Abstract
Over the past two decades, advances in arthroscopic and minimally invasive surgical techniques have led to significant growth in sports medicine surgery. Implants such as suture anchors, interference screws, and endo-buttons are commonly used in these procedures. However, traditional implants made of metal or inert materials are not absorbable, leading to complications that affect treatment outcomes. To address this issue, absorbable materials with excellent mechanical properties, good biocompatibility, and controlled degradation rates have been developed and applied in clinical practice. These materials include absorbable polymers, absorbable bioceramics, and absorbable metals. In this paper, we will provide a comprehensive summary of these absorbable materials from the perspective of clinicians, and discuss their clinical applications and related research in sport medicine.
Keywords: Absorbable implants, Sport medicine, Arthroscopic surgery, Absorbable materials, Absorbable metal
Graphical abstract
1. Introduction
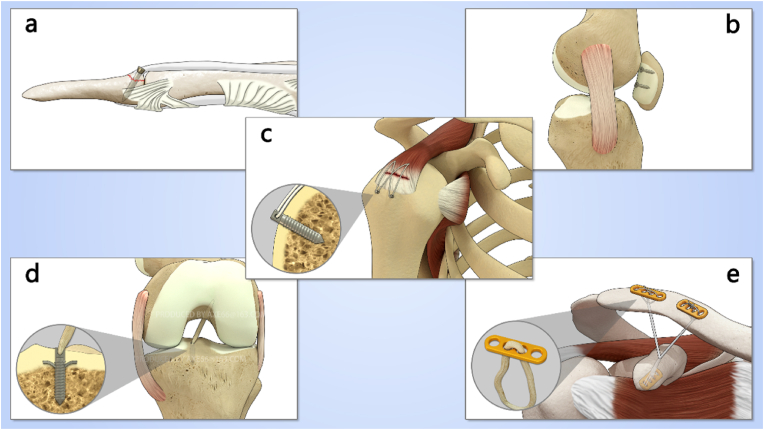
Sports medicine is a branch of orthopedics that focuses on injuries and diseases of bones, joints, and soft tissues (such as ligaments, tendons, and cartilage) that occur during sports activities. Minimally invasive arthroscopic techniques are often used for surgical repair and reconstruction, providing advantages such as minimal scarring, faster recovery, and increased safety. Implants such as suture anchors, interference screws, and endo-buttons are commonly used in arthroscopic surgery, as shown in Fig. 1. Traditionally, these implants are made of metals such as titanium alloy or stainless steel. While these materials provide effective and stable mechanical support, they also have several drawbacks: 1) Metal implants are non-absorbable and can irritate soft tissues, causing pain and discomfort if left in the body for extended periods; 2) Complications such as loosening, displacement, or breakage of metal implants can affect patient prognosis and increase the risk of reoperation; 3) Non-absorbable implants can obstruct movement or damage cartilage within the joint cavity; 4) Metal implants can interfere with follow-up imaging examinations; 5) Even if no obvious symptoms are present, some patients may still require implant removal after surgery, increasing their medical burden.
Fig. 1.
Kinds of implants used in sport medicine: (a) Screws for treating mallet fingers, (b) Screws for internal fixation of patellar cartilage fractures, (c) Suture anchors for rotator cuff injuries, (d) Interference screws used in anterior cruciate ligament reconstruction surgery, (e) Endo-buttons for treating dislocation of acromioclavicular joint.
As an alternative to metal materials, biostable polymers such as ultra-high molecular weight polyethylene (UHMWPE), polyurethane (PU), polymethyl methacrylate (PMMA), and polyether ether ketone (PEEK) have been approved by the FDA for use in orthopedic implants. These materials offer excellent mechanical properties and do not interfere with imaging. However, residual toxic small molecules or wear products can still have unpredictable effects on tissue healing [1,2]. In recent decades, advances in material science have highlighted the benefits of absorbable materials, including good biocompatibility, non-cytotoxicity, low revision rates, and no need for removal. As a result, an increasing number of scientists, clinicians, and entrepreneurs are focusing on the application of bioresorbable materials in orthopedic implants. This paper will introduce existing absorbable materials and summarize their clinical applications in sport medicine.
2. Key issues in sport medicine: the mechanisms of tendon-bone healing
Sport medicine focuses on the injury and repair of tendons, ligaments, and soft tissue. To better understand these structures, it is important to first introduce the anatomical and histological characteristics of the enthesis. Additionally, understanding the mechanisms and biomechanical characteristics of tendon-bone healing is crucial in effectively treating injuries in this area.
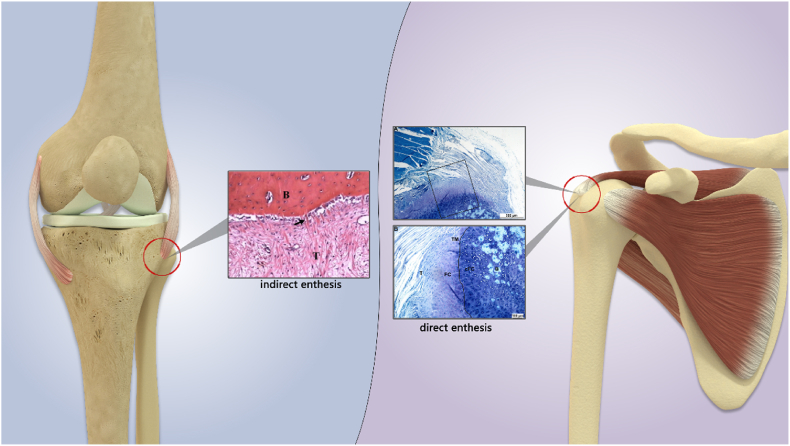
Microscopically, the enthesis, the skeletal attachment of the tendon, contains a series of tissue structures that are specifically arranged to transmit mechanical stresses between soft tissue and bone. Confocal microscopic observation shows that the main component of the ligament or bone tissue is type I collagen, while the enthesis is dominated by type II collagen [3]. There are two types of entheses: direct enthesis and indirect enthesis. The direct entheses are typically found in the epiphysis and include the attachments of the rotator cuff (RC), anterior cruciate ligament (ACL), patellar tendon, and Achilles tendon. These entheses connect soft tissue to bone through fibrocartilage and consist of four histological layers: ligament/tendon, fibrocartilage, calcified fibrocartilage, and bone [[4], [5], [6]]. The indirect enthesis connects the ligamentous tissue to the bone tissue through dense collagen fiber tissue called Sharpey's fiber, mostly located in the diaphysis of long bones. One representative example is the enthesis between the medial collateral ligament of the knee joint and the tibia [4,5], as shown in Fig. 2.
Fig. 2.
A diagram for enthesis of tendon-bone insertion. The indirect enthesis are mostly located in the diaphysis of long bones, connecting the tendon(T) to the bone(B) through dense collagen fiber tissue called Sharpey fiber(arrow) [7], for example, the enthesis between the medial collateral ligament of the knee joint and the tibia. The direct entheses are typically found in the epiphysis and include the attachments of the rotator cuff, anterior cruciate ligament, patellar tendon, and Achilles tendon. These entheses consist of four histological layers: ligament/tendon(T), fibrocartilage(FC), calcified fibrocartilage(cFC), and bone(B) [8].
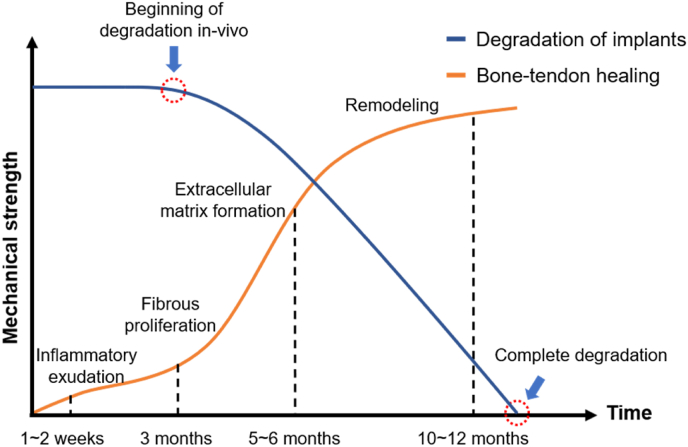
The primary goal of surgical treatment for various tendon injuries is to repair the injured tendon with sutures or to reconstruct the original anatomy using autologous or allogeneic tendon tissue or to fix the tendon to the bone tissue using implants such as suture anchors and interference screws. The strength of the reconstructed ligament depends mainly on the result of tendon-bone healing. Until the tendon-bone healing is complete, implants provide the primary mechanical support. Tendon-bone healing is a complex and dynamic biological process that involves four main stages at the cellular and molecular levels: inflammatory exudation, fibrous proliferation, extracellular matrix formation, and remodeling [4]. In 2003, Robert et al. reported histological findings of tendon-bone healing following ACL reconstruction with hamstring grafts in 12 patients. At 3 months, a fibrovascular interface was seen between the tendon and uncalcified osteoid with very few collagen fibers. At 5 and 6 months, some Sharpey-like fibers and less immature woven bone was seen. After at least 10 months, maturity of the secondary insertion was seen. And after 1 Year, an interface composed of a continuous layer of Sharpey-like fibers was found. The researchers concluded that the time to obtain a mature indirect anchorage at the bone tunnel was 10–12 months, which is much longer than in reported animal models (6–24 weeks). Likewise, Lu et al. [9] summarized the process of tendon-bone healing after ACL reconstruction into three stages. At 3 months, fibrovascular proliferation was observed between the tendon and non-calcified cartilage-like tissue, containing a small amount of Sharpey-like fibers. This corresponded to the process of inflammatory cell chemotaxis, collagen matrix exudation proliferation, and peripheral vessel formation. At 6 months, lamellar bone was visible and fibrous tissue increased between the tendon-bone interface with more Sharpey's fibers. At this stage, a large number of fibroblasts proliferated and synthesized abundant extracellular matrix. At 10 months, mature indirect entheses formed, creating stronger connections between the tendon-bone interfaces, and collagen fibers were rearranged under stress. In summary, we think the degradation rate of implants should match the process of tendon-bone healing. In ideal conditions, absorbable implants in the body should maintain mechanical integrity until the collagen fibers between the tendon bone interface are initially formed (about three months) and completely degrade after at least one year, seen in Fig. 3.
Fig. 3.
The relationship between implants degradation and tendon-bone healing process under ideal conditions. At about 3 months, collagen fibers begin to appear between the tendon-bone interface, meaning an increase in the mechanical strength of the reconstructed tissue. Therefore, the degradation of implants should not begin earlier than this point. After about 12 months, mature indirect entheses formed. Implants should be completely degraded after this point.
3. Overview of medical absorbable materials
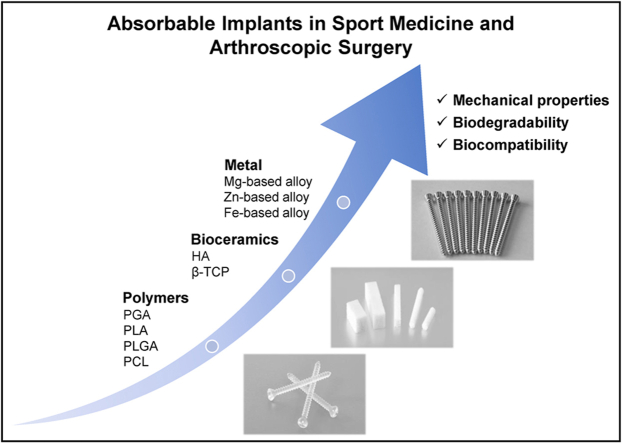
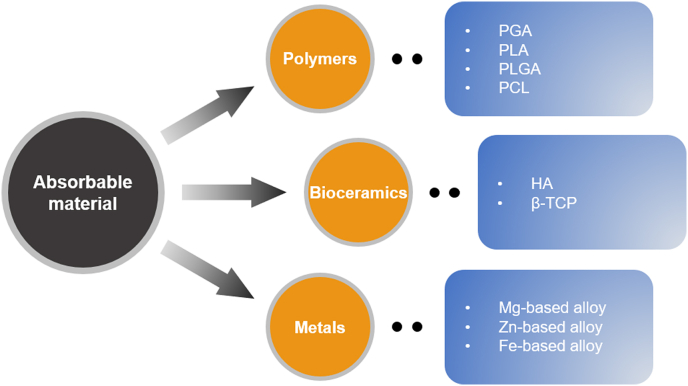
As far back as the 1990s, Speer et al. [10] proposed that absorbable materials used in orthopedic surgery should meet the following four principles: (1) The bioabsorbable implant must have adequate initial fixation strength to coapt the soft tissues to bone; (2) The implant's bioabsorption profile must enable it to retain satisfactory strength while the healing tissues are regaining mechanical integrity; (3) The implant must not bioabsorb too slowly or it will behave like its metal counterpart with breakage and migration; and (4) The implant must be made of materials that are completely safe: no toxicity, antigenicity, pyrogenicity, or carcinogenicity. According to the above principles, the mature and promising medical absorbable materials today mainly include polymer materials, bioceramics, and absorbable metals/alloys, seen in Fig. 4 and Table 1.
