Highlights
-
•
There is a lack of information about the effectiveness of foot-ankle muscle strengthening program on pain and functioning in people with KOA.
-
•
An 8-week foot-ankle muscle strengthening program decreased knee pain and enhanced physical function in individuals with KOA, presenting a potential to be clinically meaningful.
-
•
Foot-ankle muscle strengthening program can be a treatment option for individuals with knee osteoarthritis (KOA) who do not tolerate exercises with excessive load on the knee.
Keywords: Knee, Osteoarthritis, Physical-activity based intervention, Randomized controlled trial
Abstract
Background
Foot-ankle exercises could improve pain and function of individuals with KOA and need to be tested.
Objective
To investigate whether an 8-week foot-ankle muscle strengthening program is effective for individuals with KOA to reduce pain and improve function.
Methods
In this randomized controlled trial, individuals diagnosed with clinical and radiographic KOA were randomized into the intervention (supervised foot-ankle strengthening exercise program three times a week for 8 weeks) or control (usual care and recommendations of the healthcare team) group. Effectiveness was assessed by changes in clinical and functional outcomes between baseline and 8 weeks with pain as the primary outcome. ANCOVA tests using the intervention group as a reference and sex, body mass index, and baseline values as covariates assessed between-group differences.
Results
The intervention group showed lower pain scores (−4.4 units; 95%CI = −7.5, −1.1), better function (−7.1 units; 95%CI = −12.7, −1.4), higher total functional score (−11.9 units; 95%CI = −20.7, −3.1), with confidence intervals indicating a potential for the differences to be clinically meaningful, and better scores for the 30-s chair stand test (2.7 repetitions; 95%CI = 1.1, 4.1), with a confidence interval indicating a moderate clinically meaningful difference, compared to the controls.
Conclusion
The 8-week foot-ankle exercise program showed positive, and potentially clinically meaningful, effects on knee pain and physical function among individuals with KOA, when compared to usual care.
Trial registration
NCT04154059. https://clinicaltrials.gov/ct2/show/NCT04154059
Introduction
Knee osteoarthritis (KOA) is one of the most common causes of musculoskeletal pain and disability worldwide and is predicted to affect about 78.4 million older adults by 2040.1 The pathogenesis of KOA involves interaction between articular cartilage damage and incomplete repair mechanisms due to mechanical overload of the joint.2,3 Clinically, it causes pain, stiffness, and decreased functionality.4
The etiology of KOA includes poor biomechanics of the local and distal joints.5,6 A recent systematic review including 6 studies (4 classified as low quality of evidence and 2 as high quality) with a total of 403 participants (275 with KOA compared to 128 healthy control) showed that a higher percentage of individuals with KOA presented with pronated foot, which may point to the need to consider foot clinical characteristics as a factor related to KOA progression.7 Moreover, cross-sectional studies comparing foot kinematics and muscle strength between individuals with KOA and healthy participants have shown that deficits in foot mobility,8 invertor and evertor strength, and plantar flexor muscle strength are common in individuals with KOA9 and may be related to higher levels of pain and low functionality.10
Although international organizations recommend physical exercises to improve pain and functionality, only 13–48% of individuals with KOA follow these guidelines.11, 12, 13, 14, 15, 16 Knee pain and fear-avoidance of movement seem to be the main barriers to adherence to an exercise program.17,18 Therefore, an alternative to addressing the affected joint (i.e., knee), may be to provide interventions focusing on the distal joints (e.g., foot-ankle joint) of the kinetic chain to avoid local pain (i.e., knee pain) and non-adherence.
Minimalist shoes have shown substantial positive effects for reducing pain, improving function in women with KOA,19 and reducing the knee adduction moment,20 which is associated with KOA progression and severity.21,22 Moreover, foot muscle-strengthening exercise programs have improved the functionality of individuals with diabetic neuropathy23 and decreased the risk of injuries in recreational runners.24 It is hypothesized that both the effects of minimalist shoes and foot muscles exercises may be due to the better activation of the intrinsic foot muscles, better foot joint mobility, and higher capacity to absorb shock during functional activities.25 Thus, these possible mechanisms could contribute to reduction of knee pain and improvement of function in individuals with KOA. However, to our knowledge, no studies to date have determined if a specific foot-ankle strengthening exercise program would present positive effects on knee pain and physical function compared to usual care in this population.
Therefore, this study investigated the effectiveness of a supervised 8-week foot-ankle strengthening exercise program to reduce pain and improve the functionality of individuals with KOA.
Methods
Trial design
A randomized two-arm, parallel group clinical trial comprised of individuals with KOA was conducted after approval by the Universidade Federal de São Carlos Research Ethics Committee (Protocol: 16767519.2.0000.5504; Approval Report Number: 3.488.466) and registration with ClinicalTrials.gov (Identifier: NCT04154059, Registered on January 2020). The study was conducted according to the guidelines of the Declaration of Helsinki and is reported based on the CONSORT and SPIRIT Extension for RCTs Revised in Extenuating Circumstances (CONSERVE).26 All participants received oral and written instructions regarding the process and aims of the study, and they gave their written informed consent before inclusion in the study.
