Abstract
The prevalence of heart failure (HF) in the United States (U.S.) is estimated at over 6 million adults, with the incidence continuing to increase. A large proportion of the U.S. population is also at risk of HF due to the high prevalence of established HF risk factors, such as hypertension, diabetes, and obesity. Many individuals have multiple risk factors, placing them at even higher risk. In addition, these risk factors disproportionately impact various racial and ethnic groups. Recognizing the rising health and economic burden of HF in the U.S., the 2022 American Heart Association / American College of Cardiology / Heart Failure Society of America (AHA/ACC/HFSA) Heart Failure Guideline placed a strong emphasis on prevention of HF. The purpose of this review is to highlight the role of both primary and secondary prevention in HF, as outlined by the recent guideline, and address the role of the preventive cardiology community in reducing the prevalence of HF in at-risk individuals.
Keywords: Heart failure, Prevention, Guideline
1. Introduction
Heart failure (HF) continues to be an increasing health and economic burden in the United States (U.S.). New HF diagnoses rise each year with an estimated prevalence in the U.S. of over 6 million in adults, which is projected to grow to over 8 million by 2030 [1]. Given this context, it has become imperative to understand and treat those at risk for HF and prevent the development of HF.
Because of this recognition, the 2022 American Heart Association / American College of Cardiology / Heart Failure Society of America (AHA/ACC/HFSA) Heart Failure Guideline placed a strong emphasis on prevention of HF. The purpose of this review is to highlight the role of both primary and secondary prevention in HF, as outlined by the recent guideline, and address the role of the preventive cardiology community in reducing the prevalence of HF in at-risk individuals.
2. Epidemiology of HF and its risk factors
The lifetime risk of HF continues to be high, ranging from 20% to 45% in adults ages 45 to 95 years of age, and is dependent on various risk factors. For example, lifetime risk of HF is 1.6-fold higher in those with elevated BP above 160/90 mmHg compared to those with BP under 120/80 mmHg, and lifetime risk is doubled in those with BMI greater than 30 kg/m2 compared to those with a normal BMI, as well as for current smokers compared to never smokers [2,3].
A large portion of the U.S. population is at risk of HF due to the high prevalence of established HF risk factors. Approximately 115 million people in the U.S. have hypertension, 100 million have obesity, 26 million have diabetes, 92 million have pre-diabetes, and 125 million have atherosclerotic cardiovascular disease (ASCVD), all of which are associated with increased risk of developing HF [1]. Furthermore, many individuals have more than one of these modifiable HF risk factors, which further increases risk [4].
Although HF mortality has improved over the past decade, it has nonetheless remained high with the age-adjusted death rate for HF at 92 per 100,000 individuals in 2019, with significant differences between racial and ethnic groups [5]. The HF death rates in Black men and women are 2.6 and 2.9 times higher than White men and women, respectively [6]. Similarly, HF hospitalization rates for Black individuals are also about 2.5 times greater than White patients [6].
Hospitalizations for HF in the U.S. continue to rise, with 1.2 million hospitalizations in 2017, a 26% increase from 2013 [7]. If left unchanged, the total cost for HF is predicted to continue to increase from $30.7 billion dollars in 2012 (in 2010 dollars) to a staggering projected $69.8 billion by 2030 [8].
3. Re-defining the stages of HF
The 2022 AHA/ACC/HFSA HF Guideline redefined the stages of HF to include those at risk of heart failure (HF) (stage A) and pre-heart failure (stage B), in addition to the other stages where patients are symptomatic with active HF (stage C & D). This new terminology describes the stages as a continuum. Stage A includes those at-risk for HF, based on the presence of established risk factors, but without structural heart disease. Those with hypertension, ASCVD, diabetes, metabolic syndrome, obesity, exposure to cardiotoxic agents, genetic variants for cardiomyopathy, or a family history of cardiomyopathy are persons at risk for developing HF.
In contrast, stage B is defined as pre-HF where there are also no signs or symptoms of HF but structural changes, evidence of increased filling pressures, or the presence of risk factors with elevated biomarkers in the absence of competing diagnoses. It is important to identify patients in stage B, not only because they are at increased risk of developing symptomatic HF, but because they also have a higher mortality risk than those in stage A. Among asymptomatic individuals in the Framingham Heart Study, individuals with low left ventricular ejection fraction (LVEF) had a 60% increased risk of death compared to those without left ventricular dysfunction [9].
4. Controlling risk factors for HF
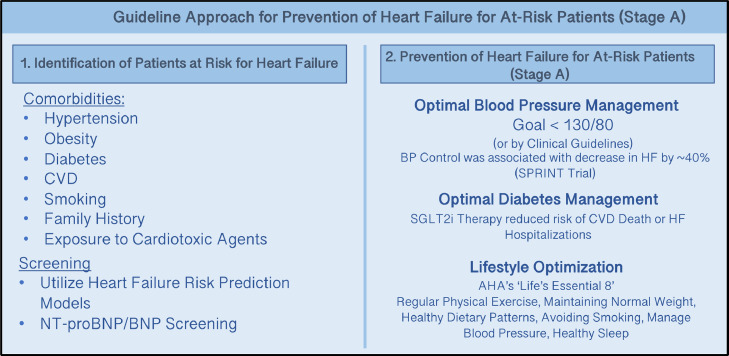
There were three Class I prevention indications in the HF Guideline for patients identified to be at-risk for HF (Fig. 1):
Fig. 1.