Fig. 4.
An overview of medical absorbable materials.
Table 1.
Summary of the three kinds of absorbable materials.
| Absorbable material | Degradation time | Degradation products | Merits | Demerits | |
|---|---|---|---|---|---|
| Polymers | PGA | 6∼12 months | Glycolic acid → Water, carbon dioxide | High elastic modulus, high mechanical strength | Rapid degradation rate, can result in inflammatory reaction at the implant site |
| PLA | Few years | Lactic acid→Water, carbon dioxide | High mechanical stability, slow degradation rate | Poor biocompatibility,acid degradation,hard to control degradation rate | |
| PLGA | More than 1–2 months | Lactic acid, Glycolic acid→Water, carbon dioxide | Good biocompatibility, controllable degradation rate, almost no inflammatory reaction | Poor mechanical strength, poor osteointegration, unable to bear too much weight | |
| PCL | 2∼4 years | Caproic acid → Water, carbon dioxide | Slow degradation rate, suitable for manufacturing scaffolds | Poor mechanical strength, unable to bear weight | |
| Bioceramics | HA and β-TCP | Few months to few years (depend on the molecular structure of materials) | Ca2+, PO34- | High mechanical stability, suitable degradation rate, good biocompatibility, good osteointegration and osteoconductive, improved environmental pH | Too brittle to be manufactured into implants independently |
| Metal | Mg- based alloy | About 1∼1.5 years (MAGNEZIX) | Mg2+, OH−, hydrogen and other metal ions | Superior mechanical properties compared with stainless steel or Ti-based alloy, good biocompatibility, good osteointegration and osteoconductive, no cytotoxicity, | Need to improve manufacturing process to control degradation rate, complications related to degradation product hydrogen |
| Zn- based alloy | More than 20 months | Zn2+, OH− and other metal ions | Similar mechanical properties to those of human bones, suitable degradation time, good biocompatibility, good osteointegration and osteoconductive, strong antibacterial activity in vivo | Uncertainty about toxicity of local high concentration zinc ions in human body | |
| Fe- based alloy | Longer than 2 years | Fe2+, Fe3+, OH−, iron oxides and hydroxides, hydrogen | Superior mechanical properties with high elasticity, strength, and plasticity | Slow degradation rate, poor biological activity, high concentration iron ions have biological toxicity | |
3.1. Polymer materials
Absorbable polymer materials share the common characteristics of polymer materials, including good mechanical strength, plasticity and elasticity. In addition, they can degrade spontaneously in a physiological environment to produce degradation products that can be absorbed or expelled from the body through metabolism. Absorbable polymer materials include natural and synthetic polymers. Natural polymers are derived from natural sources and include materials such as cellulose, chitin, and collagen. Synthetic polymers are artificially synthesized through chemical methods and their degradation rate in vivo varies depending on their structure. This rate can be regulated through improved preparation methods. Synthetic absorbable polymers that have been widely studied and applied in clinical practice include aliphatic polyesters such as polyglycolic acid, polylactic acid, polylactic-co-glycolic acid, and polycaprolactone.
3.1.1. Polyglycolic acid, PGA
PGA is the simplest linear aliphatic polyester and its degradation product, hydroxyacetic acid, is an intermediate product of the body's metabolism. It can be further decomposed into CO2 and H2O through the tricarboxylic acid cycle or excreted directly through urine [11]. PGA degrades rapidly, with its strength in tissue decreasing by more than 50% after 14 days and by 90% after 28 days under general conditions [12], and in 6–12 months PGA can be completely degraded and absorbed [13]. Due to its high crystallinity, high melting point, high elastic modulus, and controllable degradation rate, PGA has been made into the world's first absorbable surgical suture (Dexon®) [14]. However, for orthopedic products, PGA's rapid degradation rate is a significant drawback as it cannot maintain fixation strength for a sufficient amount of time. This limits its application in orthopedic or arthroscopic surgery and makes it only suitable for fixing soft tissues that do not bear tensile stress or non-displaced fractures such as non-displaced Bankart or labral injuries [15]. Therefore, since the 1990s, it has no longer been used for making orthopedic implants. In addition, excessive local accumulation of PGA degradation products will lead to unique complications, such as synovitis, bursitis, or lytic bone changes [16]. Since then, PGA has been improved by physical blending or chemical copolymerization with other materials to effectively control its degradation rate.
3.1.2. Polylactic acid, PLA
PLA has excellent mechanical properties and a controllable degradation rate. It is made from starch derived from corn and wheat, making it low-cost and carbon-neutral. As such, it is considered one of the most promising absorbable polymers. PLA was first discovered and named by Swedish chemist Scheele in 1780. In the 1960s, researchers attempted to use PLA sutures and rods to treat mandibular fractures in dogs [17,18], representing the earliest studies of PLA for clinical applications.
The first stage of PLA degradation in vivo involves hydrolysis into lactic acid and water-soluble oligomers. In the second stage, lactic acid can be metabolized into glycogen in the liver, or enter the tricarboxylic acid cycle and finally be decomposed into water and carbon dioxide and discharged from the body [19]. The degradation rate of PLA under natural conditions is quite slow, taking several years. As a result, the inflammatory reaction that occurs after PLA implantation is extremely insignificant [20]. The degradation of PLA can be accelerated by adding some hydrophilic components or by reducing the crystallinity. Since lactic acid, the monomer of PLA, has two optical isomers, there are three corresponding stereo-configurations of PLA: levo polylactic acid (PLLA), dextro polylactic acid (PDLA), and racemic polylactic acid (PDLLA). It is worth noting that L-LA is a natural metabolite in animals and is not toxic to humans. However, D-LA cannot participate in the body's metabolism and excessive use may lead to toxicity. PLLA has high tensile strength, low elongation at break and high tensile modulus of elasticity (close to 4.8 GPa), which is ideal for medical load-bearing materials, such as BioScrew®, Bio-Anchor®, MeniscalStinger®, etc. They have been common PLLA orthopedic implants. In practical applications, PLLA has a slow degradation rate because of its high crystallinity. To circumvent this problem, PLLA and PLDLA are usually copolymerized to neutralize and adjust the actual performance by using the low crystallinity and high degradation rate of PLDLA.
Primary PLA also has disadvantages such as high brittleness and poor impact resistance. For this reason, self-reinforced materials or composites have been prepared to improve the strength and control the degradation rate to further meet the needs of clinical applications. For example, the addition of hydroxyapatite (HA), which is similar to the inorganic composition of bone, can slow down the degradation rate of PLA and improve its mechanical strength, resulting in a good long-term implant material [[21], [22], [23]]. Hydroxyapatite also improves the acidic pH of the local internal environment after material degradation, increases the hydrophobicity of the material surface, and enhances the osteoinductivity and protein adsorption capacity of the material [24].
3.1.3. Polylactic-co-glycolic acid, PLGA
PLGA is the general name of a series of copolymers obtained by polymerization of glycolic acid and lactic acid in different proportions. PGA degrades rapidly, resulting in rapid mechanical strength degradation, while PLA has poor hydrophilicity and slower degradation. Copolymerization of the two monomers in different ratios can yield absorbable materials with enhanced performance. The main factors affecting the properties of PLGA are the copolymerization ratio, copolymerization morphology, and molecular weight of the two monomers. When the copolymerization ratio of LA/GA is 50/50, PLGA is a random copolymer with the fastest degradation rate, which can be completely degraded within 1∼2 months, while PLGA with other copolymerization ratios has more significant hydrolysis resistance [25,26]. PLGA exhibits good biocompatibility in clinical applications, is non-cytotoxic and does not cause inflammatory reactions [27,28], and is commonly used as surgical sutures, drug carriers, and tissue engineering materials [29]. However, due to low mechanical strength, large elasticity, and lack of osteogenic bioactivity [30], PLGA cannot be used for load-bearing applications and is rarely used in orthopedics at present [13,31].
Polylactide carbonate (PLC) is a absorbable material made from a copolymer of PDLLA and PGA mixed with carbonate. In sheep ACL reconstruction models, PCL exhibited excellent mechanical strength and osteo-conductivity [32]. However, in human applications, interference screws made from PLC were commonly associated with synovitis and knee joint swelling. At the same time, PLC would appear irregular degradation and excessive foreign body reaction in the human body, leading to clinical research failure [33,34].
3.1.4. Polycaprolactone, PCL
Compared with other medical polymers, PCL is characterized by its low glass transition temperature, low melting point, good thermal stability and rubbery state at room temperature, and it is easy to be blended with other polymers and to be molded. The higher the molecular mass, the longer the absorption time in the body. The degradation of PCL in vivo is carried out in two stages. The first stage is the continuous decrease of molecular weight, but no deformation and mass reduction. In the second stage, after the molecular weight is reduced to a certain value, the material starts to become fragments and lose mass, and is then gradually absorbed and excreted by the body. PCL can also be used in the preparation of scaffold materials, especially in bone tissue engineering [[35], [36], [37]]. For example, Nie et al. [38] found that a hydroxyapatite-coated PLLA/PCL nanofiber scaffold successfully induced healing of a circular defect in rat skull within 12 weeks. However, PCL is not currently considered suitable for fracture fixation in weight-bearing areas due to its weak mechanical properties [39].
3.2. Bioceramics
Calcium phosphate ceramics, including hydroxyapatite, tricalcium phosphate, tetracalcium phosphate and their mixtures, are important inorganic bioactive materials used in orthopedics. Hydroxyapatite (HA) is the main inorganic component of vertebrate bones and teeth, accounting for about 70% of its total weight. HA has good biological compatibility and bioactivity. After implantation, calcium and phosphorus are released from the surface of the material and absorbed by the body. HA can integrate with bone tissue and support the adhesion, proliferation and differentiation of osteoblasts, forming new bone directly on its surface [40]. Beta tricalcium phosphate (β-TCP) has a strength of 451.11–676.66 MPa and a yield strength of 137.29–156.91 MPa. It has good biocompatibility and is a good bone tissue repair and reconstruction material [41,42]. There are three main degradation pathways of β-TCP in vivo: material degradation by body fluid, phagocytosis by macrophages and multinucleated giant cells, and active absorption by osteoclasts [43]. The characteristics of calcium phosphate bioceramic materials are: they have good biocompatibility and degradability, and their mechanical properties change with porosity. With the increase of porosity, their tensile and compressive strength decreases, their brittleness increases, and their fracture toughness decreases, but their degradability will increase accordingly [44,45].
Numerous in vivo tests have demonstrated that calcium phosphate have good osteoconductivity [46]. These bioceramics bind well to the bone surface without fibrous connective tissue mediation [47] and promote bone formation and remodeling by attracting osteoblasts and osteoclasts through chemotaxis [48]. In addition, when blended with aliphatic polyester materials, the degradation of HA and β-TCP can improve the acidic environment formed in local tissues during the degradation of the former [49,50], which is more favorable for bone growth and osseointegration [51]. Nevertheless, the high brittleness of calcium phosphate makes it difficult to make suitable orthopedic internal fixation materials. Therefore, composites prepared by blending bioceramics and polymers in different ratios preserve the toughness of polymers and the osteoconductivity of bioceramics and are more promising for applications in orthopedic and arthroscopic surgery [52]. Currently, both interference screws made of β-TCP/PLLA or HA/PLLA and suture anchors made of TCP/PLGA have been put into use in adult shoulder or knee arthroscopic surgery and achieved ideal therapeutic effect [[53], [54], [55], [56]].
