Abstract
The evolution over 30 years of a population of methicillin-resistant Staphylococcus aureus (MRSA) from a tertiary referral hospital was studied by phylogenetic analysis of SmaI-generated restriction fragment length polymorphisms (RFLPs). The results suggest that a new clone of MRSA appeared at the hospital in the early 1980s, which, although usually retaining its ancestral phage-type, developed four different RFLP pulsotypes in the next 16 years. This finding indicates that multiple RFLP patterns in MRSA do not necessarily represent multiple clones deriving from different mec gene transfer events. Such variation within a clone may be significant in the interpretation of RFLP patterns during outbreaks and emphasizes the need to use two typing methods in studies of such populations. Since the appearance of new clones of MRSA is a relatively rare event, cross-infection control is paramount in the prevention of MRSA dissemination.
One of the salient characteristics of medical practice in the 1990s is the development and dissemination of bacteria resistant to multiple antibiotics, first through hospitals and, more recently, into the wider community (12, 18, 19). Methicillin-resistant Staphylococcus aureus (MRSA) may be considered the origin of this international medical problem. It continues to spread through new communities (21) wherever the methods and institutions of modern medical practice are adopted while regularly causing epidemics in places where it has been endemic for a decade or more (1, 9).
Most studies of MRSA are cross-sectional analyses of a contemporary problem, usually an outbreak. Occasionally comparisons have been made between two periods divided by a considerable number of years (6) or between selected isolates from various periods and geographical locations (10). The limitations of such sampling approaches are readily apparent, and such studies led to the erroneous belief that almost all MRSA was clonal, i.e., that there had been only one event during which the mec gene had been transferred to methicillin-sensitive S. aureus (MSSA) and that almost all MRSA strains had descended from that organism (10).
Although there was always data which seemed inconsistent with the single-clonal origin model (13), not until the sequence of the mec gene from a group of disparate isolates was compared (8) did it become generally accepted that mec gene transfer, although apparently very infrequent, has occurred more than once and that the international epidemic is not simply or predominantly monoclonal.
The study we describe here is the second (22) in a program analyzing a microbial population longitudinally over 20 years to determine how significant new MRSA clones are in clinical practice.
Nosocomial infections due to MRSA were first recorded at Royal Prince Alfred Hospital (RPAH), Sydney, Australia, in 1965 (15). Epidemiological surveillance by phage typing over the last 3 decades has identified two distinct events in MRSA evolution. From 1965 to 1972, in an analysis of their lysogenic status and phage typing patterns, four groups were found to predominate (22). However, from 1976, although a number of MRSA strains had different phage typing patterns, on the basis of their lysogenicity, the strains were a more related group, carrying a common phage. Since this observation was reported in 1986, MRSA strains of one particular phage type, (83A/85/95)wk 88//87M, and recognizable phenotypic character of grey colonies on Baird-Parker media have been persistently endemic in the hospital (24). Other MRSA phage types have come and gone. In order to implement effective cross-infection control measures, the questions arose whether these grey MRSA strains had a common source and if many generations of strains had this phenotypic characteristic.
Genotyping, restriction fragment length polymorphism (RFLP) with pulsed-field gel electrophoresis (PFGE), was the epidemiological tool used to determine the molecular relationship of isolates of this MRSA population since its first isolation 2 decades ago.
MATERIALS AND METHODS
Bacterial strains.
A total of 180 isolates of S. aureus, 174 MRSA isolates and 6 multidrug-resistant MSSA isolates, isolated at RPAH from 1967 to 1996, were included in the study. Each isolate was from an individual patient, and no repeat isolates were included. Thirty-one MRSA isolates isolated from 1967 to 1977 represented the phage patterns predominant at that time (22). One hundred grey MRSA isolates, phage type (83A/85/95)wk 88//87M (designated phage type A in this study) were randomly selected from the isolates of each year from 1978 to 1996. Thirty-four MRSA isolates were representative of the other four predominant phage patterns of this period, and 15 MRSA isolates were nontypeable or showed miscellaneous phage patterns (Table 1). The six MSSA strains isolated from 1971 to 1977 were resistant to three or more antibiotics and were nontypeable by phage typing.
TABLE 1.