Guideline Approach for Prevention of Heart Failure for At-Risk Patients (Stage A). The 2022 AHA/ACC/HFSA Heart Failure Guidelines highlight heart failure prevention by (1) identifying patients at-risk for heart failure with comorbidities and screening measures, and (2) Optimizing Blood Pressure, Diabetes, and Lifestyle in patients at-risk.
4.1. Controlling blood pressure
Elevated systolic and diastolic blood pressure are strong risk factors for the development of symptomatic HF, and a blood pressure treatment goal of <130/80 mmHg is recommended for those with a 10-year ASCVD risk of >10% and is associated with a reduced risk of HF [10,11]. The SPRINT (Systolic Blood Pressure Intervention Trial) trial found that controlling blood pressure to SBP goal of <120 mmHg decreased incident HF by 38% compared with an SBP goal of <140 mmHg [12]. A meta-analysis also demonstrated a similar reduction in HF events (40%) when blood pressure was controlled [13]. Yet, despite this robust data, awareness, treatment, and control of hypertension remains inadequate.
4.2. Controlling diabetes
Heart failure hospitalization rates are two-fold higher in individuals with diabetes than in those without diabetes [14,15]. Diabetes is also an independent risk factor for worse clinical trajectory in those with HF, including greater LVEF decline after 9 years [16]. The American Diabetes Association (ADA) has released updated evidence-based guidelines for glycemic targets in patients with diabetes and highlights diabetes management strategies for heart failure prevention as well [17,18].
Several RCTs and a meta-analysis in patients with type 2 diabetes and who are either at high risk for or who have CVD, demonstrated that when compared with placebo, sodium-glucose cotransporter-2 inhibitors (SGLT2i) therapy was associated with a 27–45% reduction in the risk of HF hospitalization [19], [20], [21], [22]. Only 10–14% of patients in these trials had HF at baseline, so this dramatic reduction in HF hospitalization reflects the impact of SGLT2i therapy in patients with diabetes as a role for primary prevention of symptomatic HF.
While higher hemoglobin A1c, as a marker of diabetes severity, is associated with increased HF risk, glycemic control alone has not been associated with lower HF risk [23]. Thus, despite the modest impact SGLT2i have on glycemia, it is likely that their impact on reducing HF risk is through some other mechanism, although such mechanisms are not yet elucidated. Of note, participants with eGFR <25 mL/min/1.73m2 were excluded from these trials, so future studies in patients with severe renal dysfunction will need to be performed to better understand the impact in this population.
4.3. Obesity management and other lifestyle modifications
The 2019 AHA/ACC Primary Prevention Guideline provides recommendations for diet, physical activity, and weight control to improve overall cardiovascular health, including HF risk reduction[24]. The AHA recommends optimizing “Life's Essential 8″ risk factors and behaviors for ideal cardiovascular health, namely blood pressure management, cholesterol control, fasting blood sugar reduction, diet and physical activity optimization, weight management, smoking cessation, and adequate sleep [25]. The Atherosclerosis Risk in Communities (ARIC) Study found that greater achievement of the AHA's “Life's Essential 8″ predecessor, “Life's Simple 7,” in middle age is associated with a lower lifetime risk of HF and greater preservation of cardiac structure and function [26].
Many other studies have shown that healthy lifestyle habits are associated with reduced HF risk [27], [28], [29], [30]. A meta-analysis of 12 prospective cohort studies including over 370,000 participants reported an inverse dose-dependent association has been associated between physical activity and risk of HF [31]. Another study found that lifestyle changes, including weight loss, smoking cessation, and physical activity, supplemented with targeted pharmacologic therapy for multimodal risk factor control was associated with a 70% reduction in incident HF in adults with type 2 diabetes and albuminuria [32].
In obese individuals and in those with diabetes, greater weight loss has been associated with greater HF risk reduction [[33], [34]]. Certain diets, such as whole grain and plant-based diets, the Mediterranean, and the DASH (Dietary Approaches to Stop Hypertension) diets are inversely associated with incident HF and should be recommended to all patients at risk of HF [35], [36], [37], [38]. In obese patients who are not achieving desired weight loss with lifestyle modifications alone, anti-obesity medications such as glucagon-like peptide-1 receptor antagonists (GLP1-RA), endoscopic procedures, and bariatric surgery should be considered given their implementation is associated with significant observed reduction in incident heart failure and cardiovascular mortality in this population [[39], [40]].