3.3. Absorbable metal
Absorbable Medical Metal Materials are considered as new materials that can potentially replace traditional titanium alloys or stainless steels in the future. These medical absorbable metals can gradually corrode and degrade under human physiological environment, with the concentration of released metal ions remaining within safe levels for the human body. Due to their excellent mechanical properties, absorbable metals are expected to overcome the limitations of bioabsorbable polymers in weight-bearing applications. Strength and Young's modulus are important indicators of mechanical properties. The tensile strength of cortical bone is 105–114 MPa, yield strength is 35–283 MPa, and elastic modulus is 14–20 GPa. When the yield strength of the metal material as an implant is greater than that of the cortical bone, it can provide sufficient mechanical support to avoid fracture displacement, and provide conditions for early fracture healing. When the elastic modulus of metal material is similar to that of cortical bone, it can effectively reduce the “stress shielding effect” after fracture healing [57]. In addition, ideal absorbable metal materials for orthopedics should also have the characteristics of complete degradation, good biosafety and no cytotoxicity after fracture healing [19,58]. Currently, absorbable metallic materials that have been intensively studied and applied include magnesium, zinc, and their alloys.
3.3.1. Magnesium and its alloys
Magnesium is an essential nutrient in the organism, and its total content in the human body is about 0.05% of body weight. Magnesium is distributed in the human body in a fixed area, with the most distribution in the bones, about 53%. The density (about 1.74 g cm−3) and modulus of elasticity (45 GPa) of magnesium are close to the density (about 1.8 g cm−3) and modulus of elasticity of bone tissue, which can be well adapted to the stress generated by human tissue and effectively avoid the “stress shielding effect” [59].
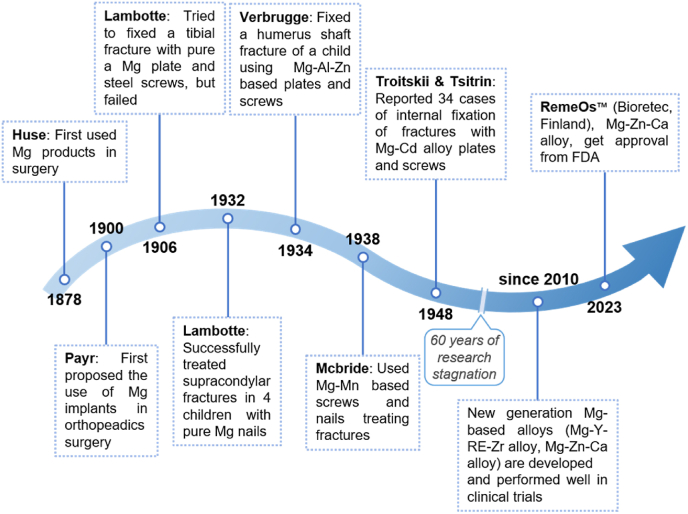
The application of magnesium in clinical medicine began in 1878 when Edward C Huse used magnesium wire for vascular ligation [60]. In 1900, Payr first proposed the idea of using Mg plates, sheets, pins, and nails in the musculoskeletal system, but the results of animal tests and clinical trials were not satisfactory [61,62]. In 1906, Lambotte first used pure magnesium plate and steel screws for fixation of a pseudarthrosis after tibial fracture, but the Mg plate was removed only on the eighth postoperative day because of extensive subcutaneous emphysema and limb swelling caused by the rapid degradation of magnesium in the body and the release of hydrogen gas [63]. He attributed this to a significant corrosion reaction between the magnesium plate and the steel screws in the body environment. After that Lambotte and his assistant Verbrugge tried to use pure magnesium plates and screws to treat children's supracondylar and intercondylar fractures of the humerus. After more than 1 year of follow-up they found good fracture healing, recovery of joint function, complete degradation of the magnesium implants, and no significant complications except for subcutaneous emphysema [64,65]. On this basis, Lambotte further used magnesium implants to treat fractures of clavicle, wrist, hand, ankle, foot and other bones [64]. Because pure magnesium has the defect of fast degradation rate in vivo, by the 1930s researchers gradually turned their attention to magnesium alloys, but the therapeutic effects reported by several scholars are different [[65], [66], [67]]. In 1948, Troitskii and Tsitrin reported 34 cases of internal fixation of fractures with magnesium alloy plates and screws containing a small amount of cadmium, and found that the alloy could stimulate callus formation. In all patients there was no increase in serum magnesium ion concentration and no significant inflammatory reaction, but the problem of gas production during degradation of the alloy remained unresolved [60]. The above early studies illustrated the characteristics of magnesium alloy materials to promote fracture healing and non-toxicity, which laid the foundation for future studies.
In recent years, a large number of studies have confirmed that magnesium and its alloys have good biocompatibility, biodegradability, and no cytotoxicity in the degradation and absorption process [[68], [69], [70], [71], [72]]. At the molecular biology level, magnesium ions were found to contribute to bone formation by participating in multiple pathways, such as the PI3K/Akt pathway [[73], [74], [75]], the osteoprotegerin (OPG)/RANKL pathway [76,77], and the Wnt signaling pathway [[78], [79], [80]]. Excessive degradation rate and hydrogen generation have been the main problems faced by magnesium alloy materials. Besides failing to maintain the fixation for sufficient time, too fast degradation of magnesium alloy also raises the local environmental alkalinity, disrupts the acid-base balance and inhibits cell growth [81,82]. And if hydrogen cannot be absorbed and dispersed in time, it will lead to emphysema in the vicinity of the implant, compressing the surrounding tissues and causing damage, which is not conducive to fracture healing. In this regard, there are two main methods to solve the excessive degradation of magnesium, one is to add a protective layer on the surface of magnesium to control the dissolution rate of magnesium, such as hydroxyapatite coating [83], calcium phosphate coating [84], natural polymer-derived bioactive coating [85] etc., and the other is to make magnesium alloy to regulate the degradation rate. The dynamic loading in human body as well as corrosive body fluid poses a significant challenge to the practical application of absorbable magnesium implants. Alloying magnesium can improve the fatigue strength of materials. Bian et al. [86] observed the corrosion fatigue behavior of Mg–Ca alloy and Mg–Zn–Ca alloy in simulated body fluid, and found that the fatigue resistance of alloy materials was better than that of pure magnesium. In addition, the addition of rare earth elements can also improve the creep resistance and corrosion resistance of magnesium alloys [87]. Currently, Mg–Y-RE-Zr alloy (MAGNEZIX®, Syntellix AG, Hanover, Germany), and Mg–Zn–Ca alloy (RESOMET™, U&I Corporation, Gyeonggi, Korea; RemeOs™, Bioretec, Tampere, Finland) have been marketed successively and have achieved good efficacy in the treatment of calcaneal valgus, ankle fractures, elbow fractures, and distal radius fractures [[88], [89], [90]]. In March 2023, Bioretec announced that it had received market authorization from the U.S. FDA for its RemeOs™ screw to be used for bone fracture healing in clinical trials. This makes Bioretec the first and currently the only medical device company to offer bioresorbable metal implants in the United States. A chronology of the development of magnesium alloys in medicine was summarized as shown in Fig. 5.
Fig. 5.
Clinical research progress of pure magnesium/magnesium-based alloy implants.
3.3.2. Zinc and its alloy
Zinc alloy has emerged as a promising absorbable metal in the field of medical implants in recent years. Zinc is a trace element that plays an essential role as a cofactor for over 300 enzymes in the human body, involved in various functions such as immune system regulation, nervous system function, and cell development [91,92]. Notably, zinc also contributes significantly to bone growth, formation, mineralization, and calcification processes [93,94]. Pure zinc has poor mechanical properties, with ultimate yield strengths ranging from 18 to 140 MPa and low ductility of 1.2%–2.1%. However, when copper, iron, and manganese are added to zinc to create alloys, the resulting materials demonstrate significantly improved yield strength and ductility, approaching the mechanical properties of cortical bone. These new zinc-based alloys surpass conventional materials like titanium and stainless steel in terms of their mechanical performance, offering promising prospects for orthopedic implant applications [95].
Theoretically, zinc alloy degrades slower than magnesium alloy, but faster than iron alloy [96,97]. Depending on the composition of the alloy, the degradation rate ranges from 9% to 11% at 12 weeks, with complete degradation typically taking longer than 20 months [95,98]. The relatively slow degradation of zinc alloys enables them to maintain their structural integrity for at least 6 months during the process of fracture healing, meeting the desired standards for absorbable metal materials used in implants. Unlike magnesium alloys, the degradation process of zinc alloys does not generate hydrogen gas, which can cause local tissue compression and emphysema formation [99].
Numerous studies have demonstrated the benefits of zinc as a bone repair material due to its complete biodegradability, ability to promote bone growth and inhibit osteoclasts, and antibacterial properties [98,100,101]. A study on a rat model of femoral defects have shown that zinc can effectively promote bone defect repair and improve the survival rate of bone graft [99]. A series of in vitro and animal tests suggest that zinc alloy implants are not cytotoxic during degradation [102], but it is still unknown whether high local concentrations of zinc ions can be toxic in humans. Currently, internal fixation using zinc alloy has demonstrated reliable therapeutic results in the treatment of tibial fractures in rabbits and mandibular fractures in dogs [102,103], but few clinical studies on human beings have been performed. Many challenges remain to be addressed before zinc alloy can be widely used in clinical applications.
3.3.3. Iron and its alloy
Iron and its alloys are also potential absorbable metal materials. Iron and its alloys have high elasticity, strength and plasticity, the elastic modulus of which is greater than that of stainless steel and magnesium-based alloys [104]. Iron is also an essential trace element for the human body, and is an important component of hemoglobin, myoglobin, cytochrome, and a variety of oxidases and metabolic enzymes [105]. Many animal experiments have confirmed that iron-based materials have good biological safety [[106], [107], [108], [109]].
Iron is a corrosive material with weaker activity compared to magnesium and zinc. However, in physiological environments, the degradation rate of pure iron is very low, and it will take 2 years or even longer for complete absorption [106,107]. Iron corrodes too slowly in body fluids, partly due to the large volume of iron oxide products that can resist biodegradation. Many scholars have attempted to improve the corrosion efficiency of iron-based materials by preparing alloys, but the improvement effect is limited [[109], [110], [111], [112]]. Similar to magnesium-based materials, iron-based materials also generate hydrogen and hydroxide ion during degradation, but their degradation rate is too slow to have an impact on human body.
The slow degradation rate and poor biological activity of iron and its alloys limit their application as orthopedic implants. In recent years, some scholars have been committed to preparing porous iron-based materials in order to increase the area of materials exposed to corrosive environments, ultimately achieving the goal of increasing the degradation rate of alloys. Li et al. [113] made topologically ordered pore iron by Direct Metal Printing (DMP). Electrochemical tests showed that the degradation rate of this material was 12 times higher than that of cold rolled steel. After 28 days of immersion testing, the weight of porous iron decreased by 3.1%, and the elastic modulus and yield strength of the material did not decrease significantly. In a simulated physiological environment, the material exhibited high fatigue resistance [114]. Wegener et al. [115] developed a porous Fe-0.8P based material with good biocompatibility. Animal tests showed that newly formed bone was growing into the porous spaces of the implant after 12 months, but no complete degradation of the implant could be observed. Grodzicka et al. [116] first reported porous iron-based scaffolds prepared by the simple replica method using polyurethane foam as a template and applying the sintering process in a tube furnace. The morphology and mechanical properties of this iron-based biomaterial were almost identical to bones, and it possessed adequate wettability, indicating its potential as biomaterial for scaffolds or implants in orthopedics.
4. Application of absorbable biomaterials in sport medicine procedures
4.1. Shoulder
Shoulder injuries commonly involve the rotator cuff and the labrum, and they are mainly treated by arthroscopic surgeries nowadays. The procedure involves fixing the injured tendon and cartilage to the bone tissue using suture anchors. Many suture anchors are made from titanium alloy or biostable polyetherether ketone (PEEK), which is mechanically strong, resistant to wear and corrosion, and stable in the body for long periods without biodegradation. However, studies have shown that PEEK is not ideal in promoting osseointegration, which is detrimental to tendon-bone healing [117]. In this regard, biocompatible resorbable polymers are more advantageous. Currently, suture anchors made of bioresorbable polymers are well established, and common products on the market include Bio-Corkscrew® (PDLLA) and Bio-Corkscrew® FT (PLLA) by Arthex (Naples, USA), SpiraLok® (PLLA) by Depuy-Mitek (Norwood, USA), Impact® and Duet® [both SR-PD(4)L(96)LA] by Linvatec (Largo, USA), BioRaptor® (PLLA) and TwinFix® AB (PLLA) by Smith & Nephew Endoscopy (Andover, USA), and BioZip® (PLLA) by Stryker Endoscopy(San Jose, USA), etc. [118].
