | R2 | n | R2 | |
| N-5 | 0.3286 | 0.9887 | 0.6524 | 0.9858 |
| M−5 | 0.3068 | 0.9848 | 0.5796 | 0.9957 |
| N-10 | 0.3714 | 0.9828 | 0.5835 | 0.9900 |
| M−10 | 0.4134 | 0.9753 | 0.6249 | 0.9865 |
| N-15 | 0.2866 | 0.9081 | 0.6012 | 0.9865 |
| M−15 | 0.4161 | 0.9661 | 0.4999 | 0.8989 |
| N-20 | 0.3828 | 0.9866 | 0.5921 | 0.9869 |
| M−20 | 0.3892 | 0.9672 | 0.5901 | 0.9868 |
Hence, we hypothesized that the β-carotene release matrix of emulsion-filled gels may be: with increasing emulsion concentration and modification, the gel matrix cross-linked to form a tightly and uniformly packed network. In the gastric phase, the water molecule invasion and matrix decomposition rate were reduced, limiting the release of β-carotene. In the intestinal phase, the gel network structure with enhanced swelling capacity and erosion resistance disintegrated at a slower rate, achieving a slow-release effect.
3.8. Bioaccessibility and stability
The biological activity of β-carotene is probably decreased owing to its low chemical instability and bioaccessibility in the gastrointestinal tract [49]. As shown in Fig. 6, the bioaccessibility of β-carotene increased with the increase of emulsion concentration. The bioaccessibility of β-carotene within the modified emulsion-filled gels was significantly better than natural emulsion gels (p < 0.05), and M−15 was the highest (72.25%). In general, the smaller the lipid droplet, the higher the bioaccessibility of β-carotene in the modified emulsion-filled gels [50]. The pH-shifting combined with ultrasound treatment reduced the particle size of the emulsion droplets, and the smaller oil droplets in the gel had a larger specific surface area, facilitating the release of β-carotene [51]. The denser gel structure reduced the aggregation of oil droplets during digestion, promoted the ability of lipase to approach the surface of oil droplets, and increased the β-carotene content in the micellar phase [50]. The stability of β-carotene in the emulsion-filled gels formed by the modification and emulsion concentration increase was significant (p < 0.05), with the highest M−15 at 89.86%. It was consistent with the trend of bioaccessibility. This phenomenon might be explained that the formation of a dense three-dimensional gel network reducing the contact of oxygen and radicals with the oil surface, and the enhanced antioxidant properties of the gel system [52].
Fig. 6.
The bioaccessibility and stability of natural or modified emulsion-filled gels with different emulsion concentrations (5, 10, 15, 20 wt%). Different letters (a-g) indicate significant differences (p < 0.05) with the same group.
4. Conclusion
In this work, pH-shifting combined with ultrasound treatment of 7S-stabilized emulsions were used as active fillers embedded in protein-based gel networks. The effects of emulsifier and emulsion concentration on the gel structure and controlled release properties were investigated. Protein modification and increased emulsion concentration improved the texture, water holding and rheological properties of the gels and the microstructure improved, resulting in a denser and more homogeneous network structure. Gel network formation was mainly dominated by hydrogen and disulfide bonds, and water migration occurred. The release of β-carotene within the gels was controlled by simulating the combination of Fickian diffusion in gastric fluid and Fickian diffusion and erosion in intestinal fluid. The β-carotene bioaccessibility and stability were significantly improved in M−15. An important extension of this research is to guide the development of functional food delivery systems containing bioactive substances.
CRediT authorship contribution statement
Ziteng Lian: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft. Sai Yang: Conceptualization, Data curation, Visualization, Writing – original draft. Xinhui Peng: Software. Xiaohong Tong: Visualization. Mengmeng Wang: Visualization. Shicheng Dai: Writing – review & editing. Tingting Zhu: Methodology. Huan Wang: Conceptualization, Data curation, Supervision, Writing – review & editing. Lianzhou Jiang: Conceptualization, Resources, Visualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (32202105 and 32230082).
Contributor Information
Huan Wang, Email: whname@neau.edu.cn.
Lianzhou Jiang, Email: jlzname@neau.edu.cn.