Though lifestyle modifications are primarily focused on in the context of patients at risk of HF, it is important to continue emphasizing these strategies to those at all stages of this disease. Healthy lifestyle modification is a key feature of cardiac rehabilitation programs, and participation in these programs is associated with a reduction in cardiovascular mortality and HF hospitalization [41].
5. Biomarker based screening
There is emerging data regarding biomarker screening and identifying persons at risk for HF. Currently the use of biomarkers are Class 2 recommendations to identify for persons at-risk for HF.
5.1. NT-proBNP/BNP screening
In the ambulatory setting, BNP and NT-proBNP, which are measures of ventricular stretch, can be useful screening tools in those with HF risk factors, an equivocal physical exam, and presenting symptoms that could be attributed to HF. A low BNP or NT-proBNP can help exclude HF as a cause of symptoms in the ambulatory setting, though BNP and NT-proBNP both tend to be lower in obese patients, and thus have lower sensitivity [[42], [43]]. The specificity of these biomarkers is lower than the sensitivity, though higher levels do have higher positive predictive values in diagnosing HF and also portend greater risk of adverse short- and long-term outcomes [44]. For example, those with stage B HF by elevated biomarkers, even in the absence of echocardiography findings, are at increased risk for HF and mortality [45].
6. Risk scores to estimate risk of subsequent HF incidence
Multivariable risk scores can be useful to estimate the subsequent risk of incident HF and were recommended in the guideline as a Class 2a recommendation.
Examples of validated multivariable risk scores include:
-
•
Framingham Heart Failure Risk Score – 1999 [46]
-
•
Health ABC Heart Failure Score – 2008 [47]
-
•
ARIC Risk Score – 2012 [48]
-
•
Pooled Cohort equations to Prevent HF (PCP-HF) – 2019 [10]
The Pooled Cohort equations to Prevent HF (PCP-HF) model provides race- and sex-specific 10-year risk of HF based on data from 7 community-based cohorts with long-term follow-up [10]. Predictors of HF in the models above included blood pressure, fasting glucose, body mass index, cholesterol, smoking status, and QRS duration [[10], [46], [47], [48]]. In the ambulatory setting, one may input these variables for patients at risk of HF to determine long-term incident HF risk, with the caveat that these models are derived from population data averages and that individual risk may vary.
Though the hope is that integrating risk scores into clinical practice will improve clinical outcomes, data thus far has been limited. The REVEAL-HF trial has been the only randomized clinical trial thus far to assess whether 1-year prognostic knowledge affects clinical decision-making and patient outcomes, and the authors did not find any evidence to support this hypothesis [49]. Further studies are needed with longer follow-up to better assess the impact of multivariable risk scores on HF outcomes.
7. Disparities and vulnerable populations
A focus area for prevention highlighted by the HF guideline is the disparity in populations affected by HF. For example, HF hospitalizations are nearly 2.5 times more prevalent in Black adults compared with White adults, with no significant improvement over the past decade [[50], [51]]. Causes for this could be related to disparities in both medical risk factors and social determinants of health, which are closely inter-related. Hypertension, obesity, and diabetes mellitus affect Black individuals significantly more than White individuals [[50], [52]]. Social determinants of health are both independent risk factors for, and inextricably linked with, these medical risk factors for HF and HF hospitalizations.
A recent study demonstrated that several social determinants of health, such as lower level of education, lower income, housing instability, and being born in a country outside the United States were independently associated with increased HF hospitalization length of stay, after adjusting for both clinical status and hospital-level factors [53]. This analysis estimated that social determinants of health accounted for about 25% of the risk for prolonged length of stay among Black patients, compared with 10% of risk for non-Black patients [53].
Other racial and ethnic disparities in HF should also be noted. A MESA (Multi-Ethnic Study of Atherosclerosis) analysis showed that incident HF is 4.6 per 1000 person-years in Hispanic Black adults, 3.5 per 1000 person-years in Hispanic adults, compared with 2.4 per 1000 person-years in Non-Hispanic White adults [54]. HF risk factors with higher prevalence in Hispanic populations, compared with non-Hispanic, include diabetes mellitus, obesity, and hypertension [[55], [56]]. South Asian individuals tend to have a higher burden of coronary artery disease, diabetes mellitus, and obesity, which are all associated with increased risk of HF [[52], [57]].
Another population of individuals with unmet HF prevention needs includes cancer survivors, particularly those who have received potentially cardiotoxic agents as part of their cancer therapy. One study found that cancer survivors were at a 1.5-fold increased risk of heart failure compared to individuals without cancer history [58]. The European Society of Cardiology has released cardio-oncology guidelines that focus on prevention of cardiovascular disease in this unique population [59].
In vulnerable patient populations at risk for health disparities, HF risk assessments and multidisciplinary management strategies should target both known risks for CVD and social determinants of health, as a means toward elimination of disparate HF outcomes [60]. Addressing structural racism and biases that lead to disparities in social determinants of health could help address primary & secondary prevention in vulnerable populations [50]. Evidence of health disparities should be monitored and addressed at the clinical practice and the health care systems levels.