Long-term clinical practice has confirmed that the above-mentioned bioabsorbable suture anchors have comparable mechanical performance to metal or PEEK anchors [[119], [120], [121]], but there are still some unavoidable complications, such as degradation of fragments into the joint cavity, soft tissue inflammation, cyst formation, and local osteolytic changes [122]. Among these, cyst formation and osteolytic changes are of particular concern to sport medicine surgeons, as they may affect tendon-bone healing and lead to failure. By retrospecting 348 patients underwent arthroscopic SLAP repair, Park et al. [123] found the use of absorbable anchors, particularly PLDLA 96L/4D anchors were independent risk factors for returning to operating room and revision surgery. In his cohort, all cases of surgical failure (n = 22, 6.3%) were treated with suture anchors made by PLDLA, while no patients treated with metal or PEEK anchors needed to repeat operation. An MRI imaging evaluation study showed that of 209 patients of rotator cuff injuries treated with PLLA or PLDLA suture anchors, cyst formation was observed in 97 cases (46.4%) after 10 months postoperatively, and severe cyst formation (defined as cyst diameter larger than twice the anchor diameter) occurred in 18 (8.6%) of these patients. Although statistical analysis showed that the cysts were not related to tendon-bone healing rate, there was a concern that suture anchors within cysts were much less mechanically stable than within bone tissue, which may put tendons at an elevated risk of re-tearing or non-healing [124]. Therefore, for the purpose of reducing bone loss and avoiding reoperations, these types of bioabsorbable suture anchors were clearly inappropriate. McCarty et al. [125] retrospectively identified 44 patients who underwent arthroscopic debridement following surgical treatment of glenoid labrum or rotator cuff lesions with PLLA or PLDLA suture anchors. The research concluded that a recurrence of tear was observed in all patients of rotator cuff injuries by preoperative MRI scan, implant debris were visible in 55% of all cases and glenohumeral cartilage damages classified as Outerbridge grade-III or IV were observed in 70% through arthroscopic exploration, and papillary synovitis in 79%, foreign body giant cell reaction in 84%, and polarized crystalline materials in 100% were found by synovial biopsy. Augusti et al. [126] observed 12 patients with traumatic shoulder instability treated with PLA or PLDLA suture anchors, and found osteolytic changes in all of them in postoperative CT imaging follow-up, with 9 (75%) having anterior glenoid fractures, some of them without even suffering high-energy trauma.
Based on the above facts, biocomposite suture anchors with a bioactive ceramic coating have a slower and more controlled degradation rate, likely to reduce the risk of treatment failure or repeat surgery. It is reported that PLGA/β-TCP is one of the materials with the highest osteoconductivity, and almost 90% of PLGA/β-TCP suture anchors are resorbed within 3 years, avoiding inflammatory reactions and cyst formation that are common with rapidly degrading materials [127]. Randelli et al. [128] evaluated the postoperative performance of 20 cases of Bankart injury treated with PLGA (70%)/β-TCP(30%) suture anchors using MRI and concluded, after a mean follow-up of 29 months, that PLGA/β-TCP suture anchors had a good biological performance with little to no cystic changes and that the signal changes on MRI appeared to be equivalent to the process of trabecular bone formation. PLGA(65%)-β-TCP(15%)-Calcium Sulfate(20%) is a newest biocomposite material with stable initial mechanical strength, good soft tissue healing, and complete degradation within 24 months, which can avoid cyst formation and joint effusion [129]. Vonhoegen et al. [129] analyzed MRI imaging of a total of 82 PLGA/β-TCP/CS suture anchors in 48 patients undergoing arthroscopic rotator cuff repair and found that only 2 anchors (2.4%) induced peripheral osteolysis during degradation, and none of the defects exceeded the anchor diameter (5.5 mm). 50% of the anchors degraded completely, and no anchor pull-out complications was observed. In recent years, all-suture anchors made entirely of non-absorbable sutures have even been developed, and have proven to be as effective as bioabsorbable suture anchors for rotator cuff injuries and Bankart injuries in terms of short-term efficacy [56,130]. In the future, whether all-suture anchors will be prepared with bioabsorbable materials is worthy of clinicians' anticipation.
Magnesium suture anchors have entered the stage of animal experiment. The researchers used 99.98% high purity magnesium suture anchors to repair sheep's infraspinatus tendon in sheep, and histological examination 12 weeks after surgery revealed good tendon-bone healing, no local inflammatory reaction, and no toxic damage to organs, during which the anchors maintained reliable strength [131]. Some scholars tried to use Magnezix ® magnesium alloy screws in Latarjet procedure for shoulder instability, but biomechanical studies on cadaveric bones have found that the screws have a risk of breakage [132].
4.2. Knee
The technique of using bioabsorbable interference screws in anterior cruciate ligament (ACL) reconstruction has been applied for more than 20 years, and a series of early studies have shown that PLA interference screws are comparable to metal interference screws and have fewer complications [133,134]. However, similar to resorbable polymer suture anchors, absorbable polymer interference screws are associated with early or late complications such as breakage of screws, displacement of screws, inflammatory reactions, cyst formation, and bone tunnel enlargement [122,135].
Cyst formation is mostly secondary to the inflammatory reaction around the interface screw and can be detected by MRI. Significant cystic changes can cause symptoms. In contrast, inflammatory reactions and cystic changes are more common and symptomatic in the tibial tunnel than in the femoral tunnel. Ramsingh et al. [136] investigated 273 cases of arthroscopic ACL reconstruction with PLLA/β-TCP interference screws. 14 (5%) patients developed anterior tibial swelling and pain after a mean follow-up of 26 months, and after arthroscopic debridement and curettage of cystic lesions, the symptoms of these patients improved. Bone tunnel enlargement is another common complication, but according to the literature, it does not cause significant symptoms and does not affect clinical outcomes nor lead to knee instability [137,138].
Hopes for reducing complications with absorbable interference screws have likewise been pinned on the development of biocomposite materials, yet some composites appear to have the opposite effect. Calaxo® interference screw, manufactured by Smith & Nephew Endoscopy (Andover, USA), is composed of 65% PLDLA(85)-co-GA(15) and 35% calcium carbonate. Despite the excellent mechanical properties, the copolymer composite degrades unstably in vivo. A RCT study led by Bourke et al. [139] showed that Calaxo® was completely degraded within 6 months after implantation, and 88% of cases had cyst formation around the tibial tunnel. Due to these complications, Calaxo® was eventually withdrawn from the market.
Over the years, magnesium interference screws have been expected for their good mechanical properties and biocompatibility. A biomechanical analysis on cadaveric bone showed that high-purity magnesium interference screws performed comparably to PLLA screws in terms of postoperative knee stability [140]. Thormann et al. [141] confirmed the good biocompatibility of magnesium alloys in vivo by observing a series of changes after implantation of interference screws made of Mg–Y alloy into the femoral condyles of sheep. In rabbit model of ACL reconstruction, Cheng et al. [142] found that High-purity magnesium interference screws promoted the expression of bone morphogenetic protein-2 (BMP-2), vascular endothelial growth factor (VEGF), and fibrocartilage markers (Aggrecan, COL2A1 and SOX-9) and the production of glycosaminoglycan (GAG), they also observed fibrocartilage formation. Diekmann et al. [143] performed ACL reconstruction in rabbits using MgYREZr alloy interference screws, and histological examination after 24 weeks showed good tendon-bone healing and adequate osseous-integration, while no tendon necrosis or inflammatory reaction was observed. Wang et al. [144] showed that Mg interference screws promote mineralization of tendon-bone insertion and bone formation around the screws, with the possible mechanism being enhanced the migration, adhesion, and osteogenic differentiation of bone marrow mesenchymal stem cells [145]. Furthermore Wang [146] and his collaborators developed a Mg–Zn–Sr alloy with better mechanical properties, which performed better in promoting bone formation than PLA interference screws. In a word, the results of the above animal experiments indicate that interference screws made of magnesium and its alloy have excellent mechanical properties, controlled degradation, good biocompatibility and ability to promote tendon-bone healing, which may have good application prospects.
Cross-pin provide an alternative method of fixation and are widely used in arthroscopic ACL and PCL reconstruction. This device fixes the tendon graft close to the anatomical position, allowing extensive contact with the bone tunnel, avoiding the “bungee effect” (referring to the telescopic movement of the tendon graft along the longitudinal axis of the bone tunnel) and “windshield wiper effect” (referring to swinging motion of the tendon graft perpendicular to the longitudinal axis of the tunnel like a wiper), and facilitating tendon-bone healing. Rigid Fix® is produced from PLLA/β-TCP by Depuy-Mitek(Norwood, USA),whom had been proved by a large number of clinical studies to have reliable mechanical effects for used in both distal femur and proximal tibia [[147], [148], [149], [150]]. However, this device is by no means perfect. In addition to the complexity of operation and high risk of causing iatrogenic neurovascular injuries [151], several complications associated with degradability are also common in clinical practice, including implant breakage, damage to articular cartilage and meniscus from degraded debris entering the joint cavity, no bone growth into the residual cavity after degradation of implants, and inflammatory reactions and osteolysis around the pin track caused by degradation [152,153]. Fu et al. [154] carried out animal trials of magnesium alloy cross-pin with positive results, but no further applications have been made. Mao et al. [155] designed a MgYREZr alloy stretch plate and applied it in an ACL reconstruction model of Beagle. The results of biomechanical tests at 6 months postoperatively showed good knee stability and degradation of the implant at an appropriate rate in vivo, as well as abundant Sharpey's fibers and new bone formation visible in the bone tunnel, suggesting satisfying tendon-bone healing. This absorbable plate was initially validated for safety and efficacy and is expected to be used in the future for clinical applications.
Absorbable materials have also been used in the development of meniscal repair devices, known as meniscal arrows. In 1988, Miller et al. [156] first reported experimental results on the repair of meniscal tears using meniscal arrows made of self-reinforced PLA (SR-PLA) material. Subsequently in 1993, Albrecht-Olsen et al. [157] first reported their experience in arthroscopic repair of meniscal injuries with absorbable meniscal arrows. The meniscus arrow, as an all-inside meniscus repair device, became more widely used in the early part of this century [158,159], but a biomechanical study found that the mechanical strength of the meniscal arrow was inferior to that of the suture repair [160], and long-term follow-up showed poor results of meniscal arrows, also [161,162]. Nowadays, meniscal arrows are only recommended for the repair of meniscal posterior root and body injuries, which can assure a high success rate [[163], [164], [165]].
Other applications of absorbable materials in the knee include the treatment of cartilage diseases and intercondylar ridge fractures. SR-PGA or SR-PLLA rods, pins, nails have been used since more than 20 years ago to treat osteochondritis dissecans of the knee in adults or children with good results [[166], [167], [168], [169]], patients occasionally suffering complications such as synovitis [167]. A latest retrospective study showed that in 58 children who underwent arthroscopic fixation of unstable osteochondral lesions in the knee joint using absorbable P-96L/4D-LA nails, nail breakage(44.8%), secondary cartilage damages (24.1%), and secondary meniscal injuries (10.3%) were also possible complications [170]. Therefore, magnesium alloy may be a safer option. Jungesblut et al. [171] used MAGNEZIX pin for arthroscopic fixation of unstable osteochondritis dissecans of the knee and found cartilage healing well with satisfactory joint function and only one internal fixation failed. Gigante et al. reported three cases of tibial intercondylar eminence avulsion fractures treated by arthroscopic internal fixation with magnesium screws, and the clinical follow-up found that the patients’ knee function recovered well without implant-related complications, and the screws were completely absorbed after 6 months and replaced by new bone after 12 months [172].