References
- 1.Maiani G., Periago Castón M.J., Catasta G., Toti E., Cambrodón I.G., Bysted A., Granado-Lorencio F., Olmedilla-Alonso B., Knuthsen P., Valoti M., Böhm V., Mayer-Miebach E., Behsnilian D., Schlemmer U. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutrit. Food Res. 2009;53(S2):S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 2.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piorkowski D.T., McClements D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014;42:5–41. doi: 10.1016/j.foodhyd.2013.07.009. [DOI] [Google Scholar]
- 4.Lin D., Kelly A.L., Miao S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020;102:123–137. doi: 10.1016/j.tifs.2020.05.024. [DOI] [Google Scholar]
- 5.Dickinson E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012;28:224–241. doi: 10.1016/j.foodhyd.2011.12.017. [DOI] [Google Scholar]
- 6.Silva K.C.G., Feltre G., Hubinger M.D., Sato A.C.K. Protection and targeted delivery of β-carotene by starch-alginate-gelatin emulsion-filled hydrogels. J. Food Eng. 2021;290 doi: 10.1016/j.jfoodeng.2020.110205. [DOI] [Google Scholar]
- 7.Ma T., Xiong Y.L., Jiang J. Calcium-aided fabrication of pea protein hydrogels with filler emulsion particles coated by pH12-shifting and ultrasound treated protein. Food Hydrocoll. 2022;125 doi: 10.1016/j.foodhyd.2021.107396. [DOI] [Google Scholar]
- 8.Yang S., Lian Z., Wang M., Liao P., Wu H., Cao J., Tong X., Tian T., Wang H., Jiang L. Molecular structural modification of β-conglycinin using pH-shifting with ultrasound to improve emulsifying properties and stability. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang C.-H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017;57:2636–2679. doi: 10.1080/10408398.2015.1067594. [DOI] [PubMed] [Google Scholar]
- 10.Peng L.-P., Tang C.-H. Outstanding antioxidant pickering high internal phase emulsions by co-assembled polyphenol-soy β-conglycinin nanoparticles. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.109509. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z., Sun J., Zhao L., He W., Liu T., Liu B. Analysis of the gel properties, microstructural characteristics, and intermolecular forces of soybean protein isolate gel induced by transglutaminase. Food Sci. Nutr. 2022;10:772–783. doi: 10.1002/fsn3.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang J., Wang Q., Xiong Y.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018;19:50–56. doi: 10.1016/j.cofs.2018.01.002. [DOI] [Google Scholar]
- 13.Tang S.-Q., Du Q.-H., Fu Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farjami T., Madadlou A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019;86:85–94. doi: 10.1016/j.tifs.2019.02.043. [DOI] [Google Scholar]
- 15.Oliver L., Scholten E., van Aken G.A. Effect of fat hardness on large deformation rheology of emulsion-filled gels. Food Hydrocoll. 2015;43:299–310. doi: 10.1016/j.foodhyd.2014.05.031. [DOI] [Google Scholar]
- 16.Feng L., Jia X., Yan J., Yan W., Yin L. Mechanical, thermal stability and microstructural properties of emulsion-filled gels: Effect of sugar beet pectin/soy protein isolate ratio. LWT. 2021;141 doi: 10.1016/j.lwt.2021.110917. [DOI] [Google Scholar]
- 17.Lu Y., Mao L., Zheng H., Chen H., Gao Y. Characterization of β-carotene loaded emulsion gels containing denatured and native whey protein. Food Hydrocoll. 2020;102 doi: 10.1016/j.foodhyd.2019.105600. [DOI] [Google Scholar]
- 18.Lv D., Zhang P., Chen F., Yin L. Effects of emulsion concentration on the physicochemical properties of wheat bran arabinoxylan-soy protein isolate emulsion-filled gels used as β-carotene carriers. LWT. 2022;153 doi: 10.1016/j.lwt.2021.112498. [DOI] [Google Scholar]
- 19.Wang X., He Z., Zeng M., Qin F., Adhikari B., Chen J. Effects of the size and content of protein aggregates on the rheological and structural properties of soy protein isolate emulsion gels induced by CaSO4. Food Chem. 2017;221:130–138. doi: 10.1016/j.foodchem.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Wu C., McClements D.J., Li L., He M., Li Y., Teng F. Fabrication of composite hydrogels by assembly of okara cellulose nanofibers and gum Arabic in ionic liquids: Structure and properties. J. Mol. Liq. 2022;349 doi: 10.1016/j.molliq.2021.118132. [DOI] [Google Scholar]