8. Guideline directed medical therapies (GDMT) for those with pre-heart failure to prevent development of symptoms
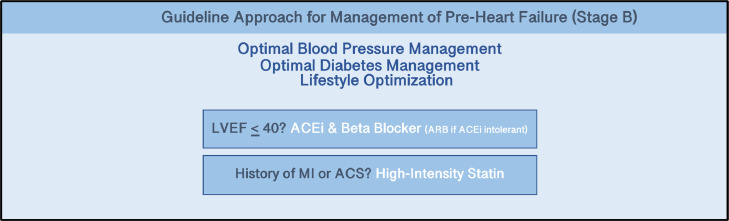
The use of standardized GDMT is emphasized throughout these guidelines. Reducing advanced stages of HF, hospitalizations and adverse outcomes requires adherence to GDMT. For those in stage B, or pre-HF, there were several classes I indications in the Guideline (Fig. 2):
Fig. 2.
Guideline Approach for Management of Pre-Heart Failure (Stage B). The 2022 AHA/ACC/HFSA Heart Failure Guidelines highlight management of Pre-Heart Failure by continued optimizing Blood Pressure, Diabetes, and Lifestyle with the addition of ACEi and Beta Blockers with patients with reduced Left Ventricular Ejection Fraction (LVEF) and high-intensity statin with patients with history of myocardial infarction and/or acute coronary syndrome (ACS).
8.1. ACE-inhibitors (ACEi) or angiotensin receptor blockers (ARB)
ACEi initiation in patients with asymptomatic reduced LVEF (<40%) after acute MI is associated with reduced HF hospitalizations, progression to severe HF, and mortality compared with placebo [[61], [62]]. Reduced HF hospitalization and mortality associated with enalapril have also been shown in patients with asymptomatic LV dysfunction, even in absence of recent MI, in the Studies of Left Ventricular Systolic Dysfunction (SOLVD) prevention trial [[63], [64]]. Thus, all patients with asymptomatic LV dysfunction, regardless of recent MI history, should be initiated on an ACEi.
The trials evaluating ARBs in patients with asymptomatic LV dysfunction have only been in the setting of previous MI. The Valsartan in Acute Myocardial Infarction (VALIANT) study, in which 25% of the study population included those with asymptomatic LV dysfunction, demonstrated that the reduction of mortality and other adverse cardiovascular outcomes of valsartan were comparable to that of captopril [[65], [66]]. Thus, those with asymptomatic LV dysfunction and recent MI who are intolerant to ACEi or develop suspected ACEi-induced cough should be initiated on an ARB instead. Care should be taken in those who previously developed ACEi-induced angioedema as some patients may also develop angioedema with ARB.
8.2. Statins
Statin therapy in patients with a history of MI, acute coronary syndrome (ACS), or high cardiovascular risk is associated with a reduced risk of incident HF [67], [68], [69]. A meta-analysis of 6 RCTs including >110,000 patients with ACS demonstrated that intensive statin therapy was associated with reduced HF hospitalizations [68]. This data was subsequently supported by evidence from another larger meta-analysis of 17 RCTs, which showed that statin therapy is associated with a reduced risk of HF hospitalization [70]. Thus, all patients with history of acute MI or ACS should be initiated on statin therapy to help prevent symptomatic HF.
8.3. Beta-blockers
Carvedilol reduces maladaptive remodeling and mortality compared to placebo in patients with reduced LVEF <40% and recent MI [71]. The SOLVD prevention trial and the Survival and Ventricular Enlargement (SAVE) trial showed beta blockers, in addition to ACEi, was associated with reduced mortality and hospitalizations in those with asymptomatic LV dysfunction [72,73]. Thus, patients with reduced LVEF <40%, regardless of history of MI, should be initiated on a beta-blocker, such as carvedilol or metoprolol succinate, to reduce symptomatic HF, mortality, and hospitalizations.
8.4. Medications to avoid
In those with LVEF <50%, thiazolidinediones and nondihydropyridine calcium channel blockers with negative inotropic effects are not recommended, as they increase the risk of HF, including hospitalizations. In RCTs of patients with type 2 diabetes who were largely free of symptomatic HF at baseline, thiazolidinediones have been associated with fluid retention and increased rates of symptomatic HF [74,75]. In patients with depressed LVEF and mild HF symptoms (NYHA class I to II), rosiglitazone was associated with worsening edema and need for increased HF medications [76]. In studies of patients with reduced LVEF after MI, diltiazem was associated with increased risk of HF (54, 55). Although nondihydropyridine calcium channel blockers have not been specifically evaluated in patients with asymptomatic LV dysfunction, they should generally be avoided due to negative inotropic effects.