The primary trial started in January 2020 and was conducted with the first 30 enrolled participants. Data were acquired in two blocks: the first block of individuals (n = 12) was recruited over a 3-week period from January to February 2020 (pre-COVID-19 pandemic), and the second block (n = 18) was recruited over a 4-week period from February to March 2020. Then, in March 2020, because the duration of the pandemic was unknown, we focused on mitigating the impact of the pandemic to participants already enrolled in the trial.
Major changes from the original protocol
The COVID-19 pandemic was an extenuating circumstance due its possible effects on the population studied that involved individuals with KOA who were mostly older adults, a population that is affected more drastically and quickly from an infection.
The most impactful methodological modifications made in the trial was that the intervention for the individuals in the second block had to be adapted due to the restriction of personal contact. Prior to the pandemic, exercises were conducted in groups of a maximum of 10-12 participants at the university's facilities. In the second block, after the second week of face-to-face treatment, social distancing recommendations began. Thus, the exercise program was provided remotely to ensure continuity and conclusion of the protocol at 8 weeks.27 All individuals allocated in the first and second block performed a face-to-face baseline assessment. The evaluation after the exercise program was performed in person in an open environment following distancing and protection recommendations for both the assessor and participants with KOA. For individuals who did not feel safe going to the post-intervention assessment in person, it was performed remotely (synchronously via videoconference) following the same standards of the face-to-face assessment with regard to clinical and functional outcomes.
In addition, assessments of “foot muscle isometric strength” and “foot kinematic and kinetics during gait” were suspended. These evaluations were originally performed in a closed environment with temperature control. Due to the risk of contamination of the study population and the intent to follow social distancing recommendations issued by the World Health Organization, we decided to suspend these evaluations.
Unfortunately, we were not able to perform the complete data collection of the follow-up at 16 weeks after the end of the exercise program. Although we also proposed to perform the assessments in an open environment at week 16, following the distancing and protection recommendations for both the researcher and the study participants, most participants did not feel safe coming to the university facilities.
One of the researchers (GD) quickly planned the changes, and contacted and explained the new procedures to the participants and the supervisors of the exercises program. The changes were reviewed and approved by researchers TFS and ICNS. The data were analyzed following the decision to suspend the study.
Participants
Recruitment occurred through public announcements and lists of local and regional orthopedic and rheumatologic health services in January and February 2020. Inclusion criteria were as follows: individuals of both sexes, age range of 40 to 75 years,28 diagnosis of medial compartment KOA grade II or III based on clinical and radiological criteria of the American College of Rheumatology,29 knee pain scores ranging between 30 and 80 mm on a 0-100 visual analog scale (VAS),30 and a body mass index (BMI) less than 35 kg/m2.31 Main exclusion criteria were the diagnosis of isolated lateral compartment KOA grade II or III;27 history of physical therapy treatment or lower limb strength training in the last three months;27 history of using minimalist shoes for at least 6 h a day 5 days a week; history of intra-articular infiltration;19 and recent changes in pharmacological treatment.27
Interventions
Participants randomly assigned to the intervention group, after baseline assessment, received a supervised foot-ankle exercise program focusing on strengthening of the extrinsic and intrinsic muscles and range of motion of the foot-ankle joints.27 Followed the criteria of the American College of Sports Medicine,32 the exercise program was performed three times a week for 8 consecutive weeks (24 sessions), with each session lasting around 1 h and 20 min. During the first 4 weeks, individuals performed isolated exercises to improve their foot-ankle range of motion and foot muscle strength. During the second 4 weeks, additional functional exercises were included to increase the strength of the extrinsic and intrinsic foot muscles.
The exercise program for the first block of individuals was supervised by a physical therapist in a face-to-face manner during the entire 8-week intervention. The second block of participants was also supervised in a face-to-face manner during the first 2 weeks of intervention and then remotely supervised for the remaining 6 weeks. Considering the potential for low adherence and compliance to the exercise program, a social media group was created to provide the individuals with access to videos about the exercise program, instructions on how to perform each exercise, number of sets, repetitions, rest time, and perceived effort. The physical therapist also encouraged the individuals to continue practice online and remained available throughout the session durations to support them when necessary.
Participants randomly allocated into the control group continued with usual care and treatment recommended by the healthcare team such as pharmacological treatment and/or physical activity, according to the Osteoarthritis Research Society International (OARSI) Clinical Trial Recommendation33 but performed no foot-ankle strengthening exercises.27 For compliance with ethical requirements, after the final assessment of the study, the individuals in the control group were invited to undergo 8 weeks of treatment using the foot-ankle exercise program.