Phage typing patterns of 172 MRSA isolates
| Phage
|
No. of isolates during the period from:
|
|||
|---|---|---|---|---|
| Type | Pattern | 1967–1977 | 1978–1986 | 1987–1996 |
| A | (83A/85/95)wk 88//87Ma | 46 | 54 | |
| B | 83A/85/95/90/88//47T/90A/13Ma | 14 | ||
| C | 83A/85/88//56C/87Ma | 8 | ||
| D | 83A/85/88//56B/56C/67R/87Ma | 4 | ||
| E | 77/83A/84/85/95/90/88//47T/90A/13Ma | 1 | 4 | 3 |
| F | 6/47/53/54/77/83A/84/85/88b | 3 | ||
| G | 88b | 12 | 1 | |
| H | 77/84b | 3 | ||
| K | Miscellaneousa | 6 | 3 | 4 |
| L | Not typeablea | 6 | 1 | 1 |
100× RTD.
RTD.
All isolates were slide coagulase positive. Isolates from 1967 to 1986 were originally stored on nutrient agar slopes at room temperature. All strains are now stored in Protect Bacterial Preserver beads (Technical Service Consultants Ltd., Heywood, Lancashire, United Kingdom) at −70°C.
Antimicrobial susceptibility.
Susceptibility tests were performed by a replica plate technique (25) and according to National Committee for Clinical Laboratory Standards methods (document M7-A2). The concentrations of antibiotics in the test agar (in milligrams per liter) were as follows: for penicillin, 0.125; for methicillin, 4; for erythromycin, 1; for tetracycline, 4; for chloramphenicol, 8; for fusidic acid, 1; for rifampin, 1; for vancomycin, 4; for trimethoprim, 1; for gentamicin, 2; and for ciprofloxacin, 1. Plates were incubated at 37°C, with duplicate methicillin plates also incubated at 30°C. Results were read after 18 h, and strains were considered resistant if more than three colonies of visible growth were present.
Tellurite reduction.
All isolates were tested for tellurite reduction on Baird-Parker Medium Oxoid CM275 by the replica plate technique. Reactions were read after 24 h of incubation at 37°C. As previously described (23), isolates were recorded as giving strong or weak reactions if they grew as black or grey colonies, respectively.
Phage typing.
Phage typing was carried out according to the method described by Blair and Williams (3). The 23 phages of the Basic International Set of Typing Phages were supplemented by three experimental phages, 187, 90, and 88, issued by the International Centre, Colindale, United Kingdom, and 9 experimental phages isolated at RPAH (2, 23). From 1967 to 1982, all phages were used at routine test dilution (RTD) and 100× RTD; thereafter, all phages were used only at 100× RTD.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was done with the enzyme SmaI as described before (9). DNA was separated on a Bio-Rad CHEF-DRII system with the following parameters: 200 V with an initial pulse time of 1.0 s and a final pulse time of 40 s for 24 h at 9°C.
The gels were stained with ethidium bromide and visualized with a transilluminator (Ultraviolet Products Inc., Sydney, Australia) and photographed with Polaroid film type 665, in a Polaroid Land camera.
A four-band difference indicated a different pulsotype (17). If isolates differed by up to three bands, then they were classified as subtypes of the pulsotype.
Strains are identified as in the following example: strain 15, phage type A, pulsotype II, isolated in 1982 is shown as 15-A/II/82.
Phylogenetic analysis.
The degree of nucleotide sequence similarity of chromosomal DNA in the isolates was estimated by the modification of the methods of Nei and Li (14) and Upholt (20) as suggested by El-Adhami et al. (7). The proportion of shared fragments between any two isolates was calculated as follows: F = 2nxy/(nx + ny), where nx is the total number of fragments from isolate X, ny is the total number of fragments from isolate Y, and nxy is the number of fragments shared by the two isolates. The fraction of nucleotides that differed between two isolates was measured by the following relationship: ρ = 1 − {[(F2 + 8F)1/2 − F]1/n/2}, where F is the coefficient of similarity, and n is the number of base pairs recognized by the restriction endonuclease, which has a value of 6 for SmaI. With the estimated ρ value, a dendrogram was constructed by the neighbor-joining algorithm of Saitou and Nei (16) and Drawtree PHYLIP accessible in the PHYLIP group of programs (distributed by J. Felsenstein, Department of Genetics, University of Washington, Seattle) through the Australian National Genome Information Service.