4.3. Other parts of body
In recent years, the application of absorbable implants has been gradually expanded to treat sports injuries in the elbow, wrist, ankle, hand and foot. Biber et al. [173] treated a osteochondral fracture of humeral capitulum with a MAGNEZIX screw and found good results after 1 year of follow-up, with no restriction of elbow movement nor pain and swelling of elbow. Aarts et al. [174] described a technique for minimally invasive treatment of the mallet finger using the P-80L/20D-LA Meniscus Arrow in an outpatient setting. The majority of the 50 cases had a good prognosis, with only one case of wound infection that improved with antibiotics and one case of spontaneous detachment of the internal fixation after frequent hand movement. In addition, several studies have used magnesium screws in modified Chevron or Youngswick osteotomies for the treatment of hallux valgus and have achieved results comparable to those of titanium screws [[175], [176], [177], [178]].
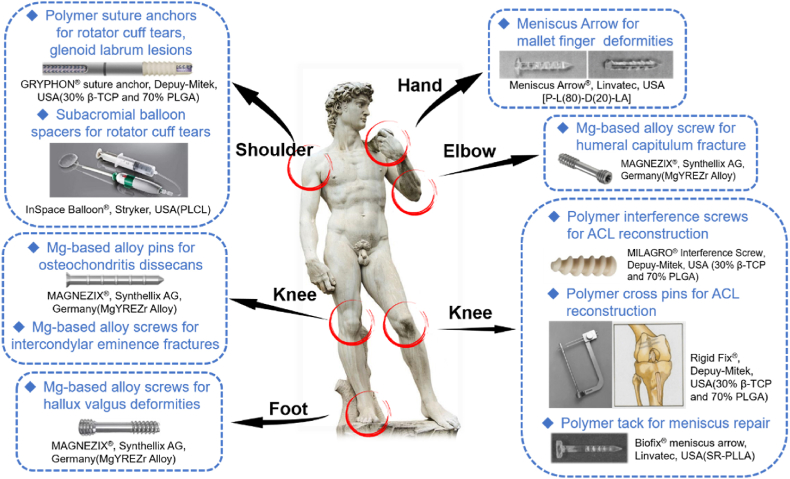
Several representative applications of absorbable implants for sport medicine are summarized in Fig. 6 and Table 2.
Fig. 6.
Several representative applications of absorbable implants for sport medicine in different parts of body.
Table 2.
Summary of clinical applications of different degradable materials in sport medicine.
| Material | Implant | Material compound | Clinical need | Some representative commercial product (Manufacturer) |
|---|---|---|---|---|
| Polymer (with/without bioceramics) | Suture anchor | PLA, PLGA, PLLA/HA, PLGA/β-TCP, PLGA/β-TCP/CS | Rotator cuff tear, Glenoid labium lesion(e.g. Bankart lesion) | Bio-Corkscrew(Arthrex, USA), Bio-Corkscrew FT(Arthrex, USA), SpiraLok(Depuy-Mitek, USA), GRYPHON(Depuy-Mitek, USA) Impact, Duet(Linvatec, USA), BioRaptor(Smith & Nephew Endoscopy, USA)、TwinFix AB(Smith & Nephew Endoscopy, USA), BioZip(Stryker, USA) |
| Interference screw | PLLA, PLLA/HA, PLLA/β-TCP | ACL/PCL reconstruction | BioRCI-HA(Smith&Nephew Endoscopy, USA), BIOSURE HA(Smith&Nephew Endoscopy, USA), Milagro(DePuy Mitek, USA), Intrafix(DePuy Mitek, USA), ComposiTCP(Zimmer Biomet, USA), BioScrew(Linvatec, USA) | |
| Cross-pin | PLLA/β-TCP | ACL/PCL reconstruction | Rigid Fix(DePuy Mitek, USA) | |
| Meniscal Repair System | PLLA, PLDLA | meniscal repair | Biofix meniscus arrow(Linvatec, USA) Rapid Loc(DePuy Mitek, USA), Fast-Fix(Smith&Nephew Endoscopy, USA) |
|
| Tack | SR-PLLA | Avulsive fracture or ligament lesion(e.g. Mallet finger) | Biofix (Linvatec, USA) | |
| Pin/nail | SR-PGA, SR-PLLA | Osteochondritis Dissecans Lesion |
Biofix (Linvatec, USA) | |
| Magnesium-based alloy | screw | Mg–Y-RE-Zr | Intercondylar eminence fracture, humeral capitulum fracture, hallux valgus | MAGNEZIX(Syntellix AG, Germany) |
| pin | Mg–Y-RE-Zr | Osteochondritis dissecans lesion | MAGNEZIX(Syntellix AG, Germany) |
5. Summary and prospect
Advances in bone, joint and soft tissue repair and reconstruction techniques have been made possible by advances in materials science. Traditional stainless steel and titanium internal fixations have shown many shortcomings in clinical applications, and absorbable materials offer a solution to these problems. The properties of absorbable materials vary depending on their composition. For example, bioresorbable polymers have excellent mechanical properties, good biocompatibility, and controlled degradation rates. Alloys made of magnesium, zinc or iron, which are essential trace elements for the human body, have the ability to promote the growth of the tendon bone system, in addition to their enhanced mechanical properties. Therefore, in the future, these metal biomaterials are expected to replace traditional stainless steel or titanium alloys as the “gold standard” for implants used in internal fixations.
Before these absorbable alloys can be used for tissue repair and reconstruction in orthopedics and sport medicine, there are still important issues to be resolved. For magnesium alloys, the high corrosion rate in vivo is still the biggest problem. The rapid degradation affects the mechanical stability of the implants, while the large amount of hydrogen released during the degradation process hinders bone healing. Zinc alloys face the opposite problem, with a slower degradation rate that cannot be matched to tendon-bone healing. Meanwhile, the in vivo degradation rate of zinc alloys should be as uniform as possible, as high concentrations of Zn2+ are cytotoxic, which still needs to be further controlled. As for iron-based materials, the slow corrosion rate still severely limits their clinical application as orthopedic implants. The rate of degradation of medical absorbable alloys in vivo can be modulated by composition adjustment, surface modification, structural modification, or composite treatment. How to regulate the in vivo corrosion rate of absorbable metallic materials to match the rate of bone formation and tendon-bone healing, thus providing a stable tissue growth environment, still needs to be further explored. Translational medicine, as a bridge between basic research and clinical applications, should play a greater role in this process.
Ethics approval and consent to participate
This paper is a review article, so informed consent and IRB approval are not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Basic Science Research Center Project of National Natural Science Foundation of China (Grant No. T2288102).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yuhui Kou, Email: yuhuikou@bjmu.edu.cn.
Baoguo Jiang, Email: jiangbaoguo@vip.sina.com.
References
- 1.Maloney W.J., Smith R.L. Periprosthetic osteolysis in total hip arthroplasty: the role of particulate wear debris[J] Instr. Course Lect. 1996;45:171–182. [PubMed] [Google Scholar]
- 2.Stratton-Powell A.A., Pasko K.M., Brockett C.L., et al. The biologic response to polyetheretherketone (PEEK) wear particles in total joint replacement: a systematic review[J] Clin. Orthop. Relat. Res. 2016;474(11):2394–2404. doi: 10.1007/s11999-016-4976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossetti L., Kuntz L.A., Kunold E., et al. The microstructure and micromechanics of the tendon-bone insertion[J] Nat. Mater. 2017;16(6):664–670. doi: 10.1038/nmat4863. [DOI] [PubMed] [Google Scholar]
- 4.Lui P., Zhang P., Chan K., et al. Biology and augmentation of tendon-bone insertion repair[J] J. Orthop. Surg. Res. 2010;5:59. doi: 10.1186/1749-799X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothrauff B.B., Tuan R.S. Cellular therapy in bone-tendon interface regeneration[J] Organogenesis. 2014;10(1):13–28. doi: 10.4161/org.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumstein M.A., Lädermann A., Raniga S., et al. The biology of rotator cuff healing[J] Orthop. Traumatol. Surg. Res. 2017;103(1s):S1–s10. doi: 10.1016/j.otsr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Huangfu X., Zhao J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model[J] Arthroscopy. 2007;23(5):455–462. doi: 10.1016/j.arthro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Zumstein MA., Lädermann A., Raniga S. The biology of rotator cuff healing[J] Orthop. Traumatol. Surg. Res. 2017;103(1S):S1–S10. doi: 10.1016/j.otsr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Lu H., Chen C., Xie S., et al. Tendon healing in bone tunnel after human anterior cruciate ligament reconstruction: a systematic review of histological results[J] J. Knee Surg. 2019;32(5):454–462. doi: 10.1055/s-0038-1653964. [DOI] [PubMed] [Google Scholar]
- 10.Speer K.P., Warren R.F. Arthroscopic shoulder stabilization. A role for biodegradable materials[J] Clin. Orthop. Relat. Res. 1993;(291):67–74. [PubMed] [Google Scholar]
- 11.Sheikh Z., Najeeb S., Khurshid Z., et al. Biodegradable materials for bone repair and tissue engineering applications[J] Materials. 2015;8(9):5744–5794. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan L., Yu X., Wan P., et al. Biodegradable materials for bone repairs: a review[J] J. Mater. Sci. Technol. 2013;29(6):503–513. [Google Scholar]
- 13.Razak S., Sharif N., Rahman W., et al. Biodegradable polymers and their bone applications: a review[J] Int. J. Basic Appl. Sci. 2012;12(01):31–49. [Google Scholar]
- 14.Reed A.M., Gilding D.K.J.P. Biodegradable polymers for use in surgery — poly(glycolic)/poly(Iactic acid) homo and copolymers: 2. In vitro degradation[J] 1981;22(4):494–498. [Google Scholar]
- 15.Speer K.P., Warren R.F., Pagnani M., et al. An arthroscopic technique for anterior stabilization of the shoulder with a bioabsorbable tack[J] J. Bone Jt. Surg. Am. 1996;78(12):1801–1807. doi: 10.2106/00004623-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Barber F.A. Biodegradable materials: anchors and interference screws[J] Sports Med. Arthrosc. Rev. 2015;23(3):112–117. doi: 10.1097/JSA.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni R.K., Pani K.C., Neuman C., et al. Polylactic acid for surgical implants[J] Arch. Surg. 1966;93(5):839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni R.K., Moore E.G., Hegyeli A.F., et al. Biodegradable poly(lactic acid) polymers[J] J. Biomed. Mater. Res. 1971;5(3):169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- 19.Eglin D., Alini M. Degradable polymeric materials for osteosynthesis: tutorial[J] Eur. Cell. Mater. 2008;16:80–91. doi: 10.22203/ecm.v016a09. [DOI] [PubMed] [Google Scholar]
- 20.Böstman O.M., Pihlajamäki H.K. Adverse tissue reactions to bioabsorbable fixation devices[J] Clin. Orthop. Relat. Res. 2000;(371):216–227. [PubMed] [Google Scholar]
- 21.Higashi S., Yamamuro T., Nakamura T., et al. Polymer-hydroxyapatite composites for biodegradable bone fillers[J] Biomaterials. 1986;7(3):183–187. doi: 10.1016/0142-9612(86)90099-2. [DOI] [PubMed] [Google Scholar]
- 22.Nejati E., Mirzadeh H., Zandi M., et al. Synthesis and characterization of nano-hydroxyapatite rods/poly(l-lactide acid) composite scaffolds for bone tissue engineering[J] Compos. Appl. Sci. Manuf. 2008;39(10):1589–1596. [Google Scholar]
- 23.Li J., Lu X.L., Zheng Y.F. Effect of surface modified hydroxyapatite on the tensile property improvement of HA/PLA composite[J] Appl. Surf. Sci. 2008;255(2):494–497. [Google Scholar]
- 24.Ge M., Ge K., Gao F., et al. vol. 13. 2018. pp. 1707–1721. (Biomimetic Mineralized Strontium-Doped Hydroxyapatite on Porous poly(L-Lactic Acid) Scaffolds for Bone Defect repair[J]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller R.A., Brady J.M., Cutright D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): rate modification with changes in PLA/PGA copolymer ratios[J] J. Biomed. Mater. Res. 1977;11(5):711–719. doi: 10.1002/jbm.820110507. [DOI] [PubMed] [Google Scholar]
- 26.Gunatillake P., Mayadunne R., Adhikari R. Recent developments in biodegradable synthetic polymers[J] Biotechnol. Annu. Rev. 2006;12:301–347. doi: 10.1016/S1387-2656(06)12009-8. [DOI] [PubMed] [Google Scholar]
- 27.Hollinger J.O. Preliminary report on the osteogenic potential of a biodegradable copolymer of polyactide (PLA) and polyglycolide (PGA)[J] J. Biomed. Mater. Res. 1983;17(1):71–82. doi: 10.1002/jbm.820170107. [DOI] [PubMed] [Google Scholar]
- 28.Nelsonjf, Stanford H.G., Cutright D.E. Evaluation and comparisons of biodegradable substances as osteogenic agents[J] Oral Surg. Oral Med. Oral Pathol. 1977;43(6):836–843. doi: 10.1016/0030-4220(77)90075-5. [DOI] [PubMed] [Google Scholar]