9. GDMT and other secondary prevention strategies in those with symptomatic (Stage C) or advanced (Stage D) heart failure
The HF Guideline also focuses on secondary prevention strategies in those with symptomatic or advanced HF. These hallmarks of secondary prevention include: a multidisciplinary approach to health care and patient education, regular physical activity for those who are able, diuretic management, and GDMT. Although this review focuses primarily on those with heart failure with reduced left ventricular ejection fraction (HFrEF), it is important to note that individuals with heart failure with preserved left ventricular ejection fraction (HFpEF) represent about 50% of the population with HF. GDMT for secondary prevention of HF, specifically HFrEF, and its ensuing complications includes: renin-angiotensin-aldosterone system (RAAS) inhibitors, angiotensin receptor neprilysin inhibitor (ARNI), beta-blockers, mineralocorticoid-receptor antagonists (MRA), SGLT2is, and in those who self-identify as Black – hydralazine and isosorbide dinitrate.
There are Class I indications listed in the Guideline that recommend up-titration of GDMT to doses shown to be efficacious in RCTs, or to maximally tolerated doses, to reduce cardiovascular mortality and HF hospitalizations. Implantable cardioverter defibrillator (ICD) implantation is also recommended in those with symptomatic HF on GDMT and LVEF <30–35% at least 40 days post-MI for primary prevention of sudden cardiac death (SCD) and to reduce total mortality. Cardiac resynchronization therapy with defibrillator (CRT-D) is recommended for those with symptomatic HF and LVEF <35% on GDMT with a left bundle branch block (LBBB) and QRS duration >150 ms to reduce total mortality, reduce hospitalizations, and improve symptoms and QOL.
10. Conclusion
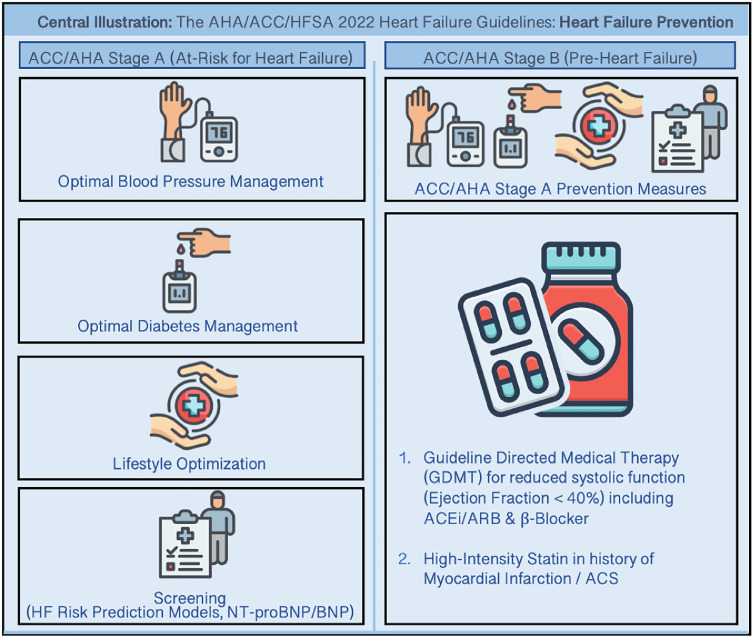
The recent AHA/ACC/HFSA HF Guideline places a strong emphasis on the prevention of HF in the setting of continued rising health and cost burden of HF in the United States [44]. The Guideline focuses on updated definitions for the stages of HF, including definitions for those who are at risk of HF and pre-HF, and places key recommendations for risk factor management and pharmacotherapy to prevent HF endpoints (Central Illustration). The implementation of prevention strategies in this population is critical to reduce the risk of developing HF and HF hospitalizations.
Central Illustration.
The 2022 AHA/ACC/HFSA Heart Failure Guidelines emphasizes Heart Failure Prevention for individuals at-risk for Heart Failure (ACC/AHA Stage A) and for individuals with Pre-Heart Failure (ACC/AHA Stage B).
Contributions
A.A, D.S.M.: initial drafting of manuscript and figures. R.C., C.E.N, R.S.B, R.B., M.G.: revising and editing manuscript, providing guidance.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Roger S. Blumenthal reports a relationship with Amgen Inc that includes: funding grants. Roger S. Blumenthal reports a relationship with Novartis that includes: funding grants. Martha Gulati reports a relationship with Novartis that includes: board membership. Martha Gulati reports a relationship with Siemens Healthcare Diagnostics Inc that includes: speaking and lecture fees. Martha Gulati is a co-investigator and site PI of the Women's IschemiA TRial to Reduce Events In Non-ObstRuctive CAD (WARRIOR) Study funded by the Department of Defense, and a co-investigator of the Women Ischemic Syndrome Evaluation (WISE) Study funded by the NHLBI
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Tsao C.W., Lyass A., Enserro D., et al. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC: Heart Failure. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson M., Dardari Z., Kianoush S., et al. Relation Between Cigarette Smoking and Heart Failure (from the Multiethnic Study of Atherosclerosis) The American Journal of Cardiology. 2019;123:1972–1977. doi: 10.1016/j.amjcard.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamo C.E., Kwak L., Wang D., et al. Heart Failure Risk Associated With Severity of Modifiable Heart Failure Risk Factors: The ARIC Study. Journal of the American Heart Association. 2022;11 doi: 10.1161/JAHA.121.021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC WONDER. Multiple Cause of Death, 1999-2020 Request Form Accessed June 5, 2022. https://wonder.cdc.gov/controller/datarequest/D77.