Reproducibility.
Each isolate was typed twice, and the pattern was interpreted by two independent observers.
RESULTS
Phenotypic characteristics of MRSA isolates. Antibiotic sensitivity.
Antibiotic sensitivity testing showed that 174 isolates were resistant to penicillin and methicillin and showed varying resistance patterns to the other antibiotics tested. One hundred sixty-six (96.5%) of these MRSA isolates were resistant to five or more antibiotics, and eight isolates, from the period from 1967 to 1977, were resistant to three antibiotics.
Tellurite reduction.
All isolates of phage type A, pattern (83A/85/95)wk 88//87M, produced grey colonies on Baird-Parker medium, while all other MRSA isolates grew as black colonies.
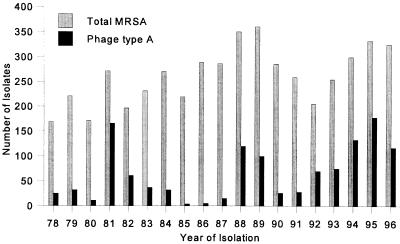
The isolation of this MRSA at RPAH, since its appearance in the late 1970s, has ranged from 61.1% of all MRSA isolates in 1981 to 1.7% in 1986 and 36.0% in 1996 (Fig. 1).
FIG. 1.
Total MRSA and phage type A isolates from the period 1978 to 1996.
Phage typing.
Table 1 shows the phage typing patterns of MRSA isolates from three decades, 1967 to 1977, 1978 to 1986, and 1987 to 1996. Nineteen of the 31 isolates from 1967 to 1977 were phage types, E, F, G, and H, which were predominant at the time. Six isolates were not typeable, and six isolates gave miscellaneous patterns. Forty-six of the 73 isolates from 1978 to 1986 were phage type A, pattern (83A/85/95)wk 88//87M. The remaining 23 isolates represented the other phage types, B, D, E, F, and G, prevalent at the time, and four isolates showed miscellaneous patterns or were not typeable at 100 × RTD. In the period from 1987 to 1996, 54 isolates belonged to phage type A and 11 isolates belonged to phage types C and E, with five isolates with miscellaneous patterns or untypeable.
The six MSSA isolates were not typeable with the phages at 100× RTD.
PFGE analysis.
Analysis of the 174 MRSA isolates (by PFGE) showed that 157 isolates could be divided into pulsotype patterns I to XIII and that 17 isolates showed miscellaneous RFLP patterns (Table 2) (Fig. 2). The six MSSA isolates also showed miscellaneous RFLP patterns and were unrelated to each other or to any other isolate. PFGE analysis identified 139 different RFLP patterns among the 180 isolates.
TABLE 2.
Pulsotypes of MRSA isolates
| Period of isolation | Phage type | No. of isolates of pulsotype:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | Misca | Total | ||
| 1967–1977 | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 3 | |
| G | 0 | 0 | 1 | 0 | 0 | 0 | 7 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 12 | |
| H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | |
| K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 6 | |
| L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 6 | |
| 1978–1986 | A | 8 | 22 | 10 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 46 |
| B | 1 | 4 | 0 | 0 | 0 | 0 | 2 | 4 | 2 | 0 | 0 | 0 | 0 | 1 | 14 | |
| C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| D | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | |
| E | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 4 | |
| F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| K | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | |
| L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 1987–1996 | A | 2 | 6 | 3 | 36 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 54 |
| B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C | 0 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8 | |
| D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| E | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| K | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | |
| L | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
Misc, miscellaneous pulsotype.
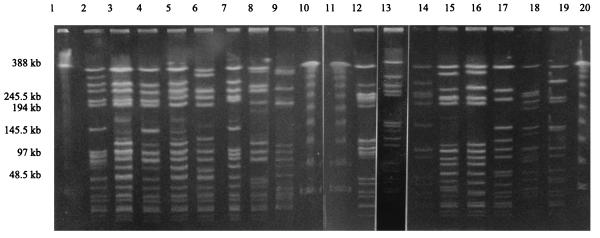
FIG. 2.