Outcomes
Assessments at baseline (T0) and 8 weeks after the intervention (T1) were performed at the university's facilities. The primary efficacy endpoint was defined as the mean difference in the Western Ontario & McMaster Universities Osteoarthritis (WOMAC) pain subscale based on the OARSI task force between T0 and T134 (Table 1). As for the secondary endpoints, the mean difference between T0 and T1 for self-reported stiffness, function, and WOMAC total index as well as physical function performance-based tests by the 30-s chair stand test, 9-step stair climb test, and 40-m fast-paced walk test were considered (Table 1).
Table 1.
Detailed description of the outcome measures.
| Outcome | Description | Scoring | MCID |
|---|---|---|---|
| Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) | WOMAC is a 24-item self-reported questionnaire that measures three domains (pain, stiffness and physical function) graded on a five-point Likert scale. | Pain: 0-20 units; Stiffness: 0-8 units; Function: 0-68 units; Total index: 0-96 units; Higher scores indicate a worse condition. |
Pain: 2-point change on a 20-point Likert scale37; Function: a score of 21 or higher indicating physical work limitations38; Total index: improvement of 12% from baseline.38 |
| 30 seconds (s) chair stand test | Individual sit in the middle of the chair placed against a wall to prevent movement, with their back straight and feet resting on the floor in line with their shoulders. The participant is requested to rise from sitting to standing as many times as possible during 30s. | Total number of repetitions within 30s; Higher number of repetitions indicates better condition. | Increase of 2 to 3 repetitions.37 |
| 9-step stair climb test | Individual is positioned in front of the stairs and, at the therapist's signal, they climb the indicated steps (nine-step stair) and descend promptly, while the researcher keeps time. | The final score was calculated based on the time the participant took to perform the test; Shorter time represents better condition. | Reduction of 5.5s in time of the test.37 |
| 40-m fast-paced walk test | After marking a distance of 10 m (using tape), cones are placed 2 m before the start and 2 m after the end of each marking. Individual is instructed to walk as quickly but as safely as possible the first 10 m (from the start mark), to turn around at the cone and walk back the 10 m again, successively until completing the distance of 40 m. |
The final score was calculated based on the speed (m/s) to perform the test; Higher speed represents better condition. | Increase of 0.2–0.3 m per second in speed of the test.37 |
MCID, minimal clinically important difference.
Randomization, allocation, and blinding
Offsite randomization was performed using RStudio v.1.1.463 (R Foundation for Statistical Computing, Vienna, Austria) in RStudio V.1.1.463 (RStudio, Boston, MA, USA) and processed in permuted blocks. Allocation concealment was ensured using sealed opaque envelopes inaccessible to blinded assessors.
After signing the informed consent form, eligible individuals were randomly allocated to the intervention or control group by another independent researcher 1 week after the baseline assessment. Clinical and functional outcomes were evaluated by an independent researcher blinded to group allocation, and individuals were asked not to reveal their allocation status during the study period.
Statistical analysis
A statistician blinded to the group allocation conducted all analyses before decoding the groups. The intention-to-treat approach was employed for the data analysis. To handle missing data, the data imputation method was utilized, specifically the Multiple Imputation by Chained Equations algorithm.35
Continuous variables were analyzed with the Shapiro–Wilk test of normality and are presented as means and standard deviations or medians (first and third quartile). Categorical variables are presented as counts and percentages. The Mann–Whitney U test was used to compare continuous variables between groups and Pearson's chi-squared test with Yates's continuity correction for categorical variables. ANCOVA was conducted using the intervention group as a reference and sex, BMI, and baseline measurements of each outcome as covariates. The coefficient of determination (R2) of the ANCOVA model was also reported to analyze the percentage of variation in the response to intervention explained by the model. All statistical significance was set at a two-sided p-value less than .05, also reporting the interpretation of the 95% confidence interval.36
Results
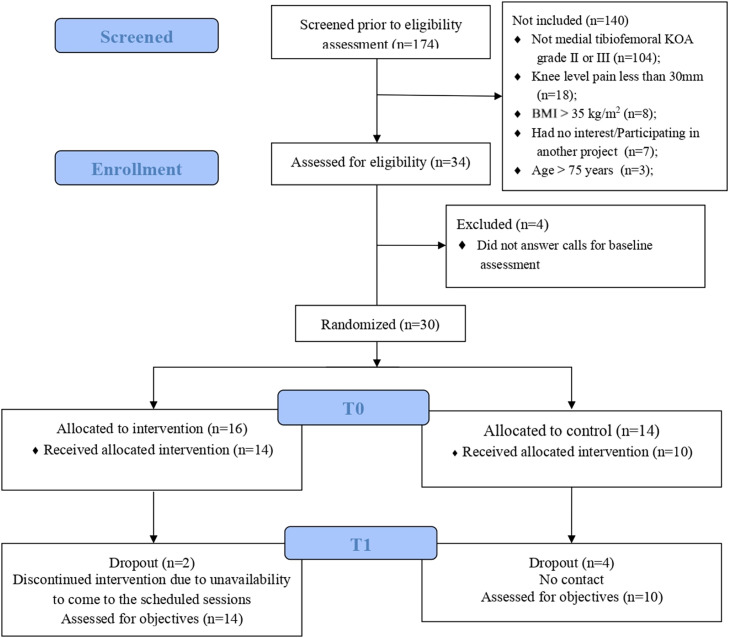
We contacted 174 individuals from public announcements and lists of local and regional orthopedic and rheumatology clinics (Fig. 1) and 140 did not met the eligibility criteria, mainly due to a lack of medial tibiofemoral KOA or low levels of knee pain (< 30 mm on VAS). 30 individuals meeting the inclusion criteria were randomized into the control group (n = 14) and intervention group (n = 16) (Fig. 1). Of these, 24 individuals (control: n = 10; intervention: n = 14) completed the 8-week program. Average attendance rate for the exercise program was 88%. Four participants from the control group dropped out due to loss of contact and two participants from the intervention group due to a lack of attendance at scheduled sessions.