- 21.Jiang J., Xiong Y.L. Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci. 2013;93:469–476. doi: 10.1016/j.meatsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Xi Z., Liu W., McClements D.J., Zou L. Rheological, structural, and microstructural properties of ethanol induced cold-set whey protein emulsion gels: Effect of oil content. Food Chem. 2019;291:22–29. doi: 10.1016/j.foodchem.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y., Sun Y., Wang Z., Zhong M., Li R., Yan S., Qi B., Li Y. Structure, rheology, and functionality of emulsion-filled gels: Effect of various oil body concentrations and interfacial compositions. Food Chem. X. 2022;16 doi: 10.1016/j.fochx.2022.100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao J.-H., Deng Y.-M., Jia N., Li R.-R., Cao J.-X., Liu D.-Y., Li J.-R. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chem. 2016;200:308–314. doi: 10.1016/j.foodchem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Liao P., Yang S., Dai S., Lian Z., Yang J., Zhang Q., Wang Y., Qi B., Wang H., Jiang L. The multilayered emulsion-filled gel microparticles: Regulated the release behavior of β-carotene. J. Food Eng. 2022;331 doi: 10.1016/j.jfoodeng.2022.111119. [DOI] [Google Scholar]
- 26.Ritger P.L., Peppas N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 27.Zou L., Zheng B., Liu W., Liu C., Xiao H., McClements D.J. Enhancing nutraceutical bioavailability using excipient emulsions: Influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. J. Funct. Foods. 2015;15:72–83. doi: 10.1016/j.jff.2015.02.044. [DOI] [Google Scholar]
- 28.Han J., Zhang Z., Shang W., Yan J., McClements D.J., Xiao H., Wu H., Zhu B. Modulation of physicochemical stability and bioaccessibility of β-carotene using alginate beads and emulsion stabilized by scallop (Patinopecten yessoensis) gonad protein isolates. Food Res. Int. 2020;129 doi: 10.1016/j.foodres.2019.108875. [DOI] [PubMed] [Google Scholar]
- 29.Alavi F., Emam-Djomeh Z., Salami M., Mohammadian M. Effect of microbial transglutaminase on the mechanical properties and microstructure of acid-induced gels and emulsion gels produced from thermal denatured egg white proteins. Int. J. Biol. Macromol. 2020;153:523–532. doi: 10.1016/j.ijbiomac.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Oner Z., Karahan A., Aydemir S., Aloglu H.S. Effect of transglutaminase on physicochemical properties of set-style yogurt. Int. J. Food Prop. 2008;11:196–205. doi: 10.1080/10942910701286346. [DOI] [Google Scholar]
- 31.Zhang B., Meng R., Li X.-L., Liu W.-J., Cheng J.-S., Wang W. Preparation of Pickering emulsion gels based on κ-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin. Food Chem. 2021;357 doi: 10.1016/j.foodchem.2021.129726. [DOI] [PubMed] [Google Scholar]
- 32.Chen H., Mao L., Hou Z., Yuan F., Gao Y. Roles of additional emulsifiers in the structures of emulsion gels and stability of vitamin E. Food Hydrocoll. 2020;99 doi: 10.1016/j.foodhyd.2019.105372. [DOI] [Google Scholar]
- 33.Nie Y., Liu Y., Jiang J., Xiong Y.L., Zhao X. Rheological, structural, and water-immobilizing properties of mung bean protein-based fermentation-induced gels: Effect of pH-shifting and oil imbedment. Food Hydrocoll. 2022;129 doi: 10.1016/j.foodhyd.2022.107607. [DOI] [Google Scholar]
- 34.Farjami T., Madadlou A., Labbafi M. Characteristics of the bulk hydrogels made of the citric acid cross-linked whey protein microgels. Food Hydrocoll. 2015;50:159–165. doi: 10.1016/j.foodhyd.2015.04.011. [DOI] [Google Scholar]
- 35.Lee W.-J., Lucey J. Formation and physical properties of yogurt. Asian Australas. J. Anim. Sci. 2010;23:1127–1136. doi: 10.5713/ajas.2010.r.05. [DOI] [Google Scholar]
- 36.Li Y., Wang Q., Guo L., Ho H., Wang B., Sun J., Xu X., Huang M. Effects of ultrafine comminution treatment on gelling properties of myofibrillar proteins from chicken breast. Food Hydrocoll. 2019;97 doi: 10.1016/j.foodhyd.2019.105199. [DOI] [Google Scholar]