PFGE patterns of SmaI digests of total DNA from representatives of each pulsotype. Lanes: 1, 10, 11, and 20, lambda DNA concatemers used as molecular size markers; 2, pulsotype I (19-A/I/83); 3, pulsotype II (27-A/II/88); 4, pulsotype III (20-A/III/83); 5, pulsotype IV (31-A/IV/90-96); 6, pulsotype V (28-A/V/89); 7, pulsotype VI (8-D/VI/78); 8, pulsotype VII (2-G/VII/67); 9, miscellaneous pulsotype (4-D/M/76); 12, pulsotype VIII (6-G/VIII/77); 13, pulsotype IX (7-E/IX/77); 14, pulsotype X (1-G/X/67); 15, pulsotype XI (5-H/XI/76); 16, pulsotype XII (13-A/XII/82); 17, pulsotype XIII (34-A/XIII/94); 18, miscellaneous pulsotype (38-A/M/96); 19, MSSA (42-L/M/74).
Eighty-seven of 100 phage type A isolates belonged to four pulsotypes, I, II, III, and IV. Two pulsotypes, II and IV, predominated with 28 and 36 isolates, respectively. Six type II isolates from 1982, 1983, 1985, and 1988 had identical RFLP patterns and are represented on the dendrogram by isolate 17-A/II/82-88. There were 10 identical type IV isolates from 1989 to 1996, represented by isolate 29-A/IV/89-96, and there were 12 identical type IV isolates from the period 1990 to 1996, represented by 31-A/IV/90-96. Pulsotypes I and III contained 10 and 13 isolates, respectively. The remaining 13 isolates of phage type A were divided among seven pulsotypes. Of the 43 contemporary MRSA isolates from the period 1978 to 1996, of other phage types, 21 (48.8%) belonged to pulsotypes I, II, III, or IV, and the remainder showed a diversity of pulsotypes.
The 31 isolates, 1967 to 1977, belonged to seven pulsotypes, with seven isolates (22.6%) of phage type G belonging to pulsotype VII. Six of these isolates had identical RFLP patterns. Only one isolate from this period belonged to pulsotype III, and there were no isolates in pulsotype I, II, or IV.
Phylogenetic analysis.
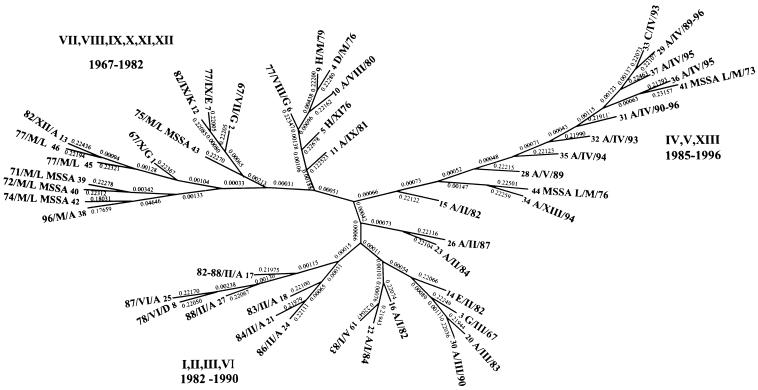
A dendrogram was constructed, as described in Materials and Methods with the estimated ρ value for all isolates. This dendrogram of 139 isolates was analyzed, and then a dendrogram of representative MRSA and MSSA isolates from all pulsotypes was constructed (Fig. 3). The isolates representing the major pulsotypes I, II, III, and IV were chosen by first constructing separate dendrograms for all isolates of these pulsotypes and then selecting the isolates from the terminal branch extremities. This selection resulted in the use of 46 isolates to construct the dendrogram. Zero genetic distance corresponds to identical PFGE patterns. Each terminal branch represents a pulsotype subtype, with phage type and year of isolation defined. The dendrogram showed that isolates of phage type A from 1982 to 1990 from pulsotypes I, II, III, and VI grouped together, while the majority of isolates of this endemic MRSA from 1990 to 1996, from pulsotype IV, grouped together on another branch. There was, however, one 1996 phage type A isolate, 38-A/M/96, which was quite different. This isolate was situated on the dendrogram with MSSA strains and 1967 to 1982 isolates of pulsotypes VII to XII, the majority of which were not phage type A. Isolates of the same pulsotype but different phage types grouped together.
FIG. 3.
Dendrogram calculated from the ρ values from 46 different strains isolated from 1967 to 1996. Pulsotypes are represented by roman numerals, and phage types are represented by uppercase letters. The genetic distance (ρ) is indicated between each node of the tree. The methods for the calculation and construction of the dendrogram are described in Materials and Methods.