Fig. 1.
Flow diagram of study.
Primary outcome
Groups were similar at baseline (Table 2). In the ANCOVA model, after adjusting for sex, BMI, and the baseline WOMAC pain score, the intervention showed a reduction of 4.4 points (95% CI = −7.5, −1.1) on the WOMAC pain score compared to the control group, indicating a potentially clinically meaningful effect (Table 3). This model explained 49% (R2) of the variability in WOMAC pain subscale score after 8 weeks of intervention (Table 3).
Table 2.
Demographic and clinical characteristics of participants, and outcome scores at baseline.
| Overall (n=30) | Control group (n=14) | Intervention group (n=16) | |
|---|---|---|---|
| Female sex [n (%)] | 15 (50) | 6 (42.9) | 9 (56.2) |
| Age [years] | 61.9 ± 8.9 | 63.1 ± 8.2 | 60.9 ± 9.5 |
| Body Mass Index [kg/m2] | 29.9 ± 3.7 | 30.8 ± 3.9 | 29.1 ± 3.5 |
| Right dominant limb [n (%)] | 26 (86.7) | 10 (71.4) | 16 (100) |
| KOA limb | |||
| Right [n (%)] | 12 (40) | 5 (35.7) | 7 (43.8) |
| Left [n (%)] | 6 (20) | 3 (21.4) | 3 (18.8) |
| Both [n (%)] | 12 (40) | 6 (42.9) | 6 (37.5) |
| Most symptomatic limb | |||
| Right [n (%)] | 17 (56.7) | 7 (50) | 10 (62.5) |
| Left [n (%)] | 13 (43.3) | 7 (50) | 6 (37.5) |
| WOMAC pain (score) | 9.4 ± 4 | 8.8 ± 4.6 | 10 ± 3.3 |
| WOMAC stiffness (score) | 2.1 ± 1.6 | 2.6 ± 1.6 | 1.7 ± 1.6 |
| WOMAC function (score) | 19.7 ± 11.5 | 20.5 ± 12.6 | 18.9 ± 10.7 |
| WOMAC Total Index (score) | 31.2 ± 15.4 | 31.9 ± 17.5 | 30.7 ± 13.7 |
| 30 seconds chair stand test (repetitions) | 10.5 ± 2.3 | 10.3 ± 3 | 10.7 ± 1.4 |
| 9-step stair climb test (seconds) | 16.6 ± 5.6 | 17.6 ± 6.6 | 15.6 ± 4.5 |
| 40-m fast-paced walk test (m/s) | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 |
Table 3.
Primary and secondary outcomes comparisons after eight-week intervention.