- 29.Sadat Tabatabaei Mirakabad F., Nejati-Koshki K., Akbarzadeh A., et al. PLGA-based nanoparticles as cancer drug delivery systems[J] Asian Pac. J. Cancer Prev. 2014;15(2):517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- 30.Mehrasa M., Asadollahi M.A., Ghaedi K., et al. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering[J] Int. J. Biol. Macromol. 2015;79:687–695. doi: 10.1016/j.ijbiomac.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Gunatillake P.A., Adhikari R. Biodegradable synthetic polymers for tissue engineering[J] Eur. Cell. Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. discussion 16. [DOI] [PubMed] [Google Scholar]
- 32.Walsh W.R., Cotton N.J., Stephens P., et al. Comparison of poly-L-lactide and polylactide carbonate interference screws in an ovine anterior cruciate ligament reconstruction model[J] Arthroscopy. 2007;23(7):757–765. doi: 10.1016/j.arthro.2007.01.030. 765.e1-765. [DOI] [PubMed] [Google Scholar]
- 33.Konan S., Haddad F.S. The unpredictable material properties of bioabsorbable PLC interference screws and their adverse effects in ACL reconstruction surgery[J] Knee Surg. Sports Traumatol. Arthrosc. 2009;17(3):293–297. doi: 10.1007/s00167-008-0684-x. [DOI] [PubMed] [Google Scholar]
- 34.Foldager C., Jakobsen B.W., Lund B., et al. Tibial tunnel widening after bioresorbable poly-lactide calcium carbonate interference screw usage in ACL reconstruction[J] Knee Surg. Sports Traumatol. Arthrosc. 2010;18(1):79–84. doi: 10.1007/s00167-009-0865-2. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal C.M., Ray R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering[J] J. Biomed. Mater. Res. 2001;55(2):141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Coombes A.G., Rizzi S.C., Williamson M., et al. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery[J] Biomaterials. 2004;25(2):315–325. doi: 10.1016/s0142-9612(03)00535-0. [DOI] [PubMed] [Google Scholar]
- 37.Porter J.R., Henson A., Popat K.C. Biodegradable poly(epsilon-caprolactone) nanowires for bone tissue engineering applications[J] Biomaterials. 2009;30(5):780–788. doi: 10.1016/j.biomaterials.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Nie W., Gao Y., Mccoul D.J., et al. Rapid mineralization of hierarchical poly(l-lactic acid)/poly(ε-caprolactone) nanofibrous scaffolds by electrodeposition for bone regeneration[J] Int. J. Nanomed. 2019;14:3929–3941. doi: 10.2147/IJN.S205194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mondrinos M.J., Dembzynski R., Lu L., et al. Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering[J] Biomaterials. 2006;27(25):4399–4408. doi: 10.1016/j.biomaterials.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 40.Yu X., Tang X., Gohil S.V., et al. Biomaterials for bone regenerative engineering[J] Adv. Healthcare Mater. 2015;4(9):1268–1285. doi: 10.1002/adhm.201400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roohani-Esfahani S.I., No Y.J., Lu Z., et al. A bioceramic with enhanced osteogenic properties to regulate the function of osteoblastic and osteocalastic cells for bone tissue regeneration[J] Biomed. Mater. 2016;11(3) doi: 10.1088/1748-6041/11/3/035018. [DOI] [PubMed] [Google Scholar]
- 42.De Wild M., Amacher F., Bradbury C.R., et al. Investigation of structural resorption behavior of biphasic bioceramics with help of gravimetry, μCT, SEM, and XRD[J] J. Biomed. Mater. Res. B Appl. Biomater. 2016;104(3):546–553. doi: 10.1002/jbm.b.33419. [DOI] [PubMed] [Google Scholar]
- 43.Daculsi G., Legeros R.Z., Heughebaert M., et al. Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics[J] Calcif. Tissue Int. 1990;46(1):20–27. doi: 10.1007/BF02555820. [DOI] [PubMed] [Google Scholar]
- 44.Dong J., Uemura T., Shirasaki Y., et al. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells[J] Biomaterials. 2002;23(23):4493–4502. doi: 10.1016/s0142-9612(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W., Wang D., Xiong J., et al. Study on clinical application of nano-hydroxyapatite bone in bone defect repair[J] Artif. Cells, Nanomed. Biotechnol. 2015;43(6):361–365. doi: 10.3109/21691401.2014.893521. [DOI] [PubMed] [Google Scholar]
- 46.El-Ghannam A. Bone reconstruction: from bioceramics to tissue engineering[J] Expet Rev. Med. Dev. 2005;2(1):87–101. doi: 10.1586/17434440.2.1.87. [DOI] [PubMed] [Google Scholar]
- 47.Kamitakahara M., Ohtsuki C., Miyazaki T. Review paper: behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition[J] J. Biomater. Appl. 2008;23(3):197–212. doi: 10.1177/0885328208096798. [DOI] [PubMed] [Google Scholar]
- 48.Polini A., Pisignano D., Parodi M., et al. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors[J] PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z., Best S.M., Cameron R.E. The influence of α-tricalcium phosphate nanoparticles and microparticles on the degradation of poly(D,L-lactide-co-glycolide)[J] Adv. Mater. 2009;21(38–39):3900–3904. [Google Scholar]
- 50.Ehrenfried L.M., Patel M.H., Cameron R.E. The effect of tri-calcium phosphate (TCP) addition on the degradation of polylactide-co-glycolide (PLGA)[J] J. Mater. Sci. Mater. Med. 2008;19(1):459–466. doi: 10.1007/s10856-006-0061-6. [DOI] [PubMed] [Google Scholar]
- 51.Suárez-González D., Lee J.S., Lan Levengood S.K., et al. Mineral coatings modulate β-TCP stability and enable growth factor binding and release[J] Acta Biomater. 2012;8(3):1117–1124. doi: 10.1016/j.actbio.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dinarvand P., Seyedjafari E., Shafiee A., et al. New approach to bone tissue engineering: simultaneous application of hydroxyapatite and bioactive glass coated on a poly(L-lactic acid) scaffold[J] ACS Appl. Mater. Interfaces. 2011;3(11):4518–4524. doi: 10.1021/am201212u. [DOI] [PubMed] [Google Scholar]
- 53.Barber F.A., Dockery W.D. Long-term absorption of beta-tricalcium phosphate poly-L-lactic acid interference screws[J] Arthroscopy. 2008;24(4):441–447. doi: 10.1016/j.arthro.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Frosch K.H., Sawallich T., Schütze G., et al. Magnetic resonance imaging analysis of the bioabsorbable Milagro interference screw for graft fixation in anterior cruciate ligament reconstruction[J] Strat. Trauma Limb Reconstr. 2009;4(2):73–79. doi: 10.1007/s11751-009-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston M., Morse A., Arrington J., et al. Resorption and remodeling of hydroxyapatite-poly-L-lactic acid composite anterior cruciate ligament interference screws[J] Arthroscopy. 2011;27(12):1671–1678. doi: 10.1016/j.arthro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.H., Park I., Hyun H.S., et al. Comparison of clinical outcomes and computed tomography analysis for tunnel diameter after arthroscopic bankart repair with the all-suture anchor and the biodegradable suture anchor[J] Arthroscopy. 2019;35(5):1351–1358. doi: 10.1016/j.arthro.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Liu X., Sun J., Yang Y., et al. Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn–Mg–Sr alloys as biodegradable metals[J] Mater. Lett. 2016;162:242–245. [Google Scholar]
- 58.Li H., Zheng Y., Qin L. Progress of biodegradable metals[J] Prog. Nat. Sci.: Mater. Int. 2014;24(5):414–422. [Google Scholar]
- 59.Kubota K., Mabuchi M., Higashi K. Review Processing and mechanical properties of fine-grained magnesium alloys[J] J. Mater. Sci. 1999;34(10):2255–2262. [Google Scholar]
- 60.Witte F. The history of biodegradable magnesium implants: a review[J] Acta Biomater. 2010;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 61.Payr E. Beitrge zur Technik der Blutgefss- und Nervennaht nebst Mittheilungen über die Verwendung eines resorbirbaren Metalles in der Chirurgie[J] Arch. Klin Chir. 1900;(62):67–93. [Google Scholar]
- 62.Payr E. Zur Verwendung des Magnesiums für resorbirbare Darmknöpfe und andere chirurgisch-technische Zwecke[J] Centralblatt Chir. 1901;28(20):513–515. [Google Scholar]
- 63.Lambotte A. Technique et indications de la prothèse perdue dans la traitement des fractures[J] Presse Med. Belge. 1909;17:321–323. [Google Scholar]
- 64.Lambotte A.L. 'utilisation du magnesium comme materiel perdu dans l'osteosynthè se[J] Bull. Mem Soc. Nat. Chir. 1932;28:1325–1334. [Google Scholar]
- 65.Verbrugge J. vol. 23. La Press Med; 1934. pp. 460–465. (Le Matériel Métallique Résorbable En Chirurgie Osseuse[J]). [Google Scholar]
- 66.Mcbride E.D. Magnesium screw and nail transfixion in fractures[J] South. Med. J. 1938;31(5):508–514. [Google Scholar]
- 67.Mcbride E.D. Absorbable metal in bone surgery - a further report on the use of magnesium alloys[J] J. Am. Med. Assoc. 1938;111(27):2464–2466. [Google Scholar]
- 68.Waizy H., Diekmann J., Weizbauer A., et al. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months[J] J. Biomater. Appl. 2014;28(5):667–675. doi: 10.1177/0885328212472215. [DOI] [PubMed] [Google Scholar]
- 69.Serre C.M., Papillard M., Chavassieux P., et al. Influence of magnesium substitution on a collagen-apatite biomaterial on the production of a calcifying matrix by human osteoblasts[J] J. Biomed. Mater. Res. 1998;42(4):626–633. doi: 10.1002/(sici)1097-4636(19981215)42:4<626::aid-jbm20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 70.Feyerabend F., Wendel H.P., Mihailova B., et al. Blood compatibility of magnesium and its alloys[J] Acta Biomater. 2015;25:384–394. doi: 10.1016/j.actbio.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 71.Farraro K.F., Kim K.E., Woo S.L., et al. Revolutionizing orthopaedic biomaterials: the potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering[J] J. Biomech. 2014;47(9):1979–1986. doi: 10.1016/j.jbiomech.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iglesias C., Bodelón O.G., Montoya R., et al. Fracture bone healing and biodegradation of AZ31 implant in rats[J] Biomed. Mater. 2015;10(2) doi: 10.1088/1748-6041/10/2/025008. [DOI] [PubMed] [Google Scholar]
- 73.Guntur A.R., Rosen C.J. The skeleton: a multi-functional complex organ: new insights into osteoblasts and their role in bone formation: the central role of PI3Kinase[J] J. Endocrinol. 2011;211(2):123–130. doi: 10.1530/JOE-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu C.M., Chen P.C., Li T.M., et al. Si-Wu-tang extract stimulates bone formation through PI3K/Akt/NF-κB signaling pathways in osteoblasts[J] BMC Compl. Alternative Med. 2013;13:277. doi: 10.1186/1472-6882-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu S.S., Liang Q.H., Liu Y., et al. Omentin-1 stimulates human osteoblast proliferation through PI3K/Akt signal pathway[J] Int. J. Endocrinol. 2013;2013 doi: 10.1155/2013/368970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoppet M., Preissner K.T., Hofbauer L.C. RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function[J] Arterioscler. Thromb. Vasc. Biol. 2002;22(4):549–553. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 77.Bae Y.J., Kim M.H. Calcium and magnesium supplementation improves serum OPG/RANKL in calcium-deficient ovariectomized rats[J] Calcif. Tissue Int. 2010;87(4):365–372. doi: 10.1007/s00223-010-9410-z. [DOI] [PubMed] [Google Scholar]
- 78.Hung C.C., Chaya A., Liu K., et al. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway[J] Acta Biomater. 2019;98:246–255. doi: 10.1016/j.actbio.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Hamushan M., Cai W., Zhang Y., et al. High-purity magnesium pin enhances bone consolidation in distraction osteogenesis via regulating Ptch protein activating Hedgehog-alternative Wnt signaling[J] Bioact. Mater. 2021;6(6):1563–1574. doi: 10.1016/j.bioactmat.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu J., Hu P., Zhang X., et al. Magnesium implantation or supplementation ameliorates bone disorder in CFTR-mutant mice through an ATF4-dependent Wnt/β-catenin signaling[J] Bioact. Mater. 2022;8:95–108. doi: 10.1016/j.bioactmat.2021.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Witte F., Kaese V., Haferkamp H., et al. In vivo corrosion of four magnesium alloys and the associated bone response[J] Biomaterials. 2005;26(17):3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 82.Gu X., Zheng Y., Cheng Y., et al. In vitro corrosion and biocompatibility of binary magnesium alloys[J] Biomaterials. 2009;30(4):484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Kim S.M., Jo J.H., Lee S.M., et al. Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response[J] J. Biomed. Mater. Res. 2014;102(2):429–441. doi: 10.1002/jbm.a.34718. [DOI] [PubMed] [Google Scholar]