DISCUSSION
Just how frequently new MRSA clones arise is not merely a theoretical problem. If mec transfer is a rare event, then infection control procedures alone should prevent the dissemination of MRSA. If it is a common event, then the control of antibiotic usage to limit the selection pressure favoring multidrug-resistant organisms is paramount. There is even anecdotal evidence that a revertant population, having lost the mec gene, may become the dominant species once selective pressure is removed (11).
The MRSA and multidrug-resistant MSSA isolates we analyzed in this study come from a population of bacteria that has been subject to constant selective antibiotic pressure similar to that in any comparable, tertiary referral hospital in the developed world. Understanding how such populations develop is essential in preventing new emergences of multidrug- and eventually pan-drug-resistant bacteria and in controlling current problems.
The recommended methods (17) of analyzing RFLP data popularly applied in routine hospital epidemiology are not sufficiently systematic to cope with longitudinal data and, as we have previously suggested, may be inadequate for studying limited outbreaks in the complex MRSA populations characteristic of late-20th-century hospitals (9). Sequence, especially of the mec gene site, is probably more reliable, but its cost for this number of historic isolates is prohibitive.
Our analysis is consistent with the hypothesis that a new clone of MRSA emerged in the early 1980s (A/II) and that while its descendants almost always preserved the ancestral phage type, their RFLP pattern gradually varied through time (I, III, V, and most recently, IV).
The clonality of the separate MRSA populations is suggested by the nonrandom distribution of MSSA isolates. Despite their variety, with two exceptions, isolates 41 and 44, they group together, suggesting that although they are a very varied population, they are different from the surrounding, more-homogenous MRSA clusters.
Our data and analysis also underline the utility of using two, preferably unrelated, typing methods in the analysis of bacterial populations which may be clonal (4, 9, 17). At every moment in the history of this hospital’s dominant clone, phage typing indicated the genuine relatedness of isolates, but, as the clone became endemic, was incapable of providing adequate discriminatory data in outbreaks.
It would have been equally wrong, however, to suggest that multiple RFLP types necessarily indicated multiple clones in the strict sense, i.e., descendants of different mec gene transfer events (5). Again, our data and analysis show that RFLP patterns may be stable through long periods, but random variation over more than a decade has resulted in the appearance of six different pulsotype patterns in what are most likely the descendants of a single mec gene transfer event.
The conclusion we draw from this study is that cross-infection has been the primary factor in the persistence of MRSA in this hospital. Longitudinal studies of the MRSA population may provide us with information we need to design effective control measures for the growing number of novel multidrug-resistant microbial species.
ACKNOWLEDGMENTS
This work was supported by a donation from D. Heggie.
We thank Anna Heron for help in preparing the manuscript.
REFERENCES
- 1.Anonymous. Epidemic methicillin-resistant Staphylococcus aureus. Commun Dis Rep. 1996;6:197. [PubMed] [Google Scholar]
- 2.Beard-Pegler M A, Vickery A M. Lysogenicity of methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1985;20:147–155. doi: 10.1099/00222615-20-2-147. [DOI] [PubMed] [Google Scholar]
- 3.Blair J E, Williams R E O. Phage typing of staphylococci. Bull W H O. 1961;24:771–784. [PMC free article] [PubMed] [Google Scholar]