| Outcomes | Control group (n=10) | Intervention group (n=14) | Unstandardized coefficient of ANCOVA (95% CI) | R2 |
|---|---|---|---|---|
| WOMAC pain | ||||
| T0 | 8.8 ± 4.6 | 10.0 ± 3.3 | −4.4 (−7.5, −1.1) | 0.49 |
| T1 | 8.6 ± 5.0 | 4.8 ± 3.3 | ||
| T1 - T0 change | −0.2 ± 4.0 | −5.6 ± 4.0 | ||
| WOMAC stiffness | ||||
| T0 | 2.6 ± 1.6 | 1.7 ± 1.6 | −0.9 (−2.5, 0.7) | 0.18 |
| T1 | 2.2 ± 1.8 | 1.1 ± 1.5 | ||
| T1 - T0 change | −0.4 ± 1.8 | −0.6 ± 2.1 | ||
| WOMAC function | ||||
| T0 | 20.5 ± 12.6 | 18.9 ± 10.7 | −7.1 (−12.7, −1.4) | 0.72 |
| T1 | 18.7 ± 10.4 | 9.1 ± 8.9 | ||
| T1 - T0 change | −3.2 ± 5.6 | −10.4 ± 7.9 | ||
| WOMAC Total Index | ||||
| T0 | 31.9 ± 17.5 | 30.7 ± 13.7 | −11.9 (−20.7, −3.1) | 0.69 |
| T1 | 29.5 ± 16.0 | 15 ± 13.0 | ||
| T1 - T0 change | −3.4 ± 8.2 | −16.8 ± 12.0 | ||
| 30 seconds (s) chair stand test | ||||
| T0 | 10.3 ± 3.0 | 10.7 ± 1.4 | 2.7 (1.1, 4.1) | 0.65 |
| T1 | 9.3 ± 1.9 | 12.1 ± 2.3 | ||
| T1 - T0 change | −1.4 ± 2 | 1.6 ± 1.9 | ||
| 9-step stair climb test | ||||
| T0 | 17.6 ± 6.6 | 15.6 ± 4.5 | −2.7 (−5.7, 0.1) | 0.62 |
| T1 | 17.7 ± 7.0 | 12.1 ± 2.0 | ||
| T1 - T0 change | 0.1 ± 4.6 | −3.6 ± 3.2 | ||
| 40-m fast-paced walk test | ||||
| T0 | 1.4 ± 0.2 | 1.3 ± 0.2 | 0.2 (−0.0, 0.4) | 0.29 |
| T1 | 1.4 ± 0.3 | 1.6 ± 0.1 | ||
| T1 - T0 change | −0.0 ± 0.2 | 0.3 ± 0.2 |
Data based on Intention-to-treat analysis. Continuous data are mean ± standard deviation. ANCOVA analysis using covariates sex, body mass index, and baseline measurements of each outcome.
Abbreviations: 95% CI, 95% Confidence Interval; T0, baseline assessment; T1, post-intervention assessment.
Secondary outcomes
The ANCOVA model showed a reduction of 7.1 (95%CI = −12.7, −1.4) and 11.9 (95%CI = −20.7, −3.1) points on the WOMAC function and total index scores, respectively, in favor of the intervention. The width of confidence intervals suggests a potentially clinically meaningful effect for both secondary outcomes (Table 3). In addition, each model explained 72% and 69% of the variability of WOMAC function and total index scores, respectively (Table 3). Regarding 30-s chair stand scores, the intervention demonstrated an improvement of 2.7 (95%CI = 1.1, 4.1) repetitions compared to the control group, with a confidence interval indicating a moderate clinically meaningful effect and the model explaining 65% of variability (Table 3).
Discussion
Our findings showed that a foot-ankle exercise strengthening program might be effective in improving knee pain and physical function of individuals with KOA, with a potential to be clinically meaningful. In addition to demonstrating effectiveness for pain and function, the proposed foot-ankle exercise program could have the advantage of being low cost and easy to perform (most of the exercises can be performed sitting down), allowing individuals with stability problems or poor cardiovascular health to participate.
The proposed foot-ankle exercise program appears to be a good treatment option for reducing knee pain and improving function in individuals with KOA because it (i) reduced WOMAC pain by 4.4 points, a value twice the minimal clinically important difference (MCID) of 2 points;37 (ii) improved self-reported functionality (WOMAC function subscale) by 7.1 points; (iii) increased physical function of sitting to standing by 2.7 repetitions (MCID = 2 to 3). In addition, WOMAC total index scores improved by 11.9 points (12.40% of the total index score), also reaching MCID levels which is 12% from baseline assessment.38 Despite reaching MCID values, the small sample size and some wide confidence interval values may generate some uncertainty about the effects of the intervention.36,39 Even with the small sample size, the R2 values of the ANCOVA model also could suggest a positive effect of the intervention potentially clinically meaningful.40,41
A meta-analysis conducted by Goh et al.,42 which included trials with primary outcomes of pain (69 trials) and function (64 trials), demonstrated that physical exercises, compared to usual care, are effective for improving knee pain and physical function for up to 8 weeks for individuals with mild to moderate KOA. However, the exercises proposed in those trials did not include foot-ankle exercises. Another meta-analysis that included 26 studies comparing different types of physical exercise (individual or in groups; isokinetic, isotonic, and isometric; high and low loads; and combined with aerobic or proprioceptive) with control groups confirmed the efficacy of those general exercises, regardless of type, for reducing pain and improving function.43 Our results showed that foot-ankle exercises could be considered in the treatment of KOA.