- 6.Nayak A., Hicks A.J., Morris A.A. Understanding the Complexity of Heart Failure Risk and Treatment in Black Patients. Circulation: Heart Failure. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkurt B., Coats A.J., Tsutsui H., et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Journal of Cardiac Failure. 2021;27:387–413. [Google Scholar]
- 8.Heidenreich P.A., Albert N.M., Allen L.A., et al. Forecasting the Impact of Heart Failure in the United States. Circulation: Heart Failure. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H., Negishi K., Wang Y., Nolan M., Saito M., Marwick T.H. Echocardiographic screening for non-ischaemic stage B heart failure in the community. European Journal of Heart Failure. 2016;18:1331–1339. doi: 10.1002/ejhf.643. [DOI] [PubMed] [Google Scholar]
- 10.Khan S.S., Ning H., Shah S.J., et al. 10-Year Risk Equations for Incident Heart Failure in the General Population. Journal of the American College of Cardiology. 2019;73:2388–2397. doi: 10.1016/j.jacc.2019.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Upadhya B., Rocco M., Lewis C.E., et al. Effect of Intensive Blood Pressure Treatment on Heart Failure Events in the Systolic Blood Pressure Reduction Intervention Trial. Circulation: Heart Failure. 2017 doi: 10.1161/CIRCHEARTFAILURE.116.003613. Published onlineApril 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomopoulos C., Parati G., Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure–meta-analyses of randomized trials. J Hypertens. 2016;34:373–384. doi: 10.1097/HJH.0000000000000848. discussion 384. [DOI] [PubMed] [Google Scholar]
- 14.McAllister D.A., Read S.H., Kerssens J., et al. Incidence of Hospitalization for Heart Failure and Case-Fatality Among 3.25 Million People With and Without Diabetes Mellitus. Circulation. 2018;138:2774–2786. doi: 10.1161/CIRCULATIONAHA.118.034986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavender M.A., PhG Steg, Smith S.C., et al. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death. Circulation. 2015;132:923–931. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 16.Julián M.T., Alonso N., Lupón J., et al. Long-term LVEF trajectories in patients with type 2 diabetes and heart failure: diabetic cardiomyopathy may underlie functional decline. Cardiovascular Diabetology. 2020;19:38. doi: 10.1186/s12933-020-01011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ElSayed N.A., Aleppo G., Aroda V.R., et al. 6. Glycemic Targets: Standards of Care in Diabetes—2023. Diabetes Care. 2022;46:S97–S110. doi: 10.2337/dc23-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ElSayed N.A., Aleppo G., Aroda V.R., et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2023. Diabetes Care. 2022;46:S158–S190. doi: 10.2337/dc23-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 20.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 21.Zinman B., Wanner C., Lachin J.M., Empagliflozin et al. Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 22.Zelniker T.A., Wiviott S.D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 23.Iribarren C., Karter A.J., Go A.S., et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 24.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., et al. Life's Essential 8: Updating and Enhancing the American Heart Association's Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folsom A.R., Shah A.M., Lutsey P.L., et al. American Heart Association's Life's Simple 7: Avoiding Heart Failure and Preserving Cardiac Structure and Function. Am J Med. 2015;128:970–976.e2. doi: 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Gobbo L.C., Kalantarian S., Imamura F., et al. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults: The Cardiovascular Health Study. JACC: Heart Failure. 2015;3:520–528. doi: 10.1016/j.jchf.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Tuomilehto J., Jousilahti P., et al. Lifestyle factors in relation to heart failure among Finnish men and women. Circ Heart Fail. 2011;4:607–612. doi: 10.1161/CIRCHEARTFAILURE.111.962589. [DOI] [PubMed] [Google Scholar]
- 29.Young D.R., Reynolds K., Sidell M., et al. Effects of physical activity and sedentary time on the risk of heart failure. Circ Heart Fail. 2014;7:21–27. doi: 10.1161/CIRCHEARTFAILURE.113.000529. [DOI] [PubMed] [Google Scholar]
- 30.Hu G., Tuomilehto J., Silventoinen K., Barengo N., Jousilahti P. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J. 2004;25:2212–2219. doi: 10.1016/j.ehj.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Pandey A., Garg S., Khunger M., et al. Dose–Response Relationship Between Physical Activity and Risk of Heart Failure. Circulation. 2015;132:1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 32.Oellgaard J., Gæde P., Rossing P., et al. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia. 2018;61:1724–1733. doi: 10.1007/s00125-018-4642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundström J., Bruze G., Ottosson J., Marcus C., Näslund I., Neovius M. Weight Loss and Heart Failure. Circulation. 2017;135:1577–1585. doi: 10.1161/CIRCULATIONAHA.116.025629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Group Look AHEAD Research, E Gregg, Jakicic J., et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. doi: 10.1016/S2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tektonidis T.G., Åkesson A., Gigante B., Wolk A., Larsson S.C. Adherence to a Mediterranean diet is associated with reduced risk of heart failure in men. Eur J Heart Fail. 2016;18:253–259. doi: 10.1002/ejhf.481. [DOI] [PubMed] [Google Scholar]