- 37.Cen K., Yu X., Gao C., Yang Y., Tang X., Feng X. Effects of quinoa protein pickering emulsion on the properties, structure and intermolecular interactions of myofibrillar protein gel. Food Chem. 2022:133456. doi: 10.1016/j.foodchem.2022.133456. [DOI] [PubMed] [Google Scholar]
- 38.Xue H., Zhang G., Han T., Li R., Liu H., Gao B., Tu Y., Zhao Y. Improvement of gel properties and digestibility of the water-soluble polymer of tea polyphenol-egg white under thermal treatment. Food Chem. 2022;372 doi: 10.1016/j.foodchem.2021.131319. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y., Shan A., Wang R., Zhao Y., Chi Y. Characterization of egg white powder gel structure and its relationship with gel properties influenced by pretreatment with dry heat. Food Hydrocoll. 2021;110 doi: 10.1016/j.foodhyd.2020.106149. [DOI] [Google Scholar]
- 40.Farag K., Duggan E., Morgan D., Cronin D., Lyng J. A comparison of conventional and radio frequency defrosting of lean beef meats: Effects on water binding characteristics. Meat Sci. 2009;83:278–284. doi: 10.1016/j.meatsci.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Zhang R., Jiang H., Yan Z., Zhang Y., Zhang T., Liu X. Network structure of response to freeze-thaw cycles in egg white protein gels filled with emulsion: Digestive kinetics regulated by the state of water and embedded oil. Food Hydrocoll. 2023;135 doi: 10.1016/j.foodhyd.2022.108135. [DOI] [Google Scholar]
- 42.Pearce K.N., Kinsella J.E. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- 43.Zhao J., Bhandari B., Gaiani C., Prakash S. Physicochemical and microstructural properties of fermentation-induced almond emulsion-filled gels with varying concentrations of protein, fat and sugar contents. Curr. Res. Food Sci. 2021;4:577–587. doi: 10.1016/j.crfs.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo J., Zhang Y., Yang X.-Q. A novel enzyme cross-linked gelation method for preparing food globular protein-based transparent hydrogel. Food Hydrocoll. 2012;26:277–285. doi: 10.1016/j.foodhyd.2011.06.005. [DOI] [Google Scholar]
- 45.Zhang X., Zhang S., Zhong M., Qi B., Li Y. Soy and whey protein isolate mixture/calcium chloride thermally induced emulsion gels: Rheological properties and digestive characteristics. Food Chem. 2022;380 doi: 10.1016/j.foodchem.2022.132212. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Zhao J., Zhang S., Zhao X., Liu Y., Jiang J., Xiong Y.L. Structural and rheological properties of mung bean protein emulsion as a liquid egg substitute: The effect of pH shifting and calcium. Food Hydrocoll. 2022;126 doi: 10.1016/j.foodhyd.2022.107485. [DOI] [Google Scholar]
- 47.Rai U., Thrimawithana T.R., Dharmadana D., Valery C., Young S.A. Release kinetics of somatostatin from self‐assembled nanostructured hydrogels. Peptide Sci. 2018;110:e23085. doi: 10.1002/bip.23085. [DOI] [PubMed] [Google Scholar]
- 48.Caillard R., Petit A., Subirade M. Design and evaluation of succinylated soy protein tablets as delayed drug delivery systems. Int. J. Biol. Macromol. 2009;45:414–420. doi: 10.1016/j.ijbiomac.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Cornacchia L., Roos Y.H. Stability of β-carotene in protein-stabilized oil-in-water delivery systems. J. Agric. Food Chem. 2011;59:7013–7020. doi: 10.1021/jf200841k. [DOI] [PubMed] [Google Scholar]
- 50.Soukoulis C., Cambier S., Hoffmann L., Bohn T. Chemical stability and bioaccessibility of β-carotene encapsulated in sodium alginate o/w emulsions: Impact of Ca2+ mediated gelation. Food Hydrocoll. 2016;57:301–310. doi: 10.1016/j.foodhyd.2016.02.001. [DOI] [Google Scholar]
- 51.Liu W., Gao H., McClements D.J., Zhou L., Wu J., Zou L. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocoll. 2019;88:210–217. doi: 10.1016/j.foodhyd.2018.10.012. [DOI] [Google Scholar]
- 52.Zhang X., Chen X., Gong Y., Li Z., Guo Y., Yu D., Pan M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105756. [DOI] [PMC free article] [PubMed] [Google Scholar]