To the best of our knowledge, this is the first study to investigate the effects of a foot-ankle exercise program on knee pain and function in individuals with KOA. We postulate that a stronger foot structure should better dissipate cumulative loads by actively supporting the lower limb. Some studies have demonstrated the benefits of strengthening the foot-ankle muscles in various populations and musculoskeletal conditions.24,44, 45, 46, 47 Mølgaard et al.48 demonstrated that exercises for the foot muscles combined associated with the use of foot orthoses were more effective than performing exercises directed at the knee in individuals with patellofemoral pain. We suggest that better control of foot-ankle motion and alignment through strengthening of the foot-ankle musculature has the potential to reduce knee pain by decreasing cumulative loads at the knee during locomotion.49, 50, 51 Further studies should assess foot-ankle kinematics and knee adduction moments to verify the increased motion and functioning of the foot joints in addition to the supposed reduction of the knee impacts represented by the knee adduction moment, which is highly associated with KOA progression and pain.52
In addition to the exercise program's biomechanical mechanism of action, improvements in general fitness, psychosocial, and neurophysiological aspects53,54 could also explain the pain reduction and physical function improvement in the intervention group. Even though KOA is considered a local (joint) musculoskeletal disease, central sensitization may be one of the pathophysiological mechanisms explaining the pain in these individuals.55 The foot-ankle exercise program could have been beneficial for pain and function due to its exercise-induced antinociceptive effects (opioid release) and hypoalgesia.54 It is also important to consider the placebo effect of the care provided, in addition to the natural history of KOA, and the regression to the mean56 that may have occurred during the program and may also explain the observed effects. However, our results strongly suggest the beneficial effects of the exercise program regardless of the mechanism of action, and such changes in the course of the disease are the ultimate goals in this population.
The main limitation of the present study is the small sample size which limits the external validity (generalizability) of the results. However, the control group, who received usual care, presented with high levels of pain and worse function during the study period, indicating the beneficial effect of the exercise program. Additionally, due to the type of intervention (physical exercises), it was impossible to blind participants or the researchers responsible for the treatment. But, to maximize the methodological quality of the study, the researchers responsible for the evaluations and data analysis remained blinded to group allocation throughout the study period.
Conclusion
An 8-week foot-ankle strengthening exercise program showed positive, potentially clinically meaningful, effects of decreasing knee pain and improving physical function among individuals with KOA, when compared to the usual care.
The findings suggest that foot–ankle exercises could be an effective treatment strategy for improving pain and functional deficits related to KOA. Larger trials are needed to confirm the efficacy of this exercise program and investigate the effects of foot–ankle exercises program on gait biomechanics parameters.
Conflicts of interest
None.
Acknowledgments
This study was supported by the Sao Paulo Research Foundation (FAPESP, Process No. 2019/21814-2; 2019/20672-0; 2020/02725-6; 2020/00456-8). TFS and ICNS are Researchers for the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Processes 302169/2018-0 and 304124/2018-4, respectively). GD is PhD fellow from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001).
The study was financially supported by Sao Paulo Research Foundation (FAPESP, Process number 2019/21814-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Processes 302169/2018-0 and 304124/2018-4).
References
- 1.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US Adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582. doi: 10.1002/ART.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthr Cartil. 2013;21(1):10–15. doi: 10.1016/J.JOCA.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerman IN, Kemp JL, Crossley KM, Culvenor AG, Hinman RS. Hip and knee osteoarthritis affects younger people, too. J Orthop Sport Phys Ther. 2017;47(2):67–79. doi: 10.2519/JOSPT.2017.7286. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286(2):188–195. doi: 10.1001/JAMA.286.2.188. [DOI] [PubMed] [Google Scholar]