- 84.Dorozhkin S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys[J] Acta Biomater. 2014;10(7):2919–2934. doi: 10.1016/j.actbio.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Kunjukunju S., Roy A., Ramanathan M., et al. A layer-by-layer approach to natural polymer-derived bioactive coatings on magnesium alloys[J] Acta Biomater. 2013;9(10):8690–8703. doi: 10.1016/j.actbio.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 86.Bian D., Zhou W., Liu Y., et al. Fatigue behaviors of HP-Mg, Mg-Ca and Mg-Zn-Ca biodegradable metals in air and simulated body fluid[J] Acta Biomater. 2016;41:351–360. doi: 10.1016/j.actbio.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 87.Raman R.K.S., Harandi S.E. Resistance of magnesium alloys to corrosion fatigue for biodegradable implant applications: current status and challenges[J] Materials. 2017;10(11) doi: 10.3390/ma10111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antoniac I., Miculescu M., Mănescu Păltânea V., et al. Magnesium-based alloys used in orthopedic surgery[J] Materials. 2022;15(3) doi: 10.3390/ma15031148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun Y., Wu H., Wang W., et al. Translational status of biomedical Mg devices in China[J] Bioact. Mater. 2019;4:358–365. doi: 10.1016/j.bioactmat.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holweg P., Herber V., Ornig M., et al. A lean bioabsorbable magnesium-zinc-calcium alloy ZX00 used for operative treatment of medial malleolus fractures: early clinical results of a prospective non-randomized first in man study[J] Bone Jt. Res. 2020;9(8):477–483. doi: 10.1302/2046-3758.98.BJR-2020-0017.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brand O-Neto J., Stefan V., Mendon A.B.B., et al. Vol. 15. 1995. pp. 335–358. (The essential role of zinc in growth[J]). 3. [Google Scholar]
- 92.Kumar V., Kumar A., Singh K., et al. Neurobiology of zinc and its role in neurogenesis[J] Eur. J. Nutr. 2021;60(1):55–64. doi: 10.1007/s00394-020-02454-3. [DOI] [PubMed] [Google Scholar]
- 93.Huang T., Yan G., Guan M. Zinc homeostasis in bone: zinc transporters and bone diseases[J] Int. J. Mol. Sci. 2020;21(4) doi: 10.3390/ijms21041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamaguchi M. Role of nutritional zinc in the prevention of osteoporosis[J] Mol. Cell. Biochem. 2010;338(1–2):241–254. doi: 10.1007/s11010-009-0358-0. [DOI] [PubMed] [Google Scholar]
- 95.Kabir H., Munir K., Wen C., et al. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives[J] Bioact. Mater. 2021;6(3):836–879. doi: 10.1016/j.bioactmat.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Sun J., Yang Y., et al. vol. 162. 2016. pp. 242–245. (Microstructure, Mechanical Properties, in Vitro Degradation Behavior and Hemocompatibility of Novel Zn-Mg-Sr Alloys as Biodegradable metals[J]). JAN.1. [Google Scholar]
- 97.Alves, Marta M., et al. 2017. Evolution of the in Vitro Degradation of Zn-Mg Alloys under Simulated Physiological conditions[J] [DOI] [PubMed] [Google Scholar]
- 98.Yang H., Jia B., Zhang Z., et al. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications[J] Nat. Commun. 2020;11(1):401. doi: 10.1038/s41467-019-14153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanjilal D., Grieg C., Culbertson M.D., et al. Improved osteogenesis in rat femur segmental defects treated with human allograft and zinc adjuvants[J] Exp. Biol. Med. (Maywood, NJ, U. S.) 2021;246(16):1857–1868. doi: 10.1177/15353702211019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yusa K., Yamamoto O., Iino M., et al. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering[J] Arch. Oral Biol. 2016;71:162–169. doi: 10.1016/j.archoralbio.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 101.An S., Gong Q., Huang Y. Promotive effect of zinc ions on the vitality, migration, and osteogenic differentiation of human dental pulp cells[J] Biol. Trace Elem. Res. 2017;175(1):112–121. doi: 10.1007/s12011-016-0763-7. [DOI] [PubMed] [Google Scholar]
- 102.Klíma K., Ulmann D., Bartoš M., et al. Zn-0.8Mg-0.2Sr (wt.%) absorbable screws-an in-vivo biocompatibility and degradation pilot study on a rabbit model. J. Mater. 2021;14(12) doi: 10.3390/ma14123271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Shao X., Dai T., et al. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system[J] Acta Biomater. 2019;92:351–361. doi: 10.1016/j.actbio.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Gąsior G., Szczepański J., Radtke A. Biodegradable iron-based materials-what was done and what more can Be done? J. Mater. 2021;14(12) doi: 10.3390/ma14123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Purnama A., Hermawan H., Couet J., et al. Assessing the biocompatibility of degradable metallic materials: state-of-the-art and focus on the potential of genetic regulation[J] Acta Biomater. 2010;6(5):1800–1807. doi: 10.1016/j.actbio.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 106.Peuster M., Wohlsein P., Brügmann M., et al. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6-18 months after implantation into New Zealand white rabbits[J] Heart. 2001;86(5):563–569. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peuster M., Hesse C., Schloo T., et al. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta[J] Biomaterials. 2006;27(28):4955–4962. doi: 10.1016/j.biomaterials.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 108.Waksman R., Pakala R., Baffour R., et al. Short-term effects of biocorrodible iron stents in porcine coronary arteries[J] J. Intervent. Cardiol. 2008;21(1):15–20. doi: 10.1111/j.1540-8183.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 109.Kraus T., Moszner F., Fischerauer S., et al. Biodegradable Fe-based alloys for use in osteosynthesis: outcome of an in vivo study after 52 weeks[J] Acta Biomater. 2014;10(7):3346–3353. doi: 10.1016/j.actbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 110.Hermawan H., Dubé D., Mantovani D. Degradable metallic biomaterials: design and development of Fe-Mn alloys for stents[J] J. Biomed. Mater. Res. 2010;93(1):1–11. doi: 10.1002/jbm.a.32224. [DOI] [PubMed] [Google Scholar]
- 111.Liu B., Zheng Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron[J] Acta Biomater. 2011;7(3):1407–1420. doi: 10.1016/j.actbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Wegener B., Sievers B., Utzschneider S., et al. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials[J] Mater. Sci. Eng., B. 2011;176(20):1789–1796. [Google Scholar]
- 113.Li Y., Jahr H., Lietaert K., et al. Additively manufactured biodegradable porous iron[J] Acta Biomater. 2018;77:380–393. doi: 10.1016/j.actbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 114.Li Y., Lietaert K., Li W., et al. Corrosion fatigue behavior of additively manufactured biodegradable porous iron[J] Corrosion Sci. 2019;156:106–116. doi: 10.1016/j.actbio.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Wegener B., Sichler A., Milz S., et al. Development of a novel biodegradable porous iron-based implant for bone replacement[J] Sci. Rep. 2020;10(1):9141. doi: 10.1038/s41598-020-66289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grodzicka M., Gąsior G., Wiśniewski M., et al. A simple replica method as the way to obtain a morphologically and mechanically bone-like iron-based biodegradable Material[J] Materials (Basel) 2022;15(13) doi: 10.3390/ma15134552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kurtz S.M., Devine J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants[J] Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cho C.H., Bae K.C., Kim D.H. Biomaterials used for suture anchors in orthopedic surgery[J] Clin. Orthop. Surg. 2021;13(3):287–292. doi: 10.4055/cios20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Milano G., Grasso A., Santagada D.A., et al. Comparison between metal and biodegradable suture anchors in the arthroscopic treatment of traumatic anterior shoulder instability: a prospective randomized study[J] Knee Surg. Sports Traumatol. Arthrosc. 2010;18(12):1785–1791. doi: 10.1007/s00167-010-1212-3. [DOI] [PubMed] [Google Scholar]
- 120.Milano G., Grasso A., Salvatore M., et al. Arthroscopic rotator cuff repair with metal and biodegradable suture anchors: a prospective randomized study[J] Arthroscopy. 2010;26(9 Suppl):S112–S119. doi: 10.1016/j.arthro.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 121.Saccomanno M.F., Cerciello S., Adriani M., et al. Knotless PEEK and double-loaded biodegradable suture anchors ensure comparable clinical outcomes in the arthroscopic treatment of traumatic anterior shoulder instability: a prospective randomized study[J] Knee Surg. Sports Traumatol. Arthrosc. 2022 doi: 10.1007/s00167-022-06969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barber F.A. Complications of biodegradable materials: anchors and interference screws[J] Sports Med. Arthrosc. Rev. 2015;23(3):149–155. doi: 10.1097/JSA.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 123.Park M.J., Hsu J.E., Harper C., et al. Poly-L/D-lactic acid anchors are associated with reoperation and failure of SLAP repairs[J] Arthroscopy. 2011;27(10):1335–1340. doi: 10.1016/j.arthro.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 124.Kim S.H., Oh J.H., Lee O.S., et al. Postoperative imaging of bioabsorbable anchors in rotator cuff repair[J] Am. J. Sports Med. 2014;42(3):552–557. doi: 10.1177/0363546513517538. [DOI] [PubMed] [Google Scholar]
- 125.Mccarty L.P., 3rd, Buss D.D., Datta M.W., et al. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants[J] J. Bone Jt. Surg. Am. 2013;95(6):507–511. doi: 10.2106/JBJS.L.00314. [DOI] [PubMed] [Google Scholar]
- 126.Augusti C.A., Paladini P., Campi F., et al. Anterior glenoid rim fracture following use of resorbable devices for glenohumeral stabilization[J] Orthop. J. Sports Med. 2015;3(6) doi: 10.1177/2325967115586559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Böstman O., Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review[J] Biomaterials. 2000;21(24):2615–2621. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 128.Randelli P., Compagnoni R., Aliprandi A., et al. Long-term degradation of poly-lactic co-glycolide/β-tricalcium phosphate biocomposite anchors in arthroscopic bankart repair: a prospective study[J] Arthroscopy. 2014;30(2):165–171. doi: 10.1016/j.arthro.2013.09.082. [DOI] [PubMed] [Google Scholar]
- 129.Vonhoegen J., John D., Hägermann C. Osteoconductive resorption characteristics of a novel biocomposite suture anchor material in rotator cuff repair[J] J. Orthop. Surg. Res. 2019;14(1):12. doi: 10.1186/s13018-018-1049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ro K., Pancholi S., Son H.S., et al. Perianchor cyst formation after arthroscopic rotator cuff repair using all-suture-type, bioabsorbable-type, and PEEK-type anchors[J] Arthroscopy. 2019;35(8):2284–2292. doi: 10.1016/j.arthro.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y., Sun Y., Wu X., et al. Rotator cuff repair with biodegradable high-purity magnesium suture anchor in sheep model[J] J. Orthop. Translat. 2022;35:62–71. doi: 10.1016/j.jot.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bockmann B., Jaeger E., Dankl L., et al. A biomechanical comparison of steel screws versus PLLA and magnesium screws for the Latarjet procedure[J] Arch. Orthop. Trauma Surg. 2022;142(6):1091–1098. doi: 10.1007/s00402-021-03898-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barber F.A., Elrod B.F., Mcguire D.A., et al. Preliminary results of an absorbable interference screw[J] Arthroscopy. 1995;11(5):537–548. doi: 10.1016/0749-8063(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 134.Sthelin A.C., Feinstein R., Friederich N.F. 1995. Clinical Experience Using a Bioabsorbable Interference Screw for ACL reconstruction[J] [Google Scholar]