- 36.Levitan E.B., Wolk A., Mittleman M.A. Consistency With the DASH Diet and Incidence of Heart Failure. Archives of Internal Medicine. 2009;169:851–857. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levitan E.B., Wolk A., Mittleman M.A. Relation of Consistency with the Dietary Approaches to Stop Hypertension Diet and Incidence of Heart Failure in Men Aged 45 to 79 Years. Am J Cardiol. 2009;104:1416–1420. doi: 10.1016/j.amjcard.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lara K.M., Levitan E.B., Gutierrez O.M., et al. Dietary Patterns and Incident Heart Failure in U.S. Adults Without Known Coronary Disease. J Am Coll Cardiol. 2019;73:2036–2045. doi: 10.1016/j.jacc.2019.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hritani R., Al Rifai M., Mehta A., German C. Obesity management for cardiovascular disease prevention. Obesity Pillars. 2023;7 doi: 10.1016/j.obpill.2023.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. The Lancet. 2021;398:262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor C.M., Whellan D.J., Lee K.L., et al. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horwich T.B., Hamilton M.A., Fonarow G.C. B-Type Natriuretic Peptide Levels in Obese Patients With Advanced Heart Failure. Journal of the American College of Cardiology. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 43.Mehra M.R., Uber P.A., Park M.H., et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. Journal of the American College of Cardiology. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 44.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Jia X., Al Rifai M., Ndumele C.E., et al. Reclassification of Pre-Heart Failure Stages Using Cardiac Biomarkers: Atherosclerosis Risk in Communities (ARIC) Study. JACC Heart Fail. 2023 doi: 10.1016/j.jchf.2022.12.005. S2213-1779(22)00723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannel W.B., D'Agostino R.B., Silbershatz H., Belanger A.J., Wilson P.W.F., Levy D. Profile for Estimating Risk of Heart Failure. Archives of Internal Medicine. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 47.Butler J., Kalogeropoulos A., Georgiopoulou V., et al. Incident Heart Failure Prediction in the Elderly. Circulation: Heart Failure. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal S.K., Chambless L.E., Ballantyne C.M., et al. Prediction of Incident Heart Failure in General Practice: The ARIC Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad T., Desai N.R., Yamamoto Y., et al. Alerting Clinicians to 1-Year Mortality Risk in Patients Hospitalized With Heart Failure: The REVEAL-HF Randomized Clinical Trial. JAMA Cardiology. 2022;7:905–912. doi: 10.1001/jamacardio.2022.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piña I.L., Jimenez S., Lewis E.F., et al. Race and Ethnicity in Heart Failure: JACC Focus Seminar 8/9. Journal of the American College of Cardiology. 2021;78:2589–2598. doi: 10.1016/j.jacc.2021.06.058. [DOI] [PubMed] [Google Scholar]
- 51.Ziaeian B., Kominski G.F., Ong M.K., Mays V.M., Brook R.H., Fonarow G.C. National Differences in Trends for Heart Failure Hospitalizations by Sex and Race/Ethnicity. Circulation: Cardiovascular Quality and Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawson C.A., Zaccardi F., Squire I., et al. Risk Factors for Heart Failure. Circulation: Heart Failure. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006472. [DOI] [PubMed] [Google Scholar]
- 53.Segar M.W., Keshvani N., Rao S., Fonarow G.C., Das S.R., Race Pandey A. Social Determinants of Health, and Length of Stay Among Hospitalized Patients With Heart Failure: An Analysis From the Get With The Guidelines-Heart Failure Registry. Circulation: Heart Failure. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.009401. [DOI] [PubMed] [Google Scholar]
- 54.Bahrami H., Kronmal R., Bluemke D.A., et al. Differences in the Incidence of Congestive Heart Failure by Ethnicity: The Multi-Ethnic Study of Atherosclerosis. Archives of Internal Medicine. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daviglus M.L., Pirzada A., Durazo-Arvizu R., et al. Prevalence of Low Cardiovascular Risk Profile Among Diverse Hispanic/Latino Adults in the United States by Age, Sex, and Level of Acculturation: The Hispanic Community Health Study/Study of Latinos. Journal of the American Heart Association. 5:e003929. [DOI] [PMC free article] [PubMed]