- 6.Gross KD, Felson DT, Niu J, et al. Association of flat feet with knee pain and cartilage damage in older adults. Arthritis Care Res. 2011;63(7):937–944. doi: 10.1002/ACR.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeheyawi RN, Bricca A, Riskowski JL, Barn R, Steultjens M. Foot characteristics and mechanics in individuals with knee osteoarthritis: systematic review and meta-analysis. J Foot Ankle Res. 2021;14(1) doi: 10.1186/S13047-021-00462-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold J, Mackintosh S, Jones S, Thewlis D. Altered dynamic foot kinematics in people with medial knee osteoarthritis during walking: a cross-sectional study. Knee. 2014;21(6):1101–1106. doi: 10.1016/j.knee.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Vårbakken K, Lorås H, Nilsson KG, Engdal M, Stensdotter AK. Relative difference in muscle strength between patients with knee osteoarthritis and healthy controls when tested bilaterally and joint-inclusive: an exploratory cross-sectional study. BMC Musculoskelet Disord. 2019;20(1):1–13. doi: 10.1186/s12891-019-2957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Røsland T, Gregersen LS, Eskehave TN, Kersting UG, Arendt-Nielsen L. Pain sensitization and degenerative changes are associated with aberrant plantar loading in patients with painful knee osteoarthritis. Scand J Rheumatol. 2015;44(1):61–69. doi: 10.3109/03009742.2014.923038. [DOI] [PubMed] [Google Scholar]
- 11.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–474. doi: 10.1002/ACR.21596. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 14.Kelley GA, Kelley KS, Hootman JM, Jones DL. Effects of community-deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: a meta-analysis. Arthritis Care Res. 2011;63(1):79–93. doi: 10.1002/ACR.20347. [DOI] [PubMed] [Google Scholar]
- 15.Wallis JA, Webster KE, Levinger P, Taylor NF. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthr Cartil. 2013;21(11):1648–1659. doi: 10.1016/J.JOCA.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop DD, Semanik P, Song J, Manheim LM, Shih V, Chang RW. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52(4):1274. doi: 10.1002/ART.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson F, Bennell KL, French SD, et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am J Phys Med Rehabil. 2016;95(5):372–389. doi: 10.1097/PHM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 18.Kanavaki AM, Rushton A, Efstathiou N, et al. Barriers and facilitators of physical activity in knee and hip osteoarthritis: a systematic review of qualitative evidence. BMJ Open. 2017;7(12) doi: 10.1136/BMJOPEN-2017-017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trombini-Souza F, Matias AB, Yokota M, et al. Long-term use of minimal footwear on pain, self-reported function, analgesic intake, and joint loading in elderly women with knee osteoarthritis: a randomized controlled trial. Clin Biomech. 2015;30(10):1194–1201. doi: 10.1016/j.clinbiomech.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Trombini-Souza F, Fuller R, Goldenstein-Schainberg C, Sacco ICN. Long-term use of minimal footwear in older adult women with knee osteoarthritis: mechanisms of action in the knee adduction moment. J Biomech. 2020;108 doi: 10.1016/j.jbiomech.2020.109885. [DOI] [PubMed] [Google Scholar]
- 21.Andriacchi TP, Favre J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr Rheumatol Rep. 2014;16(11):1–8. doi: 10.1007/s11926-014-0463-2. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DR, McWalter EJ, Johnston JD. The measurement of joint mechanics and their role in osteoarthritis genesis and progression. Rheum Dis Clin North Am. 2008;34(3):605–622. doi: 10.1016/j.rdc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Sartor CD, Hasue RH, Cacciari LP, et al. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: results of a randomized controlled trial. BMC Musculoskelet Disord. 2014;15(1):137. doi: 10.1186/1471-2474-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taddei UT, Matias AB, Duarte M, Sacco ICN. Foot core training to prevent running-related injuries: a survival analysis of a single-blind, randomized controlled trial. Am J Sports Med. 2020;48(14):3610–3619. doi: 10.1177/0363546520969205. [DOI] [PubMed] [Google Scholar]
- 25.Ferenczi MA, Bershitsky SY, Koubassova NA, et al. Why muscle is an efficient shock absorber. PLoS One. 2014;9(1):e85739. doi: 10.1371/JOURNAL.PONE.0085739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA - J Am Med Assoc. 2021;326(3):257–265. doi: 10.1001/jama.2021.9941. [DOI] [PubMed] [Google Scholar]
- 27.Dantas G, Sacco ICN, Dos Santos AF, et al. Effects of a foot-ankle strengthening programme on clinical aspects and gait biomechanics in people with knee osteoarthritis: protocol for a randomised controlled trial. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-039279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorge A, Dantas L, Serrão P, Alburquerque-Sendín F, Salvini T. Photobiomodulation therapy associated with supervised therapeutic exercises for people with knee osteoarthritis: a randomised controlled trial protocol. BMJ Open. 2020;0:35711. doi: 10.1136/bmjopen-2019-035711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 30.Erhart-Hledik J, Elspas B, Giori N, Andriacchi T. Effect of variable-stiffness walking shoes on knee adduction moment, pain, and function in subjects with medial compartment knee osteoarthritis after 1 year. J Orthop Res. 2012;30(4):514–521. doi: 10.1002/JOR.21563. [DOI] [PubMed] [Google Scholar]
- 31.Shakoor N, Block JA. Walking barefoot decreases loading on the lower extremity joints in knee osteoarthritis. Arthritis Rheum. 2006;54(9):2923–2927. doi: 10.1002/art.22123. [DOI] [PubMed] [Google Scholar]