- 4.Carles-Nurit M J, Christophle B, Broche S, Gouby A, Bouziges N, Ramuz M. DNA polymorphisms in methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1992;30:2092–2096. doi: 10.1128/jcm.30.8.2092-2096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couto I, Melo-Cristino J, Fernandes M L, Garcia T, Serrano N, Salgado M J, Torres-Pereira A, Sanches I S, de Lencestre H. Unusually large number of methicillin-resistant Staphylococcus aureus clones in a Portuguese hospital. J Clin Microbiol. 1995;33:2032–2035. doi: 10.1128/jcm.33.8.2032-2035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lencestre H, de Lencestre A, Tomascz A. Methicillin-resistant Staphylococcus aureus isolates recovered from a New York hospital: analysis by molecular fingerprinting techniques. J Clin Microbiol. 1996;34:2121–2124. doi: 10.1128/jcm.34.9.2121-2124.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Adhami W, Roberts L, Vickery A, Inglis B, Gibbs A, Stewart P R. Epidemiological analysis of a methicillin-resistant Staphylococcus aureus outbreak using restriction fragment length polymorphisms of genomic DNA. J Gen Microbiol. 1991;137:2713–2720. doi: 10.1099/00221287-137-12-2713. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu K. Molecular evolution of MRSA. Microbiol Immunol. 1995;39:531–543. doi: 10.1111/j.1348-0421.1995.tb02239.x. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen M, Givney R, Pegler M, Vickery A, Funnell G. Typing multidrug-resistant Staphylococcus aureus: conflicting epidemiological data produced by phenotypic and genotypic methods clarified by phylogenetic analysis. J Clin Microbiol. 1996;34:398–403. doi: 10.1128/jcm.34.2.398-403.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreiswirth B, Kornblum J, Arbeit W E, Maslow J N, McGeer A, Low D E, Novick R P. Evidence for a clonal origin of methicillin-resistance in Staphylococcus aureus. Science. 1993;259:227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence A, Cosseron M, Durand P, Costa Y, Leclercq R. Consecutive isolation of homologous strains of methicillin-resistant and methicillin-susceptible Staphylococcus aureus from a hospitalized child. J Hosp Infect. 1996;3:349–353. doi: 10.1016/s0195-6701(96)90028-6. [DOI] [PubMed] [Google Scholar]
- 12.Moreno F, Crisp C, Jorgensen J H, Patterson J E. Methicillin-resistant Staphylococcus aureus as a community organism. Clin Infect Dis. 1995;21:1308–1312. doi: 10.1093/clinids/21.5.1308. [DOI] [PubMed] [Google Scholar]
- 13.Musser J M, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nei M, Li W H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rountree P M, Beard M A. Hospital strains of Staphylococcus aureus, with particular reference to methicillin-resistant strains. Med J Aust. 1968;2:1163–1168. doi: 10.5694/j.1326-5377.1968.tb83502.x. [DOI] [PubMed] [Google Scholar]
- 16.Saitou N, Nei M. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 17.Tenover F C, Arbeit R, Goering R, Mickelsen P, Murray B, Persing D, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udo E E, Grubb W B. Genetic analysis of methicillin-resistant Staphylococcus aureus from a Nigerian hospital. J Med Microbiol. 1993;38:203–208. doi: 10.1099/00222615-38-3-203. [DOI] [PubMed] [Google Scholar]
- 19.Udo E E, Pearman J, Grubb W B. Emergence of high-level resistance in methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect. 1994;26:157–165. doi: 10.1016/0195-6701(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 20.Upholt W B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4:1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Belkum A, van Leeuwen W, Verkooyen R, Sacilik S C, Cokmus C, Verbrugh H. Dissemination of a single clone of methicillin-resistant Staphylococcus aureus among Turkish hospitals. J Clin Microbiol. 1997;35:978–981. doi: 10.1128/jcm.35.4.978-981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vickery A M, Beard-Pegler M A, Stubbs E. Phage-typing patterns and lysogenicity of methicillin-resistant strains of Staphylococcus aureus from Sydney, Australia, 1965–85. J Med Microbiol. 1986;22:209–216. doi: 10.1099/00222615-22-3-209. [DOI] [PubMed] [Google Scholar]
- 23.Vickery A M, Beard-Pegler M A, Rountree P M. Strain differentiation in methicillin-resistant Staphylococcus aureus. Pathology. 1983;15:235–240. doi: 10.3109/00313028309083499. [DOI] [PubMed] [Google Scholar]
- 24.Vickery A M The Australian Group for Antimicrobial Resistance. Strains of methicillin-resistant Staphylococcus aureus isolated in Australian hospitals from 1986 to 1990. J Hosp Infect. 1993;24:139–151. doi: 10.1016/0195-6701(93)90076-c. [DOI] [PubMed] [Google Scholar]
- 25.Washington J A, Sutter V L. The dilution susceptibility test: agar and macro-broth dilution procedures. In: Lennette E H, Balows A, Hausler W J Jr, Truant J P, editors. Manual of clinical microbiology. 3rd ed. Washington, D.C: American Society for Microbiology; 1980. pp. 453–458. [Google Scholar]