- 56.Daviglus M.L., Talavera G.A., Avilés-Santa M.L., et al. Prevalence of Major Cardiovascular Risk Factors and Cardiovascular Diseases Among Hispanic/Latino Individuals of Diverse Backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jose P.O., Frank A.T.H., Kapphahn K.I., et al. Cardiovascular Disease Mortality in Asian Americans. Journal of the American College of Cardiology. 2014;64:2486–2494. doi: 10.1016/j.jacc.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Florido R., Daya N.R., Ndumele C.E., et al. Cardiovascular Disease Risk Among Cancer Survivors: The Atherosclerosis Risk In Communities (ARIC) Study. J Am Coll Cardiol. 2022;80:22–32. doi: 10.1016/j.jacc.2022.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC) European Heart Journal. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 60.Javed Z., Haisum Maqsood M., Yahya T., et al. Race, Racism, and Cardiovascular Health: Applying a Social Determinants of Health Framework to Racial/Ethnic Disparities in Cardiovascular Disease. Circulation: Cardiovascular Quality and Outcomes. 2022;15 doi: 10.1161/CIRCOUTCOMES.121.007917. [DOI] [PubMed] [Google Scholar]
- 61.Pfeffer M.A., Braunwald E., Moyé L.A., et al. Effect of Captopril on Mortality and Morbidity in Patients with Left Ventricular Dysfunction after Myocardial Infarction. New England Journal of Medicine. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 62.Køber L., Torp-Pedersen C., Carlsen J.E., et al. A Clinical Trial of the Angiotensin-Converting–Enzyme Inhibitor Trandolapril in Patients with Left Ventricular Dysfunction after Myocardial Infarction. New England Journal of Medicine. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 63.Yusuf S., Pitt B., Davis C.E., Hood W.B., Jr., Cohn J.N., SOLVD Investigators Effect of Enalapril on Mortality and the Development of Heart Failure in Asymptomatic Patients with Reduced Left Ventricular Ejection Fractions. New England Journal of Medicine. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 64.Jong P., Yusuf S., Rousseau M.F., Ahn S.A., Bangdiwala S.I. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. The Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 65.Pfeffer M.A., McMurray J.J.V., Velazquez E.J., et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 66.Velazquez E.J., Pfeffer M.A., McMurray J.V., et al. VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context. Eur J Heart Fail. 2003;5:537–544. doi: 10.1016/s1388-9842(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 67.Scirica B.M., Morrow D.A., Cannon C.P., et al. Intensive statin therapy and the risk of hospitalization for heart failure after an acute coronary syndrome in the PROVE IT-TIMI 22 study. J Am Coll Cardiol. 2006;47:2326–2331. doi: 10.1016/j.jacc.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 68.Afilalo J., Majdan A.A., Eisenberg M.J. Intensive statin therapy in acute coronary syndromes and stable coronary heart disease: a comparative meta-analysis of randomised controlled trials. Heart. 2007;93:914–921. doi: 10.1136/hrt.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kjekshus J., Pedersen T.R., Olsson A.G., Faergeman O., Pyörälä K. The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail. 1997;3:249–254. doi: 10.1016/s1071-9164(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 70.Preiss D., Campbell R.T., Murray H.M., et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J. 2015;36:1536–1546. doi: 10.1093/eurheartj/ehv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dargie H.J. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 72.Exner D.V., Dries D.L., Waclawiw M.A., Shelton B., Domanski M.J. Beta-adrenergic blocking agent use and mortality in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a post hoc analysis of the studies of left ventricular dysfunction. Journal of the American College of Cardiology. 1999;33:916–923. doi: 10.1016/s0735-1097(98)00675-5. [DOI] [PubMed] [Google Scholar]
- 73.Vantrimpont P., Rouleau J.L., Wun C.C., et al. Additive Beneficial Effects of Beta-Blockers to Angiotensin-Converting Enzyme Inhibitors in the Survival and Ventricular Enlargement (SAVE) Study fn1fn1This study was supported by a University-Industry grant from the Medical Research Council, Ottawa, Ontario, Canada and Bristol Myers Squibb, Montreal, Quebec, Canada. Journal of the American College of Cardiology. 1997;29:229–236. doi: 10.1016/s0735-1097(96)00489-5. [DOI] [PubMed] [Google Scholar]
- 74.Erdmann E., Charbonnel B., Wilcox R.G., et al. Pioglitazone Use and Heart Failure in Patients With Type 2 Diabetes and Preexisting Cardiovascular Disease: Data from the PROactive Study (PROactive 08) Diabetes Care. 2007;30:2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 75.Komajda M., McMurray J.J.V., Beck-Nielsen H., et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. European Heart Journal. 2010;31:824–831. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dargie H.J., Hildebrandt P.R., Riegger G.A.J., et al. A Randomized, Placebo-Controlled Trial Assessing the Effects of Rosiglitazone on Echocardiographic Function and Cardiac Status in Type 2 Diabetic Patients With New York Heart Association Functional Class I or II Heart Failure. Journal of the American College of Cardiology. 2007;49:1696–1704. doi: 10.1016/j.jacc.2006.10.077. [DOI] [PubMed] [Google Scholar]