- 135.Marinescu R., Antoniac I., Laptoiu D., et al. vol. 52. 2015. pp. 340–344. (Complications Related to Biocomposite Screw Fixation in ACL Reconstruction Based on Clinical Experience and Retrieval analysis[J]). 3. [Google Scholar]
- 136.Ramsingh V., Prasad N., Lewis M. Pre-tibial reaction to biointerference screw in anterior cruciate ligament reconstruction. J]. Knee. 2014;21(1):91–94. doi: 10.1016/j.knee.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 137.Barber F.A. Flipped patellar tendon autograft anterior cruciate ligament reconstruction[J] Arthroscopy. 2000;16(5):483–490. doi: 10.1053/jars.2000.4384. [DOI] [PubMed] [Google Scholar]
- 138.Barber F.A., Spruill B., Sheluga M. The effect of outlet fixation on tunnel widening[J] Arthroscopy. 2003;19(5):485–492. doi: 10.1053/jars.2003.50170. [DOI] [PubMed] [Google Scholar]
- 139.Bourke H.E., Salmon L.J., Waller A., et al. Randomized controlled trial of osteoconductive fixation screws for anterior cruciate ligament reconstruction: a comparison of the Calaxo and Milagro screws[J] Arthroscopy. 2013;29(1):74–82. doi: 10.1016/j.arthro.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 140.Song B., Li W., Chen Z., et al. Biomechanical comparison of pure magnesium interference screw and polylactic acid polymer interference screw in anterior cruciate ligament reconstruction-A cadaveric experimental study[J] J. Orthop. Translat. 2017;8:32–39. doi: 10.1016/j.jot.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thormann U., Alt V., Heimann L., et al. The biocompatibility of degradable magnesium interference screws: an experimental study with sheep[J] BioMed Res. Int. 2015;2015 doi: 10.1155/2015/943603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheng P., Han P., Zhao C., et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF[J] Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 143.Diekmann J., Bauer S., Weizbauer A., et al. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: a pilot in vivo study in rabbits[J] Mater. Sci. Eng. C Mater. Biol. Appl. 2016;59:1100–1109. doi: 10.1016/j.msec.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 144.Wang J., Xu J., Fu W., et al. Biodegradable magnesium screws accelerate fibrous tissue mineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit[J] Sci. Rep. 2017;7 doi: 10.1038/srep40369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J., Xu J., Song B., et al. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits[J] Acta Biomater. 2017;63:393–410. doi: 10.1016/j.actbio.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 146.Wang J., Wu Y., Li H., et al. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits[J] Biomaterials. 2018;157:86–97. doi: 10.1016/j.biomaterials.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 147.Persson A., Gifstad T., Lind M., et al. Graft fixation influences revision risk after ACL reconstruction with hamstring tendon autografts[J] Acta Orthop. 2018;89(2):204–210. doi: 10.1080/17453674.2017.1406243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang H., Ma G., Li Q., et al. Cortical button versus cross-pin femoral fixation for hamstring anterior cruciate ligament reconstruction: a meta-analysis of randomized controlled trials[J] Am. J. Sports Med. 2018;46(9):2277–2284. doi: 10.1177/0363546517717672. [DOI] [PubMed] [Google Scholar]
- 149.Spragg L.M., Prentice H.A., Morris A., et al. Femoral-tibial fixation affects risk of revision and reoperation after anterior cruciate ligament reconstruction using hamstring autograft[J] Knee Surg. Sports Traumatol. Arthrosc. 2019;27(11):3518–3526. doi: 10.1007/s00167-019-05431-4. [DOI] [PubMed] [Google Scholar]
- 150.Liu Y.J., Li H.F., Wang J.L., et al. [RIGIDfix tibial and femur cross pin system used for hamstring grafted anterior cruciate ligament reconstruction][J] Zhonghua Yixue Zazhi. 2009;89(29):2034–2037. [PubMed] [Google Scholar]
- 151.Lee G.C., Kim D.H., Park S.H. Popliteal artery pseudoaneurysm after anterior cruciate ligament re-revision using a rigidfix cross pin[J] Knee Surg. Relat. Res. 2014;26(2):121–124. doi: 10.5792/ksrr.2014.26.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Papastergiou S.G., Koukoulias N.E., Ziogas E., et al. Broken bioabsorbable femoral cross-pin as a cause of a chondral lesion after anterior cruciate Ligament reconstruction[J] BMJ Case Rep. 2009;vol. 2009 doi: 10.1136/bcr.09.2008.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Studler U., White L.M., Naraghi A.M., et al. Anterior cruciate ligament reconstruction by using bioabsorbable femoral cross pins: MR imaging findings at follow-up and comparison with clinical findings[J] Radiology. 2010;255(1):108–116. doi: 10.1148/radiol.09091119. [DOI] [PubMed] [Google Scholar]
- 154.Fu Y.M., Yin Y., Guan J.W., et al. The evaluation of a degradable Magnesium alloy Bio-Transfix nail system compounded with bone morphogenetic protein-2 in a beagle anterior cruciate ligament reconstruction model[J] J. Biomater. Appl. 2019;34(5):687–698. doi: 10.1177/0885328219865069. [DOI] [PubMed] [Google Scholar]
- 155.Mao G.W., Gong H.B., Wang Y., et al. Special biodegradable fixation device for anterior cruciate ligament reconstruction-safety and efficacy in a beagle model[J] ACS Biomater. Sci. Eng. 2018;4(10):3600–3609. doi: 10.1021/acsbiomaterials.8b00426. [DOI] [PubMed] [Google Scholar]
- 156.Miller D.B., Jr. Arthroscopic meniscus repair[J] Am. J. Sports Med. 1988;16(4):315–320. doi: 10.1177/036354658801600401. [DOI] [PubMed] [Google Scholar]
- 157.Albrecht-Olsen P., Kristensen G., Törmälä P. Meniscus bucket-handle fixation with an absorbable Biofix tack: development of a new technique[J] Knee Surg. Sports Traumatol. Arthrosc. 1993;1(2):104–106. doi: 10.1007/BF01565462. [DOI] [PubMed] [Google Scholar]
- 158.Hürel C., Mertens F., Verdonk R. Biofix resorbable meniscus arrow for meniscal ruptures: results of a 1-year follow-up[J] Knee Surg. Sports Traumatol. Arthrosc. 2000;8(1):46–52. doi: 10.1007/s001670050010. [DOI] [PubMed] [Google Scholar]
- 159.Al-Othman A.A. Biodegradable arrows for arthroscopic repair of meniscal tears[J] Int. Orthop. 2002;26(4):247–249. doi: 10.1007/s00264-002-0359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brucker P.U., Favre P., Puskas G.J., et al. Tensile and shear loading stability of all-inside meniscal repairs: an in vitro biomechanical evaluation[J] Am. J. Sports Med. 2010;38(9):1838–1844. doi: 10.1177/0363546510368131. [DOI] [PubMed] [Google Scholar]
- 161.Kurzweil P.R., Tifford C.D., Ignacio E.M. Unsatisfactory clinical results of meniscal repair using the meniscus arrow[J] Arthroscopy. 2005;21(8):905. doi: 10.1016/j.arthro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 162.Lee G.P., Diduch D.R. Deteriorating outcomes after meniscal repair using the Meniscus Arrow in knees undergoing concurrent anterior cruciate ligament reconstruction: increased failure rate with long-term follow-up[J] Am. J. Sports Med. 2005;33(8):1138–1141. doi: 10.1177/0363546505275348. [DOI] [PubMed] [Google Scholar]
- 163.Haas A.L., Schepsis A.A., Hornstein J., et al. Meniscal repair using the FasT-Fix all-inside meniscal repair device[J] Arthroscopy. 2005;21(2):167–175. doi: 10.1016/j.arthro.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 164.Barber F.A., Coons D.A., Ruiz-Suarez M. Meniscal repair with the RapidLoc meniscal repair device[J] Arthroscopy. 2006;22(9):962–966. doi: 10.1016/j.arthro.2006.04.109. [DOI] [PubMed] [Google Scholar]
- 165.Billante M.J., Diduch D.R., Lunardini D.J., et al. Meniscal repair using an all-inside, rapidly absorbing, tensionable device[J] Arthroscopy. 2008;24(7):779–785. doi: 10.1016/j.arthro.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 166.Matsusue Y., Nakamura T., Suzuki S., et al. Biodegradable pin fixation of osteochondral fragments of the knee[J] Clin. Orthop. Relat. Res. 1996;(322):166–173. [PubMed] [Google Scholar]
- 167.Tuompo P., Arvela V., Partio E.K., et al. Osteochondritis dissecans of the knee fixed with biodegradable self-reinforced polyglycolide and polylactide rods in 24 patients[J] Int. Orthop. 1997;21(6):355–360. doi: 10.1007/s002640050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dervin G.F., Keene G.C., Chissell H.R. Biodegradable rods in adult osteochondritis dissecans of the knee[J] Clin. Orthop. Relat. Res. 1998;(356):213–221. doi: 10.1097/00003086-199811000-00029. [DOI] [PubMed] [Google Scholar]
- 169.Weckström M., Parviainen M., Kiuru M.J., et al. Comparison of bioabsorbable pins and nails in the fixation of adult osteochondritis dissecans fragments of the knee: an outcome of 30 knees[J] Am. J. Sports Med. 2007;35(9):1467–1476. doi: 10.1177/0363546507300692. [DOI] [PubMed] [Google Scholar]
- 170.Nguyen J.C., Green D.W., Lin B.F., et al. Magnetic resonance evaluation of the pediatric knee after arthroscopic fixation of osteochondral lesions with biodegradable nails[J] Skeletal Radiol. 2020;49(1):65–73. doi: 10.1007/s00256-019-03258-1. [DOI] [PubMed] [Google Scholar]
- 171.Jungesblut O.D., Moritz M., Spiro A.S., et al. Fixation of unstable osteochondritis dissecans lesions and displaced osteochondral fragments using new biodegradable magnesium pins in adolescents. J]. Cartilage. 2021;13(1_suppl):302s–310s. doi: 10.1177/1947603520942943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gigante A., Setaro N., Rotini M., et al. Intercondylar eminence fracture treated by resorbable magnesium screws osteosynthesis: a case series[J] Injury. 2018;49(Suppl 3):S48–s53. doi: 10.1016/j.injury.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 173.Biber R., Pauser J., Geßlein M., et al. Magnesium-Based Absorbable Metal Screws for Intra-articular Fracture Fixation[J] Case Rep. Orthop. 2016;vol. 2016 doi: 10.1155/2016/9673174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aarts F.L., Derks R., Wouters D.B. The Meniscus Arrow® as a fixation device for the treatment of mallet fractures: results of 50 cases[J] Hand (N Y) 2014;9(4):499–503. doi: 10.1007/s11552-014-9619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Plaass C., Ettinger S., Sonnow L., et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies[J] J. Orthop. Res. 2016;34(12):2207–2214. doi: 10.1002/jor.23241. [DOI] [PubMed] [Google Scholar]
- 176.Plaass C., Von Falck C., Ettinger S., et al. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies - 3 year results of a randomized clinical trial[J] J. Orthop. Sci. 2018;23(2):321–327. doi: 10.1016/j.jos.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 177.Acar B., Kose O., Turan A., et al. Comparison of bioabsorbable magnesium versus titanium screw fixation for modified distal chevron osteotomy in hallux valgus[J] Biomed. Res. Int. 2018;2018 doi: 10.1155/2018/5242806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klauser H. Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws[J] Foot Ankle Surg. 2019;25(3):398–405. doi: 10.1016/j.fas.2018.02.005. [DOI] [PubMed] [Google Scholar]