- 32.9th ed. 2014. ACSM's Guidelines for Exercise Testing and Prescription. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald GK, Hinman RS, Zeni J, Risberg MA, Snyder-Mackler L, Bennell KL. OARSI clinical trials recommendations: design and conduct of clinical trials of rehabilitation interventions for osteoarthritis. Osteoarthr Cartil. 2015;23(5):803–814. doi: 10.1016/J.JOCA.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Whittaker J, Truong L, Dhiman K, Beck C. Osteoarthritis year in review 2020: rehabilitation and outcomes. Osteoarthr Cartil. 2021;29(2):190–207. doi: 10.1016/J.JOCA.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamper SJ. Confidence intervals: linking evidence to practice. J Orthop Sports Phys Ther. 2019;49(10):763–764. doi: 10.2519/jospt.2019.0706. [DOI] [PubMed] [Google Scholar]
- 37.Dougados M, Leclaire P, van der Heijde D, Bloch D, Bellamy N, Altman R. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthr Cartil. 2000;8(6):395–403. doi: 10.1053/JOCA.2000.0361. [DOI] [PubMed] [Google Scholar]
- 38.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower ex. Arthritis Care Res. 2001:384–391. doi: 10.1002/1529-0131(200108)45:4. Published online. [DOI] [PubMed] [Google Scholar]
- 39.Kamper SJ. Sample size: linking evidence to practice. J Orthop Sports Phys Ther. 2022;52(8):563–564. doi: 10.2519/jospt.2022.0702. [DOI] [PubMed] [Google Scholar]
- 40.Field A, Miles J, Field Z. Sage; 2012. Discovering Statistics Using R. [Google Scholar]
- 41.Aiken LS, West SG. Sage; 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- 42.Goh S-L, Persson MSM, Stocks J, et al. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62(5):356. doi: 10.1016/J.REHAB.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imoto AM, Pardo JP, Brosseau L, et al. Evidence synthesis of types and intensity of therapeutic land-based exercises to reduce pain in individuals with knee osteoarthritis. Rheumatol Int. 2019 doi: 10.1007/s00296-019-04289-6. Published online. [DOI] [PubMed] [Google Scholar]
- 44.Mulligan EP, Cook PG. Effect of plantar intrinsic muscle training on medial longitudinal arch morphology and dynamic function. Man Ther. 2013;18(5):425–430. doi: 10.1016/j.math.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Mickle KJ, Caputi P, Potter JM, Steele JR. Efficacy of a progressive resistance exercise program to increase toe flexor strength in older people. Clin Biomech. 2016;40:14–19. doi: 10.1016/j.clinbiomech.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Abdalbary SA. Foot mobilization and exercise program combined with toe separator improves outcomes in women with moderate Hallux Valgus at 1-year follow-upA randomized clinical trial. J Am Podiatr Med Assoc. 2018;108(6):478–486. doi: 10.7547/17-026. [DOI] [PubMed] [Google Scholar]
- 47.Unver B, Erdem EU, Akbas E. Effects of short-foot exercises on foot posture, pain, disability, and plantar pressure in pes planus. J Sport Rehabil. 2019;29(4):436–440. doi: 10.1123/JSR.2018-0363. [DOI] [PubMed] [Google Scholar]
- 48.Mølgaard CM, Rathleff MS, Andreasen J, et al. Foot exercises and foot orthoses are more effective than knee focused exercises in individuals with patellofemoral pain. J Sci Med Sport. 2018;21(1):10–15. doi: 10.1016/j.jsams.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 49.McKeon PO, Hertel J, Bramble D, Davis I. The foot core system: a new paradigm for understanding intrinsic foot muscle function. Br J Sports Med. 2015;49(5):290. doi: 10.1136/bjsports-2013-092690. [DOI] [PubMed] [Google Scholar]
- 50.Nigg BM, Balich J, Federolf P, Manz S, Nigg S. Functional relevance of the small muscles crossing the ankle joint – the bottom-up approach. Curr Issues Sport Sci. 2017;2(003) doi: 10.15203/CISS_2017.003. [DOI] [Google Scholar]
- 51.Riddick R, Farris DJ, Kelly LA. The foot is more than a spring: human foot muscles perform work to adapt to the energetic requirements of locomotion. J R Soc Interface. 2019;16(150) doi: 10.1098/RSIF.2018.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall M, Bennell K, Wrigley T, et al. The knee adduction moment and knee osteoarthritis symptoms: relationships according to radiographic disease severity. Osteoarthr Cartil. 2017;25(1):34–41. doi: 10.1016/J.JOCA.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Bartholdy C, Juhl C, Christensen R, Lund H, Zhang W, Henriksen M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: a systematic review and meta-regression analysis of randomized trials. Semin Arthritis Rheum. 2017;47(1):9–21. doi: 10.1016/j.semarthrit.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Vaegter HB, Jones MD. Exercise-induced hypoalgesia after acute and regular exercise: experimental and clinical manifestations and possible mechanisms in individuals with and without pain. PAIN Rep. 2020;5(5):e823. doi: 10.1097/pr9.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fingleton C, Smart K, Moloney N, Fullen B, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(7):1043–1056. doi: 10.1016/J.JOCA.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 56.Englund M. Bout of the corner men and not the boxers? Contextual effects flex their muscles. Ann Rheum Dis. 2018;77(2):159–161. doi: 10.1136/ANNRHEUMDIS-2017-211664. [DOI] [PubMed] [Google Scholar]